Greater Biomass Production Under Elevated CO2 Is Attributed to Physiological Optimality, Trade-Offs in Nutrient Allocation, and Oxidative Defense in Drought-Stressed Mulberry
Abstract
1. Introduction
2. Materials and Methods
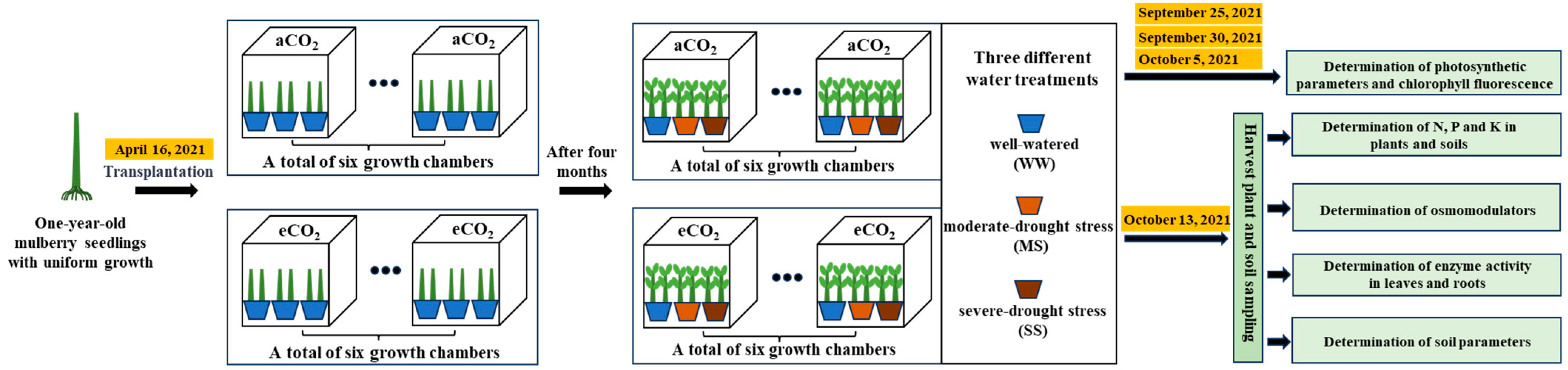
2.1. Growth Chamber Experiments
2.2. Determination of Photosynthetic Parameters
2.3. Determination of Chlorophyll Fluorescence
2.4. Plant and Soil Sampling
2.5. Determination of N, P, and K in Plants and Soils
2.6. Determination of Osmomodulators
2.7. Determination of Enzyme Activity in Leaves and Roots
2.8. Determination of Soil Enzyme Activity
2.9. Statistical Analysis
3. Results
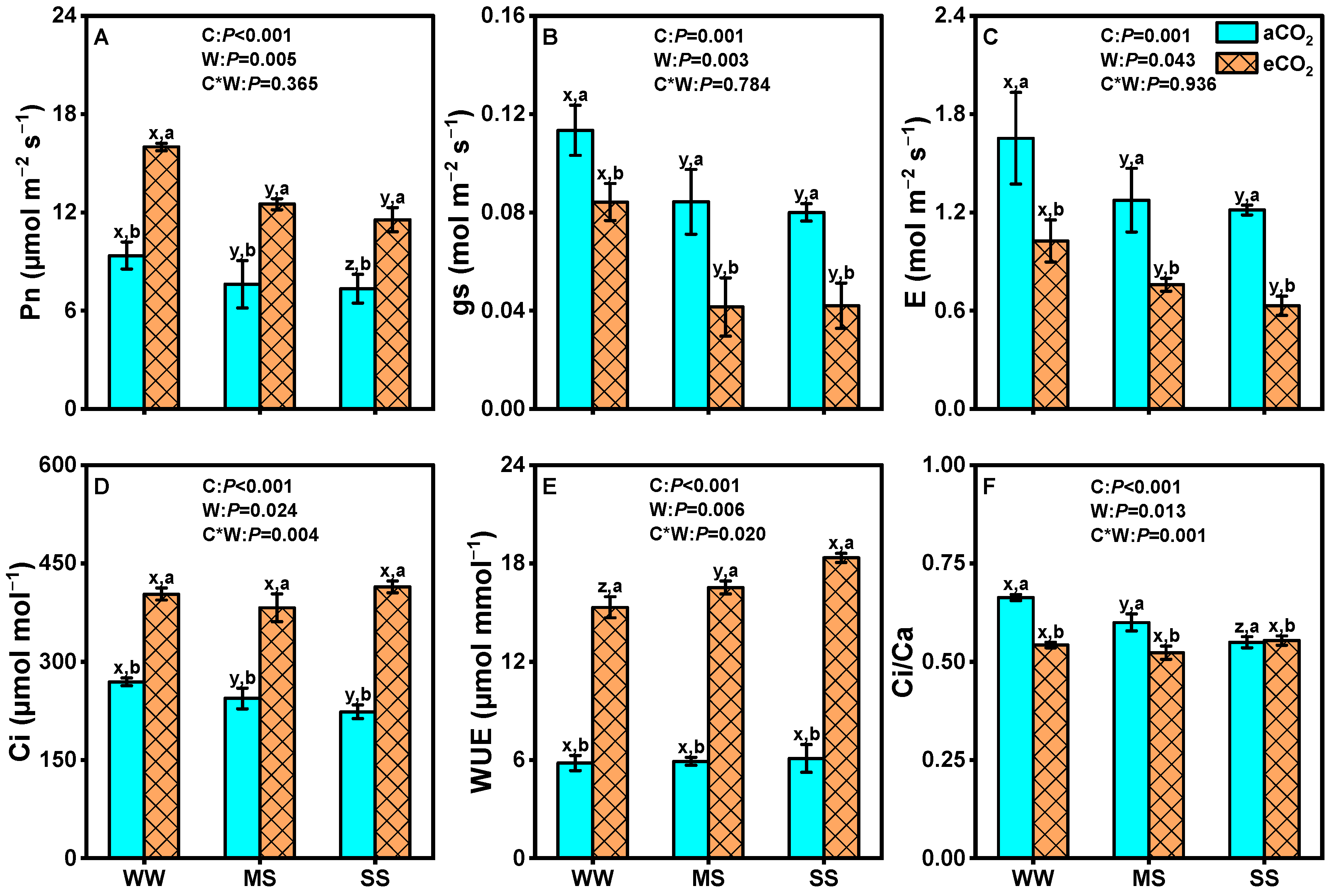
3.1. Effects of eCO2 and Drought on Photosynthetic Parameters of Leaves of Mulberry Plants
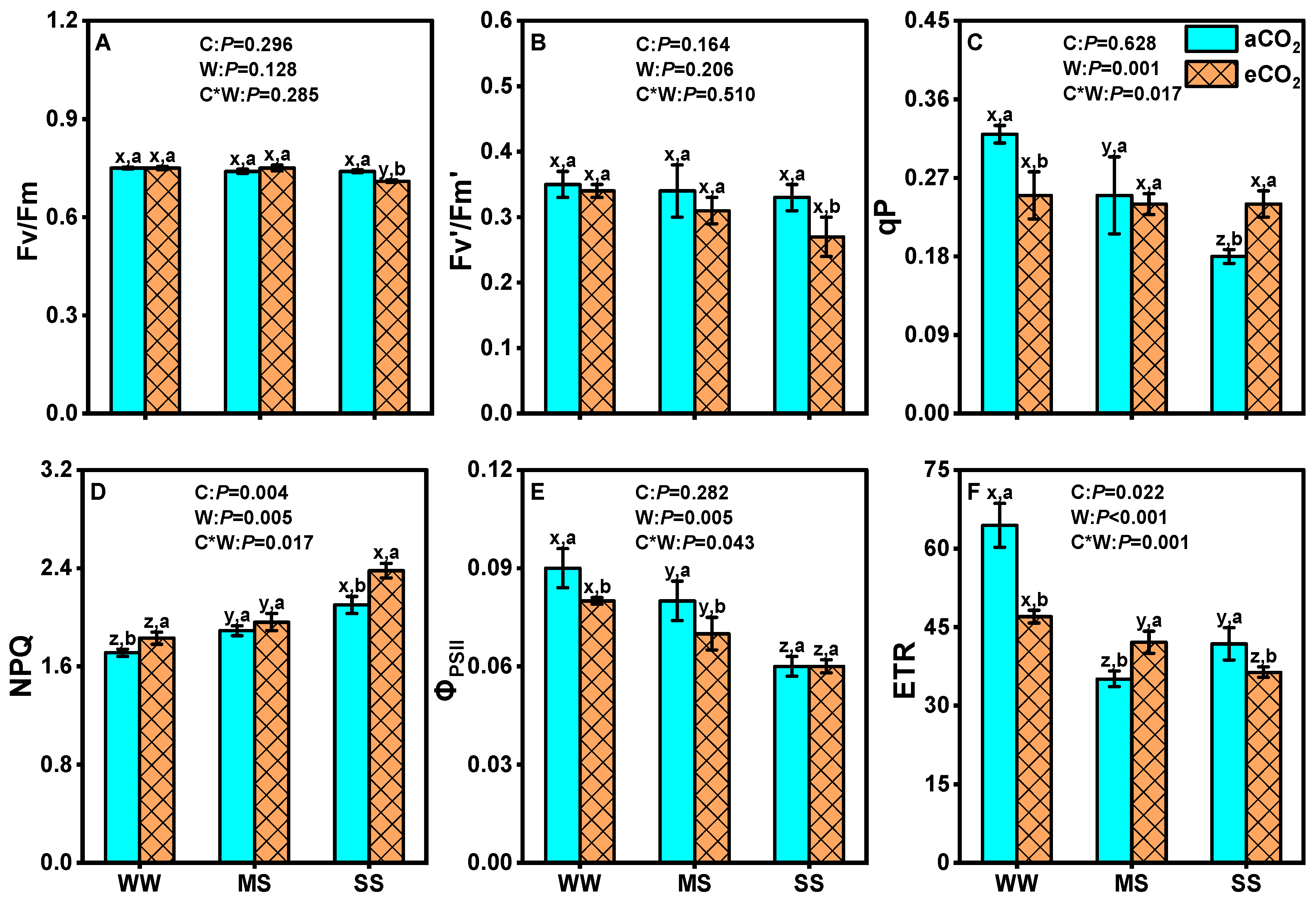
3.2. Effects of eCO2 and Drought on Leaf Chlorophyll Fluorescence of Mulberry Plants
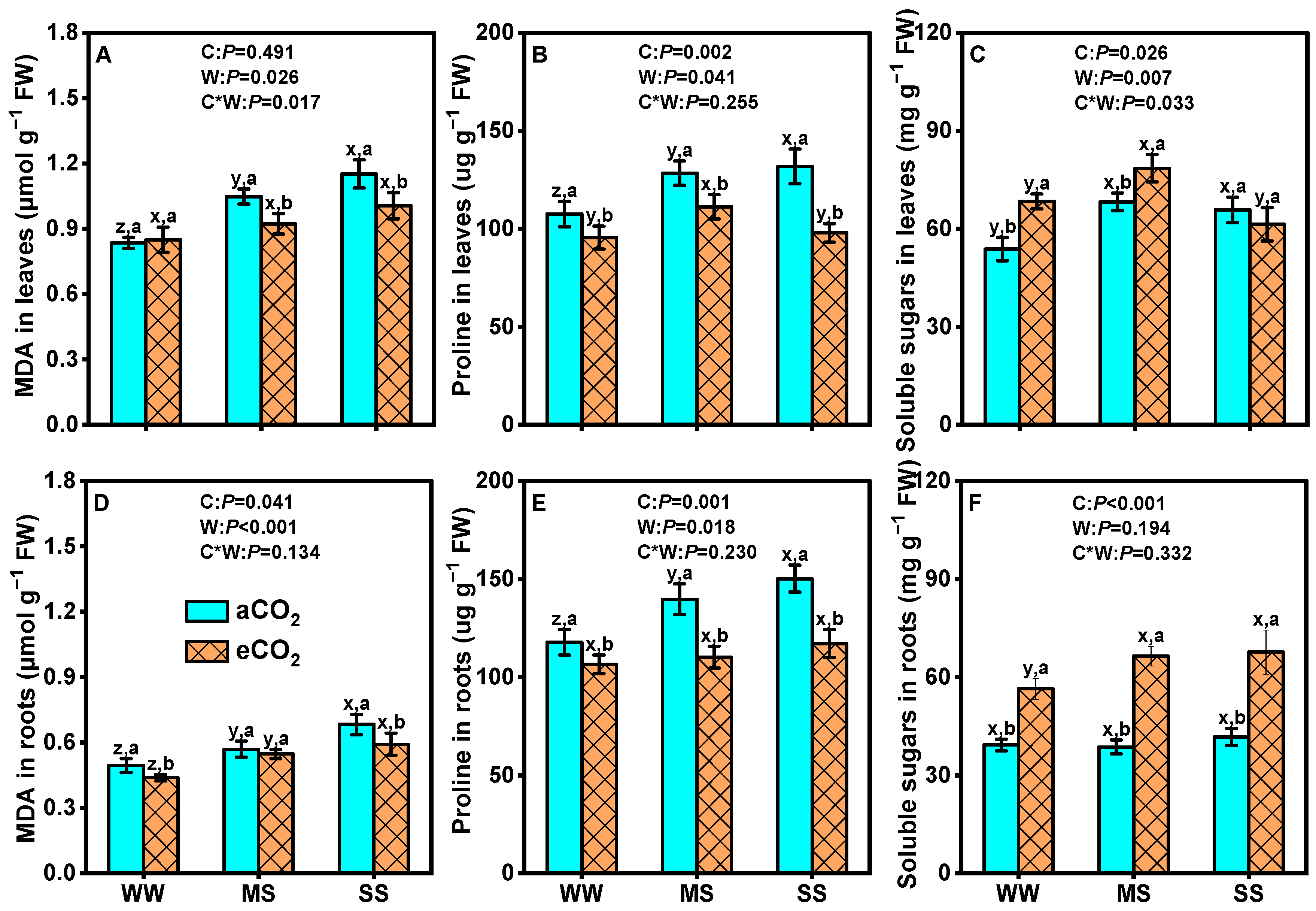
3.3. Effects of eCO2 and Drought on Osmotic Regulators of Mulberry Plants
3.4. Effects of eCO2 and Drought on Antioxidant Enzyme Activities in Mulberry Plants
3.5. Effects of eCO2 and Drought on Tissue N, P, and K in Mulberry Plants
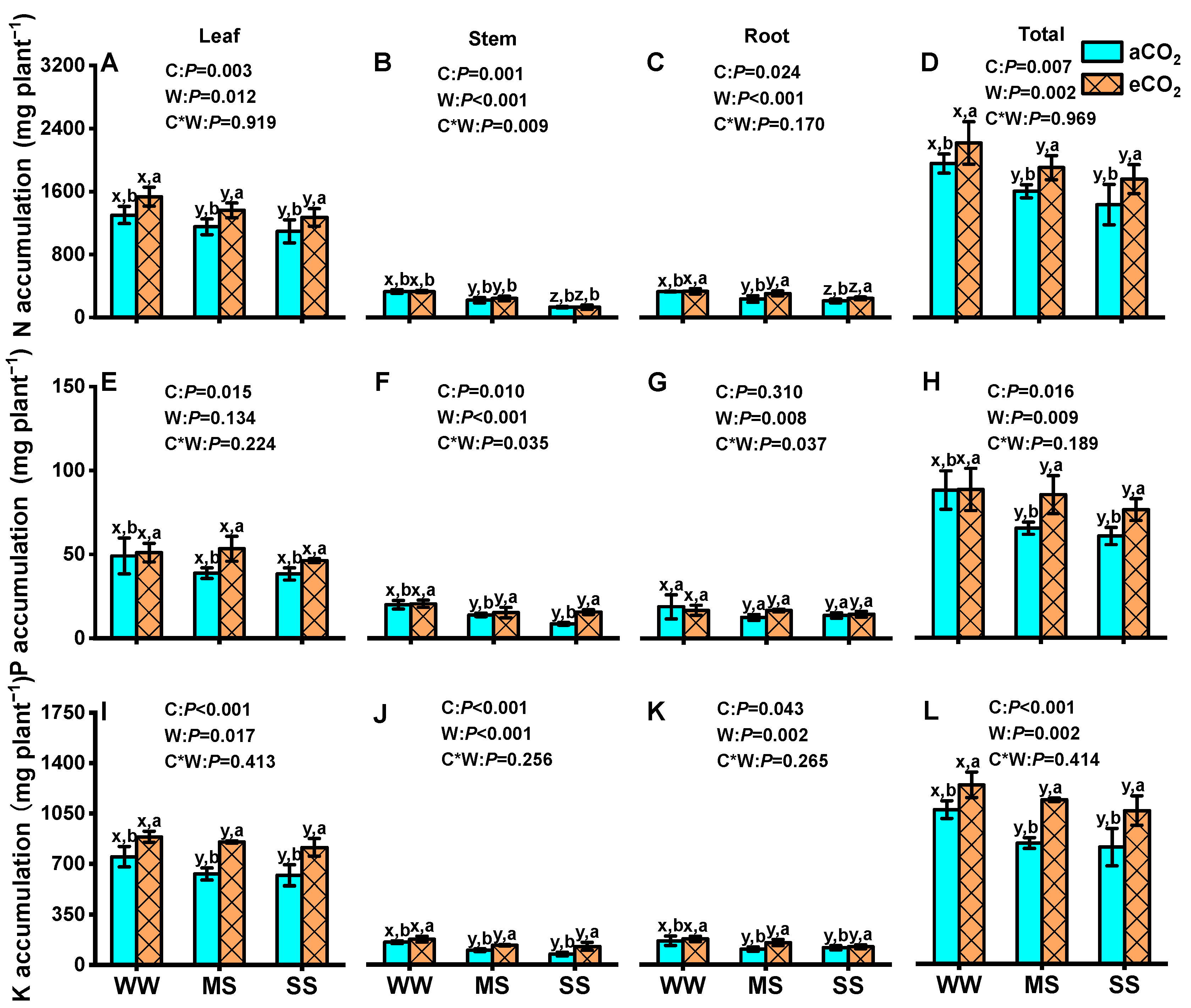
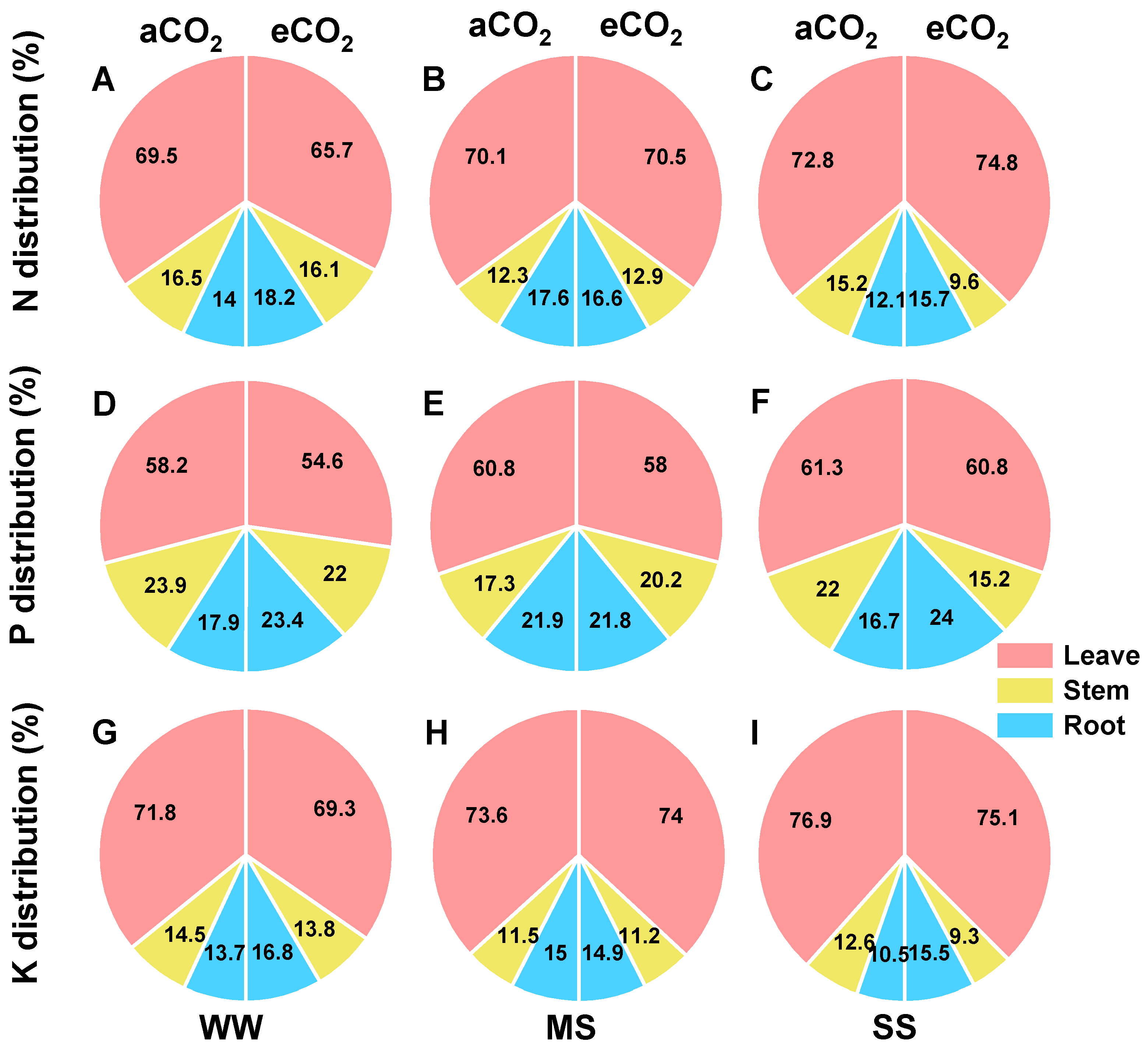
3.6. Effects of eCO2 and Drought on Tissue Accumulations and Allocation of N, P, and K in Mulberry Plants
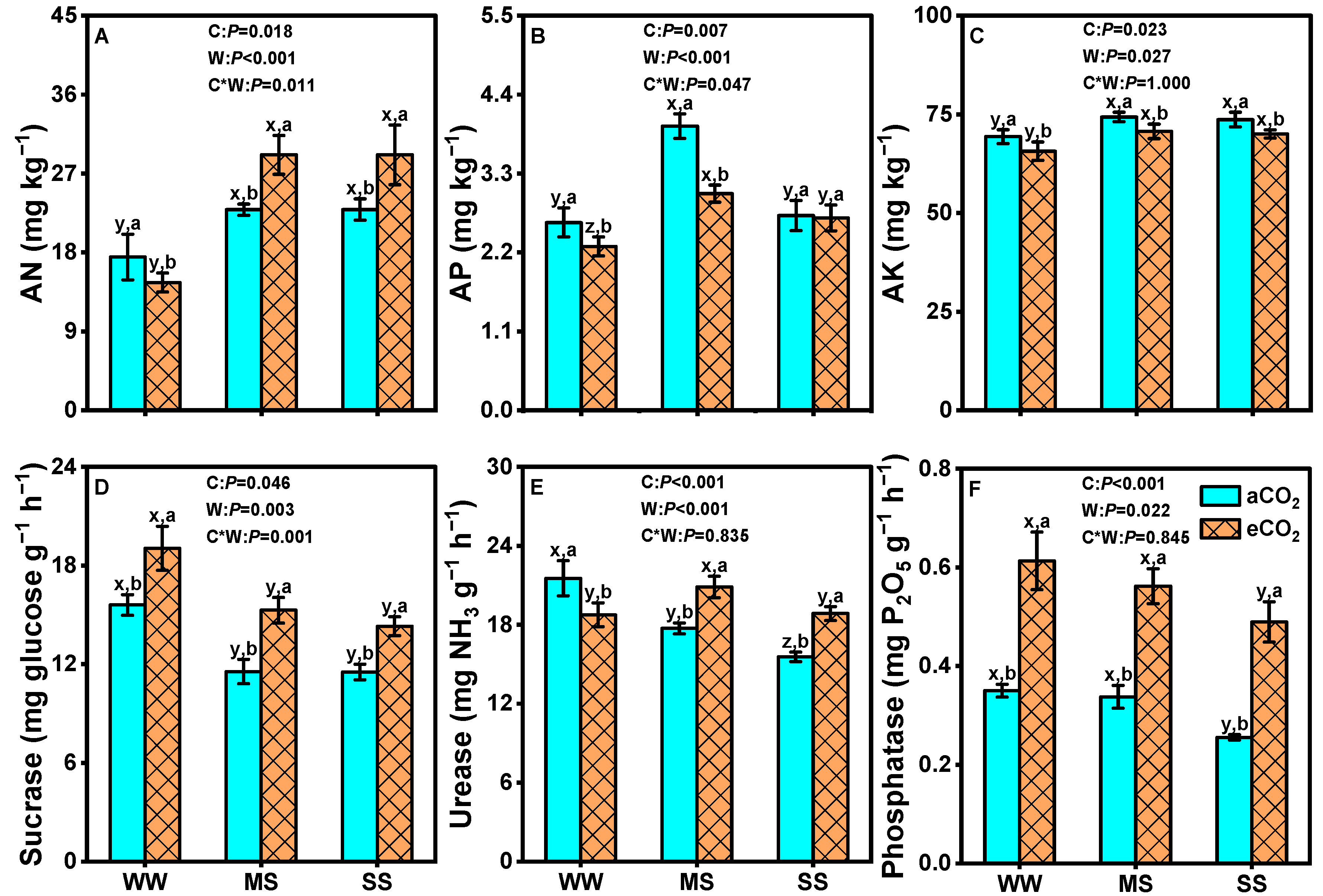
3.7. Effects of eCO2 and Drought on Soil Nutrients and Soil Enzyme Activities
3.8. Effects of eCO2 and Drought on Biomass Production in Mulberry Plants
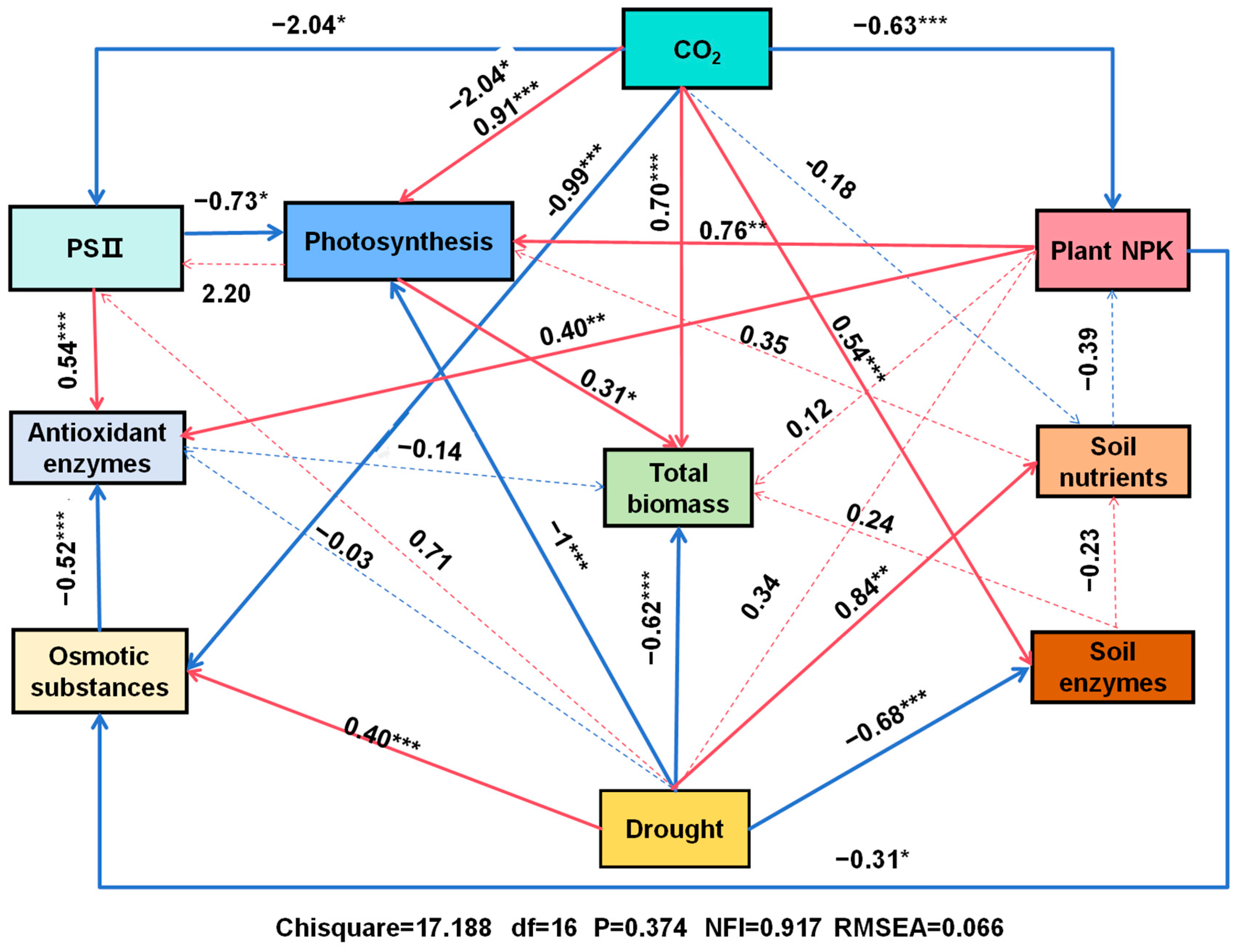
3.9. Multivariate Analyses
4. Discussion
4.1. eCO2 Alleviates Adverse Effects of Drought on Plant Biomass Production and Photosynthesis
4.2. eCO2 Mitigated the Negative Effects of Drought by Improving Water Use Efficiency
4.3. eCO2 Mitigated the Negative Effects of Drought by Improving Photosystem II Efficiency
4.4. eCO2 Mitigated the Negative Effects of Drought by Boosting Mulberry’s Defenses
4.5. eCO2 Mitigated the Negative Effects of Drought by Regulating NPK Partitioning
4.6. eCO2 Mitigated the Negative Effects of Drought by Improving Nutrient Uptake and Stimulating Soil Enzyme Activities
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| aCO2 | Ambient CO2 |
| eCO2 | Elevated CO2 |
| Ci | Intercellular CO2 concentration |
| N | Nitrogen |
| P | Phosphorus |
| K | Potassium |
| WW | Well-watered |
| MS | Moderate drought stress |
| SS | Severe drought stress |
| WUE | Water use efficiency |
| ROS | Reactive oxygen species |
| CAT | Catalase |
| POD | Peroxidase |
| SOD | Superoxide dismutase |
| MAD | Malondialdehyde |
| TAB | Thiobarbituric acid |
| NBT | Nitrogen blue tetrazolium |
| EDTA-Na2 | Ethylenediaminetetraacetic acid disodium salt |
| KMnO4 | Potassium permanganate |
| SOC | Soil organic carbon |
| AN | Soil available nitrogen |
| AP | Soil available phosphorus |
| AK | Soil available potassium |
| F0 | Initial fluorescence yield under the dark-adapted stage |
| Fm | Maximum fluorescence yield under the dark-adapted stage |
| F0′ | Minimum fluorescence yield under the light-adapted stage |
| Fm′ | Maximum fluorescence under the light-adapted stage |
| ΦPSII | Effective quantum yield of PSII photochemistry |
| Fv/Fm | Maximal quantum yield of PSII photochemistry |
| Fv′/Fm′ | Actual photosynthetic efficiency of PSII under illumination |
| qP | Photochemical quenching coefficient |
| NPQ | Non-photochemical quenching coefficient |
| ETR | PSII electron transport rate |
| SEM | Structural equation modeling |
References
- IPCC. Summary for Policymakers-Global Warming of 1.5 °C. An IPCC Special Peport; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2018; pp. 3–24. [Google Scholar] [CrossRef]
- Schewe, J.; Heinke, J.; Gerten, D.; Haddeland, I.; Arnell, N.W.; Clark, D.B.; Dankers, R.; Eisner, S.; Fekete, B.M.; Colón-González, F.J.; et al. Multimodel assessment of water scarcity under climate change. Proc. Natl. Acad. Sci. USA 2014, 111, 3245–3250. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhou, T.; Wu, P. Anthropogenic amplification of precipitation variability over the past century. Science 2024, 385, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Cohen, I.; Zandalinas, S.I.; Huck, C.; Fritschi, F.B.; Mittler, R. Meta-analysis of drought and heat stress combination impact on crop yield and yield components. Physiol. Plant. 2020, 171, 66–76. [Google Scholar] [CrossRef]
- Patel, J.; Mishra, A. Plant aquaporins alleviate drought tolerance in plants by modulating cellular biochemistry, root-architecture, and photosynthesis. Physiol. Plant. 2021, 172, 1030–1044. [Google Scholar] [CrossRef]
- Liu, Q.; Peng, C.; Schneider, R.; Cyr, D.; McDowell, N.G.; Kneeshaw, D. Drought-induced increase in tree mortality and corresponding decrease in the carbon sink capacity of Canada’s boreal forests from 1970 to 2020. Glob. Change Biol. 2023, 29, 2274–2285. [Google Scholar] [CrossRef] [PubMed]
- Birami, B.; Nägele, T.; Gattmann, M.; Preisler, Y.; Gast, A.; Arneth, A.; Ruehr, N.K. Hot drought reduces the effects of elevated CO2 on tree water-use efficiency and carbon metabolism. New Phytol. 2020, 226, 1607–1621. [Google Scholar] [CrossRef]
- Zhang, D.; Li, A.; Lam, S.K.; Li, P.; Zong, Y.; Gao, Z.; Hao, X. Increased carbon uptake under elevated CO2 concentration enhances water-use efficiency of C4 broomcorn millet under drought. Agric. Water Manag. 2021, 245, 106631. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, C.; Liu, S. Elevated CO2 alleviates adverse effects of drought on plant water relations and photosynthesis: A global meta-analysis. J. Ecol. 2022, 110, 2836–2849. [Google Scholar] [CrossRef]
- Zhai, Z.; Fang, Y.; Cheng, J.; Tian, Y.; Liu, L.; Cao, X. Intrinsic morphology and spatial distribution of non-structural carbohydrates contribute to drought resistance of two mulberry cultivars. Plant Biol. 2023, 25, 771–784. [Google Scholar] [CrossRef]
- Roy, S.; Kapoor, R.; Mathur, P. Revisiting changes in growth, physiology and stress responses of plants under the effect of enhanced CO2 and temperature. Plant Cell Physiol. 2023, 65, 4–19. [Google Scholar] [CrossRef]
- Zhang, X.; Duan, Y.; Xing, Q.; Duan, R.; Shen, J.; Zong, Y.; Zhang, D.; Shi, X.; Li, P.; Hao, X. Elevated CO2 concentration enhances drought tolerance by mitigating oxidative stress and enhancing carbon assimilation in foxtail millet (Setaria italica). J. Agron. Crop. Sci. 2024, 210, e12778. [Google Scholar] [CrossRef]
- Tankari, M.; Wang, C.; Ma, H.; Li, X.; Li, L.; Soothar, R.K.; Cui, N.; Zaman-Allah, M.; Hao, W.; Liu, F.; et al. Drought priming improved water status, photosynthesis and water productivity of cowpea during post-anthesis drought stress. Agric. Water. Manag. 2021, 245, 106565. [Google Scholar] [CrossRef]
- Martin-StPaul, N.; Delzon, S.; Cochard, H. Plant resistance to drought depends on timely stomatal closure. Ecol. Lett. 2017, 20, 1437–1447. [Google Scholar] [CrossRef]
- Ding, L.; Lu, Z.; Gao, L.; Guo, S.; Shen, Q. Is Nitrogen a key determinant of water transport and photosynthesis in higher plants upon drought stress? Front. Plant Sci. 2018, 9, 1143. [Google Scholar] [CrossRef]
- Bota, J.; Medrano, H.; Flexas, J. Is photosynthesis limited by decreased Rubisco activity and RuBP content under progressive water stress? New Phytol. 2004, 162, 671–681. [Google Scholar] [CrossRef]
- Filek, M.; Łabanowska, M.; Kościelniak, J.; Biesaga-Kościelniak, J.; Kurdziel, M.; Szarejko, I.; Hartikainen, H. Characterization of barley leaf tolerance to drought stress by chlorophyll fluorescence and electron paramagnetic resonance studies. J. Agron. Crop. Sci. 2014, 201, 228–240. [Google Scholar] [CrossRef]
- Yuan, H.; Wang, Q.; Qi, A.; Li, S.; Hu, Y.; Hu, Z.; Guo, L.; Liang, C.; Li, W.; Liu, C.; et al. Morphological, physiological, and photosynthetic differences of Tartary buckwheat induced by post-anthesis drought. Plants 2024, 13, 2161. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Feng, Y.; Zong, Y.; Zhang, D.; Hao, X.; Li, P. Elevated CO2-induced changes in photosynthesis, antioxidant enzymes and signal transduction enzyme of soybean under drought stress. Plant Physiol. Biochem. 2020, 154, 105–114. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, Q.; Yong, X.; Wu, M.; Jiang, B.; Jia, Y.; Ma, J.; Mou, L.; Tang, S.; Pan, Y. Effects of water deficit on two cultivars of Hibiscus mutabilis: A comprehensive study on morphological, physiological, and metabolic responses. Plant Physiol. Bioc. 2024, 217, 109269. [Google Scholar] [CrossRef]
- Avila, R.T.; de Almeida, W.L.; Costa, L.C.; Machado, K.L.; Barbosa, M.L.; de Souza, R.P.; Martino, P.B.; Juárez, M.A.; Marçal, D.M.; Martins, S.C.; et al. Elevated air [CO2] improves photosynthetic performance and alters biomass accumulation and partitioning in drought-stressed coffee plants. Environ. Exp. Bot. 2020, 177, 104137. [Google Scholar] [CrossRef]
- Haverd, V.; Smith, B.; Canadell, J.G.; Cuntz, M.; Mikaloff-Fletcher, S.; Farquhar, G.; Woodgate, W.; Briggs, P.R.; Trudinger, C.M. Higher than expected CO2 fertilization inferred from leaf to global observations. Glob. Change Biol. 2020, 26, 2390–2402. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Long, S.P. 30 years of free-air carbon dioxide enrichment (FACE): What have we learned about future crop productivity and its potential for adaptation? Glob. Change Biol. 2020, 27, 27–49. [Google Scholar] [CrossRef]
- Song, L.; Tao, Y.; van Groenigen, K.J.; Chang, S.X.; Peñuelas, J.; Zhang, J.; You, L.; Cai, C.; Wang, S.; Jiang, Y.; et al. Rising atmospheric carbon dioxide concentrations increase gaps of rice yields between low- and middle-to-high-income countries. Nat. Food 2024, 5, 754–763. [Google Scholar] [CrossRef]
- Busch, F.A.; Sage, R.F. The sensitivity of photosynthesis to O2 and CO2 concentration identifies strong Rubisco control above the thermal optimum. New Phytol. 2016, 213, 1036–1051. [Google Scholar] [CrossRef] [PubMed]
- Ancín, M.; Gámez, A.L.; Jauregui, I.; Galmes, J.; E Sharwood, R.; Erice, G.; A Ainsworth, E.; Tissue, D.T.; Sanz-Sáez, A.; Aranjuelo, I. Does the response of Rubisco and photosynthesis to elevated [CO2] change with unfavourable environmental conditions? J. Exp. Bot. 2024, 75, 7351–7364. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Cai, C.; He, J.; Gu, J.; Zhu, G.; Zhang, W.; Zhu, J.; Liu, G. Yield, dry matter distribution and photosynthetic characteristics of rice under elevated CO2 and increased temperature conditions. Field Crop. Res. 2020, 248, 107605. [Google Scholar] [CrossRef]
- Robredo, A.; Pérez-López, U.; de la Maza, H.S.; González-Moro, B.; Lacuesta, M.; Mena-Petite, A.; Muñoz-Rueda, A. Elevated CO2 alleviates the impact of drought on barley improving water status by lowering stomatal conductance and delaying its effects on photosynthesis. Environ. Exp. Bot. 2007, 59, 252–263. [Google Scholar] [CrossRef]
- Pazzagli, P.T.; Weiner, J.; Liu, F. Effects of CO2 elevation and irrigation regimes on leaf gas exchange, plant water relations, and water use efficiency of two tomato cultivars. Agric. Water Manag. 2016, 169, 26–33. [Google Scholar] [CrossRef]
- Avila, R.T.; Cardoso, A.A.; de Almeida, W.L.; Costa, L.C.; Machado, K.L.; Barbosa, M.L.; de Souza, R.P.; Oliveira, L.A.; Batista, D.S.; Martins, S.C.; et al. Coffee plants respond to drought and elevated [CO2] through changes in stomatal function, plant hydraulic conductance, and aquaporin expression. Environ. Exp. Bot. 2020, 177, 104148. [Google Scholar] [CrossRef]
- Li, S.; Li, X.; Wei, Z.; Liu, F. ABA-mediated modulation of elevated CO2 on stomatal response to drought. Curr. Opin. Plant Biol. 2020, 56, 174–180. [Google Scholar] [CrossRef]
- Baker, J.T.; Allen, L.H. Rice growth, yield and photosynthetic responses to elevated atmospheric carbon dioxide concentration and drought. J. Crop. Improv. 2005, 13, 7–30. [Google Scholar] [CrossRef]
- Li, A.; Lv, D.; Zhang, Y.; Zhang, D.; Zong, Y.; Shi, X.; Li, P.; Hao, X. Elevated CO2 concentration enhances drought resistance of soybean by regulating cell structure, cuticular wax synthesis, photosynthesis, and oxidative stress response. Plant Physiol. Biochem. 2023, 206, 108266. [Google Scholar] [CrossRef]
- Ulfat, A.; Shokat, S.; Li, X.; Fang, L.; Großkinsky, D.K.; Majid, S.A.; Roitsch, T.; Liu, F. Elevated carbon dioxide alleviates the negative impact of drought on wheat by modulating plant metabolism and physiology. Agric. Water Manag. 2021, 250, 106804. [Google Scholar] [CrossRef]
- Liu, W.; Liu, L.; Yan, R.; Gao, J.; Wu, S.; Liu, Y. A comprehensive meta-analysis of the impacts of intensified drought and elevated CO2 on forage growth. J. Environ. Manag. 2022, 327, 116885. [Google Scholar] [CrossRef] [PubMed]
- Miranda-Apodaca, J.; Pérez-López, U.; Lacuesta, M.; Mena-Petite, A.; Muñoz-Rueda, A. The interaction between drought and elevated CO 2 in water relations in two grassland species is species-specific. J. Plant Physiol. 2018, 220, 193–202. [Google Scholar] [CrossRef]
- Wei, Z.; Abdelhakim, L.O.A.; Fang, L.; Peng, X.; Liu, J.; Liu, F. Elevated CO2 effect on the response of stomatal control and water use efficiency in amaranth and maize plants to progressive drought stress. Agric. Water Manag. 2022, 266, 107609. [Google Scholar] [CrossRef]
- Fan, X.D.; Cao, X.; Zhou, H.R.; Hao, L.H.; Dong, W.; He, C.L.; Xu, M.; Wu, H.X.; Wang, L.S.; Chang, Z.J. Carbon dioxide fertilization effect on plant growth under soil water stress associates with changes in stomatal traits, leaf photosynthesis, and foliar nitrogen of bell pepper (Capsicum annuum L.). Environ. Exp. Bot. 2020, 179, 104203. [Google Scholar] [CrossRef]
- Wu, D.-X.; Wang, G.-X.; Bai, Y.-F.; Liao, J.-X. Effects of elevated CO2 concentration on growth, water use, yield and grain quality of wheat under two soil water levels. Agric. Ecosyst. Environ. 2004, 104, 493–507. [Google Scholar] [CrossRef]
- Ottman, M.J.; Kimball, B.A.; Pinter, P.J.; Wall, G.W.; Vanderlip, R.L.; Leavitt, S.W.; Lamorte, R.L.; Matthias, A.D.; Brooks, T.J. Elevated CO2 increases sorghum biomass under drought conditions. New Phytol. 2001, 150, 261–273. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Long, S.P. What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytol. 2005, 165, 351–372. [Google Scholar] [CrossRef]
- Leakey, A.D.B.; Ainsworth, E.A.; Bernacchi, C.J.; Rogers, A.; Long, S.P.; Ort, D.R. Elevated CO2 effects on plant carbon, nitrogen, and water relations: Six important lessons from FACE. J. Exp. Bot. 2009, 60, 2859–2876. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Dijkstra, F.A. Drought effect on plant nitrogen and phosphorus: A meta-analysis. New Phytol. 2014, 204, 924–931. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Guo, J.; Shu, M.; Wang, P.; Hu, S. Impacts of drought and nitrogen enrichment on leaf nutrient resorption and root nutrient allocation in four Tibetan plant species. Sci. Total. Environ. 2020, 723, 138106. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Min, X.; Liu, G.; Li, P.; Xue, S. Effect of plant–plant interactions and drought stress on the response of soil nutrient contents, enzyme activities and microbial metabolic limitations. Appl. Soil Ecol. 2022, 181, 104666. [Google Scholar] [CrossRef]
- Shi, S.; Luo, X.; Wen, M.; Dong, X.; Sharifi, S.; Xie, D.; He, X. Funneliformis mosseae improve growth and nutrient accumulation in wheat by facilitating soil nutrient uptake under elevated CO2 at daytime, not nighttime. J. Fungi 2021, 7, 458. [Google Scholar] [CrossRef]
- Singh, A.K.; Rai, A.; Kushwaha, M.; Chauhan, P.S.; Pandey, V.; Singh, N. Tree growth rate regulate the influence of elevated CO2 on soil biochemical responses under tropical condition. J. Environ. Manag. 2019, 231, 1211–1221. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Horwath, W.R.; Dorodnikov, M.; Blagodatskaya, E. Review and synthesis of the effects of elevated atmospheric CO2 on soil processes: No changes in pools, but increased fluxes and accelerated cycles. Soil Biol. Biochem. 2019, 128, 66–78. [Google Scholar] [CrossRef]
- Kumar, U.; Nayak, A.; Sahoo, S.; Kumar, A.; Kaviraj, M.; Shahid, M. Combined effects of elevated CO2, N fertilizer and water deficit stress on diazotrophic community in sub-humid tropical paddy soiltropical paddy soil. Appl. Soil Ecol. 2020, 155, 103682. [Google Scholar] [CrossRef]
- Guenet, B.; Lenhart, K.; Leloup, J.; Giusti-Miller, S.; Pouteau, V.; Mora, P.; Nunan, N.; Abbadie, L. The impact of long-term CO2 enrichment and moisture levels on soil microbial community structure and enzyme activities. Geoderma 2012, 170, 331–336. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Chen, Y.; Chen, H.; Li, X.; Ding, Z.; Han, X.; Tang, X. Spatial and temporal characteristics of drought events in southwest China over the past 120 years. Remote. Sens. 2023, 15, 3008. [Google Scholar] [CrossRef]
- Reddy, K.S.; Sekhar, K.M.; Sreeharsha, R.V.; Reddy, A.R. Hydraulic dynamics and photosynthetic performance facilitate rapid screening of field grown mulberry (Morus spp.) genotypes for drought tolerance. Environ. Exp. Bot. 2019, 157, 320–330. [Google Scholar] [CrossRef]
- Kaushal, R.; Kumar, A.; Mandal, D.; Tomar, J.; Jinger, D.; Islam, S.; Panwar, P.; Jayaprakash, J.; Uthappa, A.; Singhal, V.; et al. Mulberry based agroforestry system and canopy management practices to combat soil erosion and enhancing carbon sequestration in degraded lands of Himalayan foothills. Environ. Sustain. Indic. 2024, 24, 100467. [Google Scholar] [CrossRef]
- Manzoor, S.; Qayoom, K. Environmental importance of mulberry: A review. J. Exp. Agric. Int. 2024, 46, 95–105. [Google Scholar] [CrossRef]
- Sarkar, T.; Doss, S.G.; Sivaprasad, V.; Teotia, R.S. Stress Tolerant Traits in Mulberry (Morus spp.) Resilient to Climate Change, Mulberry: Genetic Improvement in Context of Climate Change; CRC Press: Boca Raton, FL, USA, 2021; pp. 165–179. [Google Scholar] [CrossRef]
- Sasmita, N.; Purba, J.H.; Yuniti, I.G.A.D. Adaptation of Morus alba and Morus cathayana plants in a different climate and environment conditions in Indonesia. Biodiversitas J. Biol. Divers. 2019, 20, 544–554. [Google Scholar] [CrossRef]
- Ghosh, A.; Gangopadhyay, D.; Chowdhury, T. Economical and environmental importance of mulberry: A review. Int. J. Plant Environ. 2017, 3, 67–72. [Google Scholar] [CrossRef]
- Bajwa, G.A.; Umair, M.; Nawab, Y.; Rizwan, Z. Morphometry of leaf and shoot variables to assess aboveground biomass structure and carbon sequestration by different varieties of white mulberry (Morus alba L.). J. For. Res. 2021, 32, 2291–2300. [Google Scholar] [CrossRef]
- Durgadevi, R.; Vijayalakshmi, D. Mulberry with increased stomatal frequency regulates gas exchange traits for improved drought tolerance. Plant Physiol. Rep. 2020, 25, 24–32. [Google Scholar] [CrossRef]
- Sun, Z.; Yin, Y.; Zhu, W.; Zhou, Y. Morphological, physiological, and biochemical composition of mulberry (Morus spp.) under drought stress. Forests 2023, 14, 949. [Google Scholar] [CrossRef]
- Shi, S.; Qiu, Y.; Wen, M.; Xu, X.; Dong, X.; Xu, C.; He, X. Daytime, not nighttime, elevated atmospheric carbon dioxide exposure improves plant growth and leaf quality of mulberry (Morus alba L.) seedlings. Front. Plant Sci. 2021, 11, 609031. [Google Scholar] [CrossRef]
- Shi, S.; Xu, X.; Dong, X.; Xu, C.; Qiu, Y.; He, X. Photosynthetic acclimation and growth responses to elevated CO2 associate with leaf nitrogen and phosphorus concentrations in mulberry (Morus multicaulis Perr.). Forests 2021, 12, 660. [Google Scholar] [CrossRef]
- Sekhar, K.M.; Rachapudi, V.S.; Mudalkar, S.; Reddy, A.R. Persistent stimulation of photosynthesis in short rotation coppice mulberry under elevated CO2 atmosphere. J. Photochem. Photobiol. B Biol. 2014, 137, 21–30. [Google Scholar] [CrossRef]
- Sekhar, K.M.; Sreeharsha, R.V.; Reddy, A.R. Differential responses in photosynthesis, growth and biomass yields in two mulberry genotypes grown under elevated CO 2 atmosphere. J. Photochem. Photobiol. B Biol. 2015, 151, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, H.; Wang, J.; Wu, X.; Ma, S.; Xu, Z.; Zhou, T.; Xu, N.; Tang, X.; An, B. Increased CO2 concentrations increasing water use efficiency and improvement PSII function of mulberry seedling leaves under drought stress. J. Plant Interact. 2019, 14, 213–223. [Google Scholar] [CrossRef]
- Kramer, D.M.; Johnson, G.; Kiirats, O.; Edwards, G.E. New fluorescence parameters for the determination of QA redox state and excitation energy fluxes. Photosynth. Res. 2004, 79, 209–218. [Google Scholar] [CrossRef]
- Yang, J.; Wang, C.; Dai, H. Soil Agrochemical Analysis and Environmental Monitoring Techniques; Chinese Dadi Press: Beijing, China, 2008; pp. 18–64. [Google Scholar]
- Li, H.S. The Experiment Principle and Technique on Plant Physiology and Biochemistry; Higher Education Press: Beijing, China, 2000; pp. 78–102. [Google Scholar]
- Guan, S.Y.; Zhang, D.; Zhang, Z. Soil Enzyme and Its Research Methods; Agricultural Press: Beijing, China, 1986; pp. 274–340. [Google Scholar]
- Huang, X.; Liu, Y.; Li, J.; Xiong, X.; Chen, Y.; Yin, X.; Feng, D. The response of mulberry trees after seedling hardening to summer drought in the hydro-fluctuation belt of Three Gorges Reservoir Areas. Environ. Sci. Pollut. Res. 2012, 20, 7103–7111. [Google Scholar] [CrossRef]
- Deng, J.; Zhang, Y.; Yin, Y.; Zhu, X.; Zhu, W.; Zhou, Y. Comparison of soil bacterial community and functional characteristics following afforestation in the semi-arid areas. PeerJ 2019, 7, e7141. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.S.; Sekhar, K.M.; Reddy, A.R. Genotypic variation in tolerance to drought stress is highly coordinated with hydraulic conductivity–photosynthesis interplay and aquaporin expression in field-grown mulberry (Morus spp.). Tree Physiol. 2017, 37, 926–937. [Google Scholar] [CrossRef]
- Lauriks, F.; Salomón, R.L.; De Roo, L.; Sobrino-Plata, J.; Rodríguez-García, A.; Steppe, K. Limited mitigating effects of elevated CO2 in young aspen trees to face drought stress. Environ. Exp. Bot. 2022, 201, 104942. [Google Scholar] [CrossRef]
- Ofori-Amanfo, K.K.; Klem, K.; Veselá, B.; Holub, P.; Agyei, T.; Juráň, S.; Grace, J.; Marek, M.V.; Urban, O. The effect of elevated CO2 on photosynthesis is modulated by nitrogen supply and reduced water availability in Picea abies. Tree Physiol. 2023, 43, 925–937. [Google Scholar] [CrossRef]
- Ayub, G.; Zaragoza-Castells, J.; Griffin, K.L.; Atkin, O.K. Leaf respiration in darkness and in the light under pre-industrial, current and elevated atmospheric CO2 concentrations. Plant Sci. 2014, 226, 120–130. [Google Scholar] [CrossRef]
- Kumar, A.; Nayak, A.; Das, B.; Panigrahi, N.; Dasgupta, P.; Mohanty, S.; Kumar, U.; Panneerselvam, P.; Pathak, H. Effects of water deficit stress on agronomic and physiological responses of rice and greenhouse gas emission from rice soil under elevated atmospheric CO2. Sci. Total Environ. 2018, 650, 2032–2050. [Google Scholar] [CrossRef] [PubMed]
- Liberloo, M.; Tulva, I.; Raïm, O.; Kull, O.; Ceulemans, R. Photosynthetic stimulation under long-term CO2 enrichment and fertilization is sustained across a closed Populus canopy profile (EUROFACE). New Phytol. 2006, 173, 537–549. [Google Scholar] [CrossRef]
- Kelly, J.W.G.; Duursma, R.A.; Atwell, B.J.; Tissue, D.T.; Medlyn, B.E. Drought × CO2 interactions in trees: A test of the low-intercellular CO2 concentration (Ci) mechanism. New Phytol. 2015, 209, 1600–1612. [Google Scholar] [CrossRef] [PubMed]
- Regier, N.; Streb, S.; Cocozza, C.; Schaub, M.; Cherubini, P.; Zeeman, S.C.; Frey, B. Drought tolerance of two black poplar (Populus nigra L.) clones: Contribution of carbohydrates and oxidative stress defence. Plant Cell Environ. 2009, 32, 1724–1736. [Google Scholar] [CrossRef]
- Semedo, J.N.; Rodrigues, A.P.; Lidon, F.C.; Pais, I.P.; Marques, I.; Gouveia, D.; Armengaud, J.; Silva, M.J.; Martins, S.; Semedo, M.C.; et al. Intrinsic non-stomatal resilience to drought of the photosynthetic apparatus in Coffea spp. is strengthened by elevated air [CO2]. Tree Physiol. 2020, 41, 708–727. [Google Scholar] [CrossRef]
- Halpern, M.; Bar-Tal, A.; Lugassi, N.; Egbaria, A.; Granot, D.; Yermiyahu, U. The role of nitrogen in photosynthetic acclimation to elevated [CO2] in tomatoes. Plant Soil 2018, 434, 397–411. [Google Scholar] [CrossRef]
- Sanches, R.F.E.; Centeno, D.d.C.; Braga, M.R.; da Silva, E.A. Impact of high atmospheric CO2 concentrations on the seasonality of water-related processes, gas exchange, and carbohydrate metabolism in coffee trees under field conditions. Clim. Change 2020, 162, 1231–1248. [Google Scholar] [CrossRef]
- Zheng, Y.; He, C.; Guo, L.; Hao, L.; Cheng, D.; Li, F.; Peng, Z.; Xu, M. Soil water status triggers CO2 fertilization effect on the growth of winter wheat (Triticum aestivum). Agric. For. Meteorol. 2020, 291, 108097. [Google Scholar] [CrossRef]
- Yousefvand, P.; Pilehvar, B.; Nasrolahi, A.H. Morphological, physiological, and biochemical responses of Pistacia atlantica seedlings to elevated CO2 concentration and drought stress. Eur. J. For. Res. 2023, 142, 657–670. [Google Scholar] [CrossRef]
- Woodward, F.I. Stomatal numbers are sensitive to increases in CO2 from pre-industrial levels. Nature 1987, 327, 617–618. [Google Scholar] [CrossRef]
- Morison, J. Stomatal response to increased CO2 concentration. J. Exp. Bot. 1998, 49, 443–452. [Google Scholar] [CrossRef]
- Correia, B.; Hancock, R.D.; Amaral, J.; Gomez-Cadenas, A.; Valledor, L.; Pinto, G. Combined drought and heat activates protective responses in eucalyptus globulus that are not activated when subjected to drought or heat stress alone. Front. Plant Sci. 2018, 9, 819. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Liu, B.; Wu, Y.; Zou, Z. Interactive effects of drought stresses and elevated CO2 concentration on photochemistry efficiency of cucumber seedlings. J. Integr. Plant Biol. 2008, 50, 1307–1317. [Google Scholar] [CrossRef] [PubMed]
- Sommer, S.G.; Han, E.; Li, X.; Rosenqvist, E.; Liu, F. The chlorophyll fluorescence parameter Fv/Fm correlates with loss of grain yield after severe drought in three wheat genotypes grown at two CO2 concentrations. Plants 2023, 12, 436. [Google Scholar] [CrossRef]
- Flexas, J.; Medrano, H. Drought-inhibition of photosynthesis in C3 plants: Stomatal and non-stomatal limitations revisited. Ann. Bot. 2002, 89, 183–189. [Google Scholar] [CrossRef]
- Chaves, M.M.; Pereira, J.S.; Marôco, J.; Rodrigues, M.L.; Ricardo, C.P.P.; Osório, M.L.; Carvalho, I.; Faria, T.; Pinheiro, C. How plants cope with water stress in the field? photosynthesis and growth. Ann. Bot. 2002, 89, 907–916. [Google Scholar] [CrossRef]
- Muller, P.; Li, X.-P.; Niyogi, K.K. Non-photochemical quenching. A response to excess light energy. Plant Physiol. 2001, 125, 1558–1566. [Google Scholar] [CrossRef]
- Murchie, E.H.; Niyogi, K.K. Manipulation of photoprotection to improve plant photosynthesis. Plant Physiol. 2011, 155, 86–92. [Google Scholar] [CrossRef]
- Long, S.P.; Ainsworth, E.A.; Leakey, A.D.B.; Nosberger, J.; Ort, D.R. Food for thought: Lower-than-expected crop yield stimulation with rising CO 2 concentrations. Science 2006, 312, 1918–1921. [Google Scholar] [CrossRef]
- Wujeska, A.; Bossinger, G.; Tausz, M. Responses of foliar antioxidative and photoprotective defence systems of trees to drought: A meta-analysis. Tree Physiol. 2013, 33, 1018–1029. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Al Mahmud, J.; Fujita, M.; Fotopoulos, V. Reactive oxygen species and antioxidant defense in plants under abiotic stress: Revisiting the crucial role of a universal defense regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, M.; Unal, B.T.; García-Caparrós, P.; Khursheed, A.; Gul, A.; Hasanuzzaman, M. Osmoregulation and its actions during the drought stress in plants. Physiol. Plant. 2020, 172, 1321–1335. [Google Scholar] [CrossRef] [PubMed]
- Gayathri, T.; Mogili, T.; Krishnan, R.; Doss, S.G.; Sarkar, T.; Teotia, R.S.; Tewary, P. Physio-biochemical evaluation and in situ localization of reactive oxygen species (ROS) in mulberry genotypes under optimal growth conditions. Acta Physiol. Plant. 2022, 44, 141. [Google Scholar] [CrossRef]
- Asada, K. The water-water cycle in chloroplasts: Scavenging of active oxygens and dissipation of excess photons. Annu. Rev. Plant Biol. 1999, 50, 601–639. [Google Scholar] [CrossRef]
- Silva, E.N.; Ferreira-Silva, S.L.; Fontenele, A.d.V.; Ribeiro, R.V.; Viégas, R.A.; Silveira, J.A.G. Photosynthetic changes and protective mechanisms against oxidative damage subjected to isolated and combined drought and heat stresses in Jatropha curcas plants. J. Plant Physiol. 2010, 167, 1157–1164. [Google Scholar] [CrossRef]
- Rodrigues, T.d.S.; Arge, L.W.P.; Guedes, F.A.d.F.; Travassos-Lins, J.; de Souza, A.P.; Cocuron, J.; Buckeridge, M.S.; Grossi-De-Sá, M.F.; Alves-Ferreira, M. Elevated CO2 increases biomass of Sorghum bicolor green prop roots under drought conditions via soluble sugar accumulation and photosynthetic activity. Physiol. Plant. 2023, 175, e13984. [Google Scholar] [CrossRef]
- Yamada, M.; Morishita, H.; Urano, K.; Shiozaki, N.; Yamaguchi-Shinozaki, K.; Shinozaki, K.; Yoshiba, Y. Effects of free proline accumulation in petunias under drought stress. J. Exp. Bot. 2005, 56, 1975–1981. [Google Scholar] [CrossRef]
- Nader, A.A.; Hauka, F.I.A.; Afify, A.H.; El-Sawah, A.M. Drought-tolerant bacteria and arbuscular mycorrhizal fungi mitigate the detrimental effects of drought stress induced by withholding irrigation at critical growth stages of soybean (Glycine max, L.). Microorganisms 2024, 12, 1123. [Google Scholar] [CrossRef]
- Verbruggen, N.; Hermans, C. Proline accumulation in plants: A review. Amino Acids 2008, 35, 753–759. [Google Scholar] [CrossRef]
- Sardans, J.; Peñuuelas, J.; Estiarte, M.; Prieto, P. Warming and drought alter C and N concentration, allocation and accumulation in a Mediterranean shrubland. Glob. Change Biol. 2008, 14, 2304–2316. [Google Scholar] [CrossRef]
- Xu, S.; Sardans, J.; Zhang, J.; Peñuelas, J. Variations in foliar carbon:nitrogen and nitrogen:phosphorus ratios under global change: A meta-analysis of experimental field studies. Sci. Rep. 2020, 10, 12156. [Google Scholar] [CrossRef]
- Xu, Z.; Zhou, G.; Wang, Y. Combined effects of elevated CO2 and soil drought on carbon and nitrogen allocation of the desert shrub Caragana intermedia. Plant Soil 2007, 301, 87–97. [Google Scholar] [CrossRef]
- Wujeska-Klause, A.; Crous, K.Y.; Ghannoum, O.; Ellsworth, D.S. Lower photorespiration in elevated CO2 reduces leaf N concentrations in mature Eucalyptus trees in the field. Glob. Change Biol. 2019, 25, 1282–1295. [Google Scholar] [CrossRef] [PubMed]
- Bista, D.R.; Heckathorn, S.A.; Jayawardena, D.M.; Boldt, J.K. Effect of drought and carbon dioxide on nutrient uptake and levels of nutrient-uptake proteins in roots of barley. Am. J. Bot. 2020, 107, 1401–1409. [Google Scholar] [CrossRef]
- Loladze, I.; Korea, R.O. Hidden shift of the ionome of plants exposed to elevated CO2 depletes minerals at the base of human nutrition. eLife 2014, 3, e02245. [Google Scholar] [CrossRef] [PubMed]
- Franklin, O. Optimal nitrogen allocation controls tree responses to elevated CO2. New Phytol. 2007, 174, 811–822. [Google Scholar] [CrossRef]
- Sekhar, K.M.; Kota, V.R.; Reddy, T.P.; Rao, K.V.; Reddy, A.R. Amelioration of plant responses to drought under elevated CO2 by rejuvenating photosynthesis and nitrogen use efficiency: Implications for future climate-resilient crops. Photosynth. Res. 2020, 150, 21–40. [Google Scholar] [CrossRef]
- Lambers, H.; Chapin, F.S.; Pons, T.L. Plant Physiological Ecology; Springer: New York, NY, USA, 2008. [Google Scholar]
- Bassirirad, H. Kinetics of nutrient uptake by roots: Responses to global change. New Phytol. 2000, 147, 155–169. [Google Scholar] [CrossRef]
- Alster, C.J.; German, D.P.; Lu, Y.; Allison, S.D. Microbial enzymatic responses to drought and to nitrogen addition in a southern California grassland. Soil Biol. Biochem. 2013, 64, 68–79. [Google Scholar] [CrossRef]
- Gao, W.; Reed, S.C.; Munson, S.M.; Rui, Y.; Fan, W.; Zheng, Z.; Li, L.; Che, R.; Xue, K.; Du, J.; et al. Responses of soil extracellular enzyme activities and bacterial community composition to seasonal stages of drought in a semiarid grassland. Geoderma 2021, 401, 115327. [Google Scholar] [CrossRef]
- Ahmed, M.A.; Sanaullah, M.; Blagodatskaya, E.; Mason-Jones, K.; Jawad, H.; Kuzyakov, Y.; Dippold, M.A. Soil microorganisms exhibit enzymatic and priming response to root mucilage under drought. Soil Biol. Biochem. 2018, 116, 410–418. [Google Scholar] [CrossRef]
- Wang, Y.; Yan, D.; Wang, J.; Ding, Y.; Song, X. Effects of elevated CO2 and drought on plant physiology, soil carbon and soil enzyme activities. Pedosphere 2017, 27, 846–855. [Google Scholar] [CrossRef]
- Yu, H.; Deng, Y.; He, Z.; Van Nostrand, J.D.; Wang, S.; Jin, D.; Wang, A.; Wu, L.; Wang, D.; Tai, X.; et al. Elevated CO2 and warming altered grassland microbial communities in soil top-layers. Front. Microbiol. 2018, 9, 1790. [Google Scholar] [CrossRef]
- Bhattacharyya, P.; Roy, K.S.; Neogi, S.; Manna, M.C.; Adhya, T.K.; Rao, K.S.; Nayak, A.K. Influence of elevated carbon dioxide and temperature on belowground carbon allocation and enzyme activities in tropical flooded soil planted with rice. Environ. Monit. Assess. 2013, 185, 8659–8671. [Google Scholar] [CrossRef] [PubMed]
- van Groenigen, K.J.; Qi, X.; Osenberg, C.W.; Luo, Y.; Hungate, B.A. Faster decomposition under increased atmospheric CO2 limits soil carbon storage. Science 2014, 344, 508–509. [Google Scholar] [CrossRef]
- Phillips, R.P.; Finzi, A.C.; Bernhardt, E.S. Enhanced root exudation induces microbial feedbacks to N cycling in a pine forest under long-term CO2 fumigation. Ecol. Lett. 2010, 14, 187–194. [Google Scholar] [CrossRef]


| Variable | p-Value | CO2 Treatment | Water Treatment | Moderate Water Deficit Effect (%) | Severe Water Deficit Effect (%) | ||||
|---|---|---|---|---|---|---|---|---|---|
| CO2 | Water | C*W | Well-Watered | Moderate Drought | Severe Drought | ||||
| Leaf N concentration (mg g−1) | 0.001 | 0.055 | 0.276 | aCO2 | 34.5 ± 0.6 y,a | 35.0 ± 1.1 y,a | 38.0 ± 0.1 x,a | +1.3 | +10.1 |
| eCO2 | 32.4 ± 0.7 x,b | 31.4 ± 0.6 x,b | 33.0 ± 1.4 x,b | −3.2 | +1.8 | ||||
| CO2 effect (%) | −6.1 | −10.1 | −13.0 | ||||||
| Stem N concentration (mg g−1) | 0.042 | 0.001 | 0.159 | aCO2 | 16.2 ± 0.4 x,a | 13.3 ± 0.5 y,a | 13.4 ± 0.8 y,a | −17.9 | −17.2 |
| eCO2 | 14.5 ± 0.4 x,b | 11.3 ± 0.5 y,b | 13.11 ± 0.8 x,a | −21.7 | −9.6 | ||||
| CO2 effect (%) | −10.5 | −15.9 | −2.2 | ||||||
| Root N concentration (mg g−1) | 0.308 | 0.526 | 0.045 | aCO2 | 16.5 ± 0.6 x,a | 14.0 ± 0.9 y,a | 14.6 ± 0.3 y,a | −15.6 | −11.2 |
| eCO2 | 13.9 ± 0.5 x,b | 15.0 ± 0.9 x,a | 14.5 ± 0.3 x,a | +8.3 | +4.8 | ||||
| CO2 effect (%) | −15.9 | +7.6 | −0.9 | ||||||
| Leaf P concentration (mg g−1) | 0.007 | 0.136 | 0.283 | aCO2 | 1.30 ± 0.06 x,a | 1.18 ± 0.04 y,a | 1.28 ± 0.04 x,a | −9.1 | −0.9 |
| eCO2 | 1.08 ± 0.08 y,b | 1.10 ± 0.03 y,b | 1.20 ± 0.03 x,b | +3.2 | +8.2 | ||||
| CO2 effect (%) | −16.7 | −6.6 | −5.8 | ||||||
| Stem P concentration (mg g−1) | 0.012 | 0.010 | 0.226 | aCO2 | 0.99 ± 0.04 x,a | 0.84 ± 0.02 y,a | 0.86 ± 0.04 y,a | −15.1 | −13.1 |
| eCO2 | 0.85 ± 0.04 x,b | 0.72 ± 0.04 y,b | 0.85 ± 0.06 x,a | −14.7 | −0.8 | ||||
| CO2 effect (%) | −14.5 | −14.2 | −0.9 | ||||||
| Root P concentration (mg g−1) | 0.044 | 0.039 | 0.005 | aCO2 | 0.95 ± 0.04 x,a | 0.74 ± 0.05 y,a | 0.95 ± 0.06 x,a | −22.0 | −0.4 |
| eCO2 | 0.70 ± 0.03 y,b | 0.84 ± 0.06 x,a | 0.87 ± 0.02 x,b | +20.8 | +26.1 | ||||
| CO2 effect (%) | −26.6 | +12.6 | −8.4 | ||||||
| Leaf K concentration (mg g−1) | 0.294 | 0.003 | 0.271 | aCO2 | 20.0 ± 0.4 y,a | 19.2 ± 0.8 y,a | 21.6 ± 0.3 x,a | −3.7 | +8.4 |
| eCO2 | 18.8 ± 0.3 z,b | 19.7 ± 0.3 y,a | 21.15 ± 0. 6 x,a | +4.8 | +10.2 | ||||
| CO2 effect (%) | −6.1 | +2.7 | −2.7 | ||||||
| Stem K concentration (mg g−1) | 0.677 | 0.106 | 0.785 | aCO2 | 7.6 ± 0.6 x,a | 6.0 ± 0.2 y,a | 7.2 ± 0.7 x,a | −21.1 | −5.6 |
| eCO2 | 7.2 ± 0.8 x,a | 6.3 ± 0.1 x,a | 6.8 ± 0.6 x,a | −12.7 | −6.0 | ||||
| CO2 effect (%) | −5.3 | +4.5 | −5.8 | ||||||
| Root K concentration (mg g−1) | 0.802 | 0.150 | 0.108 | aCO2 | 8.3 ± 0.6 x,a | 6.5 ± 0.7 y,a | 8.2 ± 0.1 x,a | −22.2 | −1.1 |
| eCO2 | 7.5 ± 0.4 x,a | 7.3 ± 0.5 x,a | 7.6 ± 0.5 x,a | −2.0 | +1.8 | ||||
| CO2 effect (%) | −10.0 | 13.1 | −7.2 | ||||||
| Variable | p-Value | CO2 Treatment | Water Treatment | Moderate Water Deficit Effect (%) | Severe Water Deficit Effect (%) | ||||
|---|---|---|---|---|---|---|---|---|---|
| CO2 | Water | C*W | Well-Watered | Moderate Drought | Severe Drought | ||||
| Leaf biomass (g plant−1) | 0.002 | 0.000 | 0.981 | ACO2 | 37.5 ± 1.8 x,b | 32.9 ± 1.0 y,b | 28.6 ± 2.9 z,b | −12.4 | −23.8 |
| ECO2 | 47.3 ± 0.4 x,a | 43.4 ± 0.5 y,a | 38.8 ± 2.7 z,a | −8.3 | −18.0 | ||||
| CO2 effect (%) | +26.1 | +32.0 | +35.7 | ||||||
| Stem biomass (g plant−1) | 0.000 | 0.000 | 0.037 | ACO2 | 20.3 ± 0.9 x,b | 16.5 ± 0.9 y,b | 10.0 ± 0.6 z,b | −18.9 | −50.6 |
| ECO2 | 24.4 ± 1.4 x,a | 21.3 ± 0.8 y,a | 18.4 ± 0.9 z,a | −12.6 | −24.4 | ||||
| CO2 effect (%) | +20.1 | +29.4 | +83.7 | ||||||
| Root biomass (g plant−1) | 0.000 | 0.001 | 0.574 | ACO2 | 19.8 ± 0.9 x,b | 16.9 ± 0.8 y,b | 14.3 ± 1.2 z,b | −14.8 | −27.8 |
| ECO2 | 23.9 ± 0.4 x,a | 20.1 ± 0.8 y,a | 16.5 ± 0.8 z,a | −16.0 | −30.9 | ||||
| CO2 effect (%) | +20.4 | +18.8 | +15.3 | ||||||
| Total biomass (g plant−1) | 0.000 | 0.000 | 0.876 | ACO2 | 77.7 ± 3.4 x,b | 66.2 ± 1.3 y,b | 53.0 ± 3.8 z,b | −14.7 | −31.8 |
| ECO2 | 95.6 ± 2.1 x,a | 84.8 ± 1.1 y,a | 73.8 ± 14.2 z,a | −11.3 | −22.8 | ||||
| CO2 effect (%) | +23.1 | +28.0 | +39.2 | ||||||
| Root : shoot ratio | 0.027 | 0.164 | 0.080 | ACO2 | 0.34 ± 0.01 x,a | 0.34 ± 0.01 x,a | 0.37 ± 0.04 x,a | −0.3 | +9.4 |
| ECO2 | 0.33 ± 0.01 x,a | 0.31 ± 0.02 x,b | 0.28 ± 0.02 x,b | −6.8 | −13.1 | ||||
| CO2 effect (%) | −2.9 | −9.2 | −22.9 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, S.; Li, H.; Wang, X.; Wang, Z.; Xu, J.; He, X.; Yang, Z. Greater Biomass Production Under Elevated CO2 Is Attributed to Physiological Optimality, Trade-Offs in Nutrient Allocation, and Oxidative Defense in Drought-Stressed Mulberry. Antioxidants 2025, 14, 383. https://doi.org/10.3390/antiox14040383
Shi S, Li H, Wang X, Wang Z, Xu J, He X, Yang Z. Greater Biomass Production Under Elevated CO2 Is Attributed to Physiological Optimality, Trade-Offs in Nutrient Allocation, and Oxidative Defense in Drought-Stressed Mulberry. Antioxidants. 2025; 14(4):383. https://doi.org/10.3390/antiox14040383
Chicago/Turabian StyleShi, Songmei, Huakang Li, Xinju Wang, Ziran Wang, Junqiang Xu, Xinhua He, and Zheng’an Yang. 2025. "Greater Biomass Production Under Elevated CO2 Is Attributed to Physiological Optimality, Trade-Offs in Nutrient Allocation, and Oxidative Defense in Drought-Stressed Mulberry" Antioxidants 14, no. 4: 383. https://doi.org/10.3390/antiox14040383
APA StyleShi, S., Li, H., Wang, X., Wang, Z., Xu, J., He, X., & Yang, Z. (2025). Greater Biomass Production Under Elevated CO2 Is Attributed to Physiological Optimality, Trade-Offs in Nutrient Allocation, and Oxidative Defense in Drought-Stressed Mulberry. Antioxidants, 14(4), 383. https://doi.org/10.3390/antiox14040383






