Addition of Chlorogenic Acid to Human Semen: Effects on Sperm Motility, DNA Integrity, Oxidative Stress, and Nrf2 Expression
Abstract
1. Introduction
2. Materials and Methods
2.1. Human Semen Samples
- Ethics Committee of the Federal Institute for Drugs and Medical Devices, ID German Clinical Trials Register DKRS00035009 (step 1);
- Ethics Committee of Siena University Hospital, ID CEAVSE 25612 (step 2).
2.2. Semen Analysis
2.3. Study Design
2.3.1. Step 1: Effect of CGA on Human Sperm Motility and F2-Isoprostane Determination
F2-Isoprostane Determination by ELISA Assay
2.3.2. Step 2: Effects of CGA on Human Sperm Motility, DNA Integrity, Seminal F2-Isoprostane Levels, and Nrf2 mRNA Expression in Samples Treated In Vitro with 1 mM H2O2
- untreated sample as control (CTR);
- sample treated with 1 mM H2O2 to induce OS (H2O2);
- sample treated with 100 µM CGA (CGA);
- sperm treated with both 1 mM H2O2 and 100 µM CGA (H2O2+CGA).
Acridine Orange Test: DNA Integrity Evaluation
Sperm RNA Extraction and qRT-PCR for Nrf2 Expression
2.4. Statistical Analysis
3. Results
3.1. Step 1: Effect of CGA on Human Sperm Motility and F2-Isoprostane Determination
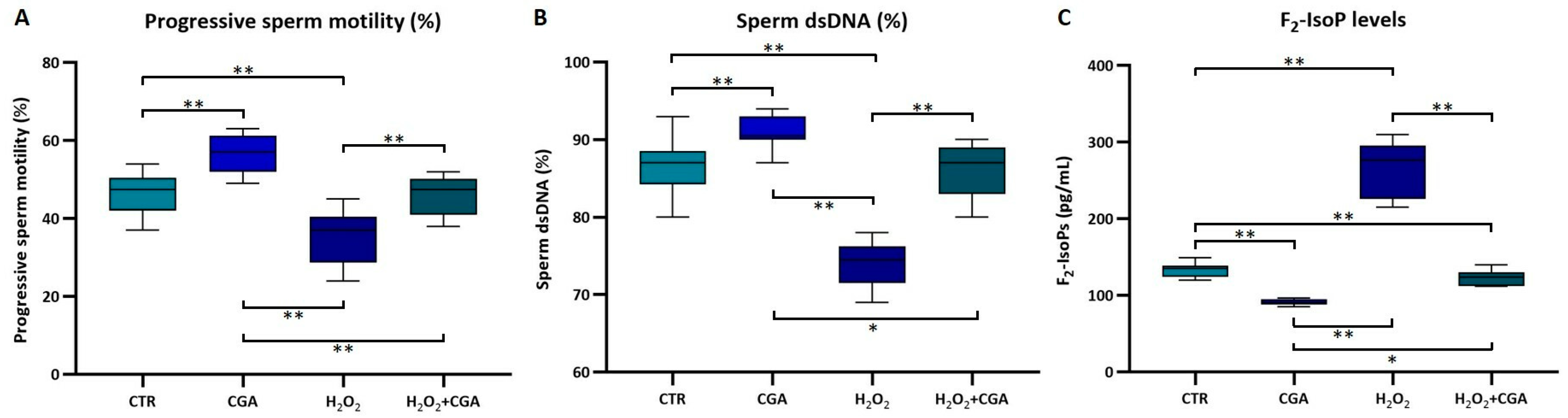
3.2. Step 2: Effects of CGA on Human Sperm Motility, DNA Integrity, Seminal F2-Isoprostane Levels, and Nrf2 mRNA Expression in Samples Treated In Vitro with H2O2
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vander Borght, M.; Wyns, C. Fertility and infertility: Definition and epidemiology. Clin. Biochem. 2018, 62, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Baskaran, S.; Parekh, N.; Cho, C.L.; Henkel, R.; Vij, S.; Arafa, M.; Panner Selvam, M.K.; Shah, R. Male infertility. Lancet 2021, 397, 319–333. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef]
- Aitken, R.J. Reactive oxygen species as mediators of sperm capacitation and pathological damage. Mol. Reprod. Dev. 2017, 84, 1039–1052. [Google Scholar] [CrossRef]
- Gupta, S.; Finelli, R.; Agarwal, A.; Henkel, R. Total antioxidant capacity-Relevance, methods and clinical implications. Andrologia 2021, 53, e13624. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J.; Gibb, Z.; Baker, M.A.; Drevet, J.; Gharagozloo, P. Causes and consequences of oxidative stress in spermatozoa. Reprod. Fertil. Dev. 2016, 28, 1–10. [Google Scholar] [CrossRef]
- Aitken, R.J.; Drevet, J.R.; Moazamian, A.; Gharagozloo, P. Male Infertility and Oxidative Stress: A Focus on the Underlying Mechanisms. Antioxidants 2022, 11, 306. [Google Scholar] [CrossRef]
- Razi, M.; Tavalaee, M.; Sarrafzadeh-Rezaei, F.; Moazamian, A.; Gharagozloo, P.; Drevet, J.R.; Nasr-Eshafani, M.H. Varicocoele and oxidative stress: New perspectives from animal and human studies. Andrology 2021, 9, 546–558. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, K.; Yao, Y.; Li, J.; Deng, S. Bacterial Infections Affect Male Fertility: A Focus on the Oxidative Stress-Autophagy Axis. Front. Cell. Dev. Biol. 2021, 9, 727812. [Google Scholar] [CrossRef]
- Agarwal, A.; Parekh, N.; Panner Selvam, M.K.; Henkel, R.; Shah, R.; Homa, S.T.; Ramasamy, R.; Ko, E.; Tremellen, K.; Esteves, S.; et al. Male Oxidative Stress Infertility (MOSI): Proposed Terminology and Clinical Practice Guidelines for Management of Idiopathic Male Infertility. World J. Men’s Health 2019, 37, 296–312. [Google Scholar] [CrossRef]
- Takalani, N.B.; Monageng, E.M.; Mohlala, K.; Monsees, T.K.; Henkel, R.; Opuwari, C.S. Role of oxidative stress in male infertility. Reprod. Fertil. 2023, 4, e230024. [Google Scholar] [CrossRef]
- Agarwal, A.; Said, T.M.; Bedaiwy, M.A.; Banerjee, J.; Alvarez, J.G. Oxidative stress in an assisted reproductive technique setting. Fertil. Steril. 2006, 86, 503–512. [Google Scholar] [CrossRef]
- Gualtieri, R.; Kalthur, G.; Barbato, V.; Longobardi, S.; Di Rella, F.; Adiga, S.K.; Talevi, R. Sperm Oxidative Stress during In Vitro Manipulation and Its Effects on Sperm Function and Embryo Development. Antioxidants 2021, 10, 1025. [Google Scholar] [CrossRef] [PubMed]
- Noto, D.; Collodel, G.; Cerretani, D.; Signorini, C.; Gambera, L.; Menchiari, A.; Moretti, E. Protective effect of chlorogenic acid on human sperm: In vitro studies and frozen-thawed protocol. Antioxidants 2021, 10, 744. [Google Scholar] [CrossRef]
- Hezavehei, M.; Sharafi, M.; Kouchesfahani, H.M.; Henkel, R.; Agarwal, A.; Esmaeili, V.; Shahverdi, A. Sperm cryopreservation: A review on current molecular cryobiology and advanced approaches. Reprod. Biomed. Online 2018, 37, 327–339. [Google Scholar]
- Moretti, E.; Signorini, C.; Corsaro, R.; Giamalidi, M.; Collodel, G. Human Sperm as an In Vitro Model to Assess the Efficacy of Antioxidant Supplements during Sperm Handling: A Narrative Review. Antioxidants 2023, 12, 1098. [Google Scholar] [CrossRef] [PubMed]
- Santana-Gálvez, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Chlorogenic acid: Recent advances on its dual role as a food additive and a nutraceutical against metabolic syndrome. Molecules 2017, 22, 358. [Google Scholar] [CrossRef] [PubMed]
- Meng, S.; Cao, J.; Feng, Q.; Peng, J.; Hu, Y. Roles of chlorogenic acid on regulating glucose and lipids metabolism: A review. Evid. Based Complement. Alternat. Med. 2013, 2013, 801457. [Google Scholar] [CrossRef]
- Miao, M.; Xiang, L. Pharmacological action and potential targets of chlorogenic acid. Adv. Pharmacol. 2020, 87, 71–88. [Google Scholar] [CrossRef]
- Abedpour, N.; Zeinali, A.; Karimipour, M.; Pourheidar, B.; Farjah, G.H.; Abak, A.; Shoorei, H. Protective effects of chlorogenic acid against ionizing radiation-induced testicular toxicity. Heliyon 2022, 8, e10798, Erratum in Heliyon 2023, 9, e14739. https://doi.org/10.1016/j.heliyon.2023.e14739. [Google Scholar] [CrossRef]
- Jia, Z.C.; Liu, S.J.; Chen, T.F.; Shi, Z.Z.; Li, X.L.; Gao, Z.W.; Zhang, Q.; Zhong, C.F. Chlorogenic acid can improve spermatogenic dysfunction in rats with varicocele by regulating mitochondrial homeostasis and inhibiting the activation of NLRP3 inflammasomes by oxidative mitochondrial DNA and cGAS/STING pathway. Bioorg. Chem. 2024, 150, 107571. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.X.; Xu, Y.M.; Fan, S.C.; Qi, S.S.; Jia, F.F.; Wu, W.; Chen, C. Potential protective role of chlorogenic acid against cyclophosphamide-induced reproductive damage in male mice. Toxicol. Res. 2024, 13, tfae176. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Sohail, T.; Kang, Y.; Sun, X.; Li, Y. Chlorogenic Acid Improves Quality of Chilled Ram Sperm by Mitigating Oxidative Stress. Animals 2022, 12, 163. [Google Scholar] [CrossRef]
- Namula, Z.; Hirata, M.; Wittayarat, M.; Tanihara, F.; Thi Nguyen, N.; Hirano, T.; Nii, M.; Otoi, T. Effects of chlorogenic acid and caffeic acid on the quality of frozen-thawed boar sperm. Reprod. Domest. Anim. 2018, 53, 1600–1604. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Hou, J.; Wan, J.; Yang, Y.; Liu, S.; Li, X.; Li, W.; Dai, X.; Zhou, P.; Liu, W.; et al. Dietary chlorogenic acid ameliorates oxidative stress and improves endothelial function in diabetic mice via Nrf2 activation. J. Int. Med. Res. 2021, 49, 300060520985363. [Google Scholar] [CrossRef]
- Wang, L.; Pan, X.; Jiang, L.; Chu, Y.; Gao, S.; Jiang, X.; Zhang, Y.; Chen, Y.; Luo, S.; Peng, C. The biological activity mechanism of chlorogenic acid and its applications in food industry: A review. Front. Nutr. 2022, 9, 943911. [Google Scholar] [CrossRef]
- Signorini, C.; Saso, L.; Ghareghomi, S.; Telkoparan-Akillilar, P.; Collodel, G.; Moretti, E. Redox homeostasis and Nrf2-regulated mechanisms are relevant to male infertility. Antioxidants 2024, 13, 193. [Google Scholar] [CrossRef]
- World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen, 6th ed.; WHO Press: Geneva, Switzerland, 2021. [Google Scholar]
- Tejada, R.I.; Mitchell, J.C.; Norman, A.; Marik, J.J.; Friedman, S. A Test for the practical evaluation of male fertility by acridine orange (AO) fluorescence. Fertil. Steril. 1984, 42, 87–91. [Google Scholar] [CrossRef]
- Moretti, E.; Mazzi, L.; Bonechi, C.; Salvatici, M.C.; Iacoponi, F.; Rossi, C.; Collodel, G. Effect of Quercetin-loaded liposomes on induced oxidative stress in human spermatozoa. Reprod. Toxicol. 2016, 60, 140–147. [Google Scholar] [CrossRef]
- Aitken, R.J.; Drevet, J.R. The Importance of Oxidative Stress in Determining the Functionality of Mammalian Spermatozoa: A Two-Edged Sword. Antioxidants 2020, 9, 111. [Google Scholar] [CrossRef]
- Baldi, E.; Tamburrino, L.; Muratori, M.; Degl’Innocenti, S.; Marchiani, S. Adverse effects of in vitro manipulation of spermatozoa. Anim. Reprod. Sci. 2020, 220, 106314. [Google Scholar] [CrossRef]
- Tomar, G.; Joshi, T.; Varghes, A.; Sasidharan, S.; Kural, M.R. Relationship of antioxidant system and reactive oxygen species with clinical semen parameters in infertile men. J. Family Med. Prim. Care. 2017, 6, 574–577. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, S.; Bashiri, R.; Ghadiri-Anari, A.; Nadjarzadeh, A. Antioxidant supplements and semen parameters: An evidence-based review. Int. J. Reprod. Med. 2016, 14, 729. [Google Scholar]
- Kallinikas, G.; Tsoporis, J.N.; Haronis, G.; Zarkadas, A.; Bozios, D.; Konstantinopoulos, V.; Kozyrakis, D.; Mitiliniou, D.; Rodinos, E.; Filios, A.; et al. The role of oral antioxidants in the improvement of sperm parameters in infertile men. World J. Urol. 2024, 42, 71. [Google Scholar] [CrossRef] [PubMed]
- Signorini, C.; Moretti, E.; Collodel, G. Role of isoprostanes in human male infertility. Syst. Biol. Reprod. Med. 2020, 66, 291–299. [Google Scholar] [CrossRef]
- Governini, L.; Ponchia, R.; Artini, P.G.; Casarosa, E.; Marzi, I.; Capaldo, A.; Luddi, A.; Piomboni, P. Respiratory Mitochondrial Efficiency and DNA Oxidation in Human Sperm after In Vitro Myo-Inositol Treatment. J. Clin. Med. 2020, 9, 1638. [Google Scholar] [CrossRef]
- Ghafarizadeh, A.A.; Malmir, M.; Naderi Noreini, S.; Faraji, T.; Ebrahimi, Z. The Effect of vitamin E on sperm motility and viability in asthenoteratozoospermic men: In vitro study. Andrologia 2021, 53, E13891. [Google Scholar] [CrossRef]
- Yilmazer, Y.; Moshfeghi, E.; Cetin, F.; Findikli, N. In vitro effects of the combination of serotonin, selenium, zinc, and vitamins D and E supplementation on human sperm motility and reactive oxygen species production. Zygote 2024, 32, 154–160. [Google Scholar] [CrossRef]
- Berger, G.K.; Smith-Harrison, L.I.; Sandlow, J.I. Sperm agglutination: Prevalence and contributory factors. Andrologia 2019, 51, e13254. [Google Scholar] [CrossRef]
- Marinaro, J.A. Sperm DNA fragmentation and its interaction with female factors. Fertil. Steril. 2023, 120, 715–719. [Google Scholar] [CrossRef]
- Tandara, M.; Bajić, A.; Tandara, L.; Bilić-Zulle, L.; Šunj, M.; Kozina, V.; Goluža, T.; Jukić, M. Sperm DNA integrity testing: Big halo is a good predictor of embryo quality and pregnancy after conventional IVF. Andrology 2014, 2, 678–686. [Google Scholar] [CrossRef] [PubMed]
- Bibi, R.; Jahan, S.; Razak, S.; Hammadeh, M.E.; Almajwal, A.; Amor, H. Protamines and DNA integrity as a biomarkers of sperm quality and assisted conception outcome. Andrologia 2022, 54, e14418. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Chen, X.; Wang, Z.; Wang, D. Is transcription in sperm stationary or dynamic? J. Reprod. Dev. 2017, 63, 439–443. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Peng, S.; Xu, J.; Fang, J. Reversing ROS-mediated neurotoxicity by chlorogenic acid involves its direct antioxidant activity and activation of Nrf2-ARE signaling pathway. Biofactors 2019, 45, 616–626. [Google Scholar] [CrossRef] [PubMed]
- Cicek, B.; Hacimuftuoglu, A.; Yeni, Y.; Danisman, B.; Ozkaraca, M.; Mokhtare, B.; Kantarci, M.; Spanakis, M.; Nikitovic, D.; Lazopoulos, G.; et al. Chlorogenic acid attenuates doxorubicin-induced oxidative stress and markers of apoptosis in cardiomyocytes via Nrf2/HO-1 and dityrosine signaling. J. Pers. Med. 2023, 13, 649. [Google Scholar] [CrossRef] [PubMed]
- Ling, X.; Yan, W.; Yang, F.; Jiang, S.; Chen, F.; Li, N. Research progress of chlorogenic acid in improving inflammatory diseases. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2023, 48, 1611–1620. [Google Scholar] [CrossRef]
- Santonastaso, M.; Mottola, F.; Iovine, C.; Colacurci, N.; Rocco, L. Protective Effects of Curcumin on the Outcome of Cryopreservation in Human Sperm. Reprod. Sci. 2021, 28, 2895–2905. [Google Scholar] [CrossRef]
- Deng, S.L.; Sun, T.C.; Yu, K.; Wang, Z.P.; Zhang, B.L.; Zhang, Y.; Wang, X.X.; Lian, Z.X.; Liu, Y.X. Melatonin reduces oxidative damage and upregulates heat shock protein 90 expression in cryopreserved human semen. Free Radic. Biol. Med. 2017, 113, 347–354. [Google Scholar] [CrossRef]


| Variables | Median (IQR) |
|---|---|
| Age | 33.0 (29.0–36.0) |
| Volume (mL) | 4.5 (3.8–5.0) |
| Sperm concentration (106 per mL) | 51.5 (38.0–75.0) |
| Normal morphology (%) | 7.0 (5.0–10.0) |
| Rapid progressive motility (%) | 5.5 (3.0–17.5) |
| Slow progressive motility (%) | 34.5 (25.0–40.0) |
| Vitality (%) | 78.0 (67.3–83.8) |
| CTR | CGA | Statistics | |
|---|---|---|---|
| Rapid progressive motility (%) | 5.0 (2.3–13.5) | 14.0 (7.3–25.0) | p < 0.01 |
| Slow progressive motility (%) | 31.0 (22.0–40.0) | 37.0 (30.0–42.0) | p < 0.01 |
| F2-IsoPs (pg/mL) | 119.5 (95.7–150.6) | 10.2 (7.6–14.4) | p < 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Signorini, C.; Corsaro, R.; Collodel, G.; Maettner, R.; Sterzik, K.; Strehler, E.; Liguori, L.; Moretti, E. Addition of Chlorogenic Acid to Human Semen: Effects on Sperm Motility, DNA Integrity, Oxidative Stress, and Nrf2 Expression. Antioxidants 2025, 14, 382. https://doi.org/10.3390/antiox14040382
Signorini C, Corsaro R, Collodel G, Maettner R, Sterzik K, Strehler E, Liguori L, Moretti E. Addition of Chlorogenic Acid to Human Semen: Effects on Sperm Motility, DNA Integrity, Oxidative Stress, and Nrf2 Expression. Antioxidants. 2025; 14(4):382. https://doi.org/10.3390/antiox14040382
Chicago/Turabian StyleSignorini, Cinzia, Roberta Corsaro, Giulia Collodel, Robert Maettner, Karl Sterzik, Erwin Strehler, Laura Liguori, and Elena Moretti. 2025. "Addition of Chlorogenic Acid to Human Semen: Effects on Sperm Motility, DNA Integrity, Oxidative Stress, and Nrf2 Expression" Antioxidants 14, no. 4: 382. https://doi.org/10.3390/antiox14040382
APA StyleSignorini, C., Corsaro, R., Collodel, G., Maettner, R., Sterzik, K., Strehler, E., Liguori, L., & Moretti, E. (2025). Addition of Chlorogenic Acid to Human Semen: Effects on Sperm Motility, DNA Integrity, Oxidative Stress, and Nrf2 Expression. Antioxidants, 14(4), 382. https://doi.org/10.3390/antiox14040382








