Unexplored Mechanisms of Photoprotection: Synergistic Light Absorption and Antioxidant Activity of Melanin
Abstract
1. Introduction
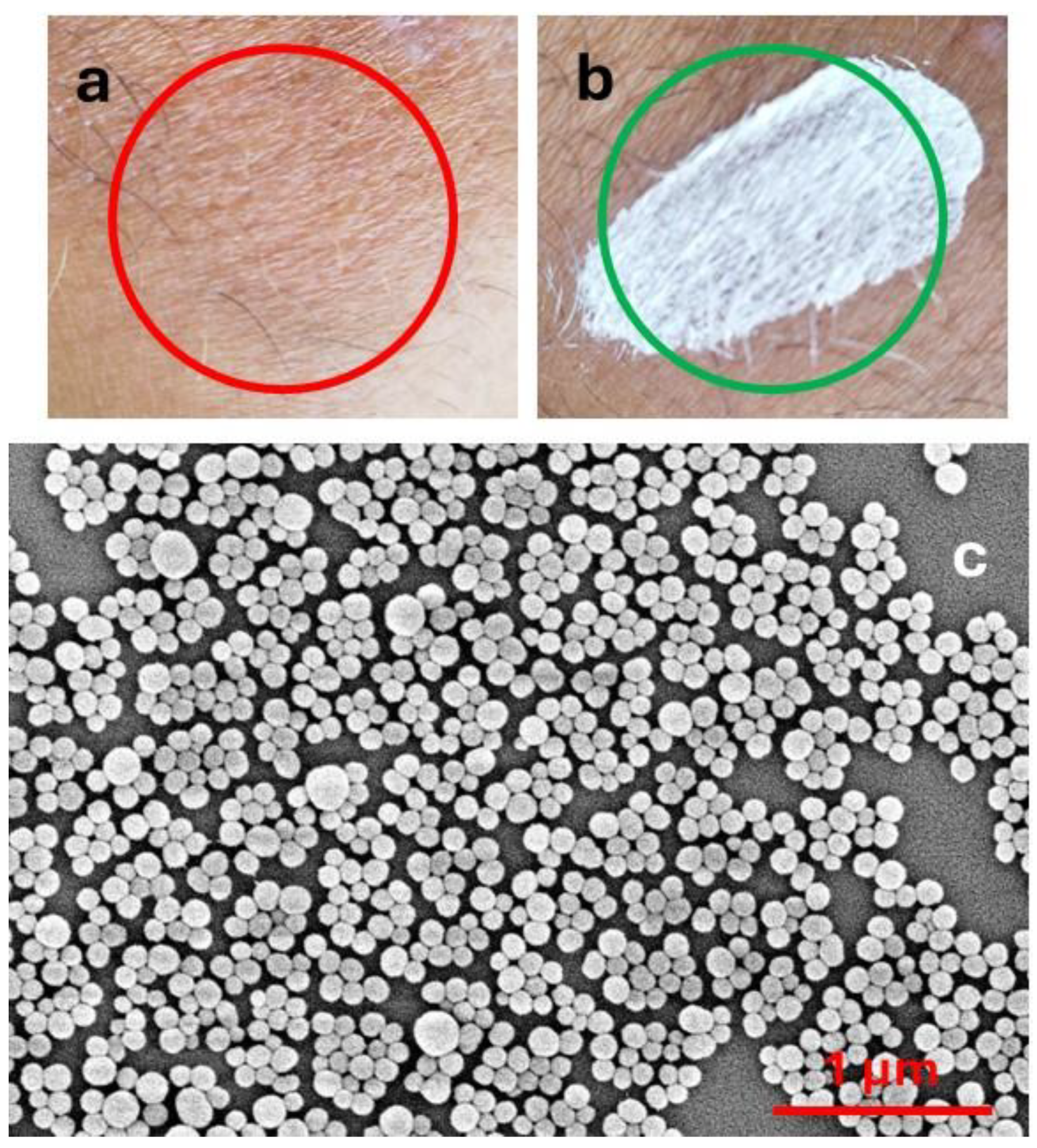
2. Natural and Biomimetic Melanin
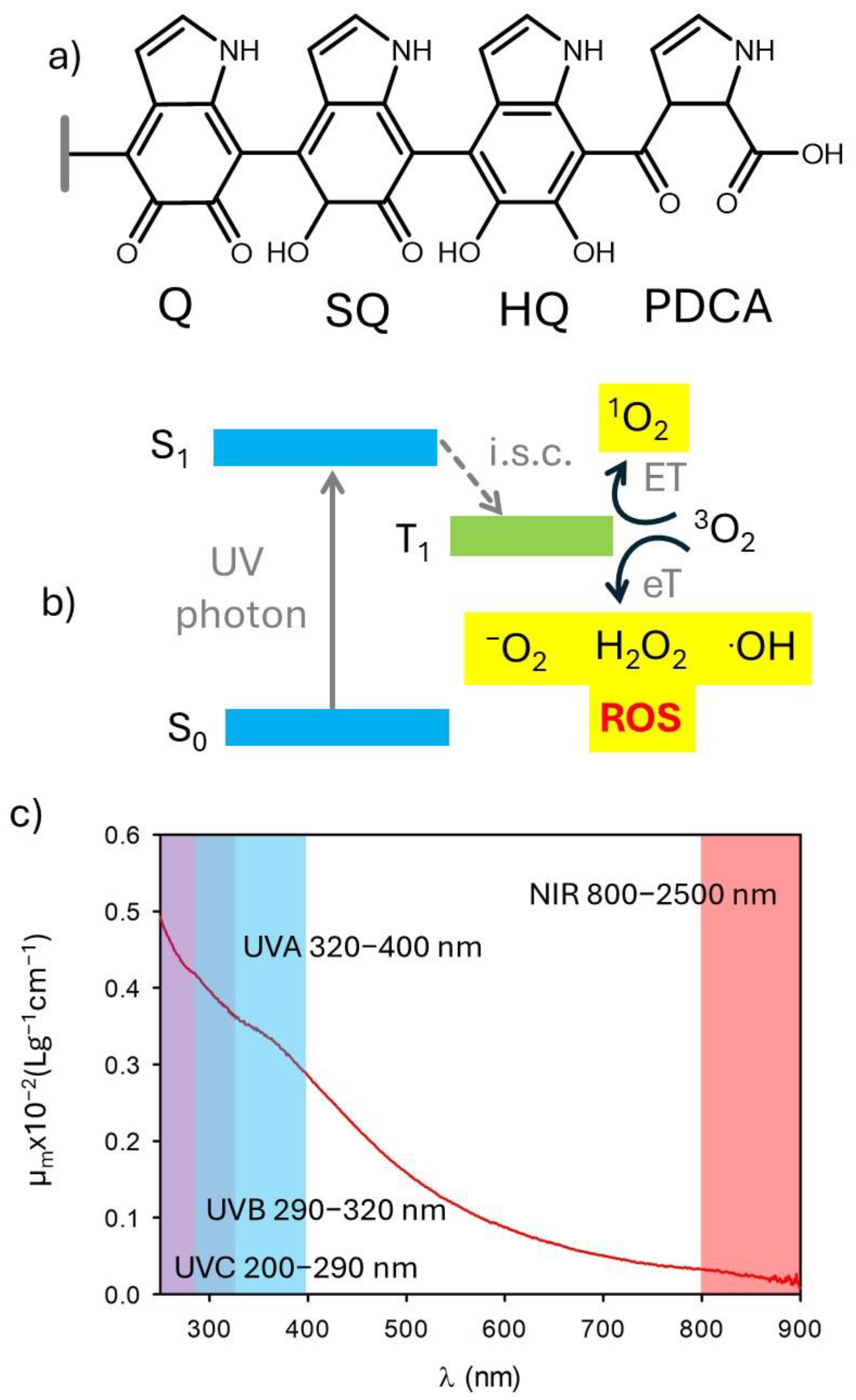
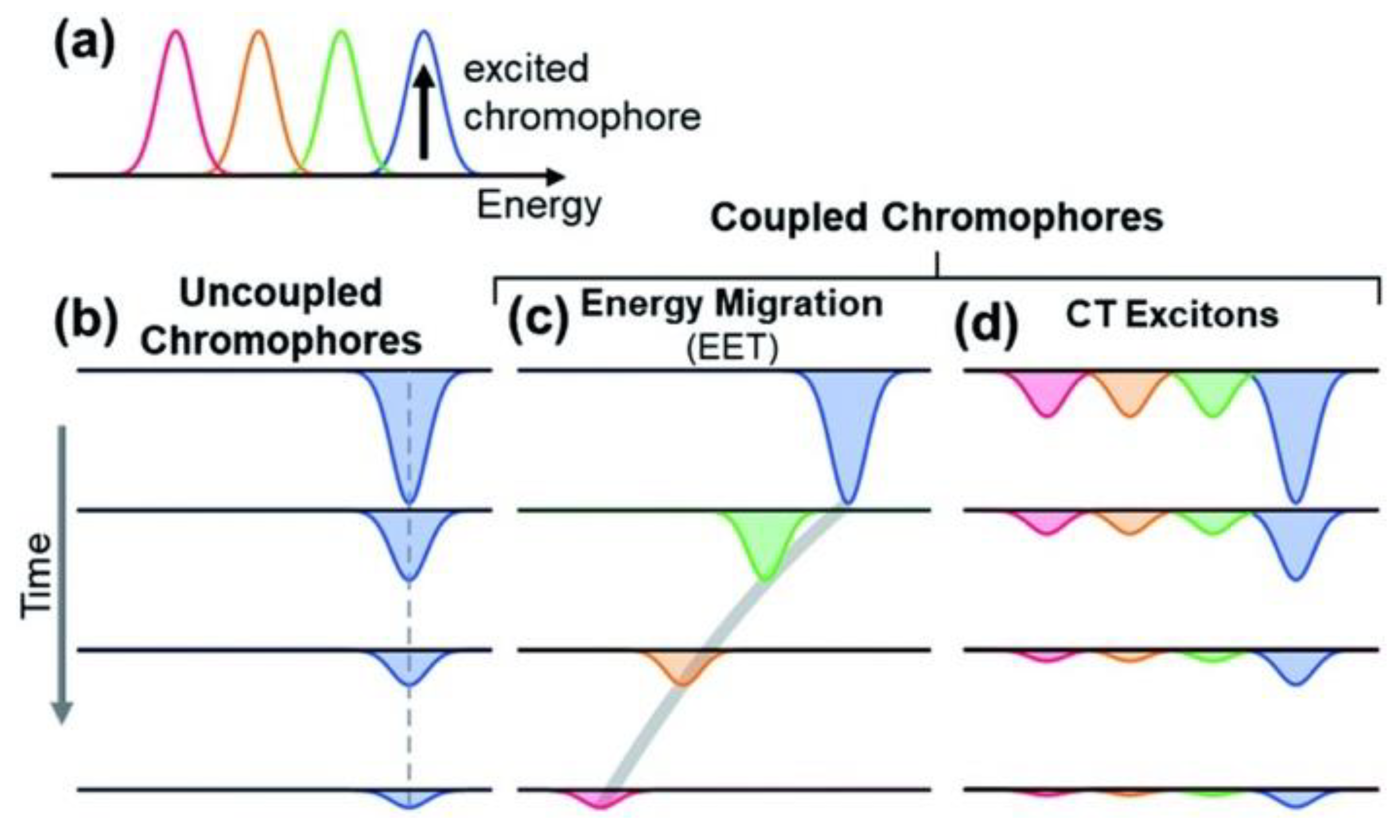
3. Light Absorption by Melanin
4. Antioxidant Activity of Melanin
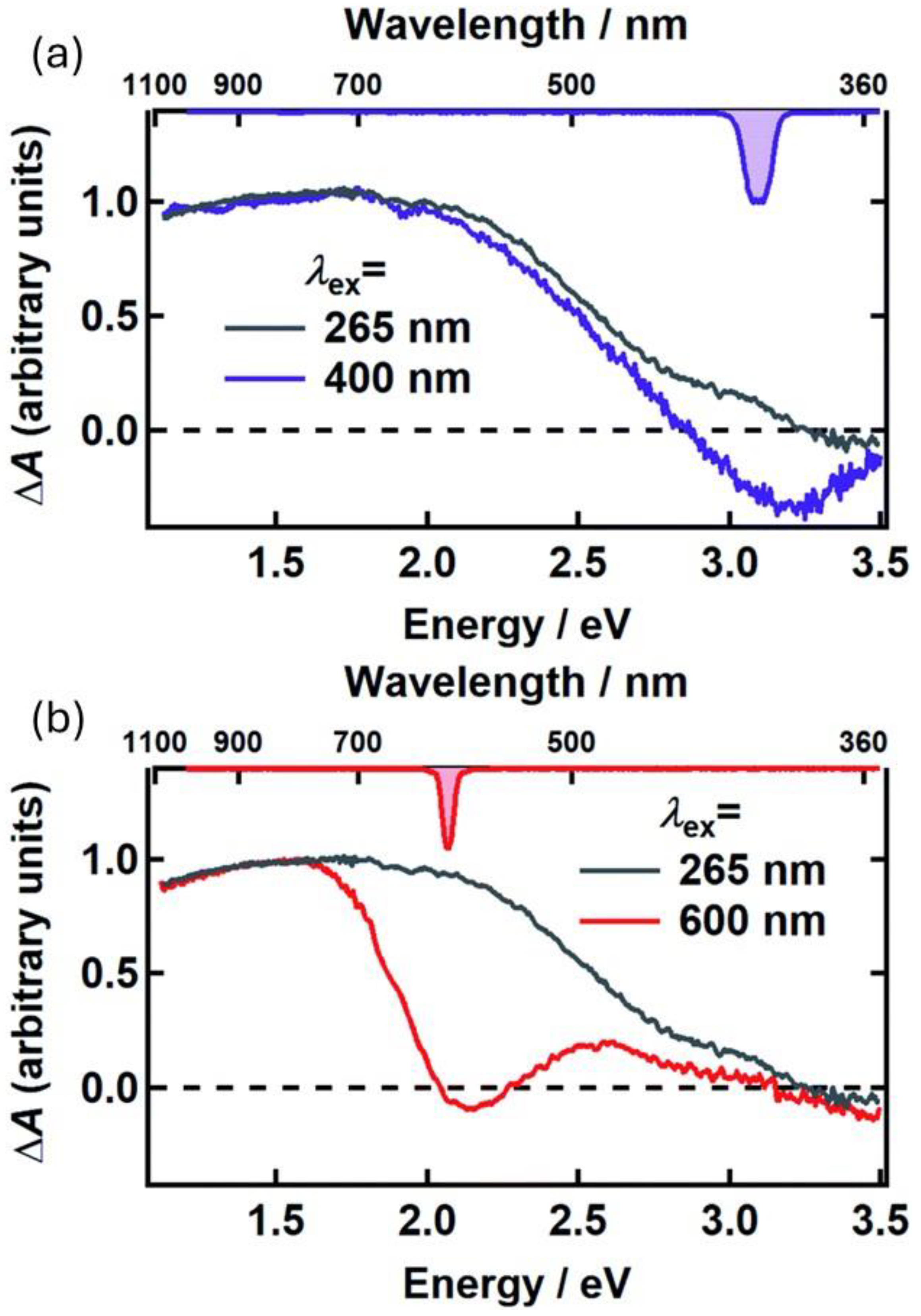
5. Light-Enhanced Radical Content in Melanin
6. Perspectives
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| UV | Ultraviolet |
| NPs | Nanoparticles |
| ROS | Reactive oxygen species |
| NIR | Near infrared |
| HQ | Di-hydroxyquinones |
| SQ | Semiquinones |
| Q | Quinones |
| PDCA | Pyrrole-2,3-dicarboxylic acid |
| PDA | Polydopamine |
| UFTA | Ultrafast transient absorption |
| RONS | Reactive oxygen and nitrogen species |
| EPR | Electron paramagnetic resonance |
| CCR | Carbon centered radical |
| HAT | Hydrogen atom transfer |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
References
- Huang, Y.; Li, Y.; Hu, Z.; Yue, X.; Proetto, M.T.; Jones, Y.; Gianneschi, N.C. Mimicking Melanosomes: Polydopamine Nanoparticles as Artificial Microparasols. ACS Cent. Sci. 2017, 3, 564–569. [Google Scholar] [CrossRef] [PubMed]
- Ichihashi, M.; Ueda, M.; Budiyanto, A.; Bito, T.; Oka, M.; Fukunaga, M.; Tsuru, K.; Horikawa, T. UV-induced skin damage. Toxicology 2003, 189, 21–39. [Google Scholar] [CrossRef] [PubMed]
- Rinnerthaler, M.; Bischof, J.; Streubel, M.K.; Trost, A.; Richter, K. Oxidative stress in aging human skin. Biomolecules 2015, 5, 545–589. [Google Scholar] [CrossRef] [PubMed]
- Green, A.C.; Williams, G.M.; Logan, V.; Strutton, G.M. Reduced melanoma after regular sunscreen use: Randomized trial follow-up. J. Clin. Oncol. 2011, 29, 257–263. [Google Scholar] [CrossRef]
- Thompson, S.C.; Jolley, D.; Marks, R. Reduction of Solar Keratoses by Regular Sunscreen Use. N. Engl. J. Med. 1993, 329, 1147–1151. [Google Scholar] [CrossRef]
- Serpone, N.; Dondi, D.; Albini, A. Inorganic and organic UV filters: Their role and efficacy in sunscreens and suncare products. Inorganica Chim. Acta 2007, 360, 794–802. [Google Scholar] [CrossRef]
- Giokas, D.L.; Salvador, A.; Chisvert, A. UV filters: From sunscreens to human body and the environment. TrAC-Trends Anal. Chem. 2007, 26, 360–374. [Google Scholar] [CrossRef]
- Mavridi-Printezi, A.; Menichetti, A.; Guernelli, M.; Montalti, M. Extending photocatalysis to the visible and NIR: The molecular strategy. Nanoscale 2021, 13, 9147–9159. [Google Scholar] [CrossRef]
- Agati, G.; Tattini, M. Multiple functional roles of flavonoids in photoprotection. New Phytol. 2010, 186, 786–793. [Google Scholar] [CrossRef]
- Graf, E. Antioxidant potential of ferulic acid. Free Radic. Biol. Med. 1992, 13, 435–448. [Google Scholar] [CrossRef]
- Cheng, Y.; Cai, S.; Wu, H.; Pan, J.; Su, M.; Wei, X.; Ye, J.; Ke, L.; Liu, G.; Chu, C. Revolutionizing eye care: The game-changing applications of nano-antioxidants in ophthalmology. Nanoscale 2024, 16, 7307–7322. [Google Scholar] [CrossRef] [PubMed]
- Weir, A.; Westerhoff, P.; Fabricius, L.; Hristovski, K.; von Goetz, N. Titanium Dioxide Nanoparticles in Food and Personal Care Products. Environ. Sci. Technol. 2012, 46, 2242–2250. [Google Scholar] [CrossRef]
- de Sá, R.G.; Arantes, T.M.; de Macedo, E.F.; Dona, L.M.; Pereira, J.C.F.; Hurtado, C.R.; Varghese, R.J.; Oluwafemi, O.S.; Tada, D.B. Photoprotective activity of zirconia nanoparticles. Colloids Surf. B Biointerfaces 2021, 202, 111636. [Google Scholar] [CrossRef]
- Aguilera, J.; Gracia-Cazaña, T.; Gilaberte, Y. New developments in sunscreens. Photochem. Photobiol. Sci. 2023, 22, 2473–2482. [Google Scholar] [CrossRef]
- Jacques, S.L. Optical properties of biological tissues: A review. Phys. Med. Biol. 2013, 58, R37–R61. [Google Scholar] [CrossRef] [PubMed]
- Mavridi-Printezi, A.; Menichetti, A.; Guernelli, M.; Montalti, M. The Photophysics and Photochemistry of Melanin- Like Nanomaterials Depend on Morphology and Structure. Chem. A Eur. J. 2021, 27, 16309–16319. [Google Scholar] [CrossRef]
- Guernelli, M.; Menichetti, A.; Guidetti, G.; Costantini, P.E.; Calvaresi, M.; Danielli, A.; Mazzaro, R.; Morandi, V.; Montalti, M. pH Switchable Water Dispersed Photocatalytic Nanoparticles. Chem. A Eur. J. 2022, 28, e202200118. [Google Scholar] [CrossRef] [PubMed]
- Guidetti, G.; Pogna, E.A.A.; Lombardi, L.; Tomarchio, F.; Polishchuk, I.; Joosten, R.R.M.; Ianiro, A.; Soavi, G.; Sommerdijk, N.A.J.M.; Friedrich, H.; et al. Photocatalytic activity of exfoliated graphite–TiO2 nanoparticle composites. Nanoscale 2019, 11, 19301–19314. [Google Scholar] [CrossRef]
- Aguilar-Ferrer, D.; Szewczyk, J.; Coy, E. Recent developments in polydopamine-based photocatalytic nanocomposites for energy production: Physico-chemical properties and perspectives. Catal. Today 2022, 397–399, 316–349. [Google Scholar] [CrossRef]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 Photocatalysis: Mechanisms and Materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef]
- Yang, Y.; Sun, W. Recent advances in redox-responsive nanoparticles for combined cancer therapy. Nanoscale Adv. 2022, 4, 3504–3516. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, J.; Fan, J.; Chao, H.; Peng, X. Recent progress in photosensitizers for overcoming the challenges of photodynamic therapy: From molecular design to application. Chem. Soc. Rev. 2021, 50, 4185–4219. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, Y.; Zhao, Z.; Qiu, J. Oxidative stress in the skin: Impact and related protection. Int. J. Cosmet. Sci. 2021, 43, 495–509. [Google Scholar] [CrossRef]
- Meredith, P.; Sarna, T. The physical and chemical properties of eumelanin. Pigment Cell Res. 2006, 19, 572–594. [Google Scholar] [CrossRef]
- Biyashev, D.; Siwicka, Z.E.; Onay, U.V.; Demczuk, M.; Xu, D.; Ernst, M.K.; Evans, S.T.; Nguyen, C.V.; Son, F.A.; Paul, N.K.; et al. Topical application of synthetic melanin promotes tissue repair. npj Regen. Med. 2023, 8, 61. [Google Scholar] [CrossRef] [PubMed]
- Mavridi-Printezi, A.; Menichetti, A.; Mordini, D.; Amorati, R.; Montalti, M. Recent Applications of Melanin-like Nanoparticles as Antioxidant Agents. Antioxidants 2023, 12, 863. [Google Scholar] [CrossRef] [PubMed]
- Mavridi-Printezi, A.; Giordani, S.; Menichetti, A.; Mordini, D.; Zattoni, A.; Roda, B.; Ferrazzano, L.; Reschiglian, P.; Marassi, V.; Montalti, M. The dual nature of biomimetic melanin. Nanoscale 2024, 16, 299–308. [Google Scholar] [CrossRef]
- Guo, Y.; Baschieri, A.; Mollica, F.; Valgimigli, L.; Cedrowski, J.; Litwinienko, G.; Amorati, R. Hydrogen Atom Transfer from HOO. to ortho-Quinones Explains the Antioxidant Activity of Polydopamine. Angew. Chem. Int. Ed. 2021, 60, 15220–15224. [Google Scholar] [CrossRef]
- Mostert, A.B.; Rienecker, S.B.; Noble, C.; Hanson, G.R.; Meredith, P. The photoreactive free radical in eumelanin. Sci. Adv. 2018, 4, eaaq1293. [Google Scholar] [CrossRef]
- Huang, L.; Liu, M.; Huang, H.; Wen, Y.; Zhang, X.; Wei, Y. Recent Advances and Progress on Melanin-like Materials and Their Biomedical Applications. Biomacromolecules 2018, 19, 1858–1868. [Google Scholar] [CrossRef]
- D’Ischia, M.; Napolitano, A.; Pezzella, A.; Meredith, P.; Sarna, T. Chemical and structural diversity in eumelanins: Unexplored bio-optoelectronic materials. Angew. Chem. Int. Ed. 2009, 48, 3914–3921. [Google Scholar] [CrossRef]
- d’Ischia, M.; Napolitano, A.; Pezzella, A.; Meredith, P.; Buehler, M. Melanin Biopolymers: Tailoring Chemical Complexity for Materials Design. Angew. Chem. Int. Ed. 2020, 59, 11196–11205. [Google Scholar] [CrossRef]
- Cao, H.; Yang, Y.; Liang, M.; Ma, Y.; Sun, N.; Gao, X.; Li, J. Pt@polydopamine nanoparticles as nanozymes for enhanced photodynamic and photothermal therapy. Chem. Commun. 2021, 57, 255–258. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ai, K.; Lu, L. Polydopamine and Its Derivative Materials: Synthesis and Promising Applications in Energy, Environmental, and Biomedical Fields. Chem. Rev. 2014, 114, 5057–5115. [Google Scholar] [CrossRef]
- Jiao, L.; Xu, W.; Yan, H.; Wu, Y.; Gu, W.; Li, H.; Du, D.; Lin, Y.; Zhu, C. A dopamine-induced Au hydrogel nanozyme for enhanced biomimetic catalysis. Chem. Commun. 2019, 55, 9865–9868. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Zhou, X.; McCallum, N.C.; Hu, Z.; Ni, Q.Z.; Kapoor, U.; Heil, C.M.; Cay, K.S.; Zand, T.; Mantanona, A.J.; et al. Unraveling the Structure and Function of Melanin through Synthesis. J. Am. Chem. Soc. 2021, 143, 2622–2637. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Q.; Han, X.; Mu, J.; Shi, X.; Zheng, Y.; Wang, T.; Cao, T.; Xi, Y.; Weng, Z.; Wang, X.; et al. Oxygen-evolving hollow polydopamine alleviates tumour hypoxia for enhancing photodynamic therapy in cancer treatment. Nanoscale Adv. 2022, 35, 5021–5026. [Google Scholar] [CrossRef]
- Ryu, J.H.; Messersmith, P.B.; Lee, H. Polydopamine Surface Chemistry: A Decade of Discovery. ACS Appl. Mater. Interfaces 2018, 10, 7523–7540. [Google Scholar] [CrossRef]
- Saiz-Poseu, J.; Mancebo-Aracil, J.; Nador, F.; Busqué, F.; Ruiz-Molina, D. The Chemistry behind Catechol-Based Adhesion. Angew. Chem. Int. Ed. 2019, 58, 696–714. [Google Scholar] [CrossRef]
- Cao, W.; Mantanona, A.J.; Mao, H.; McCallum, N.C.; Jiao, Y.; Battistella, C.; Caponetti, V.; Zang, N.; Thompson, M.P.; Montalti, M.; et al. Radical-Enriched Artificial Melanin. Chem. Mater. 2020, 32, 5759–5767. [Google Scholar] [CrossRef]
- Zhou, X.; McCallum, N.C.; Hu, Z.; Cao, W.; Gnanasekaran, K.; Feng, Y.; Stoddart, J.F.; Wang, Z.; Gianneschi, N.C. Artificial Allomelanin Nanoparticles. ACS Nano 2019, 13, 10980–10990. [Google Scholar] [CrossRef]
- Pralea, I.E.; Moldovan, R.C.; Petrache, A.M.; Ilieș, M.; Hegheș, S.C.; Ielciu, I.; Nicoară, R.; Moldovan, M.; Ene, M.; Radu, M.; et al. From extraction to advanced analytical methods: The challenges of melanin analysis. Int. J. Mol. Sci. 2019, 20, 3943. [Google Scholar] [CrossRef]
- Mavridi-Printezi, A.; Menichetti, A.; Mordini, D.; Montalti, M. Functionalization of and through Melanin: Strategies and Bio-Applications. Int. J. Mol. Sci. 2023, 24, 9689. [Google Scholar] [CrossRef]
- Liebscher, J. Chemistry of Polydopamine—Scope, Variation, and Limitation. Eur. J. Org. Chem. 2019, 2019, 4976–4994. [Google Scholar] [CrossRef]
- Diffey, B.L. Sources and measurement of ultraviolet radiation. Methods 2002, 28, 4–13. [Google Scholar] [CrossRef]
- Kohl, F.R.; Grieco, C.; Kohler, B. Ultrafast spectral hole burning reveals the distinct chromophores in eumelanin and their common photoresponse. Chem. Sci. 2020, 11, 1248–1259. [Google Scholar] [CrossRef] [PubMed]
- Ju, K.-Y.; Fischer, M.C.; Warren, W.S. Understanding the Role of Aggregation in the Broad Absorption Bands of Eumelanin. ACS Nano 2018, 12, 12050–12061. [Google Scholar] [CrossRef]
- Petropoulos, V.; Mavridi-Printezi, A.; Menichetti, A.; Mordini, D.; Kabacinski, P.; Gianneschi, N.C.; Montalti, M.; Maiuri, M.; Cerullo, G. Sub-50 fs Formation of Charge Transfer States Rules the Fate of Photoexcitations in Eumelanin-Like Materials. J. Phys. Chem. Lett. 2024, 15, 3639–3645. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Chen, X.; Yang, P.; Liang, G.; Yang, Y.; Gu, Z.; Li, Y. Regulating the absorption spectrum of polydopamine. Sci. Adv. 2020, 6, eabb4696. [Google Scholar] [CrossRef]
- Premi, S.; Wallisch, S.; Mano, C.M.; Weiner, A.B.; Bacchiocchi, A.; Wakamatsu, K.; Bechara, E.J.H.; Halaban, R.; Douki, T.; Brash, D.E. Chemiexcitation of melanin derivatives induces DNA photoproducts long after UV exposure. Science 2015, 347, 842–847. [Google Scholar] [CrossRef]
- Sarna, T.; Sealy, R.C. Photoinduced Oxygen Consumption in Melanin Systems. Action Spectra and Quantum Yields for Eumelanin and Synthetic Melanin. Photochem. Photobiol. 1984, 39, 69–74. [Google Scholar] [CrossRef]
- Alamed, J.; Chaiyasit, W.; McClements, D.J.; Decker, E.A. Relationships between Free Radical Scavenging and Antioxidant Activity in Foods. J. Agric. Food Chem. 2009, 57, 2969–2976. [Google Scholar] [CrossRef] [PubMed]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized Methods for the Determination of Antioxidant Capacity and Phenolics in Foods and Dietary Supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.-Q. Chemical Methods To Evaluate Antioxidant Ability. Chem. Rev. 2010, 110, 5675–5691. [Google Scholar] [CrossRef] [PubMed]
- Munteanu, I.G.; Apetrei, C. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef]
- Pratt, D.A.; Tallman, K.A.; Porter, N.A. Free radical oxidation of polyunsaturated lipids: New mechanistic insights and the development of peroxyl radical clocks. Acc. Chem. Res. 2011, 44, 458–467. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Gulcin, İ. Antioxidants and antioxidant methods: An updated overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef]
- Jang, M.; Cai, L.; Udeani, G.O.; Slowing, K.V.; Thomas, C.F.; Beecher, C.W.W.; Fong, H.H.S.; Farnsworth, N.R.; Kinghorn, A.D.; Mehta, R.G.; et al. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science 1997, 275, 218–220. [Google Scholar] [CrossRef]
- Mecocci, P.; Polidori, M.C.; Troiano, L.; Cherubini, A.; Cecchetti, R.; Pini, G.; Straatman, M.; Monti, D.; Stahl, W.; Sies, H.; et al. Plasma antioxidants and longevity: A study on healthy centenarians. Free Radic. Biol. Med. 2000, 28, 1243–1248. [Google Scholar] [CrossRef]
- Andrady, A.L. Microplastics in the marine environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef] [PubMed]
- Ingold, K.U.; Pratt, D.A. Advances in Radical-Trapping Antioxidant Chemistry in the 21st Century: A Kinetics and Mechanisms Perspective. Chem. Rev. 2014, 114, 9022–9046. [Google Scholar] [CrossRef]
- Shah, R.; Shchepinov, M.S.; Pratt, D.A. Resolving the Role of Lipoxygenases in the Initiation and Execution of Ferroptosis. ACS Cent. Sci. 2018, 4, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Mavridi-Printezi, A.; Mollica, F.; Lucernati, R.; Montalti, M.; Amorati, R. Insight into the Antioxidant Activity of 1,8-Dihydroxynaphthalene Allomelanin Nanoparticles. Antioxidants 2023, 12, 1511. [Google Scholar] [CrossRef]
- Hu, J.; Yang, L.; Yang, P.; Jiang, S.; Liu, X.; Li, Y. Polydopamine free radical scavengers. Biomater. Sci. 2020, 8, 4940–4950. [Google Scholar] [CrossRef]
- Solano, F. Melanin and melanin-related polymers as materials with biomedical and biotechnological applications— Cuttlefish ink and mussel foot proteins as inspired biomolecules. Int. J. Mol. Sci. 2017, 18, 1561. [Google Scholar] [CrossRef]
- Manini, P.; Lino, V.; Franchi, P.; Gentile, G.; Sibillano, T.; Giannini, C.; Picardi, E.; Napolitano, A.; Valgimigli, L.; Chiappe, C.; et al. A Robust Fungal Allomelanin Mimic: An Antioxidant and Potent π-Electron Donor with Free-Radical Properties that can be Tuned by Ionic Liquids. ChemPlusChem 2019, 84, 1331–1337. [Google Scholar] [CrossRef]
- Bao, X.; Zhao, J.; Sun, J.; Hu, M.; Yang, X. Polydopamine Nanoparticles as Efficient Scavengers for Reactive Oxygen Species in Periodontal Disease. ACS Nano 2018, 12, 8882–8892. [Google Scholar] [CrossRef]
- Liu, Y.; Ai, K.; Ji, X.; Askhatova, D.; Du, R.; Lu, L.; Shi, J. Comprehensive Insights into the Multi-Antioxidative Mechanisms of Melanin Nanoparticles and Their Application To Protect Brain from Injury in Ischemic Stroke. J. Am. Chem. Soc. 2017, 139, 856–862. [Google Scholar] [CrossRef]
- Sarna, T.; Pilas, B.; Land, E.J.; Truscott, T.G. Interaction of radicals from water radiolysis with melanin. BBA-Gen. Subj. 1986, 883, 162–167. [Google Scholar] [CrossRef]
- Mavridi-Printezi, A.; Menichetti, A.; Ferrazzano, L.; Montalti, M. Reversible Supramolecular Noncovalent Self-Assembly Determines the Optical Properties and the Formation of Melanin-like Nanoparticles. J. Phys. Chem. Lett. 2022, 13, 9829–9833. [Google Scholar] [CrossRef]
- Ito, S.; Wakamatsu, K.; Sarna, T. Photodegradation of Eumelanin and Pheomelanin and Its Pathophysiological Implications. Photochem. Photobiol. 2018, 94, 409–420. [Google Scholar] [CrossRef]
- Różanowska, M.; Sarna, T.; Land, E.J.; Truscott, T.G. Free radical scavenging properties of melanin: Interaction of eu- and pheo-melanin models with reducing and oxidising radicals. Free Radic. Biol. Med. 1999, 26, 518–525. [Google Scholar] [CrossRef]
- Burke, J.M.; Kaczara, P.; Skumatz, C.M.; Zareba, M.; Raciti, M.W.; Sarna, T. Dynamic analyses reveal cytoprotection by RPE melanosomes against non-photic stress. Mol. Vis. 2011, 17, 2864–2877. [Google Scholar]
- Kaczara, P.; Zaręba, M.; Herrnreiter, A.; Skumatz, C.M.; Ządło, A.; Sarna, T.; Burke, J.M. Melanosome-iron interactions within retinal pigment epithelium-derived cells. Pigment Cell Melanoma Res. 2012, 25, 804–814. [Google Scholar] [CrossRef]
- Mollica, F.; Lucernati, R.; Amorati, R. Expanding the spectrum of polydopamine antioxidant activity by nitroxide conjugation. J. Mater. Chem. B 2021, 9, 9980–9988. [Google Scholar] [CrossRef]
- Zareba, M.; Sarna, T.; Szewczyk, G.; Burke, J.M. Photobleaching of melanosomes from retinal pigment epithelium: II. Effects on the response of living cells to photic stress. Photochem. Photobiol. 2007, 83, 925–930. [Google Scholar] [CrossRef]
- Abrahamse, H.; Hamblin, M.R. New photosensitizers for photodynamic therapy. Biochem. J. 2016, 473, 347–364. [Google Scholar] [CrossRef]
- Zadlo, A.; Burke, J.M.; Sarna, T. Effect of untreated and photobleached bovine RPE melanosomes on the photoinduced peroxidation of lipids. Photochem. Photobiol. Sci. 2009, 8, 830–837. [Google Scholar] [CrossRef]
- Chiarelli-Neto, O.; Pavani, C.; S. Ferreira, A.; Uchoa, A.F.; Severino, D.; Baptista, M.S. Generation and suppression of singlet oxygen in hair by photosensitization of melanin. Free Radic. Biol. Med. 2011, 51, 1195–1202. [Google Scholar] [CrossRef]
- Chiarelli-Neto, O.; Ferreira, A.S.; Martins, W.K.; Pavani, C.; Severino, D.; Faião-Flores, F.; Maria-Engler, S.S.; Aliprandini, E.; Martinez, G.R.; Di Mascio, P.; et al. Melanin photosensitization and the effect of visible light on epithelial cells. PLoS ONE 2014, 9, e113266. [Google Scholar] [CrossRef]
- Szewczyk, G.; Zadlo, A.; Sarna, M.; Ito, S.; Wakamatsu, K.; Sarna, T. Aerobic photoreactivity of synthetic eumelanins and pheomelanins: Generation of singlet oxygen and superoxide anion. Pigment Cell Melanoma Res. 2016, 29, 669–678. [Google Scholar] [CrossRef]
- Ito, S.; Kikuta, M.; Koike, S.; Szewczyk, G.; Sarna, M.; Zadlo, A.; Sarna, T.; Wakamatsu, K. Roles of reactive oxygen species in UVA-induced oxidation of 5,6-dihydroxyindole-2-carboxylic acid-melanin as studied by differential spectrophotometric method. Pigment Cell Melanoma Res. 2016, 29, 340–351. [Google Scholar] [CrossRef] [PubMed]
- Petropoulos, V.; Mordini, D.; Montorsi, F.; Akturk, M.; Menichetti, A.; Olivati, A.; Petrozza, A.; Morandi, V.; Maiuri, M.; Gianneschi, N.C.; et al. Photochemical Pathways and Light-Enhanced Radical Scavenging Activity of 1,8-Dihydroxynaphthalene Allomelanin. J. Am. Chem. Soc. 2025, 147, 10031–10043. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Menichetti, A.; Mordini, D.; Vicenzi, S.; Pane, A.; Montalti, M. Unexplored Mechanisms of Photoprotection: Synergistic Light Absorption and Antioxidant Activity of Melanin. Antioxidants 2025, 14, 376. https://doi.org/10.3390/antiox14040376
Menichetti A, Mordini D, Vicenzi S, Pane A, Montalti M. Unexplored Mechanisms of Photoprotection: Synergistic Light Absorption and Antioxidant Activity of Melanin. Antioxidants. 2025; 14(4):376. https://doi.org/10.3390/antiox14040376
Chicago/Turabian StyleMenichetti, Arianna, Dario Mordini, Silvia Vicenzi, Agata Pane, and Marco Montalti. 2025. "Unexplored Mechanisms of Photoprotection: Synergistic Light Absorption and Antioxidant Activity of Melanin" Antioxidants 14, no. 4: 376. https://doi.org/10.3390/antiox14040376
APA StyleMenichetti, A., Mordini, D., Vicenzi, S., Pane, A., & Montalti, M. (2025). Unexplored Mechanisms of Photoprotection: Synergistic Light Absorption and Antioxidant Activity of Melanin. Antioxidants, 14(4), 376. https://doi.org/10.3390/antiox14040376





