Abstract
Plants face various stresses, particularly water deficit, which negatively impacts photosynthesis, growth, and development, thereby limiting agricultural production. Utilizing growth regulators, such as amino acids and polyamines, to enhance osmotic stress tolerance is a crucial area of research in sustainable agriculture. This study investigates the impact of arginine and spermine treatments on various growth attributes, enzymatic and non-enzymatic antioxidants, photosynthetic pigments, protein and lipid peroxidation, and yield traits of fenugreek plants under both normal and drought conditions. The results indicate that drought conditions significantly reduce morphological characteristics, leaf pigments, and yield traits. However, the application of arginine and spermine enhances these parameters, with spermine showing a more pronounced effect. Additionally, treatments boost antioxidant enzymes activities and improve the levels of non-enzymatic antioxidants and osmolytes, contributing to better stress tolerance and growth performance. Principal component analysis confirms that drought significantly alters plant physiology, increasing proline and malondialdehyde levels, while arginine and spermine alleviate drought stress by enhancing antioxidant activity and osmolyte accumulation. The current investigation aims to evaluate the effectiveness of spermine and arginine treatments on various growth attributes and stress tolerance of fenugreek plants under normal and drought conditions, focusing on their comparative efficacy.
1. Introduction
Fenugreek (Trigonella foenum-graecum L.) is a versatile annual herb from the Fabaceae family, extensively grown in India and across the Mediterranean region, Northern Africa, China, parts of Europe, Australia, and recently North America [1]. Traditionally used as a food and medicine, fenugreek seeds are incorporated into wheat and maize flour in Egypt and serve as a spice, a vegetable, forage for cattle, and for medicinal purposes [2]. The plant is rich in biochemical constituents such as steroids, saponins, polysaccharides, alkaloids, volatile oils, fixed oil, proteins, sugars, mucilage, and flavonoids, which contribute to its medicinal and pharmaceutical significance [3]. Its known for its immunological, anticarcinogenic, antioxidant, antidiabetic, and hypocholesterolemic activities, making it valuable in treating ailments like diabetes and hyperglycemia [4]. Additionally, it is a rich source of protein, lysine, essential nutrients, dietary fiber, and steroid saponins, which are commercially useful for steroid hormone synthesis [5]. Fenugreek’s diverse applications and health benefits underscore its importance as a medicinal and economical plant.
Plant growth and productivity are significantly impacted by various abiotic and biotic stress factors, including low temperature, salt, drought, flooding, heat, oxidative stress, heavy metal toxicity, and pathogens [6,7,8,9,10,11,12]. Drought stress, in particular, poses a major challenge, affecting approximately one-third of the potentially viable land globally due to inadequate water supply. This stress leads to various physiological and biochemical effects on plants, disrupting growth, metabolism, development, productivity, and molecular expressions [13,14,15]. It induces disturbances in the photosynthetic process and carbon metabolism and causes partial stomatal closure, reducing carbon dioxide availability and causing imbalances in nitrogen and carbon metabolism [16]. The upcoming water shortage in certain regions around the world presents a significant challenge to agricultural development and crop production. Under drought conditions, plants store osmolytes like sugars and amino acids to regulate water uptake [17]. Sugars are more efficient than proline in replacing water, forming a hydration shell around biomolecules. Proteins are essential for all physiological activities in plant cells, and their levels rise under drought stress [18]. Drought also increases phenols and flavonoids in plants, enhancing their stress tolerance [19]. Stress-induced reactive oxygen species (ROS) deactivate enzyme functionality and cause oxidative disruption to lipids, proteins, and nucleic acids [20], leading to disruptions in water relationships and membrane stability [21]. Also, it has been shown that fenugreek plants experienced significant reductions in growth, photosynthetic pigments, and proteins under water stress [5].
Plants possess various methods to face environmental stressors and enhance their physiological activity. One effective strategy is the application of chemical compounds, such as plant growth regulators like amino acids and polyamines. These regulators are not only cost-effective but also play a crucial role in boosting plant stress tolerance.
L-arginine is a highly versatile amino acid in living cells, serving as a constituent of proteins and a precursor for the biosynthesis of polyamines, proline, agmatine, and cell signaling molecules like nitric oxide and glutamine [22]. It plays a fundamental role in stress tolerance due to its involvement in numerous physiological processes, including protein synthesis, osmotic potential, stomatal activity, and vegetative growth [23,24]. Investigations have confirmed that L-arginine is a vital modulator in various processes within higher plants and in their response to stress factors like salinity, water deficiency, and disease [25,26,27]. Arginine is particularly important in nitrogen metabolism in germinating seeds and developing seedlings [24]. It is widely used to increase growth, chemical constituents, and yields of crops. Arginine induces enzymes responsible for antioxidation, such as ascorbate peroxidase, superoxide dismutase, and glutathione reductase, thereby alleviating several stressors effects [28].
Polyamines, such as spermine, spermidine, and putrescine, are low-molecular-weight aliphatic amines found in plant cells [29]. They are essential for various fundamental processes, including cell division, differentiation, root elongation, floral development, leaf senescence, fruit ripening, protein translation, transcript expression, and chromatin organization [30]. Several studies have clarified the essential role of polyamines in enhancing plant tolerance to abiotic stresses. They protect against oxidative damage by preventing lipid peroxidation and neutralizing free radicals [31,32]. Spermine, in particular, modulates several physio-biochemical processes to reduce oxidative damage and enhance plant protection against multiple abiotic and biotic stresses [33]. It acts as a secondary messenger in signaling pathways, regulating plant development and boosting tolerance mechanisms [34]. Spermine applications on various plants have been found to positively impact drought responses by boosting osmolyte accumulation, elevating free polyamine levels, and regulating polyamine biosynthetic genes [35].
The aim of this study is to evaluate the effects of arginine and spermine treatments on the growth attributes, enzymatic and non-enzymatic antioxidants, pigments, osmolytes, and yield traits of fenugreek plants under both normal and drought conditions. This study seeks to determine how these treatments can mitigate the adverse effects of drought stress and enhance plant performance, with a particular focus on the comparative efficacy of arginine and spermine.
2. Materials and Methods
2.1. Layout of the Experiment
The experiment was carried out at the Botanical Research Station, Botany and Microbiology Department, Faculty of Science, Al-Azhar University, Cairo, Egypt. Seeds of the fenugreek, Giza 3 variety, obtained from the Institute of Crops Research, Agricultural Research Centre, Giza, Egypt, were utilized. Thirty fenugreek seeds were sown in 40 cm diameter earthenware pots containing 7 kg of soil. These pots were divided into two groups: control (unstressed) and drought-stressed (induced by PEG-stimulated drought stress). Each group was further divided into three sub-groups: control (untreated), foliar-treated with 1 mM arginine, and foliar-treated with 1 mM spermine. Seven plants remained in each pot after thinning and were irrigated as needed. Arginine and spermine were applied as a foliar spray twice, on the 25th and 35th days after sowing. Randomized plant samples were harvested on the 45th day after sowing for morphological, biochemical, and physiological analysis.
2.2. Plant Lengths and Biomass
Five fenugreek plants from each group were randomly collected to measure growth characteristics, including shoot length (cm), root length (cm), number of leaves, fresh and dry weights of shoots (g), and fresh and dry weights of roots (g).
2.3. Enzymatic Antioxidants
Fenugreek buds were used to extract superoxide dismutase (SOD), peroxidase (POD), polyphenol oxidase (PPO), and catalase (CAT), as described by [36]. SOD activity was determined using the method of Marklund and Marklund [37], which involves measuring pyrogallol reduction at 325 nm. POD activity was measured using the method outlined by Bergmeyer [38], focusing on the increase in pyrogallol absorbance at 470 nm. PPO activity was assessed using the method of Matta [39], which measures changes in catechol absorbance at 495 nm. CAT activity was estimated by measuring the cleavage of hydrogen peroxide following the technique of Aebi [40], with spectrophotometric readings at 240 nm.
2.4. Non-Enzymatic Antioxidants and Osmolytes
Free proline was determined using the method of Bates et al. [41], with proline levels measured at 520 nm. Total soluble sugars were determined following the described method in [42], with absorbance measured at 620 nm. Phenolic compounds were determined using the procedure in [43], with absorbance measured at 725 nm. Free amino acids were extracted and estimated using a series of standard solutions and a ninhydrin reagent, with absorbance measured at 570 nm [44].
2.5. Leaf Pigments
Chlorophylls and carotenoids in fenugreek leaves were quantified according to the described method by Vernon and Seely [45], with absorbance readings taken at 470 nm, 649 nm, and 665 nm.
2.6. Proteins and Malondialdehyde
Total soluble proteins were assayed using the technique in [46], with absorbance measured at 750 nm. Malondialdehyde (MDA) was estimated using the method in [47], with absorbance read at 532 nm, 600 nm, and 450 nm.
2.7. Yield
At the yield stage, five fenugreek plants were randomly selected to assess yield attributes, including weight of pods/plant, number of pods/plant, weight of seeds/plant, number of seeds/plant, and 100-seed weight.
2.8. Statistical Analysis
A two-way ANOVA followed by Tukey’s test was conducted to assess the significance among treatments at a significance level of α = 0.05, ensuring data normality and homogeneity of variances using Shapiro–Wilk’s and Levene’s Median tests, respectively [48]. When assumptions were violated, data transformations were applied. Principal component analysis (PCA) was carried out to illustrate the relationship between treatments and physiological parameters using PC-ORD version 5.
3. Results
3.1. Morphological Attributes
The provided results in Table 1 illustrate the response of various morphological traits of fenugreek plants, including root length, root fresh weight, root dry weight, shoot length, shoot fresh weight, shoot dry weight, and number of leaves, to arginine and spermine treatments under both normal and drought conditions.

Table 1.
Effect of arginine and spermine treatments on the morphological growth characteristics of fenugreek plants under normal and drought conditions. Values are expressed as mean ± standard error. Distinct letters denote significant variations among the means.
Subjecting fenugreek plants to drought conditions caused notable reductions in various morphological characteristics. Specifically, there were decreases of approximately 12.5% in root length, 33.05% in root fresh weight, 50% in root dry weight, 13.71% in shoot length, 31.32% in shoot fresh weight, 36.15% in shoot dry weight, and 29.33% in the number of leaves, compared to plants under normal conditions.
The treatment with arginine and spermine resulted in noticeable improvements in the measured morphological parameters of fenugreek plants under normal conditions. These improvements included increases of approximately 0.8% and 2.7% in root length, 8.15% and 9.01% in root fresh weight, 25% and 41.67% in root dry weight, 13.31% and 17.74% in shoot length, 16.39% and 60.27% in shoot fresh weight, 12.21% and 41.78% in shoot dry weight, and 14.67% and 34.66% in the number of leaves in response to arginine and spermine, respectively.
Both under normal and adverse (drought) conditions, treating fenugreek plants with arginine and spermine showed significant responses in the morphological growth parameters. These included improvements of approximately 5% and 9.8% in root length, 14.1% and 28.21% in root fresh weight, 50% and 94.44% in root dry weight, 9.35% and 11.68% in shoot length, 35.64% and 43.32% in shoot fresh weight, 38.97% and 54.78% in shoot dry weight, and 32.08% and 33.96% in the number of leaves, respectively, when compared with untreated plants under stress.
3.2. Enzymatic Antioxidants
The data presented in Figure 1 demonstrate how different antioxidant enzymes of fenugreek plants, such as superoxide dismutase, peroxidase, polyphenol oxidase, and catalase, respond to treatments with arginine and spermine under both normal and drought conditions.
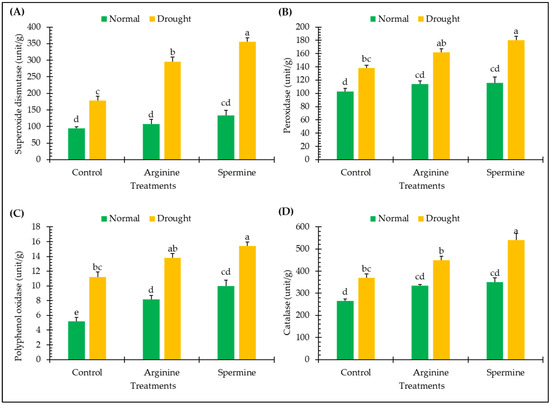
Figure 1.
Effect of arginine and spermine treatments on the activities of superoxide dismutase (A), peroxidase (B), polyphenol oxidase (C), and catalase (D) in fenugreek plants under normal and drought conditions. Bars are expressed as the mean ± the standard error. Distinct letters denote significant variations among the means.
The activities of enzymes of fenugreek plants, such as superoxide dismutase, peroxidase, polyphenol oxidase, and catalase, boosted significantly by approximately 89.36%, 33.98%, 115.38%, 39.62%, respectively, when subjected to drought conditions, as opposed to normal conditions.
Administering arginine and spermine to fenugreek plants led to substantial inductions in the activities of various enzymatic antioxidants under normal conditions. Specifically, superoxide dismutase increased by approximately 14.89% and 42.55%, peroxidase increased by 10.68% and 12.62%, polyphenol oxidase increased by 57.69% and 92.31%, and catalase increased by 26.42% and 32.08%, respectively, in response to arginine and spermine.
Moreover, under drought conditions, fenugreek plants treated with arginine and spermine exhibited notable improvements in the activities of various antioxidant enzymes. These included enhancements of approximately 66.29% and 100% in superoxide dismutase, 17.39% and 30.43% in peroxidase, 23.21% and 37.5% in polyphenol oxidase, and 21.62% and 45.95% in catalase, respectively, in comparison with untreated plants under stress.
3.3. Non-Enzymatic Antioxidants and Osmolytes
The findings in Figure 2 illustrate the impact of arginine and spermine treatments on various non-enzymatic antioxidants and osmolytes of fenugreek plants, such as sugars, proline, amino acids, and phenolics, under both normal and drought conditions.
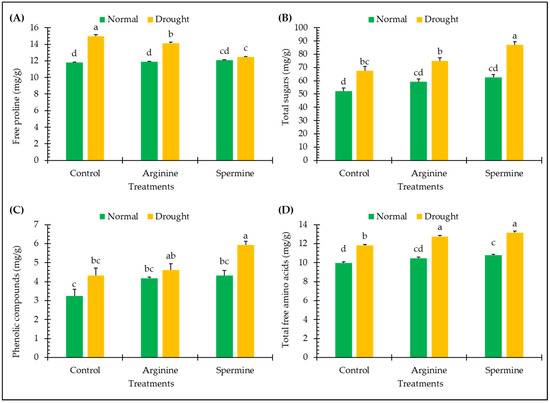
Figure 2.
Effect of arginine and spermine treatments on the levels of free proline (A), total sugars (B), phenolic compounds (C), and total free amino acids (D) in fenugreek plants under normal and drought conditions. Bars are expressed as the mean ± the standard error. Distinct letters denote significant variations among the means.
Under drought conditions, fenugreek plants exhibited significant promotions in the levels of free proline, total sugars, phenolic compounds, and total free amino acids by approximately 26.84%, 29.16%, 33.57%, and 18.38%, respectively, in comparison to plants grown under normal conditions.
Application of arginine and spermine resulted in noticeable improvements in the levels of free proline, total sugars, phenolic compounds, and total free amino acids in fenugreek plants under normal conditions. These enhancements included increases of approximately 0.92% and 2.43% in proline, 13.33% and 19.63% in sugars, 29.18% and 33.38% in phenolics, and 5% and 8.23% in amino acids, respectively, in response to arginine and spermine.
Treating fenugreek plants with arginine and spermine showed significant variations in the abovementioned attributes not only under normal conditions but also under unfavorable (drought) conditions. These included decreases of approximately 5.55% and 16.79% in proline levels and increases of approximately 11.06% and 28.81% in sugars, 6.77% and 37.06% in phenolics, and 7.96% and 11.21% in amino acids, respectively, compared to untreated plants under stress.
3.4. Photosynthetic Pigments
Figure 3 presents the effects of arginine and spermine treatments on certain leaf pigments of fenugreek plants, including chlorophyll a, chlorophyll b, total chlorophylls, and carotenoids, in normal and drought conditions.
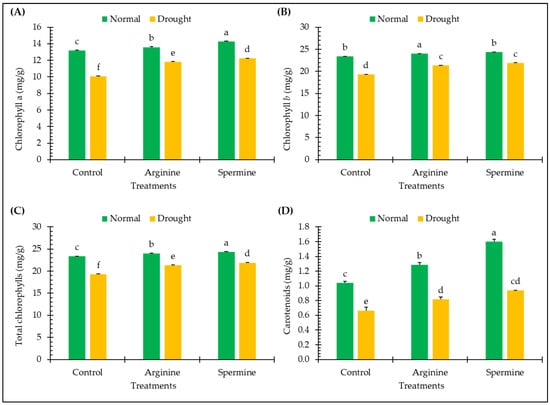
Figure 3.
Effect of arginine and spermine treatments on the contents of chlorophyll a (A), chlorophyll b (B), total chlorophylls (C), and carotenoids (D) in fenugreek plants under normal and drought conditions. Bars are expressed as the mean ± the standard error. Distinct letters denote significant variations among the means.
Drought exposure resulted in substantial decreases in the levels of photosynthetic pigments of fenugreek leaves, including chlorophyll a (23.60%), chlorophyll b (9.69%), total chlorophylls (17.52%), and carotenoids (36.06%), in comparison to plants under normal conditions.
The treatment of fenugreek plants with arginine and spermine under normal conditions led to slight enhancements in leaf pigments especially in carotenoids which were promoted by approximately 23.69% and 53.7% in carotenoids. Furthermore, treating the tested plants with arginine and spermine resulted in significant increases of approximately 17.37% and 21.45% in chlorophyll a, 3.18% and 4.92% in chlorophyll b, 10.59% and 13.55% in total chlorophylls, and 22.67% and 41.14% in carotenoids, respectively, compared to untreated plants under stress.
3.5. Proteins and Malondialdehyde
The results displayed in Figure 4 highlight the impact of arginine and spermine treatments on the amounts of total proteins and malondialdehyde in fenugreek plants in both normal and drought conditions.
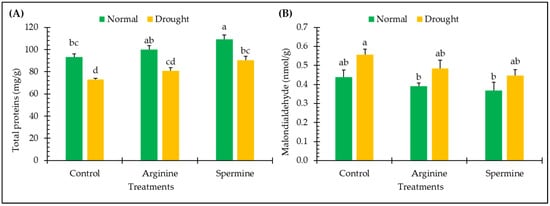
Figure 4.
Effect of arginine and spermine treatments on the amounts of total proteins (A) and malondialdehyde (B) in fenugreek plants under normal and drought conditions. Bars are expressed as the mean ± the standard error. Distinct letters denote significant variations among the means.
The drought stress led to notable variations, with reductions of about 21.68% in total proteins contents, while increments of about 27.06% were observed for the malondialdehyde contents of fenugreek plants, relative to unstressed plants.
Remarkable enhancements of 6.98% and 17.19% for protein contents and noticeable inhibitions of 10.87% and 16.02% for malondialdehyde levels in fenugreek plants were observed under normal conditions following treatment with arginine and spermine. Furthermore, under drought conditions, fenugreek plants treated with arginine and spermine showed obvious accumulations in protein amounts by about 10.55% and 23.91%, while observed suppressions in malondialdehyde contents (as an indicator for the suppression of lipid peroxidation) reached about 12.84% and 19.55%, respectively, compared to untreated plants under stress.
3.6. Yield Attributes
The data in Table 2 show how fenugreek plants’ yield indices, such as pod weight, pod number, seed weight, seed number, and 100-seed weight, are affected by arginine and spermine treatments under both normal and drought conditions.

Table 2.
Effect of arginine and spermine treatments on the yield characteristics of fenugreek plants under normal and drought conditions. Values are expressed as the mean ± the standard error. Distinct letters denote significant variations among the means.
When exposed to drought conditions, fenugreek plants showed significant declines in yield traits, such as weight of pods/plant, number of pods/plant, weight of seeds/plant, number of seeds/plant, and 100-seed weight, by approximately 34.72%, 27.08%, 30.51%, 24.55%, and 18.18%, respectively, compared to those under normal conditions.
The application of arginine and spermine led to noticeable improvements in the yield characteristics of fenugreek plants under normal conditions. These enhancements included increases of approximately 29.27% and 36.63% in the weight of pods, 22.91% and 75% in the number of pods, 41.2% and 64.75% in the weight of seeds, 30.9% and 41.14% in the number of seeds, and 11.9% and 24.69% in the 100-seed weight, respectively, in response to arginine and spermine.
Both under normal and adverse (drought) conditions, treating fenugreek plants with arginine and spermine showed significant responses in yield parameters. These included increases of approximately 12.48% and 29.64% in the weight of pods, 11.43% and 27.14% in the number of pods, 14.95% and 28.16% in the weight of seeds, 14.57% and 23.99% in the number of seeds, 13.17% and 21.81% in the 100-seed weight, respectively, compared to untreated plants under stress.
3.7. Principal Component Analysis
The provided image (Figure 5) from the PCA plot shows distinct clustering of treatments, with drought-stressed treatments (T4, T5, and T6) clearly separated from non-drought treatments (T1, T2, and T3), indicating different physiological responses. Drought alone (T4) induces unique physiological responses, while arginine (T5) and spermine (T6) treatments mitigate drought effects, leading to similar but distinct profiles compared to untreated plants. Non-drought treatments cluster closely, suggesting minimal changes in plant physiology under normal conditions. Physiological parameters such as proline and malondialdehyde (MDA) are strongly associated with drought-induced stress (T4), while antioxidant enzymes (POD, SOD, and CAT), sugars, phenols, and amino acids are linked to T5 and T6, highlighting their role in mitigating drought stress. Growth-related parameters are associated with non-drought treatments, reflecting better growth performance under normal irrigation.
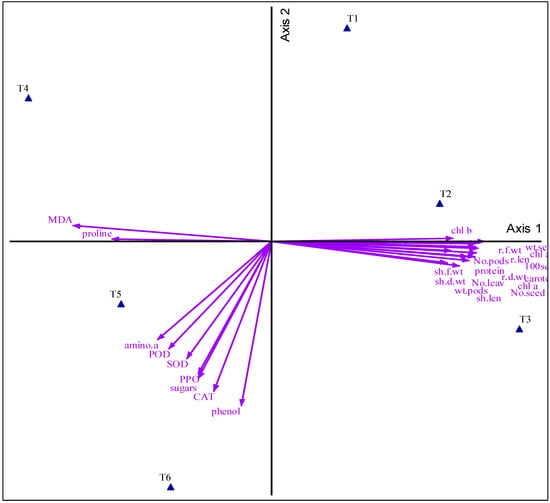
Figure 5.
Principal component analysis visualizes the relationships of fenugreek growth attributes with treatments. Different abbreviations used in the chart are as follows: no treatment under normal irrigation conditions (control) (T1), arginine without drought (T2), spermine without drought (T3), drought (T4), arginine with drought (T5), spermine with drought (T6), r.len (root length), r.f.wt (root fresh weight), r.d.wt (root dry weight), sh.len (shoot length), sh.f.wt (shoot fresh weight), sh.d.wt (shoot dry weight), No.leav (number of leaves), wt.pods (weight of pods), No.pods (number of pods), wt.seed (weight of seeds), No.seed (number of seeds), 100 seed (100-seeds weight), chl a (chlorophyll a), chl b (chlorophyll b), chl a + b (total chlorophyll), caroten (carotenoids), protein, amino.a (amino acids), proline, phenol (phenolic compounds), sugars, MDA (malondialdehyde), SOD (speroxide dismutase), POD (peroxidase), PPO (polyphenol oxidase), and CAT (catalase).
4. Discussion
Enhancing plant growth, development, productivity, and resistance to climatic stress are crucial areas in agriculture and plant-based biotechnologies [49]. Throughout their life cycle, plants face numerous biotic and abiotic stresses, with drought being one of the most severe, leading to significant reductions in agricultural productivity and posing a threat to global food security [50,51]. Drought stress affects plants on both the morphological and molecular levels, decreasing growth and productivity [13]. Addressing plant drought tolerance is a major challenge in modern agriculture, where biostimulants, like amino acids and polyamines, play a vital role [52]. These substances help mitigate the harmful effects of stress and offer essential protection against oxidative damage, ultimately enhancing plant development and productivity [53].
In the current study, drought stress significantly reduced the number of fenugreek morphological parameters, including plant height, root depth, fresh and dry weights of shoots and roots, and the number of leaves. This aligns with previous studies on various crops, showing decreased germination and growth under drought stress [54,55,56,57]. The growth inhibition is attributed to reduced cell turgor, suppressing cell elongation and development, and tissue water loss, hindering cell division and elongation [58,59]. Arginine is a crucial amino acid that significantly contributes to plant growth. Research indicates that its application results in notable enhancements in the morphological growth attributes of various crops [28,60,61]. Our results indicate that foliar application of arginine mitigates drought effects on fenugreek plant growth. Arginine may enhance plant responses to drought stress through its conversion into proline and nitric oxide, which are essential for drought adaptation. Furthermore, arginine’s ability to counteract abiotic stresses could be linked to the production of polyamines, which play significant roles in various biological processes such as growth, metabolism, and stress responses [26,27]. Similarly, the application of spermine in this study resulted in marked improvements in the growth indices of fenugreek under normal or drought conditions, consistent with previous findings on other crops [62,63,64]. Spermine plays a significant role in cell division, elongation, and protein synthesis [65]. It is particularly involved in shoot and root development, floral induction, fruit set, leaf senescence, DNA synthesis, osmolyte balance, chlorophyll protection, gene transcription, and protein translation [62,66]. Spermine plays a vital role in enabling plants to effectively respond to environmental stresses, including drought [35,67].
The activation of enzymatic antioxidants is essential for mitigating stress [68,69]. Enzymatic antioxidant activities increase when mung bean plants face water deficit conditions [70]. Additionally, previous investigations reported that drought stress induces antioxidant enzyme activities in various plants [71,72,73], aligning with our findings. Enhanced antioxidant enzyme activities under water stress are attributed to elevated hydrogen peroxide and singlet oxygen levels. In response, plants typically elevate their antioxidant activities to scavenge reactive oxygen species and mitigate stress [74]. Arginine pretreatment increased enzyme activity in different plants under normal and stress conditions [61,75]. Arginine, an amino acid, plays a significant role in alleviating drought stress [76]. Its application promotes enzyme activity, aiding in converting free radicals into water and oxygen, protecting the cell [26]. Similarly, spermine treatment ameliorated drought-induced osmotic stress by increasing catalase, superoxide dismutase, peroxidase, and polyphenol oxidase activities in several crops [14,77]. Enhanced antioxidant activities resulting from polyamine treatments are associated with improved molecular signaling, which supports adaptive plant responses to water stress [70]. Earlier studies indicate that polyamines help stabilize membranes and neutralize free radicals by boosting antioxidant activities [31].
The current study demonstrated that water scarcity in fenugreek plants led to the accumulation of certain osmolytes and non-enzymatic antioxidants, including proline, sugars, phenolics, and amino acids. These findings align with several studies reporting high levels of osmo-protectants in different crops under drought stress [78,79,80,81]. Additionally, the results showed that arginine and spermine applications increased the content of amino acids, sugars, and phenolics while reducing proline levels, as previously documented in various investigations [32,61,76,82]. The accumulation of these biomolecules serves as a tolerance strategy to reduce the oxidative damage caused by stress. Increases in soluble sugars, proline, and free amino acids in stressed plants help the cells adapt to drought conditions [83,84]. These osmolytes can scavenge free radicals, inhibit cellular redox potential, adjust osmotic pressure, stabilize membranes and proteins, and maintain the relative water content necessary for plant growth and metabolism [6,85,86]. It is noteworthy that proline levels decreased when drought-stressed plants were treated with arginine and spermine, indicating reduced plant sensitivity to drought. This indicates that polyamines and their precursor arginine play crucial roles as modulators in higher plants, influencing growth, physiological processes, development, and responses to stress factors [24,87].
Consistent with our findings, drought stress led to a reduction in chlorophyll and carotenoid levels in cotton [88], wheat [89], peanut [79], barley [28], and rice [90] and other important crops. Drought conditions have been reported to damage the photosynthetic system, reduce gas exchange, and decrease growth parameters and productivity [91]. The decline in the net photosynthetic rate under drought stress is due to biochemical disruptions caused by lipid oxidation and protein denaturation, which are crucial for pigment and chloroplast structures [92]. Conversely, the application of arginine to both normal and stressed fenugreek plants was found to significantly enhance leaf pigment content. Our findings align with previous studies on different plants [60,61,78]. The role of arginine in boosting pigment content can be attributed to its function as an amino acid that serves as a nitrogen source for chlorophyll formation [23]. The ability of arginine to alleviate stress and enhance growth characteristics is likely due to the production of polyamines, which participate in various biological processes such as growth, development, and responses to abiotic stresses [26]. Moreover, spermine greatly enhances the biosynthesis of chlorophyll pigments and PSII function through stomatal regulation, modulation of electron transfer chains to PSI receptors, and improvement in CO2 assimilation rates, plant growth, and biomass yield under stress conditions [93]. This enhancement is linked to the increased stability of thylakoid membranes, plastid biogenesis, and the prevention of chlorophyll degradation [94,95]. Additionally, spermine promotes chlorophyll synthesis by increasing the uptake of magnesium ions, which are essential components of chlorophyll [96].
In the current investigation, drought conditions led to a reduction in protein content and an increase in malondialdehyde (a product of membrane lipid peroxidation). These findings are consistent with studies on maize [97], barley [55], wheat [62], lettuce [67], and soybean [98], regarding lipids, as well as fenugreek [5], soybean [33,98], and cowpea [99], regarding proteins, under various stress conditions. Under stress, plants may enhance proteolytic enzymes, leading to protein degradation, and accumulate excessive reactive oxygen species (ROS), which destabilize cell membranes and cause damage to DNA, pigments, proteins, and lipids [64,100]. The inhibitory effects of drought may be linked to reduced photosynthesis, as carbohydrates, the primary photosynthetic product, are essential for forming vital biomolecules [101]. Supplementing fenugreek plants, whether under natural or stress conditions, with arginine or spermine enhances protein content and reduces malondialdehyde accumulation from lipid oxidation, as documented in previous studies [27,75,77,97]. L-arginine plays a crucial role in physiological processes by modulating polypeptides involved in oxidative stress. It contributes to polyamine synthesis, membrane stability, osmotic balance, signal transduction, and electron transport [25,102]. Arginine’s role in counteracting abiotic stresses may involve polyamine production, which supports growth, metabolism, and stress responses [26]. Spermine also acts a substantial role in post-transcriptional protein modifications, stabilizing protein conformation and function [103]. Exogenous spermine application alleviates stress effects by reducing lipid peroxidation and increasing total polyphenols, catalase, and superoxide dismutase activities [64,104]. Polyamines are essential for protein homeostasis, ROS detoxification, and antioxidative machinery activation under stress conditions [66]. They maintain membrane stability and permeability, enhance catalase activity, and reduce H2O2 content, ROS markers, and lipid peroxidation, thereby providing broad-spectrum tolerance against various stresses [62,105].
A water deficit significantly reduces yield attributes in crops such as tomatoes [106], wheat [107,108], and cotton [80] by negatively impacting growth and productivity. The reduction in growth and yield is associated with the excessive production of reactive oxygen species, which cause damage to cell membranes and components [97,109]. However, the application of arginine significantly increases plant yield under drought stress by promoting protein, proline, and polyamine biosynthesis, enhancing stomatal activity, osmotic potential, and overall growth [23,110]. Amino acids also provide essential substances for protein formation and function as osmo-regulators, increasing cellular osmotic components [31,111]. In this respect, polyamines play a crucial role in physiological processes such as reproductive organ development, tuberization, floral initiation, fruit development, and ripening [66], in addition to their role in maintaining turgor pressure [70,80].
PCA analysis confirms that drought significantly alters plant physiology, increasing proline and MDA levels. Arginine and spermine effectively alleviate drought stress by enhancing antioxidant activity and osmolyte accumulation, supporting their role as protective agents. These substances have a vital impact, highlighting their potential as targeted treatments for enhancing crop resilience to water deficit stress.
5. Conclusions
Applying arginine and spermine has been shown to effectively alleviate the adverse effects of drought stress on fenugreek plants. These treatments enhance both morphological growth characteristics and yield traits, while also improving the activities of antioxidant enzymes, as well as the levels of non-enzymatic antioxidants and osmolytes. Notably, spermine exhibits greater efficacy in promoting growth and stress tolerance. This study underscores the potential of utilizing arginine and spermine as eco-friendly and cost-effective solutions for enhancing plant performance under both normal and drought conditions.
Author Contributions
Conceptualization, A.A.B., N.F.G.S. and H.-A.A.H.; methodology, A.A.B. and N.F.G.S.; software, A.A.B. and W.K.A.; validation, A.A.B. and W.S.A.; formal analysis, A.A.B. and N.F.G.S.; investigation, A.A.B. and W.S.A.; resources, A.A.B. and W.K.A.; data curation, A.A.B. and H.-A.A.H.; writing—original draft preparation, A.A.B., N.F.G.S. and H.-A.A.H.; writing—review and editing, A.A.B., W.K.A., N.F.G.S., W.S.A. and H.-A.A.H.; visualization, A.A.B., W.K.A. and W.S.A.; supervision, A.A.B. and N.F.G.S.; project administration, A.A.B. and H.-A.A.H.; funding acquisition, A.A.B., W.K.A., N.F.G.S., W.S.A. and H.-A.A.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Acknowledgments
The authors would like to thank the faculty members who helped with advice, materials, or methods in producing this research in its final form.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Acharya, K.; Chakraborty, N.; Chatterjee, S. Fungal Diseases of Fenugreek. Am. J. Soc. Issues Humaities 2014, 56–59. [Google Scholar]
- Pandey, H.; Awasthi, P. Effect of Processing Techniques on Nutritional Composition and Antioxidant Activity of Fenugreek (Trigonella foenum-graecum) Seed Flour. J. Food Sci. Technol. 2015, 52, 1054–1060. [Google Scholar] [CrossRef] [PubMed]
- Hanafy, R.S.; Akladious, S.A. Physiological and Molecular Studies on the Effect of Gamma Radiation in Fenugreek (Trigonella foenum-graecum L.) Plants. J. Genet. Eng. Biotechnol. 2018, 16, 683–692. [Google Scholar] [CrossRef]
- Wani, S.A.; Kumar, P. Fenugreek: A Review on Its Nutraceutical Properties and Utilization in Various Food Products. J. Saudi Soc. Agric. Sci. 2018, 17, 97–106. [Google Scholar] [CrossRef]
- Ahmed, M.A.; Shalaby, M.A.F.; El-housini, E.A.; Khater, M.A. Alleviation of Drought Stress on Fenugreek (Trigonella foenum-graecum L.) Plants by Foliar Application of Polyamines Compounds. Middle East J. Appl. Sci. 2018, 8, 883–894. [Google Scholar]
- Badawy, A.A.; Alamri, A.A.; Hussein, H.-A.A.; Mashlawi, A.M.; Kenawy, S.K.M.; El-Shabasy, A. Glycine Betaine Mitigates Heavy Metal Toxicity in Beta vulgaris (L.): An Antioxidant-Driven Approach. Agronomy 2024, 14, 797. [Google Scholar] [CrossRef]
- Attia, M.S.; Hashem, A.H.; Badawy, A.A.; Abdelaziz, A.M. Biocontrol of Early Blight Disease of Eggplant Using Endophytic Aspergillus terreus: Improving Plant Immunological, Physiological and Antifungal Activities. Bot. Stud. 2022, 63, 26. [Google Scholar] [CrossRef]
- Osman, M.S.; Badawy, A.A.; Osman, A.I.; Abdel Latef, A.A.H. Ameliorative Impact of an Extract of the Halophyte Arthrocnemum Macrostachyum on Growth and Biochemical Parameters of Soybean under Salinity Stress. J. Plant Growth Regul. 2021, 40, 1245–1256. [Google Scholar] [CrossRef]
- Ewais, E.A.; Ismail, M.A.; Amin, M.A.; Badawy, A.A. Efficiency of Salicylic Acid and Glycine on Sugar Beet Plants Grown under Heavy Metals Pollution. Egypt. J. Biotechnol. 2015, 48, 112–126. [Google Scholar]
- Sayed, A.I.; Hanafy, M.S. Impact of Sea Salt Stress on Growth and Some Physiological Attributes of Two Soybean (Glycine max L.) Cultivars. Al-Azhar J. Agric. Res. 2021, 46, 88–100. [Google Scholar]
- Hussein, H.-A.A.; Darwesh, O.M.; Mekki, B.B. Environmentally Friendly Nano-Selenium to Improve Antioxidant System and Growth of Groundnut Cultivars under Sandy Soil Conditions. Biocatal. Agric. Biotechnol. 2019, 18, 101080. [Google Scholar] [CrossRef]
- Omer, A.M.; Osman, M.S.; Badawy, A.A. Inoculation with Azospirillum brasilense and/or Pseudomonas geniculata Reinforces Flax (Linum usitatissimum) Growth by Improving Physiological Activities under Saline Soil Conditions. Bot. Stud. 2022, 63, 15. [Google Scholar] [CrossRef] [PubMed]
- Timmusk, S.; Abd El-Daim, I.A.; Copolovici, L.; Tanilas, T.; Kännaste, A.; Behers, L.; Nevo, E.; Seisenbaeva, G.; Stenström, E.; Niinemets, Ü.; et al. Drought-Tolerance of Wheat Improved by Rhizosphere Bacteria from Harsh Environments: Enhanced Biomass Production and Reduced Emissions of Stress Volatiles. PLoS ONE 2014, 9, e96086. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.; Wahid, A.; Lee, D.-J. Exogenously Applied Polyamines Increase Drought Tolerance of Rice by Improving Leaf Water Status, Photosynthesis and Membrane Properties. Acta Physiol. Plant. 2009, 31, 937–945. [Google Scholar] [CrossRef]
- Eid, A.M.; Salim, S.S.; Hassan, S.E.D.; Ismail, M.A.; Fouda, A. Role of Endophytes in Plant Health and Abiotic Stress Management. In Microbiome in Plant Health and Disease; Springer: Singapore, 2019. [Google Scholar] [CrossRef]
- Kasim, W.A.; Hamada, E.A.M.; El-Din, N.G.S.; Eskander, S. Influence of Seaweed Extracts on the Growth, Some Metabolic Activities and Yield of Wheat Grown under Drought Stress. Int. J. Agron. Agric. Res. 2015, 7, 173–189. [Google Scholar]
- Hassanein, R.A.; Amin, A.A.E.-S.; Rashad, E.-S.M.; Hussein, H.-A.H. Effect of Thiourea and Salicylic Acid on Antioxidant Defense of Wheat Plants under Drought Stress. Int. J. ChemTech Res. 2015, 7, 346–354. [Google Scholar]
- Badawy, A.A.; Husen, A.; Salem, S.S. Proteomic Study on the Effects of Silver Nanoparticles Under Abiotic Stress. In Plant Response to Silver Nanoparticles: Plant Growth, Development, Production, and Protection; Husen, A., Ed.; Springer Nature: Singapore, 2025; pp. 79–91. ISBN 978-981-97-7352-7. [Google Scholar]
- Sallam, A.; Alqudah, A.M.; Dawood, M.F.A.A.; Baenziger, P.S.; Börner, A. Drought Stress Tolerance in Wheat and Barley: Advances in Physiology, Breeding and Genetics Research. Int. J. Mol. Sci. 2019, 20, 3137. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive Oxygen Species and Antioxidant Machinery in Abiotic Stress Tolerance in Crop Plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Abid, M.; Ali, S.; Qi, L.K.; Zahoor, R.; Tian, Z.; Jiang, D.; Snider, J.L.; Dai, T. Physiological and Biochemical Changes during Drought and Recovery Periods at Tillering and Jointing Stages in Wheat (Triticum aestivum L.). Sci. Rep. 2018, 8, 4615. [Google Scholar] [CrossRef]
- Liu, J.-H.; Nada, K.; Honda, C.; Kitashiba, H.; Wen, X.-P.; Pang, X.-M.; Moriguchi, T. Polyamine Biosynthesis of Apple Callus under Salt Stress: Importance of the Arginine Decarboxylase Pathway in Stress Response. J. Exp. Bot. 2006, 57, 2589–2599. [Google Scholar] [CrossRef]
- Hamid, Z.H.; Amer, M.; Wahab, A. Effect of Arginine on Growth and Yield of Tomato Plant (Lycopersicon esculentum) under Drought Stress. Plant Arch. 2019, 19, 4441–4444. [Google Scholar]
- Winter, G.; Todd, C.D.; Trovato, M.; Forlani, G.; Funck, D. Physiological Implications of Arginine Metabolism in Plants. Front. Plant Sci. 2015, 6, 534. [Google Scholar] [CrossRef] [PubMed]
- Matysiak, K.; Kierzek, R.; Siatkowski, I.; Kowalska, J.; Krawczyk, R.; Miziniak, W. Effect of Exogenous Application of Amino Acids L-Arginine and Glycine on Maize under Temperature Stress. Agronomy 2020, 10, 769. [Google Scholar] [CrossRef]
- Nasibi, F.; Yaghoobi, M.M.; Kalantari, K.M. Effect of Exogenous Arginine on Alleviation of Oxidative Damage in Tomato Plant Underwater Stress. J. Plant Interact. 2011, 6, 291–296. [Google Scholar] [CrossRef]
- Hussein, H.-A.A.; Alshammari, S.O.; Kenawy, S.K.M.; Elkady, F.M.; Badawy, A.A. Grain-Priming with L-Arginine Improves the Growth Performance of Wheat (Triticum aestivum L.) Plants under Drought Stress. Plants 2022, 11, 1219. [Google Scholar] [CrossRef]
- Shalaby, M.A.F.; Ahmed, M.A.; Khater, M.A. Physiological Responses of Some Barley Cultivars to Foliar Treatments with Arginine under Water Stress Conditions. Middle East J. Agric. Res. 2018, 7, 1102–1123. [Google Scholar]
- Alcázar, R.; Bueno, M.; Tiburcio, A.F. Polyamines: Small Amines with Large Effects on Plant Abiotic Stress Tolerance. Cells 2020, 9, 2373. [Google Scholar] [CrossRef]
- Pascual, L.S.; López-Climent, M.F.; Segarra-Medina, C.; Gómez-Cadenas, A.; Zandalinas, S.I. Exogenous Spermine Alleviates the Negative Effects of Combined Salinity and Paraquat in Tomato Plants by Decreasing Stress-Induced Oxidative Damage. Front. Plant Sci. 2023, 14, 1193207. [Google Scholar] [CrossRef]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative Stress and Some Antioxidant Systems in Acid Rain-Treated Bean Plants: Protective Role of Exogenous Polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Talaat, N.B.; Shawky, B.T. Dual Application of 24-Epibrassinolide and Spermine Confers Drought Stress Tolerance in Maize (Zea mays L.) by Modulating Polyamine and Protein Metabolism. J. Plant Growth Regul. 2016, 35, 518–533. [Google Scholar] [CrossRef]
- Basit, F.; Bhat, J.A.; Guan, Y.; Jan, B.L.; Tyagi, A.; Ahmad, P. Nitric Oxide and Spermine Revealed Positive Defense Interplay for the Regulation of the Chromium Toxicity in Soybean (Glycine max L.). Environ. Pollut. 2022, 308, 119602. [Google Scholar] [CrossRef]
- Diao, Q.; Song, Y.; Qi, H. Exogenous Spermidine Enhances Chilling Tolerance of Tomato (Solanum lycopersicum L.) Seedlings via Involvement in Polyamines Metabolism and Physiological Parameter Levels. Acta Physiol. Plant. 2015, 37, 230. [Google Scholar] [CrossRef]
- Ebeed, H.T.; Hassan, N.M.; Aljarani, A.M. Exogenous Applications of Polyamines Modulate Drought Responses in Wheat through Osmolytes Accumulation, Increasing Free Polyamine Levels and Regulation of Polyamine Biosynthetic Genes. Plant Physiol. Biochem. 2017, 118, 438–448. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.P.; Choudhuri, M.A. Implications of Water Stress-Induced Changes in the Levels of Endogenous Ascorbic Acid and Hydrogen Peroxide in Vigna Seedlings. Physiol. Plant. 1983, 58, 166–170. [Google Scholar] [CrossRef]
- Marklund, S.; Marklund, G. Involvement of the Superoxide Anion Radical in the Autoxidation of Pyrogallol and a Convenient Assay for Superoxide Dismutase. Eur. J. Biochem. 1974, 47, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Bergmeyer, H.U. Methods of Enzymatic Analysis, 2nd ed.; Academic Press Inc.: New York, NY, USA, 1974; Volume 1, p. 974. [Google Scholar]
- Matta, A. Accumulation of Phenols in Tomato Plants Infected by Different Forms of Fusarium oxysporum. Phytopathology 1969, 59, 512–513. [Google Scholar]
- Aebi, H. Catalase in Vitro. Methods Enzimol. 1984, 105, 121–126. [Google Scholar]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid Determination of Free Proline for Water-Stress Studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Umbreit, W.W.; Burris, R.H.; Stauffer, J.F. Manometric Techniques: A Manual Describing Methods Applicable to the Study of Tissue Metabolism; Burgess Publishing Company: Minneapolis, MN, USA, 1964. [Google Scholar]
- Dai, G.H.; Andary, C.; Cosson-Mondolot, L.; Boubals, D. Polyphenols and Resistance of Grapevines to Downy Mildew. Int. Symp. Nat. Phenols Plant Resist. 1993, 381, 763–766. [Google Scholar] [CrossRef]
- Sadasivam, S.; Manickam, A. Biochemical Methods, 3rd ed.; New Age International: New Delhi, India, 2008. [Google Scholar]
- Vernon, L.P.; Seely, G.R. (Eds.) The Chlorophylls; Academic Press: New York, NY, USA, 1966. [Google Scholar]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein Measurement with the Folin Phenol Reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in Isolated Chloroplasts: I. Kinetics and Stoichiometry of Fatty Acid Peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Snedecor, G.W.; Cochran, W.G. Statistical Methods, 8th ed.; The Iowa State University Press: Ames, IA, USA, 1989. [Google Scholar]
- Badawy, A.A.; Husen, A.; Salem, S.S. Use of Nanobiotechnology in Augmenting Soil–Plant System Interaction for Higher Plant Growth and Production. In Essential Minerals in Plant-Soil Systems; Husen, A., Ed.; Plant Biology, Sustainability and Climate Change; Elsevier: Amsterdam, The Netherlands, 2024; pp. 423–443. ISBN 978-0-443-16082-0. [Google Scholar]
- Ma, Y.; Dias, M.C.; Freitas, H. Drought and Salinity Stress Responses and Microbe-Induced Tolerance in Plants. Front. Plant Sci. 2020, 11, 591911. [Google Scholar] [CrossRef] [PubMed]
- Helalia, A.A.; Hassuba, M.M. Efficacy of Several Chemical Fungicides and Biofungicides for Controlling Damping-off and Root Rot Diseases in Common Bean under Field Conditions. Al-Azhar J. Agric. Res. 2021, 46, 154–167. [Google Scholar]
- Gupta, S.; Agarwal, V.P.; Gupta, N.K. Efficacy of Putrescine and Benzyladenine on Photosynthesis and Productivity in Relation to Drought Tolerance in Wheat (Triticum aestivum L.). Physiol. Mol. Biol. Plants 2012, 18, 331–336. [Google Scholar] [CrossRef]
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural Uses of Plant Biostimulants. Plant Soil 2014, 383, 3–41. [Google Scholar] [CrossRef]
- Fathi, A.; Tari, D.B. Effect of Drought Stress and Its Mechanism in Plants. Int. J. Life Sci. 2016, 10, 1–6. [Google Scholar] [CrossRef]
- Ahmad, M.A.; Saleem, A.; Tahir, M.; Khilji, S.A.; Sajid, Z.A.; Landry, K.B.; El-Sheikh, M.A.; Ahmad, P. Modulation of the Polyamines, Osmolytes and Antioxidant Defense System to Ameliorate Drought Stress Tolerance in Hordeum vulgare L. Using Ascorbic Acid. S. Afr. J. Bot. 2024, 171, 726–736. [Google Scholar] [CrossRef]
- Saikia, J.; Sarma, R.K.; Dhandia, R.; Yadav, A.; Bharali, R.; Gupta, V.K.; Saikia, R. Alleviation of Drought Stress in Pulse Crops with ACC Deaminase Producing Rhizobacteria Isolated from Acidic Soil of Northeast India. Sci. Rep. 2018, 8, 3560. [Google Scholar] [CrossRef]
- Faraji, J.; Sepehri, A. Ameliorative Effects of TiO2 Nanoparticles and Sodium Nitroprusside on Seed Germination and Seedling Growth of Wheat under PEG-Stimulated Drought Stress. J. Seed Sci. 2019, 41, 309–317. [Google Scholar] [CrossRef]
- Bogati, K.; Walczak, M. The Impact of Drought Stress on Soil Microbial Community, Enzyme Activities and Plants. Agronomy 2022, 12, 189. [Google Scholar] [CrossRef]
- Hellal, F.; Abdel-Hady, M.; Khatab, I.; El-Sayed, S.; Abdelly, C. Yield Characterization of Mediterranean Barley under Drought Stress Condition. AIMS Agric. Food 2019, 4, 518–533. [Google Scholar] [CrossRef]
- Nejadalimoradi, H.; Nasibi, F.; Kalantari, K.M.; Zanganeh, R. Effect of Seed Priming with L-Arginine and Sodium Nitroprusside on Some Physiological Parameters and Antioxidant Enzymes of Sunflower Plants Exposed to Salt Stress. Agric. Commun. 2014, 2, 23–30. [Google Scholar]
- Ramadan, A.A.; Abd Elhamid, E.M.; Sadak, M.S.; Elhamid, E.M.A.; Sadak, M.S. Comparative Study for the Effect of Arginine and Sodium Nitroprusside on Sunflower Plants Grown under Salinity Stress Conditions. Bull. Natl. Res. Cent. 2019, 43, 118. [Google Scholar] [CrossRef]
- Hassan, N.; Ebeed, H.; Aljaarany, A. Exogenous Application of Spermine and Putrescine Mitigate Adversities of Drought Stress in Wheat by Protecting Membranes and Chloroplast Ultra-Structure. Physiol. Mol. Biol. Plants 2020, 26, 233–245. [Google Scholar] [CrossRef]
- Ekıncı, M.; Yildirim, E.; Dursun, A.; Mohamedsrajaden, N.S. Putrescine, Spermine and Spermidine Mitigated the Salt Stress Damage on Pepper (Capsicum annum L.) Seedling. Yuz. Yıl Univ. J. Agric. Sci. 2019, 29, 290–299. [Google Scholar] [CrossRef]
- Radhakrishnan, R.; Lee, I.J. Ameliorative Effects of Spermine against Osmotic Stress through Antioxidants and Abscisic Acid Changes in Soybean Pods and Seeds. Acta Physiol. Plant. 2013, 35, 263–269. [Google Scholar] [CrossRef]
- Gull, A.; Lone, A.A.; Wani, N.U.I. Biotic and Abiotic Stresses in Plants; IntechOpen: London, UK, 2019; Volume 7, pp. 1–9. [Google Scholar] [CrossRef]
- Tiburcio, A.F.; Altabella, T.; Bitrián, M.; Alcázar, R. The Roles of Polyamines during the Lifespan of Plants: From Development to Stress. Planta 2014, 240, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.J.; Wang, H.R.; Wang, L.; Han, Y.Y.; Hao, J.H.; Fan, S.X. Effects of Different Types of Polyamine on Growth, Physiological and Biochemical Nature of Lettuce under Drought Stress. IOP Conf. Ser. Earth Environ. Sci. 2018, 185, 012010. [Google Scholar] [CrossRef]
- Amin, M.A.; Ismail, M.A.; Badawy, A.A.; Awad, M.A.; Hamza, M.F.; Awad, M.F.; Fouda, A. The Potency of Fungal-Fabricated Selenium Nanoparticles to Improve the Growth Performance of Helianthus annuus L. and Control of Cutworm Agrotis Ipsilon. Catalysts 2021, 11, 1551. [Google Scholar] [CrossRef]
- Osman, M.S.; Badawy, A.A. Influence of Arbuscular Mycorrhizal Fungi (Glomus mosseae) on Enhancing Salinity Tolerance of Two Common Bean Cultivars. Egypt. J. Biotechnol. 2020, 60, 145–162. [Google Scholar]
- Ghassemi, S.; Farhangi-Abriz, S.; Faegi-Analou, R.; Ghorbanpour, M.; Lajayer, B.A. Monitoring Cell Energy, Physiological Functions and Grain Yield in Field-Grown Mung Bean Exposed to Exogenously Applied Polyamines under Drought Stress. J. Soil Sci. Plant Nutr. 2018, 18, 1108–1125. [Google Scholar] [CrossRef]
- Mahdavian, M.; Sarikhani, H.; Hadadinejad, M.; Dehestani, A. Exogenous Application of Putrescine Positively Enhances the Drought Stress Response in Two Citrus Rootstocks by Increasing Expression of Stress-Related Genes. J. Soil Sci. Plant Nutr. 2021, 21, 1934–1948. [Google Scholar] [CrossRef]
- Cui, G.; Zhao, Y.; Zhang, J.; Chao, M.; Xie, K.; Zhang, C.; Sun, F.; Liu, S.; Xi, Y. Proteomic Analysis of the Similarities and Differences of Soil Drought and Polyethylene Glycol Stress Responses in Wheat (Triticum aestivum L.). Plant Mol. Biol. 2019, 100, 391–410. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Gu, W.; Li, C.C.; Li, W.; Li, C.C.; Li, J.; Wei, S. Exogenous Spermidine Improves Drought Tolerance in Maize by Enhancing the Antioxidant Defence System and Regulating Endogenous Polyamine Metabolism. Crop Pasture Sci. 2018, 69, 1076–1091. [Google Scholar] [CrossRef]
- Mosa, K.A.; El-Naggar, M.; Ramamoorthy, K.; Alawadhi, H.; Elnaggar, A.; Wartanian, S.; Ibrahim, E.; Hani, H. Copper Nanoparticles Induced Genotoxicty, Oxidative Stress, and Changes in Superoxide Dismutase (SOD) Gene Expression in Cucumber (Cucumis sativus) Plants. Front. Plant Sci. 2018, 9, 872. [Google Scholar] [CrossRef] [PubMed]
- Nasibi, F.; Barand, A.; Kalantari, K.M. The Effect of Arginine Pretreatment on Germination, Growth and Physiological Parameters in the Increase of Low Temperature Tolerance in Pistacia vera L. in Vitro Culture. Int. J. Agric. Crop Sci. 2013, 5, 1918–1925. [Google Scholar]
- Feiz, F.S.; Hakimi, L.; Mousavi, A.; Jahromi, M.G. The Effects of Glycine Betaine and L-Arginine on Biochemical Properties of Pot Marigold (Calendula officinalis L.) under Water Stress. Iran. J. Plant Physiol. 2019, 9, 2795–2805. [Google Scholar]
- Lee, R.R.I. Spermine Promotes Acclimation to Osmotic Stress by Modifying Antioxidant, Abscisic Acid, and Jasmonic Acid Signals in Soybean. J. Plant Growth Regul. 2013, 32, 22–30. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Rahman, A.; Inafuku, M.; Oku, H.; Fujita, M. Exogenous Nitric Oxide Donor and Arginine Provide Protection against Short-Term Drought Stress in Wheat Seedlings. Physiol. Mol. Biol. Plants 2018, 24, 993–1004. [Google Scholar] [CrossRef]
- El-sayed, S.; Ramadan, A.A.E.; Hellal, F. Drought Stress Mitigation by Application of Algae Extract on Peanut Grown under Sandy Soil Conditions. Asian J. Plant Sci. 2020, 19, 230–239. [Google Scholar] [CrossRef]
- Ahmed, A.H.H.; Darwish, E.; Alobaidy, M.G. Impact of Putrescine and 24-Epibrassinolide on Growth, Yield and Chemical Constituents of Cotton (Gossypium barbadense L.) Plant Grown under Drought Stress Conditions. Asian J. Plant Sci. 2017, 16, 9–23. [Google Scholar] [CrossRef]
- Elewa, T.A.; Sadak, M.S.; Saad, A.M. Proline Treatment Improves Physiological Responses in Quinoa Plants under Drought Stress. Biosci. Res. 2017, 14, 21–33. [Google Scholar]
- Abass, M.; Qin, C.; Maodong, Q.; Xue, X.; Ahmad, P. Spermine Application Alleviates Salinity Induced Growth and Photosynthetic Inhibition in Solanum lycopersicum by Modulating Osmolyte and Secondary Metabolite Accumulation and Di Ff Erentially Regulating Antioxidant Metabo. Plant Physiol. Biochem. 2019, 144, 1–13. [Google Scholar] [CrossRef]
- Tan, Y.; Liang, Z.; Shao, H.; Du, F. Effect of Water Deficits on the Activity of Anti-Oxidative Enzymes and Osmoregulation among Three Different Genotypes of Radix Astragali at Seeding Stage. Colloids Surf. B Biointerfaces 2006, 49, 60–65. [Google Scholar] [CrossRef]
- Badawy, A.A.; Alotaibi, M.O.; Abdelaziz, A.M.; Osman, M.S.; Khalil, A.M.A.; Saleh, A.M.; Mohammed, A.E.; Hashem, A.H. Enhancement of Seawater Stress Tolerance in Barley by the Endophytic Fungus Aspergillus ochraceus. Metabolites 2021, 11, 428. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, S. Physiology of Wheat (Triticum aestivum L.) Accessions and the Role of Phytohormones under Water Stress; Quaid-I-Azam University: Islamabad, Pakistan, 2009. [Google Scholar]
- Abdel Latef, A.A.H.; Omer, A.M.; Badawy, A.A.; Osman, M.S.; Ragaey, M.M. Strategy of Salt Tolerance and Interactive Impact of Azotobacter Chroococcum and/or Alcaligenes Faecalis Inoculation on Canola (Brassica napus L.) Plants Grown in Saline Soil. Plants 2021, 10, 110. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.-H.; Wang, W.; Wu, H.; Gong, X.; Moriguchi, T. Polyamines Function in Stress Tolerance: From Synthesis to Regulation. Front. Plant Sci. 2015, 6, 827. [Google Scholar] [CrossRef] [PubMed]
- Shallan, M.A.; Hassan, H.M.M.; Namich, A.A.M.; Ibrahim, A.A. Effect of Sodium Niroprusside, Putrescine and Glycine Betaine on Alleviation of Drought Stress in Cotton Plant. Am. J. Agric. Environ. Sci. 2012, 12, 1252–1265. [Google Scholar]
- Hammad, S.A.R.; Ali, O.A.M. Physiological and Biochemical Studies on Drought Tolerance of Wheat Plants by Application of Amino Acids and Yeast Extract. Ann. Agric. Sci. 2014, 59, 133–145. [Google Scholar] [CrossRef]
- Eissa, A.A.; Mourad, S.S.B.; Ibrahim, S.D. Evaluation of Some Rice (Oryza sativa L.) Genotypes under Drought Stress Conditions by Using Morphological, Physiological and Molecular Characteristics. Al-Azhar J. Agric. Res. 2023, 48, 179–193. [Google Scholar]
- Anjum, S.A.; Wang, L.; Farooq, M.; Xue, L.; Ali, S. Fulvic Acid Application Improves the Maize Performance under Well-Watered and Drought Conditions. J. Agron. Crop Sci. 2011, 197, 409–417. [Google Scholar] [CrossRef]
- Marcińska, I.; Czyczyło-Mysza, I.; Skrzypek, E.; Filek, M.; Grzesiak, S.; Grzesiak, M.T.; Janowiak, F.; Hura, T.; Dziurka, M.; Dziurka, K.; et al. Impact of Osmotic Stress on Physiological and Biochemical Characteristics in Drought-Susceptible and Drought-Resistant Wheat Genotypes. Acta Physiol. Plant. 2013, 35, 451–461. [Google Scholar] [CrossRef]
- Li, Y.; Han, D.; Hu, G.; Sommerfeld, M.; Hu, Q. Inhibition of Starch Synthesis Results in Overproduction of Lipids in Chlamydomonas reinhardtii. Biotechnol. Bioeng. 2010, 107, 258–268. [Google Scholar] [CrossRef]
- Borrell, A.; Carbonell, L.; Farras, R.; Puig-Parellada, P.; Tiburcio, A.F. Polyamines Inhibit Lipid Peroxidation in Senescing Oat Leaves. Physiol. Plant. 1997, 99, 385–390. [Google Scholar] [CrossRef]
- Pandey, S.; Ranade, S.A.; Nagar, P.K.; Kumar, N. Role of Polyamines and Ethylene as Modulators of Plant Senescence. J. Biosci. 2000, 25, 291–299. [Google Scholar] [CrossRef]
- Puyang, X.; An, M.; Xu, L.; Han, L.; Zhang, X. Protective Effect of Exogenous Spermidine on Ion and Polyamine Metabolism in Kentucky Bluegrass under Salinity Stress. Hortic. Environ. Biotechnol. 2016, 57, 11–19. [Google Scholar] [CrossRef]
- Talaat, N.B.; Shawky, B.T.; Ibrahim, A.S. Alleviation of Drought-Induced Oxidative Stress in Maize (Zea mays L.) Plants by Dual Application of 24-Epibrassinolide and Spermine. Environ. Exp. Bot. 2015, 113, 47–58. [Google Scholar] [CrossRef]
- Demirtas, Ç.; Yazgan, S.; Candogan, B.N.; Sincik, M.; Büyükcangaz, H.; Göksoy, A.T. Quality and Yield Response of Soybean (Glycine max L. Merrill) to Drought Stress in Sub--Humid Environment. Afr. J. Biotechnol. 2010, 9, 6873–6881. [Google Scholar]
- Alsokari, S.S. Synergistic Effect of Kinetin and Spermine on Some Physiological Aspects of Seawater Stressed Vigna sinensis Plants. Saudi J. Biol. Sci. 2011, 18, 37–44. [Google Scholar] [CrossRef]
- Miller, G.A.D.; Suzuki, N.; Ciftci-Yilmaz, S.; Mittler, R.O.N. Reactive Oxygen Species Homeostasis and Signalling during Drought and Salinity Stresses. Plant Cell Environ. 2010, 33, 453–467. [Google Scholar] [CrossRef]
- Kaur, G.; Asthir, B. Molecular Responses to Drought Stress in Plants. Biol. Plant. 2017, 61, 201–209. [Google Scholar] [CrossRef]
- Wang, T.; Liu, Q.; Wang, N.; Dai, J.; Lu, Q.; Jia, X.; Lin, L.; Yu, F.; Zuo, Y. Foliar Arginine Application Improves Tomato Plant Growth, Yield, and Fruit Quality via Nitrogen Accumulation. Plant Growth Regul. 2021, 95, 421–428. [Google Scholar] [CrossRef]
- Huang, X.; Zhou, G.; Yang, W.; Wang, A.; Hu, Z.; Lin, C.; Chen, X. Drought-Inhibited Ribulose-1, 5-Bisphosphate Carboxylase Activity Is Mediated through Increased Release of Ethylene and Changes in the Ratio of Polyamines in Pakchoi. J. Plant Physiol. 2014, 171, 1392–1400. [Google Scholar] [CrossRef]
- Nayyar, H.; Chander, S. Protective Effects of Polyamines against Oxidative Stress Induced by Water and Cold Stress in Chickpea. J. Agron. Crop Sci. 2004, 190, 355–365. [Google Scholar] [CrossRef]
- Takahashi, T.; Kakehi, J.-I. Polyamines: Ubiquitous Polycations with Unique Roles in Growth and Stress Responses. Ann. Bot. 2010, 105, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Abdeldaym, E.A.; Sabra, M.A. The Root Endophytic Fungus Piriformospora indica Improves Growth Performance, Physiological Parameters and Yield of Tomato under Water Stress Condition. Middle East J. Agric. Res. 2018, 7, 1090–1101. [Google Scholar]
- Raza, M.A.S.S.; Saleem, M.F.; Shah, G.M.; Khan, I.H.; Raza, A. Exogenous Application of Glycinebetaine and Potassium for Improving Water Relations and Grain Yield of Wheat under Drought. J. Soil Sci. Plant Nutr. 2014, 14, 348–364. [Google Scholar] [CrossRef]
- Hussein, H.-A.A.; Alshammari, S.O.; Abd El-sadek, M.E.; Kenawy, S.K.M.; Badawy, A.A. The Promotive Effect of Putrescine on Growth, Biochemical Constituents, and Yield of Wheat (Triticum aestivum L.) Plants under Water Stress. Agriculture 2023, 13, 587. [Google Scholar] [CrossRef]
- Shemi, R.; Wang, R.; Gheith, E.-S.M.S.; Hussain, H.A.; Hussain, S.; Irfan, M.; Cholidah, L.; Zhang, K.; Zhang, S.; Wang, L. Effects of Salicylic Acid, Zinc and Glycine Betaine on Morpho-physiological Growth and Yield of Maize under Drought Stress. Sci. Rep. 2021, 11, 3195. [Google Scholar] [CrossRef]
- Sadak, M.S.; Abdelhamid, M.T.; Schmidhalter, U. Effect of Foliar Application of Amino Acids on Plant Yield and Some Physiological Parameters in Bean Plants Irrigated with Seawater. Acta Biol. Colomb. 2015, 20, 141–152. [Google Scholar] [CrossRef]
- El-Bassiouny, H.M.; Mostafa, H.A.; El-Khawas, S.A.; Hassanein, R.A.; Khalil, S.I.; Abd El-Monem, A.A. Physiological Responses of Wheat Plant to Foliar Treatments with Arginine or Putrescine. Aust. J. Basic Appl. Sci. 2008, 2, 1390–1403. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).