Arginine and Spermine Ameliorate Water Deficit Stress in Fenugreek (Trigonella foenum-graecum L.) by Enhancing Growth and Physio-Biochemical Processes
Abstract
1. Introduction
2. Materials and Methods
2.1. Layout of the Experiment
2.2. Plant Lengths and Biomass
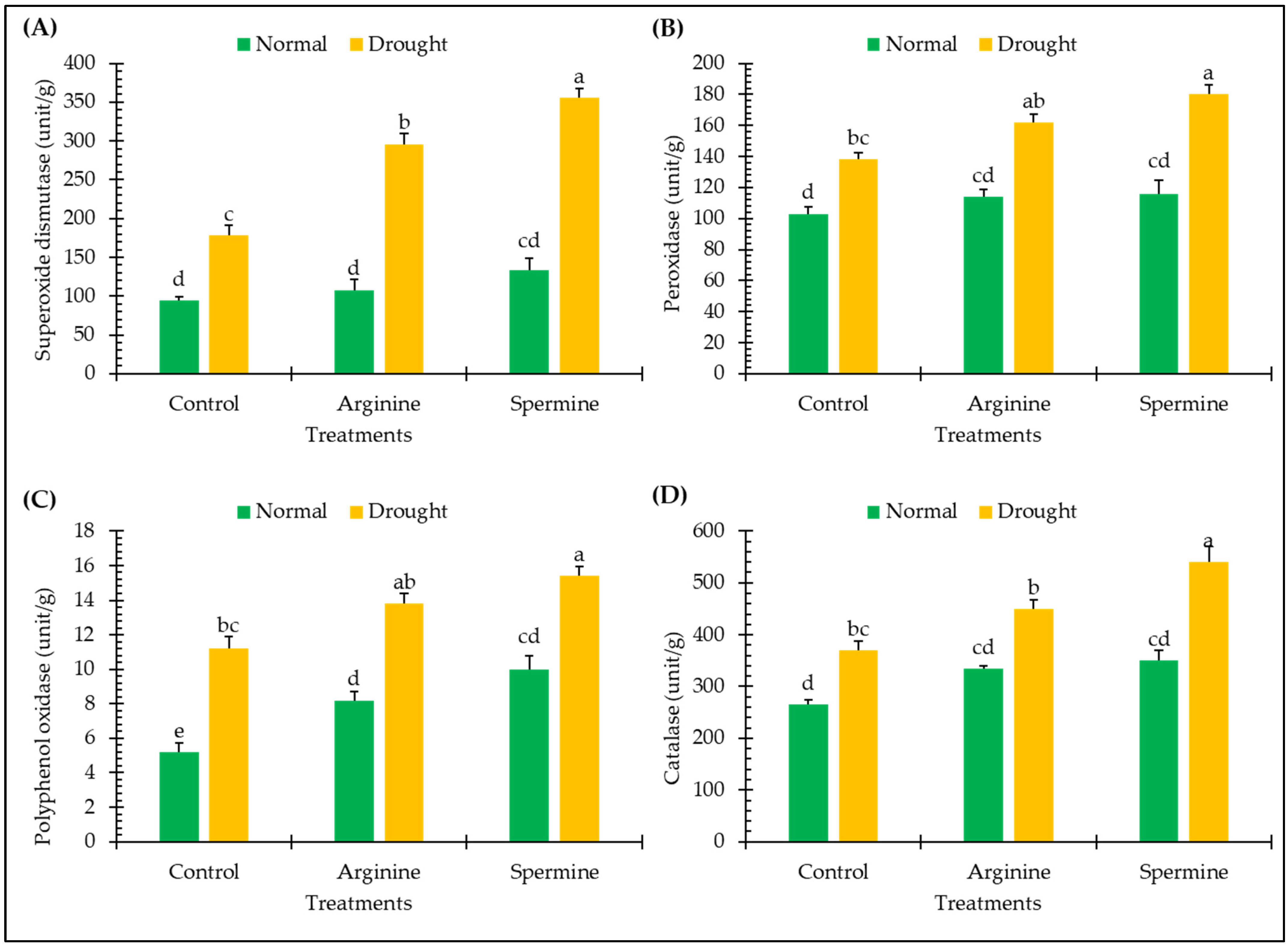
2.3. Enzymatic Antioxidants
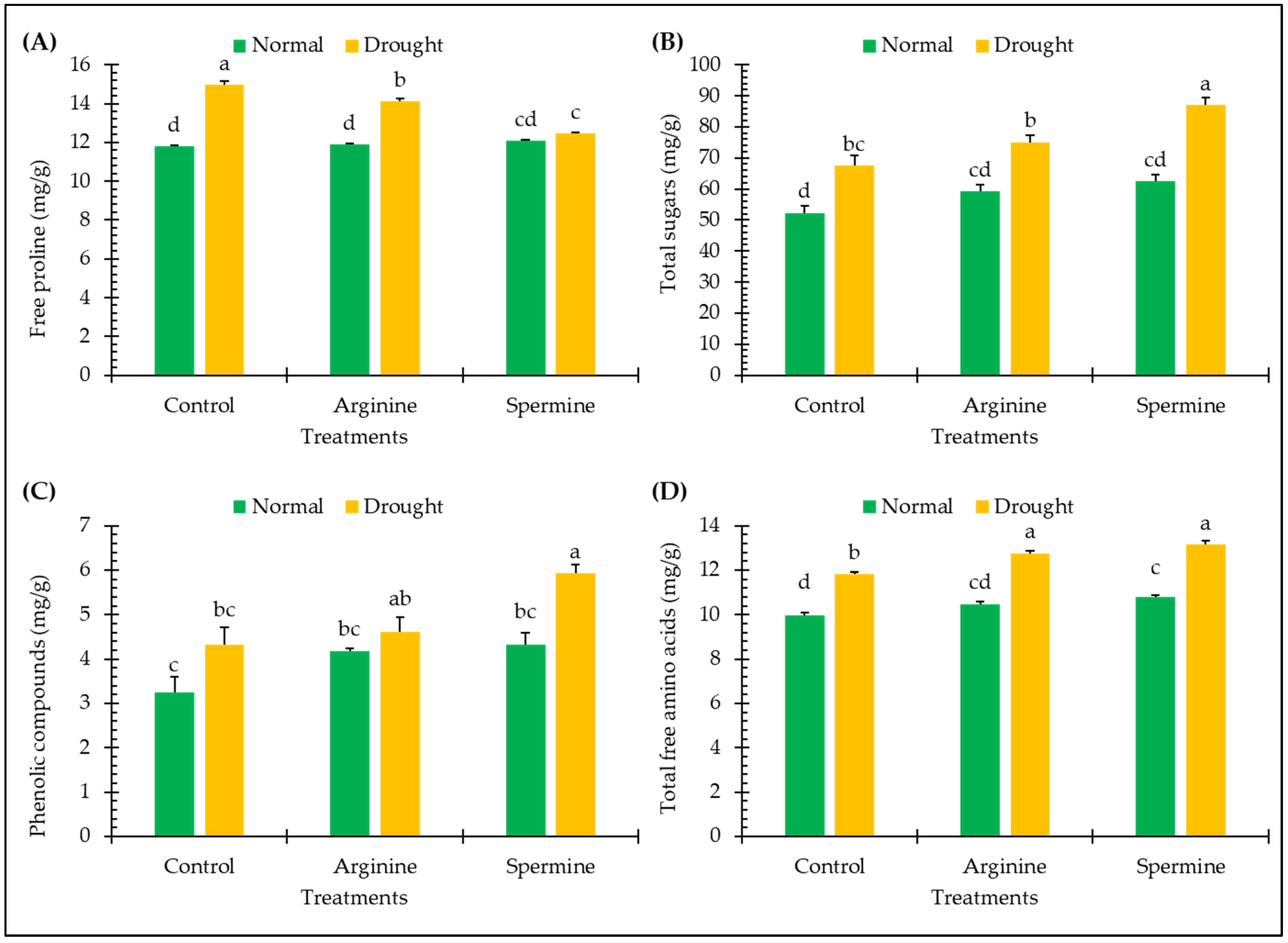
2.4. Non-Enzymatic Antioxidants and Osmolytes
2.5. Leaf Pigments
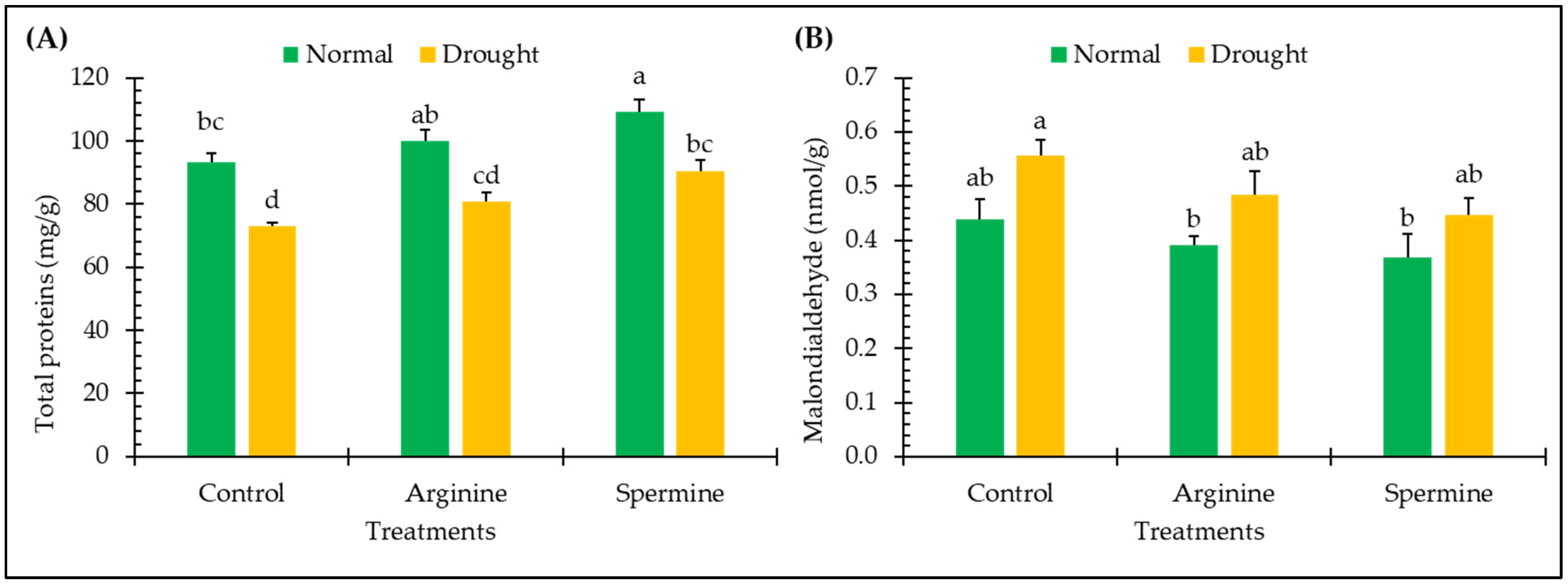
2.6. Proteins and Malondialdehyde
2.7. Yield
2.8. Statistical Analysis
3. Results
3.1. Morphological Attributes
3.2. Enzymatic Antioxidants
3.3. Non-Enzymatic Antioxidants and Osmolytes
3.4. Photosynthetic Pigments
3.5. Proteins and Malondialdehyde
3.6. Yield Attributes
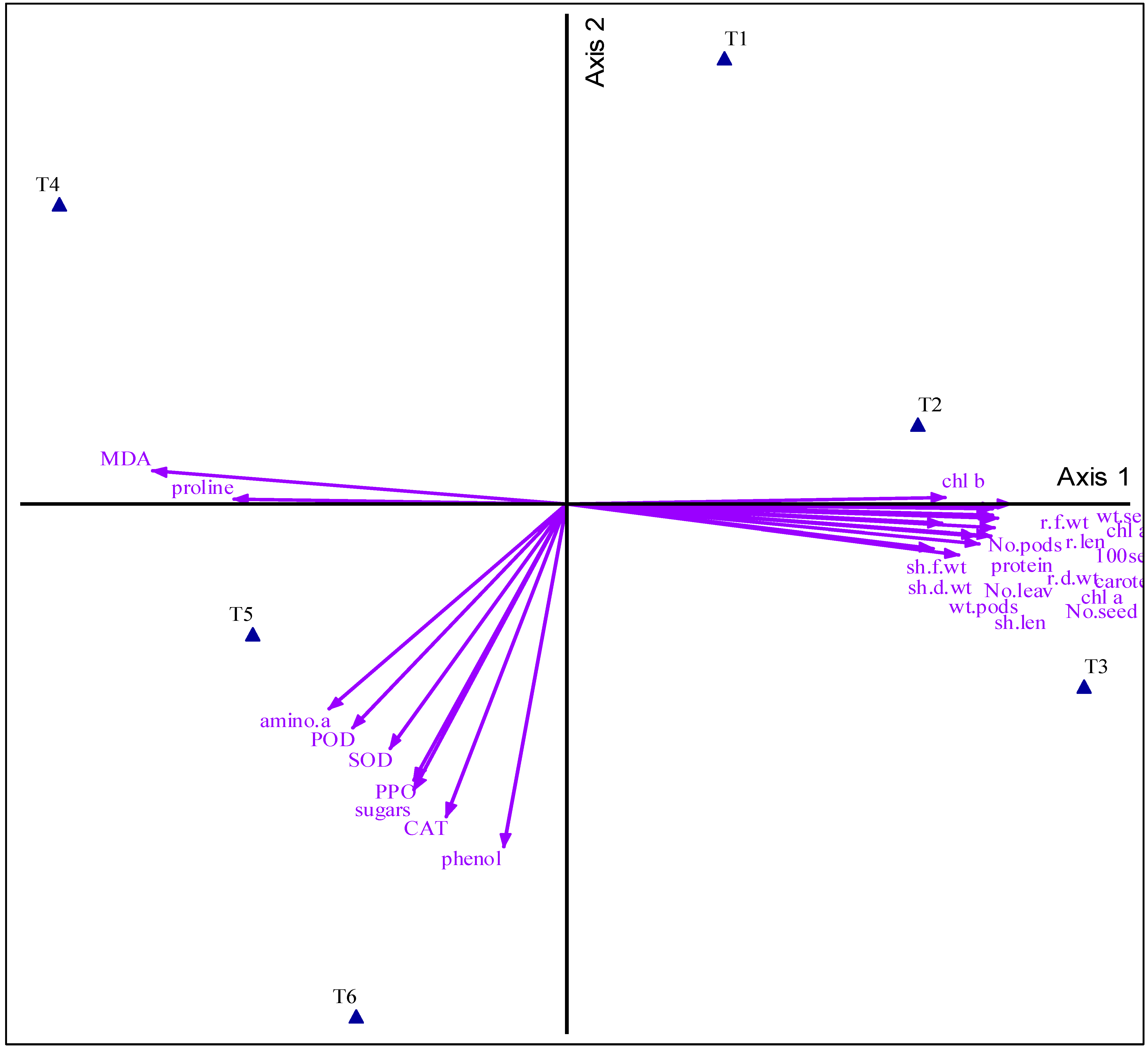
3.7. Principal Component Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Acharya, K.; Chakraborty, N.; Chatterjee, S. Fungal Diseases of Fenugreek. Am. J. Soc. Issues Humaities 2014, 56–59. [Google Scholar]
- Pandey, H.; Awasthi, P. Effect of Processing Techniques on Nutritional Composition and Antioxidant Activity of Fenugreek (Trigonella foenum-graecum) Seed Flour. J. Food Sci. Technol. 2015, 52, 1054–1060. [Google Scholar] [CrossRef] [PubMed]
- Hanafy, R.S.; Akladious, S.A. Physiological and Molecular Studies on the Effect of Gamma Radiation in Fenugreek (Trigonella foenum-graecum L.) Plants. J. Genet. Eng. Biotechnol. 2018, 16, 683–692. [Google Scholar] [CrossRef]
- Wani, S.A.; Kumar, P. Fenugreek: A Review on Its Nutraceutical Properties and Utilization in Various Food Products. J. Saudi Soc. Agric. Sci. 2018, 17, 97–106. [Google Scholar] [CrossRef]
- Ahmed, M.A.; Shalaby, M.A.F.; El-housini, E.A.; Khater, M.A. Alleviation of Drought Stress on Fenugreek (Trigonella foenum-graecum L.) Plants by Foliar Application of Polyamines Compounds. Middle East J. Appl. Sci. 2018, 8, 883–894. [Google Scholar]
- Badawy, A.A.; Alamri, A.A.; Hussein, H.-A.A.; Mashlawi, A.M.; Kenawy, S.K.M.; El-Shabasy, A. Glycine Betaine Mitigates Heavy Metal Toxicity in Beta vulgaris (L.): An Antioxidant-Driven Approach. Agronomy 2024, 14, 797. [Google Scholar] [CrossRef]
- Attia, M.S.; Hashem, A.H.; Badawy, A.A.; Abdelaziz, A.M. Biocontrol of Early Blight Disease of Eggplant Using Endophytic Aspergillus terreus: Improving Plant Immunological, Physiological and Antifungal Activities. Bot. Stud. 2022, 63, 26. [Google Scholar] [CrossRef]
- Osman, M.S.; Badawy, A.A.; Osman, A.I.; Abdel Latef, A.A.H. Ameliorative Impact of an Extract of the Halophyte Arthrocnemum Macrostachyum on Growth and Biochemical Parameters of Soybean under Salinity Stress. J. Plant Growth Regul. 2021, 40, 1245–1256. [Google Scholar] [CrossRef]
- Ewais, E.A.; Ismail, M.A.; Amin, M.A.; Badawy, A.A. Efficiency of Salicylic Acid and Glycine on Sugar Beet Plants Grown under Heavy Metals Pollution. Egypt. J. Biotechnol. 2015, 48, 112–126. [Google Scholar]
- Sayed, A.I.; Hanafy, M.S. Impact of Sea Salt Stress on Growth and Some Physiological Attributes of Two Soybean (Glycine max L.) Cultivars. Al-Azhar J. Agric. Res. 2021, 46, 88–100. [Google Scholar]
- Hussein, H.-A.A.; Darwesh, O.M.; Mekki, B.B. Environmentally Friendly Nano-Selenium to Improve Antioxidant System and Growth of Groundnut Cultivars under Sandy Soil Conditions. Biocatal. Agric. Biotechnol. 2019, 18, 101080. [Google Scholar] [CrossRef]
- Omer, A.M.; Osman, M.S.; Badawy, A.A. Inoculation with Azospirillum brasilense and/or Pseudomonas geniculata Reinforces Flax (Linum usitatissimum) Growth by Improving Physiological Activities under Saline Soil Conditions. Bot. Stud. 2022, 63, 15. [Google Scholar] [CrossRef] [PubMed]
- Timmusk, S.; Abd El-Daim, I.A.; Copolovici, L.; Tanilas, T.; Kännaste, A.; Behers, L.; Nevo, E.; Seisenbaeva, G.; Stenström, E.; Niinemets, Ü.; et al. Drought-Tolerance of Wheat Improved by Rhizosphere Bacteria from Harsh Environments: Enhanced Biomass Production and Reduced Emissions of Stress Volatiles. PLoS ONE 2014, 9, e96086. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.; Wahid, A.; Lee, D.-J. Exogenously Applied Polyamines Increase Drought Tolerance of Rice by Improving Leaf Water Status, Photosynthesis and Membrane Properties. Acta Physiol. Plant. 2009, 31, 937–945. [Google Scholar] [CrossRef]
- Eid, A.M.; Salim, S.S.; Hassan, S.E.D.; Ismail, M.A.; Fouda, A. Role of Endophytes in Plant Health and Abiotic Stress Management. In Microbiome in Plant Health and Disease; Springer: Singapore, 2019. [Google Scholar] [CrossRef]
- Kasim, W.A.; Hamada, E.A.M.; El-Din, N.G.S.; Eskander, S. Influence of Seaweed Extracts on the Growth, Some Metabolic Activities and Yield of Wheat Grown under Drought Stress. Int. J. Agron. Agric. Res. 2015, 7, 173–189. [Google Scholar]
- Hassanein, R.A.; Amin, A.A.E.-S.; Rashad, E.-S.M.; Hussein, H.-A.H. Effect of Thiourea and Salicylic Acid on Antioxidant Defense of Wheat Plants under Drought Stress. Int. J. ChemTech Res. 2015, 7, 346–354. [Google Scholar]
- Badawy, A.A.; Husen, A.; Salem, S.S. Proteomic Study on the Effects of Silver Nanoparticles Under Abiotic Stress. In Plant Response to Silver Nanoparticles: Plant Growth, Development, Production, and Protection; Husen, A., Ed.; Springer Nature: Singapore, 2025; pp. 79–91. ISBN 978-981-97-7352-7. [Google Scholar]
- Sallam, A.; Alqudah, A.M.; Dawood, M.F.A.A.; Baenziger, P.S.; Börner, A. Drought Stress Tolerance in Wheat and Barley: Advances in Physiology, Breeding and Genetics Research. Int. J. Mol. Sci. 2019, 20, 3137. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive Oxygen Species and Antioxidant Machinery in Abiotic Stress Tolerance in Crop Plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Abid, M.; Ali, S.; Qi, L.K.; Zahoor, R.; Tian, Z.; Jiang, D.; Snider, J.L.; Dai, T. Physiological and Biochemical Changes during Drought and Recovery Periods at Tillering and Jointing Stages in Wheat (Triticum aestivum L.). Sci. Rep. 2018, 8, 4615. [Google Scholar] [CrossRef]
- Liu, J.-H.; Nada, K.; Honda, C.; Kitashiba, H.; Wen, X.-P.; Pang, X.-M.; Moriguchi, T. Polyamine Biosynthesis of Apple Callus under Salt Stress: Importance of the Arginine Decarboxylase Pathway in Stress Response. J. Exp. Bot. 2006, 57, 2589–2599. [Google Scholar] [CrossRef]
- Hamid, Z.H.; Amer, M.; Wahab, A. Effect of Arginine on Growth and Yield of Tomato Plant (Lycopersicon esculentum) under Drought Stress. Plant Arch. 2019, 19, 4441–4444. [Google Scholar]
- Winter, G.; Todd, C.D.; Trovato, M.; Forlani, G.; Funck, D. Physiological Implications of Arginine Metabolism in Plants. Front. Plant Sci. 2015, 6, 534. [Google Scholar] [CrossRef] [PubMed]
- Matysiak, K.; Kierzek, R.; Siatkowski, I.; Kowalska, J.; Krawczyk, R.; Miziniak, W. Effect of Exogenous Application of Amino Acids L-Arginine and Glycine on Maize under Temperature Stress. Agronomy 2020, 10, 769. [Google Scholar] [CrossRef]
- Nasibi, F.; Yaghoobi, M.M.; Kalantari, K.M. Effect of Exogenous Arginine on Alleviation of Oxidative Damage in Tomato Plant Underwater Stress. J. Plant Interact. 2011, 6, 291–296. [Google Scholar] [CrossRef]
- Hussein, H.-A.A.; Alshammari, S.O.; Kenawy, S.K.M.; Elkady, F.M.; Badawy, A.A. Grain-Priming with L-Arginine Improves the Growth Performance of Wheat (Triticum aestivum L.) Plants under Drought Stress. Plants 2022, 11, 1219. [Google Scholar] [CrossRef]
- Shalaby, M.A.F.; Ahmed, M.A.; Khater, M.A. Physiological Responses of Some Barley Cultivars to Foliar Treatments with Arginine under Water Stress Conditions. Middle East J. Agric. Res. 2018, 7, 1102–1123. [Google Scholar]
- Alcázar, R.; Bueno, M.; Tiburcio, A.F. Polyamines: Small Amines with Large Effects on Plant Abiotic Stress Tolerance. Cells 2020, 9, 2373. [Google Scholar] [CrossRef]
- Pascual, L.S.; López-Climent, M.F.; Segarra-Medina, C.; Gómez-Cadenas, A.; Zandalinas, S.I. Exogenous Spermine Alleviates the Negative Effects of Combined Salinity and Paraquat in Tomato Plants by Decreasing Stress-Induced Oxidative Damage. Front. Plant Sci. 2023, 14, 1193207. [Google Scholar] [CrossRef]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative Stress and Some Antioxidant Systems in Acid Rain-Treated Bean Plants: Protective Role of Exogenous Polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Talaat, N.B.; Shawky, B.T. Dual Application of 24-Epibrassinolide and Spermine Confers Drought Stress Tolerance in Maize (Zea mays L.) by Modulating Polyamine and Protein Metabolism. J. Plant Growth Regul. 2016, 35, 518–533. [Google Scholar] [CrossRef]
- Basit, F.; Bhat, J.A.; Guan, Y.; Jan, B.L.; Tyagi, A.; Ahmad, P. Nitric Oxide and Spermine Revealed Positive Defense Interplay for the Regulation of the Chromium Toxicity in Soybean (Glycine max L.). Environ. Pollut. 2022, 308, 119602. [Google Scholar] [CrossRef]
- Diao, Q.; Song, Y.; Qi, H. Exogenous Spermidine Enhances Chilling Tolerance of Tomato (Solanum lycopersicum L.) Seedlings via Involvement in Polyamines Metabolism and Physiological Parameter Levels. Acta Physiol. Plant. 2015, 37, 230. [Google Scholar] [CrossRef]
- Ebeed, H.T.; Hassan, N.M.; Aljarani, A.M. Exogenous Applications of Polyamines Modulate Drought Responses in Wheat through Osmolytes Accumulation, Increasing Free Polyamine Levels and Regulation of Polyamine Biosynthetic Genes. Plant Physiol. Biochem. 2017, 118, 438–448. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.P.; Choudhuri, M.A. Implications of Water Stress-Induced Changes in the Levels of Endogenous Ascorbic Acid and Hydrogen Peroxide in Vigna Seedlings. Physiol. Plant. 1983, 58, 166–170. [Google Scholar] [CrossRef]
- Marklund, S.; Marklund, G. Involvement of the Superoxide Anion Radical in the Autoxidation of Pyrogallol and a Convenient Assay for Superoxide Dismutase. Eur. J. Biochem. 1974, 47, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Bergmeyer, H.U. Methods of Enzymatic Analysis, 2nd ed.; Academic Press Inc.: New York, NY, USA, 1974; Volume 1, p. 974. [Google Scholar]
- Matta, A. Accumulation of Phenols in Tomato Plants Infected by Different Forms of Fusarium oxysporum. Phytopathology 1969, 59, 512–513. [Google Scholar]
- Aebi, H. Catalase in Vitro. Methods Enzimol. 1984, 105, 121–126. [Google Scholar]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid Determination of Free Proline for Water-Stress Studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Umbreit, W.W.; Burris, R.H.; Stauffer, J.F. Manometric Techniques: A Manual Describing Methods Applicable to the Study of Tissue Metabolism; Burgess Publishing Company: Minneapolis, MN, USA, 1964. [Google Scholar]
- Dai, G.H.; Andary, C.; Cosson-Mondolot, L.; Boubals, D. Polyphenols and Resistance of Grapevines to Downy Mildew. Int. Symp. Nat. Phenols Plant Resist. 1993, 381, 763–766. [Google Scholar] [CrossRef]
- Sadasivam, S.; Manickam, A. Biochemical Methods, 3rd ed.; New Age International: New Delhi, India, 2008. [Google Scholar]
- Vernon, L.P.; Seely, G.R. (Eds.) The Chlorophylls; Academic Press: New York, NY, USA, 1966. [Google Scholar]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein Measurement with the Folin Phenol Reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in Isolated Chloroplasts: I. Kinetics and Stoichiometry of Fatty Acid Peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Snedecor, G.W.; Cochran, W.G. Statistical Methods, 8th ed.; The Iowa State University Press: Ames, IA, USA, 1989. [Google Scholar]
- Badawy, A.A.; Husen, A.; Salem, S.S. Use of Nanobiotechnology in Augmenting Soil–Plant System Interaction for Higher Plant Growth and Production. In Essential Minerals in Plant-Soil Systems; Husen, A., Ed.; Plant Biology, Sustainability and Climate Change; Elsevier: Amsterdam, The Netherlands, 2024; pp. 423–443. ISBN 978-0-443-16082-0. [Google Scholar]
- Ma, Y.; Dias, M.C.; Freitas, H. Drought and Salinity Stress Responses and Microbe-Induced Tolerance in Plants. Front. Plant Sci. 2020, 11, 591911. [Google Scholar] [CrossRef] [PubMed]
- Helalia, A.A.; Hassuba, M.M. Efficacy of Several Chemical Fungicides and Biofungicides for Controlling Damping-off and Root Rot Diseases in Common Bean under Field Conditions. Al-Azhar J. Agric. Res. 2021, 46, 154–167. [Google Scholar]
- Gupta, S.; Agarwal, V.P.; Gupta, N.K. Efficacy of Putrescine and Benzyladenine on Photosynthesis and Productivity in Relation to Drought Tolerance in Wheat (Triticum aestivum L.). Physiol. Mol. Biol. Plants 2012, 18, 331–336. [Google Scholar] [CrossRef]
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural Uses of Plant Biostimulants. Plant Soil 2014, 383, 3–41. [Google Scholar] [CrossRef]
- Fathi, A.; Tari, D.B. Effect of Drought Stress and Its Mechanism in Plants. Int. J. Life Sci. 2016, 10, 1–6. [Google Scholar] [CrossRef]
- Ahmad, M.A.; Saleem, A.; Tahir, M.; Khilji, S.A.; Sajid, Z.A.; Landry, K.B.; El-Sheikh, M.A.; Ahmad, P. Modulation of the Polyamines, Osmolytes and Antioxidant Defense System to Ameliorate Drought Stress Tolerance in Hordeum vulgare L. Using Ascorbic Acid. S. Afr. J. Bot. 2024, 171, 726–736. [Google Scholar] [CrossRef]
- Saikia, J.; Sarma, R.K.; Dhandia, R.; Yadav, A.; Bharali, R.; Gupta, V.K.; Saikia, R. Alleviation of Drought Stress in Pulse Crops with ACC Deaminase Producing Rhizobacteria Isolated from Acidic Soil of Northeast India. Sci. Rep. 2018, 8, 3560. [Google Scholar] [CrossRef]
- Faraji, J.; Sepehri, A. Ameliorative Effects of TiO2 Nanoparticles and Sodium Nitroprusside on Seed Germination and Seedling Growth of Wheat under PEG-Stimulated Drought Stress. J. Seed Sci. 2019, 41, 309–317. [Google Scholar] [CrossRef]
- Bogati, K.; Walczak, M. The Impact of Drought Stress on Soil Microbial Community, Enzyme Activities and Plants. Agronomy 2022, 12, 189. [Google Scholar] [CrossRef]
- Hellal, F.; Abdel-Hady, M.; Khatab, I.; El-Sayed, S.; Abdelly, C. Yield Characterization of Mediterranean Barley under Drought Stress Condition. AIMS Agric. Food 2019, 4, 518–533. [Google Scholar] [CrossRef]
- Nejadalimoradi, H.; Nasibi, F.; Kalantari, K.M.; Zanganeh, R. Effect of Seed Priming with L-Arginine and Sodium Nitroprusside on Some Physiological Parameters and Antioxidant Enzymes of Sunflower Plants Exposed to Salt Stress. Agric. Commun. 2014, 2, 23–30. [Google Scholar]
- Ramadan, A.A.; Abd Elhamid, E.M.; Sadak, M.S.; Elhamid, E.M.A.; Sadak, M.S. Comparative Study for the Effect of Arginine and Sodium Nitroprusside on Sunflower Plants Grown under Salinity Stress Conditions. Bull. Natl. Res. Cent. 2019, 43, 118. [Google Scholar] [CrossRef]
- Hassan, N.; Ebeed, H.; Aljaarany, A. Exogenous Application of Spermine and Putrescine Mitigate Adversities of Drought Stress in Wheat by Protecting Membranes and Chloroplast Ultra-Structure. Physiol. Mol. Biol. Plants 2020, 26, 233–245. [Google Scholar] [CrossRef]
- Ekıncı, M.; Yildirim, E.; Dursun, A.; Mohamedsrajaden, N.S. Putrescine, Spermine and Spermidine Mitigated the Salt Stress Damage on Pepper (Capsicum annum L.) Seedling. Yuz. Yıl Univ. J. Agric. Sci. 2019, 29, 290–299. [Google Scholar] [CrossRef]
- Radhakrishnan, R.; Lee, I.J. Ameliorative Effects of Spermine against Osmotic Stress through Antioxidants and Abscisic Acid Changes in Soybean Pods and Seeds. Acta Physiol. Plant. 2013, 35, 263–269. [Google Scholar] [CrossRef]
- Gull, A.; Lone, A.A.; Wani, N.U.I. Biotic and Abiotic Stresses in Plants; IntechOpen: London, UK, 2019; Volume 7, pp. 1–9. [Google Scholar] [CrossRef]
- Tiburcio, A.F.; Altabella, T.; Bitrián, M.; Alcázar, R. The Roles of Polyamines during the Lifespan of Plants: From Development to Stress. Planta 2014, 240, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.J.; Wang, H.R.; Wang, L.; Han, Y.Y.; Hao, J.H.; Fan, S.X. Effects of Different Types of Polyamine on Growth, Physiological and Biochemical Nature of Lettuce under Drought Stress. IOP Conf. Ser. Earth Environ. Sci. 2018, 185, 012010. [Google Scholar] [CrossRef]
- Amin, M.A.; Ismail, M.A.; Badawy, A.A.; Awad, M.A.; Hamza, M.F.; Awad, M.F.; Fouda, A. The Potency of Fungal-Fabricated Selenium Nanoparticles to Improve the Growth Performance of Helianthus annuus L. and Control of Cutworm Agrotis Ipsilon. Catalysts 2021, 11, 1551. [Google Scholar] [CrossRef]
- Osman, M.S.; Badawy, A.A. Influence of Arbuscular Mycorrhizal Fungi (Glomus mosseae) on Enhancing Salinity Tolerance of Two Common Bean Cultivars. Egypt. J. Biotechnol. 2020, 60, 145–162. [Google Scholar]
- Ghassemi, S.; Farhangi-Abriz, S.; Faegi-Analou, R.; Ghorbanpour, M.; Lajayer, B.A. Monitoring Cell Energy, Physiological Functions and Grain Yield in Field-Grown Mung Bean Exposed to Exogenously Applied Polyamines under Drought Stress. J. Soil Sci. Plant Nutr. 2018, 18, 1108–1125. [Google Scholar] [CrossRef]
- Mahdavian, M.; Sarikhani, H.; Hadadinejad, M.; Dehestani, A. Exogenous Application of Putrescine Positively Enhances the Drought Stress Response in Two Citrus Rootstocks by Increasing Expression of Stress-Related Genes. J. Soil Sci. Plant Nutr. 2021, 21, 1934–1948. [Google Scholar] [CrossRef]
- Cui, G.; Zhao, Y.; Zhang, J.; Chao, M.; Xie, K.; Zhang, C.; Sun, F.; Liu, S.; Xi, Y. Proteomic Analysis of the Similarities and Differences of Soil Drought and Polyethylene Glycol Stress Responses in Wheat (Triticum aestivum L.). Plant Mol. Biol. 2019, 100, 391–410. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Gu, W.; Li, C.C.; Li, W.; Li, C.C.; Li, J.; Wei, S. Exogenous Spermidine Improves Drought Tolerance in Maize by Enhancing the Antioxidant Defence System and Regulating Endogenous Polyamine Metabolism. Crop Pasture Sci. 2018, 69, 1076–1091. [Google Scholar] [CrossRef]
- Mosa, K.A.; El-Naggar, M.; Ramamoorthy, K.; Alawadhi, H.; Elnaggar, A.; Wartanian, S.; Ibrahim, E.; Hani, H. Copper Nanoparticles Induced Genotoxicty, Oxidative Stress, and Changes in Superoxide Dismutase (SOD) Gene Expression in Cucumber (Cucumis sativus) Plants. Front. Plant Sci. 2018, 9, 872. [Google Scholar] [CrossRef] [PubMed]
- Nasibi, F.; Barand, A.; Kalantari, K.M. The Effect of Arginine Pretreatment on Germination, Growth and Physiological Parameters in the Increase of Low Temperature Tolerance in Pistacia vera L. in Vitro Culture. Int. J. Agric. Crop Sci. 2013, 5, 1918–1925. [Google Scholar]
- Feiz, F.S.; Hakimi, L.; Mousavi, A.; Jahromi, M.G. The Effects of Glycine Betaine and L-Arginine on Biochemical Properties of Pot Marigold (Calendula officinalis L.) under Water Stress. Iran. J. Plant Physiol. 2019, 9, 2795–2805. [Google Scholar]
- Lee, R.R.I. Spermine Promotes Acclimation to Osmotic Stress by Modifying Antioxidant, Abscisic Acid, and Jasmonic Acid Signals in Soybean. J. Plant Growth Regul. 2013, 32, 22–30. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Rahman, A.; Inafuku, M.; Oku, H.; Fujita, M. Exogenous Nitric Oxide Donor and Arginine Provide Protection against Short-Term Drought Stress in Wheat Seedlings. Physiol. Mol. Biol. Plants 2018, 24, 993–1004. [Google Scholar] [CrossRef]
- El-sayed, S.; Ramadan, A.A.E.; Hellal, F. Drought Stress Mitigation by Application of Algae Extract on Peanut Grown under Sandy Soil Conditions. Asian J. Plant Sci. 2020, 19, 230–239. [Google Scholar] [CrossRef]
- Ahmed, A.H.H.; Darwish, E.; Alobaidy, M.G. Impact of Putrescine and 24-Epibrassinolide on Growth, Yield and Chemical Constituents of Cotton (Gossypium barbadense L.) Plant Grown under Drought Stress Conditions. Asian J. Plant Sci. 2017, 16, 9–23. [Google Scholar] [CrossRef]
- Elewa, T.A.; Sadak, M.S.; Saad, A.M. Proline Treatment Improves Physiological Responses in Quinoa Plants under Drought Stress. Biosci. Res. 2017, 14, 21–33. [Google Scholar]
- Abass, M.; Qin, C.; Maodong, Q.; Xue, X.; Ahmad, P. Spermine Application Alleviates Salinity Induced Growth and Photosynthetic Inhibition in Solanum lycopersicum by Modulating Osmolyte and Secondary Metabolite Accumulation and Di Ff Erentially Regulating Antioxidant Metabo. Plant Physiol. Biochem. 2019, 144, 1–13. [Google Scholar] [CrossRef]
- Tan, Y.; Liang, Z.; Shao, H.; Du, F. Effect of Water Deficits on the Activity of Anti-Oxidative Enzymes and Osmoregulation among Three Different Genotypes of Radix Astragali at Seeding Stage. Colloids Surf. B Biointerfaces 2006, 49, 60–65. [Google Scholar] [CrossRef]
- Badawy, A.A.; Alotaibi, M.O.; Abdelaziz, A.M.; Osman, M.S.; Khalil, A.M.A.; Saleh, A.M.; Mohammed, A.E.; Hashem, A.H. Enhancement of Seawater Stress Tolerance in Barley by the Endophytic Fungus Aspergillus ochraceus. Metabolites 2021, 11, 428. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, S. Physiology of Wheat (Triticum aestivum L.) Accessions and the Role of Phytohormones under Water Stress; Quaid-I-Azam University: Islamabad, Pakistan, 2009. [Google Scholar]
- Abdel Latef, A.A.H.; Omer, A.M.; Badawy, A.A.; Osman, M.S.; Ragaey, M.M. Strategy of Salt Tolerance and Interactive Impact of Azotobacter Chroococcum and/or Alcaligenes Faecalis Inoculation on Canola (Brassica napus L.) Plants Grown in Saline Soil. Plants 2021, 10, 110. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.-H.; Wang, W.; Wu, H.; Gong, X.; Moriguchi, T. Polyamines Function in Stress Tolerance: From Synthesis to Regulation. Front. Plant Sci. 2015, 6, 827. [Google Scholar] [CrossRef] [PubMed]
- Shallan, M.A.; Hassan, H.M.M.; Namich, A.A.M.; Ibrahim, A.A. Effect of Sodium Niroprusside, Putrescine and Glycine Betaine on Alleviation of Drought Stress in Cotton Plant. Am. J. Agric. Environ. Sci. 2012, 12, 1252–1265. [Google Scholar]
- Hammad, S.A.R.; Ali, O.A.M. Physiological and Biochemical Studies on Drought Tolerance of Wheat Plants by Application of Amino Acids and Yeast Extract. Ann. Agric. Sci. 2014, 59, 133–145. [Google Scholar] [CrossRef]
- Eissa, A.A.; Mourad, S.S.B.; Ibrahim, S.D. Evaluation of Some Rice (Oryza sativa L.) Genotypes under Drought Stress Conditions by Using Morphological, Physiological and Molecular Characteristics. Al-Azhar J. Agric. Res. 2023, 48, 179–193. [Google Scholar]
- Anjum, S.A.; Wang, L.; Farooq, M.; Xue, L.; Ali, S. Fulvic Acid Application Improves the Maize Performance under Well-Watered and Drought Conditions. J. Agron. Crop Sci. 2011, 197, 409–417. [Google Scholar] [CrossRef]
- Marcińska, I.; Czyczyło-Mysza, I.; Skrzypek, E.; Filek, M.; Grzesiak, S.; Grzesiak, M.T.; Janowiak, F.; Hura, T.; Dziurka, M.; Dziurka, K.; et al. Impact of Osmotic Stress on Physiological and Biochemical Characteristics in Drought-Susceptible and Drought-Resistant Wheat Genotypes. Acta Physiol. Plant. 2013, 35, 451–461. [Google Scholar] [CrossRef]
- Li, Y.; Han, D.; Hu, G.; Sommerfeld, M.; Hu, Q. Inhibition of Starch Synthesis Results in Overproduction of Lipids in Chlamydomonas reinhardtii. Biotechnol. Bioeng. 2010, 107, 258–268. [Google Scholar] [CrossRef]
- Borrell, A.; Carbonell, L.; Farras, R.; Puig-Parellada, P.; Tiburcio, A.F. Polyamines Inhibit Lipid Peroxidation in Senescing Oat Leaves. Physiol. Plant. 1997, 99, 385–390. [Google Scholar] [CrossRef]
- Pandey, S.; Ranade, S.A.; Nagar, P.K.; Kumar, N. Role of Polyamines and Ethylene as Modulators of Plant Senescence. J. Biosci. 2000, 25, 291–299. [Google Scholar] [CrossRef]
- Puyang, X.; An, M.; Xu, L.; Han, L.; Zhang, X. Protective Effect of Exogenous Spermidine on Ion and Polyamine Metabolism in Kentucky Bluegrass under Salinity Stress. Hortic. Environ. Biotechnol. 2016, 57, 11–19. [Google Scholar] [CrossRef]
- Talaat, N.B.; Shawky, B.T.; Ibrahim, A.S. Alleviation of Drought-Induced Oxidative Stress in Maize (Zea mays L.) Plants by Dual Application of 24-Epibrassinolide and Spermine. Environ. Exp. Bot. 2015, 113, 47–58. [Google Scholar] [CrossRef]
- Demirtas, Ç.; Yazgan, S.; Candogan, B.N.; Sincik, M.; Büyükcangaz, H.; Göksoy, A.T. Quality and Yield Response of Soybean (Glycine max L. Merrill) to Drought Stress in Sub--Humid Environment. Afr. J. Biotechnol. 2010, 9, 6873–6881. [Google Scholar]
- Alsokari, S.S. Synergistic Effect of Kinetin and Spermine on Some Physiological Aspects of Seawater Stressed Vigna sinensis Plants. Saudi J. Biol. Sci. 2011, 18, 37–44. [Google Scholar] [CrossRef]
- Miller, G.A.D.; Suzuki, N.; Ciftci-Yilmaz, S.; Mittler, R.O.N. Reactive Oxygen Species Homeostasis and Signalling during Drought and Salinity Stresses. Plant Cell Environ. 2010, 33, 453–467. [Google Scholar] [CrossRef]
- Kaur, G.; Asthir, B. Molecular Responses to Drought Stress in Plants. Biol. Plant. 2017, 61, 201–209. [Google Scholar] [CrossRef]
- Wang, T.; Liu, Q.; Wang, N.; Dai, J.; Lu, Q.; Jia, X.; Lin, L.; Yu, F.; Zuo, Y. Foliar Arginine Application Improves Tomato Plant Growth, Yield, and Fruit Quality via Nitrogen Accumulation. Plant Growth Regul. 2021, 95, 421–428. [Google Scholar] [CrossRef]
- Huang, X.; Zhou, G.; Yang, W.; Wang, A.; Hu, Z.; Lin, C.; Chen, X. Drought-Inhibited Ribulose-1, 5-Bisphosphate Carboxylase Activity Is Mediated through Increased Release of Ethylene and Changes in the Ratio of Polyamines in Pakchoi. J. Plant Physiol. 2014, 171, 1392–1400. [Google Scholar] [CrossRef]
- Nayyar, H.; Chander, S. Protective Effects of Polyamines against Oxidative Stress Induced by Water and Cold Stress in Chickpea. J. Agron. Crop Sci. 2004, 190, 355–365. [Google Scholar] [CrossRef]
- Takahashi, T.; Kakehi, J.-I. Polyamines: Ubiquitous Polycations with Unique Roles in Growth and Stress Responses. Ann. Bot. 2010, 105, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Abdeldaym, E.A.; Sabra, M.A. The Root Endophytic Fungus Piriformospora indica Improves Growth Performance, Physiological Parameters and Yield of Tomato under Water Stress Condition. Middle East J. Agric. Res. 2018, 7, 1090–1101. [Google Scholar]
- Raza, M.A.S.S.; Saleem, M.F.; Shah, G.M.; Khan, I.H.; Raza, A. Exogenous Application of Glycinebetaine and Potassium for Improving Water Relations and Grain Yield of Wheat under Drought. J. Soil Sci. Plant Nutr. 2014, 14, 348–364. [Google Scholar] [CrossRef]
- Hussein, H.-A.A.; Alshammari, S.O.; Abd El-sadek, M.E.; Kenawy, S.K.M.; Badawy, A.A. The Promotive Effect of Putrescine on Growth, Biochemical Constituents, and Yield of Wheat (Triticum aestivum L.) Plants under Water Stress. Agriculture 2023, 13, 587. [Google Scholar] [CrossRef]
- Shemi, R.; Wang, R.; Gheith, E.-S.M.S.; Hussain, H.A.; Hussain, S.; Irfan, M.; Cholidah, L.; Zhang, K.; Zhang, S.; Wang, L. Effects of Salicylic Acid, Zinc and Glycine Betaine on Morpho-physiological Growth and Yield of Maize under Drought Stress. Sci. Rep. 2021, 11, 3195. [Google Scholar] [CrossRef]
- Sadak, M.S.; Abdelhamid, M.T.; Schmidhalter, U. Effect of Foliar Application of Amino Acids on Plant Yield and Some Physiological Parameters in Bean Plants Irrigated with Seawater. Acta Biol. Colomb. 2015, 20, 141–152. [Google Scholar] [CrossRef]
- El-Bassiouny, H.M.; Mostafa, H.A.; El-Khawas, S.A.; Hassanein, R.A.; Khalil, S.I.; Abd El-Monem, A.A. Physiological Responses of Wheat Plant to Foliar Treatments with Arginine or Putrescine. Aust. J. Basic Appl. Sci. 2008, 2, 1390–1403. [Google Scholar]

| Treatments | Control | Arginine | Spermine | |
|---|---|---|---|---|
| Conditions | ||||
| Root length (cm) | ||||
| Normal | 12.8 ± 0.44 ab | 12.9 ± 0.43 ab | 13.14 ± 0.48 a | |
| Drought | 11.2 ± 0.60 b | 11.76 ± 0.57 ab | 12.3 ± 0.48 ab | |
| Root fresh weight (g) | ||||
| Normal | 0.47 ± 0.014 a | 0.504 ± 0.016 a | 0.508 ± 0.021 a | |
| Drought | 0.31 ± 0.021 c | 0.356 ± 0.013 bc | 0.4 ± 0.02 b | |
| Root dry weight (g) | ||||
| Normal | 0.07 ± 0.008 bc | 0.09 ± 0.007 ab | 0.10 ± 0.006 a | |
| Drought | 0.04 ± 0.007 d | 0.05 ± 0.007 cd | 0.07 ± 0.006 bc | |
| Shoot length (cm) | ||||
| Normal | 24.8 ± 0.48 a | 28.1 ± 1.01 a | 29.2 ± 1.03 a | |
| Drought | 21.4 ± 1.05 a | 23.4 ± 1.13 a | 23.9 ± 0.88 a | |
| Shoot fresh weight (g) | ||||
| Normal | 5.64 ± 0.42 bc | 6.56 ± 0.39 b | 9.04 ± 0.45 a | |
| Drought | 3.87 ± 0.23 d | 5.25 ± 0.29 c | 5.55 ± 0.36 bc | |
| Shoot dry weight (g) | ||||
| Normal | 0.85 ± 0.065 b | 0.96 ± 0.062 b | 1.21 ± 0.055 a | |
| Drought | 0.54 ± 0.067 c | 0.76 ± 0.058 b | 0.84 ± 0.049 b | |
| Number of leaves | ||||
| Normal | 30 ± 1.68 c | 34.4 ± 1.20 b | 40.4 ± 1.33 a | |
| Drought | 21.2 ± 0.95 d | 28 ± 1.08 c | 28.4 ± 1.20 c | |
| Treatments | Control | Arginine | Spermine | |
|---|---|---|---|---|
| Conditions | ||||
| Weight of pods/plant (g) | ||||
| Normal | 6.09 ± 0.27 ab | 7.87 ± 0.31 a | 8.32 ± 0.28 a | |
| Drought | 3.97 ± 0.20 c | 4.47 ± 0.41 c | 5.15 ± 0.38 ab | |
| Number of pods/plant | ||||
| Normal | 19.2 ± 1.11 c | 23.6 ± 1.20 b | 33.6 ± 0.66 a | |
| Drought | 14 ± 1.08 c | 15.6 ± 0.88 de | 17.8 ± 1.25 cd | |
| Weight of seeds/plant (g) | ||||
| Normal | 3.97 ± 0.22 b | 5.6 ± 0.27 a | 5.82 ± 0.30 a | |
| Drought | 2.76 ± 0.33 c | 3.17 ± 0.29 bc | 3.53 ± 0.23 bc | |
| Number of seeds/plant | ||||
| Normal | 211 ± 8.76 b | 276.2 ± 11.54 a | 297.8 ± 13.11 a | |
| Drought | 159.2 ± 8.83 c | 182.4 ± 13.30 bc | 197.4 ± 5.94 b | |
| 100-seeds weight (g) | ||||
| Normal | 1.78 ± 0.08 bc | 1.99 ± 0.06 ab | 2.22 ± 0.07 a | |
| Drought | 1.46 ± 0.05 d | 1.65 ± 0.11 cd | 1.78 ± 0.09 bc | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Badawy, A.A.; Alshammari, W.K.; Salem, N.F.G.; Alshammari, W.S.; Hussein, H.-A.A. Arginine and Spermine Ameliorate Water Deficit Stress in Fenugreek (Trigonella foenum-graecum L.) by Enhancing Growth and Physio-Biochemical Processes. Antioxidants 2025, 14, 329. https://doi.org/10.3390/antiox14030329
Badawy AA, Alshammari WK, Salem NFG, Alshammari WS, Hussein H-AA. Arginine and Spermine Ameliorate Water Deficit Stress in Fenugreek (Trigonella foenum-graecum L.) by Enhancing Growth and Physio-Biochemical Processes. Antioxidants. 2025; 14(3):329. https://doi.org/10.3390/antiox14030329
Chicago/Turabian StyleBadawy, Ali A., Wadha Kh. Alshammari, Noura F. G. Salem, Woroud S. Alshammari, and Hebat-Allah A. Hussein. 2025. "Arginine and Spermine Ameliorate Water Deficit Stress in Fenugreek (Trigonella foenum-graecum L.) by Enhancing Growth and Physio-Biochemical Processes" Antioxidants 14, no. 3: 329. https://doi.org/10.3390/antiox14030329
APA StyleBadawy, A. A., Alshammari, W. K., Salem, N. F. G., Alshammari, W. S., & Hussein, H.-A. A. (2025). Arginine and Spermine Ameliorate Water Deficit Stress in Fenugreek (Trigonella foenum-graecum L.) by Enhancing Growth and Physio-Biochemical Processes. Antioxidants, 14(3), 329. https://doi.org/10.3390/antiox14030329






