Effect of Block Freeze Concentration on Bioactive Compound Content and Antioxidant Capacity When Applied to Peppermint (Mentha Piperita L.) Infusion
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
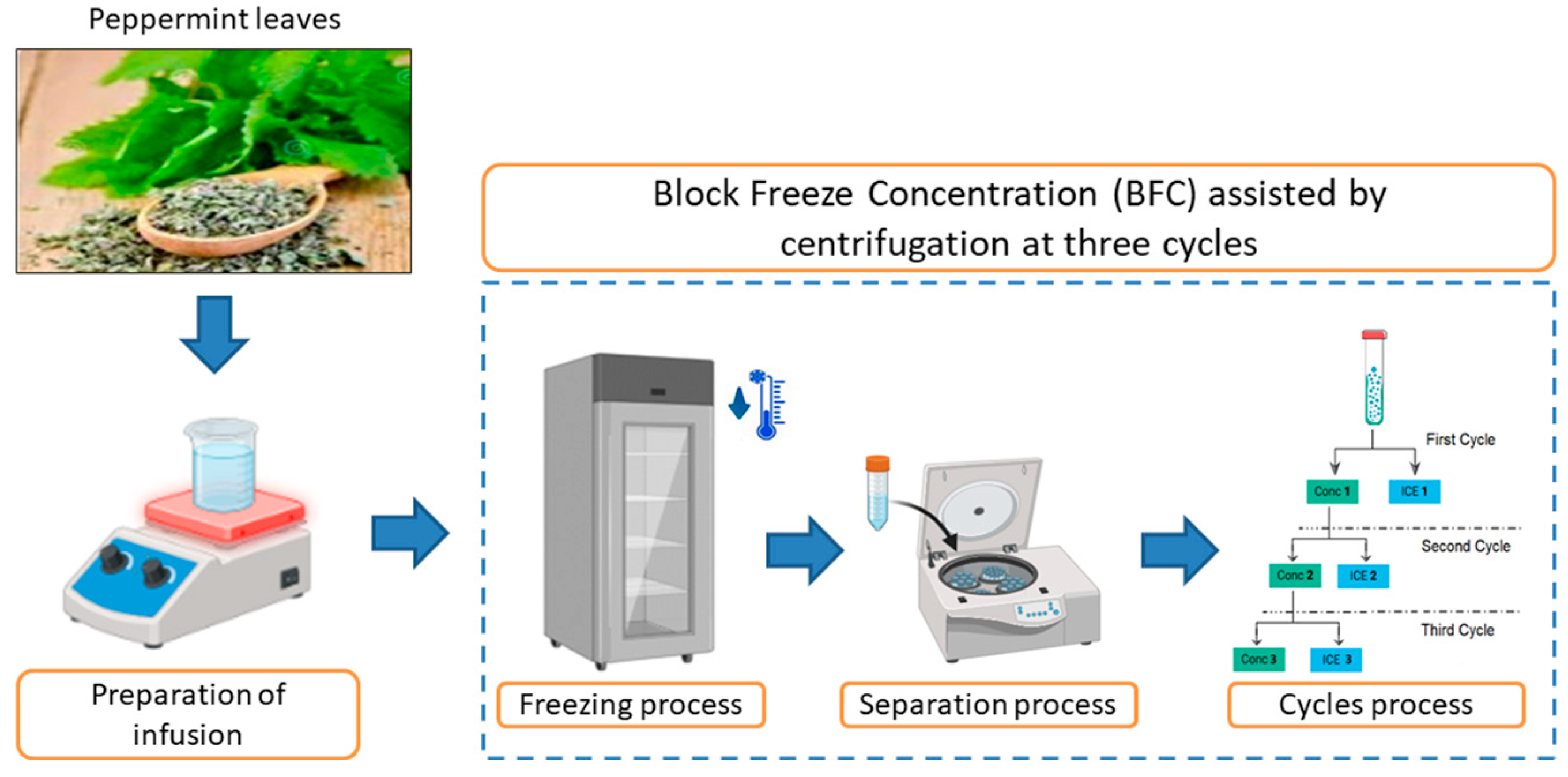
2.2. Preparation of Aqueous Solution (Infusion)
2.3. BFC Assisted by Centrifugation Procedure
2.4. Box–Behnken Design (BBD) and Response Surface Methodology (RSM)
2.5. Response Variable (Efficiency of Separation, η)
2.6. Physicochemical Analysis
2.7. Determination of Total and Individual Bioactive Components
2.7.1. Total Polyphenol Content (TPC)
2.7.2. Total Flavonoid Content (TFC)
2.7.3. Polyphenolic Profile Using HPLC
2.8. Determination of Antioxidant Capacity
2.8.1. 2,2-Diphenyl-1-picrylhydrazyl (DPPH) Assay
2.8.2. Ferric Reducing Antioxidant Power (FRAP) Assay
2.9. Statistical Analysis
3. Results and Discussion
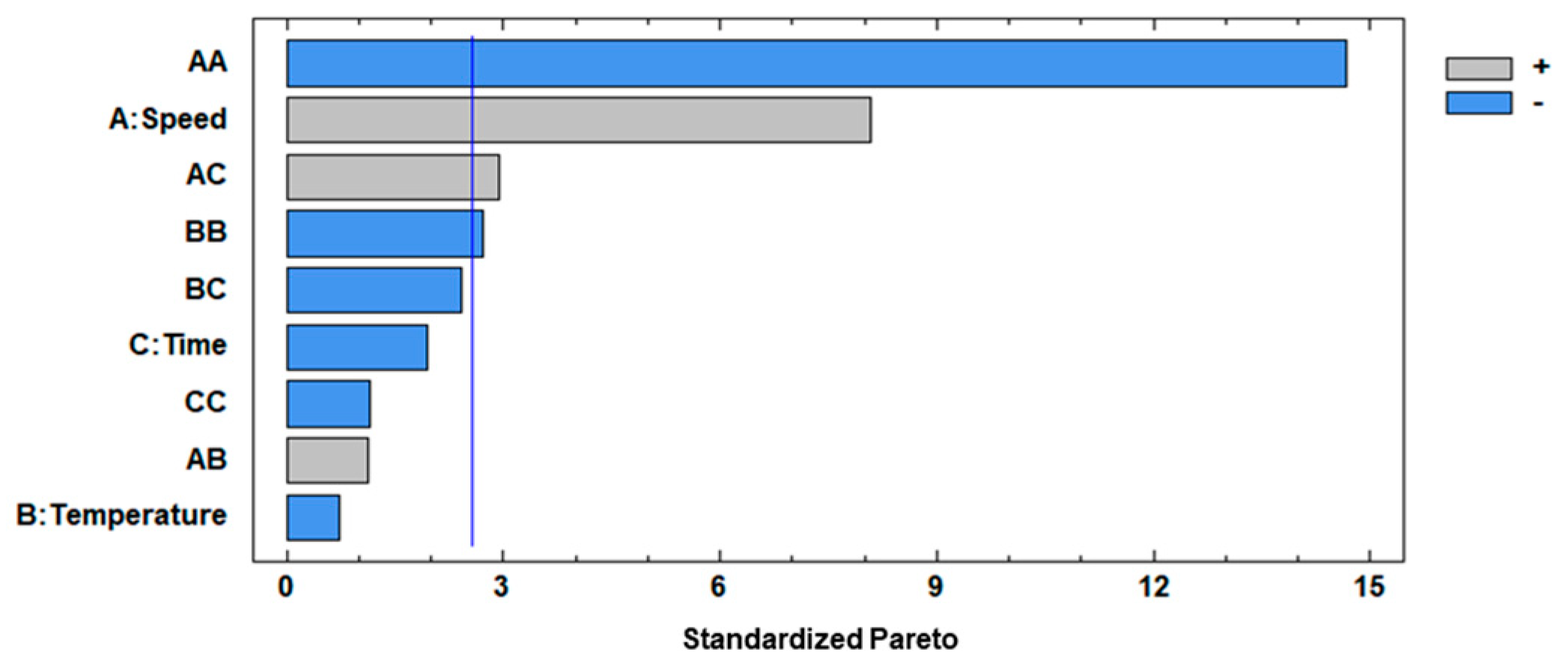
3.1. Optimization of BFC Procedure by BBD
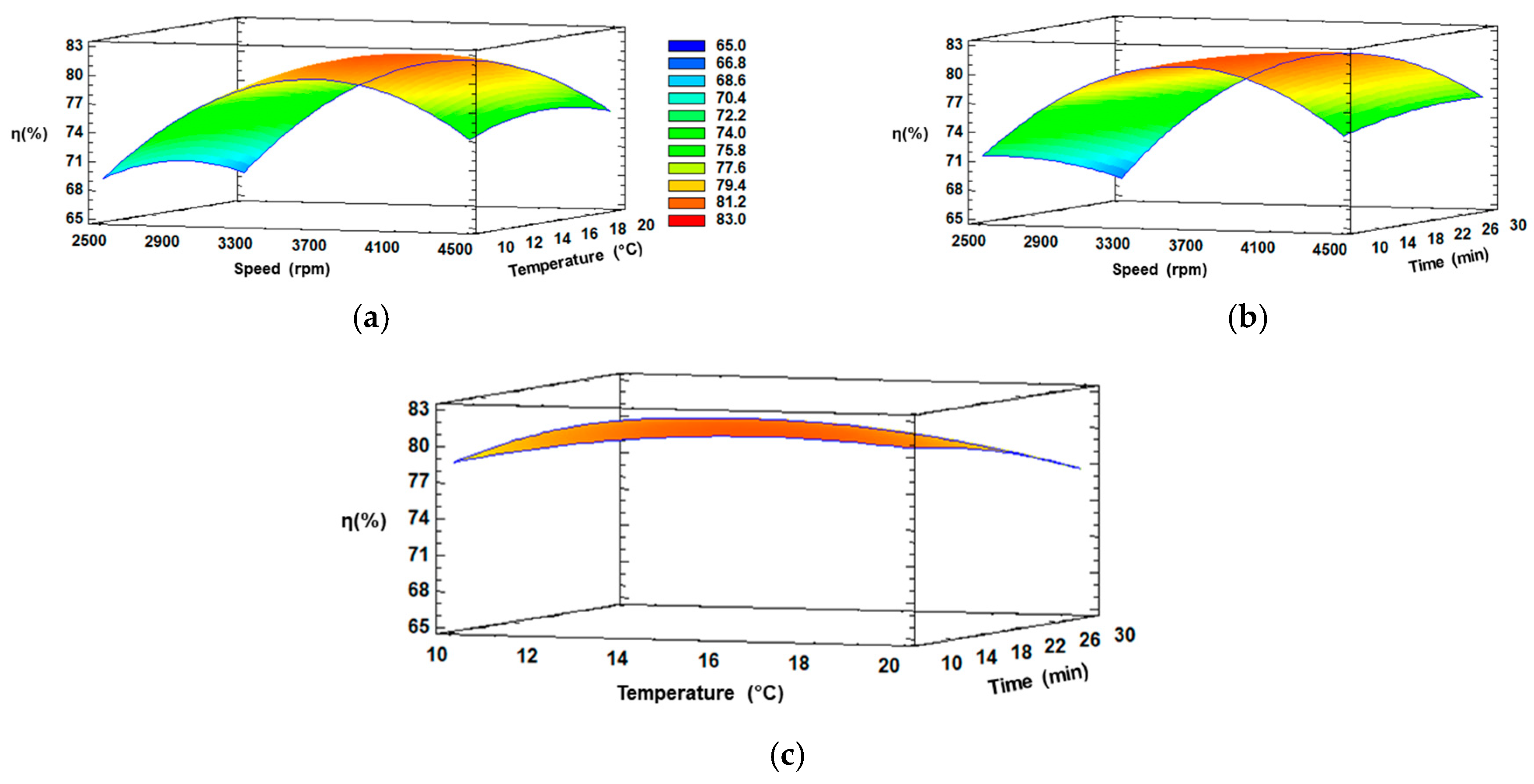
3.2. Response Surface Methodology
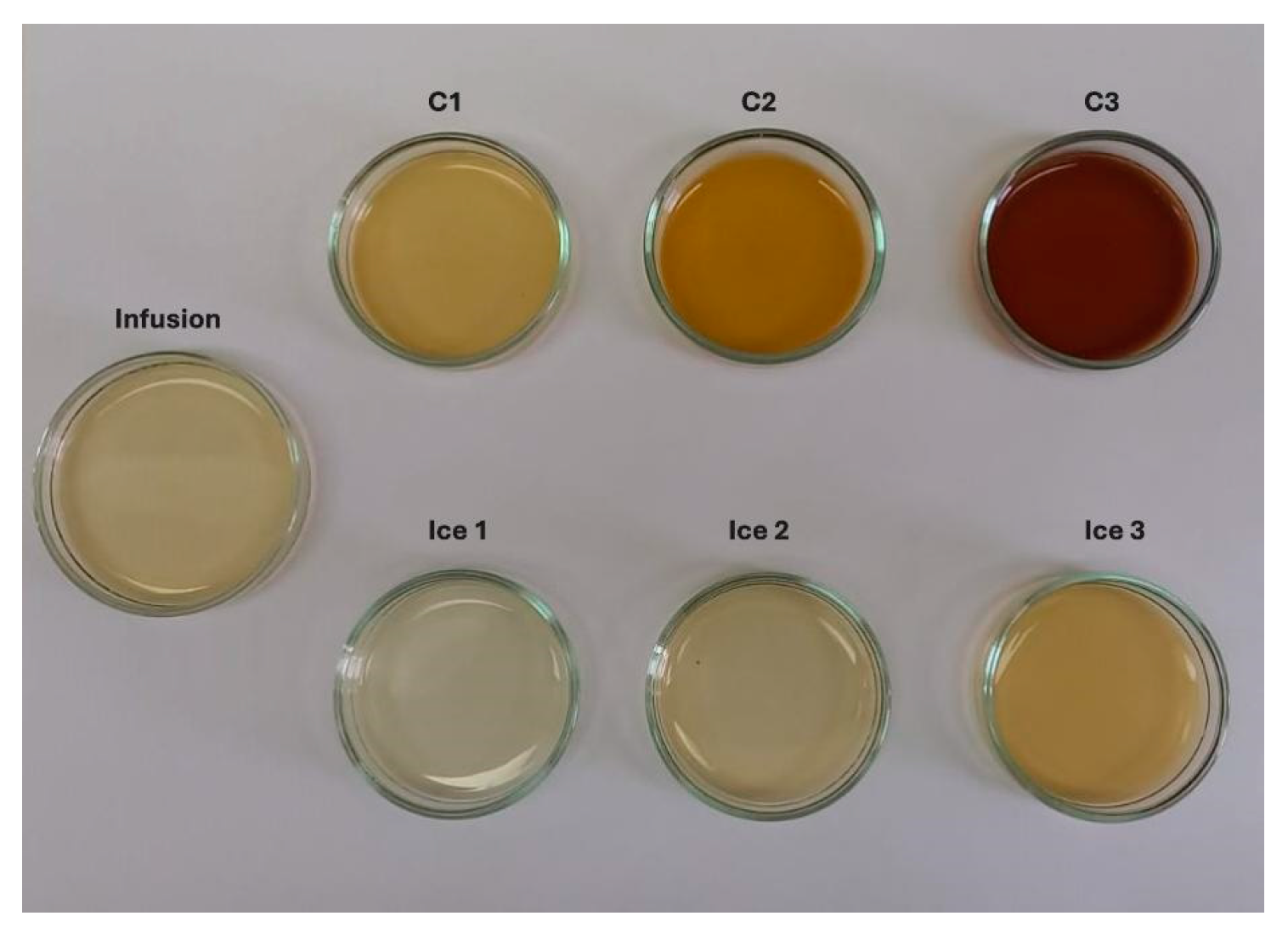
3.3. BFC Assisted by Centrifugation Procedure at Three Cycles
3.3.1. Physicochemical Analysis
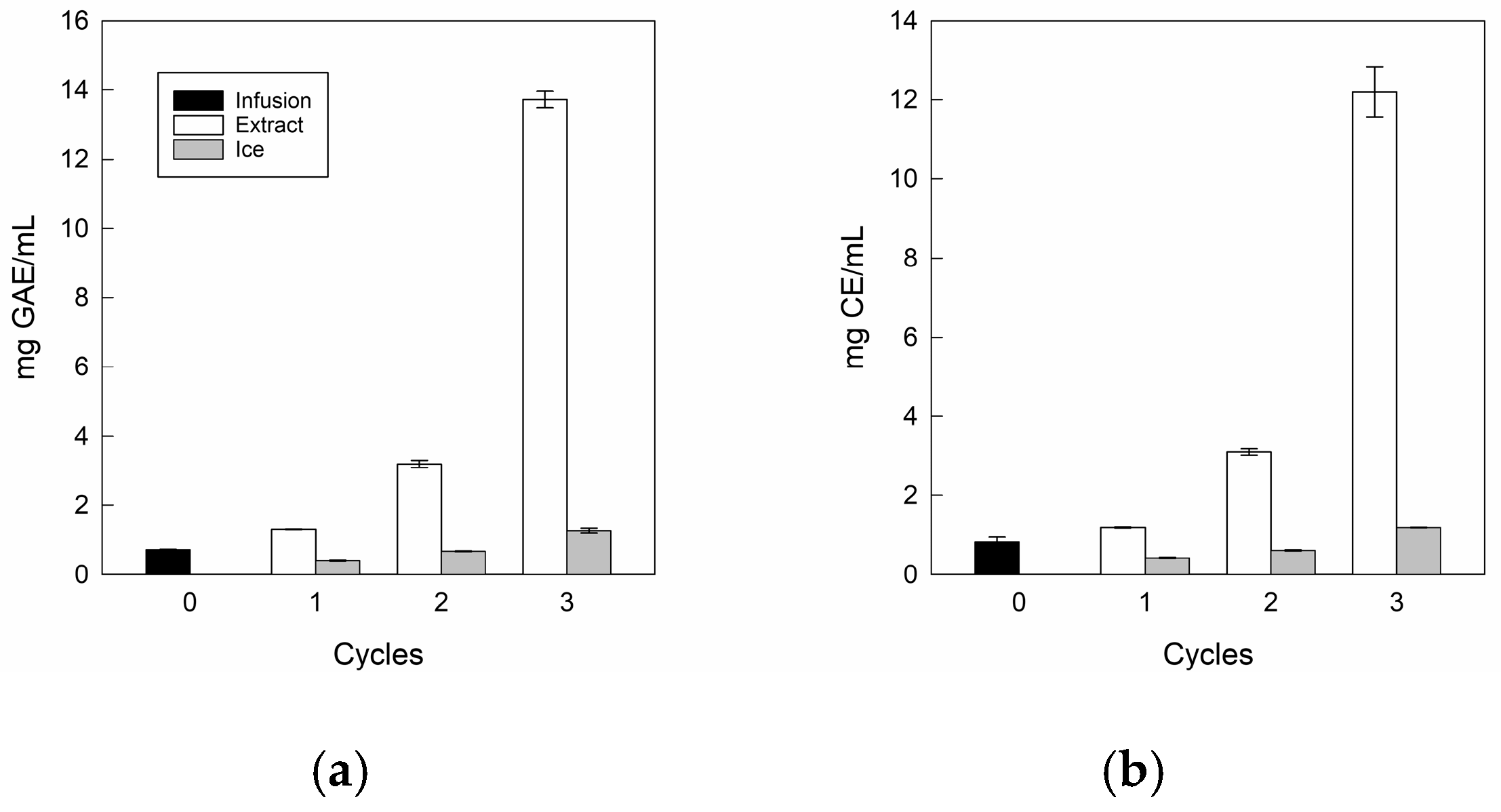
3.3.2. Total and Individual Bioactive Components
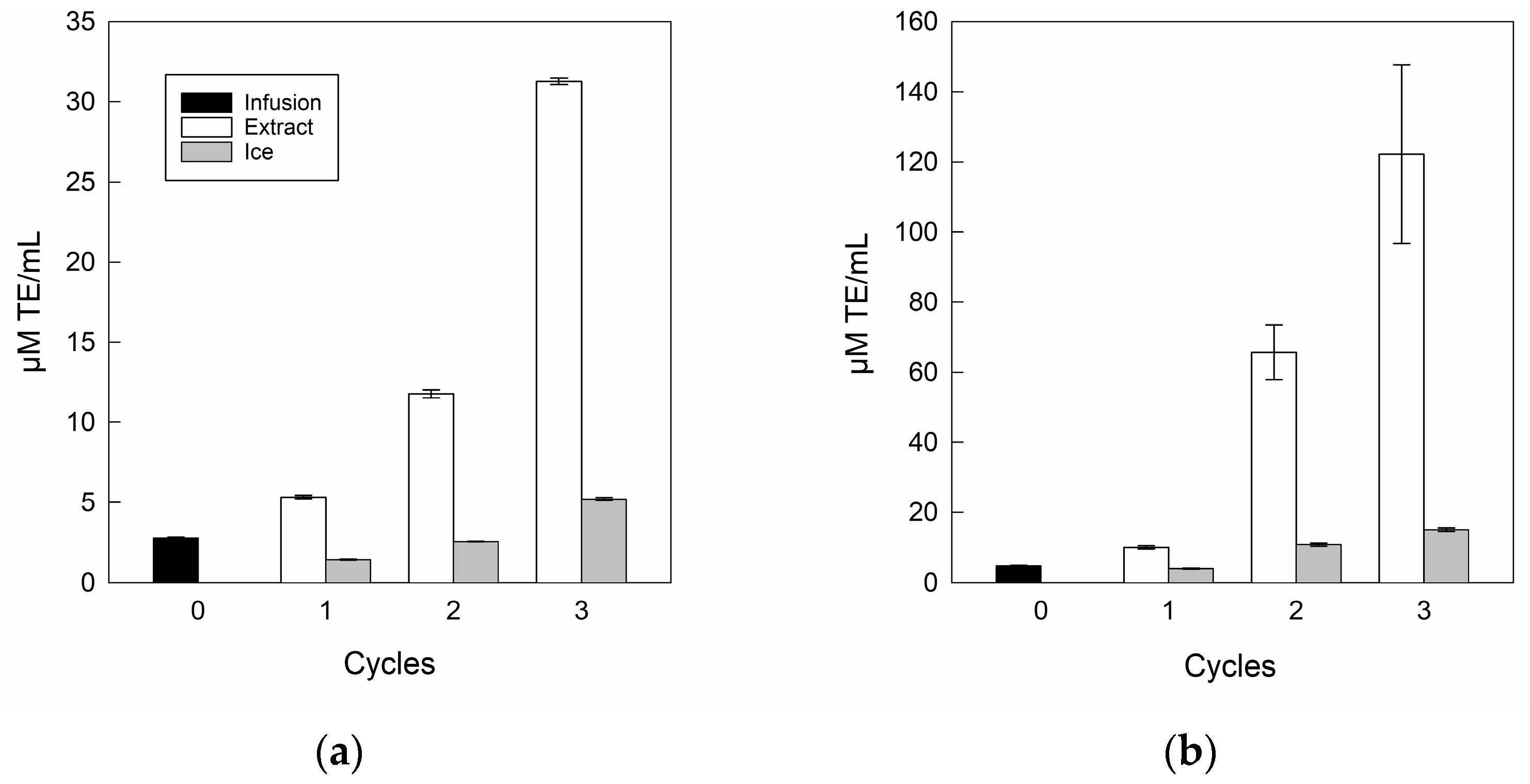
3.3.3. Antioxidant Capacity of Infusion and Extracts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, Q.; Tu, Y.; Zhu, C.; Luo, W.; Huang, W.; Liu, W.; Li, Y. Cholinesterase, β-amyloid aggregation inhibitory and antioxidant capacities of Chinese medicinal plants. Ind. Crops Prod. 2017, 108, 512–519. [Google Scholar] [CrossRef]
- Pilipović, K.; Jurišić Grubešić, R.; Dolenec, P.; Kučić, N.; Juretić, L.; Mršić-Pelčić, J. Plant-based antioxidants for prevention and treatment of neurodegenerative diseases: Phytotherapeutic potential of Laurus nobilis, Aronia melanocarpa, and celastrol. Antioxidants 2023, 12, 746. [Google Scholar] [CrossRef]
- Jamshidi-Kia, F.; Lorigooini, Z.; Amini-Khoei, H. Medicinal plants: Past history and future perspective. J. Herbmed Pharmacol. 2018, 7, 1–7. [Google Scholar] [CrossRef]
- Elansary, H.O.; Mahmoud, E.A. Egyptian herbal tea infusions antioxidants and their antiproliferative and cytotoxic activities against cancer cells. Nat. Prod. Res. 2015, 29, 474–479. [Google Scholar] [CrossRef] [PubMed]
- Uritu, C.M.; Mihai, C.T.; Stanciu, G.D.; Dodi, G.; Alexa-Stratulat, T.; Luca, A.; Leon-Constantin, M.M.; Stefanescu, R.; Bild, V.; Melnic, S.; et al. Medicinal plants of the family Lamiaceae in pain therapy: A review. Pain Res. Manag. 2018, 2018, 7801543. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Gouvinhas, I.; Rocha, J. Phytochemical and antioxidant analysis of medicinal and food plants towards bioactive food and pharmaceutical resources. Sci. Rep. 2021, 11, 10041. [Google Scholar] [CrossRef] [PubMed]
- Shahrajabian, M.H.; Sun, W.; Shen, H.; Cheng, Q. Chinese herbal medicine for SARS and SARS-CoV-2 treatment and prevention, encouraging using herbal medicine for COVID19 outbreak. Acta Agric. Scand. Sect. B 2020, 70, 437–443. [Google Scholar] [CrossRef]
- Saba, I.; Anwar, F.; Ahmad, N.; Iqbal, M.; Abbas, A.; Iqbal, S.; Nazir, A.; Al-Mijalli, S.H. Spearmint (Mentha spicata L.) leaves essential oil: Comparative compositional and biological attributes as a function of different agroclimatic regions. Biocatal. Agric. Biotechnol. 2024, 56, 102984. [Google Scholar] [CrossRef]
- Shahrajabian, M.H.; Sun, W.; Cheng, Q. Milk thistle, myrrh and mint: Herbal plants as natural medicines. Nutr. Food Sci. Res. 2021, 8, 59–65. [Google Scholar] [CrossRef]
- Amador-Luna, V.M.; Montero, L.; Herrero, M. Compressed fluids for the extraction of bioactive compounds from plants, food by-products, seaweeds and microalgae–an update from 2019 to 2023. Trends Analyt. Chem. 2023, 169, 117410. [Google Scholar] [CrossRef]
- Chaughule, R.S.; Barve, R.S. Role of herbal medicines in the treatment of infectious diseases. Vegetos 2024, 37, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Anand, U.; Jacobo-Herrera, N.; Altemimi, A.; Lakhssassi, N. A comprehensive review on medicinal plants as antimicrobial therapeutics: Potential avenues of biocompatible drug discovery. Metabolites 2019, 9, 258. [Google Scholar] [CrossRef]
- Eftekhari, A.; Khusro, A.; Ahmadian, E.; Dizaje, S.M.; Hasanzadeh, A.; Cucchiarinif, M. Phytochemical and nutra-pharmaceutical attributes of Mentha spp.: A comprehensive review. Arab. J. Chem. 2021, 14, 103106. [Google Scholar] [CrossRef]
- Wani, S.; Naik, H.R.; Wagay, J.; Ganie, N.; Mulla, M.; Dar, B.N. Mentha: A review on its bioactive compounds and potential health benefits. Qual. Assur. Saf. Crops Foods 2022, 14, 154–168. [Google Scholar] [CrossRef]
- Bahadori, M.B.; Zengin, G.; Bahadori, S.; Dinparast, L.; Movahhedin, N. Phenolic composition and functional properties of wild mint (Mentha longifolia var. calliantha (Stapf) Briq.). Int. J. Food Prop. 2018, 21, 183–193. [Google Scholar] [CrossRef]
- Adamczyk-Szabela, D.; Wolf, W.M. The influence of copper and zinc on photosynthesis and phenolic levels in basil (Ocimum basilicum L.), borage (Borago officinalis L.), common nettle (Urtica dioica L.) and peppermint (Mentha piperita L.). Int. J. Mol. Sci. 2024, 25, 3612. [Google Scholar] [CrossRef]
- Anwar, F.; Abbas, A.; Mehmood, T.; Gilani, A.H.; Rehman, N.U. Mentha: A genus rich in vital nutra-pharmaceuticals-a review. Phytother. Res. 2019, 33, 2548–2570. [Google Scholar] [CrossRef] [PubMed]
- Zeljkovic, S.C.; Šišková, J.; Komzáková, K.; De Diego, N.; Kaffková, K.; Tarkowski, P. Phenolic compounds and biological activity of selected Mentha Specie. Plants 2021, 10, 550. [Google Scholar] [CrossRef] [PubMed]
- Dousari, A.S.; Hosseininasab, S.S.; Akbarizadeh, M.R.; Naderifar, M.; Satarzadeh, N. Mentha pulegium as a source of green synthesis of nanoparticles with antibacterial, antifungal, anticancer, and antioxidant applications. Sci. Hortic. 2023, 320, 112215. [Google Scholar] [CrossRef]
- Abayechaw, D.M.; Yoseph, T. Review on health benefits of spearmint (Mentha spicata L.) and its inter-cropping advantage with maize (Zea mays L.). J. Nutr. Food Process. 2021, 4, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Faisal, S.; Tariq, M.H.; Ullah, R.; Zafar, S.; Rizwan, M.; Bibi, N.; Khattak, A.; Amir, N.; Abdullah. Exploring the antibacterial, antidiabetic, and anticancer potential of Mentha arvensis extract through in-silico and in-vitro analysis. BMC Complement. Med. Ther. 2023, 23, 267. [Google Scholar] [CrossRef] [PubMed]
- Madhura, B.; Lakshmipathy, K.; Chidanand, D.V.; Sunil, C.K.; Baskaran, N.; Rawson, A.; Dhivya, R. Evaluation of mint (Mentha spicata) hydrodistillation aqueous byproducts and its utilization in development of bioactive-rich functional drink. Food Humanit. 2024, 2, 100263. [Google Scholar] [CrossRef]
- Bhattacharjee, C.; Saxena, V.K.; Dutta, S. Fruit juice processing using membrane technology: A review. Innov. Food Sci. Emerg. Technol. 2017, 43, 136–153. [Google Scholar] [CrossRef]
- Nazir, A.; Khan, K.; Maan, A.; Zia, R.; Giorno, L.; Schroën, K. Membrane separation technology for the recovery of nutraceuticals from food industrial streams. Trends Food Sci. Technol. 2019, 86, 426–438. [Google Scholar] [CrossRef]
- Miyawaki, O.; Omote, C.; Gunathilake, M.; Ishisaki, K.; Miwa, S.; Tagami, A.; Kitano, S. Integrated system of progressive freeze-concentration combined with partial ice-melting for yield improvement. J. Food Eng. 2016, 184, 38–43. [Google Scholar] [CrossRef]
- Miyawaki, O.; Inakuma, T. Development of progressive freeze concentration and its application: A review. Food Bioprocess Technol. 2021, 14, 39–51. [Google Scholar] [CrossRef]
- Pérez-Bermúdez, I.; Castillo-Suero, A.; Cortés-Inostroza, A.; Jeldrez, C.; Dantas, A.; Hernández, E.; Orellana-Palma, P.; Petzold, G. Observation and measurement of ice morphology in foods: A review. Foods 2023, 12, 3987. [Google Scholar] [CrossRef]
- Boaventura, B.C.B.; Murakami, A.N.N.; Prudêncio, E.S.; Maraschin, M.; Murakami, F.S.; Amante, E.R.; Amboni, R.D.D.M.C. Enhancement of bioactive compounds content and antioxidant activity of aqueous extract of mate (Ilex paraguariensis A. St. Hil.) through freeze concentration technology. Food Res. Int. 2013, 53, 686–692. [Google Scholar] [CrossRef]
- Meneses, D.L.; Ruiz, Y.; Hernández, E.; Moreno, F.L. Multi-stage block freeze-concentration of green tea (Camellia sinensis) extract. J. Food Eng. 2021, 293, 110381. [Google Scholar] [CrossRef]
- Almeida, É.D.S.; Arend, G.D.; Knapp, M.A.; Rezzadori, K.; Verruck, S.; Hotza, D.; de Oliveira, D. Effect of the multi-stage block freeze concentration process on the physicochemical and biological properties of noni tea (Morinda citrifolia L.): A case study in Brazil to obtain a promising functional food. J. Food Meas. Charact. 2023, 17, 2519–2536. [Google Scholar] [CrossRef]
- Almeida, É.D.S.; Knapp, M.A.; da Rocha, J.D.G.; Hotza, D.; de Oliveira, D. Multi-stage block freeze concentration via gravitational method applied to increase of the nutritional content of Morinda citrifolia L. tea. Food Biosci. 2023, 51, 102295. [Google Scholar] [CrossRef]
- Rezzadori, K.; Arend, G.D.; Jaster, H.; Díaz-de-Cerio, E.; Verardo, V.; Segura-Carretero, A.; Verruck, S.; Prudêncio, E.S.; Petrus, J.C.C. Bioavailability of bioactive compounds of guava leaves (Psidium guajava) aqueous extract concentrated by gravitational and microwave-assisted cryoconcentration. J. Food Process. Preserv. 2022, 46, e16241. [Google Scholar] [CrossRef]
- Vuong, Q.V.; Golding, J.B.; Stathopoulos, C.E.; Nguyen, M.H.; Roach, P.D. Optimizing conditions for the extraction of catechins from green tea using hot water. J. Sep. Sci. 2011, 34, 3099–3106. [Google Scholar] [CrossRef]
- Orellana-Palma, P.; Macias-Bu, L.; Carvajal-Mena, N.; Petzold, G.; Guerra-Valle, M. Encapsulation of concentrated solution obtained by block freeze concentration in calcium alginate and corn starch calcium alginate hydrogel beads. Gels 2023, 9, 374. [Google Scholar] [CrossRef] [PubMed]
- Orellana-Palma, P.; Guerra-Valle, M.; Gianelli, M.P.; Petzold, G. Evaluation of freeze crystallization on pomegranate juice quality in comparison with conventional thermal processing. Food Biosci. 2021, 41, 101106. [Google Scholar] [CrossRef]
- AOAC (Association of Official Agricultural Chemists). The Official Methods of Analysis of AOAC International, 20th ed.; Horwitz, W., Latimer, G., Eds.; AOAC International: Gaithersburg, MD, USA, 2016. [Google Scholar]
- Baibuch, S.; Zema, P.; Bonifazi, E.; Cabrera, G.; Mondragón-Portocarrero, A.D.C.; Campos, C.; Malec, L. Effect of the drying method and optimization of extraction on antioxidant activity and phenolic of rose petals. Antioxidants 2023, 12, 681. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Gouvinhas, I.; Barros, A. Variation of the polyphenolic composition and antioxidant capacity of freshly prepared pomegranate leaf infusions over one-day storage. Antioxidants 2021, 10, 1187. [Google Scholar] [CrossRef] [PubMed]
- Dewanto, V.; Wu, X.; Adom, K.K.; Liu, R.H. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J. Agric. Food Chem. 2002, 50, 3010–3014. [Google Scholar] [CrossRef] [PubMed]
- Erdoğan, S.; Erdemoğlu, S. Evaluation of polyphenol contents in differently processed apricots using accelerated solvent extraction followed by high-performance liquid chromatography-diode array detector. Int. J. Food Sci. Nutr. 2011, 62, 729–739. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Vásquez-Castillo, F.M.; Hernández, E.; Ureña-Peralta, M.O.; Achaerandio, I. Comparative study of block cryoconcentration in pomegranate juice: Centrifugation versus vacuum. J. Food Process Eng. 2023, 46, e14435. [Google Scholar] [CrossRef]
- Santana, T.; Moreno, J.; Petzold, G.; Santana, R.; Sáez, G. Evaluation of the temperature and time in centrifugation-assisted freeze concentration. Appl. Sci. 2020, 10, 9130. [Google Scholar] [CrossRef]
- Casas-Forero, N.; Orellana, P.; Petzold, G. Recovery of solutes from ice and concentrated fractions in centrifugal block cryoconcentration applied to blueberry juice. Food Bioprocess Technol. 2021, 14, 1155–1168. [Google Scholar] [CrossRef]
- Aider, M.; Ben, O.W. Skim milk cryoconcentration as affected by the thawing mode: Gravitational vs. microwave-assisted. Int. J. Food Sci. Technol. 2012, 47, 195–202. [Google Scholar] [CrossRef]
- Dyab, A.S.; Aly, A.M.; Matuk, H. Enhancement and evaluation of peppermint (Mentha piperita L.) beverage. Int. J. Life Sci. Res. 2015, 3, 175–185. [Google Scholar]
- Mahendran, G.; Rahman, L.U. Ethnomedicinal, phytochemical and pharmacological updates on peppermint (Mentha × piperita L.)—A review. Phytother. Res. 2020, 34, 2088–2139. [Google Scholar] [CrossRef] [PubMed]
- Vásquez-Castillo, F.M.; Hernández, E.; Achaerandio, I. Centrifugal filtration enhances the block freeze concentration and recovery of bioactive compounds from pomegranate juice. LWT-Food Sci. Technol. 2024, 214, 117153. [Google Scholar] [CrossRef]
- Orellana-Palma, P.; Tobar, G.; Casas, N.; Zúñiga, R.N.; Petzold, G. Quality attributes of cryoconcentrated calafate (Berberis microphylla) juice during refrigerated storage. Foods 2020, 9, 1314. [Google Scholar] [CrossRef] [PubMed]
- Guerra, M.; Lillo, S.; Petzold, G.; Orellana, P. Effect of freeze crystallization on quality properties of two endemic patagonian berries juices: Murta (Ugni molinae) and arrayan (Luma apiculata). Foods 2021, 10, 466. [Google Scholar] [CrossRef]
- Adorno, W.T.; Rezzadori, K.; Arend, G.D.; Chaves, V.C.; Reginatto, F.H.; Di Luccio, M.; Petrus, J.C. Enhancement of phenolic compounds content and antioxidant activity of strawberry (Fragaria × ananassa) juice by block freeze concentration technology. Int. J. Food Sci. Technol. 2017, 52, 781–787. [Google Scholar] [CrossRef]
- Moreno, F.L.; Quintanilla-Carvajal, M.X.; Sotelo, L.I.; Osorio, C.; Raventós, M.; Hernández, E.; Ruiz, Y. Volatile compounds, sensory quality and ice morphology in falling-film and block freeze concentration of coffee extract. J. Food Eng. 2015, 166, 64–71. [Google Scholar] [CrossRef]
- Casas-Forero, N.; Orellana-Palma, P.; Petzold, G. Influence of block freeze concentration and evaporation on physicochemical properties, bioactive compounds and antioxidant activity in blueberry juice. Food Sci. Technol. 2020, 40, 387–394. [Google Scholar] [CrossRef]
- Wu, W.; Xiao, G.; Yu, Y.; Xu, Y.; Wu, J.; Peng, J.; Li, L. Effects of high pressure and thermal processing on quality properties and volatile compounds of pineapple fruit juice. Food Control 2021, 130, 108293. [Google Scholar] [CrossRef]
- Krapfenbauer, G.; Kinner, M.; Gössinger, M.; Schönlechner, R.; Berghofer, E. Effect of thermal treatment on the quality of cloudy apple juice. J. Agric. Food Chem. 2006, 54, 5453–5460. [Google Scholar] [CrossRef]
- Zielinski, A.A.F.; Haminiuk, C.W.I.; Alberti, A.; Nogueira, A.; Demiate, I.M.; Granato, D. A comparative study of the phenolic compounds and the in vitro antioxidant activity of different Brazilian teas using multivariate statistical techniques. Food Res. Int. 2014, 60, 246–254. [Google Scholar] [CrossRef]
- Sadowska, U.; Jewiarz, K.; Kopak, M.; Dziadek, K.; Francik, R.; Kopeć, A. Proximate analysis and antioxidant properties of young plants of Sinapis alba L. depend on the time of harvest and variety. Appl. Sci. 2023, 13, 7980. [Google Scholar] [CrossRef]
- Orellana-Palma, P.; Lazo-Mercado, V.; Gianelli, M.P.; Hernandez, E.; Zúñiga, R.N.; Petzold, G. Influence of cryoconcentration on quality attributes of apple juice (Malus domestica cv. Red Fuji). Appl. Sci. 2020, 10, 959. [Google Scholar] [CrossRef]
- Chanukya, B.S.; Rastogi, N.K. A comparison of thermal processing, freeze drying and forward osmosis for the downstream processing of anthocyanin from rose petals. J. Food Process. Preserv. 2016, 40, 1289–1296. [Google Scholar] [CrossRef]
- Bredun, M.A.; Prestes, A.A.; Panceri, C.P.; Prudêncio, E.S.; Burin, V.M. Bioactive compounds recovery by freeze concentration process from winemaking by-product. Food Res. Int. 2023, 173, 113220. [Google Scholar] [CrossRef] [PubMed]
- Orellana-Palma, P.; Petzold, G.; Guerra-Valle, M.; Astudillo-Lagos, M. Impact of block cryoconcentration on polyphenol retention in blueberry juice. Food Biosci. 2017, 20, 149–158. [Google Scholar] [CrossRef]
- Mehdizadeh, L.; Moghaddam, M.; Ganjeali, A.; Rahimmalek, M. Salicylic acid elicitation effect on phenolic profile and antioxidant activity of Mentha piperita L. in relation to zinc concentration under soilless culture. S. Afr. J. Bot. 2024, 168, 509–517. [Google Scholar] [CrossRef]
- Jurić, T.; Mićić, N.; Potkonjak, A.; Milanov, D.; Dodić, J.; Trivunović, Z.; Popović, B.M. The evaluation of phenolic content, in vitro antioxidant and antibacterial activity of Mentha piperita extracts obtained by natural deep eutectic solvents. Food Chem. 2021, 362, 130226. [Google Scholar] [CrossRef] [PubMed]
- Rouxinol, M.I.; Martins, M.R.; Salgueiro, V.; Costa, M.J.; Barroso, J.M.; Rato, A.E. Climate effect on morphological traits and polyphenolic composition of red wine grapes of Vitis vinifera. Beverages 2023, 9, 8. [Google Scholar] [CrossRef]
- Bastías-Montes, J.M.; Vidal-San-Martín, C.; Tamarit-Pino, Y.; Muñoz-Fariña, O.; García-Figueroa, O.; Quevedo-León, R.; Wei, Z.-J.; Lv, X.; Cespedes-Acuña, C.L. Cryoconcentration by centrifugation–filtration: A simultaneous, efficient and innovative method to increase thermosensitive bioactive compounds of aqueous maqui (Aristotelia chilensis (Mol.) Stuntz) Extract. Processes 2021, 10, 25. [Google Scholar] [CrossRef]
- Marafon, K.; Pereira-Coelho, M.; Haas, I.C.D.S.; Wanderley, B.R.D.S.M.; de Gois, J.S.; Vitali, L.; Luna, A.S.; Canella, M.H.M.; Hernández, E.; Amboni, R.D.D.M.C.; et al. An opportunity for acerola pulp (Malpighia emarginata DC) valorization evaluating its performance during the block cryoconcentration by physicochemical, bioactive compounds, HPLC–ESI-MS/MS, and multi-elemental profile analysis. Food Res. Int. 2024, 176, 113793. [Google Scholar] [CrossRef]
- Huynh, D.T.; Vo, M.T.N.; Kha, T.C. Enriching the bioactive components and antioxidant capacity of concentrated lime juices prepared by cryogenic and vacuum processes. Processes 2023, 11, 1883. [Google Scholar] [CrossRef]
- Wang, X.; Chen, F.; Ma, L.; Liao, X.; Hu, X. Non-volatile and volatile metabolic profiling of tomato juice processed by high-hydrostatic-pressure and high-temperature short-time. Food Chem. 2022, 371, 131161. [Google Scholar] [CrossRef]
- Prestes, A.A.; Helm, C.V.; Esmerino, E.A.; Silva, R.; da Cruz, A.G.; Prudencio, E.S. Freeze concentration techniques as alternative methods to thermal processing in dairy manufacturing: A review. J. Food Sci. 2022, 87, 488–502. [Google Scholar] [CrossRef]
- Zielinski, A.A.; Zardo, D.M.; Alberti, A.; Bortolini, D.G.; Benvenutti, L.; Demiate, I.M.; Nogueira, A. Effect of cryoconcentration process on phenolic compounds and antioxidant activity in apple juice. J. Sci. Food Agric. 2019, 99, 2786–2792. [Google Scholar] [CrossRef]
- Márquez-Montes, C.A.; Gallegos-Infante, J.A.; Petzold-Maldonado, G.R.; Orellana-Palma, P.A.; González-Laredo, R.F.; Rocha-Guzmán, N.E.; Ochoa-Martínez, L.A. Centrifugal cryoconcentration of prickly pear juice: Effect on the polyphenolic content and their antioxidant activity. Lett. Appl. NanoBioSci. 2022, 12, 57. [Google Scholar] [CrossRef]
- Abdelouhab, K.; Guemmaz, T.; Karamać, M.; Kati, D.E.; Amarowicz, R.; Arrar, L. Phenolic composition and correlation with antioxidant properties of various organic fractions from Hertia cheirifolia extracts. J. Pharm. Biomed. Anal. 2023, 235, 115673. [Google Scholar] [CrossRef]
| Factors 1 | Code Units | Levels | ||
|---|---|---|---|---|
| −1 | 0 | +1 | ||
| CS (rpm) | X1 | 2500 | 3500 | 4500 |
| CT (°C) | X2 | 10 | 15 | 20 |
| Ct (min) | X3 | 10 | 20 | 30 |
| Analysis Time (min) | Eluent A | Eluent B | Flow Rate (mL/min) | Temperature (°C) |
|---|---|---|---|---|
| 1–5 | 100 | 0 | 1 | 25 |
| 5–40 1 | 30 | 70 | 1 | 25 |
| 40–45 | 20 | 80 | 1 | 25 |
| 45–55 | 15 | 85 | 1.2 | 25 |
| 55–57 | 10 | 90 | 1.2 | 25 |
| 57–75 | 10 | 90 | 1.2 | 25 |
| Run | Factors 1 | η (%) | |||
|---|---|---|---|---|---|
| X1 (rpm) | X2 (°C) | X3 (min) | Experimental | Predicted | |
| 1 | 3500 | 20 | 30 | 76.8 | 76.6 |
| 2 | 2500 | 15 | 30 | 65.8 | 66.9 |
| 3 | 2500 | 15 | 10 | 71.4 | 71.5 |
| 4 | 4500 | 10 | 20 | 73.2 | 74.1 |
| 5 | 2500 | 10 | 20 | 69.5 | 69.2 |
| 6 | 2500 | 20 | 20 | 68.4 | 67.5 |
| 7 | 3500 | 10 | 10 | 78.5 | 78.7 |
| 8 | 3500 | 10 | 30 | 80.6 | 79.8 |
| 9 | 3500 | 20 | 10 | 79.9 | 80.7 |
| 10 | 4500 | 20 | 20 | 74.5 | 74.8 |
| 11 | 4500 | 15 | 10 | 75.6 | 74.5 |
| 12 | 4500 | 15 | 30 | 76.3 | 76.2 |
| 13 | 3500 | 15 | 20 | 81.1 | 81.1 |
| 14 | 3500 | 15 | 20 | 81.1 | 81.1 |
| 15 | 3500 | 15 | 20 | 81.1 | 81.1 |
| Factors | Coefficient Estimate | Standard Error | F Value | p-Value | Significance 1 |
|---|---|---|---|---|---|
| X1 | 6.13 | 0.76 | 65.27 | 0.0005 | S |
| X2 | −0.55 | 0.76 | 0.53 | 0.5007 | NS |
| X3 | −1.48 | 0.76 | 3.79 | 0.1093 | NS |
| X12 | −16.38 | 1.12 | 215.32 | 0.0000 | S |
| X1X2 | 1.2 | 1.07 | 1.25 | 0.3139 | NS |
| X1X3 | 3.15 | 1.07 | 8.63 | 0.0323 | S |
| X22 | −3.03 | 1.12 | 7.35 | 0.0422 | S |
| X2X3 | −2.60 | 1.07 | 5.88 | 0.0597 | NS |
| X32 | −1.28 | 1.12 | 1.31 | 0.3050 | NS |
| Sample | TSC (% w/w) | TSSC (°Brix) | L* | a* | b* | ΔE* |
|---|---|---|---|---|---|---|
| Infusion | 1.41 ± 0.06 a | 1.81 ± 0.00 a | 64.26 ± 2.13 a | 15.30 ± 1.97 a | 70.72 ± 0.49 a | - |
| C1 | 1.96 ± 0.19 b | 2.53 ± 0.15 b | 50.58 ± 0.37 b | 26.92 ± 0.27 b | 76.81 ± 0.04 b | 18.97 ± 3.24 a |
| C2 | 2.89 ± 0.24 c | 3.71 ± 0.71 c | 24.38 ± 3.56 c | 34.60 ± 0.44 c | 44.99 ± 2.67 c | 51.30 ± 5.57 b |
| C3 | 6.50 ± 0.08 d | 8.69 ± 1.01 d | 1.10 ± 0.72 d | 0.28 ± 0.74 d | 1.05 ± 0.74 d | 94.97 ± 1.99 c |
| Phenolic Compounds (mg/L) | Feed Extract | C1 | C2 | C3 |
|---|---|---|---|---|
| Chlorogenic acid | 0.87 ± 0.00 a | 0.73 ± 0.66 a | 1.16 ± 0.10 a | 4.41 ± 0.22 b |
| Gallic acid | ND | 1.25 ± 0.42 a | 4.17 ± 0.42 b | 15.26 ± 5.48 c |
| Protocatechuic acid | 0.77 ± 0.25 a | 1.05 ± 0.01 b | 3.87 ± 0.39 c | 12.98 ± 0.45 d |
| Catechin | 1.35 ± 0.81 a | 1.71 ±0.44 a,b | 4.01 ± 1.70 c | 18.26 ± 6.16 d |
| Caffeic acid | 2.67 ± 0.11 a | 4.54 ± 1.06 b | 11.70 ± 1.22 c | 43.88 ± 1.25 d |
| Vanillic acid | 2.20 ± 1.38 a | 2.70 ± 0.05 a | 7.44 ± 0.83 b | 30.91 ± 1.25 c |
| Syringic acid | 1.40 ± 0.03 a | 2.40 ± 0.15 b | 5.87 ± 0.89 c | 29.46 ± 1.11 d |
| p-coumaric acid | 2.28 ± 0.12 a | 4.97 ± 1.73 b | 12.87 ± 2.81 c | 40.82 ± 0.92 d |
| Ellagic acid | 0.26 ± 0.05 a | 0.40 ± 0.00 b | 1.18 ± 0.11 c | 5.00 ± 0.32 d |
| Transferulic acid | 3.68 ± 0.14 a | 5.93 ± 0.12 b | 17.06 ± 2.09 c | 68.68 ± 1.08 d |
| Rutin | 1.65 ± 0.21 a | 3.18 ± 0.13 b | 15.27 ± 10.91 c | 28.82 ± 6.64 c,d |
| p-hydroxybenzoic acid | 7.36 ± 0.05 a | 11.87 ± 0.62 b | 39.66 ± 4.60 c | 89.95 ± 0.07 d |
| Myricetin | 2.82 ± 2.58 a | 2.61 ± 1.99 a | 18.19 ± 5.08 b | 45.31 ± 7.14 c |
| Quercetin | 1.00 ± 0.06 a | 1.14 ± 0.37 a | 3.83 ± 0.34 b | 16.04 ± 0.09 c |
| Kaempferol | 10.04 ± 0.68 a | 18.55 ± 1.20 b | 58.99 ± 12.60 c | 177.40 ± 4.57 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Bermúdez, I.; Castillo-Suero, A.; Jara-Leiva, C.; Cortés-Valdivia, A.; Rojas-Rojas, K.; García-Rojas, V.; Opazo-Navarrete, M.; Guerra-Valle, M.; Petzold, G.; Orellana-Palma, P. Effect of Block Freeze Concentration on Bioactive Compound Content and Antioxidant Capacity When Applied to Peppermint (Mentha Piperita L.) Infusion. Antioxidants 2025, 14, 129. https://doi.org/10.3390/antiox14020129
Pérez-Bermúdez I, Castillo-Suero A, Jara-Leiva C, Cortés-Valdivia A, Rojas-Rojas K, García-Rojas V, Opazo-Navarrete M, Guerra-Valle M, Petzold G, Orellana-Palma P. Effect of Block Freeze Concentration on Bioactive Compound Content and Antioxidant Capacity When Applied to Peppermint (Mentha Piperita L.) Infusion. Antioxidants. 2025; 14(2):129. https://doi.org/10.3390/antiox14020129
Chicago/Turabian StylePérez-Bermúdez, Indira, Alison Castillo-Suero, Constanza Jara-Leiva, Axel Cortés-Valdivia, Karol Rojas-Rojas, Vivian García-Rojas, Mauricio Opazo-Navarrete, María Guerra-Valle, Guillermo Petzold, and Patricio Orellana-Palma. 2025. "Effect of Block Freeze Concentration on Bioactive Compound Content and Antioxidant Capacity When Applied to Peppermint (Mentha Piperita L.) Infusion" Antioxidants 14, no. 2: 129. https://doi.org/10.3390/antiox14020129
APA StylePérez-Bermúdez, I., Castillo-Suero, A., Jara-Leiva, C., Cortés-Valdivia, A., Rojas-Rojas, K., García-Rojas, V., Opazo-Navarrete, M., Guerra-Valle, M., Petzold, G., & Orellana-Palma, P. (2025). Effect of Block Freeze Concentration on Bioactive Compound Content and Antioxidant Capacity When Applied to Peppermint (Mentha Piperita L.) Infusion. Antioxidants, 14(2), 129. https://doi.org/10.3390/antiox14020129










