Lycopene Protects Deoxynivalenol-Induced Intestinal Barrier Dysfunction and NLRP3 Inflammasome Activation by Targeting the ERK Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Treatment
2.2. Cell Viability Detection
2.3. Immunofluorescence
2.4. Cell Permeability Assay
2.5. Enzyme-Linked Immunosorbent Assay (ELISA)
2.6. Detection of Oxidative Stress Markers
2.7. Quantitative Real-Time PCR (qRT-PCR)
2.8. Western Blot Analysis
2.9. Data Statistics and Analysis
3. Results
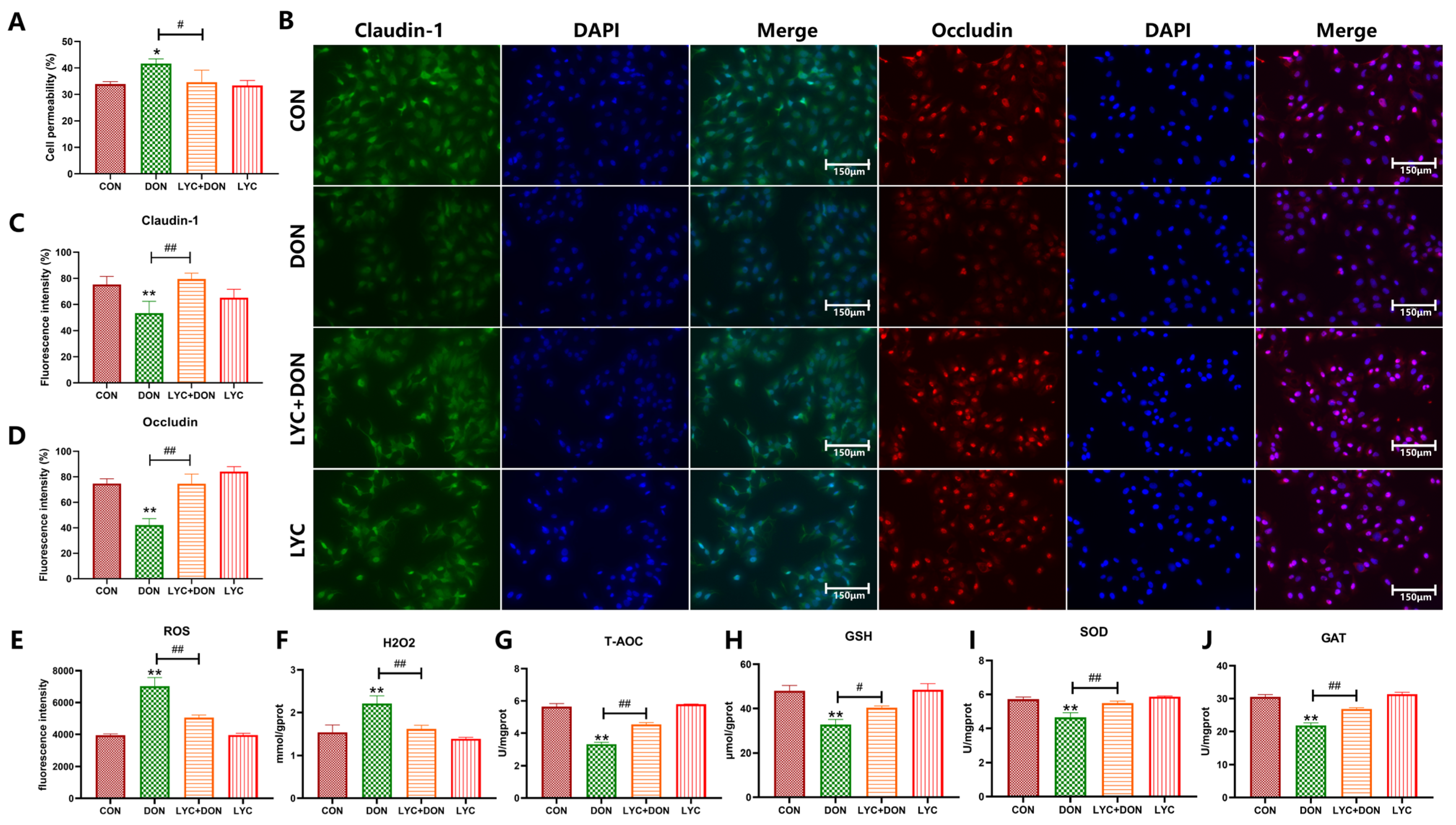
3.1. LYC Alleviated DON-Induced Intestinal Epithelial Barrier Impairment and Oxidative Stress in IPEC-J2 Cells
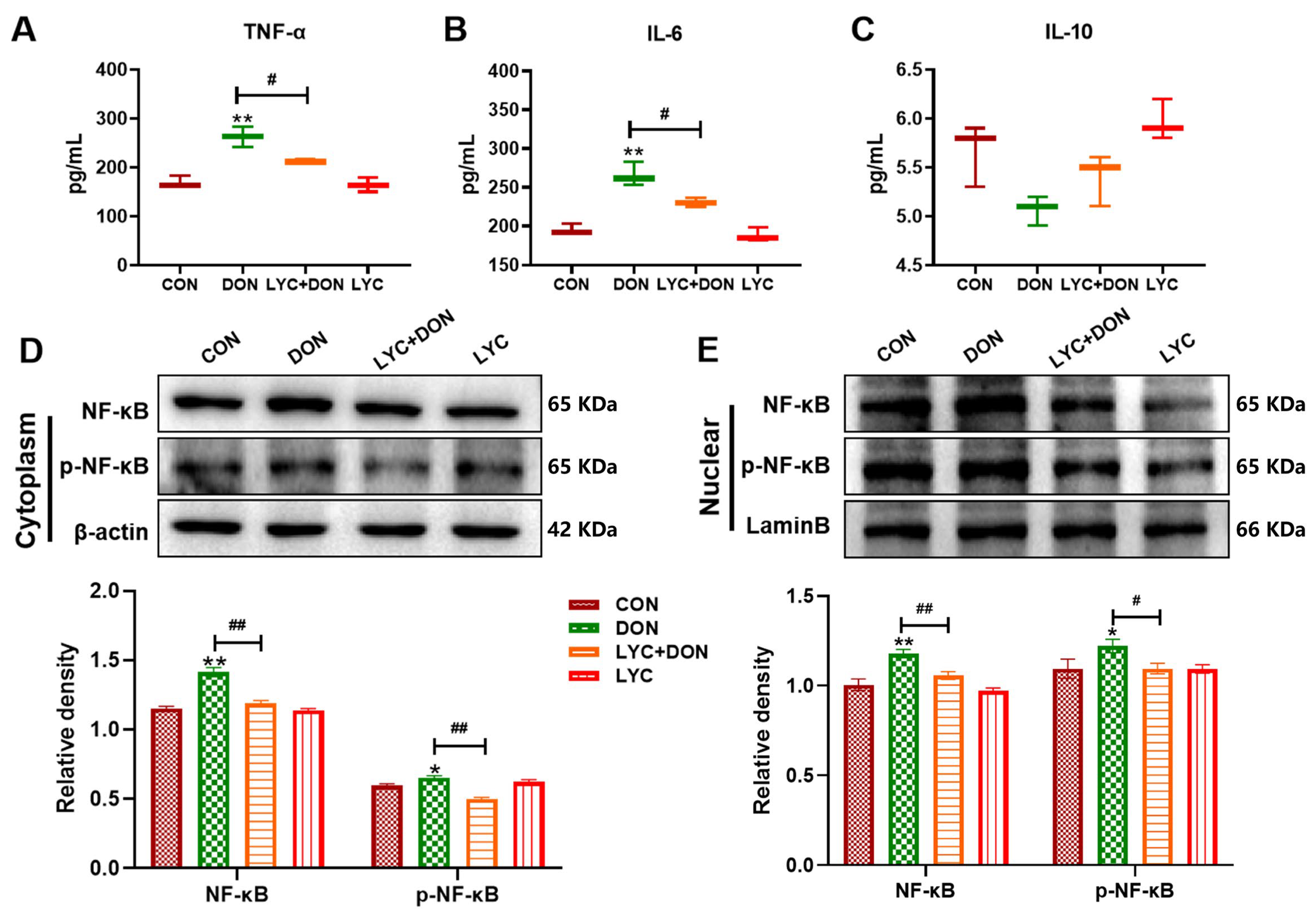
3.2. LYC Alleviated DON-Induced Inflammatory Injury and NF-κB Translocation in IPEC-J2 Cells
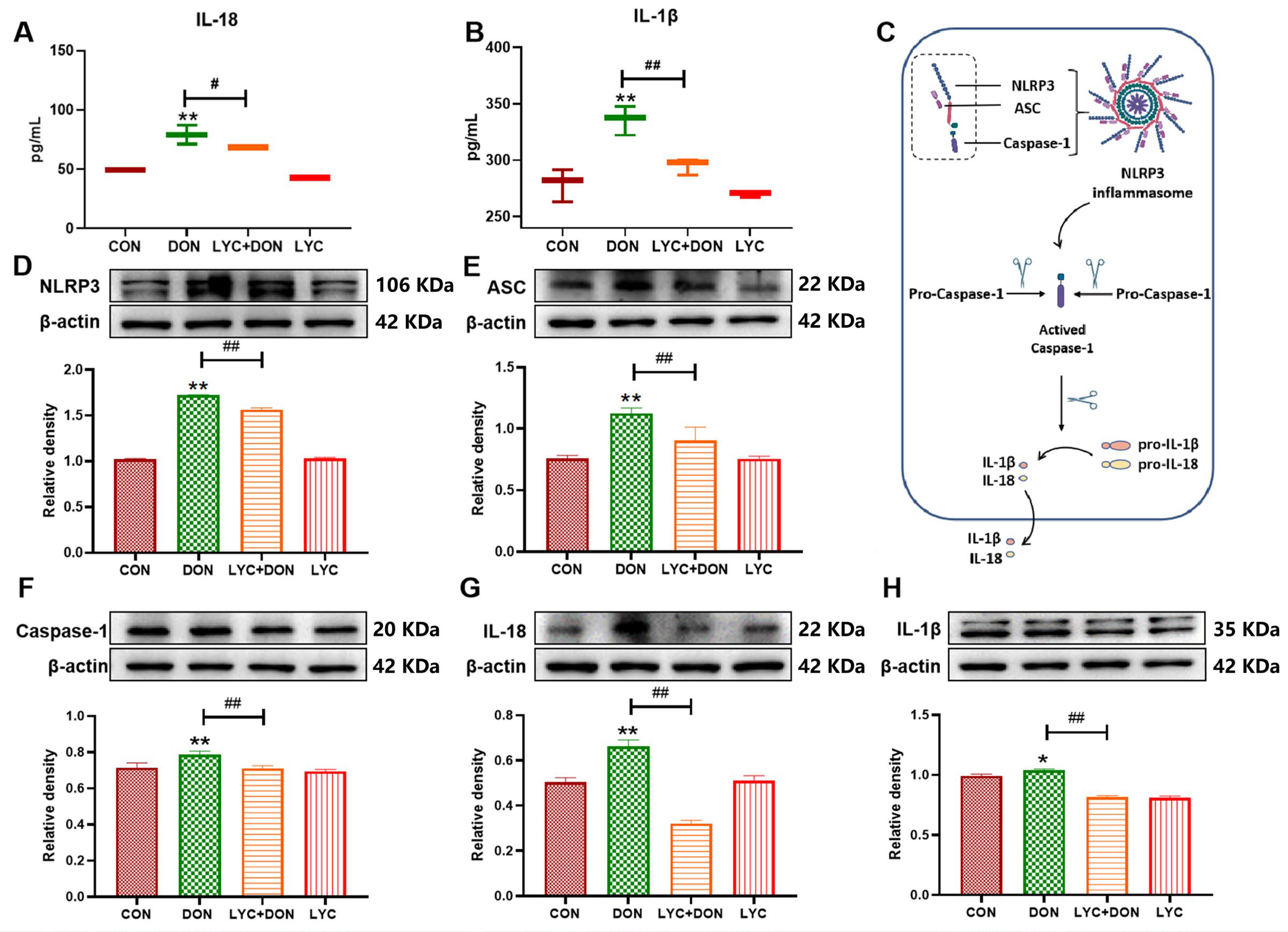
3.3. LYC Inhibited DON-Induced the Activation of NLRP3 Inflammasome in IPEC-J2 Cells
3.4. LYC Inhibited DON-Induced the Activation of the MAPK Signaling Pathway in IPEC-J2 Cells

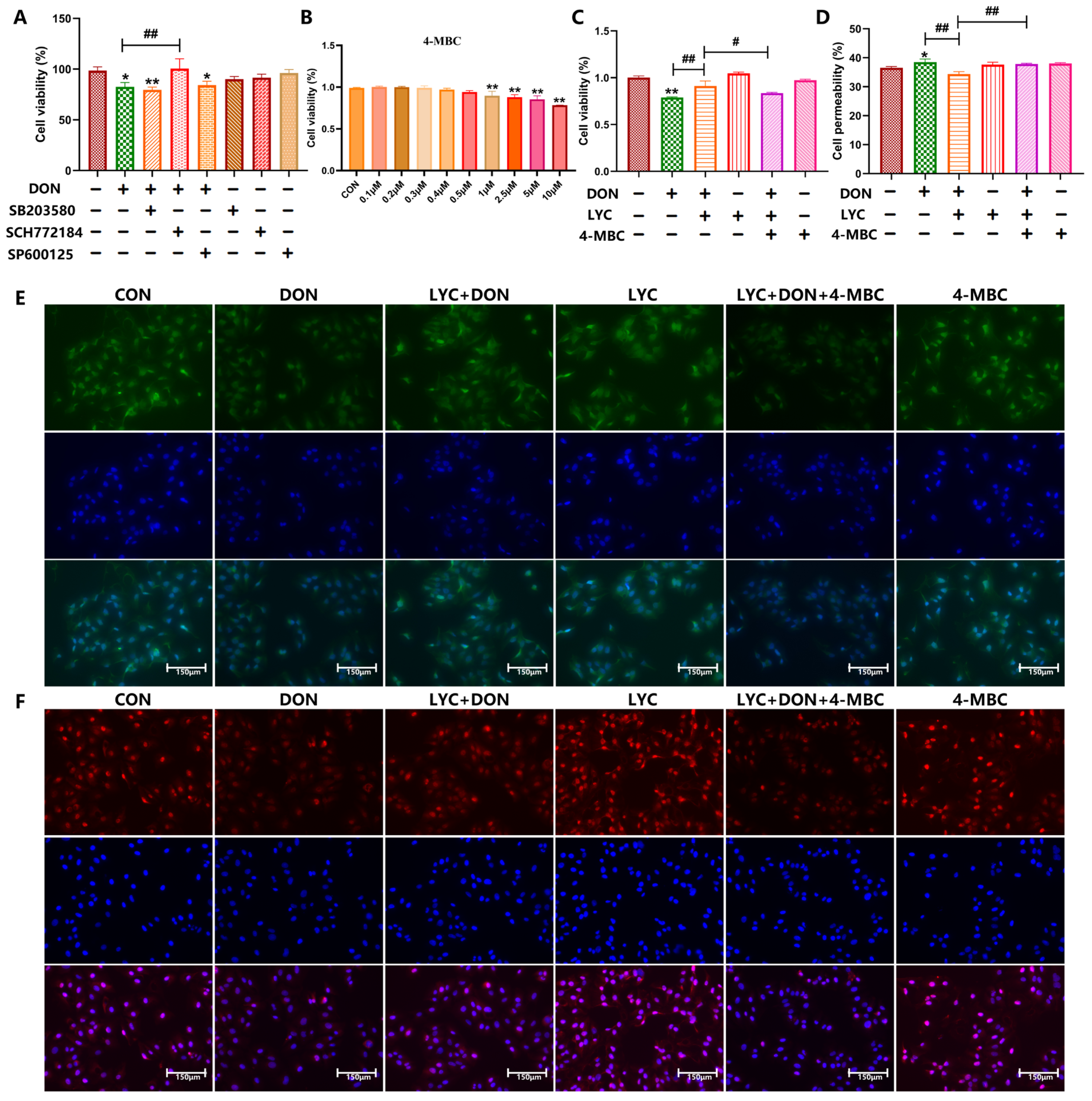
3.5. Protective Effect of LYC on Intestinal Barrier Is Mediated by ERK in the MAPK Signaling Pathway
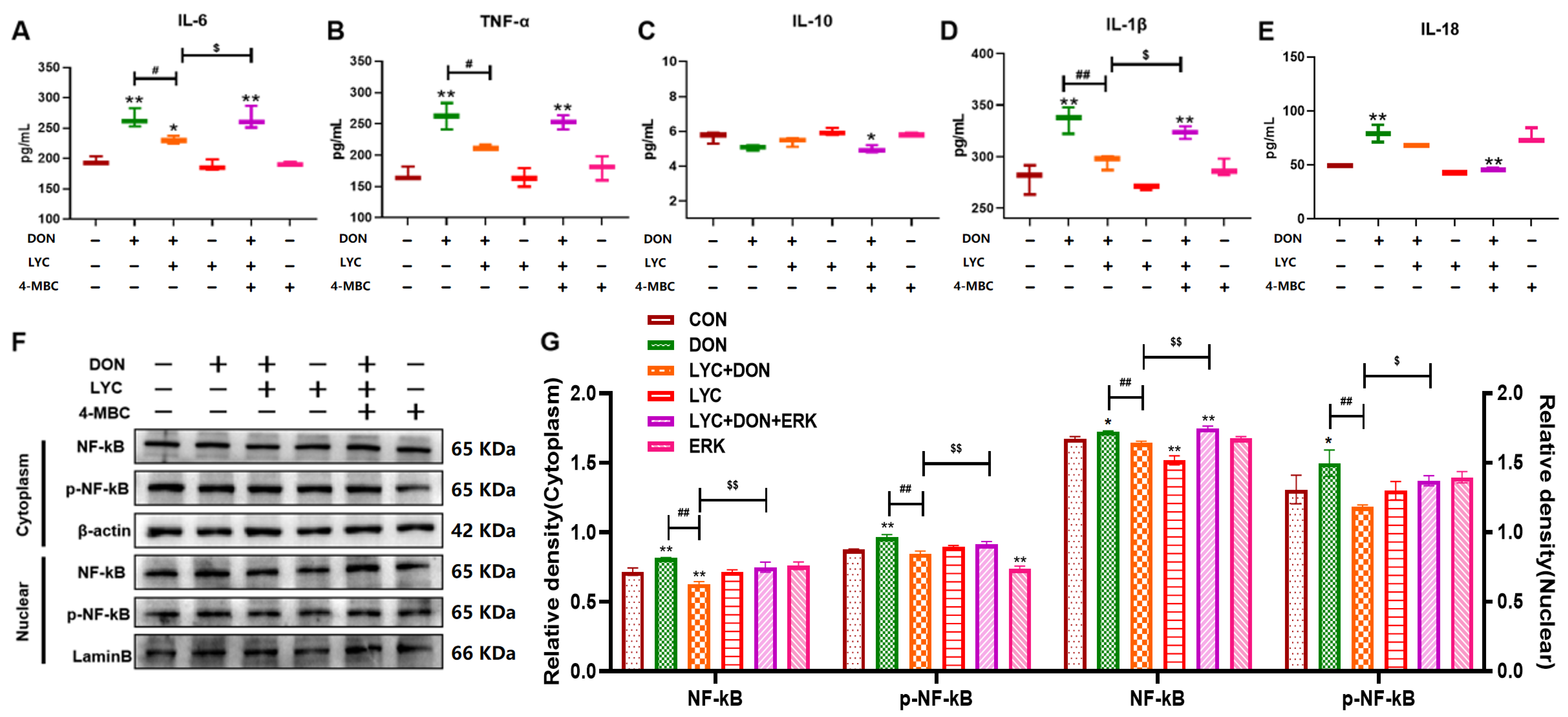
3.6. LYC Alleviated DON-Induced Inflammatory Injury and NF-κB Translocation by Targeting ERK in IPEC-J2 Cells
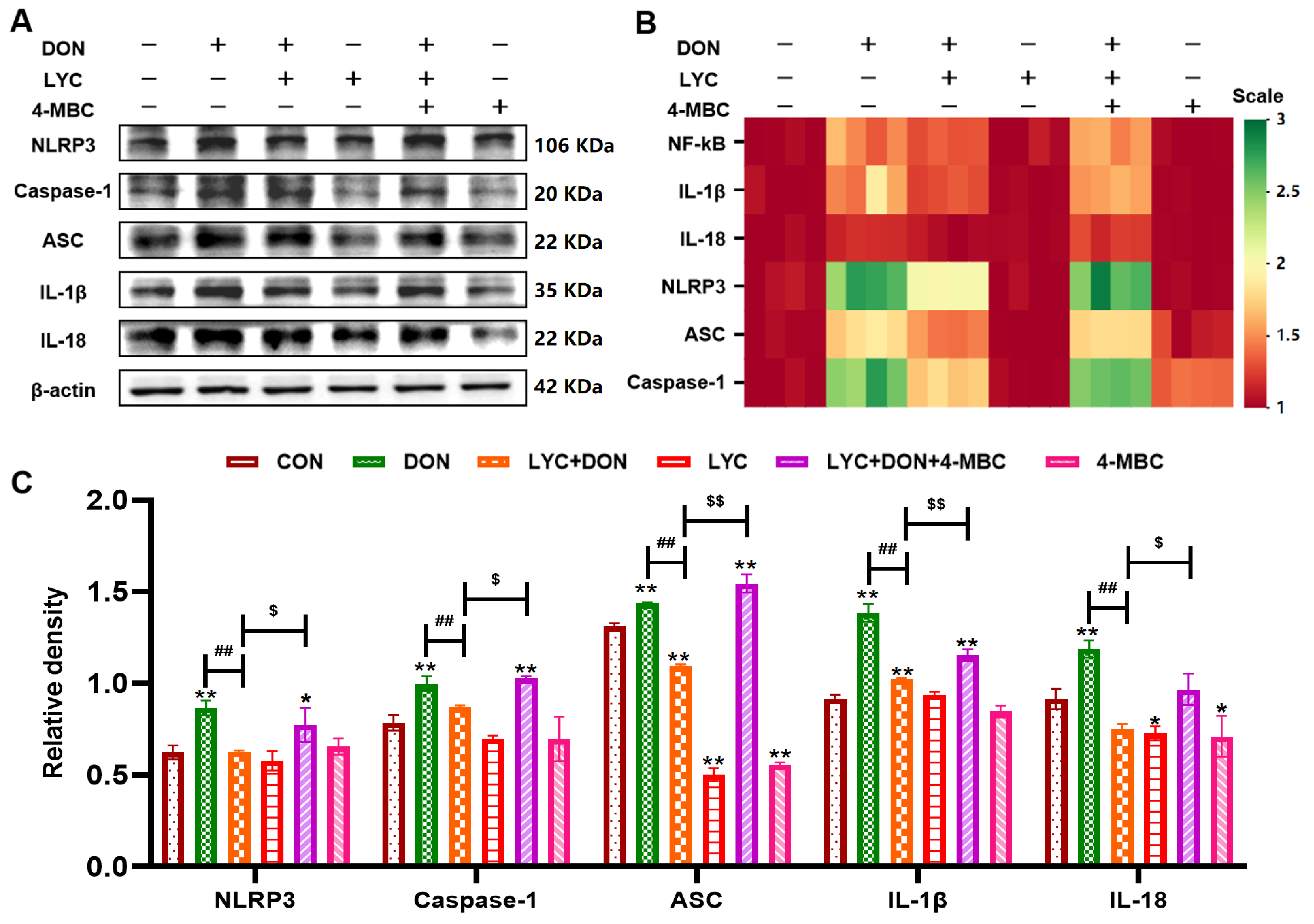
3.7. LYC Inhibited DON-Induced NLRP3 Inflammasome Activation by Targeting ERK in IPEC-J2 Cells
4. Discussion
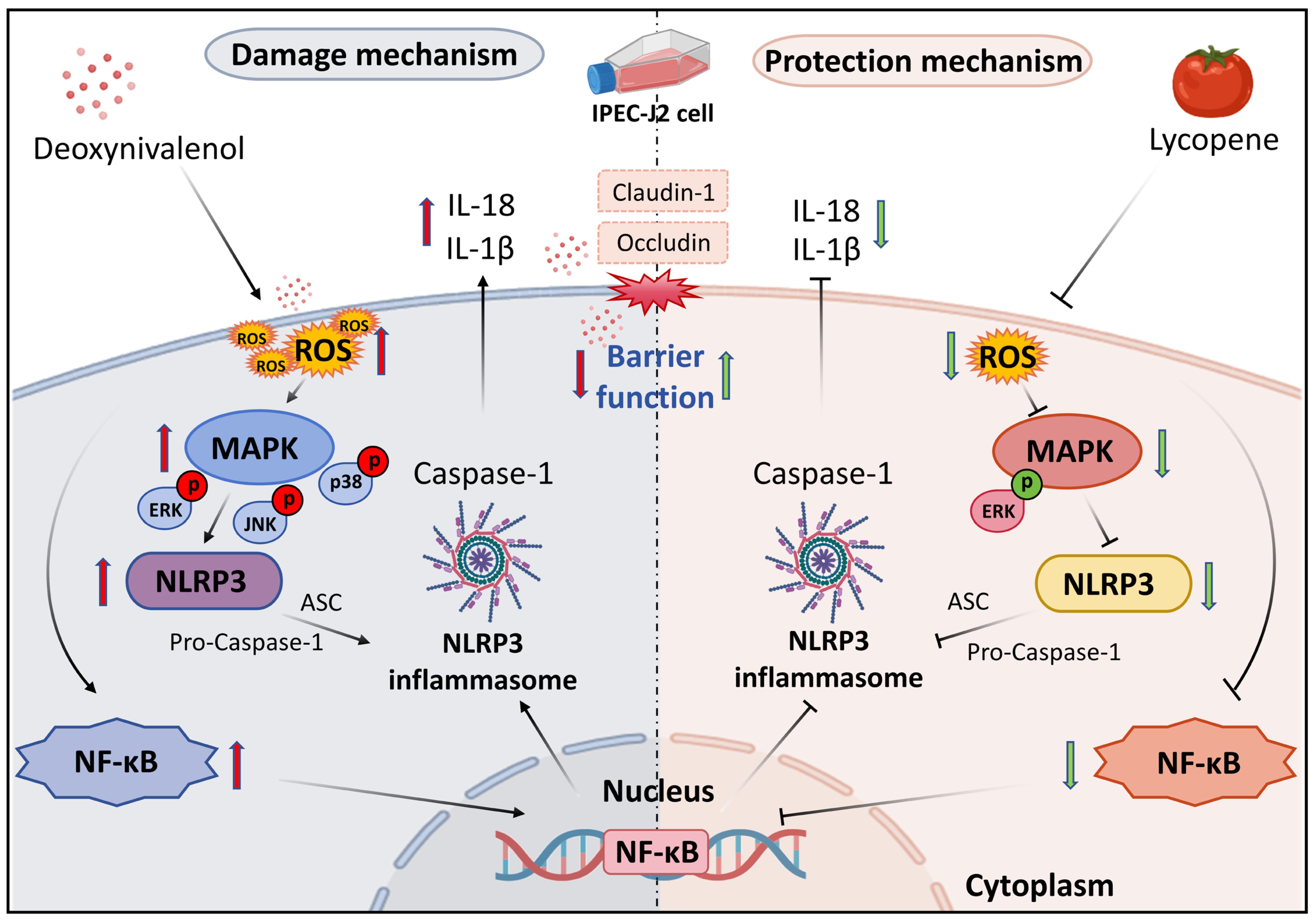
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pleadin, J.; Frece, J.; Markov, K. Mycotoxins in food and feed. Adv. Food Nutr. Res. 2019, 89, 297–345. [Google Scholar] [CrossRef]
- Sumarah, M.W. The Deoxynivalenol Challenge. J. Agric. Food Chem. 2022, 70, 9619–9624. [Google Scholar] [CrossRef] [PubMed]
- Streit, E.; Naehrer, K.; Rodrigues, I.; Schatzmayr, G. Mycotoxin occurrence in feed and feed raw materials worldwide: Long-term analysis with special focus on Europe and Asia. J. Sci. Food Agric. 2013, 93, 2892–2899. [Google Scholar] [CrossRef] [PubMed]
- Gruber-Dorninger, C.; Jenkins, T.; Schatzmayr, G. Global Mycotoxin Occurrence in Feed: A Ten-Year Survey. Toxins 2019, 11, 375. [Google Scholar] [CrossRef]
- van der Fels-Klerx, H.J.; Olesen, J.E.; Madsen, M.S.; Goedhart, P.W. Climate change increases deoxynivalenol contamination of wheat in north-western Europe. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2012, 29, 1593–1604. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Jiang, J.; Mu, P.; Lin, R.; Wen, J.; Deng, Y. Toxicokinetics and metabolism of deoxynivalenol in animals and humans. Arch. Toxicol. 2022, 96, 2639–2654. [Google Scholar] [CrossRef]
- Qu, J.; Zhang, S.; He, W.; Liu, S.; Mao, X.; Yin, L.; Yue, D.; Zhang, P.; Huang, K.; Chen, X. Crucial Function of Caveolin-1 in Deoxynivalenol-Induced Enterotoxicity by Activating ROS-Dependent NLRP3 Inflammasome-Mediated Pyroptosis. J. Agric. Food Chem. 2022, 70, 12968–12981. [Google Scholar] [CrossRef]
- Pierron, A.; Alassane-Kpembi, I.; Oswald, I. Impact of two mycotoxins deoxynivalenol and fumonisin on pig intestinal health. Porc. Health Manag. 2016, 2, 21. [Google Scholar] [CrossRef] [PubMed]
- Murtaza, B.; Wang, L.; Li, X.; Nawaz, M.Y.; Saleemi, M.K.; Khatoon, A.; Yongping, X. Recalling the reported toxicity assessment of deoxynivalenol, mitigating strategies and its toxicity mechanisms: Comprehensive review. Chem.-Biol. Interact. 2024, 387, 110799. [Google Scholar] [CrossRef]
- Broekaert, N.; Devreese, M.; Demeyere, K.; Berthiller, F.; Michlmayr, H.; Varga, E.; Adam, G.; Meyer, E.; Croubels, S. Comparative in vitro cytotoxicity of modified deoxynivalenol on porcine intestinal epithelial cells. Food Chem. Toxicol. 2016, 95, 103–109. [Google Scholar] [CrossRef]
- Qiu, Y.; Yang, J.; Wang, L.; Yang, X.; Gao, K.; Zhu, C.; Jiang, Z. Dietary resveratrol attenuation of intestinal inflammation and oxidative damage is linked to the alteration of gut microbiota and butyrate in piglets challenged with deoxynivalenol. J. Anim. Sci. Biotechnol. 2021, 12, 71. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, K.F.; Chen, F.J.; Chen, Y.H.; Yang, X.; Cai, Z.H.; Jiang, Y.B.; Wang, X.B.; Zhang, G.P.; Wang, F.Y. Deoxynivalenol triggers porcine intestinal tight junction disorder: Insights from mitochondrial dynamics and mitophagy. Ecotoxicol. Environ. Saf. 2022, 248, 114291. [Google Scholar] [CrossRef]
- Xiao, K.; Liu, C.; Qin, Q.; Zhang, Y.; Wang, X.; Zhang, J.; Odle, J.; Lin, X.; Hu, C.A.; Liu, Y. EPA and DHA attenuate deoxynivalenol-induced intestinal porcine epithelial cell injury and protect barrier function integrity by inhibiting necroptosis signaling pathway. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2020, 34, 2483–2496. [Google Scholar] [CrossRef]
- Jia, R.; Liu, W.; Zhao, L.; Cao, L.; Shen, Z. Low doses of individual and combined deoxynivalenol and zearalenone in naturally moldy diets impair intestinal functions via inducing inflammation and disrupting epithelial barrier in the intestine of piglets. Toxicol. Lett. 2020, 333, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Gan, F.; Lin, Z.; Tang, J.; Chen, X.; Huang, K. Deoxynivalenol at No-Observed Adverse-Effect Levels Aggravates DSS-Induced Colitis through the JAK2/STAT3 Signaling Pathway in Mice. J. Agric. Food Chem. 2023, 71, 4144–4152. [Google Scholar] [CrossRef]
- Miao, C.; Wu, Z.; Sun, Y.; Cao, Z. Deoxynivalenol Induces Intestinal Epithelial Barrier Damage through RhoA/ROCK Pathway-Mediated Apoptosis and F-Actin-Associated Tight Junction Disruption. J. Agric. Food Chem. 2024, 72, 9411–9423. [Google Scholar] [CrossRef]
- Gao, Y.; Meng, L.; Liu, H.; Wang, J.; Zheng, N. The Compromised Intestinal Barrier Induced by Mycotoxins. Toxins 2020, 12, 619. [Google Scholar] [CrossRef]
- Wang, X.Q.; Chang, Y.H.; Wang, X.C.; Liu, R.Q.; Yang, S.J.; Hu, Z.Y.; Jiang, F.W.; Chen, M.S.; Wang, J.X.; Liu, S.; et al. SIRT1 Regulates Fumonisin B1-Induced LMH Cell PANoptosis and Antagonism of Lycopene. J. Agric. Food Chem. 2025, 73, 4923–4935. [Google Scholar] [CrossRef]
- Wiertsema, S.P.; Van Bergenhenegouwen, J.; Garssen, J.; Knippels, L.M.J. The Interplay between the Gut Microbiome and the Immune System in the Context of Infectious Diseases throughout Life and the Role of Nutrition in Optimizing Treatment Strategies. Nutrients 2021, 13, 886. [Google Scholar] [CrossRef] [PubMed]
- Recharla, N.; Park, S.; Kim, M.; Kim, B.; Jeong, J.Y. Protective effects of biological feed additives on gut microbiota and the health of pigs exposed to deoxynivalenol: A review. J. Anim. Sci. Technol. 2022, 64, 640–653. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Wang, Q.; He, W.; Ge, L.; Huang, K. Deoxynivalenol aggravates the immunosuppression in piglets and PAMs under the condition of PEDV infection through inhibiting TLR4/NLRP3 signaling pathway. Ecotoxicol. Environ. Saf. 2022, 231, 113209. [Google Scholar] [CrossRef]
- Liao, C.; Xu, F.; Yu, Z.; Ding, K.; Jia, Y. The Novel Role of the NLRP3 Inflammasome in Mycotoxin-Induced Toxicological Mechanisms. Vet. Sci. 2024, 11, 291. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, F.; Wang, Y.; Zhang, K.; Yang, X.; Wang, X. Tanshinone IIA protects intestinal epithelial cells from deoxynivalenol-induced pyroptosis. Ecotoxicol. Environ. Saf. 2024, 269, 115743. [Google Scholar] [CrossRef] [PubMed]
- Akhter, N.; Wilson, A.; Arefanian, H.; Thomas, R.; Kochumon, S.; Al-Rashed, F.; Abu-Farha, M.; Al-Madhoun, A.; Al-Mulla, F.; Ahmad, R.; et al. Endoplasmic Reticulum Stress Promotes the Expression of TNF-α in THP-1 Cells by Mechanisms Involving ROS/CHOP/HIF-1α and MAPK/NF-κB Pathways. Int. J. Mol. Sci. 2023, 24, 15186. [Google Scholar] [CrossRef]
- Park, M.Y.; Ha, S.E.; Kim, H.H.; Bhosale, P.B.; Abusaliya, A.; Jeong, S.H.; Park, J.S.; Heo, J.D.; Kim, G.S. Scutellarein Inhibits LPS-Induced Inflammation through NF-κB/MAPKs Signaling Pathway in RAW264.7 Cells. Molecules 2022, 27, 3782. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Yuan, D.; Liao, P. Berberine improves intestinal barrier function and reduces inflammation, immunosuppression, and oxidative stress by regulating the NF-κB/MAPK signaling pathway in deoxynivalenol-challenged piglets. Environ. Pollut. 2021, 289, 117865. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, Z.; Wu, N.; Li, J.; Xi, N.; Xu, M.; Wu, F.; Fu, Q.; Yan, G.; Liu, Y.; et al. Chlorogenic acid attenuates deoxynivalenol-induced apoptosis and pyroptosis in human keratinocytes via activating Nrf2/HO-1 and inhibiting MAPK/NF-κB/NLRP3 pathways. Biomed. Pharmacother. 2024, 170, 116003. [Google Scholar] [CrossRef]
- Ge, L.; Lin, Z.; Le, G.; Hou, L.; Mao, X.; Liu, S.; Liu, D.; Gan, F.; Huang, K. Nontoxic-dose deoxynivalenol aggravates lipopolysaccharides-induced inflammation and tight junction disorder in IPEC-J2 cells through activation of NF-κB and LC3B. Food Chem. Toxicol. 2020, 145, 111712. [Google Scholar] [CrossRef]
- Story, E.N.; Kopec, R.E.; Schwartz, S.J.; Harris, G.K. An update on the health effects of tomato lycopene. Annu. Rev. Food Sci. Technol. 2010, 1, 189–210. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.H.; Chen, M.S.; Zhu, H.M.; Liu, R.Q.; Hu, Z.Y.; Yang, S.J.; Wang, X.Q.; Cheng, Y.; Song, Y.J.; Mao, X.Y.; et al. SIRT3 promotes mitophagy to attenuate fumonisin B1-induced chicken hepatocyte senescence and antagonism of lycopene. J. Hazard. Mater. 2025, 497, 139557. [Google Scholar] [CrossRef]
- Meng, Q.; Zhang, Y.; Li, J.; Shi, B.; Ma, Q.; Shan, A. Lycopene Affects Intestinal Barrier Function and the Gut Microbiota in Weaned Piglets via Antioxidant Signaling Regulation. J. Nutr. 2022, 152, 2396–2408, Erratum in J. Nutr. 2023, 153, 1659. [Google Scholar] [CrossRef]
- Laurindo, L.F.; Santos, A.R.O.D.; Carvalho, A.C.A.; Bechara, M.D.; Guiguer, E.L.; Goulart, R.A.; Vargas Sinatora, R.; Araújo, A.C.; Barbalho, S.M. Phytochemicals and Regulation of NF-kB in Inflammatory Bowel Diseases: An Overview of In Vitro and In Vivo Effects. Metabolites 2023, 13, 96. [Google Scholar] [CrossRef]
- Yue, Y.; Shi, M.; Song, X.; Ma, C.; Li, D.; Hu, X.; Chen, F. Lycopene Ameliorated DSS-Induced Colitis by Improving Epithelial Barrier Functions and Inhibiting the Escherichia coli Adhesion in Mice. J. Agric. Food Chem. 2024, 72, 5784–5796. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Chen, H.; Li, M.; Wang, W.; Long, F.; Fan, C. Associations between colorectal cancer risk and dietary intake of tomato, tomato products, and lycopene: Evidence from a prospective study of 101,680 US adults. Front. Oncol. 2023, 13, 1220270. [Google Scholar] [CrossRef]
- Hu, Z.Y.; Yang, S.J.; Chang, Y.H.; Wang, X.Q.; Liu, R.-Q.; Jiang, F.W.; Chen, M.-S.; Wang, J.X.; Liu, S.; Zhu, H.M.; et al. AHR activation relieves deoxynivalenol-induced disruption of porcine intestinal epithelial barrier functions. J. Hazard Mater. 2024, 480, 136095. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Chen, F.; Wang, Y.; Wang, X.; Yang, X.; Zhang, C. Lycopene Maintains Mitochondrial Homeostasis to Counteract the Enterotoxicity of Deoxynivalenol. Antioxidants 2023, 12, 1958. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Lim, W.; You, S.; Song, G. 4-Methylbenzylidene-camphor inhibits proliferation and induces reactive oxygen species-mediated apoptosis of human trophoblast cells. Reprod. Toxicol. 2019, 84, 49–58. [Google Scholar] [CrossRef]
- Tan, L.; Li, Q.; Sun, C.; Li, W.; Tang, N.; Tang, K. An Efficient HPLC-PDA Coupled with Supel™ Tox DON SPE Approach for the Analysis of Deoxynivalenol Contamination in Cereal Grains and Feedstuffs in Jiangxi Province. J. Food Prot. 2023, 86, 100022. [Google Scholar] [CrossRef]
- Djouina, M.; Waxin, C.; Caboche, S.; Lecointe, K.; Steimle, A.; Beury, D.; Desai, M.S.; Hot, D.; Dubuquoy, L.; Launay, D.; et al. Low dose dietary contamination with deoxynivalenol mycotoxin exacerbates enteritis and colorectal cancer in mice. Sci. Total Environ. 2023, 900, 165722. [Google Scholar] [CrossRef]
- Hu, P.; Zong, Q.; Zhao, Y.; Gu, H.; Liu, Y.; Gu, F.; Liu, H.Y.; Ahmed, A.A.; Bao, W.; Cai, D. Lactoferrin Attenuates Intestinal Barrier Dysfunction and Inflammation by Modulating the MAPK Pathway and Gut Microbes in Mice. J. Nutr. 2023, 152, 2451–2460. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Z.; Ma, J.; Xv, Q.; Gao, H.; Yin, H.; Yan, G.; Jiang, X.; Yu, W. Lycopene attenuates the inflammation and apoptosis in aristolochic acid nephropathy by targeting the Nrf2 antioxidant system. Redox Biol. 2022, 57, 102494. [Google Scholar] [CrossRef]
- Naser, A.N.; Lu, Q.; Chen, Y.H. Trans-Compartmental Regulation of Tight Junction Barrier Function. Tissue Barriers 2023, 11, 2133880. [Google Scholar] [CrossRef]
- Jiang, Y.; Song, J.; Xu, Y.; Liu, C.; Qian, W.; Bai, T.; Hou, X. Piezo1 regulates intestinal epithelial function by affecting the tight junction protein claudin-1 via the ROCK pathway. Life Sci. 2021, 275, 119254. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wu, K.; Zhao, X.; Yu, Q.; Li, J.; Wu, Y.; Liu, X. Dose-Response Metabolomics Unveils Liver Metabolic Disruptions and Pathway Sensitivity to Alkylimidazolium Ionic Liquids: Benchmark Dose Estimation for Health Risk Assessment. Environ. Sci. Technol. 2025, 59, 6414–6427. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Zhao, X.; Wu, K.; Yu, Q.; Wang, Q.; Li, J.; Wu, Y.; Liu, X. Benchmark Dose Estimation from Transcriptomics Data for Methylimidazolium Ionic Liquid Hepatotoxicity: Implications for Health Risk Assessment of Green Solvents. Environ. Health 2025, 3, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Liao, P.; Liao, M.; Li, L.; Tan, B.; Yin, Y. Effect of deoxynivalenol on apoptosis, barrier function, and expression levels of genes involved in nutrient transport, mitochondrial biogenesis and function in IPEC-J2 cells. Toxicol. Res. 2017, 6, 866–877. [Google Scholar] [CrossRef]
- Yang, Y.; Torbey, M.T. Angiogenesis and Blood-Brain Barrier Permeability in Vascular Remodeling after Stroke. Curr. Neuropharmacol. 2020, 18, 1250–1265. [Google Scholar] [CrossRef]
- Wang, P.; Li, T.; Niu, C.; Sun, S.; Liu, D. ROS-activated MAPK/ERK pathway regulates crosstalk between Nrf2 and Hif-1α to promote IL-17D expression protecting the intestinal epithelial barrier under hyperoxia. Int. Immunopharmacol. 2023, 116, 109763. [Google Scholar] [CrossRef]
- Zhang, J.; Fang, Y.; Fu, Y.; Jalukar, S.; Ma, J.; Liu, Y.; Guo, Y.; Ma, Q.; Ji, C.; Zhao, L. Yeast polysaccharide mitigated oxidative injury in broilers induced by mixed mycotoxins via regulating intestinal mucosal oxidative stress and hepatic metabolic enzymes. Poult. Sci. 2023, 102, 102862. [Google Scholar] [CrossRef]
- Hoesel, B.; Schmid, J.A. The complexity of NF-κB signaling in inflammation and cancer. Mol. Cancer 2013, 12, 86. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Xu, P.; Zhu, Z.; Zhou, L.; Li, J.; Zhou, R.; Kan, Y.; Li, Y.; Yu, X.; Zhao, J.; et al. Acetylation of p65Lys310 by p300 in macrophages mediates anti-inflammatory property of berberine. Redox Biol. 2023, 62, 102704. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, Y.; Zhao, J.; Cao, L.; Zhu, L.; Huang, Y.; Chen, X.; Rahman, S.U.; Feng, S.; Li, Y.; et al. Deoxynivalenol Induces Inflammatory Injury in IPEC-J2 Cells via NF-κB Signaling Pathway. Toxins 2020, 11, 733. [Google Scholar] [CrossRef] [PubMed]
- Ba, W.; Xu, W.; Deng, Z.; Zhang, B.; Zheng, L.; Li, H. The Antioxidant and Anti-Inflammatory Effects of the Main Carotenoids from Tomatoes via Nrf2 and NF-κB Signaling Pathways. Nutrients 2023, 15, 4652. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Jiang, Y.; Yu, T.T.; Hao, W.; Wang, G. Lycopene improves autophagy and attenuates carbon tetrachloride-induced hepatic fibrosis in rats. Croat. Med. J. 2023, 64, 243–255. [Google Scholar] [CrossRef]
- Feng, D.; Ling, W.H.; Duan, R.D. Lycopene suppresses LPS-induced NO and IL-6 production by inhibiting the activation of ERK, p38MAPK, and NF-kappaB in macrophages. Inflamm. Res 2010, 59, 115–121. [Google Scholar] [CrossRef]
- Xu, Z.; Liu, C.; Wang, R.; Gao, X.; Hao, C.; Liu, C. A combination of lycopene and human amniotic epithelial cells can ameliorate cognitive deficits and suppress neuroinflammatory signaling by choroid plexus in Alzheimer’s disease rat. J. Nutr. Biochem. 2021, 88, 108558. [Google Scholar] [CrossRef]
- Li, S.; Fang, Y.; Zhang, Y.; Song, M.; Zhang, X.; Ding, X.; Yao, H.; Chen, M.; Sun, Y.; Ding, J.; et al. Microglial NLRP3 inflammasome activates neurotoxic astrocytes in depression-like mice. Cell Rep. 2022, 41, 111532. [Google Scholar] [CrossRef]
- Kelley, N.; Jeltema, D.; Duan, Y.; He, Y. The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation. Int. J. Mol. Sci. 2019, 20, 3328. [Google Scholar] [CrossRef]
- Peng, L.; Wen, L.; Shi, Q.F.; Gao, F.; Huang, B.; Meng, J.; Hu, C.P.; Wang, C.M. Scutellarin ameliorates pulmonary fibrosis through inhibiting NF-κB/NLRP3-mediated epithelial-mesenchymal transition and inflammation. Cell Death Dis. 2020, 11, 978. [Google Scholar] [CrossRef]
- Zhao, W.; Ma, L.; Cai, C.; Gong, X. Caffeine Inhibits NLRP3 Inflammasome Activation by Suppressing MAPK/NF-κB and A2aR Signaling in LPS-Induced THP-1 Macrophages. Int. J. Biol. Sci. 2019, 15, 1571–1581. [Google Scholar] [CrossRef]
- Mao, X.; Zhang, P.; Du, H.; Ge, L.; Liu, S.; Huang, K.; Chen, X. The combined effect of deoxynivalenol and Fumonisin B1 on small intestinal inflammation mediated by pyroptosis in vivo and in vitro. Toxicol. Lett. 2023, 372, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Ge, L.; Liu, D.; Mao, X.; Liu, S.; Guo, J.; Hou, L.; Chen, X.; Huang, K. Low Dose of Deoxynivalenol Aggravates Intestinal Inflammation and Barrier Dysfunction Induced by Enterotoxigenic Escherichia coli Infection through Activating Macroautophagy/NLRP3 Inflammasomes. J. Agric. Food Chem. 2022, 70, 3009–3022. [Google Scholar] [CrossRef] [PubMed]
- Saleem, S. Targeting MAPK signaling: A promising approach for treating inflammatory lung disease. Pathol. Res. Pract. 2024, 254, 155122. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Deng, X.; Zhou, C.; Wu, W.; Zhang, H. Deoxynivalenol Induces Inflammation in IPEC-J2 Cells by Activating P38 Mapk and Erk1/2. Toxins 2020, 12, 180. [Google Scholar] [CrossRef]
- Yu, Y.H.; Lai, Y.H.; Hsiao, F.S.H.; Cheng, Y.H. Effects of Deoxynivalenol and Mycotoxin Adsorbent Agents on Mitogen-Activated Protein Kinase Signaling Pathways and Inflammation-Associated Gene Expression in Porcine Intestinal Epithelial Cells. Toxins 2021, 13, 301. [Google Scholar] [CrossRef]
- Wang, Y.; Fu, J.; Zhou, D.; Ren, Z.; Deng, J. The PGC-1α/SIRT3 pathway mediates the effect of DON on mitochondrial autophagy and liver injury in mice. Mycotoxin Res. 2025, 41, 499–511, Erratum in Mycotoxin Res. 2025, 41, 671–672. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, B.; Wang, P.; Hua, Z.; Zhang, S.; Wang, X.; Yang, X.; Zhang, C. Review of neurotoxicity of T-2 toxin. Mycotoxin Res. 2024, 40, 85–95. [Google Scholar] [CrossRef]
- Lv, H.; Rao, Z.; Li, Y.; Zhang, W.; Zhao, L.; Wang, Z.; Guo, Y. Dietary Supplementation of Novel Aflatoxin Oxidase CotA Alleviates Aflatoxin B1-Induced Oxidative Stress, Lipid Metabolism Disorder, and Apoptosis in the Liver of Japanese Quails. Animals 2025, 15, 1555. [Google Scholar] [CrossRef]
- Chen, F.; Wang, Y.; Chen, Y.; Fan, J.; Zhang, C.; He, X.; Yang, X. JNK molecule is a toxic target for IPEC-J2 cell barrier damage induced by T-2 toxin. Ecotoxicol. Environ. Saf. 2023, 263, 115247. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, Z.; Lu, Z.; Wang, Y.; Song, W.; Yang, X.; Zhang, C. Lycopene Protects Deoxynivalenol-Induced Intestinal Barrier Dysfunction and NLRP3 Inflammasome Activation by Targeting the ERK Pathway. Antioxidants 2025, 14, 1513. https://doi.org/10.3390/antiox14121513
Cai Z, Lu Z, Wang Y, Song W, Yang X, Zhang C. Lycopene Protects Deoxynivalenol-Induced Intestinal Barrier Dysfunction and NLRP3 Inflammasome Activation by Targeting the ERK Pathway. Antioxidants. 2025; 14(12):1513. https://doi.org/10.3390/antiox14121513
Chicago/Turabian StyleCai, Zihui, Zhi Lu, Youshuang Wang, Wenxi Song, Xu Yang, and Cong Zhang. 2025. "Lycopene Protects Deoxynivalenol-Induced Intestinal Barrier Dysfunction and NLRP3 Inflammasome Activation by Targeting the ERK Pathway" Antioxidants 14, no. 12: 1513. https://doi.org/10.3390/antiox14121513
APA StyleCai, Z., Lu, Z., Wang, Y., Song, W., Yang, X., & Zhang, C. (2025). Lycopene Protects Deoxynivalenol-Induced Intestinal Barrier Dysfunction and NLRP3 Inflammasome Activation by Targeting the ERK Pathway. Antioxidants, 14(12), 1513. https://doi.org/10.3390/antiox14121513






