Cistanche deserticola Polysaccharides Protect Against Doxorubicin-Induced Cardiotoxicity via Antioxidant and Mitochondrial Mechanisms
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Identification of CDPs
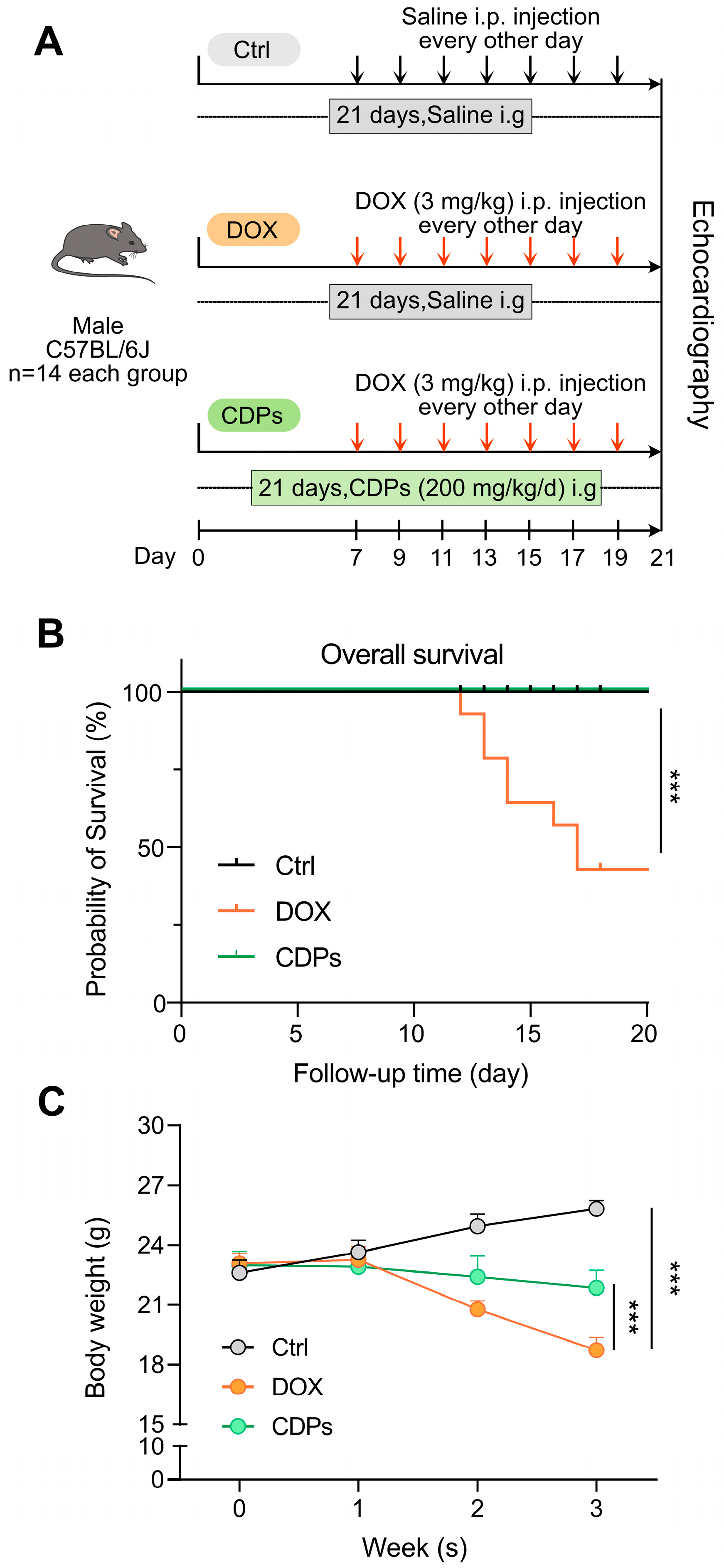
2.3. Animals and Experimental Design
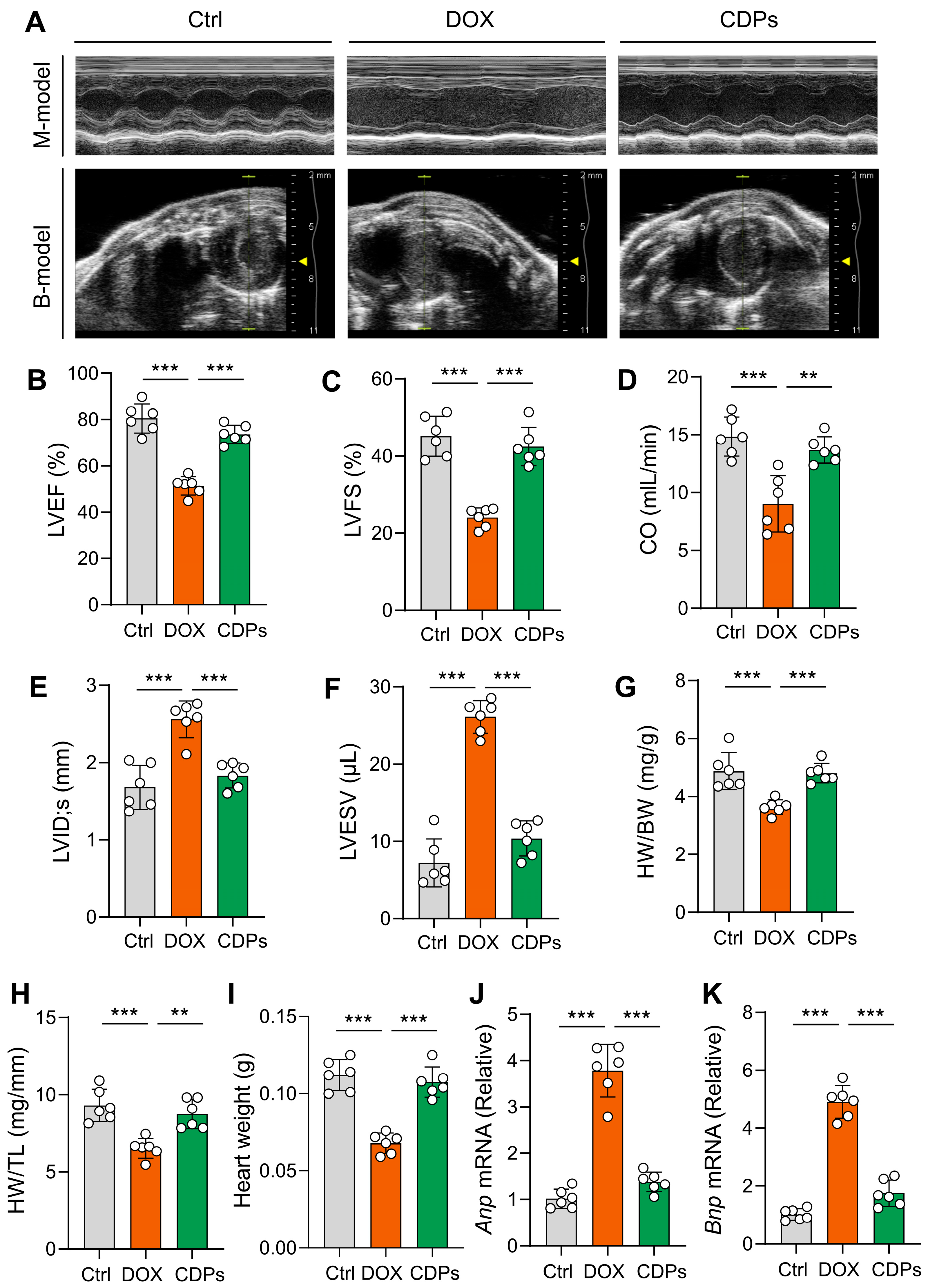
2.4. Echocardiography and Hemodynamics
2.5. Measurement of Serum Myocardial Enzymes
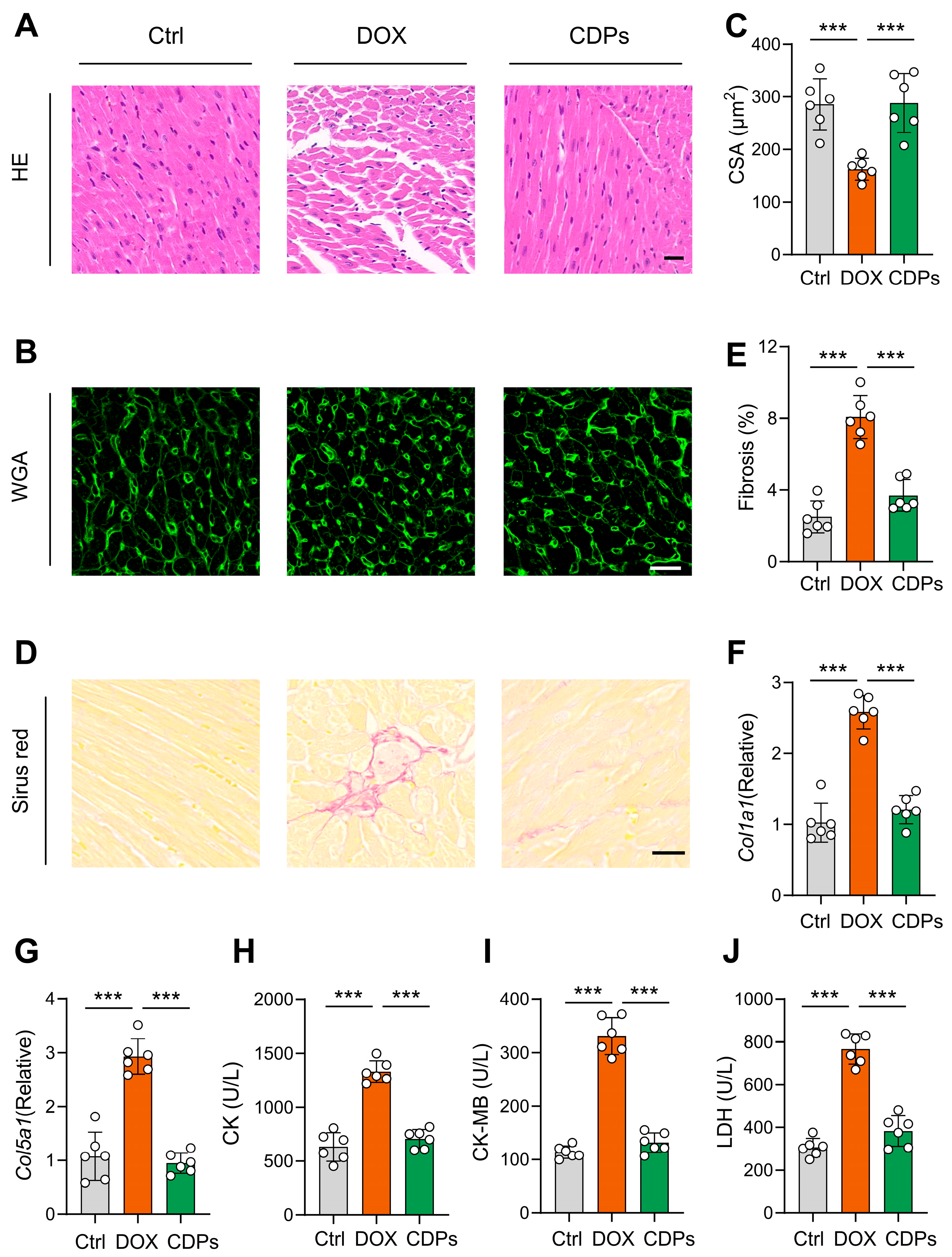
2.6. Histological Examination
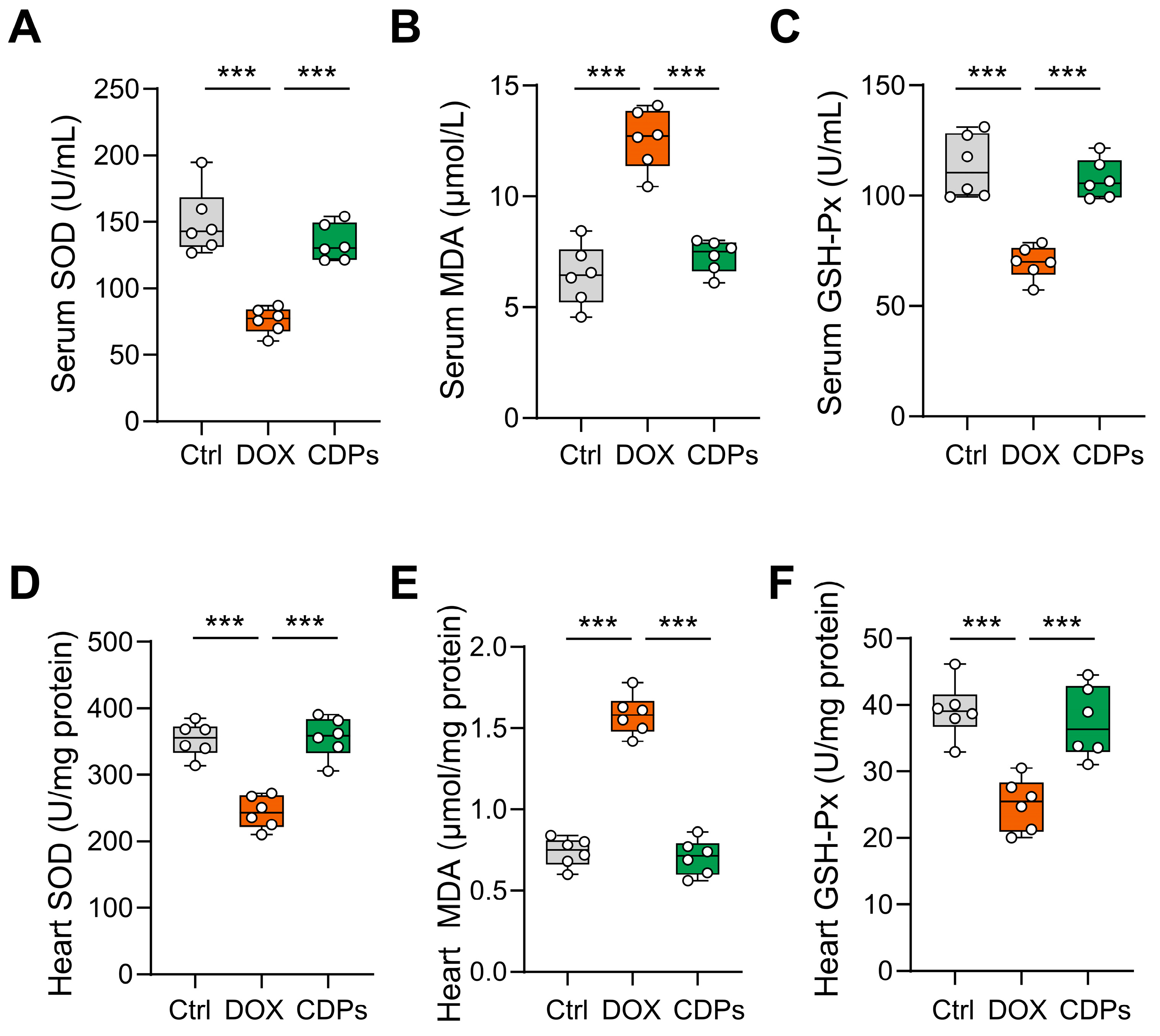
2.7. Biochemical Assays
2.8. Transmission Electron Microscopy (TEM)
2.9. RNA Extraction and Real-Time Quantitative PCR (RT-qPCR)
2.10. ATP Quantification
2.11. Statistical Analysis
3. Results
3.1. Structural Analysis of CDPs
3.2. CDPs Attenuate DOX-Induced Cardiac Dysfunction and Cardiomyocyte Injury
3.3. CDPs Alleviate the Histological Changes of Cardiac Tissue of Mice with DOX-Induced Injury
3.4. CDPs Treatment Inhibited DOX-Induced Oxidative Injury
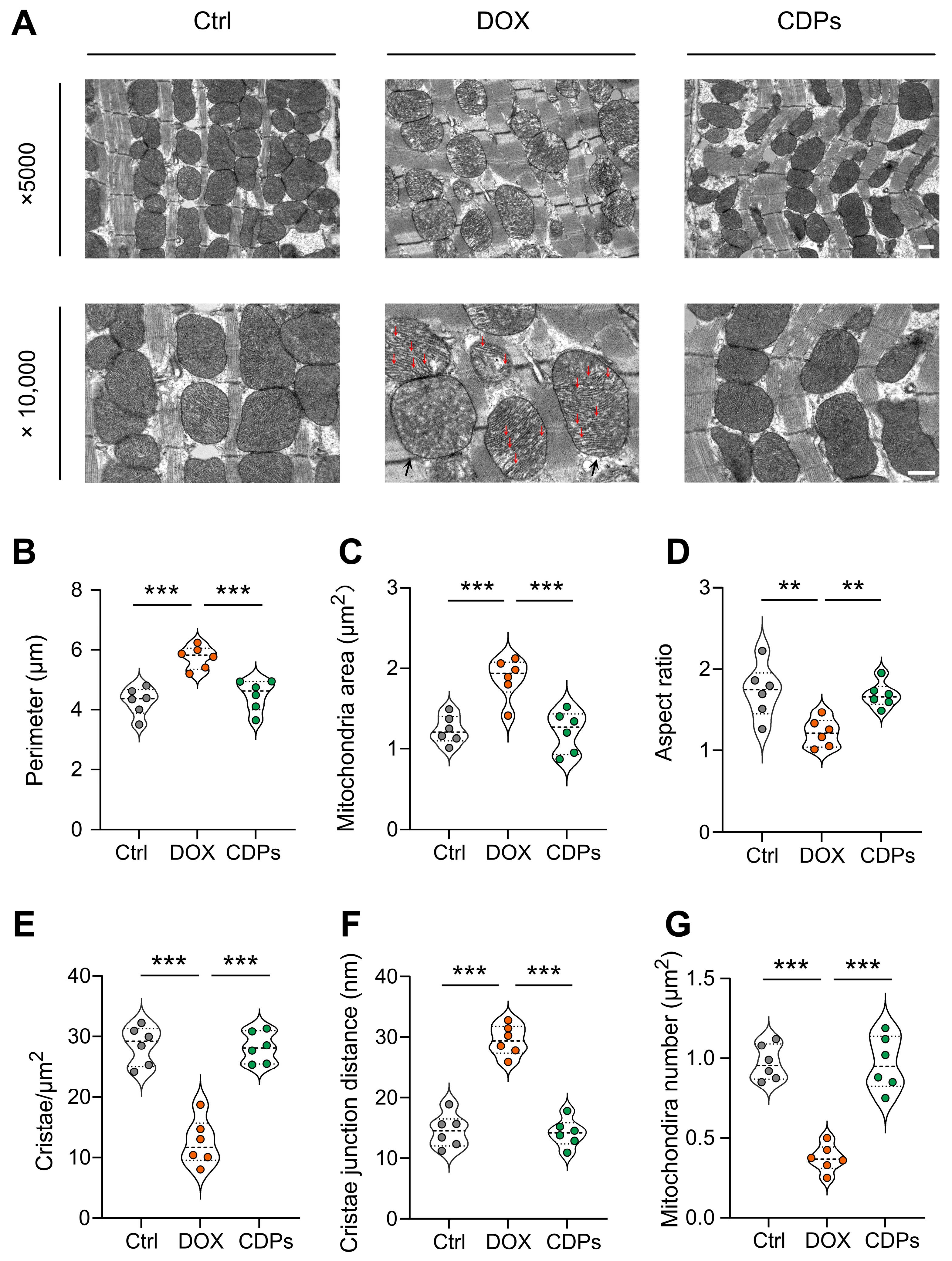
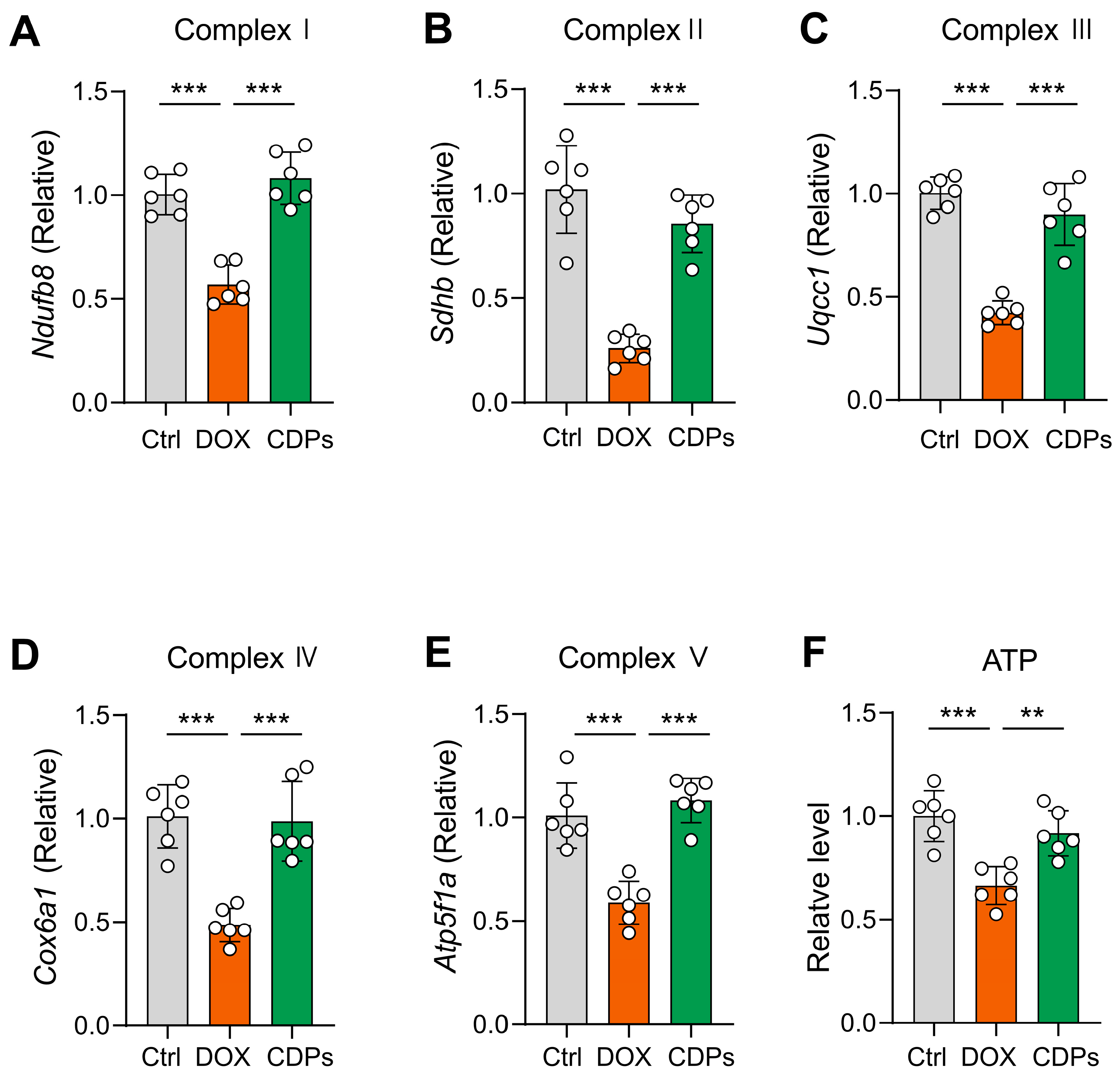
3.5. CDPs Alleviate DOX-Induced Cardiomyopathy Through Improving Mitochondrial Function
4. Discussion
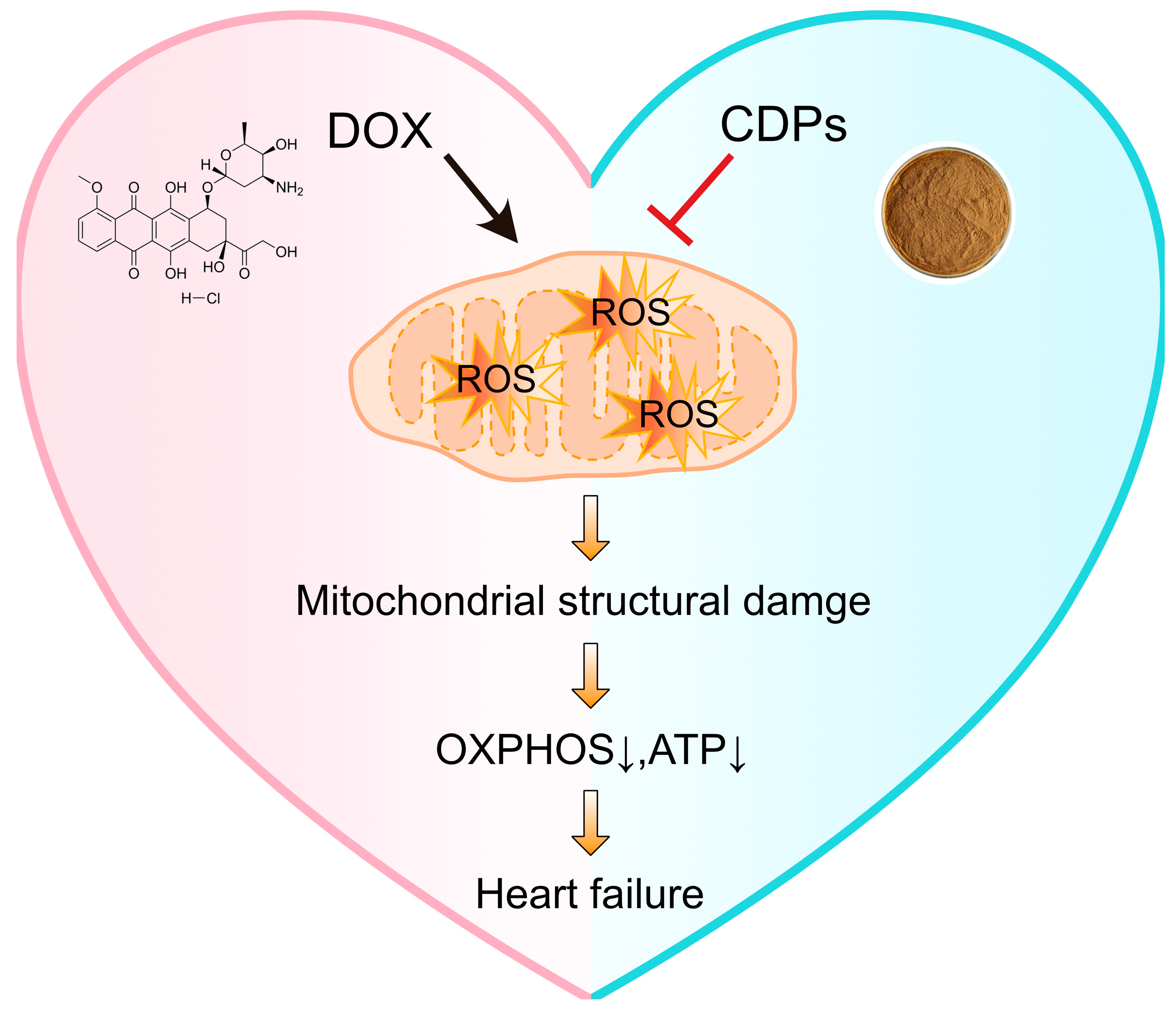
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Carvalho, C.; Santos, R.X.; Cardoso, S.; Correia, S.; Oliveira, P.J.; Santos, M.S.; Moreira, P.I. Doxorubicin: The Good, the Bad and the Ugly Effect. Curr. Med. Chem. 2009, 16, 3267–3285. [Google Scholar] [CrossRef]
- Pugazhendhi, A.; Edison, T.N.J.I.; Velmurugan, B.K.; Jacob, J.A.; Karuppusamy, I. Toxicity of Doxorubicin (Dox) to Different Experimental Organ Systems. Life Sci. 2018, 200, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Roychoudhury, S.; Kumar, A.; Bhatkar, D.; Sharma, N.K. Molecular Avenues in Targeted Doxorubicin Cancer Therapy. Future Oncol. 2020, 16, 687–700. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Shi, S.; Dai, Y. Research Progress of Therapeutic Drugs for Doxorubicin-Induced Cardiomyopathy. Biomed. Pharmacother. 2022, 156, 113903. [Google Scholar] [CrossRef]
- Robinson, E.L.; Azodi, M.; Heymans, S.; Heggermont, W. Anthracycline-Related Heart Failure: Certain Knowledge and Open Questions: Where Do We Stand with Chemotherapyinduced Cardiotoxicity? Curr. Heart Fail. Rep. 2020, 17, 357–364. [Google Scholar] [CrossRef]
- Wu, L.; Wang, L.; Du, Y.; Zhang, Y.; Ren, J. Mitochondrial Quality Control Mechanisms as Therapeutic Targets in Doxorubicin-Induced Cardiotoxicity. Trends Pharmacol. Sci. 2023, 44, 34–49. [Google Scholar] [CrossRef]
- Kong, C.-Y.; Guo, Z.; Song, P.; Zhang, X.; Yuan, Y.-P.; Teng, T.; Yan, L.; Tang, Q.-Z. Underlying the Mechanisms of Doxorubicin-Induced Acute Cardiotoxicity: Oxidative Stress and Cell Death. Int. J. Biol. Sci. 2022, 18, 760–770. [Google Scholar] [CrossRef]
- Da Dalt, L.; Cabodevilla, A.G.; Goldberg, I.J.; Norata, G.D. Cardiac Lipid Metabolism, Mitochondrial Function, and Heart Failure. Cardiovasc. Res. 2023, 119, 1905–1914. [Google Scholar] [CrossRef] [PubMed]
- Lopaschuk, G.D.; Karwi, Q.G.; Tian, R.; Wende, A.R.; Abel, E.D. Cardiac Energy Metabolism in Heart Failure. Circ. Res. 2021, 128, 1487–1513. [Google Scholar] [CrossRef]
- Yan, Q.; Liu, S.; Sun, Y.; Chen, C.; Yang, S.; Lin, M.; Long, J.; Yao, J.; Lin, Y.; Yi, F.; et al. Targeting Oxidative Stress as a Preventive and Therapeutic Approach for Cardiovascular Disease. J. Transl. Med. 2023, 21, 519. [Google Scholar] [CrossRef]
- Wallace, K.B.; Sardão, V.A.; Oliveira, P.J. Mitochondrial Determinants of Doxorubicin-Induced Cardiomyopathy. Circ. Res. 2020, 126, 926–941. [Google Scholar] [CrossRef]
- Nolfi-Donegan, D.; Braganza, A.; Shiva, S. Mitochondrial Electron Transport Chain: Oxidative Phosphorylation, Oxidant Production, and Methods of Measurement. Redox. Biol. 2020, 37, 101674. [Google Scholar] [CrossRef] [PubMed]
- Víctor, V.M.; Espulgues, J.V.; Hernández-Mijares, A.; Rocha, M. Oxidative Stress and Mitochondrial Dysfunction in Sepsis: A Potential Therapy with Mitochondria-Targeted Antioxidants. Infect. Disord. Drug Targets 2009, 9, 376–389. [Google Scholar] [CrossRef]
- Wan, S.; Qi, J.; Xia, Y.; Fan, C.; Xu, T.; Zhang, X.; Shi, J.; Wang, C.; Cheng, Y.; Zhang, D.; et al. Cardiac Slc25a49-Mediated Energy Reprogramming Governs Doxorubicin-Induced Cardiomyopathy Through the G6P–AP-1–Sln Axis. Adv. Sci. 2025, 12, 2502163. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Xiong, D.; Zhang, H.; Xiao, S.; Yi, R.; Wu, J. ETS2 Promotes Cardiomyocyte Apoptosis and Autophagy in Heart Failure by Regulating lncRNA TUG1/miR-129-5p/ATG7 Axis. FASEB J. 2023, 37, e22937. [Google Scholar] [CrossRef]
- Peng, H.; Yao, F.; Zhao, J.; Zhang, W.; Chen, L.; Wang, X.; Yang, P.; Tang, J.; Chi, Y. Unraveling Mitochondria-Targeting Reactive Oxygen Species Modulation and Their Implementations in Cancer Therapy by Nanomaterials. Exploration 2023, 3, 20220115. [Google Scholar] [CrossRef]
- Qian, K.; Gao, S.; Jiang, Z.; Ding, Q.; Cheng, Z. Recent Advances in Mitochondria-Targeting Theranostic Agents. Exploration 2024, 4, 20230063. [Google Scholar] [CrossRef] [PubMed]
- Qiang, G.-F. Natural Products Targeting Mitochondria: A Promising Strategy for Metabolic Syndrome. Chin. J. Nat. Med. 2020, 18, 801–802. [Google Scholar] [CrossRef]
- Noh, S.; Go, A.; Kim, D.B.; Park, M.; Jeon, H.W.; Kim, B. Role of Antioxidant Natural Products in Management of Infertility: A Review of Their Medicinal Potential. Antioxidants 2020, 9, 957. [Google Scholar] [CrossRef]
- Li, J.; Sun, W.; Wan, X.; Zhang, Y.; Yang, X.; Ouyang, B.; Zhang, Q.; Wang, Q.; Li, X.; Liu, X.; et al. Cistanche Deserticola Polysaccharides Protect against Cyclophosphamide-Induced Premature Ovarian Failure in Mice by Regulating the JAK-STAT Pathway. J. Ethnopharmacol. 2025, 349, 119971. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, H.; Yang, M.; Liu, N.; Zhao, Y.; Qi, X.; Niu, Y.; Sun, T.; Li, Y.; Yu, J. Cistanche Deserticola Polysaccharides Protects PC12 Cells Against OGD/RP-Induced Injury. Biomed. Pharmacother. 2018, 99, 671–680. [Google Scholar] [CrossRef]
- Jiang, H.; Ma, R.; Ji, F.; Liu, Y.; Wang, B.; Fu, S.; Ma, L.; Wang, S.; Liu, C.; Guo, Z.; et al. Structure Characterization of Polysaccharides from Cistanche Deserticola and Their Neuroprotective Effects Against Oxidative Stress in Slow Transit Constipation Mice. Int. J. Biol. Macromol. 2024, 260, 129527. [Google Scholar] [CrossRef]
- Qiao, M.; Xue, T.; Zhu, Y.; Yang, J.; Hu, J. Polysaccharides from Cistanche Deserticola Mitigate Inflammatory Bowel Disease via Modulating Intestinal Microbiota and SRC/EGFR/PI3K/AKT Signaling Pathways. Int. J. Biol. Macromol. 2025, 308, 142452. [Google Scholar] [CrossRef]
- Liu, X.; Yang, Z.; Han, M.; Zhang, Y.; Muhammad, H.; Zhong, H.; Guan, R. Bioactive Components, Pharmacological Properties, and Applications of Cistanche Deserticola Y. C. Ma: A Comprehensive Review. Nutrients 2025, 17, 1501. [Google Scholar] [CrossRef] [PubMed]
- Cheng, N.; Wang, H.; Hao, H.; Rahman, F.-U.; Zhang, Y. Research Progress on Polysaccharide Components of Cistanche Deserticola as Potential Pharmaceutical Agents. Eur. J. Med. Chem. 2023, 245, 114892. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhang, C.-S.; Liang, Y.; Gao, J.; Liu, Y.; Bu, R.; Liu, H.; Du, X.-L.; Sun, L.-J.; Li, B.; et al. Cistanche Deserticola Polysaccharides Mitigate Alzheimer’s Disease Progression by Dynamic Regulation of Gut Microbiota Composition and Metabolites. Am. J. Chin. Med. 2025, 53, 1785–1812. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Li, J.; Zhang, Y.; Sun, W.; Wan, X.; Ouyang, B.; Liu, X.; Li, X.; Zhang, Q.; Yu, X.; et al. Cistanche Deserticola Polysaccharides Enhance Female Germline Stem Cell Differentiation Through BMP/SMAD Signaling to Mitigate Premature Ovarian Failure. Int. J. Biol. Macromol. 2025, 304, 140848. [Google Scholar] [CrossRef]
- Fan, C.; Wu, J.; Hu, S.; Xia, Y.; Wei, Z.; Tang, H.; Jin, W.; Zhang, Z.; An, P.; Luo, J.; et al. Rosmarinic Acid Alleviates Doxorubicin-Induced Cellular Senescence and Cardiotoxicity by Targeting the 14-3-3/Foxo1 Signaling Axis. Phytomedicine 2025, 148, 157482. [Google Scholar] [CrossRef]
- Jenkins, G.R.; Lee, T.; Moland, C.L.; Vijay, V.; Herman, E.H.; Lewis, S.M.; Davis, K.J.; Muskhelishvili, L.; Kerr, S.; Fuscoe, J.C.; et al. Sex-Related Differential Susceptibility to Doxorubicin-Induced Cardiotoxicity in B6C3F1 Mice. Toxicol. Appl. Pharmacol. 2016, 310, 159–174. [Google Scholar] [CrossRef]
- Bustin, S.A. MIQE 2.0 and the Urgent Need to Rethink qPCR Standards. IJMS 2025, 26, 4975. [Google Scholar] [CrossRef]
- Xue, T.; Zheng, D.; Wen, L.; Hou, Q.; He, S.; Zhang, H.; Gong, Y.; Li, M.; Hu, J.; Yang, J. Advance in Cistanche Deserticola Y. C. Ma. Polysaccharides: Isolation, Structural Characterization, Bioactivities and Application: A Review. Int. J. Biol. Macromol. 2024, 278, 134786. [Google Scholar] [CrossRef]
- He, H.; Wang, L.; Qiao, Y.; Zhou, Q.; Li, H.; Chen, S.; Yin, D.; Huang, Q.; He, M. Doxorubicin Induces Endotheliotoxicity and Mitochondrial Dysfunction via ROS/eNOS/NO Pathway. Front. Pharmacol. 2019, 10, 1531. [Google Scholar] [CrossRef]
- Luanpitpong, S.; Chanvorachote, P.; Nimmannit, U.; Leonard, S.S.; Stehlik, C.; Wang, L.; Rojanasakul, Y. Mitochondrial Superoxide Mediates Doxorubicin-Induced Keratinocyte Apoptosis Through Oxidative Modification of ERK and Bcl-2 Ubiquitination. Biochem. Pharmacol. 2012, 83, 1643–1654. [Google Scholar] [CrossRef]
- Tai, P.; Chen, X.; Jia, G.; Chen, G.; Gong, L.; Cheng, Y.; Li, Z.; Wang, H.; Chen, A.; Zhang, G.; et al. WGX50 Mitigates Doxorubicin-Induced Cardiotoxicity Through Inhibition of Mitochondrial ROS and Ferroptosis. J. Transl. Med. 2023, 21, 823. [Google Scholar] [CrossRef]
- Amorim, J.A.; Coppotelli, G.; Rolo, A.P.; Palmeira, C.M.; Ross, J.M.; Sinclair, D.A. Mitochondrial and Metabolic Dysfunction in Ageing and Age-Related Diseases. Nat. Rev. Endocrinol. 2022, 18, 243–258. [Google Scholar] [CrossRef]
- Chen, W.; Zhao, H.; Li, Y. Mitochondrial Dynamics in Health and Disease: Mechanisms and Potential Targets. Signal Transduct. Target Ther. 2023, 8, 333. [Google Scholar] [CrossRef] [PubMed]
- Hinton, A.; Claypool, S.M.; Neikirk, K.; Senoo, N.; Wanjalla, C.N.; Kirabo, A.; Williams, C.R. Mitochondrial Structure and Function in Human Heart Failure. Circ. Res. 2024, 135, 372–396. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, B.C.; Neikirk, K.; Katti, P.; Claypool, S.M.; Kirabo, A.; McReynolds, M.R.; Hinton, A. Mitochondria in Disease: Changes in Shapes and Dynamics. Trends Biochem. Sci. 2024, 49, 346–360. [Google Scholar] [CrossRef] [PubMed]
- Giddings, E.L.; Champagne, D.P.; Wu, M.-H.; Laffin, J.M.; Thornton, T.M.; Valenca-Pereira, F.; Culp-Hill, R.; Fortner, K.A.; Romero, N.; East, J.; et al. Mitochondrial ATP Fuels ABC Transporter-Mediated Drug Efflux in Cancer Chemoresistance. Nat. Commun. 2021, 12, 2804. [Google Scholar] [CrossRef]
- Harrington, J.S.; Ryter, S.W.; Plataki, M.; Price, D.R.; Choi, A.M.K. Mitochondria in Health, Disease, and Aging. Physiol. Rev. 2023, 103, 2349–2422. [Google Scholar] [CrossRef]
- Ley-Ngardigal, S.; Bertolin, G. Approaches to Monitor ATP Levels in Living Cells: Where Do We Stand? FEBS J. 2022, 289, 7940–7969. [Google Scholar] [CrossRef]
- Langer, S.W. Dexrazoxane for the Treatment of Chemotherapy-Related Side Effects. Cancer Manag. Res. 2014, 6, 357–363. [Google Scholar] [CrossRef]
- Tebbi, C.K.; London, W.B.; Friedman, D.; Villaluna, D.; De Alarcon, P.A.; Constine, L.S.; Mendenhall, N.P.; Sposto, R.; Chauvenet, A.; Schwartz, C.L. Dexrazoxane-Associated Risk for Acute Myeloid Leukemia/Myelodysplastic Syndrome and Other Secondary Malignancies in Pediatric Hodgkin’s Disease. J. Clin. Oncol. 2007, 25, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.; Morsy, M.A.; Jacob, S. Dose Translation Between Laboratory Animals and Human in Preclinical and Clinical Phases of Drug Development. Drug Dev. Res. 2018, 79, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Du, W.W.; Wu, N.; Li, F.; Li, X.; Xie, Y.; Wang, S.; Yang, B.B. The Circular RNA circNlgnmediates Doxorubicin-Inducedcardiac Remodeling and Fibrosis. Mol. Ther. Nucleic Acids 2022, 28, 175–189. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, X.; Bawa-Khalfe, T.; Lu, L.-S.; Lyu, Y.L.; Liu, L.F.; Yeh, E.T.H. Identification of the Molecular Basis of Doxorubicin-Induced Cardiotoxicity. Nat. Med. 2012, 18, 1639–1642. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Nishikimi, T.; Kuwahara, K. Atrial and Brain Natriuretic Peptides: Hormones Secreted from the Heart. Peptides 2019, 111, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Samad, M.; Malempati, S.; Restini, C.B.A. Natriuretic Peptides as Biomarkers: Narrative Review and Considerations in Cardiovascular and Respiratory Dysfunctions. Yale J. Biol. Med. 2023, 96, 137–149. [Google Scholar] [CrossRef]
- Shalmi, T.W.; Jensen, A.S.B.; Goetze, J.P. Cardiac Natriuretic Peptides. Adv. Clin. Chem. 2024, 122, 115–139. [Google Scholar] [CrossRef]
- Rittié, L. Method for Picrosirius Red-Polarization Detection of Collagen Fibers in Tissue Sections. Methods Mol. Biol. 2017, 1627, 395–407. [Google Scholar] [CrossRef]
- Talman, V.; Ruskoaho, H. Cardiac Fibrosis in Myocardial Infarction-from Repair and Remodeling to Regeneration. Cell Tissue Res. 2016, 365, 563–581. [Google Scholar] [CrossRef]
- Devos, H.; Zoidakis, J.; Roubelakis, M.G.; Latosinska, A.; Vlahou, A. Reviewing the Regulators of COL1A1. Int. J. Mol. Sci. 2023, 24, 10004. [Google Scholar] [CrossRef]
- Ma, Y.-L.; Kong, C.-Y.; Guo, Z.; Wang, M.-Y.; Wang, P.; Liu, F.-Y.; Yang, D.; Yang, Z.; Tang, Q.-Z. Semaglutide Ameliorates Cardiac Remodeling in Male Mice by Optimizing Energy Substrate Utilization Through the Creb5/NR4a1 Axis. Nat. Commun. 2024, 15, 4757. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Qiu, Q.; Ju, F.; Zheng, C. Mechanisms of Doxorubicin-Induced Cardiac Inflammation and Fibrosis; Therapeutic Targets and Approaches. Arch. Biochem. Biophys. 2024, 761, 110140. [Google Scholar] [CrossRef] [PubMed]
- Quiroga, B.; Vega, A.; Abad, S.; Villaverde, M.; Reque, J.; López-Gómez, J.M. Creatine-Kinase and Dialysis Patients, a Helpful Tool for Stratifying Cardiovascular Risk? Nefrologia 2016, 36, 51–56. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Somogyi, E. Comparative Morphological and Biochemical Investigation of Healthy Human Myocardium. Tokai J. Exp. Clin. Med. 1990, 15, 135–141. [Google Scholar] [PubMed]
- Morales, C.R.; Pedrozo, Z.; Lavandero, S.; Hill, J.A. Oxidative Stress and Autophagy in Cardiovascular Homeostasis. Antioxid. Redox Signal 2014, 20, 507–518. [Google Scholar] [CrossRef]
- Tajvidi, E.; Nahavandizadeh, N.; Pournaderi, M.; Pourrashid, A.Z.; Bossaghzadeh, F.; Khoshnood, Z. Study the Antioxidant Effects of Blue-Green Algae Spirulina Extract on ROS and MDA Production in Human Lung Cancer Cells. Biochem. Biophys. Rep. 2021, 28, 101139. [Google Scholar] [CrossRef]
- Zhang, X.; Hou, L.; Guo, Z.; Wang, G.; Xu, J.; Zheng, Z.; Sun, K.; Guo, F. Lipid Peroxidation in Osteoarthritis: Focusing on 4-Hydroxynonenal, Malondialdehyde, and Ferroptosis. Cell Death Discov. 2023, 9, 320. [Google Scholar] [CrossRef]
- Chen, N.; Jiang, T.; Xu, J.; Xi, W.; Shang, E.; Xiao, P.; Duan, J.-A. The Relationship Between Polysaccharide Structure and Its Antioxidant Activity Needs to Be Systematically Elucidated. Int. J. Biol. Macromol. 2024, 270, 132391. [Google Scholar] [CrossRef]
- Valente, A.J.; Maddalena, L.A.; Robb, E.L.; Moradi, F.; Stuart, J.A. A Simple ImageJ Macro Tool for Analyzing Mitochondrial Network Morphology in Mammalian Cell Culture. Acta Histochem. 2017, 119, 315–326. [Google Scholar] [CrossRef] [PubMed]
- Rickard, B.P.; Overchuk, M.; Chappell, V.A.; Kemal Ruhi, M.; Sinawang, P.D.; Nguyen Hoang, T.T.; Akin, D.; Demirci, U.; Franco, W.; Fenton, S.E.; et al. Methods to Evaluate Changes in Mitochondrial Structure and Function in Cancer. Cancers 2023, 15, 2564. [Google Scholar] [CrossRef]
- Xiong, W.; Li, B.; Pan, J.; Li, D.; Yuan, H.; Wan, X.; Zhang, Y.; Fu, L.; Zhang, J.; Lei, M.; et al. Mitochondrial Amount Determines Doxorubicin-Induced Cardiotoxicity in Cardiomyocytes. Adv. Sci. 2025, 12, 2412017. [Google Scholar] [CrossRef]
- Agostinucci, K.; Grant, M.K.O.; Melaku, W.; Nair, C.; Zordoky, B.N. Exposure to Doxorubicin Modulates the Cardiac Response to Isoproterenol in Male and Female Mice. Pharmaceuticals 2023, 16, 391. [Google Scholar] [CrossRef] [PubMed]
- Desai, V.G.; Azevedo-Pouly, A.; Vijay, V.; Phanavanh, B.; Moland, C.L.; Han, T.; Revollo, J.; Aryal, B.; Rao, V.A.; Fuscoe, J.C. Potential Role of the Apelin-APJ Pathway in Sex-Related Differential Cardiotoxicity Induced by Doxorubicin in Mice. J. Appl. Toxicol. 2023, 43, 557–576. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, J.; Zhang, Y.; Cui, M.; Shi, Y.; Luo, X.; Fan, C.; Wan, S.; An, P.; Luo, Y.; Luo, J. Cistanche deserticola Polysaccharides Protect Against Doxorubicin-Induced Cardiotoxicity via Antioxidant and Mitochondrial Mechanisms. Antioxidants 2025, 14, 1461. https://doi.org/10.3390/antiox14121461
Qi J, Zhang Y, Cui M, Shi Y, Luo X, Fan C, Wan S, An P, Luo Y, Luo J. Cistanche deserticola Polysaccharides Protect Against Doxorubicin-Induced Cardiotoxicity via Antioxidant and Mitochondrial Mechanisms. Antioxidants. 2025; 14(12):1461. https://doi.org/10.3390/antiox14121461
Chicago/Turabian StyleQi, Jingyi, Yang Zhang, Mingyang Cui, Yufang Shi, Xinyu Luo, Chang Fan, Sitong Wan, Peng An, Yongting Luo, and Junjie Luo. 2025. "Cistanche deserticola Polysaccharides Protect Against Doxorubicin-Induced Cardiotoxicity via Antioxidant and Mitochondrial Mechanisms" Antioxidants 14, no. 12: 1461. https://doi.org/10.3390/antiox14121461
APA StyleQi, J., Zhang, Y., Cui, M., Shi, Y., Luo, X., Fan, C., Wan, S., An, P., Luo, Y., & Luo, J. (2025). Cistanche deserticola Polysaccharides Protect Against Doxorubicin-Induced Cardiotoxicity via Antioxidant and Mitochondrial Mechanisms. Antioxidants, 14(12), 1461. https://doi.org/10.3390/antiox14121461








