Linoleic Hydroperoxides Are Potent Hyperoxidative Agents of Sensitive and Robust Typical 2-Cys Peroxiredoxins
Abstract
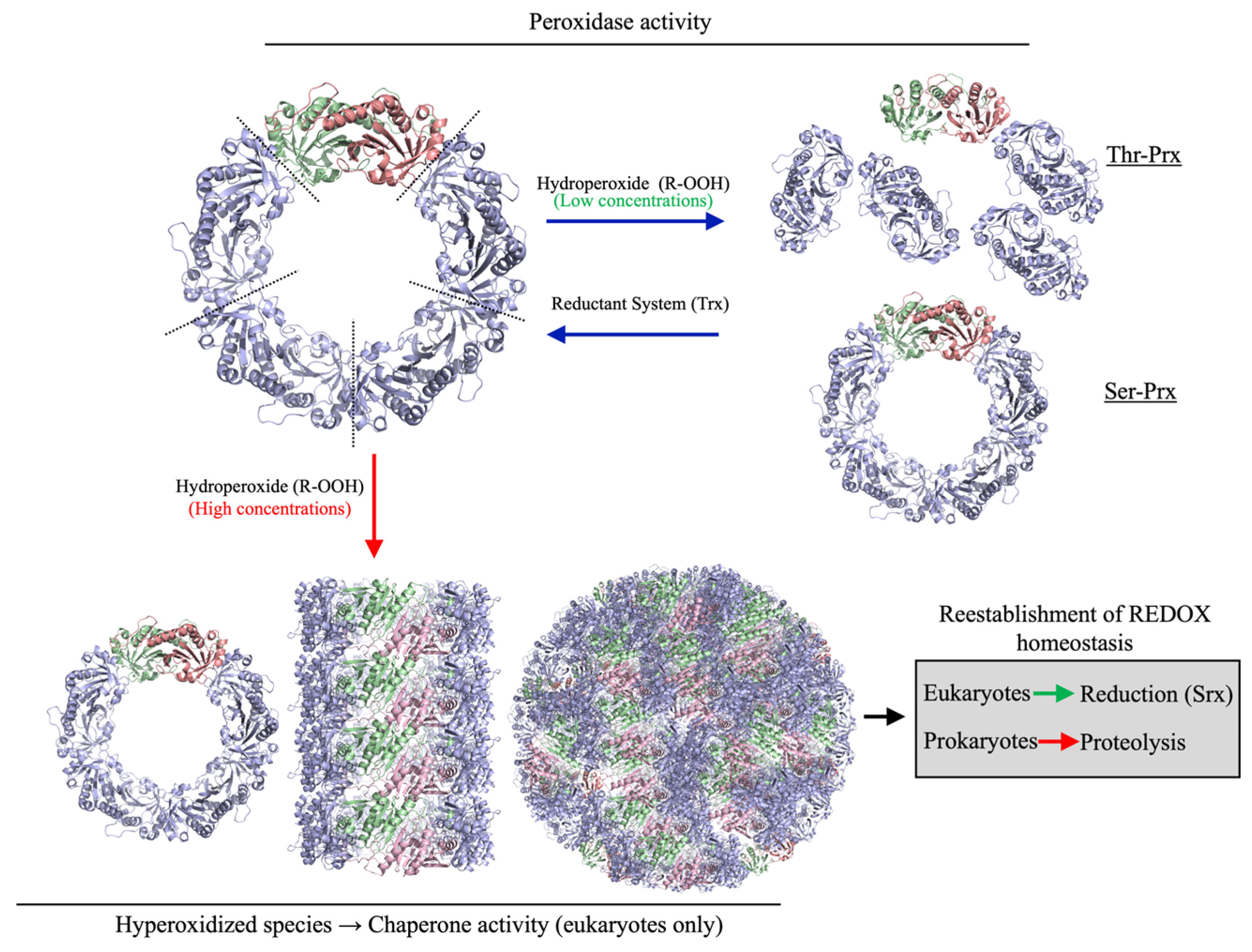
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Microbiological Culture Media
2.3. Expression, Purification and Quantification of Recombinant Proteins
2.4. Evaluation of Peroxidase Activity of Typical 2-Cys Prx by NADPH Oxidation Coupled Assays
2.5. Determination of 2-Cys Prx Free Thiol Groups
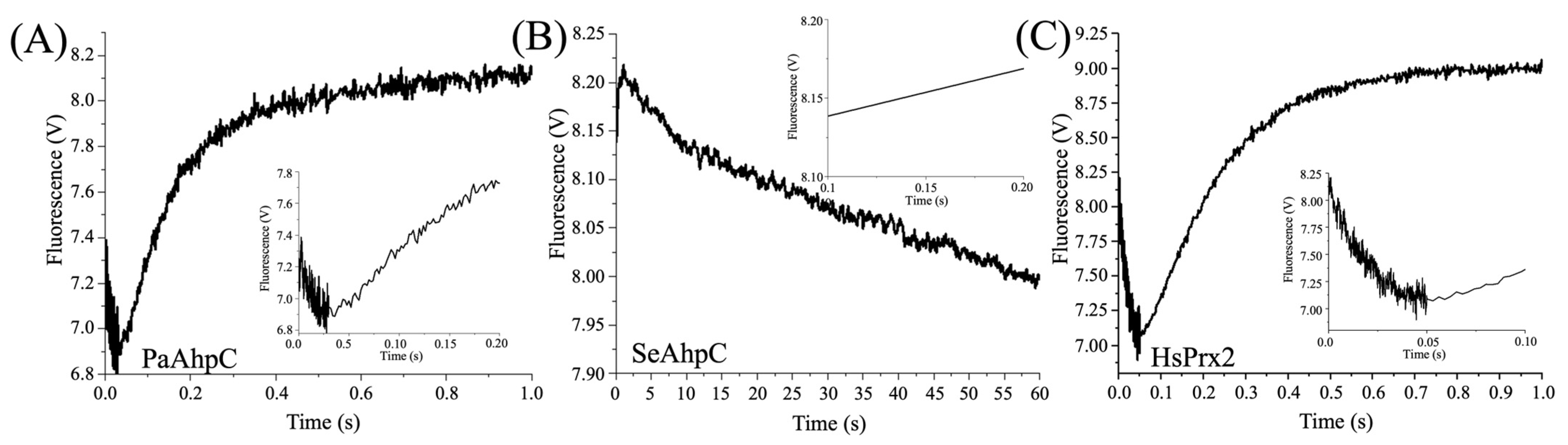
2.6. Determination of Oxidation or Hyperoxidation Rates by the Intrinsic Fluorescence of the 2-Cys Prx
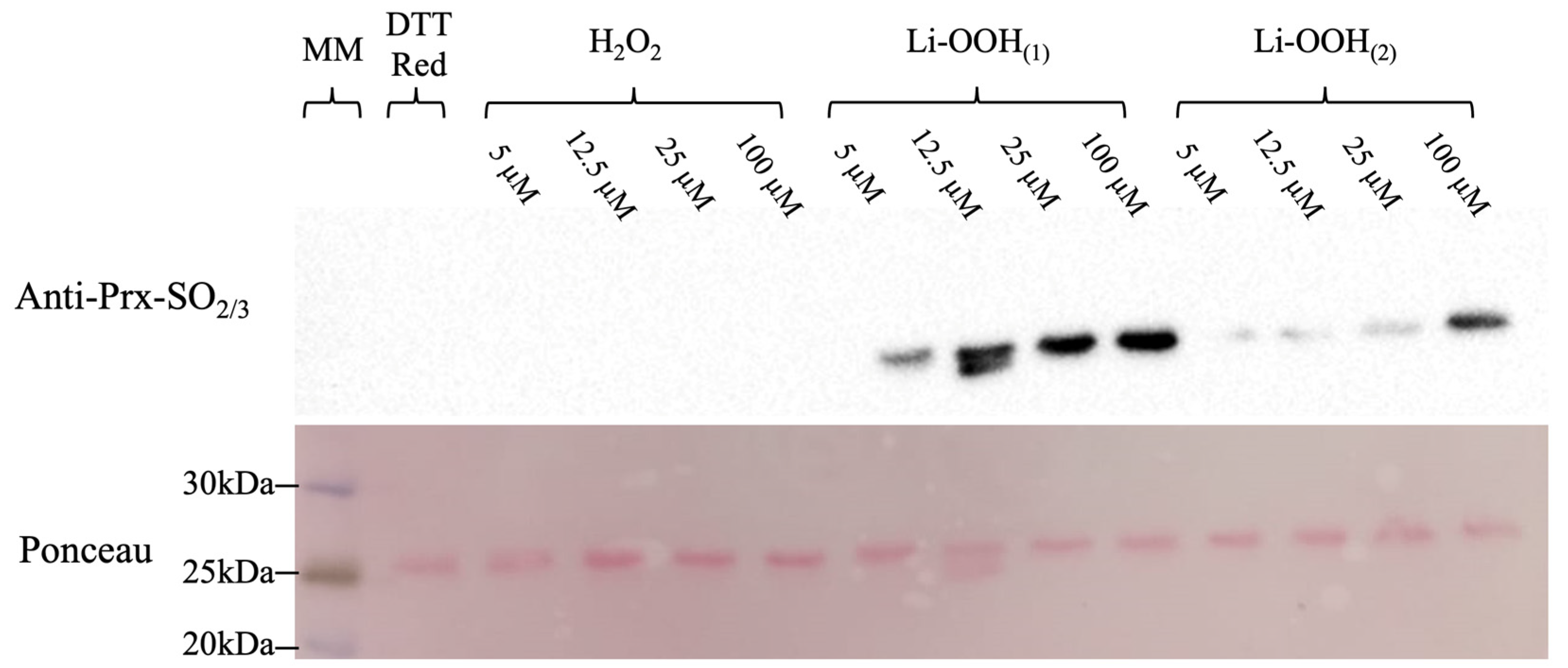
2.7. Evaluation of HsPrx2 Hyperoxidation by Western Blotting
2.8. Statistical Analysis
2.9. Structural Modeling of PaAhpC and SeAhpC
2.10. Peroxides Molecular Docking in Typical 2-Cys Prx Active Site
3. Results
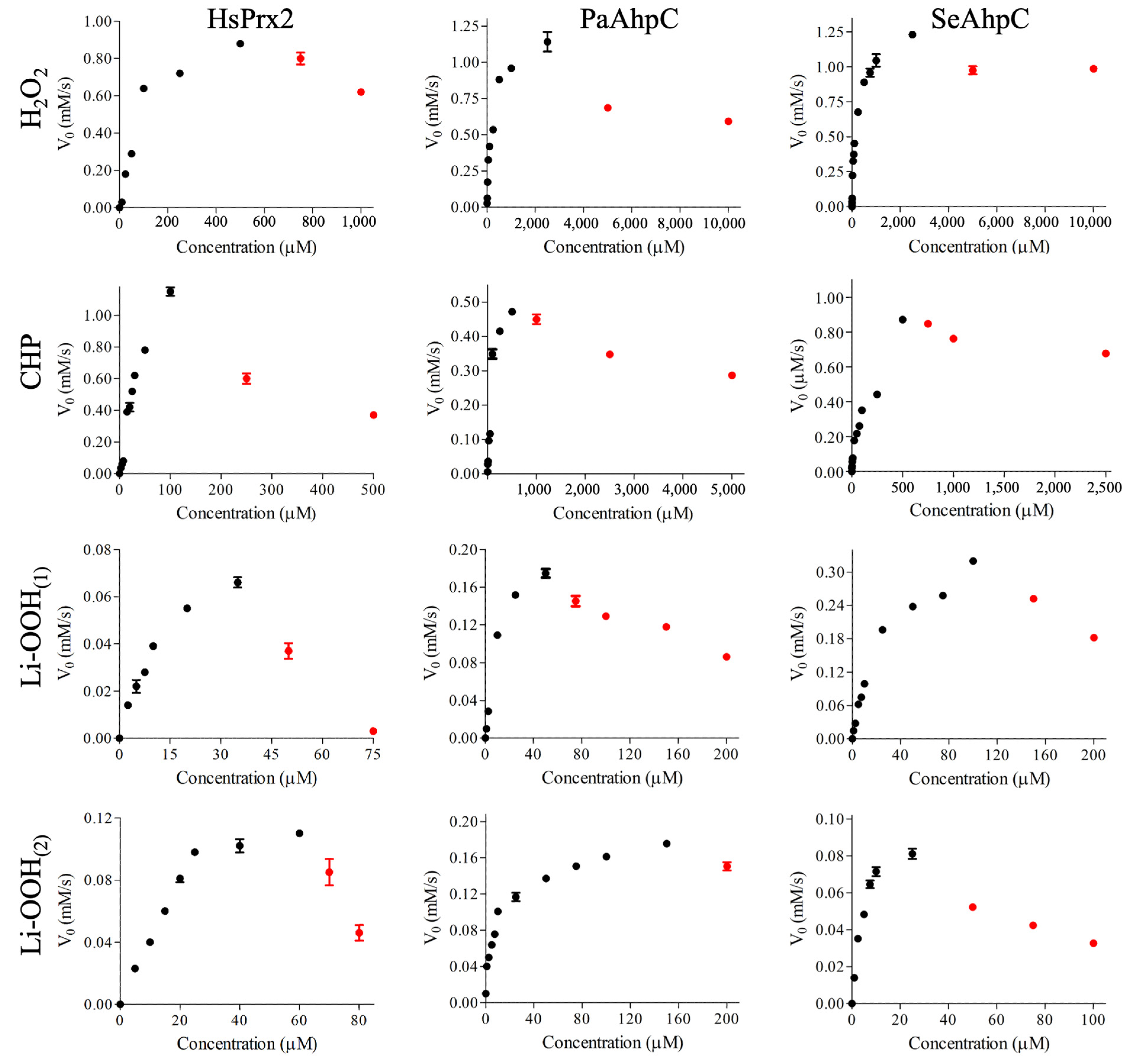
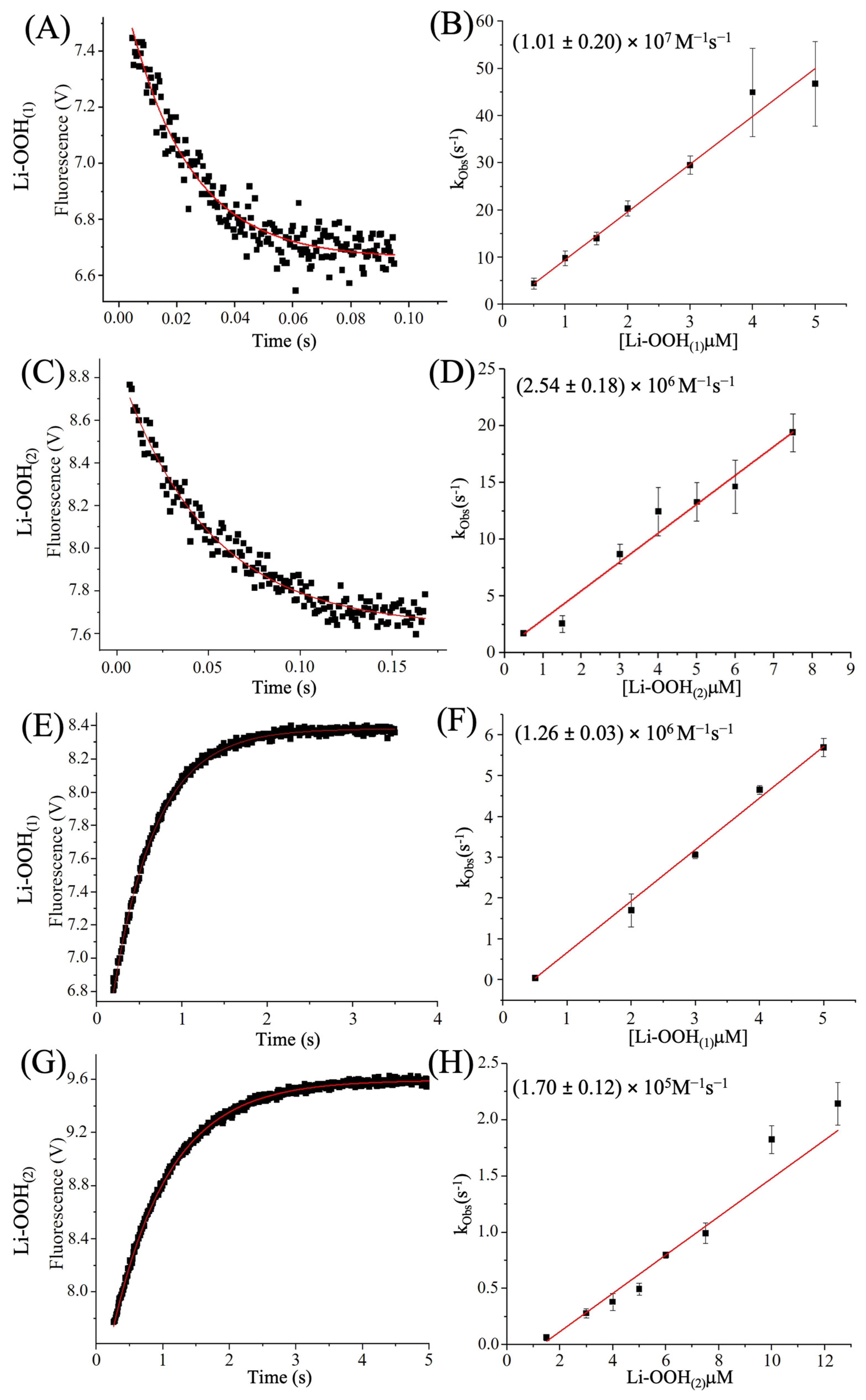
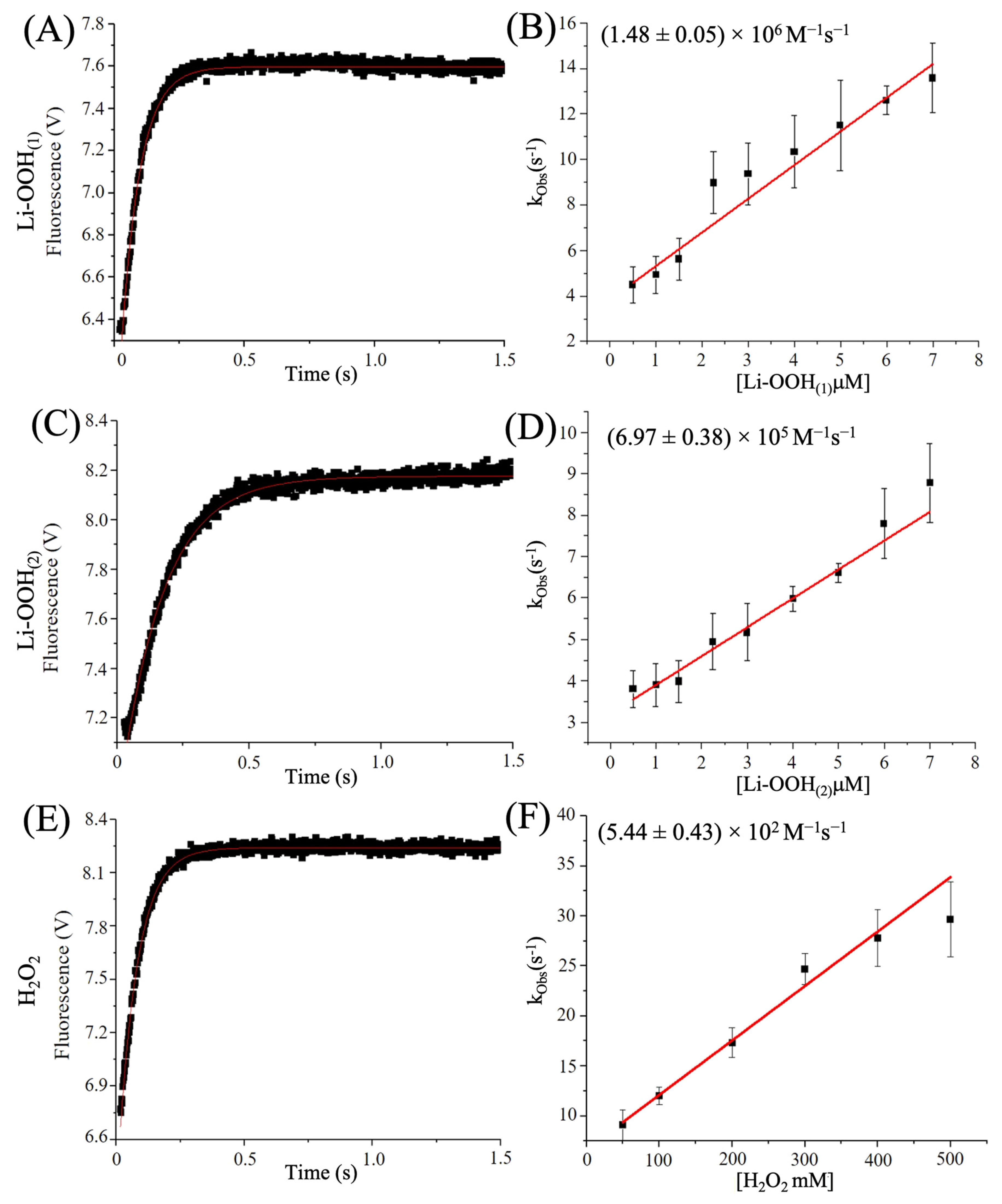
3.1. HsPrx2 and AhpCs Reduce Lipid Hydroperoxides
3.2. Assessing HsPrx2 CP Hyperoxidation by Immunoblotting
3.3. Determination of Hyperoxidation Rates by Intrinsic Trp Fluorescence
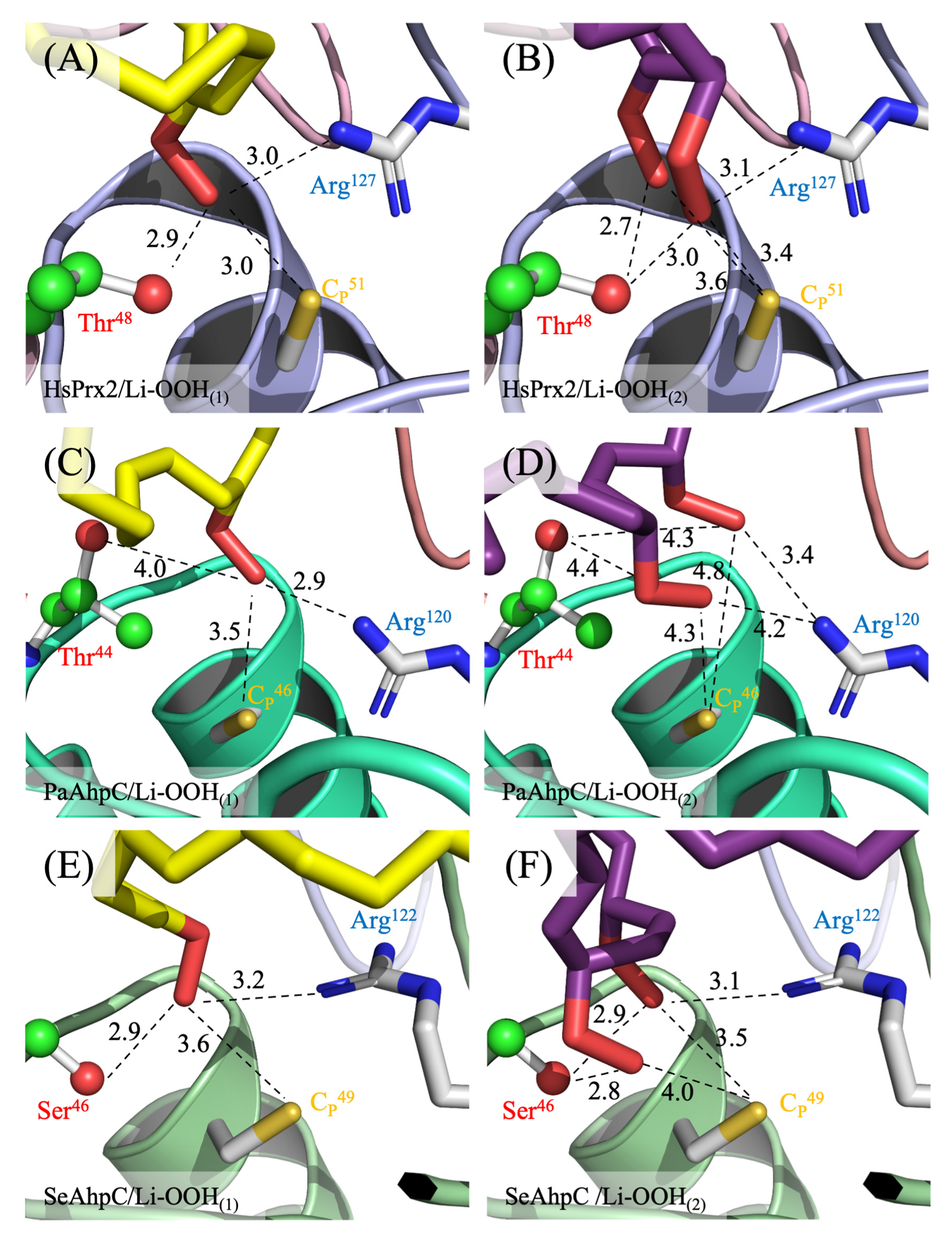
3.4. Ligand–Enzyme Interactions Simulations by Computer-Assisted Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chae, H.Z.; Chung, S.J.; Rhee, S.G. Thioredoxin-dependent peroxide reductase from yeast. J. Biol. Chem. 1994, 269, 27670–27678. [Google Scholar] [CrossRef]
- Soito, L.; Williamson, C.; Knutson, S.T.; Fetrow, J.S.; Poole, L.B.; Nelson, K.J. PREX: PeroxiRedoxin classification indEX, a database of subfamily assignments across the diverse peroxiredoxin family. Nucleic Acids Res. 2011, 39, D332–D337. [Google Scholar] [CrossRef]
- Hall, A.; Karplus, P.A.; Poole, L.B. Typical 2-Cys peroxiredoxins--structures, mechanisms and functions. FEBS J. 2009, 276, 2469–2477. [Google Scholar] [CrossRef] [PubMed]
- Poole, L.B.; Ellis, H.R. Flavin-dependent alkyl hydroperoxide reductase from Salmonella typhimurium. 1. Purification and enzymatic activities of overexpressed AhpF and AhpC proteins. Biochemistry 1996, 35, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Ferrer-Sueta, G.; Manta, B.; Botti, H.; Radi, R.; Trujillo, M.; Denicola, A. Factors affecting protein thiol reactivity and specificity in peroxide reduction. Chem. Res. Toxicol. 2011, 24, 434–450. [Google Scholar] [CrossRef] [PubMed]
- Hall, A.; Parsonage, D.; Poole, L.B.; Karplus, P.A. Structural evidence that peroxiredoxin catalytic power is based on transition-state stabilization. J. Mol. Biol. 2010, 402, 194–209. [Google Scholar] [CrossRef]
- Netto, L.E.S.; Chae, H.Z.; Kang, S.W.; Rhee, S.G.; Stadtman, E.R. Removal of hydrogen peroxide by thiol-specific antioxidant enzyme (TSA) is involved with its antioxidant properties. TSA possesses thiol peroxidase activity. J. Biol. Chem. 1996, 271, 15315–15321. [Google Scholar] [CrossRef]
- Wood, Z.A.; Schroder, E.; Robin Harris, J.; Poole, L.B. Structure, mechanism and regulation of peroxiredoxins. Trends Biochem. Sci. 2003, 28, 32–40. [Google Scholar] [CrossRef]
- Ellis, H.R.; Poole, L.B. Roles for the two cysteine residues of AhpC in catalysis of peroxide reduction by alkyl hydroperoxide reductase from Salmonella typhimurium. Biochemistry 1997, 36, 13349–13356. [Google Scholar] [CrossRef]
- Pan, A.; Balakrishna, A.M.; Nartey, W.; Kohlmeier, A.; Dip, P.V.; Bhushan, S.; Gruber, G. Atomic structure and enzymatic insights into the vancomycin-resistant Enterococcus faecalis (V583) alkylhydroperoxide reductase subunit C. Free Radic. Biol. Med. 2018, 115, 252–265. [Google Scholar] [CrossRef]
- Papinutto, E.; Windle, H.J.; Cendron, L.; Battistutta, R.; Kelleher, D.; Zanotti, G. Crystal structure of alkyl hydroperoxide-reductase (AhpC) from Helicobacter pylori. Biochim. Biophys. Acta 2005, 1753, 240–246. [Google Scholar] [CrossRef]
- Wood, Z.A.; Poole, L.B.; Karplus, P.A. Peroxiredoxin evolution and the regulation of hydrogen peroxide signaling. Science 2003, 300, 650–653. [Google Scholar] [CrossRef] [PubMed]
- Baker, L.M.; Raudonikiene, A.; Hoffman, P.S.; Poole, L.B. Essential thioredoxin-dependent peroxiredoxin system from Helicobacter pylori: Genetic and kinetic characterization. J. Bacteriol. 2001, 183, 1961–1973. [Google Scholar] [CrossRef]
- Oliveira, M.A.; Discola, K.F.; Alves, S.V.; Medrano, F.J.; Guimaraes, B.G.; Netto, L.E. Insights into the specificity of thioredoxin reductase-thioredoxin interactions. A structural and functional investigation of the yeast thioredoxin system. Biochemistry 2010, 49, 3317–3326. [Google Scholar] [CrossRef]
- Troussicot, L.; Burmann, B.M.; Molin, M. Structural determinants of multimerization and dissociation in 2-Cys peroxiredoxin chaperone function. Structure 2021, 29, 640–654. [Google Scholar] [CrossRef]
- Perkins, A.; Nelson, K.J.; Parsonage, D.; Poole, L.B.; Karplus, P.A. Peroxiredoxins: Guardians against oxidative stress and modulators of peroxide signaling. Trends Biochem. Sci. 2015, 40, 435–445. [Google Scholar] [CrossRef]
- Tairum, C.A.; Santos, M.C.; Breyer, C.A.; de Oliveira, A.L.P.; Cabrera, V.I.M.; Toledo-Silva, G.; Mori, G.M.; Toyama, M.H.; Netto, L.E.S.; de Oliveira, M.A. Effects of Serine or Threonine in the Active Site of Typical 2-Cys Prx on Hyperoxidation Susceptibility and on Chaperone Activity. Antioxidants 2021, 10, 1032. [Google Scholar] [CrossRef]
- Tairum, C.A.; Santos, M.C.; Breyer, C.A.; Geyer, R.R.; Nieves, C.J.; Portillo-Ledesma, S.; Ferrer-Sueta, G.; Toledo, J.C., Jr.; Toyama, M.H.; Augusto, O.; et al. Catalytic Thr or Ser Residue Modulates Structural Switches in 2-Cys Peroxiredoxin by Distinct Mechanisms. Sci. Rep. 2016, 6, 33133. [Google Scholar] [CrossRef]
- Nelson, K.J.; Perkins, A.; Van Swearingen, A.E.D.; Hartman, S.; Brereton, A.E.; Parsonage, D.; Salsbury, F.R., Jr.; Karplus, P.A.; Poole, L.B. Experimentally Dissecting the Origins of Peroxiredoxin Catalysis. Antioxid. Redox Signal 2018, 28, 521–536. [Google Scholar] [CrossRef] [PubMed]
- Poole, L.B. Bacterial defenses against oxidants: Mechanistic features of cysteine-based peroxidases and their flavoprotein reductases. Arch. Biochem. Biophys. 2005, 433, 240–254. [Google Scholar] [CrossRef] [PubMed]
- Forshaw, T.E.; Reisz, J.A.; Nelson, K.J.; Gumpena, R.; Lawson, J.R.; Jonsson, T.J.; Wu, H.; Clodfelter, J.E.; Johnson, L.C.; Furdui, C.M.; et al. Specificity of Human Sulfiredoxin for Reductant and Peroxiredoxin Oligomeric State. Antioxidants 2021, 10, 946. [Google Scholar] [CrossRef]
- Jang, H.H.; Lee, K.O.; Chi, Y.H.; Jung, B.G.; Park, S.K.; Park, J.H.; Lee, J.R.; Lee, S.S.; Moon, J.C.; Yun, J.W.; et al. Two enzymes in one; two yeast peroxiredoxins display oxidative stress-dependent switching from a peroxidase to a molecular chaperone function. Cell 2004, 117, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Biteau, B.; Labarre, J.; Toledano, M.B. ATP-dependent reduction of cysteine-sulphinic acid by S. cerevisiae sulphiredoxin. Nature 2003, 425, 980–984. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, F.; Tse, E.; Castro, H.; Makepeace, K.A.T.; Meinen, B.A.; Borchers, C.H.; Poole, L.B.; Bardwell, J.C.; Tomas, A.M.; Southworth, D.R.; et al. Chaperone activation and client binding of a 2-cysteine peroxiredoxin. Nat. Commun. 2019, 10, 659. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.C.; Hah, Y.S.; Kim, W.Y.; Jung, B.G.; Jang, H.H.; Lee, J.R.; Kim, S.Y.; Lee, Y.M.; Jeon, M.G.; Kim, C.W.; et al. Oxidative stress-dependent structural and functional switching of a human 2-Cys peroxiredoxin isotype II that enhances HeLa cell resistance to H2O2-induced cell death. J. Biol. Chem. 2005, 280, 28775–28784. [Google Scholar] [CrossRef]
- Noichri, Y.; Palais, G.; Ruby, V.; D’Autreaux, B.; Delaunay-Moisan, A.; Nystrom, T.; Molin, M.; Toledano, M.B. In vivo parameters influencing 2-Cys Prx oligomerization: The role of enzyme sulfinylation. Redox Biol. 2015, 6, 326–333. [Google Scholar] [CrossRef]
- de Oliveira, M.A.; Tairum, C.A.; Netto, L.E.S.; de Oliveira, A.L.P.; Aleixo-Silva, R.L.; Cabrera, V.I.M.; Breyer, C.A.; Dos Santos, M.C. Relevance of peroxiredoxins in pathogenic microorganisms. Appl. Microbiol. Biotechnol. 2021, 105, 5701–5717. [Google Scholar] [CrossRef]
- Dubois, R.N.; Abramson, S.B.; Crofford, L.; Gupta, R.A.; Simon, L.S.; Van De Putte, L.B.; Lipsky, P.E. Cyclooxygenase in biology and disease. FASEB J. 1998, 12, 1063–1073. [Google Scholar] [CrossRef]
- Sadikot, R.T.; Zeng, H.; Azim, A.C.; Joo, M.; Dey, S.K.; Breyer, R.M.; Peebles, R.S.; Blackwell, T.S.; Christman, J.W. Bacterial clearance of Pseudomonas aeruginosa is enhanced by the inhibition of COX-2. Eur. J. Immunol. 2007, 37, 1001–1009. [Google Scholar] [CrossRef]
- Conrad, D.J.; Kuhn, H.; Mulkins, M.; Highland, E.; Sigal, E. Specific inflammatory cytokines regulate the expression of human monocyte 15-lipoxygenase. Proc. Natl. Acad. Sci. USA 1992, 89, 217–221. [Google Scholar] [CrossRef]
- Conrad, M.; Kagan, V.E.; Bayir, H.; Pagnussat, G.C.; Head, B.; Traber, M.G.; Stockwell, B.R. Regulation of lipid peroxidation and ferroptosis in diverse species. Genes Dev. 2018, 32, 602–619. [Google Scholar] [CrossRef]
- Yin, H.; Xu, L.; Porter, N.A. Free radical lipid peroxidation: Mechanisms and analysis. Chem. Rev. 2011, 111, 5944–5972. [Google Scholar] [CrossRef]
- Aloulou, A.; Rahier, R.; Arhab, Y.; Noiriel, A.; Abousalham, A. Phospholipases: An Overview. Methods Mol. Biol. 2018, 1835, 69–105. [Google Scholar] [CrossRef]
- Kim, K.H.; Lee, W.; Kim, E.E. Crystal structures of human peroxiredoxin 6 in different oxidation states. Biochem. Biophys. Res. Commun. 2016, 477, 717–722. [Google Scholar] [CrossRef]
- Thomas, J.P.; Geiger, P.G.; Maiorino, M.; Ursini, F.; Girotti, A.W. Enzymatic reduction of phospholipid and cholesterol hydroperoxides in artificial bilayers and lipoproteins. Biochim. Biophys. Acta 1990, 1045, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Beavers, W.N.; Monteith, A.J.; Amarnath, V.; Mernaugh, R.L.; Roberts, L.J., 2nd; Chazin, W.J.; Davies, S.S.; Skaar, E.P. Arachidonic Acid Kills Staphylococcus aureus through a Lipid Peroxidation Mechanism. mBio 2019, 10, e01333-19. [Google Scholar] [CrossRef] [PubMed]
- Desbois, A.P.; Lawlor, K.C. Antibacterial activity of long-chain polyunsaturated fatty acids against Propionibacterium acnes and Staphylococcus aureus. Mar. Drugs 2013, 11, 4544–4557. [Google Scholar] [CrossRef] [PubMed]
- Eijkelkamp, B.A.; Begg, S.L.; Pederick, V.G.; Trapetti, C.; Gregory, M.K.; Whittall, J.J.; Paton, J.C.; McDevitt, C.A. Arachidonic Acid Stress Impacts Pneumococcal Fatty Acid Homeostasis. Front. Microbiol. 2018, 9, 813. [Google Scholar] [CrossRef]
- Knapp, H.R.; Melly, M.A. Bactericidal effects of polyunsaturated fatty acids. J. Infect. Dis. 1986, 154, 84–94. [Google Scholar] [CrossRef]
- Alegria, T.G.; Meireles, D.A.; Cussiol, J.R.; Hugo, M.; Trujillo, M.; de Oliveira, M.A.; Miyamoto, S.; Queiroz, R.F.; Valadares, N.F.; Garratt, R.C.; et al. Ohr plays a central role in bacterial responses against fatty acid hydroperoxides and peroxynitrite. Proc. Natl. Acad. Sci. USA 2017, 114, E132–E141. [Google Scholar] [CrossRef]
- Reyes, A.M.; Hugo, M.; Trostchansky, A.; Capece, L.; Radi, R.; Trujillo, M. Oxidizing substrate specificity of Mycobacterium tuberculosis alkyl hydroperoxide reductase E: Kinetics and mechanisms of oxidation and overoxidation. Free Radic. Biol. Med. 2011, 51, 464–473. [Google Scholar] [CrossRef] [PubMed]
- Cardozo, G.; Mastrogiovanni, M.; Zeida, A.; Viera, N.; Radi, R.; Reyes, A.M.; Trujillo, M. Mitochondrial Peroxiredoxin 3 Is Rapidly Oxidized and Hyperoxidized by Fatty Acid Hydroperoxides. Antioxidants 2023, 12, 408. [Google Scholar] [CrossRef] [PubMed]
- Brenot, A.; King, K.Y.; Caparon, M.G. The PerR regulon in peroxide resistance and virulence of Streptococcus pyogenes. Mol. Microbiol. 2005, 55, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Charoenlap, N.; Shen, Z.; McBee, M.E.; Muthupalani, S.; Wogan, G.N.; Fox, J.G.; Schauer, D.B. Alkyl hydroperoxide reductase is required for Helicobacter cinaedi intestinal colonization and survival under oxidative stress in BALB/c and BALB/c interleukin-10-/- mice. Infect. Immun. 2012, 80, 921–928. [Google Scholar] [CrossRef]
- Dons, L.E.; Mosa, A.; Rottenberg, M.E.; Rosenkrantz, J.T.; Kristensson, K.; Olsen, J.E. Role of the Listeria monocytogenes 2-Cys peroxiredoxin homologue in protection against oxidative and nitrosative stress and in virulence. Pathog. Dis. 2014, 70, 70–74. [Google Scholar] [CrossRef]
- Olczak, A.A.; Seyler, R.W., Jr.; Olson, J.W.; Maier, R.J. Association of Helicobacter pylori antioxidant activities with host colonization proficiency. Infect. Immun. 2003, 71, 580–583. [Google Scholar] [CrossRef]
- Rocha, L.S.; Silva, B.P.D.; Correia, T.M.L.; Silva, R.P.D.; Meireles, D.A.; Pereira, R.; Netto, L.E.S.; Meotti, F.C.; Queiroz, R.F. Peroxiredoxin AhpC1 protects Pseudomonas aeruginosa against the inflammatory oxidative burst and confers virulence. Redox Biol. 2021, 46, 102075. [Google Scholar] [CrossRef]
- Wilson, T.; de Lisle, G.W.; Marcinkeviciene, J.A.; Blanchard, J.S.; Collins, D.M. Antisense RNA to ahpC, an oxidative stress defence gene involved in isoniazid resistance, indicates that AhpC of Mycobacterium bovis has virulence properties. Microbiology 1998, 144 Pt 10, 2687–2695. [Google Scholar] [CrossRef]
- Kuhn, H.; Banthiya, S.; van Leyen, K. Mammalian lipoxygenases and their biological relevance. Biochim. Biophys. Acta 2015, 1851, 308–330. [Google Scholar] [CrossRef]
- Miyamoto, S.; Martinez, G.R.; Martins, A.P.; Medeiros, M.H.; Di Mascio, P. Direct evidence of singlet molecular oxygen [O2(1Δg)] production in the reaction of linoleic acid hydroperoxide with peroxynitrite. J. Am. Chem. Soc. 2003, 125, 4510–4517. [Google Scholar] [CrossRef]
- Miyamoto, S.; Martinez, G.R.; Medeiros, M.H.; Di Mascio, P. Singlet molecular oxygen generated from lipid hydroperoxides by the russell mechanism: Studies using 18(O)-labeled linoleic acid hydroperoxide and monomol light emission measurements. J. Am. Chem. Soc. 2003, 125, 6172–6179. [Google Scholar] [CrossRef] [PubMed]
- Buege, J.A.; Aust, S.D. Microsomal lipid peroxidation. Methods Enzymol. 1978, 52, 302–310. [Google Scholar] [CrossRef]
- Munhoz, D.C.; Netto, L.E. Cytosolic thioredoxin peroxidase I and II are important defenses of yeast against organic hydroperoxide insult: Catalases and peroxiredoxins cooperate in the decomposition of H2O2 by yeast. J. Biol. Chem. 2004, 279, 35219–35227. [Google Scholar] [CrossRef] [PubMed]
- Nagy, P.; Karton, A.; Betz, A.; Peskin, A.V.; Pace, P.; O’Reilly, R.J.; Hampton, M.B.; Radom, L.; Winterbourn, C.C. Model for the exceptional reactivity of peroxiredoxins 2 and 3 with hydrogen peroxide: A kinetic and computational study. J. Biol. Chem. 2011, 286, 18048–18055. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, M.C.; Tairum, C.A.; Cabrera, V.I.M.; Guimaraes Cauz, A.C.; Ribeiro, L.F.; Toledo Junior, J.C.; Toyama, M.H.; Lago, J.H.G.; Brocchi, M.; Netto, L.E.S.; et al. Adenanthin Is an Efficient Inhibitor of Peroxiredoxins from Pathogens, Inhibits Bacterial Growth, and Potentiates Antibiotic Activities. Chem. Res. Toxicol. 2023, 36, 570–582. [Google Scholar] [CrossRef]
- Truzzi, D.R.; Coelho, F.R.; Paviani, V.; Alves, S.V.; Netto, L.E.S.; Augusto, O. The bicarbonate/carbon dioxide pair increases hydrogen peroxide-mediated hyperoxidation of human peroxiredoxin 1. J. Biol. Chem. 2019, 294, 14055–14067. [Google Scholar] [CrossRef]
- Peshenko, I.V.; Shichi, H. Oxidation of active center cysteine of bovine 1-Cys peroxiredoxin to the cysteine sulfenic acid form by peroxide and peroxynitrite. Free Radic. Biol. Med. 2001, 31, 292–303. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Zidek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Mirdita, M.; Schutze, K.; Moriwaki, Y.; Heo, L.; Ovchinnikov, S.; Steinegger, M. ColabFold: Making protein folding accessible to all. Nat. Methods 2022, 19, 679–682. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera--a visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef]
- Nakamura, T.; Kado, Y.; Yamaguchi, T.; Matsumura, H.; Ishikawa, K.; Inoue, T. Crystal structure of peroxiredoxin from Aeropyrum pernix K1 complexed with its substrate, hydrogen peroxide. J. Biochem. 2010, 147, 109–115. [Google Scholar] [CrossRef]
- Wallace, A.C.; Laskowski, R.A.; Thornton, J.M. LIGPLOT: A program to generate schematic diagrams of protein-ligand interactions. Protein Eng. 1995, 8, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Parsonage, D.; Nelson, K.J.; Ferrer-Sueta, G.; Alley, S.; Karplus, P.A.; Furdui, C.M.; Poole, L.B. Dissecting peroxiredoxin catalysis: Separating binding, peroxidation, and resolution for a bacterial AhpC. Biochemistry 2015, 54, 1567–1575. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, L.A.C.; Truzzi, D.R.; Fallani, T.S.; Alves, S.V.; Toledo, J.C., Jr.; Augusto, O.; Netto, L.E.S.; Meotti, F.C. Urate hydroperoxide oxidizes human peroxiredoxin 1 and peroxiredoxin 2. J. Biol. Chem. 2017, 292, 8705–8715. [Google Scholar] [CrossRef]
- Peskin, A.V.; Dickerhof, N.; Poynton, R.A.; Paton, L.N.; Pace, P.E.; Hampton, M.B.; Winterbourn, C.C. Hyperoxidation of peroxiredoxins 2 and 3: Rate constants for the reactions of the sulfenic acid of the peroxidatic cysteine. J. Biol. Chem. 2013, 288, 14170–14177. [Google Scholar] [CrossRef]
- Jacobson, F.S.; Morgan, R.W.; Christman, M.F.; Ames, B.N. An alkyl hydroperoxide reductase from Salmonella typhimurium involved in the defense of DNA against oxidative damage. Purification and properties. J. Biol. Chem. 1989, 264, 1488–1496. [Google Scholar] [CrossRef]
- Kim, K.; Kim, I.H.; Lee, K.Y.; Rhee, S.G.; Stadtman, E.R. The isolation and purification of a specific “protector” protein which inhibits enzyme inactivation by a thiol/Fe(III)/O2 mixed-function oxidation system. J. Biol. Chem. 1988, 263, 4704–4711. [Google Scholar] [CrossRef]
- Bryk, R.; Griffin, P.; Nathan, C. Peroxynitrite reductase activity of bacterial peroxiredoxins. Nature 2000, 407, 211–215. [Google Scholar] [CrossRef]
- Zhang, B.; Gu, H.; Yang, Y.; Bai, H.; Zhao, C.; Si, M.; Su, T.; Shen, X. Molecular Mechanisms of AhpC in Resistance to Oxidative Stress in Burkholderia thailandensis. Front. Microbiol. 2019, 10, 1483. [Google Scholar] [CrossRef]
- Ishida, Y.I.; Ichinowatari, Y.; Nishimoto, S.; Koike, S.; Ishii, K.; Ogasawara, Y. Differential oxidation processes of peroxiredoxin 2 dependent on the reaction with several peroxides in human red blood cells. Biochem. Biophys. Res. Commun. 2019, 518, 685–690. [Google Scholar] [CrossRef]
- Green, A.R.; Freedman, C.; Tena, J.; Tourdot, B.E.; Liu, B.; Holinstat, M.; Holman, T.R. 5 S,15 S-Dihydroperoxyeicosatetraenoic Acid (5,15-diHpETE) as a Lipoxin Intermediate: Reactivity and Kinetics with Human Leukocyte 5-Lipoxygenase, Platelet 12-Lipoxygenase, and Reticulocyte 15-Lipoxygenase-1. Biochemistry 2018, 57, 6726–6734. [Google Scholar] [CrossRef]
- Nam, T.G. Lipid peroxidation and its toxicological implications. Toxicol. Res. 2011, 27, 1–6. [Google Scholar] [CrossRef]
- Frankel, E.N. Chemistry of free radical and singlet oxidation of lipids. Prog. Lipid Res. 1984, 23, 197–221. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Sun, M.; Salomon, R.G. Preparative singlet oxygenation of linoleate provides doubly allylic dihydroperoxides: Putative intermediates in the generation of biologically active aldehydes in vivo. J. Org. Chem. 2006, 71, 5607–5615. [Google Scholar] [CrossRef] [PubMed]
- Bozonet, S.M.; Findlay, V.J.; Day, A.M.; Cameron, J.; Veal, E.A.; Morgan, B.A. Oxidation of a eukaryotic 2-Cys peroxiredoxin is a molecular switch controlling the transcriptional response to increasing levels of hydrogen peroxide. J. Biol. Chem. 2005, 280, 23319–23327. [Google Scholar] [CrossRef] [PubMed]
- Day, A.M.; Brown, J.D.; Taylor, S.R.; Rand, J.D.; Morgan, B.A.; Veal, E.A. Inactivation of a peroxiredoxin by hydrogen peroxide is critical for thioredoxin-mediated repair of oxidized proteins and cell survival. Mol. Cell 2012, 45, 398–408. [Google Scholar] [CrossRef] [PubMed]
- Talwar, D.; Messens, J.; Dick, T.P. A role for annexin A2 in scaffolding the peroxiredoxin 2-STAT3 redox relay complex. Nat. Commun. 2020, 11, 4512. [Google Scholar] [CrossRef]
- Rhee, S.G.; Woo, H.A.; Kang, D. The Role of Peroxiredoxins in the Transduction of H2O2 Signals. Antioxid. Redox Signal 2018, 28, 537–557. [Google Scholar] [CrossRef]
- Montanhero Cabrera, V.I.; do Nascimento Sividanes, G.; Quintiliano, N.F.; Hikari Toyama, M.; Ghilardi Lago, J.H.; de Oliveira, M.A. Exploring functional and structural features of chemically related natural prenylated hydroquinone and benzoic acid from Piper crassinervium (Piperaceae) on bacterial peroxiredoxin inhibition. PLoS ONE 2023, 18, e0281322. [Google Scholar] [CrossRef]

| Plasmid | Antibiotic Resistance * | Reference |
|---|---|---|
| pET15b::ec_trx | Amp | [17] |
| pET15b::ec_trxr | Amp | [17] |
| pET15b::pa_ahpc | Amp | [17] |
| pET15b::se_ahpc | Amp | [17] |
| pET17b::sc_trx1 | Amp | [53] |
| pPROEX::sc_trxr1 | Kan | [53] |
| pET28a::hs_prx2 | Kan | [54] |
| Protein | Ɛ 280 nm (M−1 cm−1) | Molecular Weight (kDa) | Uniprot Entry |
|---|---|---|---|
| PaAhpC | 22.460 | 22.82 | Q02UU0 |
| SeAhpC | 26.930 | 23.36 | Q5HRY1 |
| EcTrx | 15.470 | 14.09 | P0AA25 |
| EcTrxR | 20.400 | 36.90 | P0A9P4 |
| HsPrx2 | 21.555 | 23.92 | P321194 |
| ScTrx1 | 9.970 | 11.23 | P22217 |
| ScTrxR1 | 30.370 | 37.33 | P29509 |
| Hpx | Km (µM) | kcat (s−1) | Vmax (µM/s−1) | kcat/Km (M−1 s−1) | |
|---|---|---|---|---|---|
| HsPrx2 | H2O2 | 105 (±18) | 0.35 (±0.02) | 1.07 (±0.05) | 3.4 (±0.9) × 103 |
| CHP | 57 (±17) | 0.56 (±0.09) | 1.68 (±0.09) | 9.8 (±1.3) × 103 | |
| Li-OOH(1) | 16.5 (±1) | 0.19 (±0.10) | 0.10 (±0.01) | 1.2 (±0.2) × 103 | |
| Li-OOH(2) | 23 (±6) | 0.32 (±0.01) | 0.16 (±0.01) | 1.4 (±0.4) × 103 | |
| PaAhpC (Thr) | H2O2 | 116 (±13) | 0.31 (±0.01) | 0.67 (±0.02) | 2.8 (±0.2) × 103 |
| CHP | 82 (±14) | 0.18 (±0.01) | 0.57 (±0.04) | 2.0 (±0.2) × 103 | |
| Li-OOH(1) | 12 (±1) | 0.07 (±0.01) | 0.21 (±0.01) | 6.0 (±0.5) × 103 | |
| Li-OOH(2) | 7.3 (±0.9) | 0.05 (±0.01) | 0.19(±0.01) | 8.2 (±0.4) × 103 | |
| SeAhpC (Ser) | H2O2 | 178 (±14) | 0.42 (±0.01) | 1.23 (±0.07) | 2.3 (±0.1) × 103 |
| CHP | 76 (±10) | 0.19 (±0.01) | 0.59 (±0.04) | 2.5 (±0.1) × 103 | |
| Li-OOH(1) | 27 (±3) | 0.03 (±0.01) | 0.38 (±0.02) | 4.7 (±0.5) × 103 | |
| Li-OOH(2) | 4.5 (±0.6) | 0.03 (±0.01) | 0.09 (±0.01) | 7.0 (±0.6) × 103 |
| Peroxide | koxi (M−1s−1) | khyp (M−1s−1) | Reference | |
|---|---|---|---|---|
| HsPrx2 | H2O2 | * (0.2–1.3) × 108 | † (1.2) × 104; | * [65], † [66] |
| CHP | (2.43 ± 0.05) × 108 | (5.91 ± 0.19) × 103 | This work (Figure S2) | |
| # U-OOH | (2.26 ± 0.13) × 106 | ## ND | [65] | |
| Li-OOH(1) | (1.01 ± 0.20) × 107 | (1.26 ± 0.03) × 106 | This work | |
| Li-OOH(2) | (2.54 ± 0.18) × 106 | (1.70 ± 0.12) × 105 | This work | |
| AhpC | H2O2 | ‡ (1.50 ± 0.07) × 108; | (5.44 ± 0.43) × 102 | ‡ X. fastidiosa [47], P. aeruginosa (This work) |
| # U-OOH | (2.30 ± 0.09) × 106 | ## ND | X. fastidiosa [47] | |
| Li-OOH(1) | ## ND | (1.48 ± 0.05) × 106 | P. aeruginosa (This work) | |
| Li-OOH(2) | ## ND | (6.97 ± 0.38) × 105 | P. aeruginosa (This work) |
| Enzyme | Peroxide | DSγ (Å) Best Conformation | ΔG (kcal/mol) | Residues/Interactions |
|---|---|---|---|---|
| HsPrx2 | Li-OOH(1) | 3.0 | −5.2 | Apolar = Pro44’, Leu45’, Phe49’, Val50’, Glu122’, Ile124’, Leu146’, Pro147’, Pro185”, Phe81 */Polar = CP51’, Arg127’ and Thr48’ |
| Li-OOH(2) | 3.4 | −5.7 | Apolar = Pro44’, CP51’, Val50’, Leu146’, Pro147’, Val171”, Pro185”/Polar = Arg127’ | |
| PaAhpC | Li-OOH(1) | 3.5 | −5.7 | Apolar = Cys46’, Glu115’, Leu117’, Asn139’, Val165”, Pro179”, Val 184”, Phe77 */Polar = Thr44’, Phe45’, Arg120’, Ser180” |
| Li-OOH(2) | 4.3 | −5.0 | Apolar = Pro40’, Phe45’, Glu115’, Leu117’, Val165”, Pro179”, Val184”, His76*, Phe77 */Polar = Thr44’, Asn139’ | |
| SeAhpC | Li-OOH(1) | 3.6 | −6.6 | Apolar = Pro42’, Phe47’, Val48’, Asp114’, Ala140’, Val167”, Pro181”, Gly182”, Phe79 */Polar = Ser46’, Cys49’, Arg122’, Asp141’ |
| Li-OOH(2) | 3.5 | −6.4 | Apolar = Phe40’, Pro42’, Val48’, Leu119’, Arg122’, Ala140’, Asp141’, Phe79 */Polar = Ala43’, Ser46’, Phe47’, Cys49’, Asp114’, Asn139’ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cabrera, V.I.M.; Vargas, S.; de Medeiros, N.M.; Sividanes, G.N.; da Silva, L.F.; Diniz, L.R.; Alegria, T.G.P.; Lago, J.H.G.; Toyama, M.H.; Miyamoto, S.; et al. Linoleic Hydroperoxides Are Potent Hyperoxidative Agents of Sensitive and Robust Typical 2-Cys Peroxiredoxins. Antioxidants 2025, 14, 1422. https://doi.org/10.3390/antiox14121422
Cabrera VIM, Vargas S, de Medeiros NM, Sividanes GN, da Silva LF, Diniz LR, Alegria TGP, Lago JHG, Toyama MH, Miyamoto S, et al. Linoleic Hydroperoxides Are Potent Hyperoxidative Agents of Sensitive and Robust Typical 2-Cys Peroxiredoxins. Antioxidants. 2025; 14(12):1422. https://doi.org/10.3390/antiox14121422
Chicago/Turabian StyleCabrera, Vitória Isabela Montanhero, Sabrina Vargas, Nathália Miranda de Medeiros, Gabrielle Nascimento Sividanes, Laura Fernandes da Silva, Larissa Regina Diniz, Thiago Geronimo Pires Alegria, João Henrique Ghilardi Lago, Marcos Hikari Toyama, Sayuri Miyamoto, and et al. 2025. "Linoleic Hydroperoxides Are Potent Hyperoxidative Agents of Sensitive and Robust Typical 2-Cys Peroxiredoxins" Antioxidants 14, no. 12: 1422. https://doi.org/10.3390/antiox14121422
APA StyleCabrera, V. I. M., Vargas, S., de Medeiros, N. M., Sividanes, G. N., da Silva, L. F., Diniz, L. R., Alegria, T. G. P., Lago, J. H. G., Toyama, M. H., Miyamoto, S., Truzzi, D. R., Netto, L. E. S., & de Oliveira, M. A. (2025). Linoleic Hydroperoxides Are Potent Hyperoxidative Agents of Sensitive and Robust Typical 2-Cys Peroxiredoxins. Antioxidants, 14(12), 1422. https://doi.org/10.3390/antiox14121422










