Effect of 3′,4′-Dihydroxyflavonol Eye Drops in a Rat Model of Dispase-Induced Proliferative Vitreoretinopathy
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Treatment with Pharmacological Agents
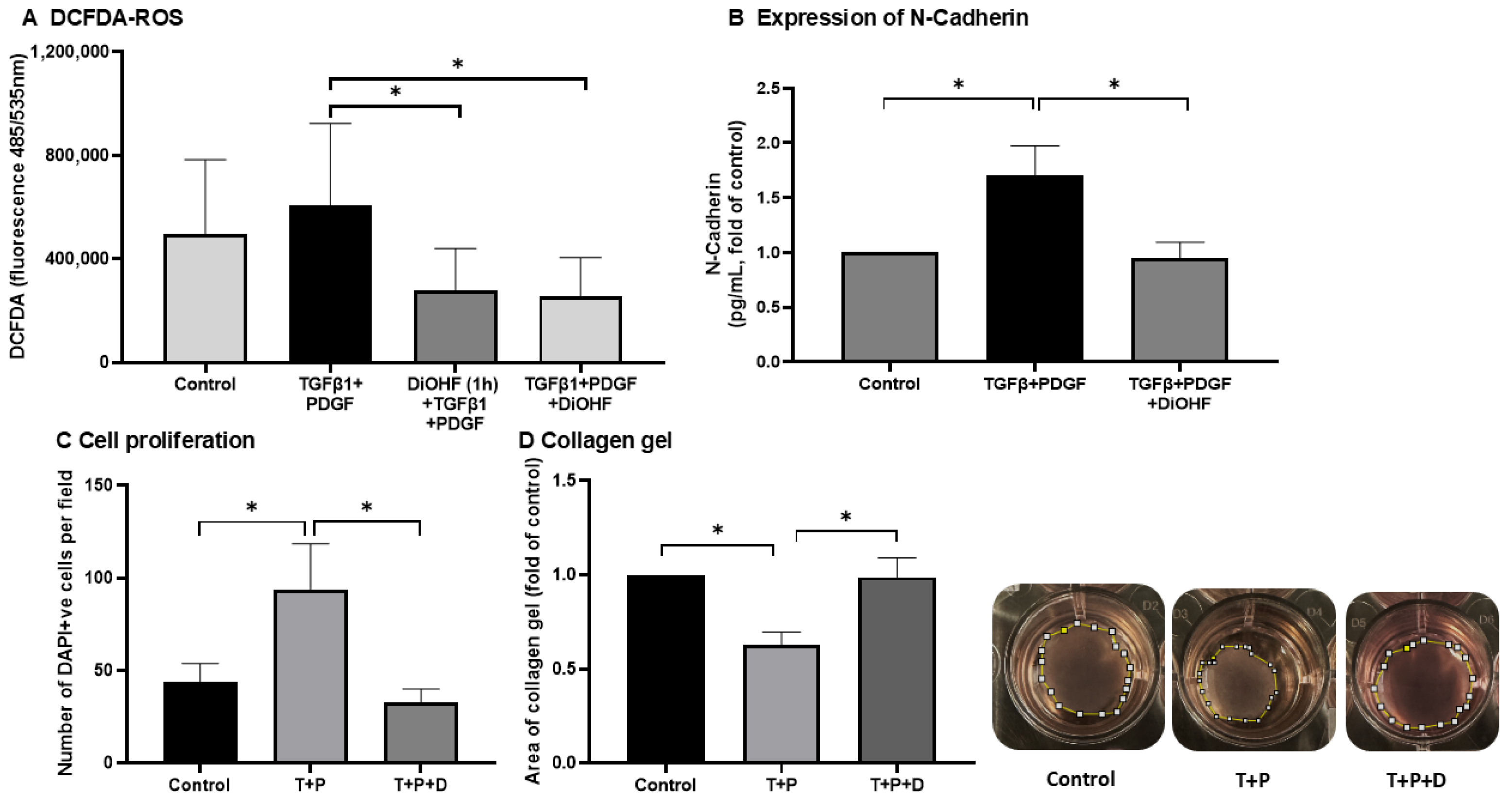
2.2. Determination of Total ROS and Expression of N-Cadherin in ARPE-19
2.3. Wound Healing Assay and Cell Proliferation
2.4. Collagen Gel Assay
2.5. Rat Model of PVR
2.6. Sample Preparation for Proteomic Analysis by Mass Spectrometry
2.7. Mass Spectrometry Data Acquisition
2.8. Mass Spectrometry Data Processing
2.9. Quantification of DiOHF in Ocular Tissues
2.10. Data Presentation and Analysis
3. Results
3.1. Effect of DiOHF on Cellular Responses Contributing to PVR Injury
3.2. Effect of DiOHF on Wound Healing in ARPE-19
3.3. Accumulation of DiOHF
3.4. In Vivo Observations of Treated Eyes
3.5. The Digestion Efficiency of Proteins Extracted from Retinas and Vitreous Humours
3.6. Protein Identification of Peptides Extracted from Rat Retinas and Vitreous Humours
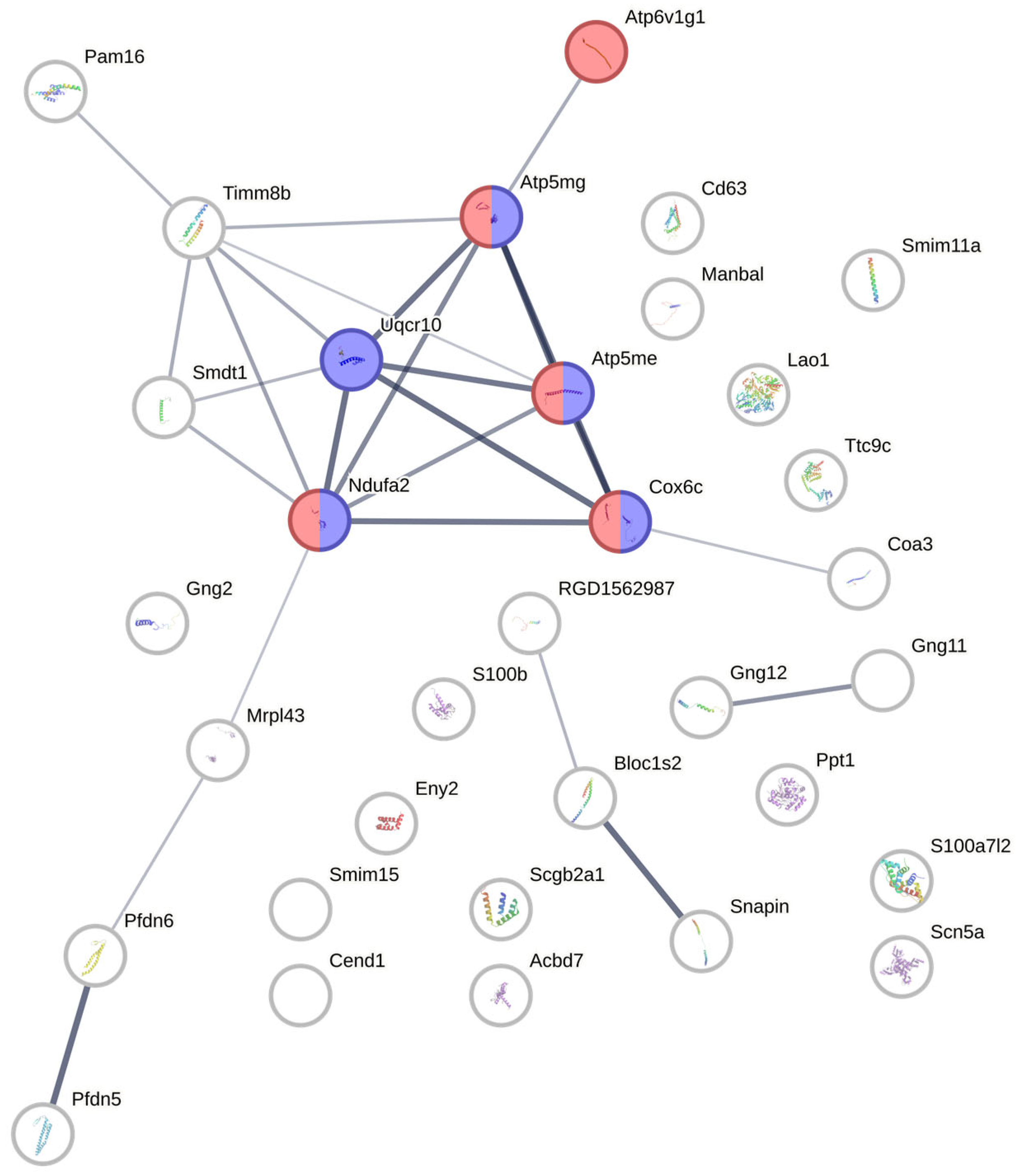
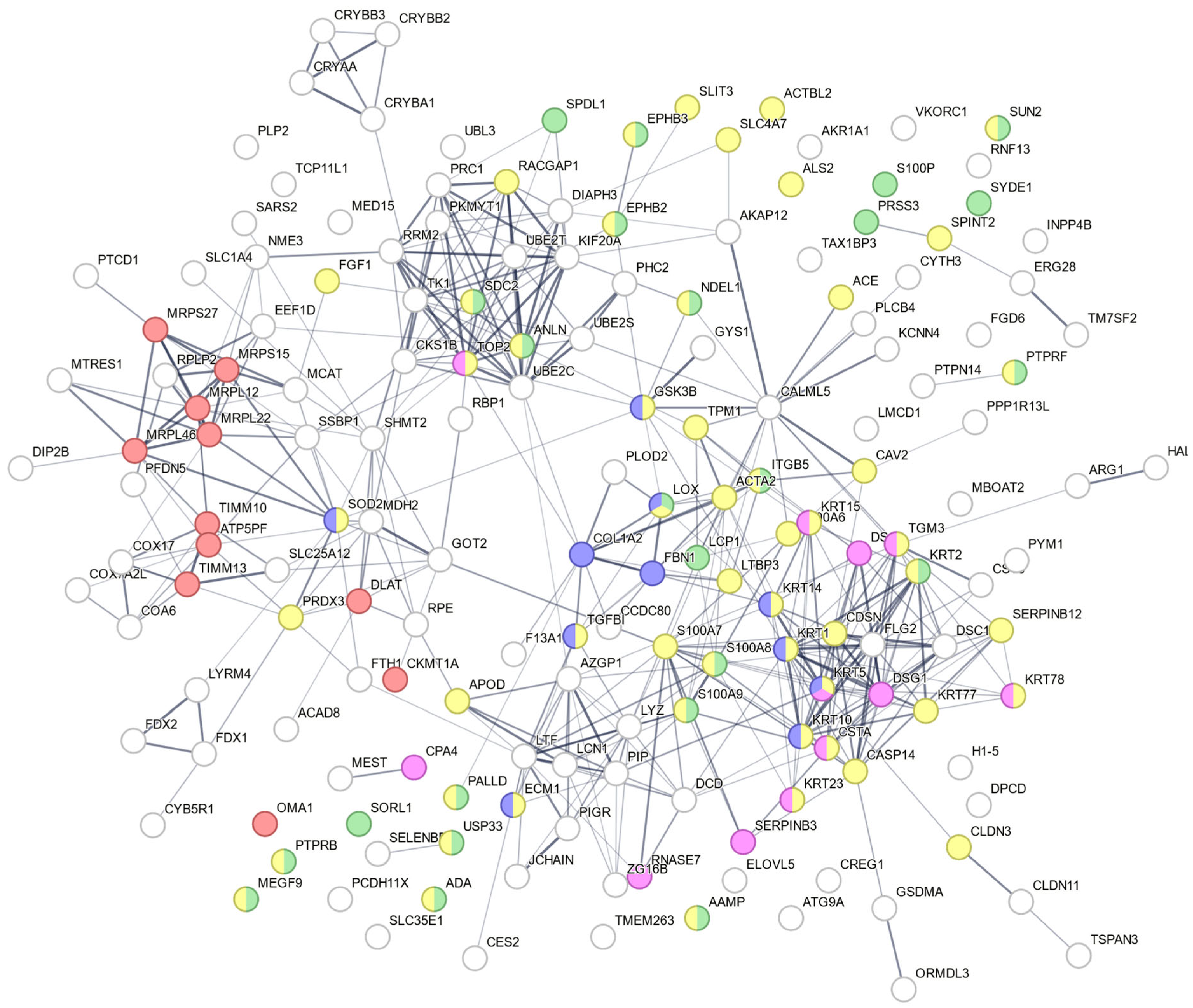
3.7. Protein–Protein Interaction Network Analysis from Candidate Proteins Identified from Retina/Vitreous Humour
3.8. Proteomic Analysis on Protein Extracted from ARPE-19
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PVR | Proliferative vitreoretinopathy |
| DiOHF | 3′,4′-dihydroxyflavonol |
| DMEM | Dulbecco’s Modified Eagle Medium |
| ROS | Reactive oxygen species |
| EMT | Epithelial–Mesenchymal transition |
| DCFDA | 2′,7′-dichlorofluorescin diacetate |
| DEP | Differentially expressed proteins |
| OCT | Optical coherence tomography |
| LTBP3 | Latent TGFβ binding protein 3 |
| COL1A2 | Collagen alpha-2(I) chain |
| FBN1 | Fibrillin-1 |
| ITGB5 | Integrin-β 5 |
| PLOD2 | Procollagen-lysine, 2-oxoglutarate 5-dioxygenase 2 |
| LOX | Protein-lysine 6-oxidase |
| CCDC80 | Coiled-coil domain-containing protein 80 |
| F13A1 | Coagulation factor XIII A chain |
| ADA | Adenosine deaminase |
| ECM1 | Extracellular matrix protein 1 |
| LYZ | Lysozyme C |
| KRT1 | Keratin type II cytoskeletal 1 |
| FGF1 | Fibroblast growth factor 1 |
| FTH1 | Ferritin heavy chain |
| ATP5PF | ATP synthase-coupling factor 6 |
| COX7A2L | Cytochrome c oxidase subunit 7A-related protein |
| MDH2 | Malate dehydrogenase |
| FDX2 | Ferredoxin-2 |
| TIMM | Inner membrane translocase |
References
- Charteris, D.G. Proliferative vitreoretinopathy: Pathobiology, surgical management, and adjunctive treatment. Br. J. Ophthalmol. 1995, 79, 953–960. [Google Scholar] [CrossRef] [PubMed]
- Wiedemann, P.; Hui, Y.N. PVR update: Pathophysiology and clinical management. Int. J. Ophthalmol. 2024, 17, 1577–1580. [Google Scholar] [CrossRef]
- Ferro Desideri, L.; Artemiev, D.; Zandi, S.; Zinkernagel, M.S.; Anguita, R. Proliferative vitreoretinopathy: An update on the current and emerging treatment options. Graefe’s Arch. Clin. Exp. Ophthalmol. 2024, 262, 679–687. [Google Scholar] [CrossRef]
- Uslubas, I.; Kanli, A.; Kasap, M.; Akpinar, G.; Karabas, L. Effect of aflibercept on proliferative vitreoretinopathy: Proteomic analysis in an experimental animal model. Exp. Eye Res. 2021, 203, 108425. [Google Scholar] [CrossRef]
- Gurelik, G.; Sul, S.; Ucgul, A.Y. Intraocular mitomycin C use in the treatment and prophylaxis of proliferative vitreoretinopathy in severe traumatic retinal detachments. Eur. J. Ophthalmol. 2021, 31, 3284–3293. [Google Scholar] [CrossRef]
- Ullah, A.; Toth, C.A.; Burnett, H.W.; Butler, J.W.; Levy, J.H.; Benner, J.D. Low-Dose Intravitreal Methotrexate for Proliferative Vitreoretinopathy. Ophthalmic Surg. Lasers Imaging Retin. 2023, 54, 139–146. [Google Scholar] [CrossRef]
- Pasvanis, Z.; Kong, R.C.K.; Shah, M.H.; Chan, E.C.; Fan Gaskin, J.C. 3′,4′-Dihydroxyflavonol Inhibits Fibrotic Response in a Rabbit Model of Glaucoma Filtration Surgery. Int. J. Mol. Sci. 2024, 25, 10767. [Google Scholar] [CrossRef]
- Fan Gaskin, J.C.; Kong, R.C.K.; Shah, M.H.; Edgley, A.J.; Peshavariya, H.M.; Chan, E.C. Inhibitory Effects of 3′,4′-Dihydroxyflavonol in a Mouse Model of Glaucoma Filtration Surgery and TGFbeta1-Induced Responses in Human Tenon’s Fibroblasts. Transl. Vis. Sci. Technol. 2022, 11, 18. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Kirsch, R.; Koutrouli, M.; Nastou, K.; Mehryary, F.; Hachilif, R.; Gable, A.L.; Fang, T.; Doncheva, N.T.; Pyysalo, S.; et al. The STRING database in 2023: Protein-protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic Acids Res. 2023, 51, D638–D646. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.; Lan, W.; Lim, R.R.; Chaurasia, S.S. S100A proteins as molecular targets in the ocular surface inflammatory diseases. Ocul. Surf. 2014, 12, 23–31. [Google Scholar] [CrossRef]
- Li, W.; Yin, L.; Shen, C.; Hu, K.; Ge, J.; Sun, A. SCN5A Variants: Association with Cardiac Disorders. Front. Physiol. 2018, 9, 1372. [Google Scholar] [CrossRef]
- Gu, M.; Jiang, H.; Tan, M.; Yu, L.; Xu, N.; Li, Y.; Wu, H.; Hou, Q.; Dai, C. Palmitoyltransferase DHHC9 and acyl protein thioesterase APT1 modulate renal fibrosis through regulating beta-catenin palmitoylation. Nat. Commun. 2023, 14, 6682. [Google Scholar] [CrossRef]
- Choi, E.S.; Lee, H.; Lee, C.H.; Goh, S.H. Overexpression of KLHL23 protein from read-through transcription of PHOSPHO2-KLHL23 in gastric cancer increases cell proliferation. FEBS Open Bio 2016, 6, 1155–1164. [Google Scholar] [CrossRef]
- Cui, H.; Xie, N.; Banerjee, S.; Dey, T.; Liu, R.M.; Antony, V.B.; Sanders, Y.Y.; Adams, T.S.; Gomez, J.L.; Thannickal, V.J.; et al. CD38 Mediates Lung Fibrosis by Promoting Alveolar Epithelial Cell Aging. Am. J. Respir. Crit. Care Med. 2022, 206, 459–475. [Google Scholar] [CrossRef] [PubMed]
- Marttila-Ichihara, F.; Elima, K.; Auvinen, K.; Veres, T.Z.; Rantakari, P.; Weston, C.; Miyasaka, M.; Adams, D.; Jalkanen, S.; Salmi, M. Amine oxidase activity regulates the development of pulmonary fibrosis. FASEB J. 2017, 31, 2477–2491. [Google Scholar] [CrossRef]
- Tri, T.T.; Lim, S.; Dang, N.N.; Chae, H.; Kim, H.; Lee, H.J.; Jo, D.S.; Cho, S.M. Maximization of cytochrome C oxidase enzyme activity by optimizing color conversion from red organic light-emitting diodes. Appl. Mater. Today 2024, 38, 102223. [Google Scholar] [CrossRef]
- Wu, L.; Yang, S.; Li, H.; Zhang, Y.; Feng, L.; Zhang, C.; Wei, J.; Gu, X.; Xu, G.; Wang, Z.; et al. TSPAN4-positive migrasome derived from retinal pigmented epithelium cells contributes to the development of proliferative vitreoretinopathy. J. Nanobiotechnol. 2022, 20, 519. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Ruan, X.; Wu, X.; Deng, Z.; Qin, S.; Jiang, H. Identification of Timm13 protein translocase of the mitochondrial inner membrane as a potential mediator of liver fibrosis based on bioinformatics and experimental verification. J. Transl. Med. 2023, 21, 188. [Google Scholar] [CrossRef]
- Kang, J.; Brajanovski, N.; Chan, K.T.; Xuan, J.; Pearson, R.B.; Sanij, E. Ribosomal proteins and human diseases: Molecular mechanisms and targeted therapy. Signal Transduct. Target. Ther. 2021, 6, 323. [Google Scholar] [CrossRef]
- Shi, L.; Feng, G.; Yang, X.; Zhang, Y.; Zhang, Y.; Cheng, J.; Lin, S. Potential of PAQosome as a therapeutic target for hepatic fibrosis. J. Gastroenterol. Hepatol. 2024, 39, 381–391. [Google Scholar] [CrossRef]
- Ge, J.Y.; Teo, Z.L.; Chee, M.L.; Tham, Y.C.; Rim, T.H.; Cheng, C.Y.; Wong, T.Y.; Group, S.S.R.R.; Wong, E.Y.M.; Lee, S.Y.; et al. International incidence and temporal trends for rhegmatogenous retinal detachment: A systematic review and meta-analysis. Surv. Ophthalmol. 2024, 69, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Idrees, S.; Sridhar, J.; Kuriyan, A.E. Proliferative Vitreoretinopathy: A Review. Int. Ophthalmol. Clin. 2019, 59, 221–240. [Google Scholar] [CrossRef] [PubMed]
- Chan, E.C.; Drummond, G.R.; Woodman, O.L. 3′,4′-dihydroxyflavonol enhances nitric oxide bioavailability and improves vascular function after ischemia and reperfusion injury in the rat. J. Cardiovasc. Pharmacol. 2003, 42, 727–735. [Google Scholar] [CrossRef]
- Woodman, O.L.; Chan, E. Vascular and anti-oxidant actions of flavonols and flavones. Clin. Exp. Pharmacol. Physiol. 2004, 31, 786–790. [Google Scholar] [CrossRef]
- Lau, Y.S.; Mustafa, M.R.; Choy, K.W.; Chan, S.M.H.; Potocnik, S.; Herbert, T.P.; Woodman, O.L. 3′,4′-dihydroxyflavonol ameliorates endoplasmic reticulum stress-induced apoptosis and endothelial dysfunction in mice. Sci. Rep. 2018, 8, 1818. [Google Scholar] [CrossRef]
- Faccia, P.; Pardini, F.; Amalvy, J. Uptake and release of Dexamethasone using pH-responsive poly(2-hydroxyethyl methacrylate-co-2-(diisopropylamino)ethyl methacrylate) hydrogels for potential use in ocular drug delivery. J. Drug Deliv. Sci. Technol. 2019, 51, 45–54. [Google Scholar] [CrossRef]
- Kallab, M.; Schuetzenberger, K.; Hommer, N.; Schafer, B.J.; Schmidl, D.; Bergmeister, H.; Zeitlinger, M.; Tan, A.; Jansook, P.; Loftsson, T.; et al. Bio-Distribution and Pharmacokinetics of Topically Administered gamma-Cyclodextrin Based Eye Drops in Rabbits. Pharmaceuticals 2021, 14, 480. [Google Scholar] [CrossRef]
- Huang, X.; Li, Q.; Xu, M.; Sun, S.; Gong, Y.; Luan, R.; Wang, M.; Shao, Y.; Li, X. The Interplay between Metabolic Reprogramming, Mitochondrial Impairment, and Steroid Response in Proliferative Vitreoretinopathy. Free Radic. Biol. Med. 2025, 229, 485–498. [Google Scholar] [CrossRef]
- Nolfi-Donegan, D.; Braganza, A.; Shiva, S. Mitochondrial electron transport chain: Oxidative phosphorylation, oxidant production, and methods of measurement. Redox Biol. 2020, 37, 101674. [Google Scholar] [CrossRef]
- Celik, H.; Kosar, M. Inhibitory effects of dietary flavonoids on purified hepatic NADH-cytochrome b5 reductase: Structure-activity relationships. Chem. Biol. Interact. 2012, 197, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Dixon, K.O.; Tabaka, M.; Schramm, M.A.; Xiao, S.; Tang, R.; Dionne, D.; Anderson, A.C.; Rozenblatt-Rosen, O.; Regev, A.; Kuchroo, V.K. TIM-3 restrains anti-tumour immunity by regulating inflammasome activation. Nature 2021, 595, 101–106. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, S. S100A4 a classical DAMP as a therapeutic target in fibrosis. Matrix Biol. 2024, 127, 1–7. [Google Scholar] [CrossRef]
- Zhang, N.; Zhao, Z.; Zhao, Y.; Yang, L.; Xue, Y.; Feng, Y.; Luo, J.; Chen, R.; Wei, W.; Qin, Y. TGF-beta changes cyto/mito-ribosome balance to target respiratory chain complex V biogenesis in pulmonary fibrosis therapy. Signal Transduct. Target. Ther. 2023, 8, 136. [Google Scholar] [CrossRef] [PubMed]
- Edmondson, S.P.; Turri, J.; Smith, K.; Clark, A.; Shriver, J.W. Structure, stability, and flexibility of ribosomal protein L14e from Sulfolobus solfataricus. Biochemistry 2009, 48, 5553–5562. [Google Scholar] [CrossRef]
- Manganiello, V.C.; Phillips, A.H. The relationship between ribosomes and the endoplasmic reticulum during protein synthesis. J. Biol. Chem. 1965, 240, 3951–3959. [Google Scholar] [CrossRef]
- Ribeiro, M.; Pasini, S.; Baratta, R.O.; Del Buono, B.J.; Schlumpf, E.; Calkins, D.J. Collagen Mimetic Peptides Promote Adherence and Migration of ARPE-19 Cells While Reducing Inflammatory and Oxidative Stress. Int. J. Mol. Sci. 2022, 23, 7004. [Google Scholar] [CrossRef]
- Kimoto, K.; Nakatsuka, K.; Matsuo, N.; Yoshioka, H. p38 MAPK mediates the expression of type I collagen induced by TGF-beta 2 in human retinal pigment epithelial cells ARPE-19. Investig. Ophthalmol. Vis. Sci. 2004, 45, 2431–2437. [Google Scholar] [CrossRef]
- Abhary, S.; Hewitt, A.W.; Burdon, K.P.; Craig, J.E. A systematic meta-analysis of genetic association studies for diabetic retinopathy. Diabetes 2009, 58, 2137–2147. [Google Scholar] [CrossRef]
- Brighenti, T.; Neri, G.; Mazzola, M.; Tome, G.; Scalfati, M.; Peroni, D.; Belli, R.; Zampedri, E.; Tebaldi, T.; Borello, U.; et al. Comparative proteomic analysis of human vitreous in rhegmatogenous retinal detachment and diabetic retinopathy reveals a common pathway and potential therapeutic target. Clin. Proteom. 2024, 21, 63. [Google Scholar] [CrossRef]
- O’Connor, T.; Ireland, L.S.; Harrison, D.J.; Hayes, J.D. Major differences exist in the function and tissue-specific expression of human aflatoxin B1 aldehyde reductase and the principal human aldo-keto reductase AKR1 family members. Biochem. J. 1999, 343, 487–504. [Google Scholar] [CrossRef] [PubMed]
- Miller, C.G.; Henderson, M.; Mantopoulos, D.; Leskov, I.; Greco, T.; Schwarzbauer, J.E.; Prenner, J.L. The Proteome of Preretinal Tissue in Proliferative Vitreoretinopathy. Ophthalmic Surg. Lasers Imaging Retin. 2021, 52, S5–S12. [Google Scholar] [CrossRef] [PubMed]
- Reyes, R.; Cardenes, B.; Machado-Pineda, Y.; Cabanas, C. Tetraspanin CD9: A Key Regulator of Cell Adhesion in the Immune System. Front. Immunol. 2018, 9, 863. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, F.M.; Ciordia, S.; Mesquita, J.; de Sousa, J.P.C.; Paradela, A.; Tomaz, C.T.; Passarinha, L.A.P. Vitreous humor proteome: Unraveling the molecular mechanisms underlying proliferative and neovascular vitreoretinal diseases. Cell. Mol. Life Sci. 2022, 80, 22. [Google Scholar] [CrossRef]
- Frenzel, E.M.; Neely, K.A.; Walsh, A.W.; Cameron, J.D.; Gregerson, D.S. A new model of proliferative vitreoretinopathy. Investig. Ophthalmol. Vis. Sci. 1998, 39, 2157–2164. [Google Scholar]
- Dai, Y.; Dai, C.; Sun, T. Inflammatory mediators of proliferative vitreoretinopathy: Hypothesis and review. Int. Ophthalmol. 2020, 40, 1587–1601. [Google Scholar] [CrossRef]
- Mirzaei, M.; Gupta, V.K.; Chitranshi, N.; Deng, L.; Pushpitha, K.; Abbasi, M.; Chick, J.M.; Rajput, R.; Wu, Y.; McKay, M.J.; et al. Retinal proteomics of experimental glaucoma model reveal intraocular pressure-induced mediators of neurodegenerative changes. J. Cell. Biochem. 2020, 121, 4931–4944. [Google Scholar] [CrossRef]
- Perez-Riverol, Y.; Bandla, C.; Kundu, D.J.; Kamatchinathan, S.; Bai, J.; Hewapathirana, S.; John, N.S.; Prakash, A.; Walzer, M.; Wang, S.; et al. The PRIDE database at 20 years: 2025 update. Nucleic Acids Res. 2025, 53, D543–D553. [Google Scholar] [CrossRef]



| Samples | N | Aqueous Humour (ng/g) | Retina/Vitreous (ng/g) |
|---|---|---|---|
| DiOHF (10 μM) | 2 | Not detected * | Not detected * |
| DiOHF (30 μM) | 3 | 0.27 ± 0.47 | 1.19 ± 0.40 |
| DiOHF (100 μM) | 3 | 1.00 ± 0.88 | 4.58 ± 2.10 |
| Gene Name | Protein Description | UniProt Primary Accession |
|---|---|---|
| Uqcr10 | Complex III subunit 9 | A0A9K3Y7E2 |
| Coa3 | Cytochrome c oxidase assembly factor 3 | A6HJA1 |
| Cox6c | Cytochrome c oxidase subunit 6C | A6HR20 |
| Eny2 | Transcription and mRNA export factor ENY2 | A6HRB0;A6HRB2;A6HRB3 |
| Smdt1 | Essential MCU regulator | A6HT67 |
| Ttc9c | Tetratricopeptide repeat domain 9C | A6HZU8 |
| Scgb2a1 | Secretoglobin | A6HZZ8 |
| Scn5a | Sodium channel protein | A6I3X4;A6I3X5 |
| Smim15 | Small integral membrane protein 15 | A6I5J6 |
| Ppt1 * | Palmitoyl-protein thioesterase | A6IS07 |
| Ndufa2 | NADH dehydrogenase [ubiquinone] 1 alpha subcomplex subunit 2 | A6J329 |
| Timm8b | Mitochondrial import inner membrane translocase TIM8B | A6J4E1 |
| Snapin | Snapin | A6J6M5 |
| Atp6v1g1 | V-type proton ATPase subunit G | A6J7Y6;B2GUV5 |
| Bloc1s2 | Biogenesis of lysosome-related organelles complex 1 subunit 2 | A6JHF0;A6JHF1 |
| Mrpl43 | Large ribosomal subunit protein mL43 | A6JHH2 |
| S100b | Protein S100 | A6JKD9 |
| Smim11 | Small integral membrane protein 11 | A6JLJ2 |
| Acbd7 | Acyl-CoA-binding protein | A6JM23 |
| Gng11 | Guanine nucleotide-binding protein subunit γ | A6K2B4 |
| Pam16 | Mitochondrial import inner membrane translocase TIM16 | A6K4S2 |
| Gng12 | Guanine nucleotide-binding protein subunit γ | A6KF41 |
| Gng2 | Guanine nucleotide-binding protein subunit γ | A6KKL4 |
| S100a7l2 | S100 calcium-binding protein | A6KMS2 |
| Cd63 | Tetraspanin | A6KSK3;A6KSK4 |
| C18orf32 ** | UPF0729 protein C18orf32 homologue | B1WC88 |
| Pfdn5 | Prefoldin 5 | B5DFN4 |
| Cend1 | C38 protein | B7X6I3 |
| Pfdn6 | Prefoldin-β | F7EQJ2 |
| Manbal | Mannosidase, beta A, lysosomal-like | F7ETW1 |
| Lao1 | Amine oxidase | F7FCK8 |
| Atp5mg | ATP synthase subunit G | Q6PDU7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chan, E.C.; Zeng, C.; Luu, C.D.; Abbott, C.J.; Chan, N.T.; Datta, K.K.; Williamson, N.; Allen, P.J.; Fan Gaskin, J.C. Effect of 3′,4′-Dihydroxyflavonol Eye Drops in a Rat Model of Dispase-Induced Proliferative Vitreoretinopathy. Antioxidants 2025, 14, 1414. https://doi.org/10.3390/antiox14121414
Chan EC, Zeng C, Luu CD, Abbott CJ, Chan NT, Datta KK, Williamson N, Allen PJ, Fan Gaskin JC. Effect of 3′,4′-Dihydroxyflavonol Eye Drops in a Rat Model of Dispase-Induced Proliferative Vitreoretinopathy. Antioxidants. 2025; 14(12):1414. https://doi.org/10.3390/antiox14121414
Chicago/Turabian StyleChan, Elsa C., Cheng Zeng, Chi D. Luu, Carla J. Abbott, Nicholas T. Chan, Keshava K. Datta, Nicholas Williamson, Penelope J. Allen, and Jennifer C. Fan Gaskin. 2025. "Effect of 3′,4′-Dihydroxyflavonol Eye Drops in a Rat Model of Dispase-Induced Proliferative Vitreoretinopathy" Antioxidants 14, no. 12: 1414. https://doi.org/10.3390/antiox14121414
APA StyleChan, E. C., Zeng, C., Luu, C. D., Abbott, C. J., Chan, N. T., Datta, K. K., Williamson, N., Allen, P. J., & Fan Gaskin, J. C. (2025). Effect of 3′,4′-Dihydroxyflavonol Eye Drops in a Rat Model of Dispase-Induced Proliferative Vitreoretinopathy. Antioxidants, 14(12), 1414. https://doi.org/10.3390/antiox14121414





