Curcumin in the Treatment of Kidney Disease: A Systematic Review with a Focus on Drug Interactions
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. Search Strategy
2.3. Analysis of Scope and Feasibility
2.4. Selection Criteria
2.5. Data Analysis
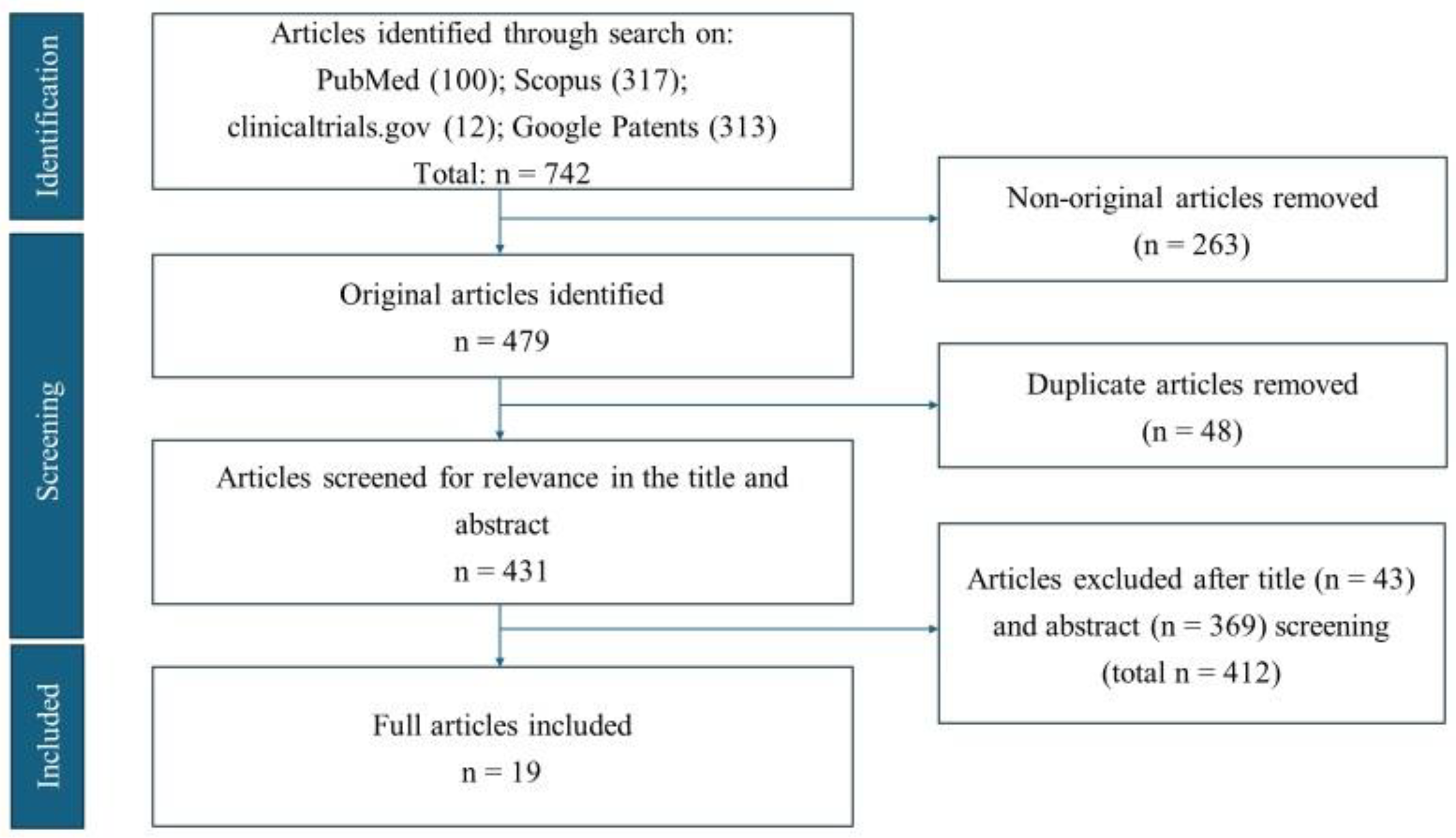
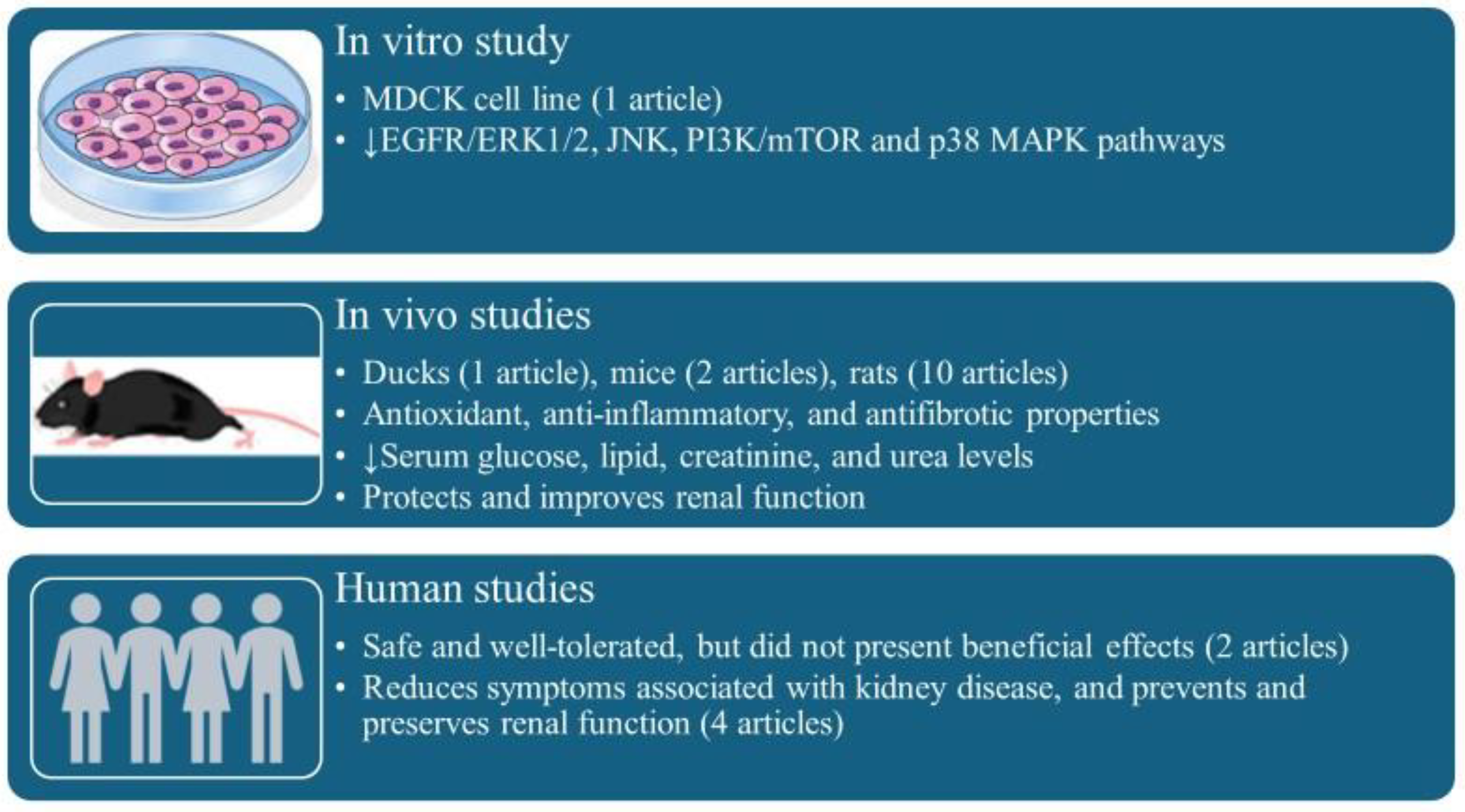
3. Results
4. Discussion
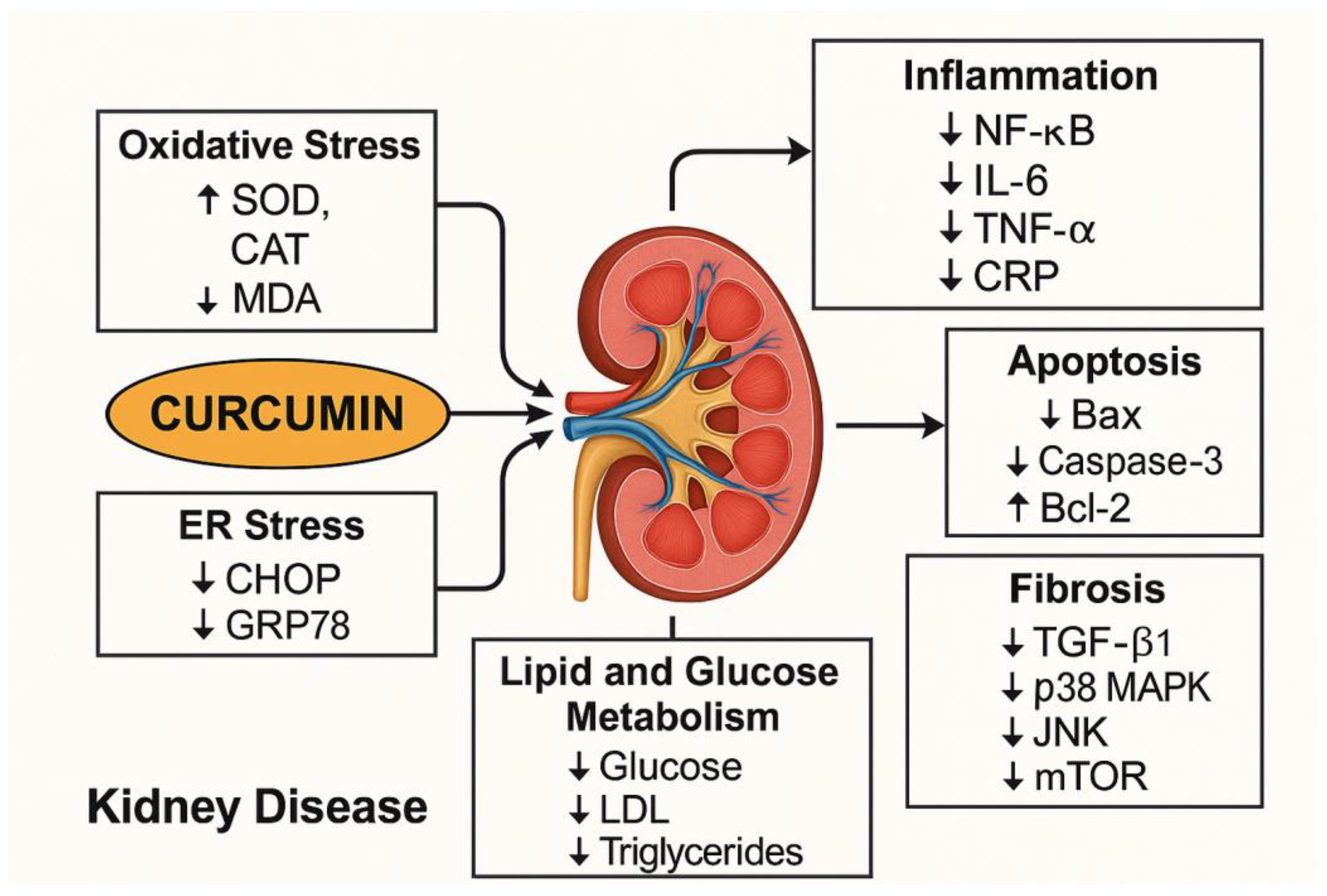
4.1. Mechanisms of Actions of Curcumin in Kidney Disease
4.2. Clinical Benefits and Adverse Effects of Curcumin in Kidney Disease
4.3. Curcumin Interactions in Kidney Disease
4.3.1. General Pharmacokinetic and Safety Interactions of Curcumin
4.3.2. Modulation of CYP Enzymes and Drug Transporters (P-gp)
4.3.3. Effects on Coagulation and Platelet Function
4.3.4. Impact on Blood Glucose Regulation
| Interaction Category | Mechanism/Effect | Clinical Implication/Risk | Ref. |
|---|---|---|---|
| Cytochrome P450 (CYP) Enzymes | Curcuminoids inhibit various CYP isoforms (CYP2C19, CYP2B6, CYP2C9, CYP3A, CYP1A2, CYP2D6). | Decreased metabolism of co-administered drugs, leading to increased systemic exposure, higher plasma concentrations, and potential for increased pharmacological effects or adverse reactions. Complex and tissue-specific modulation requires careful monitoring. | [71] |
| P-glycoprotein (P-gp) | Curcumin inhibits P-gp (efflux pump). Chronic curcumin administration can down-regulate intestinal P-gp, but up-regulate hepatic P-gp. | Altered absorption and distribution of P-gp substrate drugs, potentially increasing their bioavailability and leading to unpredictable changes in efficacy or toxicity. | [74,81] |
| Coagulation/Platelet Function | Curcumin has antiplatelet effects (inhibits platelet aggregation, interferes with clotting). | Increased risk of bleeding and bruising, especially when co-administered with anticoagulants (e.g., warfarin, clopidogrel) or antiplatelet agents. | [77,82] |
| Blood Glucose Regulation | Curcumin can lower blood glucose and HbA1c levels. | Reduce glucose level when co-administered with antidiabetic drugs (e.g., glyburide). Requires rigorous blood glucose monitoring. | [57,79] |
4.4. Systematic Analysis of Curcumin-Drug Combinations in Kidney Disease
4.4.1. Turmeric and Piperine
4.4.2. Nanoparticle-Encapsulated Curcumin and Epigallocatechin Gallate (EGCG)
4.4.3. Tetrahydrocurcumin, Polyenylphosphatidylcholine, and Losartan
4.4.4. Curcumin & Ginkgolide B
4.4.5. Curcumin & Rosuvastatin
4.4.6. Nanocurcumin Combined with Insulin
4.4.7. Curcumin-Tagged Cilostazol
4.4.8. Ginger and Curcumin
5. Limitations and Challenges
6. Conclusions and Future Directions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CKD | Chronic kidney disease |
| AKI | Acute kidney injury |
| ACE | Angiotensin-converting enzyme |
| ARBs | Angiotensin receptor blockers |
| SGLT2 | Sodium-glucose transport proteins 2 |
| NF-κB | Nuclear factor-kappa B |
| TGF-β | Transforming growth factor-beta |
| ROS | Reactive oxygen species |
| PC-AKI | Post-contrast acute kidney injury |
| SOD | Superoxide dismutase |
| BUN | Blood urea nitrogen |
| ER | Endoplasmic reticulum |
| CAT | Catalase |
| MDA | Malondialdehyde |
| CRP | C-reactive protein |
| CHOP | C/EBP homologous protein |
| GRP78 | Glucose-regulated protein 78 |
| CYP | Cytochrome P450 enzymes |
| P-gp | P-glycoprotein transporters |
| EGCG | Epigallocatechin Gallate |
| THC | Tetrahydrocurcumin |
| PPC | Polyenylphosphatidylcholine |
| PRKCA | Protein kinase C-α |
| KIM-1 | Kidney injury molecule-1 |
| GB | Ginkgolide B |
| PAFR | Platelet-Activating Factor Receptor |
| PF4 | Platelet factor 4 |
| ADPKD | Autosomal Dominant Polycystic Kidney Disease |
| COX-1 | Cyclooxygenase-1 |
References
- Francis, A.; Harhay, M.N.; Ong, A.C.; Tummalapalli, S.L.; Ortiz, A.; Fogo, A.B.; Fliser, D.; Roy-Chaudhury, P.; Fontana, M.; Nangaku, M. Chronic kidney disease and the global public health agenda: An international consensus. Nat. Rev. Nephrol. 2024, 20, 473–485. [Google Scholar] [CrossRef]
- Perico, N.; Remuzzi, G. Chronic kidney disease: A research and public health priority. Nephrol. Dial. Transplant. 2012, 27, iii19–iii26. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, K.C. Extracts from two frequently consumed spices—Cumin (Cuminum cyminum) and turmeric (Curcuma longa)—Inhibit platelet aggregation and alter eicosanoid biosynthesis in human blood platelets, Prostaglandins. Leukot. Essent. Fat. Acids 1989, 37, 57–64. [Google Scholar] [CrossRef]
- Hoste, E.A.; Kellum, J.A.; Selby, N.M.; Zarbock, A.; Palevsky, P.M.; Bagshaw, S.M.; Goldstein, S.L.; Cerdá, J.; Chawla, L.S. Global epidemiology and outcomes of acute kidney injury. Nat. Rev. Nephrol. 2018, 14, 607–625. [Google Scholar] [CrossRef]
- Kellum, J.A.; Romagnani, P.; Ashuntantang, G.; Ronco, C.; Zarbock, A.; Anders, H.-J. Acute kidney injury. Nat. Revies Dis. Primers 2021, 7, 52. [Google Scholar] [CrossRef]
- Rewa, O.; Bagshaw, S.M. Acute kidney injury—Epidemiology, outcomes and economics. Nat. Rev. Nephrol. 2014, 10, 193–207. [Google Scholar] [CrossRef]
- Kovesdy, C.P. Epidemiology of chronic kidney disease: An update 2022. Kidney Int. Suppl. 2022, 12, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.-C.; Zhang, L.-X. Prevalence and disease burden of chronic kidney disease. In Renal fibrosis: Mechanisms Therapies; Springer: Singapore, 2019; pp. 3–15. [Google Scholar] [CrossRef]
- Crews, D.C.; Bello, A.K.; Saadi, G.; Committee, W.K.D.S. Burden, access, and disparities in kidney disease. Braz. J. Med. Biol. Res. 2019, 52, e8338. [Google Scholar] [CrossRef]
- George, C.; Echouffo-Tcheugui, J.B.; Jaar, B.G.; Okpechi, I.G.; Kengne, A.P. The need for screening, early diagnosis, and prediction of chronic kidney disease in people with diabetes in low-and middle-income countries—A review of the current literature. BMC Med. 2022, 20, 247. [Google Scholar] [CrossRef]
- Turner, J.M.; Bauer, C.; Abramowitz, M.K.; Melamed, M.L.; Hostetter, T.H. Treatment of chronic kidney disease. Kidney Int. 2012, 81, 351–362. [Google Scholar] [CrossRef] [PubMed]
- Strippoli, G.F.; Bonifati, C.; Craig, M.E.; Navaneethan, S.D.; Craig, J.C. Angiotensin converting enzyme inhibitors and angiotensin II receptor antagonists for preventing the progression of diabetic kidney disease. Cochrane Database Syst. Rev. 2006, 2006, CD006257. [Google Scholar] [CrossRef]
- Peene, B.; Benhalima, K. Sodium glucose transporter protein 2 inhibitors: Focusing on the kidney to treat type 2 diabetes. Ther. Adv. Endocrinol. Metab. 2014, 5, 124–136. [Google Scholar] [CrossRef]
- Ellison, D.; Farrar, F.C. Kidney influence on fluid and electrolyte balance. Nurs. Clin. N. Am. 2018, 53, 469–480. [Google Scholar] [CrossRef]
- Radi, Z.A. Kidney pathophysiology, toxicology, and drug-induced injury in drug development. Int. J. Toxicol. 2019, 38, 215–227. [Google Scholar] [CrossRef]
- Pellegrino, D.; La Russa, D.; Marrone, A. Oxidative imbalance and kidney damage: New study perspectives from animal models to hospitalized patients. Antioxidants 2019, 8, 594. [Google Scholar] [CrossRef]
- Syed-Ahmed, M.; Narayanan, M. Immune dysfunction and risk of infection in chronic kidney disease. Adv. Chronic Kidney Dis. 2019, 26, 8–15. [Google Scholar] [CrossRef]
- Panizo, S.; Martínez-Arias, L.; Alonso-Montes, C.; Cannata, P.; Martín-Carro, B.; Fernández-Martín, J.L.; Naves-Díaz, M.; Carrillo-López, N.; Cannata-Andía, J.B. Fibrosis in chronic kidney disease: Pathogenesis and consequences. Int. J. Mol. Sci. 2021, 22, 408. [Google Scholar] [CrossRef] [PubMed]
- Irazabal, M.V.; Torres, V.E. Reactive oxygen species and redox signaling in chronic kidney disease. Cells 2020, 9, 1342. [Google Scholar] [CrossRef]
- Fakhruddin, S.; Alanazi, W.; Jackson, K.E. Diabetes-induced reactive oxygen species: Mechanism of their generation and role in renal injury. J. Diabetes Res. 2017, 2017, 8379327. [Google Scholar] [CrossRef] [PubMed]
- Duni, A.; Liakopoulos, V.; Roumeliotis, S.; Peschos, D.; Dounousi, E. Oxidative stress in the pathogenesis and evolution of chronic kidney disease: Untangling Ariadne’s thread. Int. J. Mol. Sci. 2019, 20, 3711. [Google Scholar] [CrossRef] [PubMed]
- Kocaadam, B.; Şanlier, N. Curcumin, an active component of turmeric (Curcuma longa), and its effects on health. Crit. Rev. Food Sci. 2017, 57, 2889–2895. [Google Scholar] [CrossRef]
- Ghosh, S.S.; Massey, H.D.; Krieg, R.; Fazelbhoy, Z.A.; Ghosh, S.; Sica, D.A.; Fakhry, I.; Gehr, T.W. Curcumin ameliorates renal failure in 5/6 nephrectomized rats: Role of inflammation. Am. J. Physiol.-Ren. Physiol. 2009, 296, F1146–F1157. [Google Scholar] [CrossRef]
- Ak, T.; Gülçin, I. Antioxidant and radical scavenging properties of curcumin. Chem.-Biol. Interact. 2008, 174, 27–37. [Google Scholar] [CrossRef]
- Pulido-Moran, M.; Moreno-Fernandez, J.; Ramirez-Tortosa, C.; Ramirez-Tortosa, M.J.M. Curcumin and health. Molecules 2016, 21, 264. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Gupta, S.C.; Park, B.; Yadav, V.R.; Aggarwal, B.B. Turmeric (Curcuma longa) inhibits inflammatory nuclear factor (NF)-κB and NF-κB-regulated gene products and induces death receptors leading to suppressed proliferation, induced chemosensitization, and suppressed osteoclastogenesis. J. Mol. Nutr. Food Res. 2012, 56, 454–465. [Google Scholar] [CrossRef]
- Gaedeke, J.; Noble, N.A.; Border, W.A. Curcumin blocks multiple sites of the TGF-β signaling cascade in renal cells. Kidney Int. 2004, 66, 112–120. [Google Scholar] [CrossRef]
- Ali, B.H.; Al-Salam, S.; Al Suleimani, Y.; Al Kalbani, J.; Al Bahlani, S.; Ashique, M.; Manoj, P.; Al Dhahli, B.; Al Abri, N.; Naser, H.T. Curcumin ameliorates kidney function and oxidative stress in experimental chronic kidney disease. Basic Clin. Pharmacol. Toxicol. 2018, 122, 65–73. [Google Scholar] [CrossRef]
- Akomolafe, S.F.; Olasehinde, T.A.; Adewale, O.O.; Ajayi, O.B. Curcumin improves biomolecules associated with renal function and attenuates oxidative injury and histopathological changes in potassium-induced toxicity in rats’ kidney. Biol. Trace Elem. Res. 2021, 199, 197–204. [Google Scholar] [CrossRef]
- Trujillo, J.; Chirino, Y.I.; Molina-Jijón, E.; Andérica-Romero, A.C.; Tapia, E.; Pedraza-Chaverrí, J. Renoprotective effect of the antioxidant curcumin: Recent findings. Redox Biol. 2013, 1, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhao, P.; Zhang, Y.; Zhu, L.; Zhu, J.; Luo, Y.; Li, Q. The therapeutic effects of curcumin in early septic acute kidney injury: An experimental study, Drug Design. Dev. Ther. 2021, 4243–4255. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.-Y.; Kang, D.-S.; Lee, Y.-G.; Kim, H.-S. Effects of turmeric (Curcuma longa L.) supplementation on blood urea nitrogen and enzyme activities in dyslipidemic rats. J. Environ. Sci. Int. 2019, 28, 475–483. [Google Scholar] [CrossRef]
- ALTamimi, J.Z.; AlFaris, N.A.; Al-Farga, A.M.; Alshammari, G.M.; BinMowyna, M.N.; Yahya, M.A. Curcumin reverses diabetic nephropathy in streptozotocin-induced diabetes in rats by inhibition of PKCβ/p66Shc axis and activation of FOXO-3a. J. Nutr. Biochem. 2021, 87, 108515. [Google Scholar] [CrossRef]
- Ghosh, S.; Banerjee, S.; Sil, P.C. The beneficial role of curcumin on inflammation, diabetes and neurodegenerative disease: A recent update. Food Chem. Toxicol. 2015, 83, 111–124. [Google Scholar] [CrossRef]
- Appiah-Opong, R.; Commandeur, J.N.; van Vugt-Lussenburg, B.; Vermeulen, N.P. Inhibition of human recombinant cytochrome P450s by curcumin and curcumin decomposition products. Toxicology 2007, 235, 83–91. [Google Scholar] [CrossRef]
- Hsieh, Y.-W.; Huang, C.-Y.; Yang, S.-Y.; Peng, Y.-H.; Yu, C.-P.; Chao, P.-D.L.; Hou, Y.-C. Oral intake of curcumin markedly activated CYP 3A4: In vivo and ex-vivo studies. Sci. Rep. 2014, 4, 6587. [Google Scholar] [CrossRef]
- Shamsi, S.; Tran, H.; Tan, R.S.J.; Tan, Z.J.; Lim, L.Y. Curcumin, piperine, and capsaicin: A comparative study of spice-mediated inhibition of human cytochrome P450 isozyme activities. Drug Metab. Dispos. 2017, 45, 49–55. [Google Scholar] [CrossRef]
- Gu, N.; Park, S.-I.; Chung, H.; Jin, X.; Lee, S.; Kim, T.-E. Possibility of pharmacokinetic drug interaction between a DPP-4 inhibitor and a SGLT2 inhibitor. Transl. Clin. Pharmacol. 2020, 28, 17. [Google Scholar] [CrossRef]
- Yang, R.; Luo, Z.; Liu, Y.; Sun, M.; Zheng, L.; Chen, Y.; Li, Y.; Wang, H.; Chen, L.; Wu, M. Drug interactions with angiotensin receptor blockers: Role of human cytochromes P450. Curr. Drug Metab. 2016, 17, 681–691. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Hu, Y.; Di, B.; He, P.L.; Sun, G. Effects of some antihypertensive drugs on the metabolism and pharmacokinetics of indapamide in rats. J. Pharm. Pharm. Sci. 2012, 15, 208–220. [Google Scholar] [CrossRef] [PubMed]
- May, M.; Schindler, C. Clinically and pharmacologically relevant interactions of antidiabetic drugs. Ther. Adv. Endocrinol. Metab. 2016, 7, 69–83. [Google Scholar] [CrossRef]
- Jie, Z.; Chao, M.; Jun, A.; Wei, S.; LiFeng, M. Effect of Curcumin on Diabetic Kidney Disease: A Systematic Review and Meta-Analysis of Randomized, Double-Blind, Placebo-Controlled Clinical Trials. Evid. Based Complement. Altern. Med. 2021, 2021, 6109406. [Google Scholar] [CrossRef]
- Bagherniya, M.; Soleimani, D.; Rouhani, M.H.; Askari, G.; Sathyapalan, T.; Sahebkar, A. The use of curcumin for the treatment of renal disorders: A systematic review of randomized controlled trials. In Advances in Experimental Medicine and Biology; Springer: Cham, Switzerland, 2021; pp. 327–343. [Google Scholar] [CrossRef]
- Emami, E.; Heidari-Soureshjani, S.; Sherwin, C.M.T. Anti-inflammatory response to curcumin supplementation in chronic kidney disease and hemodialysis patients: A systematic review and meta-analysis. Avicenna J. Phytomed. 2022, 12, 576. [Google Scholar] [CrossRef]
- Li, Y.; Gao, J.; Yang, X.; Li, T.; Yang, B.; Aili, A. Combination of curcumin and ginkgolide B inhibits cystogenesis by regulating multiple signaling pathways. Mol. Med. Rep. 2021, 23, 195. [Google Scholar] [CrossRef]
- Lagumdžija, D.; Mehmedbašić, A.H.; Jesenković, D.A.; Kudić, B.; Kapić, D.; Ćosović, E.; Lepara, O.; Kelle, B.P.; Prguda-Mujić, J.; Kusturica, J. Curcumin and its combination with a reduced dose of rosuvastatin: A promising therapy for chronic kidney disease and associated dyslipidemia in rat animal models. Biomol. Biomed. 2025, 25, 1377–1388. [Google Scholar] [CrossRef] [PubMed]
- Laorodphun, P.; Cherngwelling, R.; Panya, A.; Arjinajarn, P. Curcumin protects rats against gentamicin-induced nephrotoxicity by amelioration of oxidative stress, endoplasmic reticulum stress and apoptosis. Pharm. Biol. 2022, 60, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Ganugula, R.; Nuthalapati, N.K.; Dwivedi, S.; Zou, D.; Arora, M.; Friend, R.; Sheikh-Hamad, D.; Basu, R.; Kumar, M.R. Nanocurcumin combined with insulin alleviates diabetic kidney disease through P38/P53 signling axis. J. Control. Release 2023, 353, 621–633. [Google Scholar] [CrossRef] [PubMed]
- Kadhim, S.A.A.; Ghafil, F.A.; Majeed, S.A.; Hadi, N.R. Nephroprotective effects of curcumin against cyclosporine A-induced nephrotoxicity in rat model. Wiad Lek 2021, 74, 3135–3146. [Google Scholar] [CrossRef] [PubMed]
- Husain, S.; Kamal Bayoumy, N.M.; Noor, F.; Hagar, H. Nephroprotective Effects of Curcumin in Murine Models of Focal and Segmental Glomerulosclerosis. Pharmacology 2025, 110, 221–230. [Google Scholar] [CrossRef]
- Haleem, M.A.; Khan, S.A.; Khan, M.K.; Ahmad, R.S.; Naqvi, S.A.R.; Imran, M.; Ahmad, M.H.; Anwar, H.; Un Nisa, M. Potential protective role of curcumin powder to regulate arsenic-induced hepatorenal toxicity and hyperlipidemic metabolic dysfunction in rat model. Pak. J. Pharm. Sci. 2021, 34, 1535–1540. [Google Scholar] [PubMed]
- Rawat, A.; Chauhan, S.; Monika; Singh, R.P.; Gupta, S.; Jhawat, V. Preclinical pharmacology and pharmacokinetics of curcumin tagged cilostazol nanodispersion for the management of diabetic nephropathy in wister rat model. Silico Pharmacol. 2024, 12, 81. [Google Scholar] [CrossRef]
- Wu, S.; Yu, W.; Jiang, X.; Huang, R.; Zhang, X.; Lan, J.; Zhong, G.; Wan, F.; Tang, Z.; Hu, L. Protective effects of curcumin on ATO-induced nephrotoxicity in ducks in relation to suppressed autophagy, apoptosis and dyslipidemia by regulating oxidative stress. Ecotoxicol. Environ. Saf. 2021, 219, 112350. [Google Scholar] [CrossRef] [PubMed]
- Bhutia, G.T.; De, A.K.; Bhowmik, M.; Bera, T. Shellac and locust bean gum coacervated curcumin, epigallocatechin gallate nanoparticle ameliorates diabetic nephropathy in a streptozotocin-induced mouse model. Int. J. Biol. Macromol. 2024, 271, 132369. [Google Scholar] [CrossRef]
- Khazaeli, M.; Nunes, A.C.; Zhao, Y.; Khazaali, M.; Prudente, J.; Vaziri, N.D.; Singh, B.; Lau, W.L. Tetrahydrocurcumin Add-On therapy to losartan in a rat model of diabetic nephropathy decreases blood pressure and markers of kidney injury. Pharmacol. Res. Perspect. 2023, 11, e01079. [Google Scholar] [CrossRef]
- Taha, A.; Ashour, H.; Reffat, M.; Elkhawaga, O.Y. The impact of ginger and curcumin on diabetic nephropathy induced by streptozotocin in rats. Eur. J. Transl. Clin. Med. 2023, 6, 51–65. [Google Scholar] [CrossRef]
- Clinical trial:_CN117959346A; Application of Pharmaceutical Composition in Preparation of Medicine for Preventing and/or Treating Diabetic Nephropathy. Available online: https://patents.google.com/patent/CN117959346A/en?q=(curcumin)&q=(diabetic+nephropathy+renal+kidney+disease)&before=priority:20250101&after=priority:20210101&language=ENGLISH&type=PATENT&page=19 (accessed on 7 November 2025).
- Jaturapisanukul, S.; Kurathong, S.; Ngamvichchukorn, T.; Trakarnvanich, T. Curcuminoids can prevent post-contrast acute kidney injury in chronic kidney disease patients: A randomized controlled trial. Medicine 2022, 101, e30753. [Google Scholar] [CrossRef]
- Noppakun, K.; Jitraknatee, J.; Suteeka, Y.; Ruengorn, C.; Nochaiwong, S.; Gunaparn, S.; Phrommintikul, A.; Wongcharoen, W. Effect of curcuminoids on contrast-induced acute kidney injury after elective coronary angiography or intervention: A pilot randomized, double-blind, placebo-controlled study. Cardiorenal Med. 2024, 14, 160–166. [Google Scholar] [CrossRef]
- Hosseini, S.; Rahsepar, S.; Naghipour, S.; Elyasi, S. Is oral nano-curcumin formulation a safe and effective measure for preventing cisplatin-induced nephrotoxicity in cancer patients? Anti-Cancer Drugs 2024, 35, 859–866. [Google Scholar] [CrossRef] [PubMed]
- e Silva-Santana, N.C.F.; Rodrigues, H.C.N.; Martins, T.F.P.; Braga, C.C.; Silva, M.A.C.; da Cunha, L.C.; de Souza Freitas, A.T.V.; Costa, N.A.; Peixoto, M.d.R.G. Turmeric supplementation with piperine is more effective than turmeric alone in attenuating oxidative stress and inflammation in hemodialysis patients: A randomized, double-blind clinical trial. Free. Radic. Biol. Med. 2022, 193, 648–655. [Google Scholar] [CrossRef]
- Clinical trial: RU2777906C1; Specialized Food Product of Dietary Therapeutic Nutrition for Patients with Diabetic Nephropathy. Available online: https://patents.google.com/patent/RU2777906C1/en?q=curcumin+AND+(%22diabetic+nephropathy%22+OR+%22renal+disease%22+OR+%22kidney+disease%22)&before=priority:20250101&after=priority:20210101&page=8 (accessed on 7 November 2025).
- Clinical trial: CN112773877A; Pharmaceutical Composition for Treating Proteinuria Caused by Diabetic Nephropathy and Preparation Method and Application Thereof. Available online: https://patents.google.com/patent/CN112773877A/en?q=curcumin&q=diabetic+nephropathy+renal+kidney+disease&before=priority:20250101&after=priority:20210101&language=ENGLISH&type=PATENT&page=20&peid=642570beaad08%3A356%3A8398b474 (accessed on 7 November 2025).
- Ghosh, S.S.; Gehr, T.W.; Ghosh, S. Curcumin and chronic kidney disease (CKD): Major mode of action through stimulating endogenous intestinal alkaline phosphatase. Molecules 2014, 19, 20139–20156. [Google Scholar] [CrossRef] [PubMed]
- Yallapu, M.M.; Nagesh, P.K.B.; Jaggi, M.; Chauhan, S.C. Therapeutic applications of curcumin nanoformulations. AAPS J. 2015, 17, 1341–1356. [Google Scholar] [CrossRef]
- Khajehdehi, P.; Pakfetrat, M.; Javidnia, K.; Azad, F.; Malekmakan, L.; Nasab, M.H.; Dehghanzadeh, G. Oral supplementation of turmeric attenuates proteinuria, transforming growth factor-β and interleukin-8 levels in patients with overt type 2 diabetic nephropathy: A randomized, double-blind and placebo-controlled study. Scand. J. Urol. Nephrol. 2011, 45, 365–370. [Google Scholar] [CrossRef]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef]
- Lao, C.D.; Ruffin, M.T.; Normolle, D.; Heath, D.D.; Murray, S.I.; Bailey, J.M.; Boggs, M.E.; Crowell, J.; Rock, C.L.; Brenner, D.E. Dose escalation of a curcuminoid formulation. BMC Complement. Altern. Med. 2006, 6, 10. [Google Scholar] [CrossRef]
- Zhang, W.; Tan, T.; Lim, L. Impact of Curcumin-Induced Changes in P-Glycoprotein and CYP3A Expression on the Pharmacokinetics of Peroral Celiprolol and Midazolam in Rats. Drug Metab. Dispos. Biol. Fate Chem. 2007, 35, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Bahramsoltani, R.; Rahimi, R.; Farzaei, M.H. Pharmacokinetic interactions of curcuminoids with conventional drugs: A review. J. Ethnopharmacol. 2017, 209, 1–12. [Google Scholar] [CrossRef]
- Heger, M.; van Golen, R.F.; Broekgaarden, M.; Michel, M.C. The molecular basis for the pharmacokinetics and pharmacodynamics of curcumin and its metabolites in relation to cancer. Pharmacol. Rev. 2014, 66, 222–307. [Google Scholar] [CrossRef] [PubMed]
- Volak, L.P.; Ghirmai, S.; Cashman, J.R.; Court, M.H. Curcuminoids inhibit multiple human cytochromes P450, UDP-glucuronosyltransferase, and sulfotransferase enzymes, whereas piperine is a relatively selective CYP3A4 inhibitor. Drug Metab. Dispos. 2008, 36, 1594–1605. [Google Scholar] [CrossRef]
- Chearwae, W.; Anuchapreeda, S.; Nandigama, K.; Ambudkar, S.V.; Limtrakul, P. Biochemical mechanism of modulation of human P-glycoprotein (ABCB1) by curcumin I, II, and III purified from Turmeric powder. Biochem. Pharmacol. 2004, 68, 2043–2052. [Google Scholar] [CrossRef]
- Anuchapreeda, S.; Leechanachai, P.; Smith, M.M.; Ambudkar, S.V.; Limtrakul, P.-n. Modulation of P-glycoprotein expression and function by curcumin in multidrug-resistant human KB cells. Biochem. Pharmacol. 2002, 64, 573–582. [Google Scholar] [CrossRef]
- Hou, X.-L.; Takahashi, K.; Tanaka, K.; Tougou, K.; Qiu, F.; Komatsu, K.; Takahashi, K.; Azuma, J. Curcuma drugs and curcumin regulate the expression and function of P-gp in Caco-2 cells in completely opposite ways. Int. J. Pharm. 2008, 358, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Medsafe Safety Information. Early Warning System–Monitoring Communication. Beware Turmeric/ Curcumin Containing Products Can Interact with Warfarin. 2018. Available online: http://www.medsafe.govt.nz/safety/EWS/2018/Turmeric.asp (accessed on 17 June 2025).
- Liu, A.-C.; Zhao, L.-X.; Hongxiang, L. Curcumin Alters the Pharmacokinetics of Warfarin and Clopidogrel in Wistar Rats but Has No Effect on Anticoagulation or Antiplatelet Aggregation. Planta Medica 2013, 79, 971–977. [Google Scholar] [CrossRef]
- Wishart, D.S.; Knox, C.; Guo, A.C.; Shrivastava, S.; Hassanali, M.; Stothard, P.; Chang, Z.; Woolsey, J. DrugBank: A comprehensive resource for in silico drug discovery and exploration. Nucleic Acids Res. 2006, 34, D668–D672. [Google Scholar] [CrossRef]
- Marton, L.T.; Pescinini, E.S.L.M.; Camargo, M.E.C.; Barbalho, S.M.; Haber, J.; Sinatora, R.V.; Detregiachi, C.R.P.; Girio, R.J.S.; Buchaim, D.V.; Cincotto Dos Santos Bueno, P. The Effects of Curcumin on Diabetes Mellitus: A Systematic Review. Front. Endocrinol 2021, 12, 669448. [Google Scholar] [CrossRef]
- Neerati, P.; Devde, R.; Gangi, A.K. Evaluation of the effect of curcumin capsules on glyburide therapy in patients with type-2 diabetes mellitus. Phytother. Res. 2014, 28, 1796–1800. [Google Scholar] [CrossRef]
- Li, Y.; Revalde, J.; Paxton, J.W. The effects of dietary and herbal phytochemicals on drug transporters. Adv. Drug Del. Rev. 2017, 116, 45–62. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, K.; Bordia, A.; Verma, S. Curcumin, a major component of food spice turmeric (Curcuma longa) inhibits aggregation and alters eicosanoid metabolism in human blood platelets. Prostaglandins Leukot. Essent. Fat. Acids 1995, 52, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Tabanelli, R.; Brogi, S.; Calderone, V. Improving curcumin bioavailability: Current strategies and future perspectives. Pharmaceutics 2021, 13, 1715. [Google Scholar] [CrossRef] [PubMed]
- Shoba, G.; Joy, D.; Joseph, T.; Majeed, M.; Rajendran, R.; Srinivas, P.S. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Medica 1998, 64, 353–356. [Google Scholar] [CrossRef]
- Dubey, R.K.; Leeners, B.; Imthurn, B.; Merki-Feld, G.S.; Rosselli, M. Piperine Decreases Binding of Drugs to Human Plasma and Increases Uptake by Brain Microvascular Endothelial Cells. Phytother. Res. 2017, 31, 1868–1874. [Google Scholar] [CrossRef]
- Pan, Q.; Xie, L.; Zhu, H.; Zong, Z.; Wu, D.; Liu, R.; He, B.; Pu, Y. Curcumin-incorporated EGCG-based nano-antioxidants alleviate colon and kidney inflammation via antioxidant and anti-inflammatory therapy. Regen. Biomater. 2024, 11, rbae122. [Google Scholar] [CrossRef]
- Pratti, V.L.; Thomas, M.; Bhoite, R.; Satyavrat, V. Investigating bioavailability of curcumin and piperine combination in comparison to turmeric rhizomes: An in vitro study. J. Exp. Pharmacol. 2024, 16, 37–47. [Google Scholar] [CrossRef]
- Abebe, W. Review of herbal medications with the potential to cause bleeding: Dental implications, and risk prediction and prevention avenues. Epma. J. 2019, 10, 51–64. [Google Scholar] [CrossRef]
- Pan, J.; Yuan, Y.; Ailing, H.; Zhou, A.; Wu, Z.-Y. Antiplatelet aggregative activity of prodrug of Ginkgolide B. Chin. Pharmacol. Bull. 2012, 28, 1435–1438. [Google Scholar]
- Wang, L.; Xia, K.; Han, L.; Zhang, M.; Fan, J.; Song, L.; Liao, A.; Wang, W.; Guo, J. Local Administration of Ginkgolide B Using a Hyaluronan-Based Hydrogel Improves Wound Healing in Diabetic Mice. Front. Bioeng. Biotechnol. 2022, 10, 898231. [Google Scholar] [CrossRef]
- Nguyen, T.; Alzahrani, T. Ginkgo Biloba; StatPearls Publishing: Treasure Island, FL, USA, 2019. [Google Scholar] [PubMed]
- Toshniwal, S.S.; Avinash, P.; Loya, A.; Toshniwal, T.; Kumar, S.; Acharya, S. The Role of Statins in Managing Chronic Kidney Disease: A Comprehensive Review. Int. J. Nutr. Pharmacol. Neurol. Dis. 2025, 15, 10–17. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, F.; Chen, C.; Guo, Z.; Liu, J.; Yu, J.; Xu, Y.; Zhong, D.; Jiang, H. Impact of curcumin on the pharmacokinetics of rosuvastatin in rats and dogs based on the conjugated metabolites. Xenobiotica 2017, 47, 267–275. [Google Scholar] [CrossRef]
- Brutsaert, E.F. Diabetes Mellitus (DM), MSD Manual, MSD Manual Consumer Version. 2023. Available online: https://www.msdmanuals.com/home/hormonal-and-metabolic-disorders/diabetes-mellitus-dm-and-disorders-of-blood-sugar-metabolism/diabetes-mellitus-dm (accessed on 7 July 2025).
- Jha, R.; Lopez-Trevino, S.; Kankanamalage, H.R.; Jha, J.C. Diabetes and Renal Complications: An Overview on Pathophysiology, Biomarkers and Therapeutic Interventions. Biomedicines 2024, 12, 1098. [Google Scholar] [CrossRef]
- Choi, H.I.; Kim, D.Y.; Choi, S.J.; Shin, C.Y.; Hwang, S.T.; Kim, K.H.; Kwon, O. The effect of cilostazol, a phosphodiesterase 3 (PDE3) inhibitor, on human hair growth with the dual promoting mechanisms. J. Dermatol. Sci. 2018, 91, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Balinski, A.M.; Preuss, C.V. Cilostazol; StatPearls Publishing: Treasure Island, FL, USA, 2019. [Google Scholar]
- Azeez, T.B.; Lunghar, J. Antiinflammatory effects of turmeric (Curcuma longa) and ginger (Zingiber officinale). Inflamm. Nat. Prod. 2021, 83–102. [Google Scholar] [CrossRef]
- Ajanaku, C.O.; Ademosun, O.T.; Atohengbe, P.O.; Ajayi, S.O.; Obafemi, Y.D.; Owolabi, O.A.; Akinduti, P.A.; Ajanaku, K.O. Functional bioactive compounds in ginger, turmeric, and garlic. Front. Nutr. 2022, 9, 1012023. [Google Scholar] [CrossRef]
- Zhou, X.; Afzal, S.; Wohlmuth, H.; Münch, G.; Leach, D.; Low, M.; Li, C.G. Synergistic anti-inflammatory activity of ginger and turmeric extracts in inhibiting lipopolysaccharide and interferon-γ-induced proinflammatory mediators. Molecules 2022, 27, 3877. [Google Scholar] [CrossRef] [PubMed]
- Rostamkhani, H.; Veisi, P.; Niknafs, B.; Jafarabadi, M.A.; Ghoreishi, Z. The effect of zingiber officinale on prooxidant-antioxidant balance and glycemic control in diabetic patients with ESRD undergoing hemodialysis: A double-blind randomized control trial. BMC Complement. Med. Ther. 2023, 23, 52. [Google Scholar] [CrossRef] [PubMed]
- Nurtjahja-Tjendraputra, E.; Ammit, A.J.; Roufogalis, B.D.; Tran, V.H.; Duke, C.C. Effective anti-platelet and COX-1 enzyme inhibitors from pungent constituents of ginger. Thromb. Res. 2003, 111, 259–265. [Google Scholar] [CrossRef] [PubMed]
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Original articles in English | Other types of articles or languages |
| Curcumin in models of kidney disease | Different effects or models |
| Drug interactions | Only curcumin treatment |
| Humans or animals studies | Only in vitro studies |
| Curcumin Alone or in Combination | Disease Condition | Model | Mechanism of Action | Clinical Benefit | Ref. |
|---|---|---|---|---|---|
| In vitro and in vivo studies | |||||
| Curcumin & Ginkgolide B | Autosomal dominant polycystic kidney disease (ADPKD) | In vitro (MDCK) kidney cells In vivo (Pkd1 knockout mouse model) | ↓ EGFR/ERK1/2, JNK, PI3K/mTOR ↓ EGFR/ERK1/2, JNK, and p38 MAPK pathways | Reduces kidney cyst size, number, and kidney enlargement | [45] |
| Animal studies | |||||
| Curcumin & Rosuvastatin | CKD and related lipid disorders | Adenine-induced CKD in Wistar Rat | ↑ eGFR ↑ Serum albumin ↓ Triglycerides, ↓VLDL, LDL ↓ Cholesterol Improved atherogenic and coronary risk indexes | Renoprotective and lipid-lowering effects | [46] |
| Curcumin | Nephrotoxicity | Gentamicin-induced nephrotoxicity in Sprague Dawley rats | ↓ MDA ↑ SOD ↓ BUN, ↑ Creatinine clearance ↓ ER Stress markers (GRP78, CHOP, calpain-2, and caspase-12) ↓ Bax, cytochrome c, cleaved caspase-3 ↑ Bcl-2 | Exhibits protective effects by mitigating oxidative stress, ER stress, and apoptosis. | [47] |
| Nanocurcumin combined with insulin | Diabetic Nephropathy | Streptozotocin-induced nephropathy in in Sprague Dawley rats | ↓ BUN, creatinine, ↓ bilirubin, ALP. ↑ albumin, globulin ↓ blood glucose ↑ NLRP3, IL-1β, NF-κB, Caspase-3, and MAPK8 mRNA Deactivates P38 MAPK and P53. signaling pathways basement membrane thickening, tubular atrophy, and podocyte cytoskeletal impairment | Ameliorates hyperglycemia and mitigates inflammation and structural kidney damage | [48] |
| Curcumin | Nephrotoxicity | Cyclosporine A-induced toxicity in Wistar albino rats | ↓ BUN, creatinine ↓ MDA, IL-2 levels. ↑ SOD, CAT, GPx. ↓ tissue damage | Improves renal function, reduces oxidative stress, inflammation | [49] |
| Curcumin | Focal and Segmental Glomerulosclerosis | Adriamycin -induced FSGS in Wistar rats | ↓ Serum creatinine, ↓ BUN, triglycerides, ↓urinary protein ↓ TNF-α, MDA. ↑ SOD, GSH ↓ Segmental glomerulosclerosis | Anti-inflammatory and antioxidant properties reducing kidney damage and preserving renal function | [50] |
| Curcumin | Hepatorenal Toxicity | Arsenic-Induced toxicity in male albino rats | ↓ AST, ALT, ALP, bilirubin, urea, and creatinine levels. ↓ LDL, cholesterol, triglyceride levels, ↑ HDL ↓ MDA ↑ SOD, CAT, GPx, GR preserves tissue architecture. | Improves lipid imbalances, and reduces oxidative stress | [51] |
| Curcumin-Tagged Cilostazol | Diabetic nephropathy | Streptozotocin and nicotinamide-induced nephropathy in Wistar rats | ↓ blood glucose ↓ IL-6 ↓ serum creatinine ↓ BUN ↑ serum albumin levels. ↓ cholesterol, ↓ triglycerides ↓ LDL ↑ HDL | Improves glycemic control, renal function, reduces inflammation, and ameliorates lipid profiles | [52] |
| Curcumin | Nephrotoxicity | Arsenic trioxide -induced nephrotoxicity in ducks | ↓ total cholesterol, triglycerides, LDL ↑ HDL ↓ MDA ↑ SOD, CAT, GPx ↓ Bax/Bcl-2, caspase-3, LC3, Beclin-1 ↓ LC3-II/LC3- ↑ p62 ↓ kidney tissue damage | Reduces oxidative stress, apoptosis, and autophagy markers. Ameliorates dyslipidemia | [53] |
| Nanoparticle-encapsulated curcumin & epigallocatechin gallate | Diabetic nephropathy | Streptozotocin-induced diabetic nephropathy in mice | ↓ Serum creatinine, urea, proteinuria ↓ MDA ↑ SOD, CAT, GPx ↓ TNF-α, IL-6 ↓ Glomerular and tubular damage | Antioxidant, anti-inflammatory, and antifibrotic properties | [54] |
| Tetrahydrocurcumin, polyenylphosphatidylcholine & Losartan | Diabetic nephropathy and Uninephrectomy | Streptozotocin-induced diabetic nephropathy and uninephrectomy in Sprague–Dawley rats. | ↓ BP ↓ albuminuria creatinine clearance ↓ Protein kinase C-α ↓ KIM-1 ↓ Type I collagen ↓ fibrosis ↑ CuZnSOD | Improves blood pressure, reduced markers of kidney injury, and mitigated oxidative stress and fibrosis more effectively than losartan alone. | [55] |
| Ginger & Curcumin | Diabetic Nephropathy | Streptozotocin-induced diabetic nephropathy in Sprague Dawley rats | ↓ Serum creatinine ↓BUN ↓ blood glucose ↓ MDA ↓ IL-6, NF-κB ↑ CAT, SOD, GSH Histopathological Improvements: collagen deposition and glycogen accumulation | Antioxidant, anti-inflammatory, antihyperglycemic, and renal protective effect | [56] |
| Curcumin (traditional Chinese medicine) in combination with medications for preventing and/or treating diabetic nephropathy | Diabetic nephropathy | Rats | ↓ Blood sugar ↓ Serum creatinine ↓ Serum urea nitrogen | The curcumin-containing traditional Chinese medicine exhibits protective and therapeutic effects against diabetic nephropathy | [57] |
| Human studies | |||||
| Curcuminoids | Post-contrast acute kidney injury (PC-AKI) | CKD patients undergoing elective coronary angiography | ↑ eGFR | Reduces the incidence of PC-AKI and better preserve renal function | [58] |
| Curcuminoids | Contrast-Induced Acute Kidney Injury | CKD patients undergoing elective coronary angiography or percutaneous coronary intervention | No Significant effect | Curcuminoids were safe and well-tolerated | [59] |
| Nano-curcumin | Chemotherapy nephrotoxicity | Cisplatin-induced nephrotoxicity in cancer patients | No Significant effect | Nano-curcumin was well-tolerated, with no reported adverse effects | [60] |
| Turmeric & Piperine | End-Stage Renal Disease | Hemodialysis Patients | ↓ MDA ↓ CRP ↓ IL-6 ↑ Total antioxidant capacity | Reduces markers of oxidative stress and inflammation | [61] |
| Curcumin formulated in combination with taurine, docosahexaenoic acid (DHA), and essential vitamins including A, D3, E, K1, C, B1, B2, B6, B12, biotin, pantothenic acid, and folic acid | Diabetic nephropathy | Diabetic nephropathy patients | ↓ Dyslipidemia ↓ Hyperkalemia ↓ Anemia | This invention advances the optimization and personalization of therapeutic nutrition and demonstrates corrective effects on bone mineral disorders in patients | [62] |
| Curcuma, Astragalus membranaceus, Jinyingzi, Chuanxiong, and Bixie | Diabetic nephropathy | Diabetic nephropathy patients | ↓ Proteinuria | The pharmaceutical formulation alleviates clinical symptoms such as fatigue, weakness of the lumbar region and knees, poor appetite, dry mouth, edema, sore throat, and thick, greasy tongue coatings and demonstrates therapeutic efficacy in managing kidney deficiency syndrome | [63] |
| Combination | General Drug Class of Combination Drug | Observed Effects in Kidney Disease Models | Key Interaction Mechanisms/Clinical Implications | Ref. |
|---|---|---|---|---|
| Turmeric and Piperine | Herbal Supplement (Turmeric/Curcumin), Bioavailability Enhancer (Piperine/Alkaloid) | Piperine significantly enhances curcumin’s bioavailability | Piperine inhibits drug-metabolizing enzymes (CYP, P-gp), amplifying curcumin’s inherent drug interaction profile. Potentially increased systemic exposure to curcumin and other co-administered drugs | [61,87] |
| Nanoparticle-encapsulated Curcumin and Epigallocatechin Gallate (EGCG) | Polyphenol (Nano-antioxidant platform) | Enhanced bioavailability and synergistic anti-inflammatory/antioxidant effects. Mitigates acute kidney injury (AKI) in mouse models | EGCG as part of nanoparticle enhances delivery and contributes therapeutic effect. Reduces pro-inflammatory, increases anti-inflammatory cytokines | [54,86] |
| Tetrahydrocurcumin, Polyenylphosphatidylcholine and Losartan | Curcumin Metabolite, Lipid Carrier, Angiotensin II Receptor Blocker (ARB) | Add-on therapy in diabetic nephropathy rats: lowered blood pressure, enhanced antioxidant defenses, reduced kidney injury/fibrosis markers, improved renal function | Optimized delivery of THC with PPC. Renoprotective effects independent of glycemic status. Potential pharmacokinetic interaction with Losartan via CYP | [55] |
| Curcumin & Ginkgolide B | Terpene Lactone (PAFR antagonist, antiplatelet, antioxidant, anti-inflammatory) | Synergistic inhibition of cyst formation and enlargement in ADPKD models. | Pharmacodynamic synergy for ADPKD. Potential bleeding risk due to additive antiplatelet effects with other anticoagulants/antiplatelets | [45] |
| Curcumin & Rosuvastatin | HMG-CoA Reductase Inhibitor (statin) | Synergistic renal protection and antilipemic action in CKD rats | Pharmacokinetic enhancement: Curcumin allows for reduced statin dose to mitigate side effects while potentiate benefits | [46] |
| Nanocurcumin combined with Insulin | Antidiabetic hormone | Alleviates diabetic kidney disease (DKD) by inhibiting P38/P53 signaling axis, independent of glycemic control. Improved organ function and histology | Nanocurcumin formulation improves bioavailability. Targets inflammation and apoptosis pathways directly, complementing insulin’s glucose-lowering effects | [48] |
| Curcumin-Tagged Cilostazol | Phosphodiesterase-3 (PDE3) Inhibitor (antiplatelet, vasodilator) | Nanodispersion shows reno- and pancreas-protective effects in diabetic nephropathy rats (improved kidney/lipid profiles) | Advanced drug delivery for synergistic effects. Potential bleeding risk due to additive antiplatelet effects and CYP inhibition | [52] |
| Ginger and Curcumin | Herbal Supplements (Anti-inflammatory, Antioxidant, Antiplatelet, Hypoglycemic) | Combined anti-inflammatory and antioxidant effects; potential for kidney health | Potential additive antiplatelet effects increasing bleeding risk. Potential additive hypoglycemic effects increasing risk of hypoglycemia | [56,88] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ofori-Attah, E.; Aning, A.; Simón, L. Curcumin in the Treatment of Kidney Disease: A Systematic Review with a Focus on Drug Interactions. Antioxidants 2025, 14, 1369. https://doi.org/10.3390/antiox14111369
Ofori-Attah E, Aning A, Simón L. Curcumin in the Treatment of Kidney Disease: A Systematic Review with a Focus on Drug Interactions. Antioxidants. 2025; 14(11):1369. https://doi.org/10.3390/antiox14111369
Chicago/Turabian StyleOfori-Attah, Ebenezer, Abigail Aning, and Layla Simón. 2025. "Curcumin in the Treatment of Kidney Disease: A Systematic Review with a Focus on Drug Interactions" Antioxidants 14, no. 11: 1369. https://doi.org/10.3390/antiox14111369
APA StyleOfori-Attah, E., Aning, A., & Simón, L. (2025). Curcumin in the Treatment of Kidney Disease: A Systematic Review with a Focus on Drug Interactions. Antioxidants, 14(11), 1369. https://doi.org/10.3390/antiox14111369








