Enhancing Stallion Semen Cryopreservation: Selected Antioxidant Extracts and Sperm Freezability
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animals and Husbandry
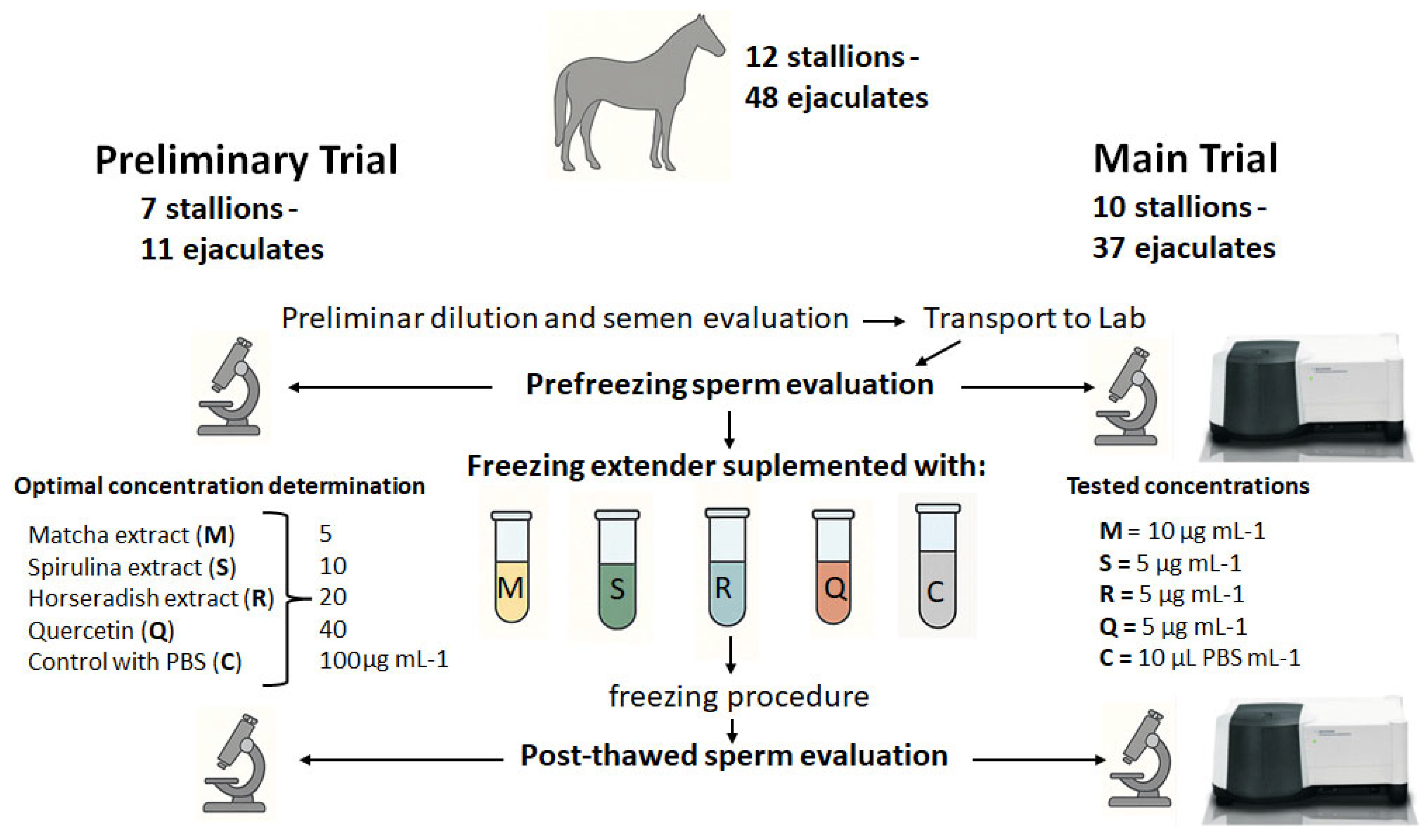
2.3. Experimental Design
2.4. Extracts of Natural Products
2.5. Phytochemical Analyses
2.6. Semen Collection and Transport
2.7. Sperm Concentration and Kinematic Evaluation
2.8. Mitochondrial Membrane Potential (MMP)
2.9. Lipid Peroxidation (LPO)
2.10. Intracellular Reactive Oxygen Species (ROS)
2.11. Intracellular Nitric Oxide (NO)
2.12. DNA Fragmentation Index (DFI)
2.13. Cryopreservation and Post-Thaw Analysis
2.14. Statistical Analysis
3. Results
3.1. Antioxidant Properties and Phytochemical Evaluation of the Extracts
3.2. Identification of the Optimal Supplement Dose in the Freezing Extender
3.3. Effect of Natural Extracts and Quercetin on Various Sperm Functional Parameters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Douglas Kinghorn, A. Pharmacognosy in the 21st century. J. Pharm. Pharmacol. 2001, 53, 135–148. [Google Scholar] [CrossRef]
- Angiolella, L.; Sacchetti, G.; Efferth, T. Antimicrobial and Antioxidant Activities of Natural Compounds. Evid. Based Complement. Alternat. Med. 2018, 2018, 1945179. [Google Scholar] [CrossRef]
- Ekiert, H.M.; Szopa, A. Biological Activities of Natural Products. Molecules 2020, 25, 5769. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zheng, P.; Yan, G.; Zhuo, Y.; Wu, J.L.; Sun, B. Chemical profiling and antioxidants screening from natural products: Using CiNingJi as an example. Food Sci. Biotechnol. 2022, 31, 407–421. [Google Scholar] [CrossRef] [PubMed]
- Miki, K. Energy metabolism and sperm function. Soc. Reprod. Fertil. Suppl. 2007, 65, 309–325. [Google Scholar]
- Wang, Y.; Fu, X.; Li, H. Mechanisms of oxidative stress-induced sperm dysfunction. Front. Endocrinol. 2025, 16, 1520835. [Google Scholar] [CrossRef]
- Catalán, J.; Yánez-Ortiz, I.; Tvarijonaviciute, A.; González-Aróstegui, L.G.; Rubio, C.P.; Barranco, I.; Yeste, M.; Miró, J. Seminal Plasma Antioxidants Are Related to Sperm Cryotolerance in the Horse. Antioxidants 2022, 11, 1279. [Google Scholar] [CrossRef]
- Del Prete, C.; Stout, T.; Montagnaro, S.; Pagnini, U.; Uccello, M.; Florio, P.; Ciani, F.; Tafuri, S.; Palumbo, V.; Pasolini, M.P.; et al. Combined addition of superoxide dismutase, catalase and glutathione peroxidase improves quality of cooled stored stallion semen. Anim. Reprod. Sci. 2019, 210, 106195. [Google Scholar] [CrossRef]
- Varela, E.; Rey, J.; Plaza, E.; Muñoz de propios, P.; Ortiz-Rodríguez, J.M.; Álvarez, M.; Anel-López, L.; Anel, L.; De Paz, P.; Gil, M.C.; et al. How does the microbial load affect the quality of equine cool-stored semen? Theriogenology 2018, 114, 212–220. [Google Scholar] [CrossRef]
- Fleming, S.D.; Thomson, L.K. The Oxidative Stress of Human Sperm Cryopreservation. Antioxidants 2025, 14, 402. [Google Scholar] [CrossRef] [PubMed]
- Aranda-Rivera, A.K.; Cruz-Gregorio, A.; Arancibia-Hernández, Y.L.; Hernández-Cruz, E.Y.; Pedraza-Chaverri, J. RONS and Oxidative Stress: An Overview of Basic Concepts. Oxygen 2022, 2, 437–478. [Google Scholar] [CrossRef]
- Bansal, A.K.; Bilaspuri, G.S. Impacts of oxidative stress and antioxidants on semen functions. Vet. Med. Int. 2011, 2011, 686137. [Google Scholar] [CrossRef]
- Andrabi, S.W.; Ara, A.; Saharan, A.; Jaffar, M.; Gugnani, N.; Esteves, S.C. Sperm DNA Fragmentation: Causes, evaluation and management in male infertility. JBRA Assist. Reprod. 2024, 28, 306–319. [Google Scholar] [CrossRef]
- Miller, D.; Brinkworth, M.; Iles, D. Paternal DNA packaging in spermatozoa: More than the sum of its parts? DNA, histones, protamines and epigenetics. Reproduction 2010, 139, 287–301. [Google Scholar] [CrossRef] [PubMed]
- Rashki Ghaleno, L.; Alizadeh, A.; Drevet, J.R.; Shahverdi, A.; Valojerdi, M.R. Oxidation of Sperm DNA and Male Infertility. Antioxidants 2021, 10, 97. [Google Scholar] [CrossRef]
- Grimsrud, P.A.; Xie, H.; Griffin, T.J.; Bernlohr, D.A. Oxidative stress and covalent modification of protein with bioactive aldehydes. J. Biol. Chem. 2008, 283, 21837–21841. [Google Scholar] [CrossRef]
- Ballou, J.D. Potential contribution of cryopreserved germ plasm to the preservation of genetic diversity and conservation of endangered species in captivity. Cryobiology 1992, 29, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Morrell, J.M.; Mayer, I. Reproduction biotechnologies in germplasm banking of livestock species: A review. Zygote 2017, 25, 545–557. [Google Scholar] [CrossRef] [PubMed]
- Castro, M.; Leal, K.; Pezo, F.; Contreras, M.J. Sperm Membrane: Molecular Implications and Strategies for Cryopreservation in Productive Species. Animals 2025, 15, 1808. [Google Scholar] [CrossRef]
- Larbi, A.; Li, C.; Quan, G. An updated review on the application of proteomics to explore sperm cryoinjury mechanisms in livestock animals. Anim. Reprod. Sci. 2024, 263, 107441. [Google Scholar] [CrossRef]
- Len, J.S.; Koh, W.S.D.; Tan, S.X. The roles of reactive oxygen species and antioxidants in cryopreservation. Biosci. Rep. 2019, 39, BSR20191601. [Google Scholar] [CrossRef]
- Holt, W.V. Fundamental aspects of sperm cryobiology: The importance of species and individual differences. Theriogenology 2000, 53, 47–58. [Google Scholar] [CrossRef]
- Beatty, R.A.; Stewart, D.L.; Spooner, R.L.; Hancock, J.L. Evaluation by the heterospermic insemination technique of the differential effect of freezing at—196 degrees C on fertility of individual bull semen. J. Reprod. Fertil. 1976, 47, 377–379. [Google Scholar] [CrossRef] [PubMed]
- Loomis, P.R.; Graham, J.K. Commercial semen freezing: Individual male variation in cryosurvival and the response of stallion sperm to customized freezing protocols. Anim. Reprod. Sci. 2008, 105, 119–128. [Google Scholar] [CrossRef]
- Martins, R.V.L.; Silva, A.M.S.; Duarte, A.P.; Socorro, S.; Correia, S.; Maia, C.J. Natural products as protective agents for male fertility. BioChem 2021, 1, 122–147. [Google Scholar] [CrossRef]
- Bazzano, M.; Laus, F.; Spaterna, A.; Marchegiani, A. Use of nutraceuticals in the stallion: Effects on semen quality and preservation. Reprod. Domest. Anim. 2021, 56, 951–957. [Google Scholar] [CrossRef]
- Abdelnour, S.A.; Sindi, R.A.; Abd El-Hack, M.E.; Khalifa, N.E.; Khafaga, A.F.; Noreldin, A.E.; Samir, H.; Tufarelli, V.; Losacco, C.; Gamal, M.; et al. Quercetin: Putative effects on the function of cryopreserved sperms in domestic animals. Reprod. Domest. Anim. 2023, 58, 191–206. [Google Scholar] [CrossRef] [PubMed]
- Hodgson, J.M.; Croft, K.D. Tea flavonoids and cardiovascular health. Mol. Asp. Med. 2010, 31, 495–502. [Google Scholar] [CrossRef]
- Gale, I.; Gil, L.; Malo, C.; González, N.; Martínez, F. Effect of Camellia sinensis supplementation and increasing holding time on quality of cryopreserved boar semen. Andrologia 2015, 47, 505–512. [Google Scholar] [CrossRef]
- Mehdipour, M.; Daghigh Kia, H.; Najafi, A.; Vaseghi Dodaran, H.; García-Álvarez, O. Effect of green tea (Camellia sinensis) extract and pre-freezing equilibration time on the post-thawing quality of ram semen cryopreserved in a soybean lecithin-based extender. Cryobiology 2016, 73, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.; Khan, M.; Qureshi, M.S.; Shakoor, A.; Gohar, A.; Ullah, H.; Hussain, A.; Khatri, P.; Shah, S.S.A.; Rehman, H. Effect of green tea extract (Camellia sinensis) on fertility indicators of post-thawed bull spermatozoa. Pak. J. Zool. 2017, 49, 1243–1249. [Google Scholar] [CrossRef]
- Setumo, M.A.; Choma, S.S.; Henkel, R.; Opuwari, C.S. Green tea (Camellia sinensis) aqueous extract improved human spermatozoa functions in vitro. J. Med. Plant. Econ. Dev. 2022, 6, 166. [Google Scholar] [CrossRef]
- Abd El-Baky, H.H.; El Baz, F.; El-Baroty, G.S. Enhancement of antioxidant production in Spirulina platensis under oxidative stress. Acta Physiol. Plant. 2009, 31, 623–631. [Google Scholar] [CrossRef]
- Spínola, M.P.; Mendes, A.R.; Prates, J.A.M. Chemical Composition, Bioactivities, and Applications of Spirulina (Limnospira platensis) in Food, Feed, and Medicine. Foods 2024, 13, 3656. [Google Scholar] [CrossRef]
- Badr, M.; Rawash, Z.; Azab, A.; Dohreg, R.; Ghattas, T.; Fathi, M. Spirulina platensis extract addition to semen extender enhances cryotolerance and fertilizing potentials of buffalo bull spermatozoa. Anim. Reprod. 2021, 18, e20200520. [Google Scholar] [CrossRef]
- Zeitoun, M.M.; Ateah, M.A.; Almaiman, A.T.; Mansour, M.M. Spirulina Supplementation to the Semen Extender Influences the Quality and Antioxidant Parameters of Chilled or Cryopreserved Arabian Stallion Spermatozoa. J. Equine Vet. Sci. 2022, 118, 104108. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.M.; Gonda, S.; Vasas, G. A review on the phytochemical composition and potential medicinal uses of horseradish (Armoracia rusticana) root. Food Rev. Int. 2013, 29, 261–275. [Google Scholar] [CrossRef]
- Agneta, R.; Möllers, C.; Rivelli, A.R. Horseradish (Armoracia rusticana), a neglected medical and condiment species with a relevant glucosinolate profile: A review. Genet. Resour. Crop Evol. 2013, 60, 1923–1943. [Google Scholar] [CrossRef]
- Atawodi, S.E.; Atawodi, J.C.; Idakwo, G.A.; Pfundstein, B.; Haubner, R.; Wurtele, G.; Bartsch, H.; Owen, R.W. Evaluation of the polyphenol content and antioxidant properties of methanol extracts of the leaves, stem, and root barks of Moringa oleifera Lam. J. Med. Food 2010, 13, 710–716. [Google Scholar] [CrossRef]
- Formica, J.V.; Regelson, W. Review of the biology of Quercetin and related bioflavonoids. Food Chem. Toxicol. 1995, 33, 1061–1080. [Google Scholar] [CrossRef]
- Afanas’Ev, I.B.; Dcrozhko, A.I.; Brodskii, A.V.; Kostyuk, V.A.; Potapovitch, A.I. Chelating and free radical scavenging mechanisms of inhibitory action of rutin and quercetin in lipid peroxidation. Biochem. Pharmacol. 1989, 38, 1763–1769. [Google Scholar] [CrossRef] [PubMed]
- Nass-Arden, L.; Breitbart, H. Modulation of mammalian sperm motility by quercetin. Mol. Reprod. Dev. 1990, 25, 369–373. [Google Scholar] [CrossRef]
- Ranawat, P.; Pathak, C.M.; Khanduja, K.L. A new perspective on the quercetin paradox in male reproductive dysfunction. Phytother. Res. 2013, 27, 802–810. [Google Scholar] [CrossRef] [PubMed]
- Sayuti, N.; Kamarudin, A.; Saad, N.; Ab Razak, N.; Esa, N.M. Optimized green extraction conditions of matcha green tea (Camellia sinensis) using central composite design for maximal polyphenol and antioxidant contents. BioResources 2021, 16, 3255. [Google Scholar] [CrossRef]
- Tavakoli, S.; Hong, H.; Wang, K.; Yang, Q.; Gahruie, H.H.; Zhuang, S.; Li, Y.; Liang, Y.; Tan, Y.; Luo, Y. Ultrasonic-assisted food-grade solvent extraction of high-value added compounds from microalgae Spirulina platensis and evaluation of their antioxidant and antibacterial properties. Algal Res. 2021, 60, 102493. [Google Scholar] [CrossRef]
- Calabrone, L.; Larocca, M.; Marzocco, S.; Martelli, G.B.G.; Rossano, R. Total phenols and flavonoids content, antioxidant capacity and lipase inhibition of root and leaf horseradish (Armoracia rusticana) extracts. Food Nutr. Sci. 2015, 6, 64–74. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Oyaizu, M. Studies on products of browning reaction antioxidative activities of products of browning reaction prepared from glucosamine. Jpn. J. Nutr. Diet. 1986, 44, 307–315. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- 14502-1:2005; Determination of Substances Characteristic of Green and Black Tea; Part 1: Content of Total Polyphenols in Tea; Colorimetric Method Using Folin–Ciocalteu Reagent. International Organization for Standardization [ISO]: Geneva, Switzerland, 2005.
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Boni, R.; Ruggiero, R.; Di Palma, T.; Ferrara, M.A.; Preziosi, G.; Cecchini Gualandi, S. Stallion Sperm Freezing with Different Extenders: Role of Antioxidant Activity and Nitric Oxide Production. Animals 2024, 14, 2465. [Google Scholar] [CrossRef]
- Boni, R.; Ruggiero, R.; De Luca, F.; Serritella, M.L.; Di Palma, T.; Cecchini Gualandi, S. Repeatability of Selected Parameters Related to Stallion Sperm Quality and Cryotolerance. Animals 2025, 15, 2805. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Iovine, C.; Agarwal, A.; Henkel, R. TUNEL assay-Standardized method for testing sperm DNA fragmentation. Andrologia 2021, 53, e13738. [Google Scholar] [CrossRef]
- Evenson, D.P. Sperm Chromatin Structure Assay (SCSA®) for Fertility Assessment. Curr. Protoc. 2022, 2, e508. [Google Scholar] [CrossRef]
- Love, C.C.; Kenney, R.M. The relationship of increased susceptibility of sperm DNA to denaturation and fertility in the stallion. Theriogenology 1998, 50, 955–972. [Google Scholar] [CrossRef]
- Peris, S.I.; Bilodeau, J.F.; Dufour, M.; Bailey, J.L. Impact of cryopreservation and reactive oxygen species on DNA integrity, lipid peroxidation, and functional parameters in ram sperm. Mol. Reprod. Dev. 2007, 74, 878–892. [Google Scholar] [CrossRef]
- Aurich, J.; Kuhl, J.; Tichy, A.; Aurich, C. Efficiency of Semen Cryopreservation in Stallions. Animals 2020, 10, 1033. [Google Scholar] [CrossRef]
- Bang, S.; Tanga, B.M.; Fang, X.; Seong, G.; Saadeldin, I.M.; Qamar, A.Y.; Lee, S.; Kim, K.J.; Park, Y.J.; Nabeel, A.H.T.; et al. Cryopreservation of Pig Semen Using a Quercetin-Supplemented Freezing Extender. Life 2022, 12, 1155. [Google Scholar] [CrossRef]
- Moichela, F.T.; Adefolaju, G.A.; Henkel, R.R.; Opuwari, C.S. Aqueous leaf extract of Moringa oleifera reduced intracellular ROS production, DNA fragmentation and acrosome reaction in Human spermatozoa in vitro. Andrologia 2021, 53, e13903. [Google Scholar] [CrossRef] [PubMed]
- Al-Essawe, E.M.; Wallgren, M.; Wulf, M.; Aurich, C.; Macías-García, B.; Sjunnesson, Y.; Morrell, J.M. Seminal plasma influences the fertilizing potential of cryopreserved stallion sperm. Theriogenology 2018, 115, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Neuhauser, S.; Gösele, P.; Handler, J. Postthaw Addition of Autologous Seminal Plasma Improves Sperm Motion Characteristics in Fair and Poor Freezer Stallions. J. Equine Vet. Sci. 2019, 72, 117–123. [Google Scholar] [CrossRef]
- Stephens, T.D.; Brooks, R.M.; Carrington, J.L.; Cheng, L.; Carrington, A.C.; Porr, C.A.; Splan, R.K. Effects of pentoxifylline, caffeine, and taurine on post-thaw motility and longevity of equine frozen semen. J. Equine Vet. Sci. 2013, 33, 615–621. [Google Scholar] [CrossRef]
- Rossi, M.; Gonzalez-Castro, R.; Falomo, M.E. Effect of Caffeine and Pentoxifylline Added Before or After Cooling on Sperm Characteristics of Stallion Sperm. J. Equine Vet. Sci. 2020, 87, 102902. [Google Scholar] [CrossRef]
- Salem, O.; Szwajkowska-Michałek, L.; Przybylska-Balcerek, A.; Szablewski, T.; Cegielska-Radziejewska, R.; Świerk, D.; Stuper-Szablewska, K. New Insights into Bioactive Compounds of Wild-Growing Medicinal Plants. Appl. Sci. 2023, 13, 13196. [Google Scholar] [CrossRef]
- Carrera-Chávez, J.M.; Jiménez-Aguilar, E.E.; Acosta-Pérez, T.P.; Núñez-Gastélum, J.A.; Quezada-Casasola, A.; Escárcega-Ávila, A.M.; Itza-Ortiz, M.F.; Orozco-Lucero, E. Effect of Moringa oleifera seed extract on antioxidant activity and sperm characteristics in cryopreserved ram semen. J. Appl. Anim. Res. 2020, 48, 114–120. [Google Scholar] [CrossRef]
- Seifi-Jamadi, A.; Kohram, H.; Shahneh, A.Z.; Ansari, M.; Macías-García, B. Quercetin ameliorate motility in frozen-thawed turkmen stallions sperm. J. Equine Vet. Sci. 2016, 45, 73–77. [Google Scholar] [CrossRef]
- Gibb, Z.; Butler, T.J.; Morris, L.H.; Maxwell, W.M.; Grupen, C.G. Quercetin improves the postthaw characteristics of cryopreserved sex-sorted and nonsorted stallion sperm. Theriogenology 2013, 79, 1001–1009. [Google Scholar] [CrossRef] [PubMed]
- Squeff Filho, J.; Corcini, C.D.; dos Santos, F.C.C.; Nobre, A.; Anciuti, N.L.S.G.; Anastácio, E.; Mielke, R.; Wayne, C.E.; Nogueira, B.d.R.C.; Junior, A.S.V. Quercetin in equine frozen semen. Cryo Lett. 2017, 38, 299–304. [Google Scholar]
- Zribi, N.; Chakroun, N.F.; Ben Abdallah, F.; Elleuch, H.; Sellami, A.; Gargouri, J.; Rebai, T.; Fakhfakh, F.; Keskes, L.A. Effect of freezing-thawing process and quercetin on human sperm survival and DNA integrity. Cryobiology 2012, 65, 326–331. [Google Scholar] [CrossRef]
- Behdarvandian, P.; Nasr-Esfahani, A.; Tavalaee, M.; Pashaei, K.; Naderi, N.; Darmishonnejad, Z.; Hallak, J.; Aitken, R.J.; Gharagozloo, P.; Drevet, J.R.; et al. Sperm chromatin structure assay (SCSA(®)) and flow cytometry-assisted TUNEL assay provide a concordant assessment of sperm DNA fragmentation as a function of age in a large cohort of approximately 10,000 patients. Basic. Clin. Androl. 2023, 33, 33. [Google Scholar] [CrossRef]
- López-Fernández, C.; Crespo, F.; Arroyo, F.; Fernández, J.L.; Arana, P.; Johnston, S.D.; Gosálvez, J. Dynamics of sperm DNA fragmentation in domestic animals II. The stallion. Theriogenology 2007, 68, 1240–1250. [Google Scholar] [CrossRef] [PubMed]
- Dowsett, K.F.; Knott, L.M. The influence of age and breed on stallion semen. Theriogenology 1996, 46, 397–412. [Google Scholar] [CrossRef] [PubMed]


| Matcha | Spirulina | Horseradish | Quercetin | Ascorbic Acid | |
|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | |
| TAC | 2186.7 ± 102.5 A | 117.6 ± 17.4 B | 139.8 ± 15.4 B | 877.8 ± 39.4 C | 1492.8 ± 90.6 D |
| TRP | 334.0 ± 37.7 A | 43.6 ± 6.9 B | 57.3 ± 8.1 B | 772.4 ± 61.4 C | - |
| FSRA | 45.7 ± 4.1 A | 8.6 ± 0.8 B | 10.5 ± 1.1 B | 50.9 ± 6.6 A | 48.6 ± 6.7 A |
| TPC | 192.7 ± 15.0 A | 24.3 ± 2.7 B | 19.6 ± 3.0 B | 505.5 ± 44.8 C | - |
| TFC | 282.9 ± 16.9 A | 46.0 ± 7.3 Ba | 14.9 ± 2.3 Bb | - | - |
| Mean ± SD | 75% Confidence Interval | Inter-Stallion Variability (p=) | Age Variability (p=) | ||
|---|---|---|---|---|---|
| Gel-free volume | mL | 48.9 ± 26.5 | 43.7 ÷ 54.0 | 0.231 | 0.158 |
| Sperm concentration | ×106 | 263 ± 172 | 269 ÷ 331 | 0.213 | 0.960 |
| Spermatozoa per ejaculate | ×109 | 13.6 ± 10.7 | 11.5 ÷ 15.7 | 0.343 | 0.587 |
| TM | % | 85.3 ± 11.0 | 83.1 ÷ 87.4 | 0.001 *** | 0.575 |
| PM | % | 34.9 ± 10.7 | 32.8 ÷ 37.0 | 0.200 | 0.706 |
| VCL | µm s−1 | 90.4 ± 17.1 | 87.1 ÷ 93.7 | 0.031 * | 0.950 |
| VSL | µm s−1 | 35.9 ± 7.3 | 34.5 ÷ 37.3 | 0.646 | 0.822 |
| VAP | µm s−1 | 48.7 ± 9.5 | 46.9 ÷ 50.6 | 0.412 | 0.891 |
| MMP | J0B/J0A | 13.6 ± 9.0 | 12.2 ÷ 15.0 | 0.288 | 0.945 |
| LPO | C0A/(C0A + C0B) | 11.8 ± 4.0 | 11.2 ÷ 12.5 | 0.012 ** | 0.009 ** |
| ROS content | a.u. | 2.6 ± 1.0 | 2.4 ÷ 2.8 | 0.005 ** | 0.359 |
| NO content | a.u. | 2.5 ± 0.8 | 2.3 ÷ 2.6 | 0.287 | 0.471 |
| C0 | Matcha | Spirulina | Horseradish | Quercetin | ||
|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | ||
| TM | % | 47.0 ± 17.8 | 48.6 ± 18.8 | 47.6 ± 18.7 | 44.1 ± 18.2 | 43.3 ± 15.8 |
| PM | % | 13.1 ± 11.4 | 14.3 ± 11.9 | 16.3 ± 13.4 | 12.1 ± 10.7 | 12.6 ± 10.1 |
| VCL | µm s−1 | 35.0 ± 16.2 | 36.2 ± 14.2 | 38.7 ± 15.3 | 36.0 ± 13.3 | 36.0 ± 13.7 |
| VSL | µm s−1 | 16.4 ± 8.1 | 16.7 ± 7.7 | 18.5 ± 8.5 | 16.6 ± 7.1 | 16.7 ± 6.9 |
| VAP | µm s−1 | 20.2 ± 8.9 | 20.7 ± 8.2 | 22.4 ± 9.0 | 20.5 ± 7.7 | 20.5 ± 7.5 |
| MMP | J0B/J0A | 7.6 ± 4.9 | 9.1 ± 5.7 | 9.8 ± 8.6 a | 9.3 ± 8.6 | 6.4 ± 4.8 b |
| LPO | C0A/(C0A + C0B) | 13.7 ± 5.8 | 14.4 ± 6.5 | 14.8 ± 7.8 | 15.8 ± 11.8 | 13.6 ± 6.6 |
| ROS content | a.u. | 2.1 ± 1.6 | 3.1 ± 4.0 | 2.6 ± 2.1 | 3.1 ± 6.3 | 2.4 ± 1.9 |
| NO content | a.u | 1.9 ± 1.0 | 2.5 ± 2.1 | 2.1 ± 1.3 | 2.2 ± 1.4 | 2.0 ± 1.0 |
| Effect of | |||||||
|---|---|---|---|---|---|---|---|
| ≤9 Years Old | >9 Years Old | Age | Treatment | Stallion | Age × Treat | ||
| Mean ± SD | Mean ± SD | p= | p= | p= | p= | ||
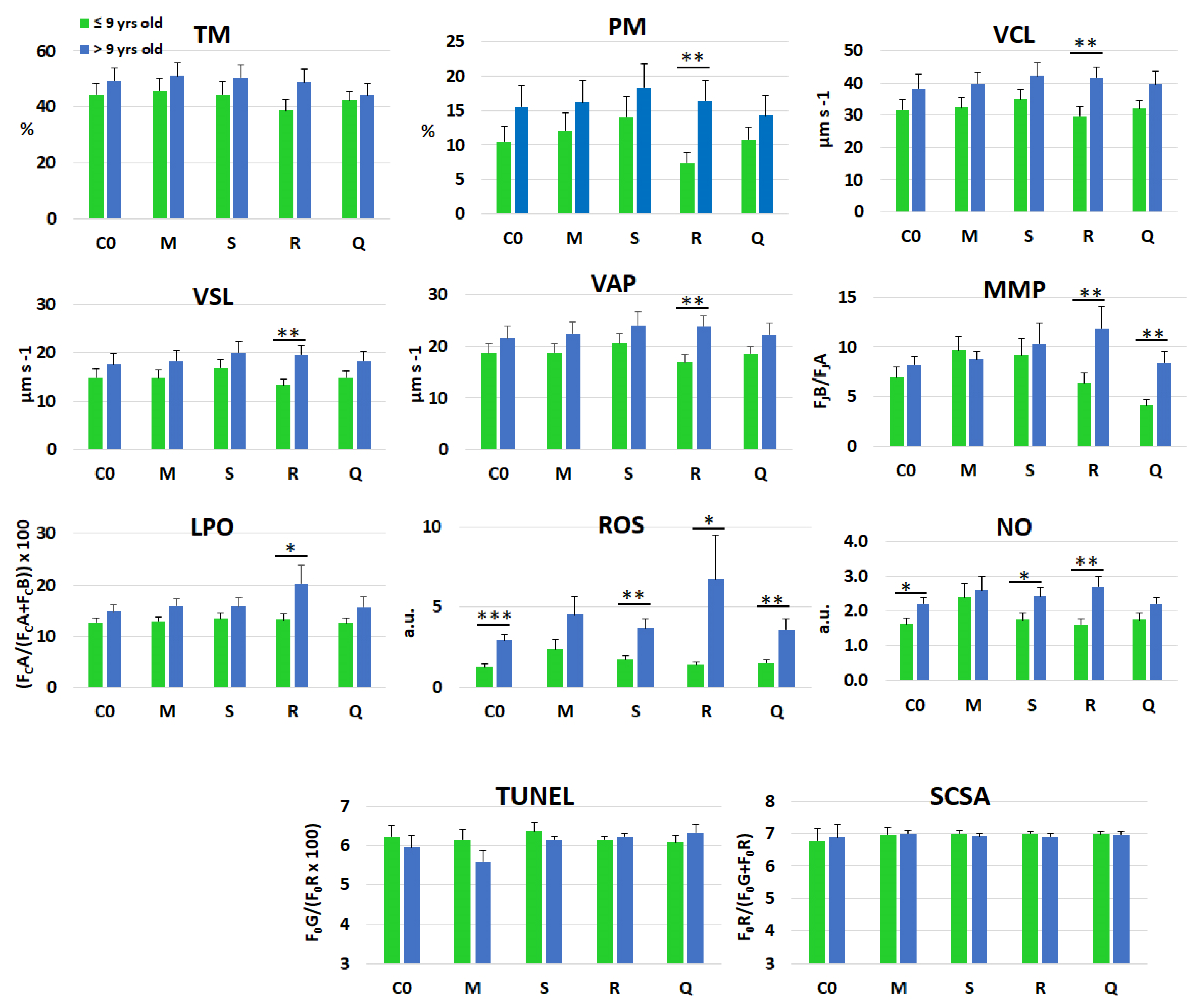
| TM | % | 43.1 ± 16.9 | 48.9 ± 18.6 | 0.030 * | 0.623 | 0.001 *** | 0.892 |
| PM | % | 11.0 ± 9.5 | 16.1 ± 12.9 | 0.002 ** | 0.436 | 0.001 *** | 0.801 |
| VCL | µm s−1 | 32.1 ± 12.2 | 40.3 ± 15.7 | 0.001 *** | 0.795 | 0.001 *** | 0.898 |
| VSL | µm s−1 | 15.0 ± 6.4 | 18.8 ± 8.5 | 0.001 *** | 0.681 | 0.001 *** | 0.832 |
| VAP | µm s−1 | 18.7 ± 6.9 | 22.8 ± 9.1 | 0.001 *** | 0.711 | 0.001 *** | 0.797 |
| MMP | J0B/J0A | 7.3 ± 6.5 | 9.5 ± 8.1 | 0.007 ** | 0.035 * | 0.001 *** | 0.081 |
| LPO | C0A/(C0A + C0B) | 13.0 ± 5.1 | 16.5 ± 11.7 | 0.001 *** | 0.385 | 0.001 *** | 0.543 |
| ROS content | a.u. | 1.7 ± 1.8 | 4.3 ± 7.3 | 0.001 *** | 0.272 | 0.027 * | 0.303 |
| NO content | a.u. | 1.8 ± 1.3 | 2.4 ± 1.5 | 0.001 *** | 0.159 | 0.240 | 0.507 |
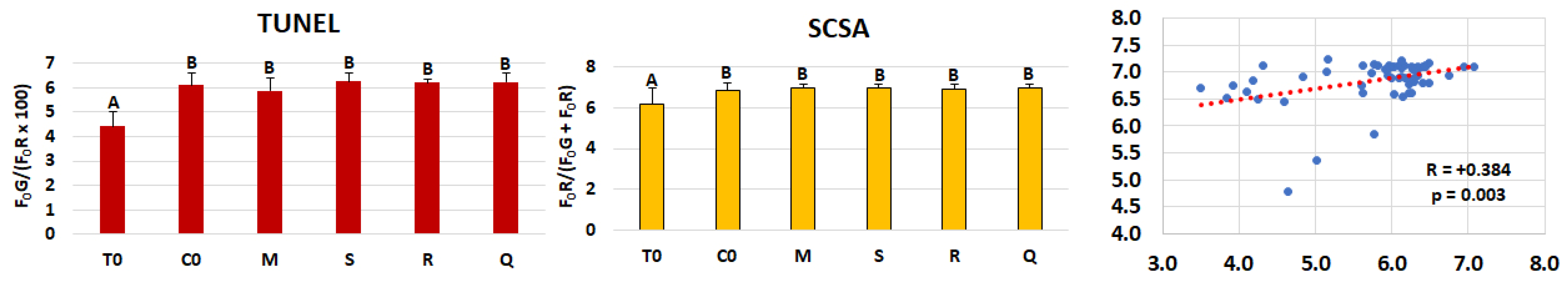
| DFI TUNEL | F0G/(F0R × 100) | 5.13 ± 0.81 | 6.52 ± 0.84 | 0.084 | 0.593 | 0.947 | 0.185 |
| DFI SCSA | (F0R/F0G + F0R) | 6.87 ± 0.49 | 7.00 ± 0.58 | 0.695 | 0.768 | 0.001 *** | 0.703 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boni, R.; Ruggiero, R.; De Luca, F.; Preziosi, G.; Ferrara, M.A.; Ostuni, A.; Guerriero, S.; Gallo, A.; Murano, C.; Cecchini Gualandi, S. Enhancing Stallion Semen Cryopreservation: Selected Antioxidant Extracts and Sperm Freezability. Antioxidants 2025, 14, 1363. https://doi.org/10.3390/antiox14111363
Boni R, Ruggiero R, De Luca F, Preziosi G, Ferrara MA, Ostuni A, Guerriero S, Gallo A, Murano C, Cecchini Gualandi S. Enhancing Stallion Semen Cryopreservation: Selected Antioxidant Extracts and Sperm Freezability. Antioxidants. 2025; 14(11):1363. https://doi.org/10.3390/antiox14111363
Chicago/Turabian StyleBoni, Raffaele, Raffaella Ruggiero, Felisia De Luca, Graziano Preziosi, Maria Antonietta Ferrara, Angela Ostuni, Simone Guerriero, Alessandra Gallo, Carola Murano, and Stefano Cecchini Gualandi. 2025. "Enhancing Stallion Semen Cryopreservation: Selected Antioxidant Extracts and Sperm Freezability" Antioxidants 14, no. 11: 1363. https://doi.org/10.3390/antiox14111363
APA StyleBoni, R., Ruggiero, R., De Luca, F., Preziosi, G., Ferrara, M. A., Ostuni, A., Guerriero, S., Gallo, A., Murano, C., & Cecchini Gualandi, S. (2025). Enhancing Stallion Semen Cryopreservation: Selected Antioxidant Extracts and Sperm Freezability. Antioxidants, 14(11), 1363. https://doi.org/10.3390/antiox14111363










