1. Introduction
Gleichenella pectinata (Willd.) Ching (‘Star fern’) is a species of fern belonging to the
Polypodiopsida class,
Gleicheniales order,
Gleicheniaceae family and
Gleichenella genus. It is a monotypic taxon. It has various botanical synonyms, including
Gleichenia brasiliana,
Gleichenia hermannii, Mertensia hookeri, Mertensia emarginata and
Dicranopteris pectinata. Members of the order
Gleicheniales are mainly found in tropical regions and comprise between 130 and 150 species, which are grouped into three to five genera [
1,
2,
3].
G. pectinata thrives in nutrient-poor soils, particularly in tropical regions, where it forms extensive colonies along roads and in other disturbed areas. Its ability to colonise new spaces efficiently is associated with the presence of creeping rhizomes and the indeterminate growth of its fronds [
4].
From a phytochemical perspective, research on
G. pectinata is limited; however, preliminary analyses have identified phenolic compounds, particularly glycosylated flavonols such as kaempferol-3-rhamnosylglucose, quercetin-3-glucose and kaempferol-3-glucose [
5]. These compounds belong to the flavonoid group, which is widely recognised for its antioxidant activity and pharmacological potential [
6].
Interest in this species increases when you consider that other members of the
Gleicheniaceae family, such as
G. linearis and
G. truncata, have a diverse phytochemical profile and are used in traditional medicine. For example,
G. linearis contains alkaloids, phenols, flavonoids, tannins, triterpenoids and steroids—compounds commonly associated with therapeutic effects [
7]. In traditional medicine,
G. linearis is used in powder form, as an aqueous extract, or as a hot juice to treat fertility problems, sore throats, coughs, asthma, bacterial infections, intestinal parasites, wounds, ulcers, and fever [
8]. Furthermore,
G. truncata has demonstrated anti-inflammatory and antimalarial properties by inhibiting glycogen synthase kinase-3, which is a key regulator of inflammatory responses [
9].
Beyond these species, ferns are used in traditional medicine to treat ailments such as fever, wounds, infections, pain, rheumatism, respiratory diseases, and gastrointestinal problems, in preparations including infusions, decoctions, lotions, and poultices. From a scientific perspective, the flavonoid, polyphenol, polyunsaturated fatty acid, carotenoid, terpenoid and steroid content of some ferns has been characterised, and these compounds are associated with antioxidant, anti-inflammatory and antimicrobial activities [
8].
Conversely, bioactive plant compounds are secondary metabolites that perform adaptive functions and have beneficial effects on human health. These include phenols, terpenoids, thiols and dietary fibres [
10,
11]. They act by regulating cellular redox balance, modulating inflammatory pathways, and interfering with gene expression through epigenetic mechanisms. Such properties have been linked to the prevention of chronic diseases such as cancer, cardiovascular disease and metabolic disorders [
12].
Several flavonoids, such as quercetin and epigallocatechin gallate, are among the compounds most studied for their biological activity. These compounds have demonstrated the ability to inhibit bacterial toxins, such as haemolysins, including the thermolabile haemolysin of
Vibrio parahaemolyticus. This reduces their haemolytic activity [
13]. Similarly, various phenolic acids, such as gallic acid and cinnamic acid, have been shown to possess antibacterial properties [
14]. In particular, quercetin is effective against
Staphylococcus aureus [
15], while rutin and catechin have been effective against
Escherichia coli and
Pseudomonas aeruginosa [
16]. Kaempferol, myricetin and quercetin have also been studied for their potential anti-tumor properties, particularly in breast and gynaecological cancers [
17].
In this context, given the reported presence of flavonoids in the genus Gleichenella and the observed therapeutic potential of related species, this study aimed to evaluate the bioactive compounds and antioxidant, antimicrobial, antitumor and anti-inflammatory activities of Gleichenella pectinata leaves. These fern species have received little scientific attention despite their promising potential, so the results will serve as a starting point for future research.
2. Materials and Methods
2.1. Plant Material and Physico-Chemical Analysis
The study collected ‘
Star fern’ (
Gleichenella pectinata) leaves in the Amazon region of the Ecuador province of Pastaza (1°44′21″ S, 77°29′1″ W) in March (
Figure 1). For botanical species identification, the plant was analysed at the herbarium at the Salesian Polytechnic University in Quito, Ecuador. Fresh plant material was stored between layers of paper and cardboard to preserve structural integrity. The leaves were collected randomly from different plants in the same area. The sample was divided into two parts; thus, the first part, when fresh, was used for physical and chemical characterisation, while the second part was kept in sterile conical centrifuge tubes and maintained at −80 °C until lyophilisation. Freezy-drying was carried out using a Christ Alpha 1–4 LDplus system (GmbH, Osterode am Harz, Germany). Once dried, the samples were ground into a homogeneous fine freeze-dried ‘Star fern’, transferred into amber glass containers under a nitrogen atmosphere, and stored at −20 °C for subsequent analysis.
Physicochemical characterisation involved the determination of various parameters, including pH, moisture and ash content, soluble solids content, and titratable acidity. The pH was measured using SevenMultiS47 (Mettler Toledo, Columbus, OH, USA). Soluble solids were assessed with a Hitech RHB-32 refractometer (G-Won Hitech Co., Ltd., Seoul, Republic of Korea). Titratable acidity was determined by acid-base titration with 0.1 M NaOH. Moisture content was calculated by oven drying at 100 °C using a Memmert Be 20 device (Memmert GmbH + Co.KG, Barcelona, Spain) until reaching constant weight. In contrast, ash content was obtained after calcination in a muffle (Thermo Fisher Scientific, Waltham, MA, USA) at 550 °C [
18].
2.2. Mineral Quantification
For the extraction of macro (potassium, calcium, sodium, and magnesium) and micro (iron) mineral content, 40 mg of freeze-dried ‘Star fern’ was transferred into vessels of a Speedwave Xpert microwave digestion system (Berghof products + Instruments GmbH, Eningen unter Achalm, Germany) [
18]. Subsequently, 5 mL of concentrated nitric acid (65%) was added to each vessel. The mixtures were allowed to stand for 10 min to ensure initial acid-sample interaction before sealing the vessel. The mixtures were allowed to stand for 10 min to ensure initial acid-sample interaction before sealing the vessels. Microwave-assisted digestion was carried out using a three-step temperature and pressure program. First, a linear ramp to 140 °C under 30 bars at 70% power for 5 min; followed by heating to 200 °C under 35 bars at 80% power for 15 min; and finally, cooling to 50 °C under 25 bars with no power input over 10 min. Upon completion, the digestion vessels were cooled to ambient temperature for 20 min. Each digested sample was diluted to a final volume of 25 mL with ultrapure water (Mili-Q) and stored in amber glass bottles until instrument analysis.
Mineral analysis was conducted using an atomic absorption spectrophotometer Varian SpectrAA-55 instrument (Agilent Technologies, Santa Clara, CA, USA) [
18]. Calcium was measured at 422.7 nm with a 0.5 nm slit width using an acetylene-nitrous oxide flame. At the same time, iron, sodium, potassium, and magnesium were analysed with an air-acetylene flame at wavelengths of 372.0, 589.6, 404.4, and 202.6 nm, respectively, with slit widths of 0.20, 0.5, 0.5, and 1.0 nm. Calibration was performed using dilutions of a 1000 ppm standard, with working ranges of 0–5 ppm for Ca, 0–20 ppm for Fe, 0–200 ppm for K, 0–10 ppm for Mg, and 0–8 ppm for Na. The minerals were extracted in triplicate and read in duplicate. Results are expressed as mean ± standard deviation. Results were expressed as milligrams per 100 g of dry weight of ‘Star fern’ (mg/100 g DW).
2.3. Screening Phytochemical
The extract was prepared by weighing 20 mg of freeze-dried ‘Star fern’ and initially mixing it with one mL of deionised water. The mixture was homogenised in a VM-300 vortex device (Interbiolab Inc., Orlando, FL, USA), followed by ultrasonication in an FS60 ultrasonic bath (Fisher Scientific Inc., Waltham, MA, USA) for 3 min at room temperature (approximately 25 °C) and at a frequency of 40 kHz. The supernatant and residue were separated in a 5430 microcentrifuge (Eppendorf AG, Hamburg, Germany) at 14,000 rpm, 4 °C, and 5 min. The supernatant was saved, and the residue was re-extracted twice more using 500 µL of water.
The final supernatant was used to detect the presence or absence of secondary metabolites according to the methodology described by León-Fernández et al. [
19]. Thus, terpenoids were analysed by reaction with chloroform and sulphuric acid; phenols and tannins with 10% ferric chloride; alkaloids with 2 N hydrochloric acid and Mayer’s reagent; flavonoids with a 10% 1 M ammonia solution and concentrated sulphuric acid; and anthraquinones with benzene and 10% ammonia. The presence of foam was used as an indicator of saponins, and acetogenins were confirmed with 3,5-dinitrobenzoic acid and potassium hydroxide. All extractions were performed in triplicate, and readings were taken in duplicate. The results were recorded qualitatively as ‘+’ for presence and ‘-’ for absence of the compound.
2.4. Bioactive Compounds
2.4.1. Vitamin C Identification
L-ascorbic acid (1 mg/mL) was used as a reference standard for vitamin C quantification [
18]. A sample of 20 mg of freeze-dried ‘Star fern’ was dissolved in 1.2 mL of 3% metaphosphoric acid and 200 µL of 0.2%
DL-homocysteine. The mixture was vortexed and sonicated for one minute. Following homogenization, 600 mL of ultrapure water was added, and the solid was separated for centrifugation (14,000 rpm, 4 °C, 5 min). The supernatant was filtered before analysis with a 0.45 µm PVDF filter. Chromatographic separation was carried out using a rapid resolution liquid chromatography (RRLC) 1200 system (Agilent Technologies, Mississauga, ON, Canada) equipped with a DAD-UV-VIS detector at 244 nm, and a Zorbax Eclipse XDB 80 AC C18 column (1.8 μm, 4.6 mm × 50 mm) (Agilent Scientific Instruments, Santa Clara, CA, USA). The mobile phase consisted of a solution prepared in a 90:10 (
v/
v) ratio of two solutions, the first being an aqueous solution of 1.5% (
w/
v) KH2PO4 and the second being a methanolic solution of 1.8% (
w/
v) n-cetyl, n, n, n-trimethylammonium bromide. The mobile phase was pumped at a flow rate of 1 mL/min. The chromatograms were recovered and analysed using Lab ChemStation software version 2.15.26 (Agilent Technologies, Santa Clara, CA, USA) comparing the retention times of the standard, the spectrum of
L-ascorbic acid and an internal standard. The extraction was performed in triplicate, and the injection was performed in duplicate for each sample. Results are expressed as mean ± standard deviation. Results were reported as milligrams of vitamin C per 100 g of dry weight of ‘Star fern’ (mg/100 g DW).
2.4.2. Organic Acid Identification
References standards at a concentration of 100 mg/mL of tartaric, malic, and citric acids were employed for the identification and quantification of individual organic acids [
18]. For the extract, 20 mg of the freeze-dried ‘Star fern’ was suspended in 1.5 mL of 0.02 M sulfuric acid supplemented with 0.05% metaphosphoric acid and 0.02%
DL-homocysteine. The mixture was vortexed and sonicated for three minutes. The supernatant recovered for centrifugation (14,000 rpm, 4 °C, 5 min) was filtered before analysis with a 0.45 µm PVDF filter and analysed with an RRLC 1200, DAD-UV-VIS detector at 210 nm and a YMC-Triart C18 column (3 µm, 4.6 mm × 150 mm) (YMC Europe GmbH, Dinslaken, Germany). The mobile phase consisted of a 0.027% sulfuric acid solution and was pumped at a flow rate of 1 mL/min. The chromatograms were recovered and analysed using Lab ChemStation software version 2.15.26, which compared the retention times of the standard, the spectrum of organic acids, and an internal standard. The extraction was performed in triplicate, and the injection was performed in duplicate for each sample. Results are expressed as mean ± standard deviation. Results were reported as milligrams of individual organic acid per 100 g of dry weight of ‘Star fern’ (mg/100 g DW).
2.4.3. Carotenoids Identification
Reference standards at a concentration of 1 mg/mL of astaxanthin, α-carotene, β-carotene, β-crytoxanthin, lutein, lycopene, trans-β-apo-8-carotenal, and zeaxanthin were employed for the identification and quantification of individual carotenoids [
18]. For extraction, 20 mg of freeze-dried ‘Star fern’ was combined with 250 µL of acetone, 500 µL of dichloromethane, and 250 µL of methanol. The mixture was vortexed and sonicated for two minutes. The coloured liquid was recovered by centrifugation (14,000 rpm, 4 °C, 5 min), and the solid was re-extracted with 500 µL of the mixture until the pigment was completely recovered from the sample. The resulting-coloured liquid was concentrated to dryness under reduced pressure using a rotary evaporator Buchi TM R-100 (Fisher Scientific, Hampton, NH, USA) at a temperature below 30 °C. The dry pigment was reconstituted in 40 µL of ethyl acetate, which was centrifuged (14,000 rpm, 4 °C, 5 min), and 20 µL was placed in a vial equipped with an insert for the respective chromatographic analysis in an RRLC 1200 equipped with a DAD-UV-VIS detector and a YMC C30 column (3 µm, 4.6 × 150 mm) (YMC Europe GmbH, Dinslaken, Germany). A ternary mobile phase composed of methanol (solvent A), methyl tert-butyl ether (solvent B), and water (solvent C) was pumped at 1 mL/min employed under a linear gradient program, thus 95% A + 5% B + 0% C (0–5 min), 89% A + 11% B + 10% C (10 min), 75% A + 25% B + 0% C (16 min), 40% A + 60% B + 0% C (20 min), 15% A + 85% B + 0% C (22.5 min), and 90% A + 5% B + 5% C (25–28 min). The chromatograms were recovered at 350 or 450 nm and analysed using Lab ChemStation software version 2.15.26, comparing the retention times of the standard, the spectrum of carotenoids and an internal standard. The extraction was performed in triplicate, and the injection was performed in duplicate for each sample. Results are expressed as mean ± standard deviation. Results were reported as milligrams of individual carotenoids per 100 g of dry weight of ‘Star fern’ (mg/100 g DW).
2.4.4. Chlorophylls and Their Derivatives
Reference standards at a concentration of 1 mg/mL of chlorophyll a, pheophytin a, and pheophytin b were employed for the identification and quantification of individual compounds [
18]. The extraction and quantification of chlorophyll pigments and their degradation derivatives were performed following the methodology previously detailed (
Section 2.4.3). The extraction was performed in triplicate, and the injection was performed in duplicate for each sample. Results are expressed as mean + standard deviation. Results were reported as milligrams of individual chlorophylls and their derivatives per 100 g of dry weight of ‘Star fern’ (mg/100 g DW).
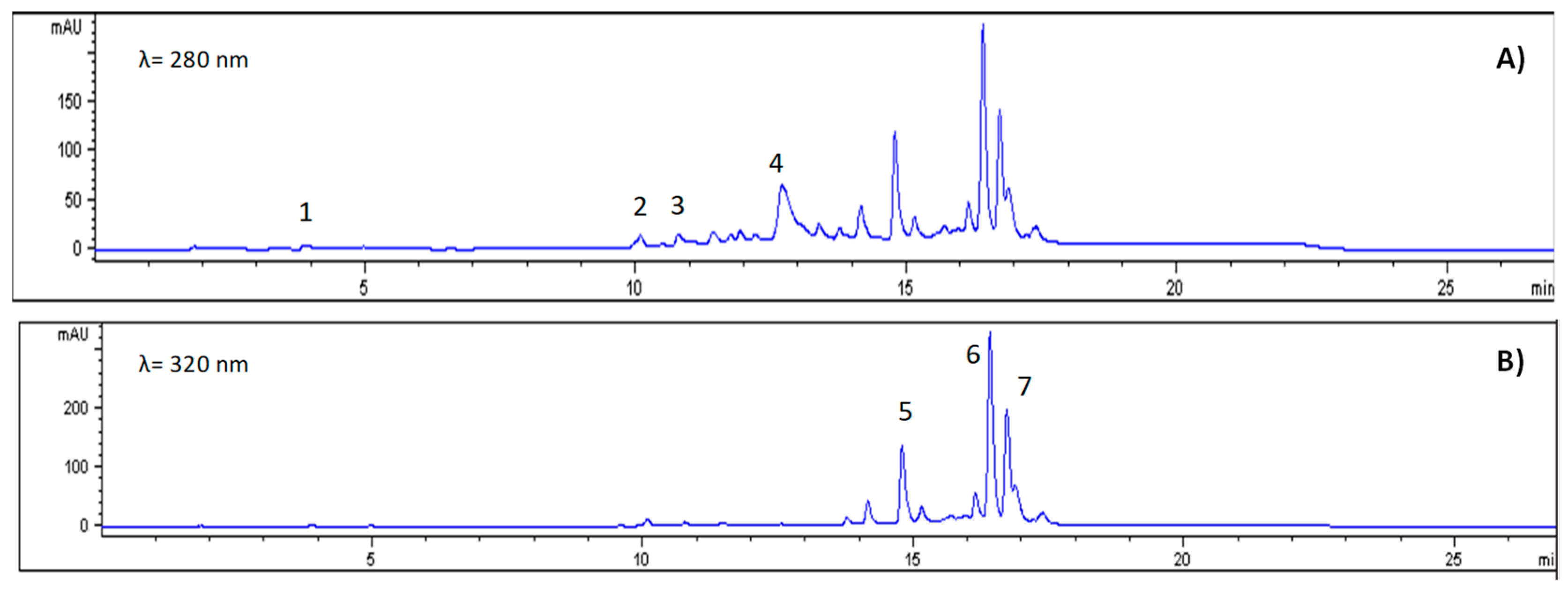
2.4.5. Phenolic Compound Identification
Reference standard at a concentration of 1 mg/mL of caffeic acid, vanillic acid, chlorogenic acid, syringic acid, chrysin, shikimic acid,
p-,
m-, and
o-coumaric acids, rutin, 2,5-dihydroxybenzoic acid, quercetin, ferulic acid, naringin, gallic acid, luteolin,
p-hydroxybenzoic acid, kaempferol, 2,5-dihydroxybenzoic acid, and 3-hydroxybenzoic acid were employed for the identification and quantification of individual phenolics [
18].
For extraction, 20 mg of freeze-dried ‘Star fern’ was combined with 1000 µL of 80% methanol acidified with 0.1% HCl. The mixture was vortexed and sonicated for three minutes. The liquid was recovered by centrifugation (14,000 rpm, 4 °C, 5 min), and the solid was re-extracted with 500 µL of methanol acidified twice more. The methanolic extract was filtered with a 0.45 µm PVDF filter and analysed with an RRLC 1200, DAD-UV-VIS detector between 220 nm and 500 nm, and a Zorbax Eclipse Plus C18 column (4.6 × 150 mm, 5 µm) (Agilent Scientific Instruments, Santa Clara, CA, USA). A binary mobile phase was pumped at 1 mL/min, consisting of an aqueous solution with 0.01% formic acid, and solvent B was acetonitrile. The elution gradient was programmed with initial conditions at 100% A, transitioning to 95% A and 5% B at 5 min, followed by a gradual shift to 50% A and 50% B at 20 min, and maintained until 30 min. After this, the column was washed for two minutes and re-equilibrated. The chromatograms were recovered at 280 nm, 320 nm, or 370 nm, depending on each compound, and analysed using Lab ChemStation software, comparing the retention times of the standard, the spectrum of phenolics and an internal standard. The extraction was performed in triplicate, and the injection was performed in duplicate for each sample. Results are expressed as mean ± standard deviation. Results were reported as milligrams of individual phenolics per 100 g of dry weight of ‘Star fern’ (mg/100 g DW).
2.5. Antimicrobial Activity
Phenolic-rich extract was prepared by suspending 2.5 g of freeze-dried ‘Star fern’ (
Gleichenella pectinate) in 25 mL of 50% ethanol, followed by homogenization and sonication for 6 min. The liquid was recovered by centrifugation (7500 rpm, 5 min, 4 °C), and the solid was re-extracted with 25 mL of ethanolic solution twice more. The ethanolic extract was filtered with filter paper, and ethanol was evaporated under reduced pressure at temperatures not exceeding 40 °C. The concentrated extracts were frozen and lyophilised. The freeze-dried ethanolic extract was stored frozen until analysis. This extraction method was previously validated by Coyago et al., maximising the concentration of phenolic compounds, which are the metabolites that mainly generate biological activities [
20].
2.5.1. Antibacterial Activity
The antibacterial activity was evaluated using the agar well diffusion method and the broth microdilution technique to determine the minimum inhibitory concentration (MIC), following protocols outlined by the Clinical and Laboratory Standards Institute (CLSI) [
21,
22,
23]. Freeze-dried extracts were tested against
Streptococcus aureus ATCC 6538P,
Streptococcus mutans ATCC 25175,
Escherichia coli ATCC 8739, and
Pseudomonas aeruginosa ATCC 9027, obtained from the American Type Culture Collection.
Before testing, 300 mg of freeze-dried ethanolic extract were reconstituted in 1 mL of sterile distilled water. Bacterial cultures were maintained on solidified Mueller–Hinton agar (MHA). For the agar well diffusion assay, microbial inoculation was adjusted to approximately 1.5 × 108 CFU/mL and spread uniformly onto agar plates. Wells with 5 mm diameters were created on the agar surface, and 80 µL of each extract was added to the wells. Streptomycin sulphate salt was used as a positive control for bacterial strains. Each test was performed in triplicate. Plates were incubated at 37 °C for 24 h, after which inhibition zones were measured in millimetres. Results are expressed as mean ± standard deviation.
For the microdilution technique, 100 µL of each freeze-dried ethanolic extract in solution was combined with 100 µL of Brain Heart Infusion (BHI) medium in a sterile 96-well microplate, and serial twofold dilutions were prepared directly within the wells. Subsequently, 20 µL of bacterial suspension, standardised to 5 × 105 CFU/mL, were added to each well, yielding a final reaction volume of 220 µL. Streptomycin sulphate salt was included as the positive control, while wells containing the bacterial suspension and sterile water served as the negative control. The microplates were incubated at 37 °C for 24 h. To visualise bacterial growth, 20 µL of 2,3,5-triphenyltetrazolium chloride (TTC) was added and incubated for an additional two hours at 37 °C. Reduction of TTC to red formazan by metabolically active bacteria enabled direct visual determination of growth inhibition. The minimum inhibitory concentration (MIC) corresponded to the lowest extract concentration showing no visible red colouration. All experiments were performed at least in triplicate.
2.5.2. Antibacterial Activity Against Multidrug-Resistant Bacteria
The antibacterial potential of the freeze-dried ethanolic extract of ‘Star fern’ was investigated against seven multidrug-resistant bacterial strains, such as Klebsiella pneumoniae, Escherichia coli, Salmonella enterica serovar Kentucky, Enterococcus faecalis, Staphylococcus epidermidis, Enterococcus faecium, and Pseudomonas aeruginosa. These isolates were obtained from the National Health Institute of Ecuador (INSPI) and are part of the institution’s External Quality Evaluation Program.
Bacterial inocula were cultured in brain heart infusion (BHI) broth and standardised to 5 × 10
5 CFU/mL. The freeze-dried ethanolic extract was prepared in DMSO at 267 mg/mL, while nourseothricin (100 µg/mL) served as the reference antimicrobial control. BHI medium alone and BHI containing the extract at stock concentration were used as blank controls. The antibacterial assay was performed using the microdilution technique, following CLSI guidelines [
23] with slight modifications. Specifically, 5 µL of the extract stock solution was added to 195 µL of bacterial suspension (5 × 10
5 CFU/mL), reaching a total volume of 200 µL per well. The microplates were incubated at 37 °C for 20 h with continuous agitation at 300 rpm (double orbital setting). Bacterial growth inhibition was determined by measuring absorbance at 600 nm. All experiments were performed at least in triplicate.
2.5.3. Antifungal Activity
The antifungal activity was evaluated using the agar well diffusion method and the broth microdilution technique to determine the minimum inhibitory concentration (MIC), following protocols outlined by the Clinical and Laboratory Standards Institute (CLSI) {Formatting Citation}. Freeze-dried ethanolic extracts were tested against Candida albicans ATCC 10231 and Candida tropicalis ATCC 13803, obtained from the American Type Culture Collection.
Before testing, 300 mg of freeze-dried ethanolic extract was reconstituted in 1 mL of sterile distilled water. Yeast strains were preserved on Sabouraud dextrose agar (SDA). For the agar well diffusion assay, microbial inoculation was adjusted to approximately 5 × 105 CFU/mL and spread uniformly onto agar plates. Wells with 5 mm diameters were created on the agar surface, and 80 µL of each extract was added to the well. Fluconazole was used as a positive control for fungal strains. Each test was performed in triplicate. Plates were incubated at 35 °C for 48 h, after which inhibition zones were measured in millimetres. Results are expressed as mean ± standard deviation.
For the microdilution technique, 100 µL of each freeze-dried ethanolic extract solution was combined with 100 µL of Yeast Peptone Dextrose Broth (YPDB) medium in a sterile 96-well microplate, and serial twofold dilutions were prepared directly within the wells. Subsequently, 20 µL of fungal suspension, standardised to 1 × 106 CFU/mL, was added to each well, yielding a final reaction volume of 220 µL. Fluconazole was included as the positive control, while wells containing the fungal suspension and sterile water served as the negative control. The microplates were incubated at 37 °C for 72 h. To visualise fungal growth, 20 µL of 2,3,5-triphenyltetrazolium chloride (TTC) was added and incubated for an additional two hours at 37 °C. Reduction of TTC to red formazan by metabolically active fungi enabled direct visual determination of growth inhibition. The minimum inhibitory concentration (MIC) corresponded to the lowest extract concentration, showing no visible red colouration. All experiments were performed at least in triplicate.
2.6. Antioxidant Activity
Reference standards at a concentration of 10 mM of Trolox were employed for quantifying antioxidant activity using the DPPH and ABTS methods. For extraction, 20 mg of freeze-dried ‘Star fern’ was combined with 2 mL of methanol. The mixture was vortexed and sonicated for three minutes. The supernatant recovered for centrifugation (14,000 rpm, 4 °C, 5 min) was filtered before analysis with a 0.45 µm PVDF (Polyvinylidene fluoride) filter (Sigma-Aldrich, Darmstadt, Germany) [
18].
The DPPH• radical solution was freshly prepared by dissolving 10 mg of DPPH in 50 mL of methanol. For calibration curve preparation, Trolox standard solutions ranging from 0.1 to 4.0 mM were prepared in methanol. For each reaction, 20 µL of sample or standard was added to 280 µL of DPPH• solution in a microplate. This was incubated in the dark for 40 min on an orbital shaker 4310 (Fisher Scientific, Waltham, MA, USA). Absorbance readings were taken at 515 nm using a BioTek H1 spectrophotometer (Agilent Scientific Instruments, Santa Clara, CA, USA).
The ABTS•+ radical cation was generated by mixing equal volumes of 7 mM ABTS and 2.45 mM potassium persulfate solutions, followed by incubation in the dark for 16 h. Before use, the radical solution was diluted tenfold with absolute methanol until reaching an absorbance of approximately 0.7 at 734 nm. For calibration curve preparation, Trolox standard solutions ranging from 0.2 to 0.7 mM were prepared in ethanol. For each reaction, 20 µL of sample or standard was added to 280 µL of ABTS•+ radical in a microplate. This was incubated in the dark for 15 min on an orbital shaker. Absorbance readings were taken at 734 nm in a spectrophotometer.
The extraction was performed in triplicate, and the quantification in duplicate. Results are expressed as mean ± standard deviation. Results were expressed as millimoles of Trolox equivalents per 100 g of dry weight of freeze-dried ‘Star fern’ (mmol TE/100 g DW).
2.7. Antitumor Activity
The cell lines HeLa (human cervical carcinoma, ATCC No. CCL-2, RRID: CVCL_0030), HCT116 (human colorectal carcinoma, ATCC No. CCL-247, RRID: CVCL_0291), HepG2 (human hepatoma, ATCC No. HB-8065, RRID: CVCL_0027), and NIH3T3 (mouse embryonic fibroblasts, ATCC No. CRL-1658, RRID: CVCL_0594) were sourced from the American Type Culture Collection (ATCC, Manassas, VA, USA). Additionally, the THJ29T cell line (human thyroid carcinoma, Cat. No. T8254, RRID: CVCL_W922) was procured from Applied Biological Materials Inc. (ABM, Richmond, BC, Canada). This panel was intentionally assembled to cover distinct tissue origins and molecular backgrounds, enabling a wider appraisal of the extract’s antiproliferative effects. All cell cultures were maintained in Dulbecco’s Modified Eagle’s Medium/Nutrient Mixture F-12 Ham (DMEM/F12) (Corning, Manassas, VA, USA) supplemented with 10% fetal bovine serum (FBS) (Eurobio, Les Ulis, France) and 1% penicillin-streptomycin (Thermo Fisher Scientific, Gibco, Miami, FL, USA). Cultures were kept at 37 °C in a humidified incubator with 5% CO2.
To assess the impact of the freeze-dried ethanolic extract of
G. pectinata (
Section 2.5) on cell proliferation, cells were plated in 96-well plates at a density of 1 × 10
4 cells per well. After allowing 24 h for cell attachment, the cultures were exposed to 100 µL of the extract, prepared in distilled water, using serial two-fold dilutions spanning from 5 mg/mL to 0.08 mg for 72 h. This range was selected considering the extract’s solubility limits and the maximal solvent load compatible with cell viability. The 72 h exposure was chosen to align with typical doubling times and to capture cytostatic effects commonly assessed in this assay window. Cisplatin (CDDP) was used as a positive control.
Cell viability was then evaluated using the MTT assay, following standard procedures. Briefly, 10 µL of MTT (M5655, Sigma-Aldrich, Darmstadt, Germany) solution (5 mg/mL) was added to each well, and the plates were incubated for 1 to 2 h (cell line-dependent) at 37 °C under humidified conditions. The supernatant was carefully removed, and 50 µL of DMSO was added to dissolve the formed formazan crystals. Plates were placed on an orbital shaker for 2 min to ensure complete solubilization before measuring absorbance at 570 nm using the Cytation 5 multi-mode plate reader (BioTek, Winooski, VT, USA). Wells containing culture medium only served as the negative control (defined as 100% proliferation), and percent inhibition was calculated relative to this control. Each concentration was tested in quadruplicate, and experiments were independently repeated at least four times. The half-maximal inhibitory concentration (IC50) was calculated from dose–response curves generated using GraphPad Prism 10.2 (GraphPad Software, Corp., San Diego, CA, USA). IC50 values were obtained by nonlinear regression using a four-parameter logistic model. The therapeutic index (TI) was determined by dividing the IC50 value of non-tumour NIH3T3 cells by the IC50 values of each tumour cell line, with TI values > 1 interpreted as preferential activity against tumour cells. NIH3T3 was selected as the non-tumour comparator as a fibroblast model of healthy tissue. Results are expressed as mean ± standard deviation.
2.8. Anti-Inflammatory Activity
RAW 264.7 murine macrophages (ATCC No. TIB-71, RRID: CVCL_0493) were grown in Dulbecco’s Modified Eagle Medium (DMEM; Corning, Manassas, VA, USA) containing 4.5 g/L glucose and L-glutamine, supplemented with 10% fetal bovine serum (FBS; Eurobio, Les Ulis, France) and 1% penicillin-streptomycin (Thermo Fisher Scientific, Gibco, Miami, FL, USA). To ensure adequate growth conditions, cells were maintained using a combination of 70% fresh medium and 30% conditioned medium.Cultures were incubated at 37 °C in a humidified atmosphere containing 5% CO2.
For the anti-inflammatory assay, RAW 264.7 cells were seeded in 24-well plates at a density of 3 × 10
5 cells per well and allowed to adhere for 18–24 h. Once attached, the culture medium was replaced with serum-free DMEM containing the different treatments. These included DMEM alone as the control, dexamethasone (DEX; Sigma-Aldrich, St. Louis, MO, USA) at 2 µg/mL as the anti-inflammatory reference, and freeze-dried ethanolic extract of
G. pectinate (
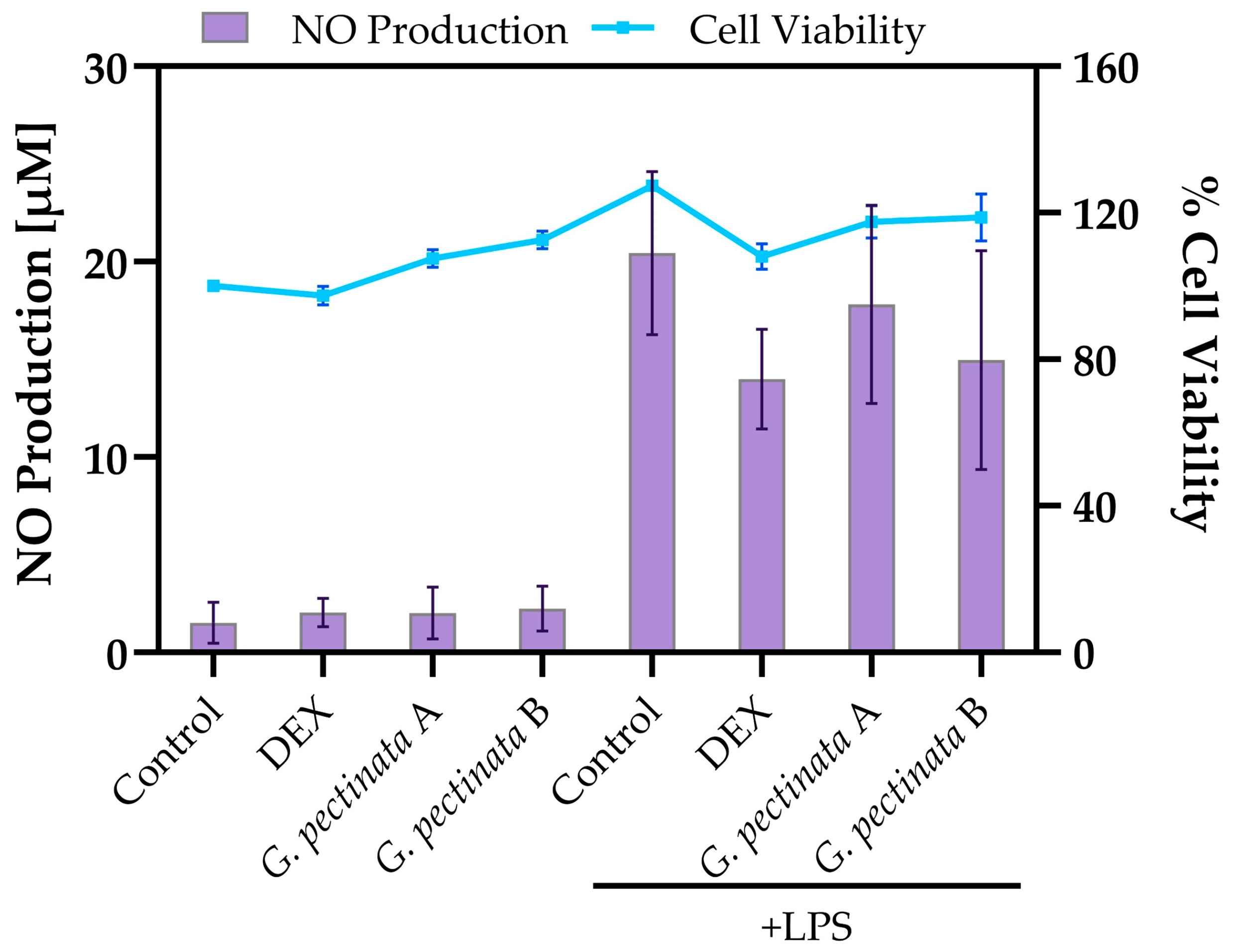
Section 2.5) at concentrations of 0.05 mg/mL and 0.1 mg/mL. After four hours of exposure, lipopolysaccharide (LPS; InvivoGen, San Diego, CA, USA) at 1 μg/mL was added to stimulate nitric oxide (NO) production, and the cultures were further incubated for 20 h. All stock solutions were freshly prepared in DMEM.
Nitric oxide levels were determined by transferring 50 µL of supernatant from each treatment into a 96-well plate, together with a NO standard curve prepared with Promega reagent (G296A) in the range of 0.78–100 µM. An equal volume of Griess reagent (Sigma-Aldrich, Darmstadt, Germany, G4410; 50 mg/mL) was then added to each well, giving a final reaction volume of 100 µL. After incubation for 10 min at room temperature in the dark, absorbance was measured at 540 nm using a Cytation 5 plate reader (BioTek, Vermont, VT, USA). The absorbance corresponding to DMEM alone was subtracted, and NO concentrations were calculated from the standard curve. All assays were performed in triplicate.
Cell viability was evaluated in parallel by fixing the cells in the 24-well plates with 4% paraformaldehyde for 20 min at room temperature, followed by staining with 0.5% (w/v) crystal violet for 30 min. Plates were then carefully washed with water to remove excess dye and air-dried. The stained plates were scanned, and the absorbance was measured at 570 nm using a Cytation 5 multimode detection system (BioTek, Vermont, VT, USA). Untreated cells were considered the reference for 100% viability.
4. Conclusions
Gleichenella pectinata, known as ‘Star fern’, is a species traditionally used by Amazonian indigenous communities to treat various diseases; however, scientific information on its composition and bioactivity is limited. In this study, the leaves showed low titratable acidity, correlated with a high concentration of organic acids, especially malic acid. In addition, high levels of potassium, calcium, and iron were detected. The screening showed the presence of acetogenins, flavonoids, and phenols. Among the pigments, β-carotene was the predominant carotenoid, and chlorophyll b had the highest concentration. In terms of phenolic compounds, ferulic acid and quercetin glucoside stood out for their high values. Antioxidant activity (ABTS and DPPH) showed similar results with significant activities. The freeze-dried ethanolic extracts were more effective against Pseudomonas aeruginosa ATCC and multi-resistant bacteria such as Escherichia coli and P. aeruginosa. Finally, moderate antitumor potential was observed in liver and cervical carcinoma cells, attributable to its profile of phenolic acids, flavonoids, acetogenins and carotenoids, positioning G. pectinata as a promising candidate for future research into natural therapies.