MitoQ as a Mitochondria-Targeted Antioxidant in Sperm Cryopreservation: An Updated Review on Its Mechanisms, Efficacy, and Future Perspectives
Abstract
1. Introduction
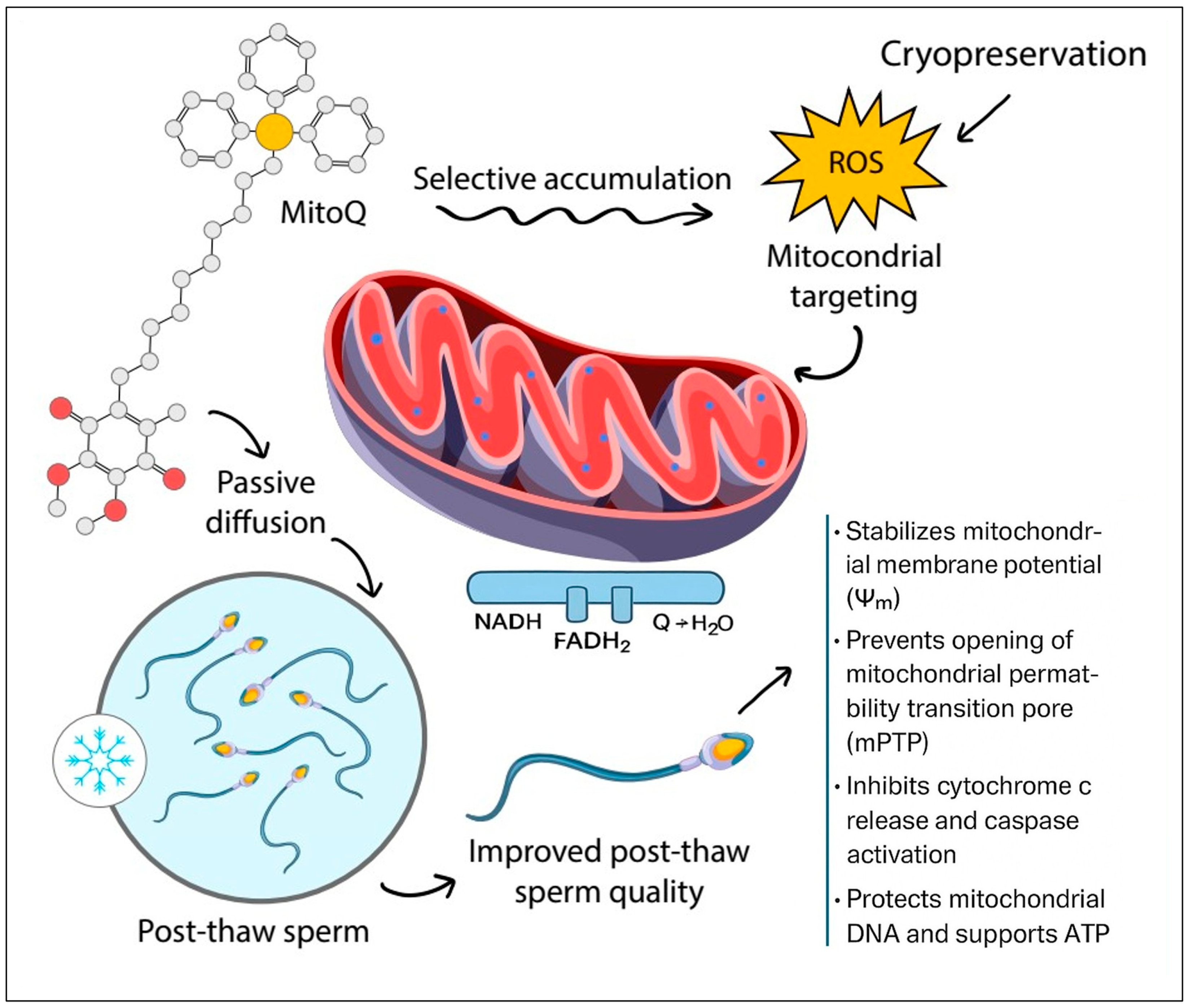
2. Mechanism of Action of MitoQ
3. Dosage and Toxicity Considerations
3.1. Applications in Assisted Reproduction and Livestock Breeding
3.2. Applications and Future Directions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Watson, P.F. The causes of reduced fertility with cryopreserved semen. Anim. Reprod. Sci. 2000, 60–61, 481–492. [Google Scholar] [CrossRef]
- Holt, W.V. Basic aspects of frozen storage of semen. Anim. Reprod. Sci. 2000, 62, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Sharafi, M.; Borghei-Rad, S.M.; Hezavehei, M.; Shahverdi, A.; Benson, J.D. Cryopreservation of semen in domestic animals: A review of current challenges, applications, and prospective strategies. Animals 2022, 12, 3271. [Google Scholar] [CrossRef]
- Farhadi, R.; Farshad, A.; Najafi, A.; Rostamzadeh, J. Improvement of cryopreserved epididymal ram sperm quality and fertility through curcumin nanoparticles. Theriogenology 2025, 243, 117462. [Google Scholar] [CrossRef]
- Farshad, A.; Diel, E.; Wehrend, A. Influence of antifreeze protein III on canine sperm cryopreservation. Theriogenology 2025, 235, 86–93. [Google Scholar] [CrossRef]
- Mazur, P. Freezing of living cells: Mechanisms and implications. Am. J. Physiol. 1984, 247, C125–C142. [Google Scholar]
- Hammerstedt, R.H.; Graham, J.K.; Nolan, J.P. Cryopreservation of mammalian sperm: What we ask them to survive. J. Androl. 1990, 11, 73–88. [Google Scholar] [CrossRef]
- Aitken, R.J.; Clarkson, J.S. Significance of reactive oxygen species and antioxidants in defining the efficacy of sperm preparation techniques. J. Androl. 1988, 9, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Sharma, R.K.; Desai, N.R.; Prabakaran, S.; Tavares, A.; Sabanegh, E. Role of oxidative stress in pathogenesis of varicocele and infertility. Urology 2009, 73, 461–469. [Google Scholar] [CrossRef]
- Koppers, A.J.; De Iuliis, G.N.; Finnie, J.M.; McLaughlin, E.A.; Aitken, R.J. Significance of mitochondrial reactive oxygen species in the generation of oxidative stress in spermatozoa. J. Clin. Endocrinol. Metab. 2008, 93, 3199–3207. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, J.G.; Storey, B.T. Role of glutathione peroxidase in protecting mammalian spermatozoa from loss of motility caused by spontaneous lipid peroxidation. Gamete Res. 1989, 23, 77–90. [Google Scholar] [CrossRef]
- St John, J.C.; Jokhi, R.P.; Barratt, C.L.R. The impact of mitochondrial genetics on male infertility. Int. J. Androl. 2005, 28, 65–73. [Google Scholar] [CrossRef]
- Sikka, S.C. Oxidative stress and role of antioxidants in normal and abnormal sperm function. Front. Biosci. 1996, 1, e78–e86. [Google Scholar] [CrossRef]
- Nenicu, A.; Lüers, G.H.; Kovacs, W.; David, M.; Zimmer, A.; Bergmann, M.; Baumgart-Vogt, E. Peroxisomes in human and mouse testis: Differential expression of peroxisomal proteins in germ cells and distinct somatic cell types of the testis. Biol. Reprod. 2007, 77, 1060–1072. [Google Scholar] [CrossRef]
- Murphy, M.P.; Smith, R.A.J. Targeting antioxidants to mitochondria by conjugation to lipophilic cations. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 629–656. [Google Scholar] [CrossRef]
- Kelso, G.F.; Porteous, C.M.; Coulter, C.V.; Hughes, G.; Porteous, W.K.; Ledgerwood, E.C.; Smith, R.A.J.; Murphy, M.P. Selective targeting of a redox-active ubiquinone to mitochondria within cells: Antioxidant and antiapoptotic properties. J. Biol. Chem. 2001, 276, 4588–4596. [Google Scholar] [CrossRef]
- Smith, R.A.J.; Murphy, M.P. Animal and human studies with the mitochondria-targeted antioxidant MitoQ. Ann. N. Y. Acad. Sci. 2010, 1201, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Gottwald, E.M.; Duss, M.; Bugarski, M.; Haenni, D.; Schuh, C.D.; Landau, E.M.; Hall, A.M. The targeted anti-oxidant MitoQ causes mitochondrial swelling and depolarization in kidney tissue. Physiol. Rep. 2018, 6, e13667. [Google Scholar] [CrossRef]
- Rossman, M.J.; Santos-Parker, J.R.; Steward, C.A.C.; Bispham, N.Z.; Cuevas, L.M.; Rosenberg, H.L.; Woodward, K.A.; Chonchol, M.; Gioscia-Ryan, R.A.; Murphy, M.P.; et al. Chronic supplementation with a mitochondrial antioxidant (MitoQ) improves vascular function in healthy older adults. Hypertension 2018, 71, 1056–1063. [Google Scholar] [CrossRef] [PubMed]
- Gualtieri, R.; Kalthur, G.; Barbato, V.; Di Nardo, M.; Adiga, S.K.; Talevi, R. Mitochondrial dysfunction and oxidative stress caused by cryopreservation in reproductive cells. Antioxidants 2021, 10, 337. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Li, Q.-Y.; Li, H.; Wang, H.-Y.; Fan, C.-X.; Dong, Q.-Y.; Pan, B.-C.; Ji, Z.-L.; Li, J.-Y. ROS-induced oxidative stress is a major contributor to sperm cryoinjury. Hum. Reprod. 2024, 39, 310–325. [Google Scholar] [CrossRef]
- Takalani, N.B.; Monageng, E.M.; Mohlala, K.; Monsees, T.K.; Henkel, R.; Opuwari, C.S. Role of oxidative stress in male infertility. Reprod. Fertil. 2023, 4, e230024. [Google Scholar] [CrossRef]
- Smith, R.A.J.; Porteous, C.M.; Gane, A.M.; Murphy, M.P. Delivery of bioactive molecules to mitochondria in vivo. Proc. Natl. Acad. Sci. USA 2003, 100, 5407–5412. [Google Scholar] [CrossRef]
- Ross, M.F.; Kelso, G.F.; Blaikie, F.H.; James, A.M.; Cochemé, H.M.; Filipovska, A.; Porteous, C.M.; Lodovici, M.; Gil, B.; Asin-Cayuela, J.; et al. Lipophilic triphenylphosphonium cations as tools in mitochondrial bioenergetics and free radical biology. Biochemistry 2005, 70, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Elkhawagah, A.R.; Donato, G.G.; Poletto, M.; Martino, N.A.; Vincenti, L.; Conti, L.; Necchi, D.; Nervo, T. Effect of Mitoquinone on Sperm Quality of Cryopreserved Stallion Semen. J. Equine Vet. Sci. 2024, 141, 105168. [Google Scholar] [CrossRef] [PubMed]
- Gohil, K.; Rothfuss, L.; Lang, J.; Packer, L. Effect of Exercise Training on Tissue Vitamin E and Ubiquinone Content. J. Appl. Physiol. 1987, 63, 1638–1641. [Google Scholar] [CrossRef]
- Bailey, D.M.; McEneny, J.; Mathieu-Costello, O.; Henry, R.R.; James, P.E.; McCord, J.M.; Pietri, S.; Young, L.S.; Richardson, R.S. Sedentary aging increases resting and exercise-induced intramuscular free radical formation. J. Appl. Physiol. 2010, 109, 449–456. [Google Scholar] [CrossRef]
- Zoladz, J.A.; Koziel, A.; Woyda-Ploszczyca, A.; Celichowski, J.; Jarmuszkiewicz, W. Endurance training increases the efficiency of rat skeletal muscle mitochondria. Pflugers Arch. 2016, 468, 1709–1724. [Google Scholar] [CrossRef]
- López-Lluch, G. Coenzyme Q homeostasis in aging: Response to non-genetic interventions. Free Radic. Biol. Med. 2021, 164, 285–302. [Google Scholar] [CrossRef] [PubMed]
- Câmara, D.R.; Ibanescu, I.; Siuda, M.; Bollwein, H. Mitoquinone does not improve sperm cryo-resistance in bulls. Reprod. Domest. Anim. 2022, 57, 10–18. [Google Scholar] [CrossRef]
- Alipour-Jenaghard, P.; Daghigh-Kia, H.; Masoudi, R.; Moghaddam, G.; Qasemi-Panahi, B. Mitochondria-targeted antioxidant “MitoQ” improves rooster’s cooled sperm quality indicators and reproductive performance. Theriogenology 2023, 197, 26–30. [Google Scholar] [CrossRef]
- Mehdipour, M.; Mohammadi, H.; Salih, S.A.; Rashidi, A. Mitochondrial specific antioxidant MitoPBN mitigates oxidative stress and improves mitochondrial function in cryopreserved ram sperm. Sci. Rep. 2025, 15, 10526. [Google Scholar] [CrossRef]
- Nazari, S.A.; Bahnamiri, H.Z.; Yazdanshenas, P.; Jahandideh-Golroodbari, P.; Sharma, M.; Tvrda, E.; Dodaran, H.V.; Mohammadi-Sangcheshmeh, A.; Sharafi, M. Beneficial effects of the mitochondria-targeted antioxidant MitoQ on bull semen post cryopreservation quality characteristics. Reprod. Domest. Anim. 2025, 60, e70118. [Google Scholar] [CrossRef]
- Rezaei, A.; Bahmani, H.R.; Mafakheri, S.; Farshad, A.; Nazari, P.; Masoudi, R. Protective effects of different doses of MitoQ separately and combined with trehalose on oxidative stress and sperm function of cryopreserved Markhoz goat semen. Cryobiology 2023, 110, 36–43. [Google Scholar] [CrossRef]
- Yi, X.; Qiu, Y.; Tang, X.; Lei, Y.; Pan, Y.; Raza, S.H.A.; Althobaiti, N.A.; Albalawi, A.E.; Al Abdulmonem, W.; Makhlof, R.T.M.; et al. Effect of five different antioxidants including MitoQ on cryopreservation of Saanen dairy goat semen. Reprod. Sci. 2024, 31, 1958–1972. [Google Scholar] [CrossRef] [PubMed]
- Farshad, A.; Diel, E.; Wehrend, A. Evaluating the protective effects of MitoQ and antifreeze protein III on cryopreserved canine sperm. Animals 2025, 15, 270. [Google Scholar] [CrossRef]
- Sun, L.; He, M.; Xu, J.; Wu, C.; Zhang, S.; Zhang, D.; Dai, J.; Gao, J. Does Antioxidant Mitoquinone (MitoQ) Ameliorate Oxidative Stress in Frozen–Thawed Rooster Sperm? Animals 2022, 12, 3181. [Google Scholar] [CrossRef] [PubMed]
- Masoudi, R.; Dadashpour-Davachi, N.; Asadzadeh, N.; Hatefi, A.; Alipour-Jenaghard, P. MitoQ Preserves the Quality and Fertility of Liquid-Preserved Ram Sperm. Theriogenology 2024, 216, 8–11. [Google Scholar] [CrossRef]
- Al-Tarayra, N.; Al-Alami, Z.M.; Battah, A.; Muhaidat, N. Addition of Mitoquinone (MitoQ) to Fresh Human Sperm Enhances Sperm Motility without Attenuating Viability. Biology 2024, 13, 653. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Zhang, Y.; Huang, X.; Shi, M.; Sun, D.; Zhang, Y.; Li, W.; Jin, T.; Feng, J.; Xing, J.; et al. Effects of mitoquinone (MitoQ) supplementation during boar semen cryopreservation on sperm quality, antioxidant status and mitochondrial proteomics. Anim. Reprod. Sci. 2022, 247, 107099. [Google Scholar] [CrossRef]
- Tauskela, J.S. MitoQ—A mitochondria-targeted antioxidant. IDrugs 2007, 10, 399–412. [Google Scholar]
- Feng, Z.; Shi, J.; Ren, J.; Luo, L.; Liu, D.; Guo, Y.; Sun, B.; Liu, G.; Deng, M.; Li, Y. Mitochondria-targeted antioxidant MitoQ improves in vitro maturation and subsequent embryonic development from culled cows. Animals 2024, 14, 2929. [Google Scholar] [CrossRef]
- Tsui, K.-H.; Li, C.-J. Mitoquinone shifts energy metabolism to reduce ROS-induced oxeiptosis in female granulosa cells and mouse oocytes. Aging 2023, 15, 246–260. [Google Scholar] [CrossRef]
- Ferreira, F.C.; Teixeira, J.; Lidon, F.; Cagide, F.; Borges, F.; Pereira, R.M.L.N. Assisted reproduction technologies (ART): Impact of mitochondrial (dys)function and antioxidant therapy. Animals 2025, 15, 289. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Murphy, M.P.; Xing, W.; Wu, H.; Zhang, R.; Sun, H. Mitochondria-targeted antioxidant MitoQ reduced renal damage caused by ischemia–reperfusion injury in rodent kidneys: Longitudinal observations of T2-weighted imaging and dynamic contrast-enhanced MRI. Magn. Reson. Med. 2018, 79, 1559–1567. [Google Scholar] [CrossRef]
- Graham, D.; Huynh, N.N.; Hamilton, C.A.; Beattie, E.; Smith, R.A.; Cochemé, H.M.; Murphy, M.P.; Dominiczak, A.F. Mitochondria-targeted antioxidant MitoQ10 improves endothelial function and attenuates cardiac hypertrophy. Hypertension 2009, 54, 322–328. [Google Scholar] [CrossRef]
- Shinn, L.J.; Lagalwar, S. Treating neurodegenerative disease with antioxidants: Efficacy of resveratrol and mitochondrial-targeted MitoQ and SkQ. Antioxidants 2021, 10, 573. [Google Scholar] [CrossRef] [PubMed]
- Najafi, A.; Sharifi, S.D.; Farhadi, R.; Bapir, S.H. Elamipretide enhances post-thaw rooster sperm quality by mitigating oxidative stress and optimizing mitochondrial function during cryopreservation. Sci. Rep. 2025, 15, 23564. [Google Scholar] [CrossRef]
- Doughan, A.K.; Dikalov, S.I. Mitochondrial redox cycling of mitoquinone leads to superoxide production and cellular apoptosis. Antioxid. Redox Signal. 2007, 9, 1825–1836. [Google Scholar] [CrossRef]
- Gane, E.J.; Weilert, F.; Orr, D.W.; Keogh, G.F.; Gibson, M.; Lockhart, M.M.; Frampton, C.M.; Taylor, K.M.; Smith, R.A.; Murphy, M.P. The mitochondria-targeted antioxidant mitoquinone decreases liver damage in a phase II study of hepatitis C patients. Liver Int. 2010, 30, 1019–1026. [Google Scholar] [CrossRef] [PubMed]
- Snow, B.J.; Rolfe, F.L.; Lockhart, M.M.; Frampton, C.M.; O’Sullivan, J.D.; Fung, V.; Smith, R.A.; Murphy, M.P.; Taylor, K.M.; Protect Study Group. A Double-Blind, Placebo-Controlled Study to Assess the Mitochondria-Targeted Antioxidant MitoQ as a Disease-Modifying Therapy in Parkinson’s Disease. Mov. Disord. 2010, 25, 1670–1674. [Google Scholar] [CrossRef] [PubMed]
- Braakhuis, A.J.; Nagulan, R.; Somerville, V. The effect of MitoQ on aging-related biomarkers: A systematic review and meta-analysis. Oxid. Med. Cell. Longev. 2018, 2018, 8575263. [Google Scholar] [CrossRef] [PubMed]
- Javaheri Barfourooshi, H.; Asadzadeh, N.; Masoudi, R. The mitochondria-targeted antioxidant “MitoQ” preserves quality and reproductive performance of ram spermatozoa cryopreserved in soybean lecithin-based extender. Theriogenology 2023, 208, 71–76. [Google Scholar] [CrossRef] [PubMed]
| Species | Application | Concentration | Outcome | |
|---|---|---|---|---|
| Rezaei et al. (2023) [34] | Goat (Frozen semen) | Cryopreservation; MitoQ ± trehalose in extender | 100–1000 nM | Improved post-thaw viability, plasma membrane integrity, mitochondrial activity; dose-dependent benefits. |
| Yi et al. (2024) [35] | Goat (Frozen semen) | Cryopreservation with five antioxidants including MitoQ | 150 nM | Enhanced viability, membrane integrity, mitochondrial activity |
| Farshad et al. (2025) [36] | Canine (Frozen semen) | Cryopreservation with MitoQ + antifreeze protein III | Not specified (nM range) | Improved motility and post-thaw survival |
| Sun et al. (2022) [37] | Rooster (Frozen semen) | Added to cryopreservation extender | 50–200 nM (optimal 150 nM) | 150 nM improved motility, viability, ATP 200 nM increased ROS |
| Masoudi et al. (2024) [38] | Ram (Chilled semen) | Cold storage | 10 nM, 100 nM | Improved motility and preserved sperm quality during chilling. |
| Câmara et al. (2022) [30] | Bull (Frozen semen) | Added to extender | 0.2, 2, 20 nM | No improvement 20 nM increased ROS. |
| Al-Tarayra et al. (2024) [39] | Human (Chilled semen) | Swim-up preparation | nM to <1 µM (experimental doses) | Increased total mobility no effect on viability. |
| Shi et al. (2022) [40] | Boar sperm (Frozen semen) | Cryopreservation protocols | <40 μM | Improved post-thaw viability Reduced lipid peroxidation. |
| Elkhawagah et al. (2024) [25] | Horse sperm (Frozen semen) | Cryopreservation protocols | 25, 50, and 100 nM | at 25 nM improved sperm motility, while 200 nM impaired |
| Application | Concentration | Outcome | |
|---|---|---|---|
| Feng et al. (2024) [42] | Bos taurus oocytes (IVM from culled cows) | 1–5 µM | Improved maturation and blastocyst rates; enhanced mitochondrial activity; reduced ROS |
| Tsui et al. (2023) [43] | Mus musculus oocytes (oxidative stress model) | µM range | Improved spindle integrity and chromosomal stability; increased survival under stress |
| Ferreira et al. (2025) [44] | Bos taurus oocytes (IVF media supplementation) | 1 µM | Enhanced embryo development and mitochondrial function; reduced oxidative damage |
| System | Outcome | |
|---|---|---|
| Liu et al. (2018) [45] | Rodent ischemia–reperfusion (liver, gut, kidney) | Reduced ROS, tissue damage, apoptosis. |
| Graham et al. (2009) [46] | Animal cardiovascular models | Improved endothelial function and reduced oxidative stress. |
| Al-Tarayra et al. (2024) [39] | Human sperm in vitro | Enhanced motility and mitochondrial activity without harming viability. |
| Shinn und Lagalwar (2021) [47] | Neurodegenerative disease models (preclinical) | MitoQ reduced oxidative stress and improved neuronal resilience. |
| Population | Dose | Outcome | |
|---|---|---|---|
| Gane et al. (2010) [50] | Chronic hepatitis C patients | 40 mg/day | No significant antiviral effect; safe. |
| Snow et al. (2010) [51] | Parkinson’s disease trial | 40–80 mg/day | No slowing of progression; well tolerated. |
| Rossman et al. (2018) [19] | Older adults (endothelial function) | 20 mg/day for 6 weeks | Improved brachial artery flow-mediated dilation. |
| Braakhuis (2018) [52] | Multiple human trials | 20–80 mg/day | Improved oxidative stress markers |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farshad, A.; Wehrend, A. MitoQ as a Mitochondria-Targeted Antioxidant in Sperm Cryopreservation: An Updated Review on Its Mechanisms, Efficacy, and Future Perspectives. Antioxidants 2025, 14, 1350. https://doi.org/10.3390/antiox14111350
Farshad A, Wehrend A. MitoQ as a Mitochondria-Targeted Antioxidant in Sperm Cryopreservation: An Updated Review on Its Mechanisms, Efficacy, and Future Perspectives. Antioxidants. 2025; 14(11):1350. https://doi.org/10.3390/antiox14111350
Chicago/Turabian StyleFarshad, Abbas, and Axel Wehrend. 2025. "MitoQ as a Mitochondria-Targeted Antioxidant in Sperm Cryopreservation: An Updated Review on Its Mechanisms, Efficacy, and Future Perspectives" Antioxidants 14, no. 11: 1350. https://doi.org/10.3390/antiox14111350
APA StyleFarshad, A., & Wehrend, A. (2025). MitoQ as a Mitochondria-Targeted Antioxidant in Sperm Cryopreservation: An Updated Review on Its Mechanisms, Efficacy, and Future Perspectives. Antioxidants, 14(11), 1350. https://doi.org/10.3390/antiox14111350






