Anti-Tumoral Treatment with Thioredoxin Reductase 1 Inhibitor Auranofin Fosters Regulatory T Cell and B16F10 Expansion in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Cell Culture
2.3. SRB NCI-60 Screen for IC50
2.4. qPCR of Genes Downstream of NRF2 Activation
2.5. Animals
2.6. In Vivo Tumor Studies
2.7. For Adoptive Transfer of DCs
2.8. Serology via MSD
2.9. Proteomics Sample Preparation
2.10. Mass Spectrometry Analysis
2.11. Proteomics Analysis and Statistical Analysis
2.12. Ex Vivo Murine Treg Expansion Studies
2.13. Ex Vivo PBMC Treg Expansion Studies
2.14. Intracellular Staining for Cytokines
2.15. Flow Cytometry Analysis
2.16. Measuring Intracellular ROS via DHR Staining
2.17. Statistics and Reproducibility
3. Results
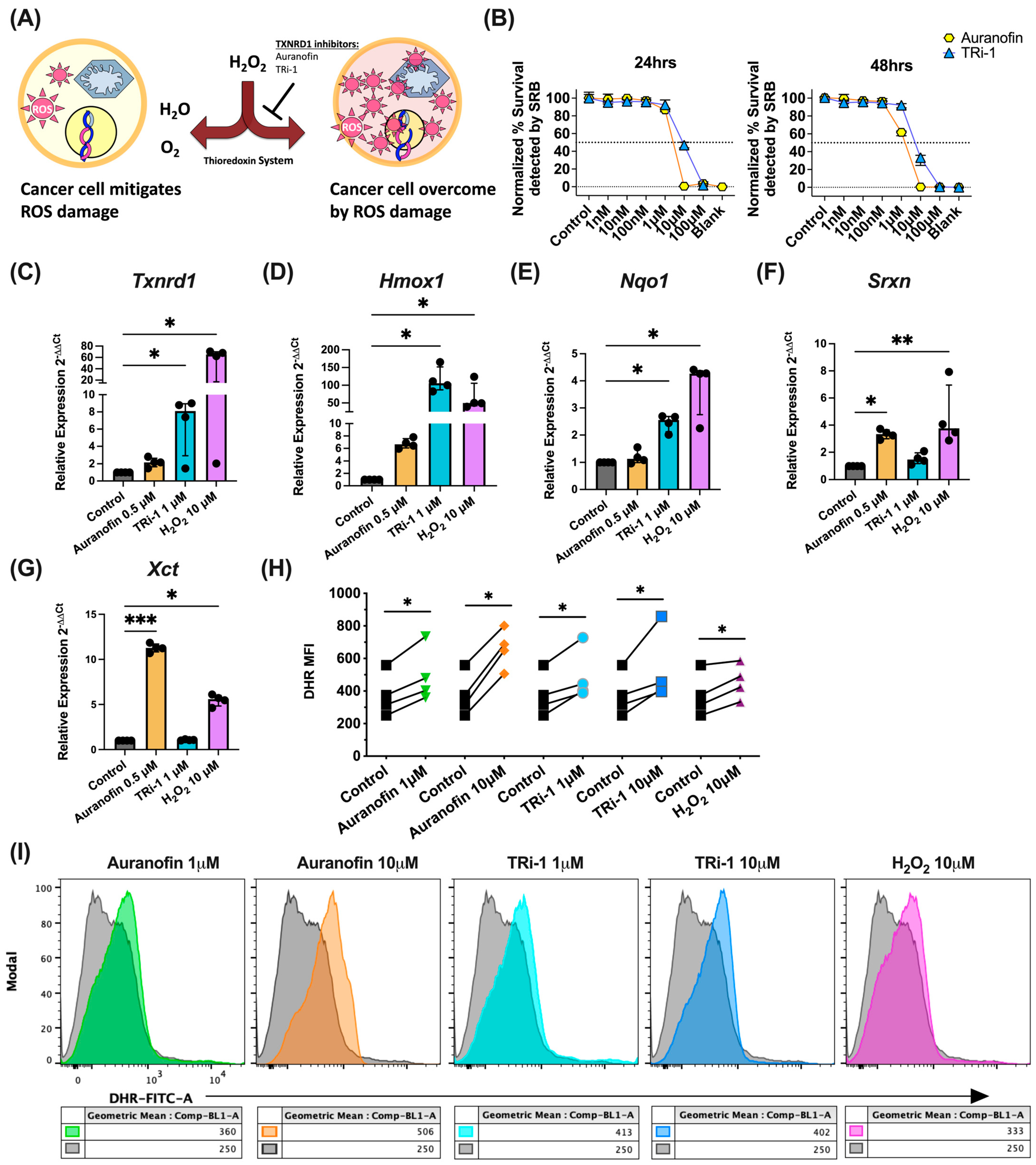
3.1. TXNRD1 Inhibitors Elevated ROS Levels, Activate NRF2, and Kill B16F10 Cells In Vitro
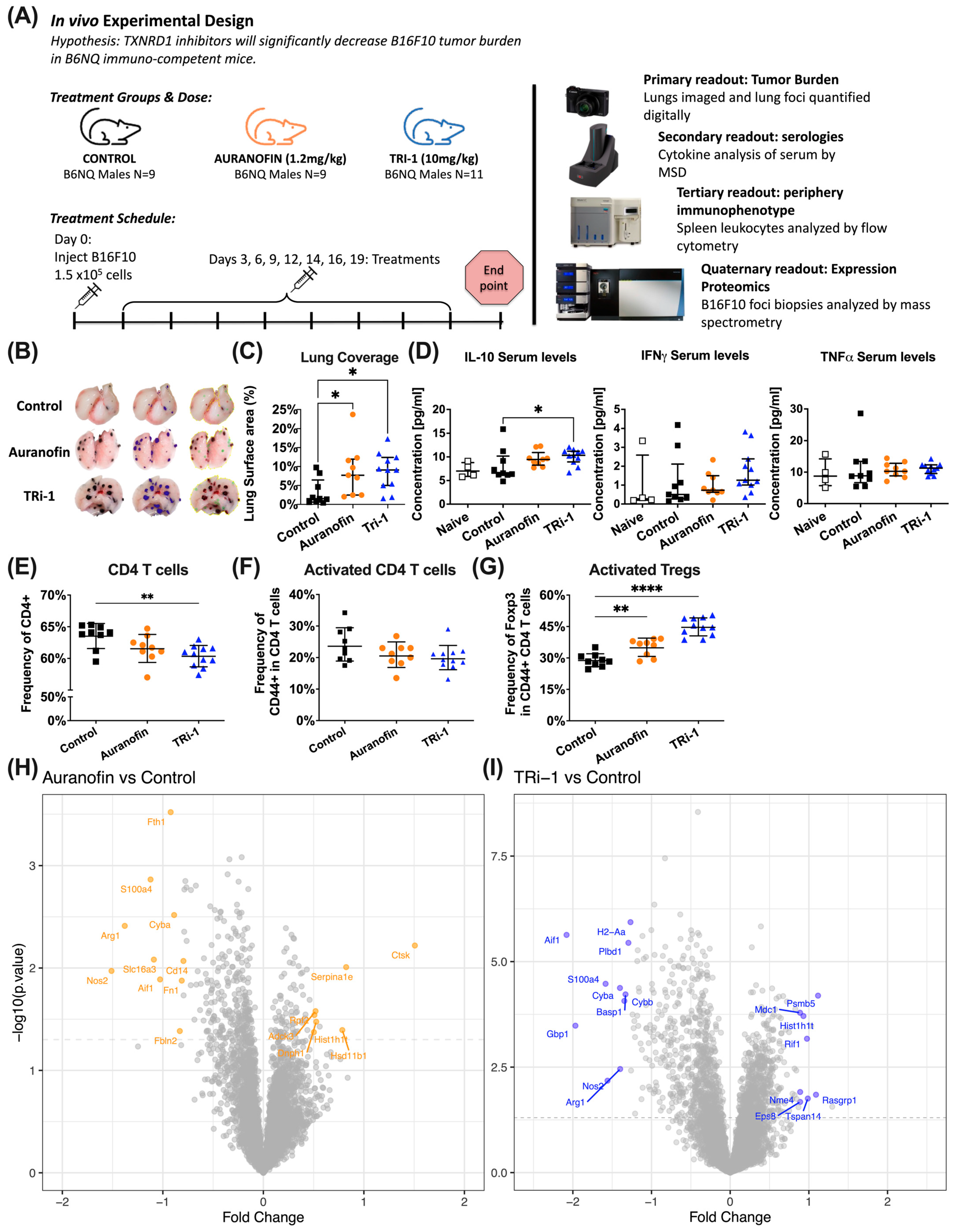
3.2. Auranofin and TRi-1 Treatment Increased Tumor Burden in Lungs of Mice Challenged with B16F10
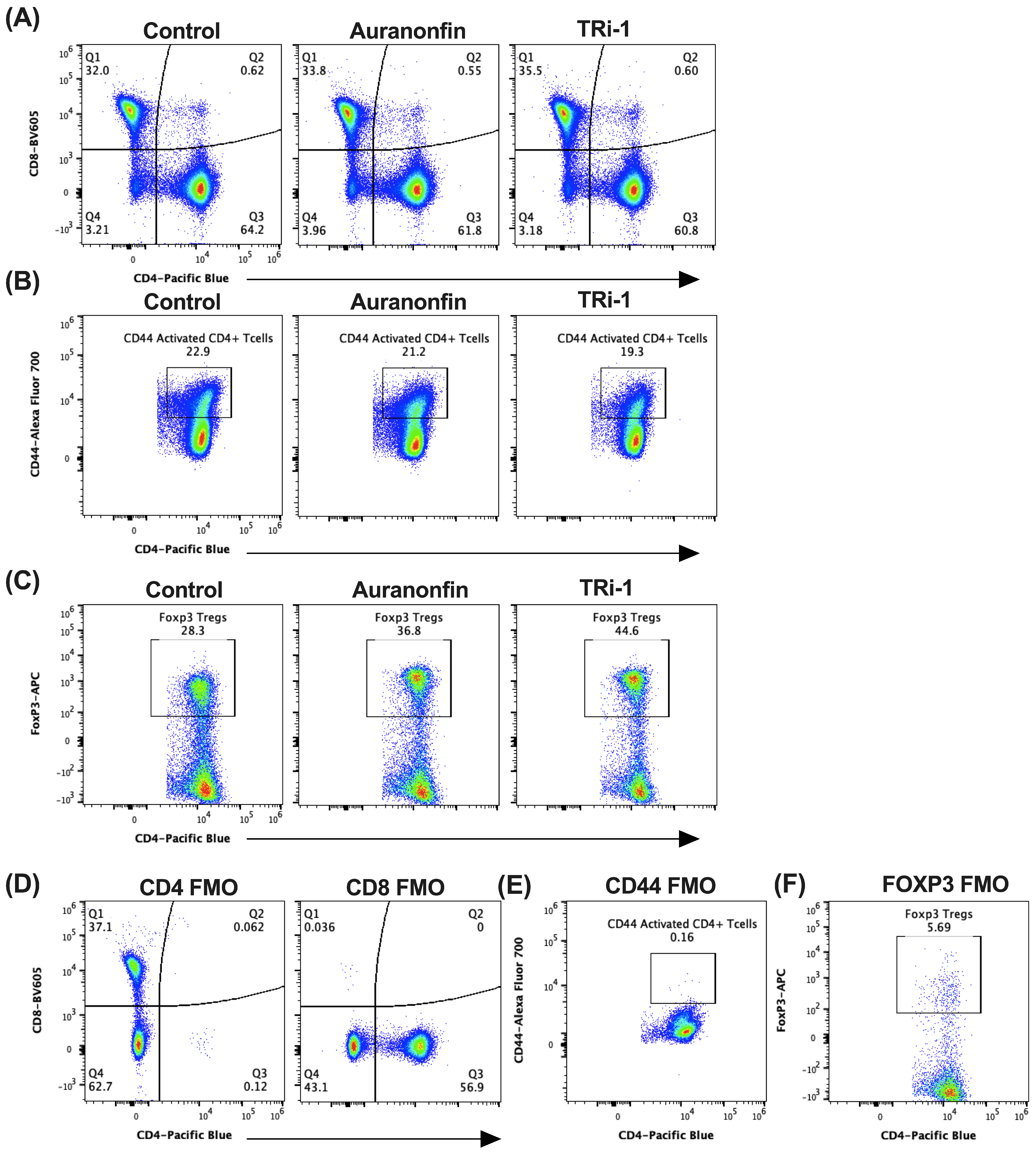
3.3. Tumor Bearing Mice Treated with Auranofin and TRi-1 Show Elevated Treg Formation When Compared to Control Treated Mice
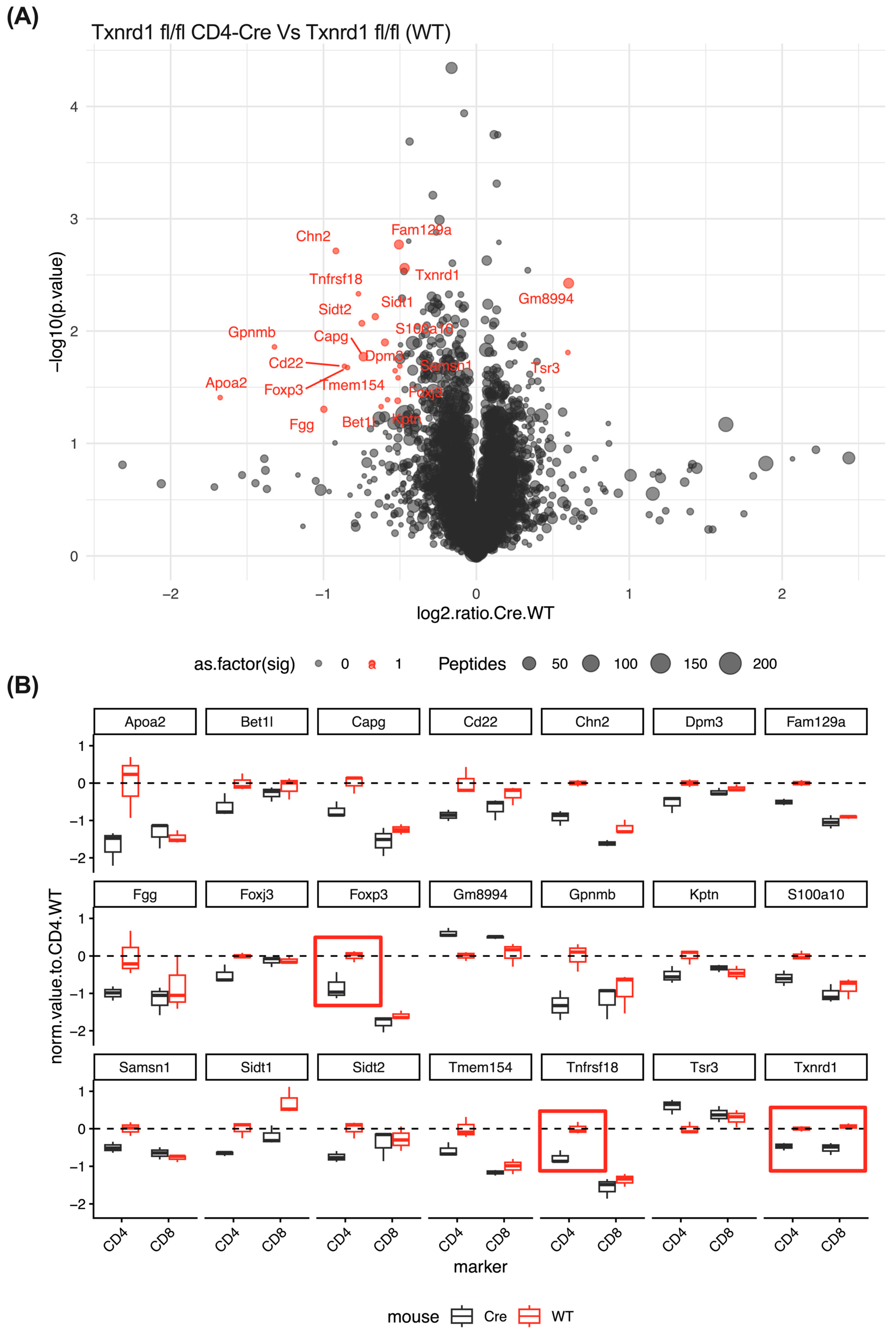
3.4. Expression Proteomic and GO Enrichment Pathway Analysis Suggest Differences in Immune Response in Auranofin and TRi-1 Treated Mice
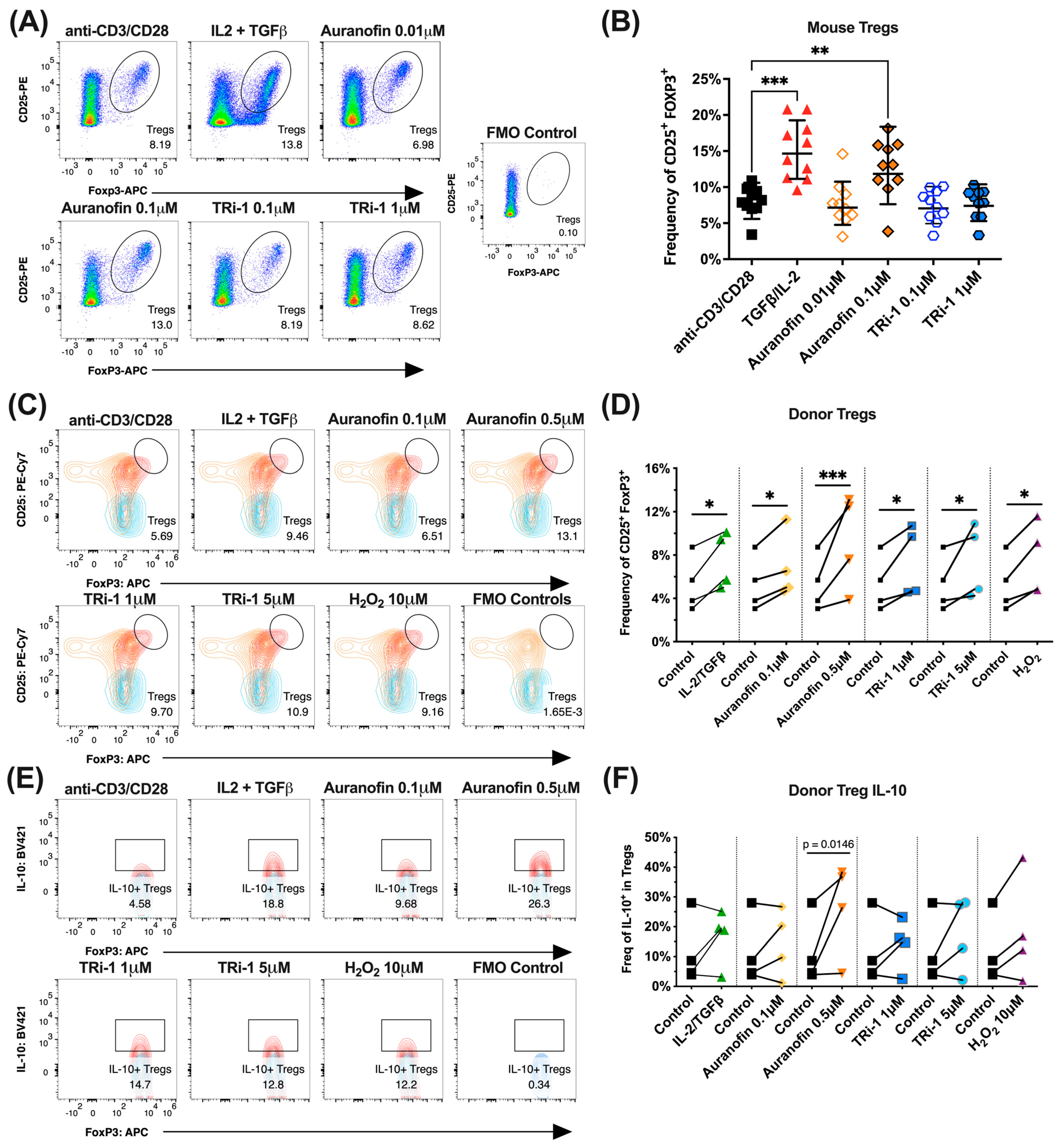
3.5. Naïve Murine Tregs Expand in Co-Cultured with Auranofin
3.6. Healthy Human Donor Tregs Expand in Co-Culture with Auranofin or TRi-1
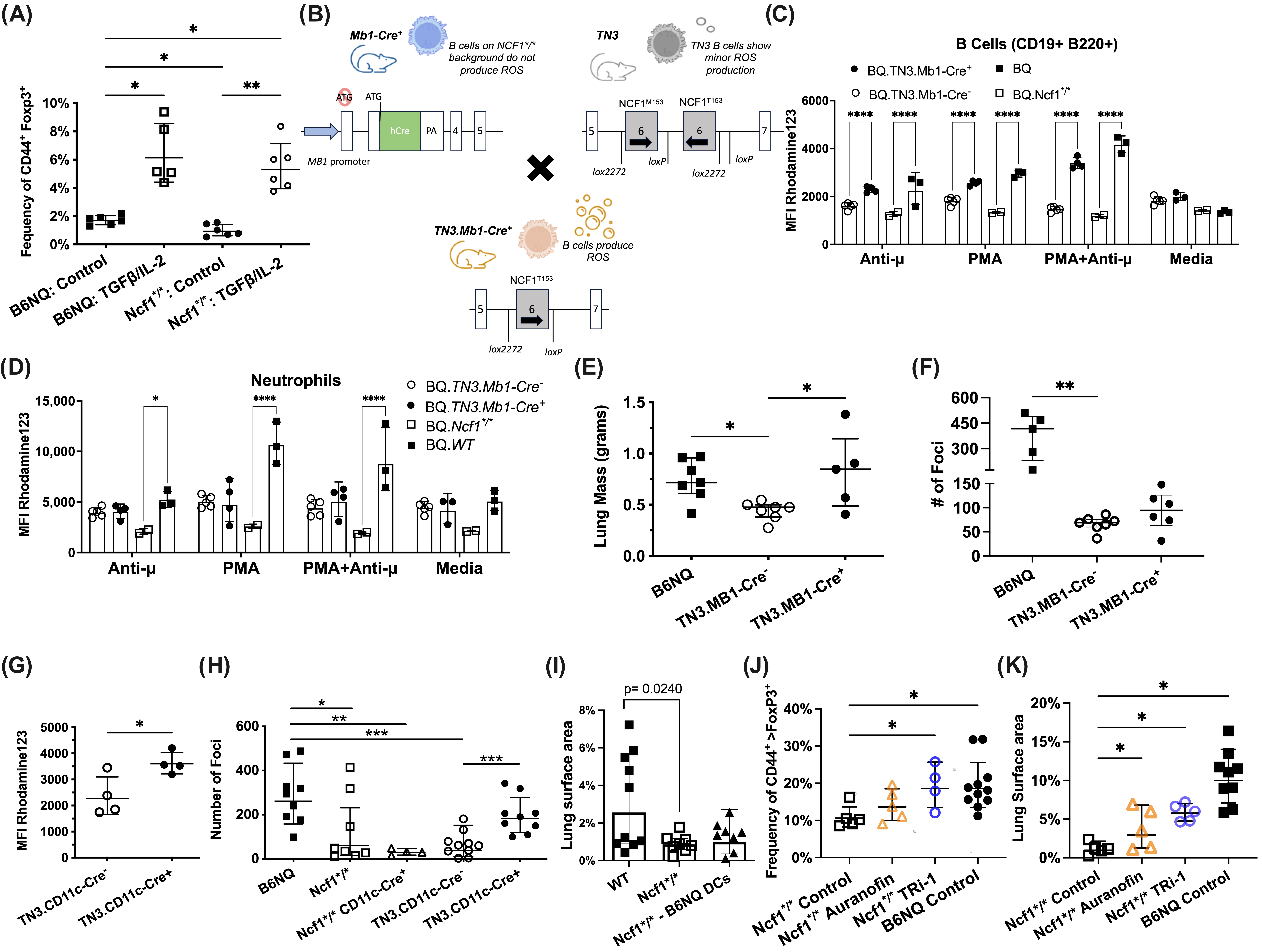
3.7. B Cell Specific NOX-2 ROS Augments B16F10 Tumor Burden
3.8. DC Specific NOX-2 ROS Augments B16F10 Tumor Burden
3.9. TXNRD1 Inhibitors Expand Tregs and Augment B16F10 Tumors in Ncf1*/* Mice
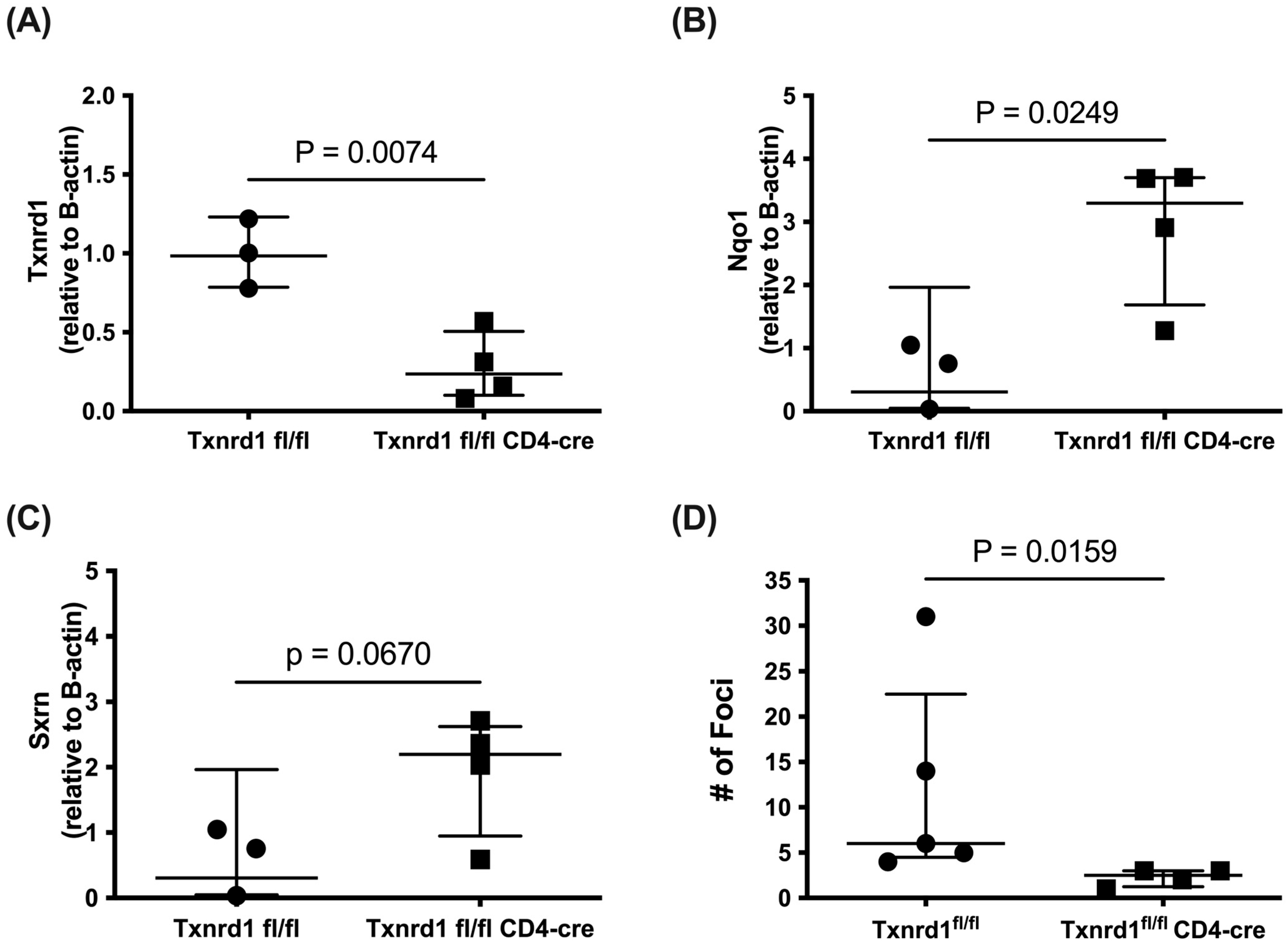
3.10. Txnrd1fl/fl CD4-Cre Mice Upregulate NRF2 Target Genes and Reduce B16F10 Tumor Burden
3.11. CD4+ T Cells from TXNRD1fl/fl CD4-Cre Mice Express Less FOXP3 and GITR
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TXNRD1 | Thioredoxin reductase 1 (TXNRD1) |
| Treg | Regulatory T cell |
| Ncf1 | neutrophil cytosolic factor 1 |
| APC | antigen-presenting cells |
| NOX2 | NADPH oxidase 2 |
Appendix A
| Reagent or Resource | Source | Cat Number | Dilution/ Concentration | RRID |
|---|---|---|---|---|
| Auranofin | R&D Systems | 4600/50 | ||
| TRi-1 | Arner Lab | |||
| Cell Dissociation Buffer, enzyme-free, PBS | Gibco | 13151014 | ||
| B16F10 | ATCC | CRL-6475 | CVCL_0159 | |
| Ionomycin | ThermoFisher | I24222 | 1 μg/mL | |
| BFA | ThermoFisher | B7450 | 5 μg/mL | |
| PMA | Sigma-Aldrich | CAS#: 16561-29-8 Cat. P8139 | 100 ng/mL | |
| dimethylsulfoxide (DMSO) | Sigma-Aldrich | CAS# 67-68-5 | ||
| DHR | ThermoFisher | D23806 | 3 μM | |
| Fixable Live/Dead NIR | ThermoFisher | L10119 | 1:1000 | |
| BD cytofix/cytoperm solution | BD Biosciences | 554714 | AB_2869008 | |
| iScript™ cDNA Synthesis Kit | Bio-Rad | 1708891 | ||
| Sulforhodamine B sodium salt | Sigma-Aldrich | S1402-1G | ||
| QIAshredder (50) | Qiagen | 79654 | ||
| RNeasy Kits for RNA Purification | Qiagen | 74106 | ||
| Iq™ SYBR, 1708882 | Bio-Rad | 1708882 | ||
| CD19 Microbeads | Miltenyi Biotec | 130-052-201 | ||
| Recombinant Mouse FLT3L (carrier-free) | Biolegend | 550704 | ||
| Recombinant Mouse DLL1 Fc Chimera Protein, CF | R&D Systems | 5026-DL-050 | ||
| DMA (N,N-Dimethylacetamide) | Sigma | CAS# 127-19-5 Cat. PHR2110 | ||
| PEG 400 | Merck | 8.17003.1000 | ||
| Cremophor | Sigma | C5135-500G | ||
| Ficoll-Paque PLUS | Cytiva | 17-1440-02 | ||
| Serologies | ||||
| MSD U-PLEX Custom Biomarker Group1 | Meso Scale Diagnostics | K15069M-2 |
| Target | Clone | Fluorophore | Source | Cat Number | Dilution/ Concentration | RRID |
|---|---|---|---|---|---|---|
| Mouse Abs | ||||||
| CD45 | 30-F11 | AlexaFluor 700 | Biolegend | 103128 | 1:200 | AB_493715 |
| TCRß | H57 597 | PE-Cyanine7 | BD Biosciences | 560729 | 1:200 | AB_1937310 |
| CD4 | RM4_5 | Pacific Blue | BD Biosciences | 558107 | 1:100 | AB_397030 |
| CD8a | 53-6.7 | Qdot 605 | BD Biosciences | 563152 | 1:100 | AB_2738030 |
| CD44 | IM7 | Alexa Fluor 700 | Biolegend | 103026 | 1:200 | AB_493713 |
| CD25 | PC61 | PE | BD Biosciences | 553866 | 1:200 | AB_395101 |
| FOXP3 | FJK-16s | APC | eBioscience | 17-5773-82 | 1:200 | AB_469457 |
| CD45R/B220 | RA3 6B2 | PB | BD Biosciences | 558108 | 1:200 | AB_397031 |
| CD19 | 6D5 | PE | Biolegend | 115520 | 1:200 | AB_313655 |
| LY6G | 1A8 | PerCP/Cy5.5 | Biolegend | 127616 | 1:200 | AB_1877271 |
| CD11c | N418 | BV711 | Biolegend | 117349 | 1:200 | AB_2563905 |
| CD11b | M1/70 (RUO) | BV605 | BD Biosciences | 563015 | 1:200 | AB_2737951 |
| MHC Class II (I-A/I-E) | M5/114.15.2 | FITC | Thermo Scientific | 11-5321-85 | 1:200 | AB_465233 |
| IL-10 | JES5-16E3 | FITC | Biolegend | 505006 | 1:200 | AB_315360 |
| IgM, µ chain specific | Polyclonal | Jackson ImmunoResearch | 115-006-075 | 1:200 | AB_2338474 | |
| CD16/CD32 Fcr RIII | 2.4G2 | Holmdahl lab | 1:50 | |||
| Human abs/PBMC ex vivo studies | ||||||
| TCRβ | H57-597 | AlexaFluor 488 | Biolegend | 109215 | 1:200 | AB_493344 |
| CD4 | OKT4 | BV605 | Biolegend | 317437 | 1:200 | AB_11204077 |
| CD25 | M-A251 | PE-Cy7 | BD Biosciences | 560920 | 1:200 | AB_396847 |
| FOXP3 | PCH101 | APC | eBioscience | 17-4776-41 | 1:200 | AB_1603281 |
| IL10 anti-human | JES3-9D7 | BV421 | BD Biosciences | 566276 | 1:200 | AB_2738566 |
| Fc Block | Fc1 | BD Biosciences | 564219 | 1:50 | AB_2728082 |
| qPCR Targets | Primer Sequences (Source: Eurofins) | ||
|---|---|---|---|
| Forward | Reverse | Product Length (bp) | |
| B-actin | GCAGGAGTACGATGAGTCCG | ACGCAGCTCAGTAACAGTCC | 74 |
| Hmox1 | CAGAACCCAGTCTATGCCCC | GTGAGGCCCATACCAGAAGG | 93 |
| Nqo1 | CTCACGGAAGTATGTGTCTCCT | CCCAGGGCTGAGGGTGTATT | 105 |
| Srxn1 | CTCATACCTTCCAGTTTGGGT | AGGCTATTGCATGGTGTGT | 149 |
| Txnrd1 | AGCGAGGAGACCATAGAGGG | CTCCAGGATGTCACCGATGG | 186 |
| Xct | AGCGAAGGCTGAAACACAC | CCCTTTGCTATCACCGACTGG | 76 |



References
- Gamberi, T.; Chiappetta, G.; Fiaschi, T.; Modesti, A.; Sorbi, F.; Magherini, F. Upgrade of an old drug: Auranofin in innovative cancer therapies to overcome drug resistance and to increase drug effectiveness. Med. Res. Rev. 2022, 42, 1111–1146. [Google Scholar] [CrossRef]
- Hochberg, M.C. Rheumatology, 5th ed.; Mosby/Elsevier: Philadelphia, PA, USA, 2011. [Google Scholar]
- Vint, I.A.; Chain, B.M.; Foreman, J.C. The interaction of auranofin and buthionine sulfoximine blocks activation of human peripheral T lymphocytes. Cell. Immunol. 1993, 152, 152–161. [Google Scholar] [CrossRef]
- Vint, I.A.; Foreman, J.C.; Chain, B.M. The gold anti-rheumatic drug auranofin governs T cell activation by enhancing oxygen free radical production. Eur. J. Immunol. 1994, 24, 1961–1965. [Google Scholar] [CrossRef]
- Salinas, G.; Gao, W.; Wang, Y.; Bonilla, M.; Yu, L.; Novikov, A.; Virginio, V.G.; Ferreira, H.B.; Vieites, M.; Gladyshev, V.N.; et al. The Enzymatic and Structural Basis for Inhibition of Echinococcus granulosus Thioredoxin Glutathione Reductase by Gold(I). Antioxid. Redox Signal. 2017, 27, 1491–1504. [Google Scholar] [CrossRef]
- Zhong, L.; Arner, E.S.; Ljung, J.; Aslund, F.; Holmgren, A. Rat and calf thioredoxin reductase are homologous to glutathione reductase with a carboxyl-terminal elongation containing a conserved catalytically active penultimate selenocysteine residue. J. Biol. Chem. 1998, 273, 8581–8591. [Google Scholar] [CrossRef] [PubMed]
- Cheff, D.M.; Cheng, Q.; Guo, H.; Travers, J.; Klumpp-Thomas, C.; Shen, M.; Arner, E.S.J.; Hall, M.D. Development of an assay pipeline for the discovery of novel small molecule inhibitors of human glutathione peroxidases GPX1 and GPX4. Redox Biol. 2023, 63, 102719. [Google Scholar] [CrossRef] [PubMed]
- Gorrini, C.; Harris, I.S.; Mak, T.W. Modulation of oxidative stress as an anticancer strategy. Nat. Rev. Drug Discov. 2013, 12, 931–947. [Google Scholar] [CrossRef]
- McLoughlin, M.R.; Orlicky, D.J.; Prigge, J.R.; Krishna, P.; Talago, E.A.; Cavigli, I.R.; Eriksson, S.; Miller, C.G.; Kundert, J.A.; Sayin, V.I.; et al. TrxR1, Gsr, and oxidative stress determine hepatocellular carcinoma malignancy. Proc. Natl. Acad. Sci. USA 2019, 116, 11408–11417. [Google Scholar] [CrossRef] [PubMed]
- Anestal, K.; Prast-Nielsen, S.; Cenas, N.; Arner, E.S. Cell death by SecTRAPs: Thioredoxin reductase as a prooxidant killer of cells. PLoS ONE 2008, 3, e1846. [Google Scholar] [CrossRef]
- Gencheva, R.; Arner, E.S.J. Thioredoxin Reductase Inhibition for Cancer Therapy. Annu. Rev. Pharmacol. Toxicol. 2022, 62, 177–196. [Google Scholar] [CrossRef]
- Stafford, W.C.; Peng, X.; Olofsson, M.H.; Zhang, X.; Luci, D.K.; Lu, L.; Cheng, Q.; Tresaugues, L.; Dexheimer, T.S.; Coussens, N.P.; et al. Irreversible inhibition of cytosolic thioredoxin reductase 1 as a mechanistic basis for anticancer therapy. Sci. Transl. Med. 2018, 10, eaaf7444. [Google Scholar] [CrossRef]
- Wang, H.; Bouzakoura, S.; de Mey, S.; Jiang, H.; Law, K.; Dufait, I.; Corbet, C.; Verovski, V.; Gevaert, T.; Feron, O.; et al. Auranofin radiosensitizes tumor cells through targeting thioredoxin reductase and resulting overproduction of reactive oxygen species. Oncotarget 2017, 8, 35728–35742. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, M.; McGrath, K.L.; Di Trapani, G.; Charoentong, P.; Shah, F.; King, M.M.; Clarke, F.M.; Tonissen, K.F. The thioredoxin system in breast cancer cell invasion and migration. Redox Biol. 2016, 8, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Yoo, M.H.; Xu, X.M.; Carlson, B.A.; Gladyshev, V.N.; Hatfield, D.L. Thioredoxin reductase 1 deficiency reverses tumor phenotype and tumorigenicity of lung carcinoma cells. J. Biol. Chem. 2006, 281, 13005–13008. [Google Scholar] [CrossRef]
- Raninga, P.V.; Lee, A.C.; Sinha, D.; Shih, Y.Y.; Mittal, D.; Makhale, A.; Bain, A.L.; Nanayakarra, D.; Tonissen, K.F.; Kalimutho, M.; et al. Therapeutic cooperation between auranofin, a thioredoxin reductase inhibitor and anti-PD-L1 antibody for treatment of triple-negative breast cancer. Int. J. Cancer 2020, 146, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Lin, E.Y.; Jones, J.G.; Li, P.; Zhu, L.; Whitney, K.D.; Muller, W.J.; Pollard, J.W. Progression to malignancy in the polyoma middle T oncoprotein mouse breast cancer model provides a reliable model for human diseases. Am. J. Pathol. 2003, 163, 2113–2126. [Google Scholar] [CrossRef]
- Sellers, R.S.; Clifford, C.B.; Treuting, P.M.; Brayton, C. Immunological variation between inbred laboratory mouse strains: Points to consider in phenotyping genetically immunomodified mice. Vet. Pathol. 2012, 49, 32–43. [Google Scholar] [CrossRef]
- Osman, G.E.; Hannibal, M.C.; Anderson, J.P.; Lasky, S.R.; Ladiges, W.C.; Hood, L. FVB/N (H2(q)) mouse is resistant to arthritis induction and exhibits a genomic deletion of T-cell receptor V beta gene segments. Immunogenetics 1999, 49, 851–859. [Google Scholar] [CrossRef]
- Gross, E.T.; Han, S.; Vemu, P.; Peinado, C.D.; Marsala, M.; Ellies, L.G.; Bui, J.D. Immunosurveillance and immunoediting in MMTV-PyMT-induced mammary oncogenesis. Oncoimmunology 2017, 6, e1268310. [Google Scholar] [CrossRef]
- Foerster, F.; Boegel, S.; Heck, R.; Pickert, G.; Russel, N.; Rosigkeit, S.; Bros, M.; Strobl, S.; Kaps, L.; Aslam, M.; et al. Enhanced protection of C57 BL/6 vs Balb/c mice to melanoma liver metastasis is mediated by NK cells. Oncoimmunology 2018, 7, e1409929. [Google Scholar] [CrossRef]
- Chen, X.; Oppenheim, J.J.; Howard, O.M. BALB/c mice have more CD4+CD25+ T regulatory cells and show greater susceptibility to suppression of their CD4+CD25− responder T cells than C57BL/6 mice. J. Leukoc. Biol. 2005, 78, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Muller, A.M.S.; Min, D.; Wernig, G.; Levy, R.B.; Perez, V.L.; Herretes, S.; Florek, M.; Burnett, C.; Weinberg, K.; Shizuru, J.A. Modeling Chronic Graft-versus-Host Disease in MHC-Matched Mouse Strains: Genetics, Graft Composition, and Tissue Targets. Biol. Blood Marrow Transplant. 2019, 25, 2338–2349. [Google Scholar] [CrossRef]
- Mirabelli, C.K.; Johnson, R.K.; Sung, C.M.; Faucette, L.; Muirhead, K.; Crooke, S.T. Evaluation of the in vivo antitumor activity and in vitro cytotoxic properties of auranofin, a coordinated gold compound, in murine tumor models. Cancer Res. 1985, 45, 32–39. [Google Scholar]
- Lee, D.; Xu, I.M.; Chiu, D.K.; Leibold, J.; Tse, A.P.; Bao, M.H.; Yuen, V.W.; Chan, C.Y.; Lai, R.K.; Chin, D.W.; et al. Induction of Oxidative Stress Through Inhibition of Thioredoxin Reductase 1 Is an Effective Therapeutic Approach for Hepatocellular Carcinoma. Hepatology 2019, 69, 1768–1786. [Google Scholar] [CrossRef]
- Kelkka, T.; Pizzolla, A.; Laurila, J.P.; Friman, T.; Gustafsson, R.; Kallberg, E.; Olsson, O.; Leanderson, T.; Rubin, K.; Salmi, M.; et al. Mice lacking NCF1 exhibit reduced growth of implanted melanoma and carcinoma tumors. PLoS ONE 2013, 8, e84148. [Google Scholar] [CrossRef]
- Kong, X.; Thimmulappa, R.; Craciun, F.; Harvey, C.; Singh, A.; Kombairaju, P.; Reddy, S.P.; Remick, D.; Biswal, S. Enhancing Nrf2 pathway by disruption of Keap1 in myeloid leukocytes protects against sepsis. Am. J. Respir. Crit. Care Med. 2011, 184, 928–938. [Google Scholar] [CrossRef] [PubMed]
- Macoch, M.; Morzadec, C.; Genard, R.; Pallardy, M.; Kerdine-Romer, S.; Fardel, O.; Vernhet, L. Nrf2-dependent repression of interleukin-12 expression in human dendritic cells exposed to inorganic arsenic. Free Radic. Biol. Med. 2015, 88, 381–390. [Google Scholar] [CrossRef]
- Hammer, A.; Waschbisch, A.; Knippertz, I.; Zinser, E.; Berg, J.; Jorg, S.; Kuhbandner, K.; David, C.; Pi, J.; Bayas, A.; et al. Role of Nuclear Factor (Erythroid-Derived 2)-Like 2 Signaling for Effects of Fumaric Acid Esters on Dendritic Cells. Front. Immunol. 2017, 8, 1922. [Google Scholar] [CrossRef]
- Suzuki, T.; Murakami, S.; Biswal, S.S.; Sakaguchi, S.; Harigae, H.; Yamamoto, M.; Motohashi, H. Systemic Activation of NRF2 Alleviates Lethal Autoimmune Inflammation in Scurfy Mice. Mol. Cell. Biol. 2017, 37, e00063-17. [Google Scholar] [CrossRef] [PubMed]
- Keleku-Lukwete, N.; Suzuki, M.; Yamamoto, M. An Overview of the Advantages of KEAP1-NRF2 System Activation During Inflammatory Disease Treatment. Antioxid. Redox Signal. 2018, 29, 1746–1755. [Google Scholar] [CrossRef]
- Kobayashi, E.H.; Suzuki, T.; Funayama, R.; Nagashima, T.; Hayashi, M.; Sekine, H.; Tanaka, N.; Moriguchi, T.; Motohashi, H.; Nakayama, K.; et al. Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. Nat. Commun. 2016, 7, 11624. [Google Scholar] [CrossRef] [PubMed]
- Monks, A.; Scudiero, D.; Skehan, P.; Shoemaker, R.; Paull, K.; Vistica, D.; Hose, C.; Langley, J.; Cronise, P.; Vaigro-Wolff, A.; et al. Feasibility of a high-flux anticancer drug screen using a diverse panel of cultured human tumor cell lines. J. Natl. Cancer Inst. 1991, 83, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Sareila, O.; Jaakkola, N.; Olofsson, P.; Kelkka, T.; Holmdahl, R. Identification of a region in p47phox/NCF1 crucial for phagocytic NADPH oxidase (NOX2) activation. J. Leukoc. Biol. 2013, 93, 427–435. [Google Scholar] [CrossRef]
- Luo, H.; Urbonaviciute, V.; Saei, A.A.; Lyu, H.; Gaetani, M.; Vegvari, A.; Li, Y.; Zubarev, R.A.; Holmdahl, R. NCF1-dependent production of ROS protects against lupus by regulating plasmacytoid dendritic cell development and functions. JCI Insight 2023, 8, e164875. [Google Scholar] [CrossRef]
- Sareila, O.; Hagert, C.; Kelkka, T.; Linja, M.; Xu, B.; Kihlberg, J.; Holmdahl, R. Reactive Oxygen Species Regulate Both Priming and Established Arthritis, but with Different Mechanisms. Antioxid. Redox Signal. 2017, 27, 1473–1490. [Google Scholar] [CrossRef]
- Bankhead, P.; Loughrey, M.B.; Fernandez, J.A.; Dombrowski, Y.; McArt, D.G.; Dunne, P.D.; McQuaid, S.; Gray, R.T.; Murray, L.J.; Coleman, H.G.; et al. QuPath: Open source software for digital pathology image analysis. Sci. Rep. 2017, 7, 16878. [Google Scholar] [CrossRef] [PubMed]
- Kress, J.K.C.; Jessen, C.; Hufnagel, A.; Schmitz, W.; Xavier da Silva, T.N.; Ferreira Dos Santos, A.; Mosteo, L.; Goding, C.R.; Friedmann Angeli, J.P.; Meierjohann, S. The integrated stress response effector ATF4 is an obligatory metabolic activator of NRF2. Cell Rep. 2023, 42, 112724. [Google Scholar] [CrossRef] [PubMed]
- Morgenstern, C.; Lastres-Becker, I.; Demirdogen, B.C.; Costa, V.M.; Daiber, A.; Foresti, R.; Motterlini, R.; Kalyoncu, S.; Arioz, B.I.; Genc, S.; et al. Biomarkers of NRF2 signalling: Current status and future challenges. Redox Biol. 2024, 72, 103134. [Google Scholar] [CrossRef]
- Sabatier, P.; Beusch, C.M.; Gencheva, R.; Cheng, Q.; Zubarev, R.; Arner, E.S.J. Comprehensive chemical proteomics analyses reveal that the new TRi-1 and TRi-2 compounds are more specific thioredoxin reductase 1 inhibitors than auranofin. Redox Biol. 2021, 48, 102184. [Google Scholar] [CrossRef]
- National Library of Medicine. NDC Code: 54766-093-06. LABEL: RIDAURA-Auranofin Capsule; FDA Boxed Warning; Dosage and Administration. 2018. Available online: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=43ba3de1-ab2e-06f6-e054-00144ff8d46c (accessed on 8 November 2024).
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef]
- Capparelli, E.V.; Bricker-Ford, R.; Rogers, M.J.; McKerrow, J.H.; Reed, S.L. Phase I Clinical Trial Results of Auranofin, a Novel Antiparasitic Agent. Antimicrob. Agents Chemother. 2017, 61, 10-1128. [Google Scholar] [CrossRef]
- Johnson, S.S.; Liu, D.; Ewald, J.T.; Robles-Planells, C.; Christensen, K.A.; Bayanbold, K.; Wels, B.R.; Solst, S.R.; O’Dorisio, M.S.; Allen, B.G.; et al. Auranofin Inhibition of Thioredoxin Reductase Sensitizes Lung Neuroendocrine Tumor Cells (NETs) and Small Cell Lung Cancer (SCLC) Cells to Sorafenib as well as Inhibiting SCLC Xenograft Growth. Cancer Biol. Ther. 2024, 25, 2382524. [Google Scholar] [CrossRef] [PubMed]
- Saei, A.A.; Gullberg, H.; Sabatier, P.; Beusch, C.M.; Johansson, K.; Lundgren, B.; Arvidsson, P.I.; Arner, E.S.J.; Zubarev, R.A. Comprehensive chemical proteomics for target deconvolution of the redox active drug auranofin. Redox Biol. 2020, 32, 101491. [Google Scholar] [CrossRef] [PubMed]
- Saei, A.A.; Lundin, A.; Lyu, H.; Gharibi, H.; Luo, H.; Teppo, J.; Zhang, X.; Gaetani, M.; Vegvari, A.; Holmdahl, R.; et al. Multifaceted Proteome Analysis at Solubility, Redox, and Expression Dimensions for Target Identification. Adv. Sci. 2024, 11, e2401502. [Google Scholar] [CrossRef]
- Forman, H.J.; Bernardo, A.; Davies, K.J. What is the concentration of hydrogen peroxide in blood and plasma? Arch. Biochem. Biophys. 2016, 603, 48–53. [Google Scholar] [CrossRef]
- Kraaij, M.D.; Savage, N.D.; van der Kooij, S.W.; Koekkoek, K.; Wang, J.; van den Berg, J.M.; Ottenhoff, T.H.; Kuijpers, T.W.; Holmdahl, R.; van Kooten, C.; et al. Induction of regulatory T cells by macrophages is dependent on production of reactive oxygen species. Proc. Natl. Acad. Sci. USA 2010, 107, 17686–17691. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Li, Q.; Luo, H.; Holmdahl, R. Neutrophil-derived reactive oxygen species promote tumor colonization. Commun. Biol. 2021, 4, 865. [Google Scholar] [CrossRef]
- Muri, J.; Heer, S.; Matsushita, M.; Pohlmeier, L.; Tortola, L.; Fuhrer, T.; Conrad, M.; Zamboni, N.; Kisielow, J.; Kopf, M. The thioredoxin-1 system is essential for fueling DNA synthesis during T-cell metabolic reprogramming and proliferation. Nat. Commun. 2018, 9, 1851. [Google Scholar] [CrossRef]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef]
- Turk, M.J.; Guevara-Patino, J.A.; Rizzuto, G.A.; Engelhorn, M.E.; Sakaguchi, S.; Houghton, A.N. Concomitant tumor immunity to a poorly immunogenic melanoma is prevented by regulatory T cells. J. Exp. Med. 2004, 200, 771–782. [Google Scholar] [CrossRef]
- Noel, S.; Lee, S.A.; Sadasivam, M.; Hamad, A.R.A.; Rabb, H. KEAP1 Editing Using CRISPR/Cas9 for Therapeutic NRF2 Activation in Primary Human T Lymphocytes. J. Immunol. 2018, 200, 1929–1936. [Google Scholar] [CrossRef]
- Kurzhagen, J.T.; Noel, S.; Lee, K.; Sadasivam, M.; Gharaie, S.; Ankireddy, A.; Lee, S.A.; Newman-Rivera, A.; Gong, J.; Arend, L.J.; et al. T Cell Nrf2/Keap1 Gene Editing Using CRISPR/Cas9 and Experimental Kidney Ischemia-Reperfusion Injury. Antioxid. Redox Signal. 2023, 38, 959–973. [Google Scholar] [CrossRef] [PubMed]
- Yagishita, Y.; Uruno, A.; Chartoumpekis, D.V.; Kensler, T.W.; Yamamoto, M. Nrf2 represses the onset of type 1 diabetes in non-obese diabetic mice. J. Endocrinol. 2019, 240, 403–416. [Google Scholar] [CrossRef]
- Holmdahl, R.; Sareila, O.; Pizzolla, A.; Winter, S.; Hagert, C.; Jaakkola, N.; Kelkka, T.; Olsson, L.M.; Wing, K.; Backdahl, L. Hydrogen peroxide as an immunological transmitter regulating autoreactive T cells. Antioxid. Redox Signal. 2013, 18, 1463–1474. [Google Scholar] [CrossRef]
- Cen, Y.; Li, F.; Li, Y.; Zhang, K.; Riaz, F.; Zhao, K.; Wei, P.; Pan, F. Dimethyl fumarate alleviates allergic asthma by strengthening the Nrf2 signaling pathway in regulatory T cells. Front. Immunol. 2024, 15, 1375340. [Google Scholar] [CrossRef] [PubMed]
- Rojo de la Vega, M.; Chapman, E.; Zhang, D.D. NRF2 and the Hallmarks of Cancer. Cancer Cell 2018, 34, 21–43. [Google Scholar] [CrossRef]
- Pant, T.; Uche, N.; Juric, M.; Zielonka, J.; Bai, X. Regulation of immunomodulatory networks by Nrf2-activation in immune cells: Redox control and therapeutic potential in inflammatory diseases. Redox Biol. 2024, 70, 103077. [Google Scholar] [CrossRef]
- Lima, A.D.R.; Ferrari, B.B.; Pradella, F.; Carvalho, R.M.; Rivero, S.L.S.; Quintiliano, R.P.S.; Souza, M.A.; Brunetti, N.S.; Marques, A.M.; Santos, I.P.; et al. Dimethyl fumarate modulates the regulatory T cell response in the mesenteric lymph nodes of mice with experimental autoimmune encephalomyelitis. Front. Immunol. 2024, 15, 1391949. [Google Scholar] [CrossRef] [PubMed]
- Hammer, A.; Waschbisch, A.; Kuhbandner, K.; Bayas, A.; Lee, D.H.; Duscha, A.; Haghikia, A.; Gold, R.; Linker, R.A. The NRF2 pathway as potential biomarker for dimethyl fumarate treatment in multiple sclerosis. Ann. Clin. Transl. Neurol. 2018, 5, 668–676. [Google Scholar] [CrossRef]
- Pilotto, F.; Chellapandi, D.M.; Puccio, H. Omaveloxolone: A groundbreaking milestone as the first FDA-approved drug for Friedreich ataxia. Trends Mol. Med. 2024, 30, 117–125. [Google Scholar] [CrossRef]
- Procaccini, C.; Garavelli, S.; Carbone, F.; Di Silvestre, D.; La Rocca, C.; Greco, D.; Colamatteo, A.; Lepore, M.T.; Russo, C.; De Rosa, G.; et al. Signals of pseudo-starvation unveil the amino acid transporter SLC7A11 as key determinant in the control of Treg cell proliferative potential. Immunity 2021, 54, 1543–1560.E6. [Google Scholar] [CrossRef] [PubMed]
- Arensman, M.D.; Yang, X.S.; Leahy, D.M.; Toral-Barza, L.; Mileski, M.; Rosfjord, E.C.; Wang, F.; Deng, S.; Myers, J.S.; Abraham, R.T.; et al. Cystine-glutamate antiporter xCT deficiency suppresses tumor growth while preserving antitumor immunity. Proc. Natl. Acad. Sci. USA 2019, 116, 9533–9542. [Google Scholar] [CrossRef]
- Woo, Y.; Lee, H.J.; Kim, J.; Kang, S.G.; Moon, S.; Han, J.A.; Jung, Y.M.; Jung, Y.J. Rapamycin Promotes ROS-Mediated Cell Death via Functional Inhibition of xCT Expression in Melanoma Under gamma-Irradiation. Front. Oncol. 2021, 11, 665420. [Google Scholar] [CrossRef]
- Tsai, J.J.; Velardi, E.; Shono, Y.; Argyropoulos, K.V.; Holland, A.M.; Smith, O.M.; Yim, N.L.; Rao, U.K.; Kreines, F.M.; Lieberman, S.R.; et al. Nrf2 regulates CD4+ T cell-induced acute graft-versus-host disease in mice. Blood 2018, 132, 2763–2774. [Google Scholar] [CrossRef]
- Klemm, P.; Rajendiran, A.; Fragoulis, A.; Wruck, C.; Schippers, A.; Wagner, N.; Bopp, T.; Tenbrock, K.; Ohl, K. Nrf2 expression driven by Foxp3 specific deletion of Keap1 results in loss of immune tolerance in mice. Eur. J. Immunol. 2020, 50, 515–524. [Google Scholar] [CrossRef]
- Benrahmoune, M.; Therond, P.; Abedinzadeh, Z. The reaction of superoxide radical with N-acetylcysteine. Free Radic. Biol. Med. 2000, 29, 775–782. [Google Scholar] [CrossRef]
- Park, J.; Kim, M.; Kang, S.G.; Jannasch, A.H.; Cooper, B.; Patterson, J.; Kim, C.H. Short-chain fatty acids induce both effector and regulatory T cells by suppression of histone deacetylases and regulation of the mTOR-S6K pathway. Mucosal Immunol. 2015, 8, 80–93. [Google Scholar] [CrossRef] [PubMed]
- Gade, W.; Brown, J.L. Purification and partial characterization of alpha-N-acylpeptide hydrolase from bovine liver. J. Biol. Chem. 1978, 253, 5012–5018. [Google Scholar] [CrossRef] [PubMed]
- Koppula, P.; Zhang, Y.; Shi, J.; Li, W.; Gan, B. The glutamate/cystine antiporter SLC7A11/xCT enhances cancer cell dependency on glucose by exporting glutamate. J. Biol. Chem. 2017, 292, 14240–14249. [Google Scholar] [CrossRef]
- Klysz, D.; Tai, X.; Robert, P.A.; Craveiro, M.; Cretenet, G.; Oburoglu, L.; Mongellaz, C.; Floess, S.; Fritz, V.; Matias, M.I.; et al. Glutamine-dependent alpha-ketoglutarate production regulates the balance between T helper 1 cell and regulatory T cell generation. Sci. Signal. 2015, 8, ra97. [Google Scholar] [CrossRef]
- Lai, Z.W.; Hanczko, R.; Bonilla, E.; Caza, T.N.; Clair, B.; Bartos, A.; Miklossy, G.; Jimah, J.; Doherty, E.; Tily, H.; et al. N-acetylcysteine reduces disease activity by blocking mammalian target of rapamycin in T cells from systemic lupus erythematosus patients: A randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2012, 64, 2937–2946. [Google Scholar] [CrossRef] [PubMed]
- Obrador, E.; Salvador-Palmer, R.; Lopez-Blanch, R.; Oriol-Caballo, M.; Moreno-Murciano, P.; Estrela, J.M. N-Acetylcysteine Promotes Metastatic Spread of Melanoma in Mice. Cancers 2022, 14, 3614. [Google Scholar] [CrossRef] [PubMed]
- Jobling, M.F.; Mott, J.D.; Finnegan, M.T.; Jurukovski, V.; Erickson, A.C.; Walian, P.J.; Taylor, S.E.; Ledbetter, S.; Lawrence, C.M.; Rifkin, D.B.; et al. Isoform-specific activation of latent transforming growth factor beta (LTGF-beta) by reactive oxygen species. Radiat. Res. 2006, 166, 839–848. [Google Scholar] [CrossRef] [PubMed]
- Amarnath, S.; Dong, L.; Li, J.; Wu, Y.; Chen, W. Endogenous TGF-beta activation by reactive oxygen species is key to Foxp3 induction in TCR-stimulated and HIV-1-infected human CD4+CD25− T cells. Retrovirology 2007, 4, 57. [Google Scholar] [CrossRef]
- Cheng, X.; Haeberle, S.; Shytaj, I.L.; Gama-Brambila, R.A.; Theobald, J.; Ghafoory, S.; Wolker, J.; Basu, U.; Schmidt, C.; Timm, A.; et al. NHC-gold compounds mediate immune suppression through induction of AHR-TGFbeta1 signalling in vitro and in scurfy mice. Commun. Biol. 2020, 3, 10. [Google Scholar] [CrossRef] [PubMed]
- Pickering, I.J.; Cheng, Q.; Rengifo, E.M.; Nehzati, S.; Dolgova, N.V.; Kroll, T.; Sokaras, D.; George, G.N.; Arner, E.S.J. Direct Observation of Methylmercury and Auranofin Binding to Selenocysteine in Thioredoxin Reductase. Inorg. Chem. 2020, 59, 2711–2718. [Google Scholar] [CrossRef]
- Ba, Y.; Sun, L.; Zuo, J.; Yu, S.Y.; Yang, S.; Ding, L.M.; Feng, Z.C.; Li, Z.Y.; Zhou, G.Y.; Yu, F.F. Association of oxidative stress and Kashin-Beck disease integrated Meta and Bioinformatics analysis. Osteoarthr. Cartil. 2022, 30, 1606–1615. [Google Scholar] [CrossRef]
- Araujo-Jorge, T.C.; Waghabi, M.C.; Hasslocher-Moreno, A.M.; Xavier, S.S.; Higuchi Mde, L.; Keramidas, M.; Bailly, S.; Feige, J.J. Implication of transforming growth factor-beta1 in Chagas disease myocardiopathy. J. Infect. Dis. 2002, 186, 1823–1828. [Google Scholar] [CrossRef]
- Barrett, C.W.; Singh, K.; Motley, A.K.; Lintel, M.K.; Matafonova, E.; Bradley, A.M.; Ning, W.; Poindexter, S.V.; Parang, B.; Reddy, V.K.; et al. Dietary selenium deficiency exacerbates DSS-induced epithelial injury and AOM/DSS-induced tumorigenesis. PLoS ONE 2013, 8, e67845. [Google Scholar] [CrossRef]
- Hiramoto, K.; Satoh, H.; Suzuki, T.; Moriguchi, T.; Pi, J.; Shimosegawa, T.; Yamamoto, M. Myeloid lineage-specific deletion of antioxidant system enhances tumor metastasis. Cancer Prev. Res. 2014, 7, 835–844. [Google Scholar] [CrossRef]
- van de Geer, A.; Cuadrado, E.; Slot, M.C.; van Bruggen, R.; Amsen, D.; Kuijpers, T.W. Regulatory T cell features in chronic granulomatous disease. Clin. Exp. Immunol. 2019, 197, 222–229. [Google Scholar] [CrossRef]
- van der Weyden, L.; Speak, A.O.; Swiatkowska, A.; Clare, S.; Schejtman, A.; Santilli, G.; Arends, M.J.; Adams, D.J. Pulmonary metastatic colonisation and granulomas in NOX2-deficient mice. J. Pathol. 2018, 246, 300–310. [Google Scholar] [CrossRef]
- Aydin, E.; Johansson, J.; Nazir, F.H.; Hellstrand, K.; Martner, A. Role of NOX2-Derived Reactive Oxygen Species in NK Cell-Mediated Control of Murine Melanoma Metastasis. Cancer Immunol. Res. 2017, 5, 804–811. [Google Scholar] [CrossRef]
- Ligtenberg, M.A.; Cinar, O.; Holmdahl, R.; Mougiakakos, D.; Kiessling, R. Methylcholanthrene-Induced Sarcomas Develop Independently from NOX2-Derived ROS. PLoS ONE 2015, 10, e0129786. [Google Scholar] [CrossRef] [PubMed]
- Chamoto, K.; Wakita, D.; Ohkuri, T.; Uchinami, Y.; Matsushima, K.; Kitamura, H.; Nishimura, T. 3-Methylcholanthrene-induced transforming growth factor-beta-producing carcinomas, but not sarcomas, are refractory to regulatory T cell-depletion therapy. Cancer Sci. 2010, 101, 855–861. [Google Scholar] [CrossRef]
- Gupta, A.; Budhu, S.; Fitzgerald, K.; Giese, R.; Michel, A.O.; Holland, A.; Campesato, L.F.; van Snick, J.; Uyttenhove, C.; Ritter, G.; et al. Isoform specific anti-TGFbeta therapy enhances antitumor efficacy in mouse models of cancer. Commun. Biol. 2021, 4, 1296. [Google Scholar] [CrossRef]
- Bendle, G.M.; Linnemann, C.; Bies, L.; Song, J.Y.; Schumacher, T.N. Blockade of TGF-beta signaling greatly enhances the efficacy of TCR gene therapy of cancer. J. Immunol. 2013, 191, 3232–3239. [Google Scholar] [CrossRef]
- Betts, G.; Twohig, J.; Van den Broek, M.; Sierro, S.; Godkin, A.; Gallimore, A. The impact of regulatory T cells on carcinogen-induced sarcogenesis. Br. J. Cancer 2007, 96, 1849–1854. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pang, Y.; Gara, S.K.; Achyut, B.R.; Li, Z.; Yan, H.H.; Day, C.P.; Weiss, J.M.; Trinchieri, G.; Morris, J.C.; Yang, L. TGF-beta signaling in myeloid cells is required for tumor metastasis. Cancer Discov. 2013, 3, 936–951. [Google Scholar] [CrossRef] [PubMed]
- Nouel, A.; Pochard, P.; Simon, Q.; Segalen, I.; Le Meur, Y.; Pers, J.O.; Hillion, S. B-Cells induce regulatory T cells through TGF-beta/IDO production in A CTLA-4 dependent manner. J. Autoimmun. 2015, 59, 53–60. [Google Scholar] [CrossRef]
- Efimova, O.; Szankasi, P.; Kelley, T.W. Ncf1 (p47phox) is essential for direct regulatory T cell mediated suppression of CD4+ effector T cells. PLoS ONE 2011, 6, e16013. [Google Scholar] [CrossRef] [PubMed]
- Assoian, R.K.; Komoriya, A.; Meyers, C.A.; Miller, D.M.; Sporn, M.B. Transforming growth factor-beta in human platelets. Identification of a major storage site, purification, and characterization. J. Biol. Chem. 1983, 258, 7155–7160. [Google Scholar] [CrossRef]
- Nakamura, K.; Kitani, A.; Strober, W. Cell contact-dependent immunosuppression by CD4+CD25+ regulatory T cells is mediated by cell surface-bound transforming growth factor beta. J. Exp. Med. 2001, 194, 629–644. [Google Scholar] [CrossRef]
- Meyer, A.; Wang, W.; Qu, J.; Croft, L.; Degen, J.L.; Coller, B.S.; Ahamed, J. Platelet TGF-beta1 contributions to plasma TGF-beta1, cardiac fibrosis, and systolic dysfunction in a mouse model of pressure overload. Blood 2012, 119, 1064–1074. [Google Scholar] [CrossRef]
- Ahamed, J.; Laurence, J. Role of Platelet-Derived Transforming Growth Factor-beta1 and Reactive Oxygen Species in Radiation-Induced Organ Fibrosis. Antioxid. Redox Signal. 2017, 27, 977–988. [Google Scholar] [CrossRef]
- Rachidi, S.; Metelli, A.; Riesenberg, B.; Wu, B.X.; Nelson, M.H.; Wallace, C.; Paulos, C.M.; Rubinstein, M.P.; Garrett-Mayer, E.; Hennig, M.; et al. Platelets subvert T cell immunity against cancer via GARP-TGFbeta axis. Sci. Immunol. 2017, 2, eaai7911. [Google Scholar] [CrossRef]
- Bodogai, M.; Lee Chang, C.; Wejksza, K.; Lai, J.; Merino, M.; Wersto, R.P.; Gress, R.E.; Chan, A.C.; Hesdorffer, C.; Biragyn, A. Anti-CD20 antibody promotes cancer escape via enrichment of tumor-evoked regulatory B cells expressing low levels of CD20 and CD137L. Cancer Res. 2013, 73, 2127–2138. [Google Scholar] [CrossRef]
- DiLillo, D.J.; Yanaba, K.; Tedder, T.F. B cells are required for optimal CD4+ and CD8+ T cell tumor immunity: Therapeutic B cell depletion enhances B16 melanoma growth in mice. J. Immunol. 2010, 184, 4006–4016. [Google Scholar] [CrossRef]
- Hu, B.; Ren, J.; Luo, Y.; Keith, B.; Young, R.M.; Scholler, J.; Zhao, Y.; June, C.H. Augmentation of Antitumor Immunity by Human and Mouse CAR T Cells Secreting IL-18. Cell Rep. 2017, 20, 3025–3033. [Google Scholar] [CrossRef] [PubMed]
- Noyes, D.; Bag, A.; Oseni, S.; Semidey-Hurtado, J.; Cen, L.; Sarnaik, A.A.; Sondak, V.K.; Adeegbe, D. Tumor-associated Tregs obstruct antitumor immunity by promoting T cell dysfunction and restricting clonal diversity in tumor-infiltrating CD8+ T cells. J. Immunother. Cancer 2022, 10, e004605. [Google Scholar] [CrossRef] [PubMed]
- Workman, C.J.; Collison, L.W.; Bettini, M.; Pillai, M.R.; Rehg, J.E.; Vignali, D.A. In vivo Treg suppression assays. Methods Mol. Biol. 2011, 707, 119–156. [Google Scholar] [CrossRef] [PubMed]
- Perez-Riverol, Y.; Bai, J.; Bandla, C.; Garcia-Seisdedos, D.; Hewapathirana, S.; Kamatchinathan, S.; Kundu, D.J.; Prakash, A.; Frericks-Zipper, A.; Eisenacher, M.; et al. The PRIDE database resources in 2022: A hub for mass spectrometry-based proteomics evidences. Nucleic Acids Res. 2022, 50, D543–D552. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonner, M.Y.; Vancsik, T.; Oliveira-Coelho, A.; Sabatier, P.; Beusch, C.M.; Zeqiraj, K.; Svensson, C.; Zubarev, R.A.; Arnér, E.S.J.; Holmdahl, R. Anti-Tumoral Treatment with Thioredoxin Reductase 1 Inhibitor Auranofin Fosters Regulatory T Cell and B16F10 Expansion in Mice. Antioxidants 2025, 14, 1351. https://doi.org/10.3390/antiox14111351
Bonner MY, Vancsik T, Oliveira-Coelho A, Sabatier P, Beusch CM, Zeqiraj K, Svensson C, Zubarev RA, Arnér ESJ, Holmdahl R. Anti-Tumoral Treatment with Thioredoxin Reductase 1 Inhibitor Auranofin Fosters Regulatory T Cell and B16F10 Expansion in Mice. Antioxidants. 2025; 14(11):1351. https://doi.org/10.3390/antiox14111351
Chicago/Turabian StyleBonner, Michael Y., Tamas Vancsik, Ana Oliveira-Coelho, Pierre Sabatier, Christian M. Beusch, Kejsi Zeqiraj, Carolin Svensson, Roman A. Zubarev, Elias S. J. Arnér, and Rikard Holmdahl. 2025. "Anti-Tumoral Treatment with Thioredoxin Reductase 1 Inhibitor Auranofin Fosters Regulatory T Cell and B16F10 Expansion in Mice" Antioxidants 14, no. 11: 1351. https://doi.org/10.3390/antiox14111351
APA StyleBonner, M. Y., Vancsik, T., Oliveira-Coelho, A., Sabatier, P., Beusch, C. M., Zeqiraj, K., Svensson, C., Zubarev, R. A., Arnér, E. S. J., & Holmdahl, R. (2025). Anti-Tumoral Treatment with Thioredoxin Reductase 1 Inhibitor Auranofin Fosters Regulatory T Cell and B16F10 Expansion in Mice. Antioxidants, 14(11), 1351. https://doi.org/10.3390/antiox14111351







