Gadolinium-Based Magnetic Resonance Theranostic Agent with Gallic Acid as an Anti-Neuroinflammatory and Antioxidant Agent
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instrument
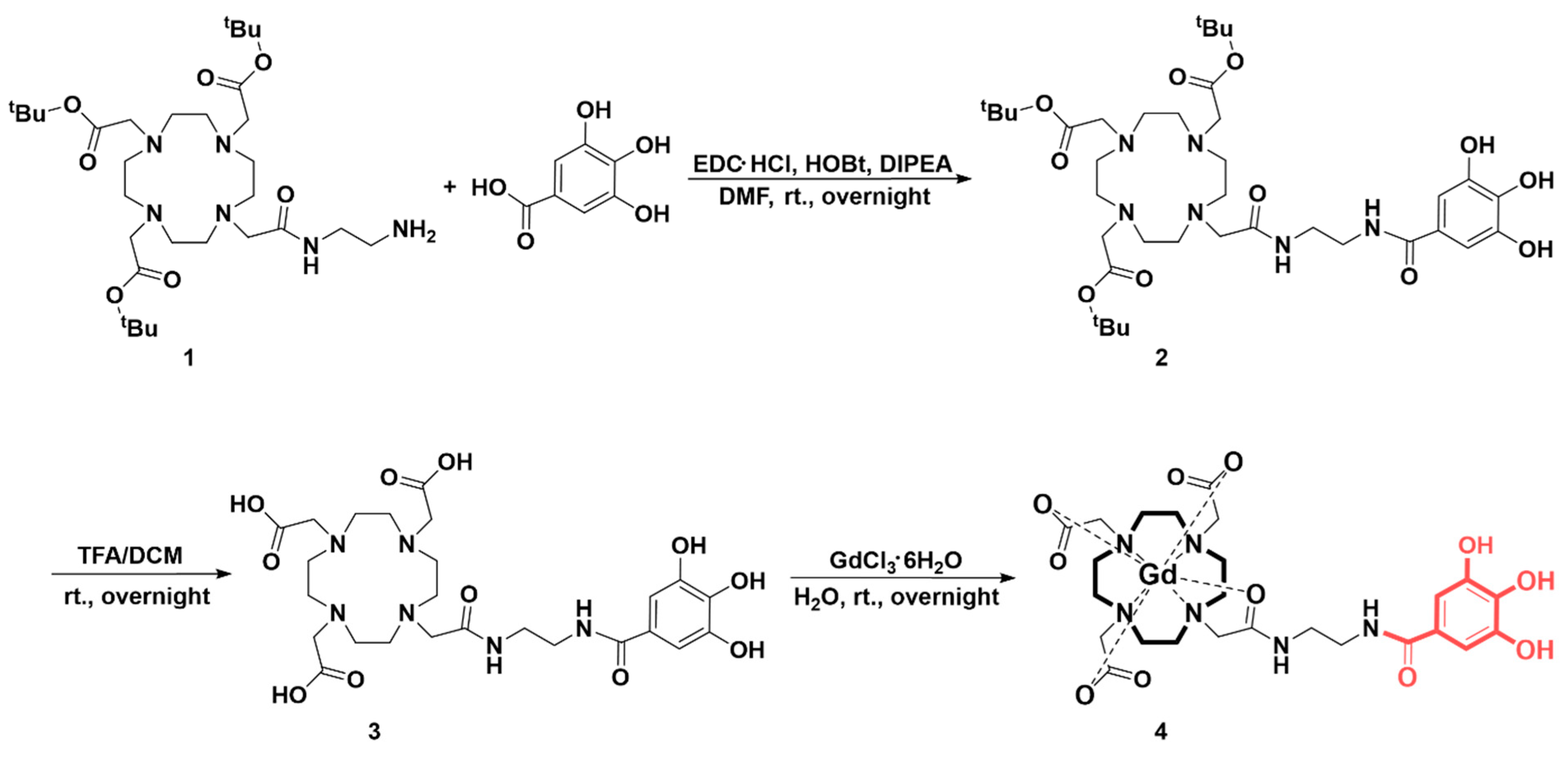
2.3. Synthesis and Characterization
2.4. Relaxivity Measurement
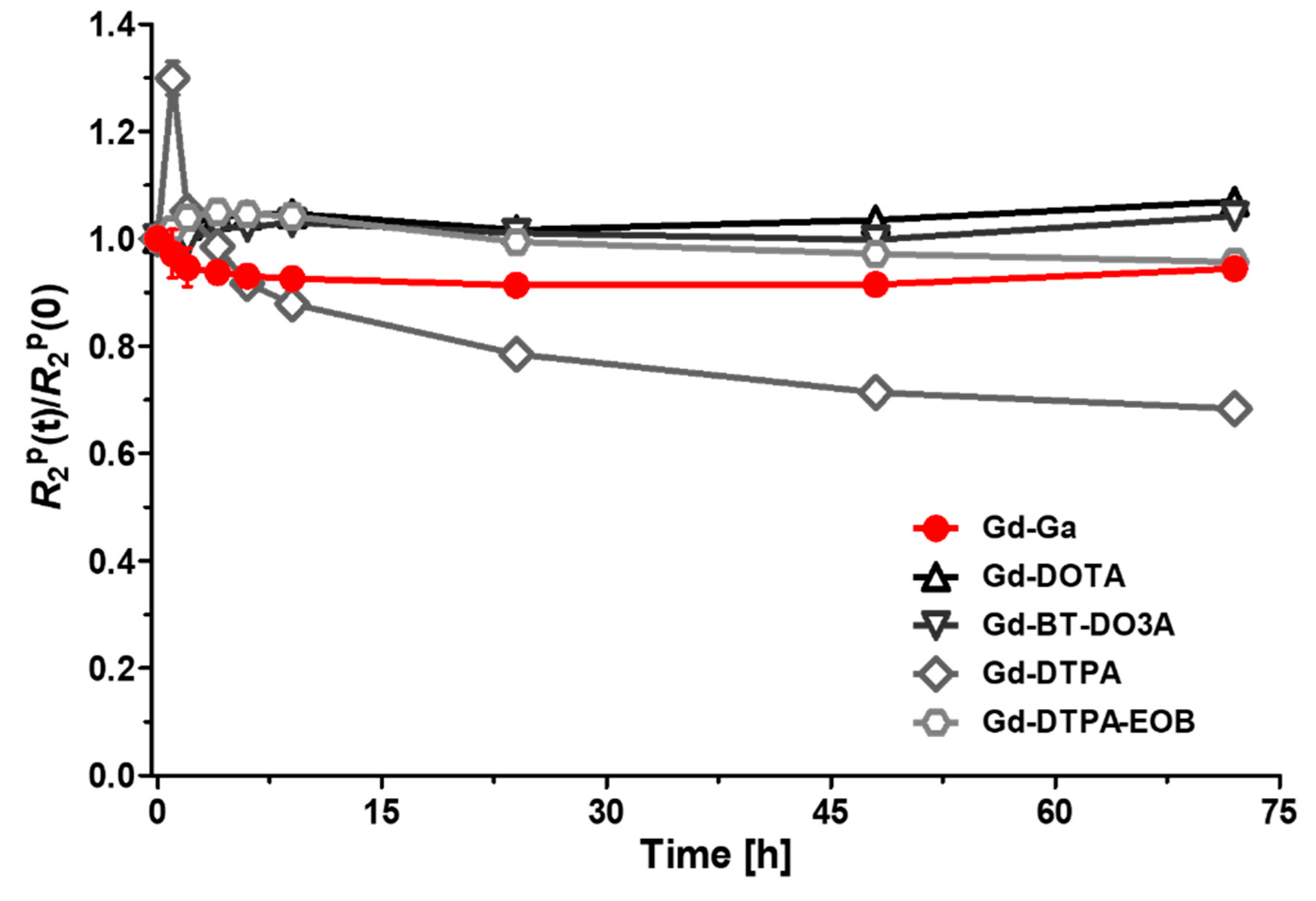
2.5. Kinetic Inertness and pH Stability Measurement
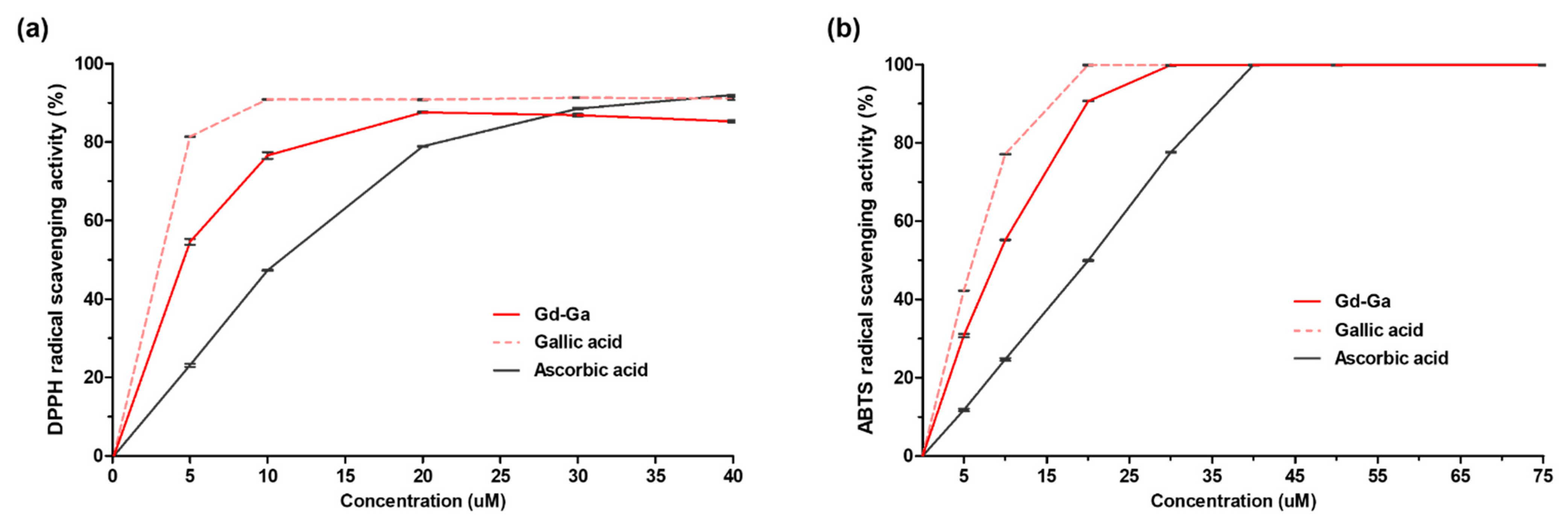
2.6. DPPH and ABTS Assay [25,26]
2.7. Cell Culture and Cell Viability Measurement
2.8. Griess Assay and DCF-DA Assay
2.9. Cytoplasmic and Nuclear Extracts for IκB and NF-κB
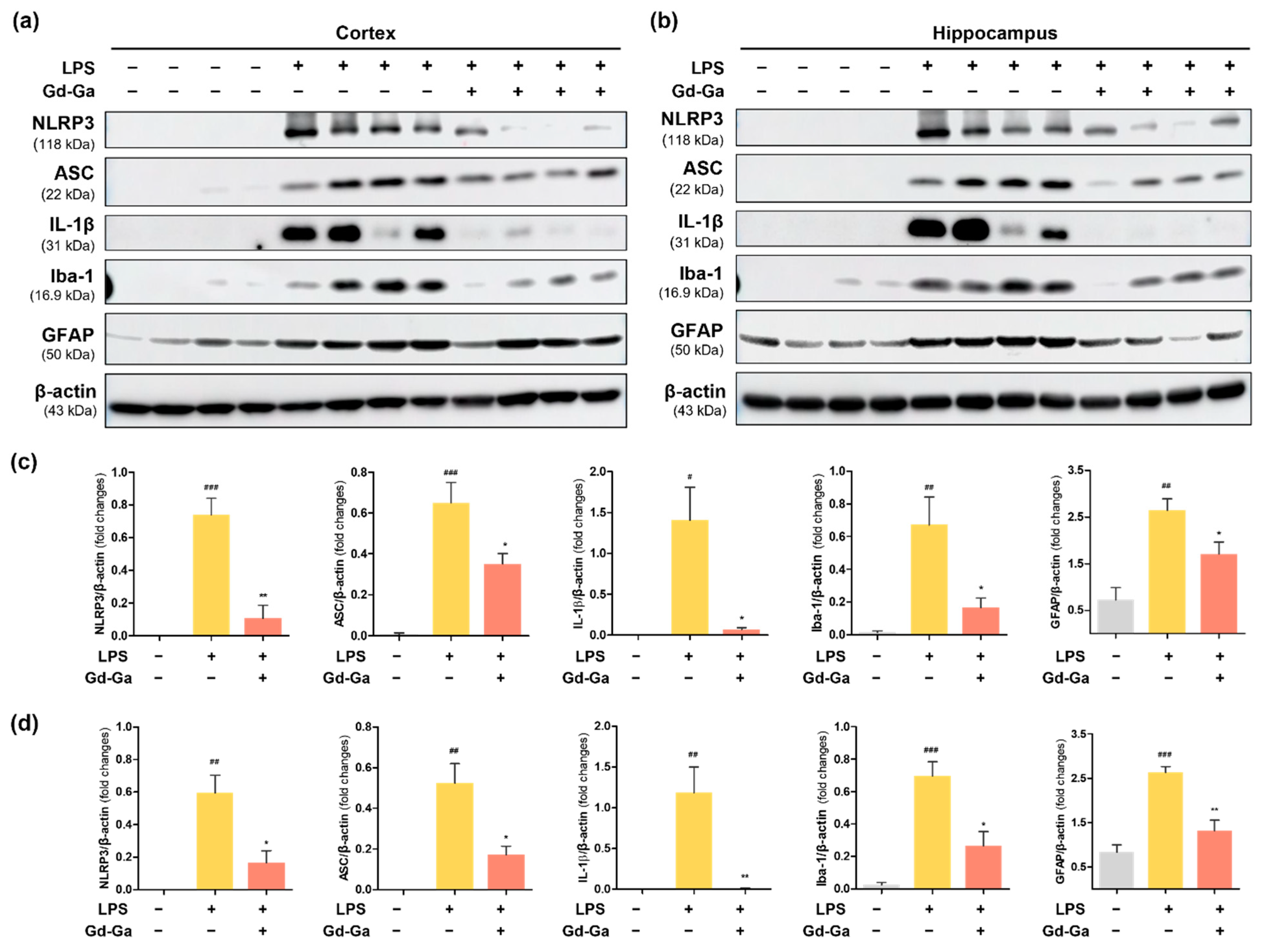
2.10. Western Blot
2.11. Biodistribution Method
2.12. LPS-Induced Meuroinflammation Mouse Model
- (I)
- i.c.v. saline group (control).
- (II)
- i.c.v. LPS group (LPS.)
- (III)
- i.c.v. LPS group treated with Gd-Ga (LPS + Gd-Ga).
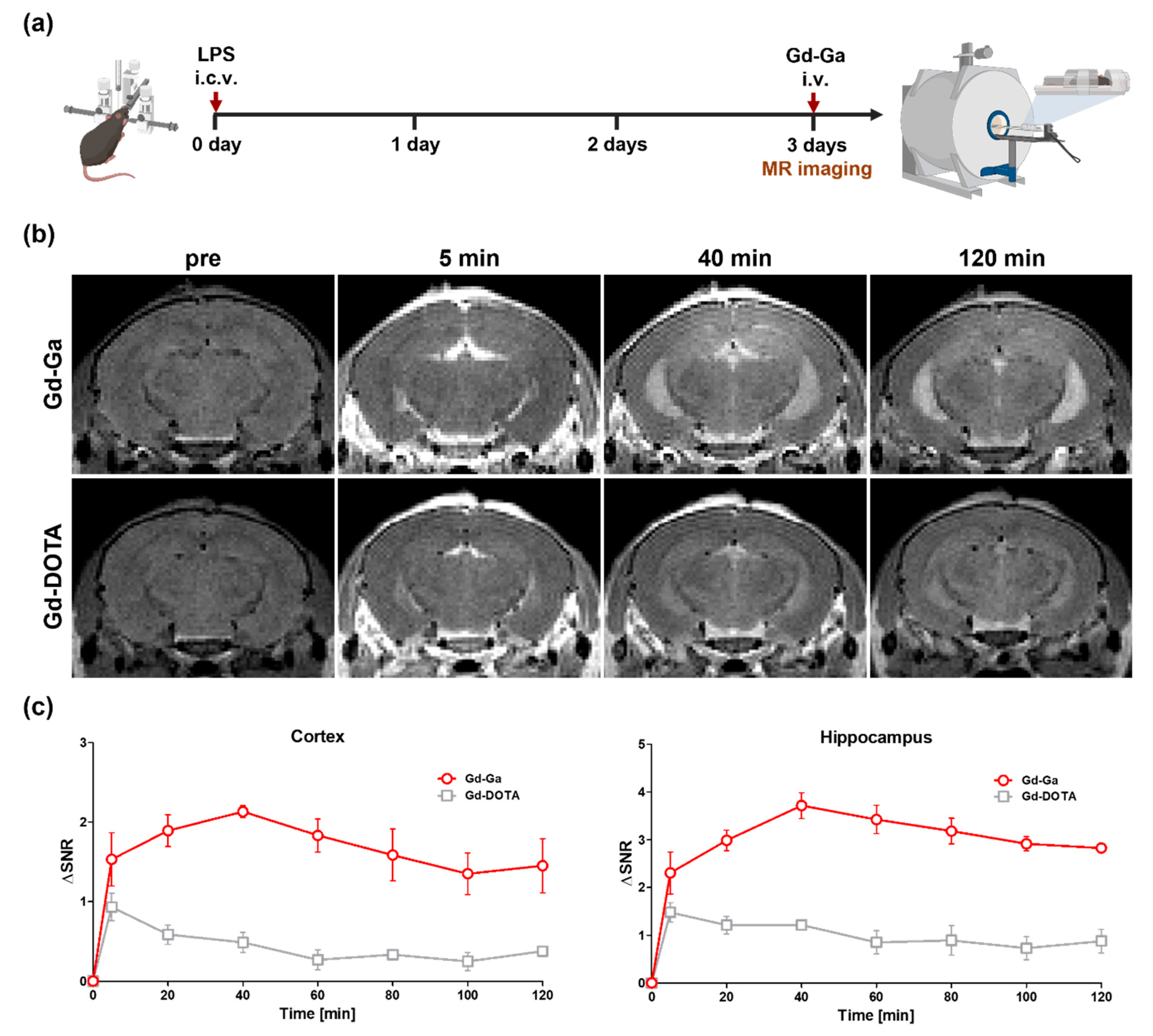
2.13. In Vivo MR Imaging Method
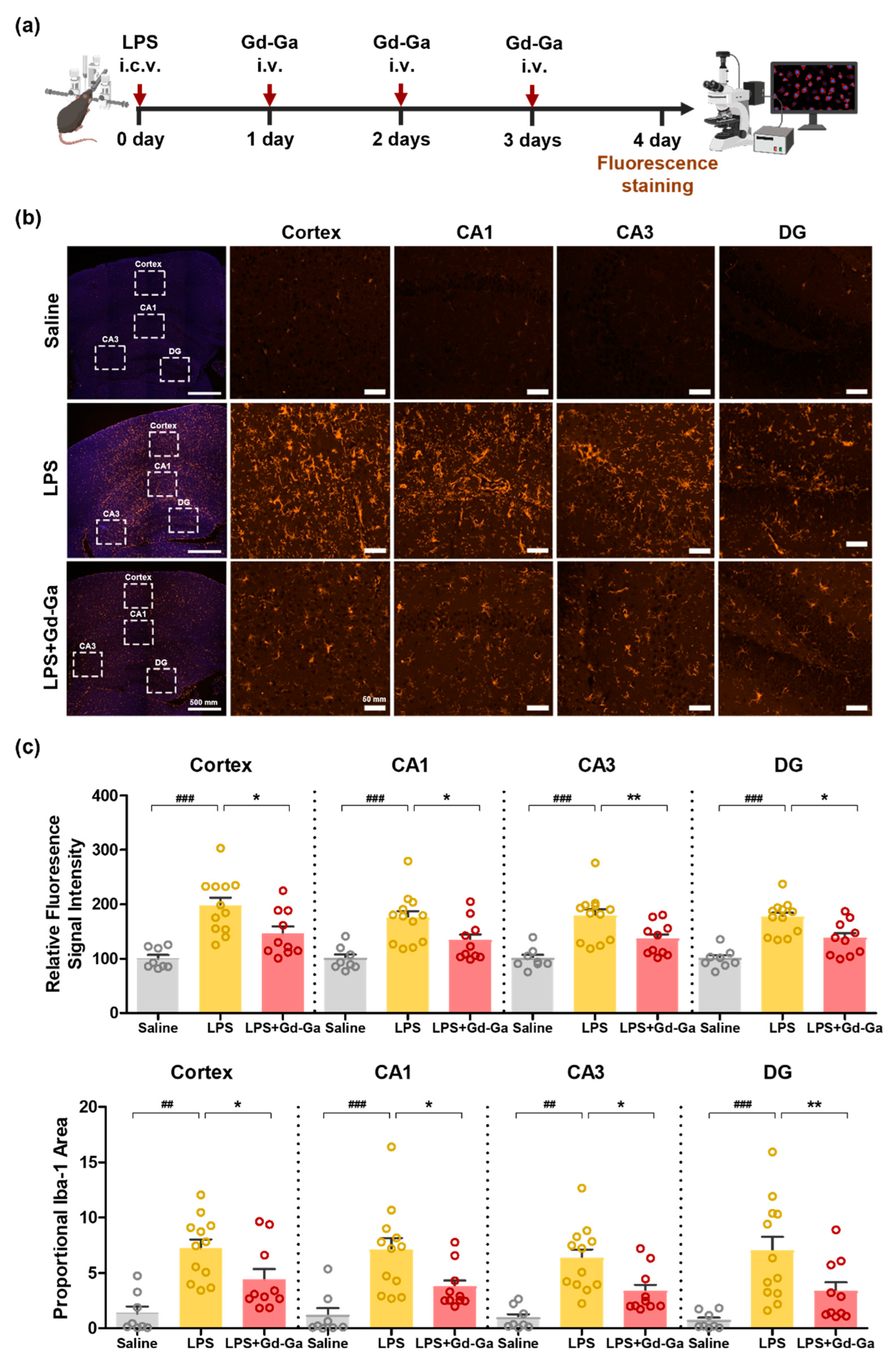
2.14. Immunofluorescence Staining
2.15. Docking Study
2.16. Binding Affitity for MD2 Protein and Gd Complex
2.17. Statistical Analysis
3. Results
3.1. Synthesis Ans Characterization
3.2. Physicochemical Characterization
3.3. Kinetic Inertness and pH Stability
3.4. Free Radical Scavenging Activity
3.5. Intracellular Antioxidant Effect of Gd-Ga on LPS-Induced BV-2 Cells
3.6. Effects of Gd-Ga on IκB Phosphorylation and NF-κB Translocation in LPS-Induced BV-2 Cells
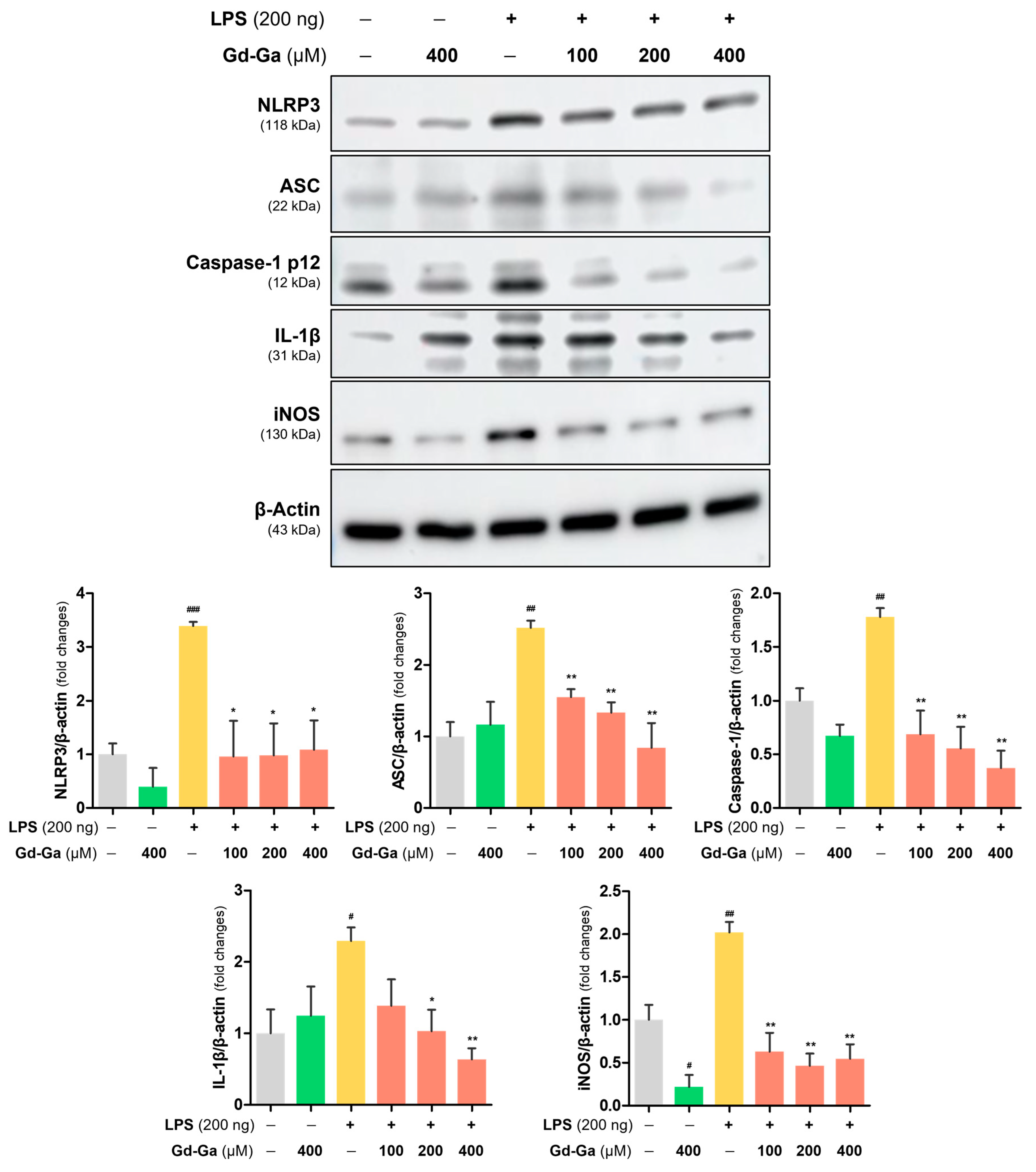
3.7. Anti-Inflammatory Effect of Gd-Ga in LPS-Induced BV-2 Cells
3.8. In Vivo MR Study on LPS-Induced Neuroinflammatory Model
3.9. In Vivo Anti-Inflammatory Effect of Gd-Ga on LPS-Induced Neuroinflammatory Model
3.10. Docking Study
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TLR4 | toll-like receptor 4 |
| PAMPs | pathogen-associated molecular patterns |
| DAMPs | damage-associated molecular patterns |
| LPS | lipopolysaccharide |
| NLRP3 | nucleotide binding and oligomerization domain-like receptor family pyrin domain-containing protein 3 |
| MD2 | myeloid differentiation protein 2 |
| SET | single electron transfer |
| ROS | reactive oxygen species |
| iNOS | inducible nitric oxide synthase |
| NO | nitric oxide |
| IL-1β | interleukin-1β |
| IκBα | nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor |
| NF-κB | nuclear transcription factor kappa B |
| ASC | apoptosis-associated speck-like protein containing a C-terminal caspase recruitment domain |
| i.c.v. | intracerebroventricular |
| i.v. | intravenous |
| MRI | magnetic resonance imaging |
| SE | spin echo |
| TE | echo time |
| NEX | number of excitations |
| FOV | field of view |
| TR | repetition time |
| SI | signal intensity |
| SNR | signal-to-noise ratio |
| Kd | equilibrium dissociation constant |
References
- Batista, C.R.A.; Gomes, G.F.; Candelario-Jalil, E.; Fiebich, B.L.; De Oliveira, A.C.P. Lipopolysaccharide-induced neuroinflammation as a bridge to understand neurodegeneration. Int. J. Mol. Sci. 2019, 20, 2293. [Google Scholar] [CrossRef]
- Rogers, J.; Mastroeni, D.; Leonard, B.; Joyce, J.; Grover, A. Neuroinflammation in Alzheimer’s disease and Parkinson’s disease: Are microglia pathogenic in either disorder? Int. Rev. Neurobiol. 2007, 82, 235–246. [Google Scholar]
- Kempuraj, D.; Thangavel, R.; Natteru, P.; Selvakumar, G.; Saeed, D.; Zahoor, H.; Zaheer, S.; Iyer, S.; Zaheer, A. Neuroinflammation induces neurodegeneration. J. Neurol. Neurosurg. Spine 2016, 1, 1003. [Google Scholar]
- Cai, Z.; Hussain, M.D.; Yan, L.-J. Microglia, neuroinflammation, and beta-amyloid protein in Alzheimer’s disease. Int. J. Neurosci. 2014, 124, 307–321. [Google Scholar] [CrossRef]
- Gao, X.; Zhang, X.; Sun, Y.; Dai, X. Mechanism of NLRP3 inflammasome activation and its role in Alzheimers disease. Explor. Immunol. 2022, 2, 229–244. [Google Scholar] [CrossRef]
- Gustin, A.; Kirchmeyer, M.; Koncina, E.; Felten, P.; Losciuto, S.; Heurtaux, T.; Tardivel, A.; Heuschling, P.; Dostert, C. NLRP3 inflammasome is expressed and functional in mouse brain microglia but not in astrocytes. PLoS ONE 2015, 10, e0130624. [Google Scholar] [CrossRef]
- Lehnardt, S.; Lachance, C.; Patrizi, S.; Lefebvre, S.; Follett, P.L.; Jensen, F.E.; Rosenberg, P.A.; Volpe, J.J.; Vartanian, T. The toll-like receptor TLR4 is necessary for lipopolysaccharide-induced oligodendrocyte injury in the CNS. J. Neurosci. 2002, 22, 2478–2486. [Google Scholar] [CrossRef]
- Falsig, J.; van Beek, J.; Hermann, C.; Leist, M. Molecular basis for detection of invading pathogens in the brain. J. Neurosci. Res. 2008, 86, 1434–1447. [Google Scholar] [CrossRef]
- Henn, A.; Kirner, S.; Leist, M. TLR2 hypersensitivity of astrocytes as functional consequence of previous inflammatory episodes. J. Immunol. 2011, 186, 3237–3247. [Google Scholar] [CrossRef]
- Akira, S.; Hemmi, H. Recognition of pathogen-associated molecular patterns by TLR family. Immunol. Lett. 2003, 85, 85–95. [Google Scholar] [CrossRef]
- Yang, J.; Wise, L.; Fukuchi, K.-I. TLR4 cross-talk with NLRP3 inflammasome and complement signaling pathways in Alzheimer’s disease. Front. Immunol. 2020, 11, 724. [Google Scholar] [CrossRef]
- Qin, Z.Y.; Gu, X.; Chen, Y.L.; Liu, J.B.; Hou, C.X.; Lin, S.Y.; Hao, N.N.; Liang, Y.; Chen, W.; Meng, H.Y. Toll-like receptor 4 activates the NLRP3 inflammasome pathway and periodontal inflammaging by inhibiting Bmi-1 expression. Int. J. Mol. Med. 2021, 47, 137–150. [Google Scholar] [CrossRef]
- Jin, X.; Liu, M.Y.; Zhang, D.F.; Zhong, X.; Du, K.; Qian, P.; Yao, W.F.; Gao, H.; Wei, M.J. Baicalin mitigates cognitive impairment and protects neurons from microglia-mediated neuroinflammation via suppressing NLRP 3 inflammasomes and TLR 4/NF-κB signaling pathway. CNS Neurosci. Ther. 2019, 25, 575–590. [Google Scholar] [CrossRef]
- Badshah, H.; Ikram, M.; Ali, W.; Ahmad, S.; Hahm, J.R.; Kim, M.O. Caffeine may abrogate LPS-induced oxidative stress and neuroinflammation by regulating Nrf2/TLR4 in adult mouse brains. Biomolecules 2019, 9, 719. [Google Scholar] [CrossRef]
- Zhang, S.-S.; Liu, M.; Liu, D.-N.; Yang, Y.-L.; Du, G.-H.; Wang, Y.-H. TLR4-IN-C34 inhibits lipopolysaccharide-stimulated inflammatory responses via downregulating TLR4/MyD88/NF-κB/NLRP3 signaling pathway and reducing ROS generation in BV2 cells. Inflammation 2022, 45, 838–850. [Google Scholar] [CrossRef]
- Farré-Alins, V.; Narros-Fernández, P.; Palomino-Antolín, A.; Decouty-Pérez, C.; Lopez-Rodriguez, A.B.; Parada, E.; Muñoz-Montero, A.; Gómez-Rangel, V.; López-Muñoz, F.; Ramos, E. Melatonin reduces NLRP3 inflammasome activation by increasing α7 nAChR-mediated autophagic flux. Antioxidants 2020, 9, 1299. [Google Scholar] [CrossRef]
- Rehman, S.U.; Ali, T.; Alam, S.I.; Ullah, R.; Zeb, A.; Lee, K.W.; Rutten, B.P.; Kim, M.O. Ferulic acid rescues LPS-induced neurotoxicity via modulation of the TLR4 receptor in the mouse hippocampus. Mol. Neurobiol. 2019, 56, 2774–2790. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Chen, D.; Chen, S. Antioxidant activity and mechanism of protocatechuic acid in vitro. Funct. Foods Health Dis. 2011, 1, 232–244. [Google Scholar] [CrossRef]
- Velderrain-Rodríguez, G.R.; Torres-Moreno, H.; Villegas-Ochoa, M.A.; Ayala-Zavala, J.F.; Robles-Zepeda, R.E.; Wall-Medrano, A.; González-Aguilar, G.A. Gallic acid content and an antioxidant mechanism are responsible for the antiproliferative activity of ‘Ataulfo’mango peel on LS180 cells. Molecules 2018, 23, 695. [Google Scholar] [CrossRef]
- Milenković, D.A.; Marković, J.M.D.; Marković, Z.S. DFT investigation of the reaction of cyanidin with hydroxyl radical. In Proceedings of the 2015 IEEE 15th International Conference on Bioinformatics and Bioengineering (BIBE), Belgrade, Serbia, 2–4 November 2015; pp. 1–5. [Google Scholar]
- Torres-Osorio, V.; Urrego, R.; Echeverri-Zuluaga, J.J.; López-Herrera, A. Oxidative stress and antioxidant use during in vitro mammal embryo production. Review. Rev. Mex. Cienc. Pecu. 2019, 10, 433–459. [Google Scholar] [CrossRef]
- Platzer, M.; Kiese, S.; Herfellner, T.; Schweiggert-Weisz, U.; Miesbauer, O.; Eisner, P. Common trends and differences in antioxidant activity analysis of phenolic substances using single electron transfer based assays. Molecules 2021, 26, 1244. [Google Scholar] [CrossRef]
- Zou, H.; Ben, T.; Wu, P.; Waterhouse, G.I.; Chen, Y. Effective anti-inflammatory phenolic compounds from dandelion: Identification and mechanistic insights using UHPLC-ESI-MS/MS, fluorescence quenching and anisotropy, molecular docking and dynamics simulation. Food Sci. Hum. Wellness 2023, 12, 2184–2194. [Google Scholar] [CrossRef]
- Qiu, Y.; Xiao, Z.; Wang, Y.; Zhang, D.; Zhang, W.; Wang, G.; Chen, W.; Liang, G.; Li, X.; Zhang, Y. Optimization and anti-inflammatory evaluation of methyl gallate derivatives as a myeloid differentiation protein 2 inhibitor. Bioorganic Med. Chem. 2019, 27, 115049. [Google Scholar] [CrossRef]
- Hwang, K.-A.; Hwang, Y.-J.; Song, J. Antioxidant activities and oxidative stress inhibitory effects of ethanol extracts from Cornus officinalis on raw 264.7 cells. BMC Complement. Altern. Med. 2016, 16, 196. [Google Scholar] [CrossRef]
- Alcalde, B.; Granados, M.; Saurina, J. Exploring the antioxidant features of polyphenols by spectroscopic and electrochemical methods. Antioxidants 2019, 8, 523. [Google Scholar] [CrossRef]
- André, J.P.; Geraldes, C.F.; Martins, J.A.; Merbach, A.E.; Prata, M.I.; Santos, A.C.; de Lima, J.J.; Tóth, É. Lanthanide (III) complexes of DOTA–glycoconjugates: A potential new class of lectin-mediated medical imaging agents. Chem. A Eur. J. 2004, 10, 5804–5816. [Google Scholar] [CrossRef]
- Jacques, V.; Dumas, S.; Sun, W.-C.; Troughton, J.S.; Greenfield, M.T.; Caravan, P. High relaxivity MRI contrast agents part 2: Optimization of inner-and second-sphere relaxivity. Investig. Radiol. 2010, 45, 613. [Google Scholar] [CrossRef]
- Kim, S.; Kim, H.-K.; Baek, A.R.; Sung, B.; Yang, B.W.; Kim, Y.-H.; Lee, J.-j.; Yang, J.-u.; Shin, C.-H.; Jung, H. Rose bengal conjugated gadolinium complex as a new multimodal imaging agent targeting presynaptic vesicular glutamate transporters. J. Ind. Eng. Chem. 2021, 95, 83–91. [Google Scholar] [CrossRef]
- Idée, J.M.; Port, M.; Robic, C.; Medina, C.; Sabatou, M.; Corot, C. Role of thermodynamic and kinetic parameters in gadolinium chelate stability. J. Magn. Reson. Imaging 2009, 30, 1249–1258. [Google Scholar] [CrossRef]
- Brücher, E. Kinetic stabilities of gadolinium (III) chelates used as MRI contrast agents. In Contrast Agents I; Springer: Berlin/Heidelberg, Germany, 2002; pp. 103–122. [Google Scholar]
- Do, Q.; Ratnakar, J.; Kovacs, Z.; Tircso, G.; Kalman, K.; Baranyai, Z.; Brucher, E.; Toth, I. Contrast Agents for MRI: Experimental Methods; The Royal Society of Chemistry: Cambridge, UK, 2018. [Google Scholar]
- Khan, M.S.; Ali, T.; Kim, M.W.; Jo, M.H.; Jo, M.G.; Badshah, H.; Kim, M.O. Anthocyanins protect against LPS-induced oxidative stress-mediated neuroinflammation and neurodegeneration in the adult mouse cortex. Neurochem. Int. 2016, 100, 1–10. [Google Scholar] [CrossRef]
- López-García, J.; Lehocký, M.; Humpolíček, P.; Sáha, P. HaCaT keratinocytes response on antimicrobial atelocollagen substrates: Extent of cytotoxicity, cell viability and proliferation. J. Funct. Biomater. 2014, 5, 43–57. [Google Scholar] [CrossRef]
- Standard, I. Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity; International Organization for Standardization: Geneve, Switzerland, 2009. [Google Scholar]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative stress: A key modulator in neurodegenerative diseases. Molecules 2019, 24, 1583. [Google Scholar] [CrossRef]
- Olufunmilayo, E.O.; Gerke-Duncan, M.B.; Holsinger, R.D. Oxidative Stress and Antioxidants in Neurodegenerative disorders. Antioxidants 2023, 12, 517. [Google Scholar] [CrossRef]
- Sierra, A.; Navascués, J.; Cuadros, M.A.; Calvente, R.; Martín-Oliva, D.; Ferrer-Martín, R.M.; Martín-Estebané, M.; Carrasco, M.-C.; Marín-Teva, J.L. Expression of inducible nitric oxide synthase (iNOS) in microglia of the developing quail retina. PLoS ONE 2014, 9, e106048. [Google Scholar] [CrossRef]
- Korhonen, R.; Lahti, A.; Kankaanranta, H.; Moilanen, E. Nitric oxide production and signaling in inflammation. Curr. Drug Targets Inflamm. Allergy 2005, 4, 471–479. [Google Scholar] [CrossRef]
- Nagata, J.; Yokodera, H.; Maeda, G. In vitro and in vivo studies on anti-inflammatory effects of traditional Okinawan vegetable methanol extracts. ACS Omega 2019, 4, 15660–15664. [Google Scholar] [CrossRef]
- Chiarini, A.; Gui, L.; Viviani, C.; Armato, U.; Dal Prà, I. NLRP3 Inflammasome’s Activation in Acute and Chronic Brain Diseases—An Update on Pathogenetic Mechanisms and Therapeutic Perspectives with Respect to Other Inflammasomes. Biomedicines 2023, 11, 999. [Google Scholar] [CrossRef]
- Li, S.; Fang, Y.; Zhang, Y.; Song, M.; Zhang, X.; Ding, X.; Yao, H.; Chen, M.; Sun, Y.; Ding, J. Microglial NLRP3 inflammasome activates neurotoxic astrocytes in depression-like mice. Cell Rep. 2022, 41, 111532. [Google Scholar] [CrossRef]
- Lee, H.-j.; Hoe, H.-S. Inhibition of CDK4/6 regulates AD pathology, neuroinflammation and cognitive function through DYRK1A/STAT3 signaling. Pharmacol. Res. 2023, 190, 106725. [Google Scholar] [CrossRef]
- Kim, H.-K.; Lee, J.-J.; Choi, G.; Sung, B.; Kim, Y.-H.; Baek, A.R.; Kim, S.; Song, H.; Kim, M.; Cho, A.E. Gadolinium-based neuroprognostic magnetic resonance imaging agents suppress COX-2 for prevention of reperfusion injury after stroke. J. Med. Chem. 2020, 63, 6909–6923. [Google Scholar] [CrossRef]
- Anand, S.; Sowbhagya, R.; Ansari, M.A.; Alzohairy, M.A.; Alomary, M.N.; Almalik, A.I.; Ahmad, W.; Tripathi, T.; Elderdery, A.Y. Polyphenols and their nanoformulations: Protective effects against human diseases. Life 2022, 12, 1639. [Google Scholar] [CrossRef]
- Souza, T.N.; Santos, F.M.; Alves, P.R.; Ferro, J.N.; Correia, A.C.C.; Melo, T.S.; Soares, W.R.; Andrade, B.S.; Lagente, V.; Barreto, E. Local administration of p-coumaric acid decreases lipopolysaccharide-induced acute lung injury in mice: In vitro and in silico studies. Eur. J. Pharmacol. 2021, 897, 173929. [Google Scholar] [CrossRef]
- Favarin, D.C.; Teixeira, M.M.; de Andrade, E.L.; Alves, C.d.F.; Chica, J.E.L.; Sorgi, C.A.; Faccioli, L.H.; Rogerio, A.P. Anti-inflammatory effects of ellagic acid on acute lung injury induced by acid in mice. Mediat. Inflamm. 2013, 2013, 164202. [Google Scholar]
- Wang, G.-F.; Shi, L.-P.; Ren, Y.-D.; Liu, Q.-F.; Liu, H.-F.; Zhang, R.-J.; Li, Z.; Zhu, F.-H.; He, P.-L.; Tang, W. Anti-hepatitis B virus activity of chlorogenic acid, quinic acid and caffeic acid in vivo and in vitro. Antivir. Res. 2009, 83, 186–190. [Google Scholar] [CrossRef]
- Abotaleb, M.; Liskova, A.; Kubatka, P.; Büsselberg, D. Therapeutic potential of plant phenolic acids in the treatment of cancer. Biomolecules 2020, 10, 221. [Google Scholar] [CrossRef]
- Li, W.; Rang, Y.; Liu, H.; Liu, C. Update on new trends and progress of natural active ingredients in the intervention of Alzheimer’s disease, based on understanding of traditional Chinese and Western relevant theories: A review. Phytother. Res. 2023, 37, 3744–3764. [Google Scholar] [CrossRef]
- Kasture, V.S.; Katti, S.A.; Mahajan, D.; Wagh, R.; Mohan, M.; Kasture, S.B. Antioxidant and antiparkinson activity of gallic acid derivatives. Pharmacologyonline 2009, 1, 385–395. [Google Scholar]
- Silva, F.A.; Borges, F.; Guimarães, C.; Lima, J.L.; Matos, C.; Reis, S. Phenolic acids and derivatives: Studies on the relationship among structure, radical scavenging activity, and physicochemical parameters. J. Agric. Food Chem. 2000, 48, 2122–2126. [Google Scholar] [CrossRef]
- Förstermann, U.; Xia, N.; Li, H. Roles of vascular oxidative stress and nitric oxide in the pathogenesis of atherosclerosis. Circ. Res. 2017, 120, 713–735. [Google Scholar] [CrossRef]
- Alhayaza, R.; Haque, E.; Karbasiafshar, C.; Sellke, F.W.; Abid, M.R. The relationship between reactive oxygen species and endothelial cell metabolism. Front. Chem. 2020, 8, 1043. [Google Scholar] [CrossRef]
- Chatterjee, S. Chapter two-oxidative stress, inflammation, and disease. In Oxidative Stress and Biomaterials; Academic Press: Cambridge, MA, USA, 2016; pp. 35–58. [Google Scholar]
- Lee, C.H.; Jeon, Y.T.; Kim, S.H.; Song, Y.S. NF-κB as a potential molecular target for cancer therapy. Biofactors 2007, 29, 19–35. [Google Scholar] [CrossRef]
- Oeckinghaus, A.; Ghosh, S. The NF-κB family of transcription factors and its regulation. Cold Spring Harb. Perspect. Biol. 2009, 1, a000034. [Google Scholar] [CrossRef]
- Shoji, S.; Hanada, K.; Takahashi, M.; Watanabe, K.; Yonemochi, M.; Tomabechi, Y.; Shirouzu, M. The NF-κB regulator IκBβ exhibits different molecular interactivity and phosphorylation status from IκBα in an IKK2-catalysed reaction. FEBS Lett. 2020, 594, 1532–1549. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Jin, C.Y.; Kim, C.H.; Yoo, Y.H.; Choi, S.H.; Kim, G.Y.; Yoon, H.M.; Park, H.T.; Choi, Y.H. Isorhamnetin alleviates lipopolysaccharide-induced inflammatory responses in BV2 microglia by inactivating NF-κB, blocking the TLR4 pathway and reducing ROS generation. Int. J. Mol. Med. 2019, 43, 682–692. [Google Scholar] [CrossRef]
- Norden, D.M.; Trojanowski, P.J.; Villanueva, E.; Navarro, E.; Godbout, J.P. Sequential activation of microglia and astrocyte cytokine expression precedes increased Iba-1 or GFAP immunoreactivity following systemic immune challenge. Glia 2016, 64, 300–316. [Google Scholar] [CrossRef]
- Herber, D.L.; Roth, L.M.; Wilson, D.; Wilson, N.; Mason, J.E.; Morgan, D.; Gordon, M.N. Time-dependent reduction in Aβ levels after intracranial LPS administration in APP transgenic mice. Exp. Neurol. 2004, 190, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Zamyatina, A.; Heine, H. Lipopolysaccharide recognition in the crossroads of TLR4 and caspase-4/11 mediated inflammatory pathways. Front. Immunol. 2020, 11, 585146. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Hu, L.; Li, D.; Wang, Y.; He, Y.; Zhu, L.; Ren, W. MD-2 regulates LPS-induced NLRP3 inflammasome activation and IL-1beta secretion by a MyD88/NF-κB-dependent pathway in alveolar macrophages cell line. Mol. Immunol. 2017, 90, 1–10. [Google Scholar] [CrossRef]
- Molagoda, I.M.N.; Athapaththu, A.M.G.K.; Choi, Y.H.; Park, C.; Jin, C.-Y.; Kang, C.-H.; Lee, M.-H.; Kim, G.-Y. Fisetin inhibits NLRP3 inflammasome by suppressing TLR4/MD2-mediated mitochondrial ROS production. Antioxidants 2021, 10, 1215. [Google Scholar] [CrossRef]
- Hengerer, A.; Grimm, J. Molecular magnetic resonance imaging. Biomed. Imaging Interv. J. 2006, 2, e8. [Google Scholar] [CrossRef]



| Cas | 3.0 T MRI | 9.4 T MRI | ||
|---|---|---|---|---|
| r1 (mM−1s−1) | r2 (mM−1s−1) | r1 (mM−1s−1) | r2 (mM−1s−1) | |
| Gd-Ga | 4.04 ± 0.17 | 4.82 ± 0.21 | 4.14 ± 0.11 | 4.78 ± 0.07 |
| Gd-DOTA | 3.40 ± 0.07 | 3.88 ± 0.16 | 3.63 ± 0.07 | 4.77 ± 0.11 |
| DPPH Assay | ABTS Assay | |||
|---|---|---|---|---|
| EC50 (μM) | R2 | EC50 (μM) | R2 | |
| Gd-Ga | 4.01 ± 0.06 | 0.9936 | 8.04 ± 0.06 | 0.9650 |
| Gallic acid | 3.04 ± 0.13 | 0.9999 | 5.75 ± 0.01 | 0.9902 |
| Ascorbic acid | 9.03 ± 0.06 | 0.9978 | 17.40 ± 0.04 | 0.9775 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sung, B.; Hwang, D.; Baek, A.; Yang, B.; Lee, S.; Park, J.; Kim, E.; Kim, M.; Lee, E.; Chang, Y. Gadolinium-Based Magnetic Resonance Theranostic Agent with Gallic Acid as an Anti-Neuroinflammatory and Antioxidant Agent. Antioxidants 2024, 13, 204. https://doi.org/10.3390/antiox13020204
Sung B, Hwang D, Baek A, Yang B, Lee S, Park J, Kim E, Kim M, Lee E, Chang Y. Gadolinium-Based Magnetic Resonance Theranostic Agent with Gallic Acid as an Anti-Neuroinflammatory and Antioxidant Agent. Antioxidants. 2024; 13(2):204. https://doi.org/10.3390/antiox13020204
Chicago/Turabian StyleSung, Bokyung, Dongwook Hwang, Ahrum Baek, Byeongwoo Yang, Sangyun Lee, Jangwoo Park, Eunji Kim, Minsup Kim, Eunshil Lee, and Yongmin Chang. 2024. "Gadolinium-Based Magnetic Resonance Theranostic Agent with Gallic Acid as an Anti-Neuroinflammatory and Antioxidant Agent" Antioxidants 13, no. 2: 204. https://doi.org/10.3390/antiox13020204
APA StyleSung, B., Hwang, D., Baek, A., Yang, B., Lee, S., Park, J., Kim, E., Kim, M., Lee, E., & Chang, Y. (2024). Gadolinium-Based Magnetic Resonance Theranostic Agent with Gallic Acid as an Anti-Neuroinflammatory and Antioxidant Agent. Antioxidants, 13(2), 204. https://doi.org/10.3390/antiox13020204






