Abstract
Oxalis corniculata L. (O. corniculata) has attracted increasing attention as a natural source of antioxidants with diverse pharmacological potential. Phytochemical studies have identified a diverse spectrum of metabolites, dominated by flavonoids, polysaccharides, and organic acids. These compounds exhibit antioxidant properties as well as related biological activities, including anti-inflammatory, antimicrobial, neuroprotective, hypoglycemic, and anticancer effects. Its long-standing use in traditional remedies, along with its incorporation into approved Chinese patent medicines, underscores its safety and translational value. This review synthesizes recent advances in the chemical composition, bioactivities, and molecular mechanisms of O. corniculata, emphasizing its antioxidant-driven pharmacological prospects. The review highlights O. corniculata as a sustainable and accessible botanical resource with significant potential for the development of pharmaceuticals, dietary supplements, and health-promoting applications.
1. Introduction
Plant-derived natural products have long served as an indispensable source of bioactive compounds, owing to their structural diversity and broad pharmacological potential [1]. In the context of increasing demand for sustainable resources in food and health industries, herbs with wide adaptability, ease of cultivation, and abundant bioactive constituents are attracting renewed scientific and industrial interest [2,3].
O. corniculata, commonly known as creeping woodsorrel, belongs to the family Oxalidaceae and is one of the most widely distributed vascular plants worldwide [4,5]. Traditionally, it has been used in various medical systems to treat digestive, dermatological, and urinary disorders, and in modern practice it is also included in several approved Chinese patent medicines for related indications [6]. Pharmacological studies have demonstrated that extracts of O. corniculata exhibit diverse biological activities, including antioxidant, anti-inflammatory, antimicrobial, hypoglycemic, and anticancer effects [7]. Flavonoids, polysaccharides, and organic acids are recognized as the major classes of bioactive compounds in O. corniculata [8,9]. Building on these findings, subsequent studies suggest that these compounds play key roles in mediating its pharmacological effects through antioxidant enhancement and regulation of inflammation, metabolism, and cellular protection [10]. In addition, emerging formulation and delivery strategies, such as nanomaterial-based systems and exosome-mediated approaches [11], have been explored to improve stability, bioavailability, and functional efficacy. Preliminary toxicological evaluations in animal models also indicate a favorable safety margin, supporting the translational potential of O. corniculata [12].
Although extensive research has been conducted, a comprehensive and up-to-date synthesis of the phytochemistry, antioxidant mechanisms, and health-related applications of O. corniculata remains lacking. By systematically summarizing recent advances, this review aims to clarify its antioxidant basis, link bioactive constituents to biological activities, and provide new perspectives on its potential roles in dietary supplements and therapeutic applications. O. corniculata also offers a sustainable source for the development of natural antioxidant-based products.
2. Botanical Features and Geographic Distribution
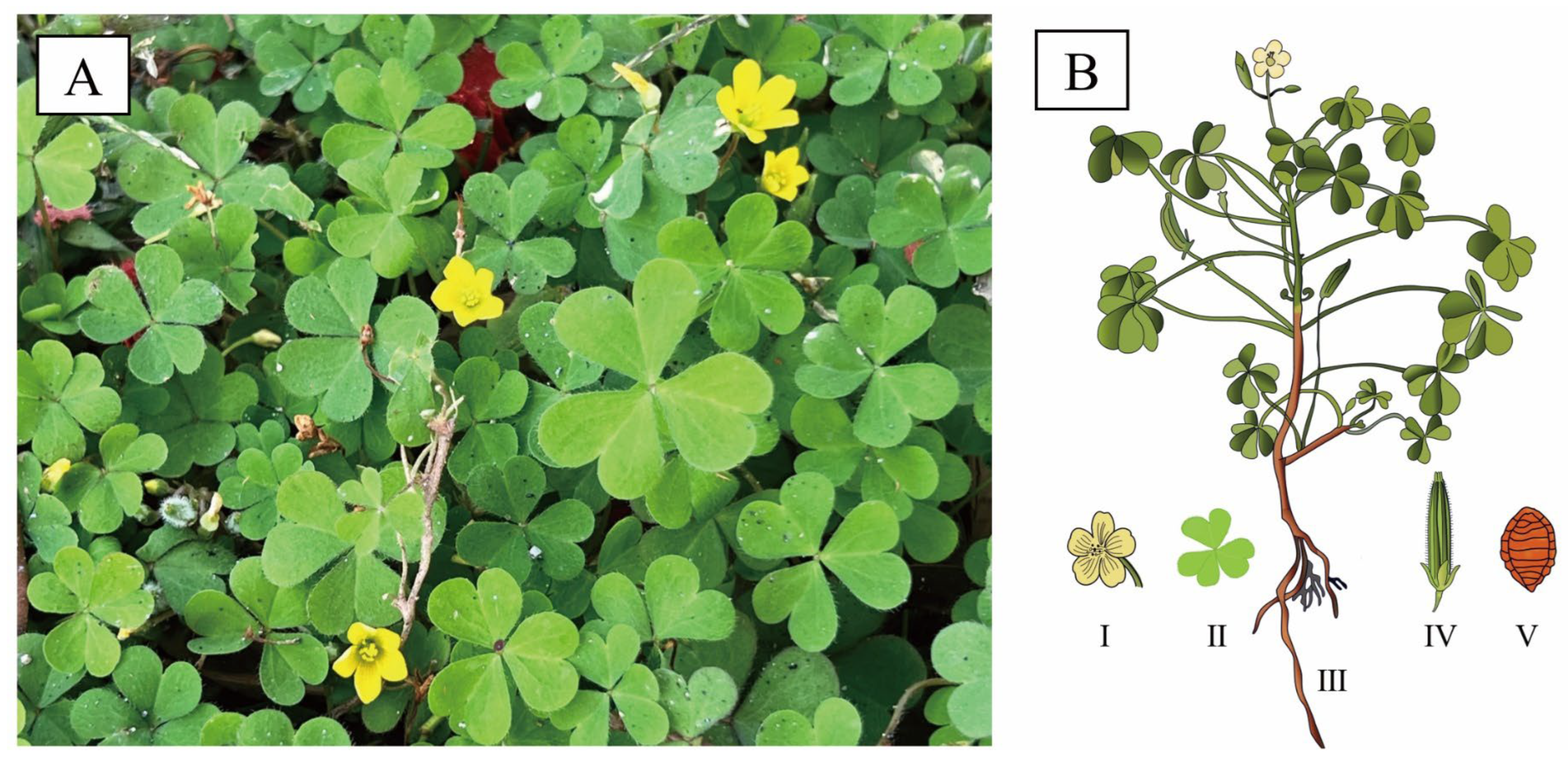
O. corniculata is a small herbaceous plant, annual to perennial in habit. It is characterized by profusely branched [13], slender, and creeping stems with reddish nodes that readily produce adventitious roots, enabling rapid vegetative propagation in environments (Figure 1) [14]. Plants typically reach 10–40 cm in height and are sparsely covered with fine hairs. The leaves are trifoliolate, bearing obcordate leaflets (4–16 mm long, 4–22 mm wide) notched at the apex and ciliate along the margins. Flowers are axillary, solitary, or borne in small umbels, each comprising five yellow petals (6–8 mm) and five lanceolate sepals (3–5 mm). The floral structure includes ten stamens of unequal lengths and a five-loculed ovary topped with capitate stigmas. The fruit is a cylindrical capsule (1–2.5 cm) that dehisces explosively at maturity, releasing ovoid seeds (1–1.5 mm) with transverse ridged-reticulate ornamentation [15]. Flowering and fruiting occur from March to September. (https://www.iplant.cn/, accessed on 1 September 2025)
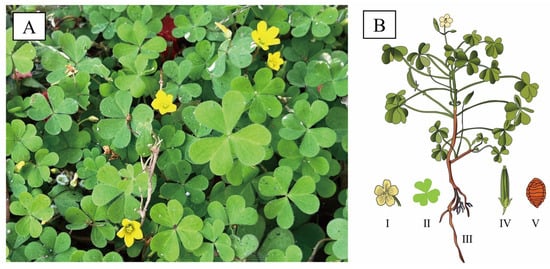
Figure 1.
Morphology of O. corniculata. (A): Field photograph of O. corniculata displaying its characteristic trifoliate leaves and yellow flowers; (B): Diagrammatic illustration of O. corniculata, displaying the whole plant (III) with detailed depictions of the flower (I), leaf (II), fruit (IV), and seed (V).
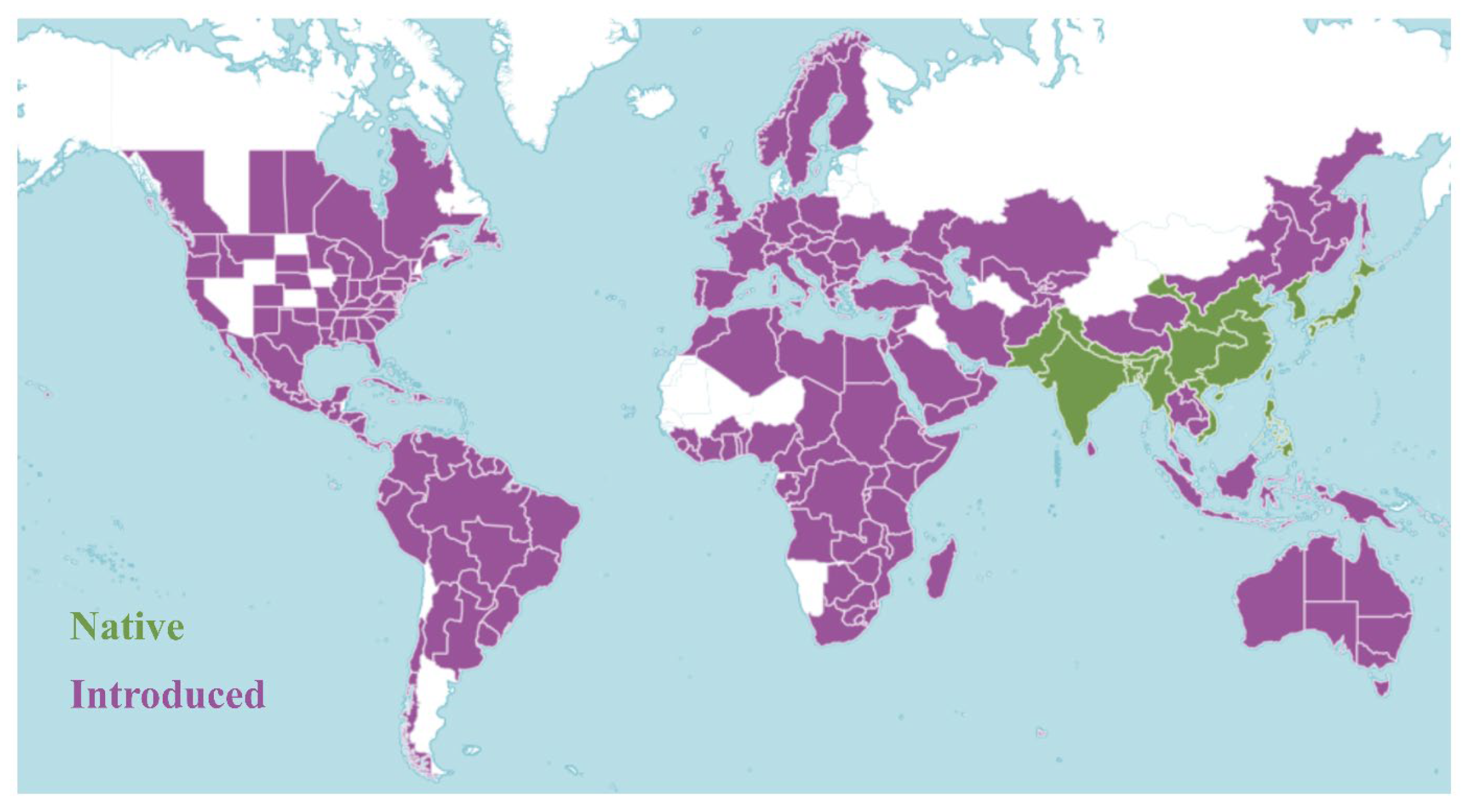
O. corniculata exhibits a nearly global distribution, thriving across tropical, subtropical, and temperate regions (Figure 2). Native to Asia, particularly East, South, and Southeast Asia, it has since naturalized across Europe, the Mediterranean, Oceania, and the Americas [16]. The species grows most vigorously at elevations of 500–1500 m, with higher density and frequency observed during the rainy season [17]. It commonly colonizes roadsides, grasslands, field margins, and forest edges, especially in moist or human-disturbed habitats. Its ecological success is largely attributed to adaptive growth habits and versatile reproductive strategies, which support survival and dispersal across diverse environments [18].
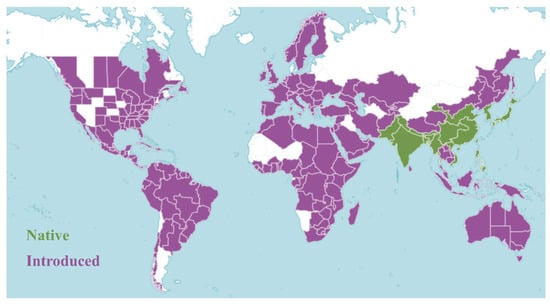
Figure 2.
Global distribution of O. corniculata. Green areas indicate regions where the species is native, while purple areas represent regions where it has been introduced (https://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:177893-2 (accessed on 1 July 2025)).
3. Phytochemistry
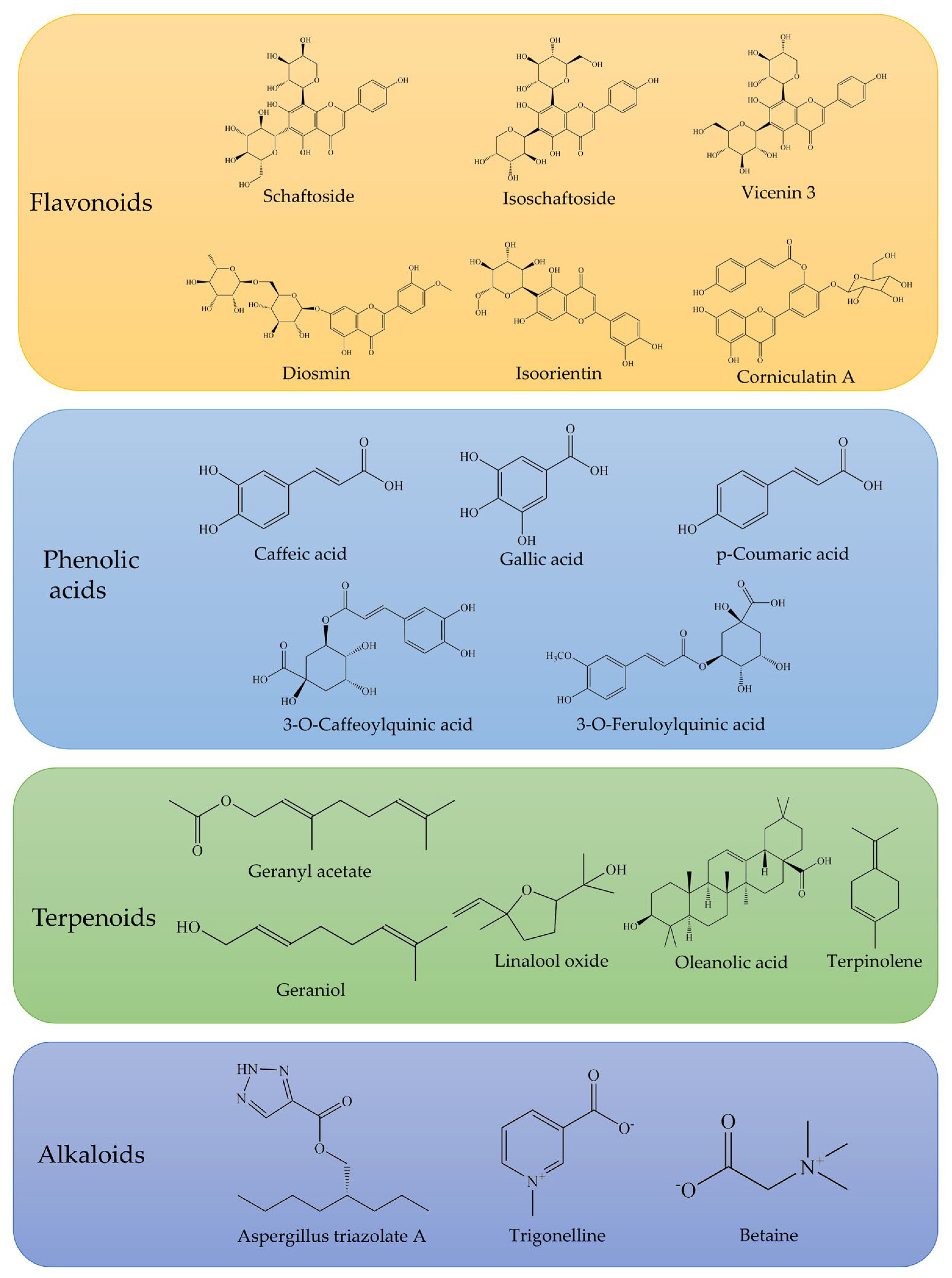
Phytochemical studies have identified 227 chemical constituents (Table S1) in O. corniculata, primarily isolated from its whole herb and leaves. Flavonoids are the predominant class of compounds, with approximately 84 identified in the plant [19], contributing to a total flavonoid content of up to 13.5 mg/g [10]. Organic acids, particularly phenolic acids, represent another major class of active constituents, with 51 organic acids identified, including 23 phenolic acids [20,21]. The total phenolic content in dry plant samples is quantified at 98.6 μg/g, with notable concentrations of hydroxybenzoic acid, p-coumaric acid, caffeic acid, and others [22]. The terpenoid fraction, primarily monoterpenes, consists of approximately 20 different compounds, with the highest concentration found in the leaves. Terpenoids such as geraniol and linalool have demonstrated antioxidant, antimicrobial, and anti-inflammatory activities [23].
Notably, compounds 47 and 48 (new flavones), compound 160 (a novel alkaloid, Aspergillus triazolate A), and compound 161 (a lignan named corniculin A) are among the novel constituents first isolated from O. corniculata. Additional constituents include coumarins, simple phenolics, fatty acid esters, anthraquinones, aldehydes, glycosides, sterols, carbohydrates, amino acids, carotenoids, pigments, and other specialized metabolites. Typical compounds are shown in Figure 3.
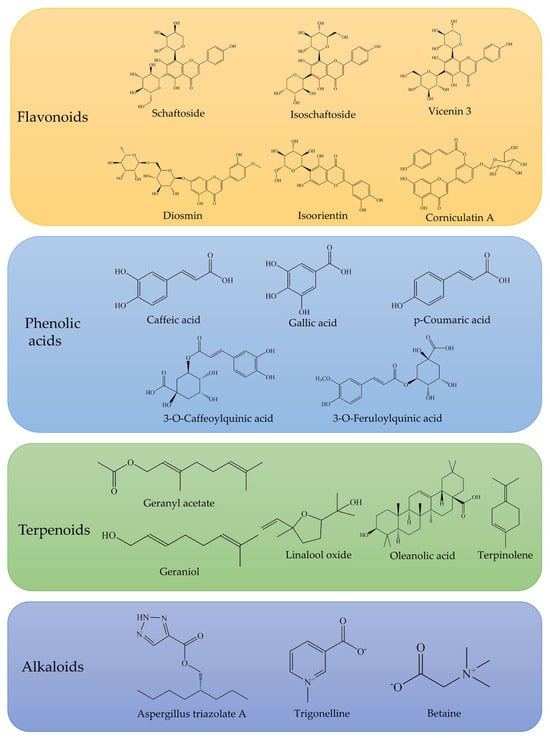
Figure 3.
Representative flavonoids, phenolic acids, terpenoids, and alkaloids identified in O. corniculata.
3.1. Flavonoids
Flavonoids are representative compounds in O. corniculata, featuring a basic 2-phenylchromen-4-one skeleton. They are categorized into several subclasses, including flavones, flavonols, dihydroflavones, isoflavones, chalcone, dihydrochalcones, and flavanols (Figures S1 and S2). The antioxidant activity of these compounds depends on both the number and position of hydroxyl groups in their structure. Hydroxylation at the 3, 5, and 7 positions and the presence of a C2=C3 double bond in the C-ring enhance radical-scavenging activity and anti-inflammatory effects [24]. For instance, flavonols possess a hydroxyl or other oxygen-containing substituent at the 3-position of the flavonoid backbone, a structural feature that has been associated with enhanced antioxidant activity [25]. In addition, glycosylation generally improves water solubility and bioavailability, which may enhance physiological efficacy [26]. These insights underscore the importance of structure-activity relationships in determining the functional properties of flavonoids.
In O. corniculata, flavonoid content may reach optimal levels when harvested in June or October, periods likely corresponding to peaks in secondary metabolite accumulation [27]. Flavonoids occur mainly as C-glycosyl and O-glycosyl flavones. C-glycosyl flavones (schaftoside, isoschaftoside, and isovitexin) demonstrate strong radical-scavenging activity and superior structural stability [28]. In contrast, O-glycosyl flavones (orientin, isoorientin and diosmin) exhibit enhanced solubility and absorption [29]. These flavonoids collectively represent the major contributors to the antioxidant and anti-inflammatory activities of O. corniculata.
3.2. Organic Acids
Organic acids constitute an important class of phytochemicals in O. corniculata, contributing both to its characteristic sour taste and to its diverse biological activities [20]. Among them, phenolic acids (85–107) are dominated by caffeic acid and its derivatives (Figure S3A). Caffeic acid content peaks in dry-area samples, reaching up to 1.28 μg/g [22], with lower concentrations observed in samples from marshy and moist areas. Caffeic acid derivatives, characterized by a catechol moiety, exhibit strong free radical scavenging capacity and protect cells against oxidative stress. Their antioxidant potential is largely determined by structural factors such as the number and position of hydroxyl groups on the aromatic ring, while modifications like methoxylation can further modulate activity [30].
In addition to phenolic acids, O. corniculata also contains low-molecular-weight organic acids such as malic and citric acids [20]. Fatty acid-type organic acids have been identified as well, with gas chromatography-mass spectrometry (GC/MS) analyses revealing oleic acid and 6-octadecenoic acid as predominant components (31.08%), followed by palmitic acid (2.55%) (Figure S3B) [31]. These fatty acids are associated with metabolic regulation and potential cardiovascular protective effects [32].
3.3. Terpenoids
Terpenoids, biosynthesized from isoprene units, constitute a structurally diverse class of natural products that contribute to plant defense, volatile signaling, and physiological regulation [33]. To date, 20 terpenoids (136–155) have been identified in O. corniculata, comprising monoterpenes (136–150), a cycloether monoterpene (151), and triterpenes (152–155).
Supercritical CO2 extraction followed by GC/MS analysis of leaf samples from Guangdong Province, China, revealed that monoterpenes dominate the essential oil fraction [23]. The most abundant constituents were geranyl acetate (13.3%), terpinolene (9.2%), linalool oxide (7.4%), and geraniol (6.4%). These compounds impart characteristic aromatic properties and exhibit antioxidant, antimicrobial, and anti-inflammatory activities [34]. In addition to volatile monoterpenes, several triterpenoids have been detected in the non-volatile fractions, including oleanolic acid, eburicoic acid, squalene, and phytol [31,35]. The chemical structures of the identified terpenoids are shown in Figure S4.
3.4. Alkaloids
Alkaloids are nitrogen-containing natural compounds with diverse chemical structures [36]. Five alkaloids (156–160) have been reported from O. corniculata. Among them, trigonelline (156), a classical pyridine alkaloid with an N+-methylated pyridine ring, displays high water solubility due to its quaternary ammonium structure [37]. Betaine (158), a naturally occurring zwitterion with a trimethylated amino group, serves as an important osmolyte and methyl donor, and has been associated with antioxidant, hepatoprotective, and metabolic regulatory effects [38].
Particularly noteworthy is compound 160, Aspergillus triazolate A (ATA), a novel triazole alkaloid first isolated from the dried whole plant of O. corniculata. Structurally, ATA consists of a hydrophilic triazole ring and a hydrophobic C8 alkyl chain, forming an amphiphilic scaffold. This structural balance is proposed to facilitate stable interactions with target enzymes, thereby enhancing inhibitory activity. Bioassays have demonstrated that ATA lowers blood glucose levels by inhibiting α-glucosidase (α-Glu), highlighting its potential as a natural lead compound for α-Glu inhibitor development [39]. The chemical structures of these alkaloids are shown in Figure S5.
3.5. Polysaccharides
Polysaccharides are natural macromolecules consisting of more than ten monosaccharide units linked through glycosidic bonds, often comprising hundreds to thousands of residues [40]. In O. corniculata, crude polysaccharides (OCP) are typically extracted using hot water and ethanol precipitation, followed by purification steps such as deproteinization and ethanol fractionation, with a total yield of 9.45% [7]. The major fraction, OCP-3, has a molecular weight of 31.5 kDa and is mainly composed of arabinose (47.83%) and galacturonic acid (17.81%), indicating its acidic nature. OCP-3 demonstrates strong free radical scavenging capacity, suppresses lipid peroxidation, and confers protection against oxidative damage in both cell-based and in vivo models [7].
3.6. Nutrients
On a dry-weight basis, O. corniculata contains considerable amounts of essential minerals, including calcium (5.63%), potassium (3.15%), and magnesium (2.63%) [41]. Trace elements are also present [41], with iron (89.16 ppm), manganese (4.21 ppm), zinc (1.59 ppm), and copper (0.12 ppm) detected. The vitamin C content reaches 0.139 mg/g. Fresh methanolic extracts provide approximately 710 μg vitamin C equivalent antioxidant capacity per gram of fresh leaves [42], reflecting strong antioxidant capacity attributable to vitamin C. This effect is likely enhanced by synergistic interactions with flavonoids, organic acids, and other bioactive constituents [43]. In addition, dried O. corniculata powder contains appreciable levels of crude protein (12.25%), crude fiber (10.64%), and ash (9.98%). The total antioxidant capacity (TAC) has been measured at 31.60 mmol ascorbic acid equivalent/kg dry weight and 31.22 mmol vitamin E equivalent/kg dry weight, indicating strong overall antioxidant potential [44].
4. Traditional Uses
O. corniculata has a long-standing history in traditional medicine, first documented in the Xin Xiu Ben Cao in 659 AD [45]. Over centuries, it has been incorporated into diverse ethnomedical systems, including Traditional Chinese Medicine (TCM), Ayurveda, and Unani. In oral applications, decoctions, teas, or fresh juices prepared from the whole plant or leaves are commonly used to relieve gastrointestinal discomfort, fever, urinary disorders, and liver ailments [6,46]. Topical preparations, such as poultices or expressed juices, are applied to treat skin conditions including warts, eczema, wounds, and abscesses, reflecting recognition of its antimicrobial and wound-healing effects [47,48].
In TCM, O. corniculata is traditionally categorized as a heat-clearing and detoxifying herb, used to eliminate damp-heat, relieve strangury, and reduce swelling and pain. It has been incorporated into several approved Chinese patent medicines, such as Mi Lin Qing Capsules (for damp-heat urinary tract infections/strangury), Fu Yan Xiao Capsules (for gynecological inflammation), and Gu Kang Capsules (for traumatic injury and swelling). These commercial formulations illustrate its compatibility within compound prescriptions and its translational potential from traditional use to standardized products. These applications reflect its traditional recognition for antimicrobial, antioxidant, and anti-inflammatory properties (Table 1).

Table 1.
Traditional uses of O. corniculata.
5. Biological Activities of O. corniculata
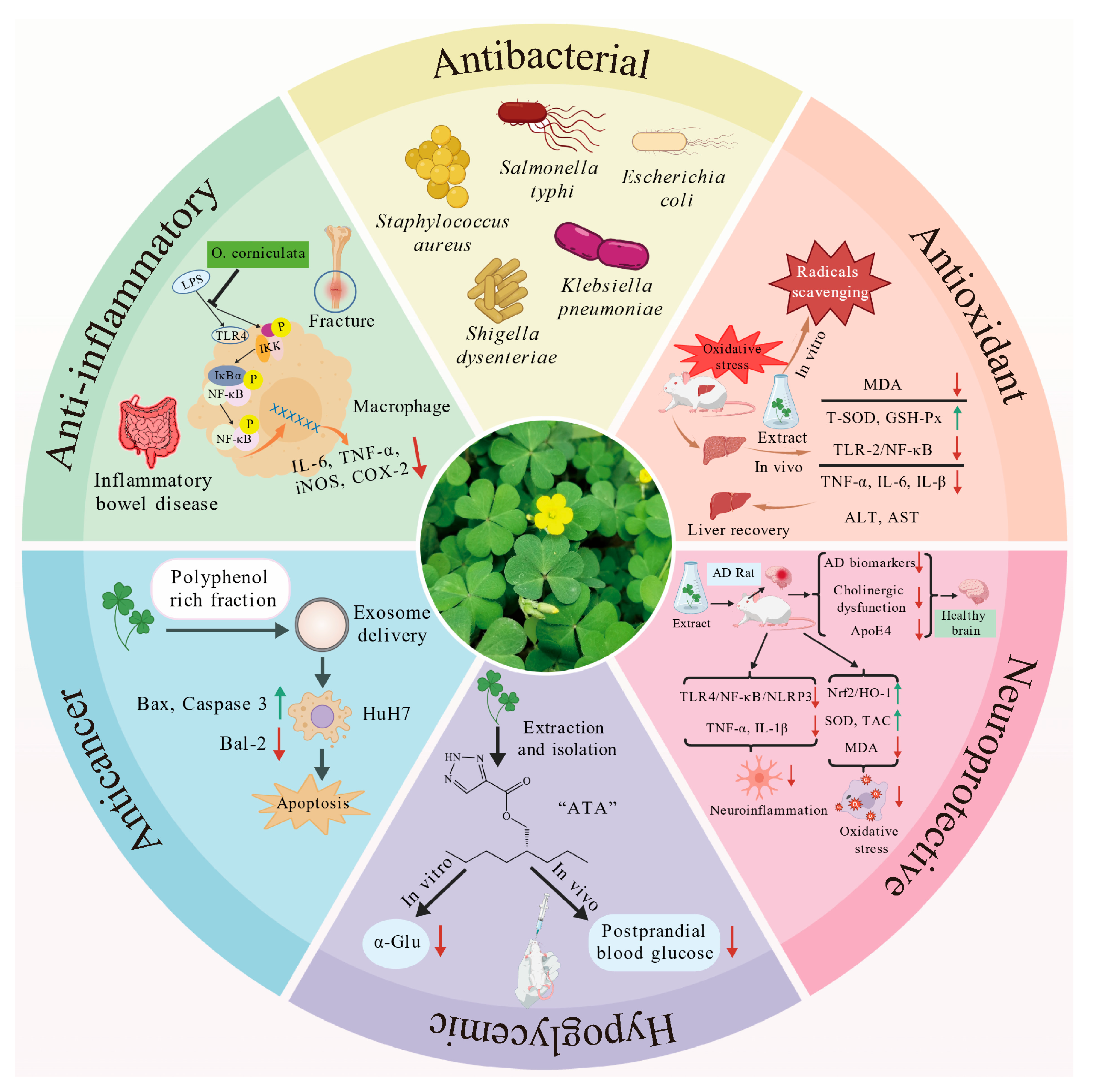
O. corniculata exhibits a wide range of biological activities, increasingly supported by experimental evidence. These effects are largely attributed to the synergistic actions among its diverse phytochemicals, particularly flavonoids, polysaccharides, and phenolic acids. The plant demonstrates notable potential in antioxidant, anti-inflammatory, antimicrobial, hepatoprotective, neuroprotective, hypoglycemic, and anticancer applications. An overview of these biological activities is presented in Figure 4, providing a foundation for the detailed discussions that follow.
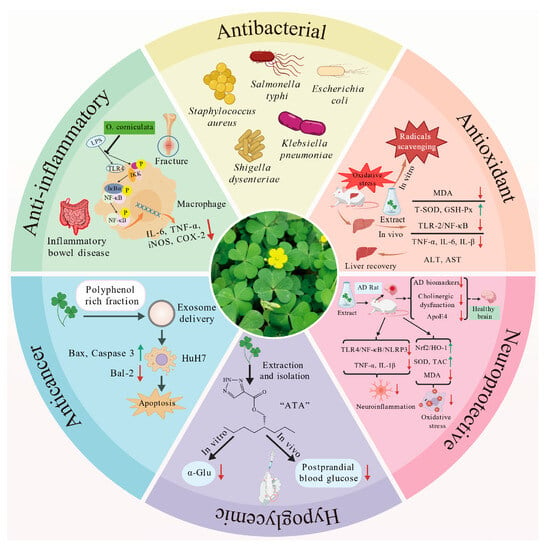
Figure 4.
Biological Activities of O. corniculata. Upward-pointing arrows denote upregulation of gene/protein expression or protective markers, while downward-pointing arrows indicate downregulation of expression levels or damage markers. Several elements in the image were obtained from https://biogdp.com (accessed on 1 September 2025) [55].
5.1. Antioxidant Activity
Oxidative stress caused by excessive reactive oxygen species (ROS) is a major contributor to lipid peroxidation, protein oxidation, and DNA damage, processes implicated in chronic diseases such as hepatic dysfunction, neurodegeneration, and cancer [56]. It suppresses enzymatic antioxidants, including superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GSH-Px), as well as non-enzymatic antioxidants such as glutathione (GSH). This imbalance leads to the accumulation of oxidative products such as malondialdehyde (MDA) and protein carbonyls (PC), which further aggravate cellular injury and inflammation [7].
O. corniculata contains abundant bioactive constituents, notably flavonoids, phenolic acids, and polysaccharides, that contribute to strong free radical scavenging and anti-oxidative stress effects (Table 2) [4]. The polysaccharide fraction OCP-3 inhibits lipid peroxidation and protects DNA from oxidative damage. In both HEK-293 cells and C. elegans, OCP-3 reduces MDA and PC levels, while enhancing antioxidant enzymes including SOD, CAT, and GSH-Px [7]. Methanol extracts of O. corniculata activate the Nrf2/HO-1 signaling pathway, enhancing antioxidant enzyme activities [57]. In carbon tetrachloride-induced hepatic and renal injury models, the extracts restore the activities of multiple antioxidant enzymes, including SOD, CAT, GSH-Px, glutathione-S-transferase (GST), glutathione reductase (GSR), peroxidase (POD), and quinone reductase (QR). The extracts also lower MDA levels, suppress lipid peroxidation, improve serum biochemical indices such as alanine aminotransferase (ALT) and aspartate aminotransferase (AST), and mitigate histopathological damage [58,59].
Dietary supplementation with O. corniculata powder has been shown to enhance antioxidant capacity under heat stress in broilers by increasing SOD, CAT, and GSH-Px activities while reducing MDA levels [60]. It also improved oxidative stability and nutritional quality of broiler meat by lowering lipid peroxidation and increasing polyunsaturated fatty acid (PUFA) content [4,5]. These findings indicate that O. corniculata supplementation effectively mitigates oxidative stress and maintains redox balance in animal systems, supporting its use as a natural antioxidant source in animal nutrition [44].

Table 2.
Summary of antioxidant activity studies of O. corniculata.
Table 2.
Summary of antioxidant activity studies of O. corniculata.
| No. | Testing Subjects | Application Part or Compounds | Doses/Duration | Effects | Ref. |
|---|---|---|---|---|---|
| 1 | Free radicals | Polyphenol-rich methanol extract | 0.5–50 μg/mL | DPPH·scavenging | [61] |
| 2 | pUC18 plasmid DNA | Polyphenol-rich methanol extract | 100–500 ng/mL | Protected DNA from·OH-induced strand breaks | [61] |
| 3 | BSA | Polyphenol-rich methanol extract | 100–500 μg/mL | Inhibited PC formation | [61] |
| 4 | Liver cells | Polyphenol-rich methanol extract | 10–50 µg/mL, 45 min incubation | Protected liver cells from oxidative damage by OH | [61] |
| 5 | Free radicals; Fe3+ | Hot water extract | 50–400 μg/mL | Free radicals scavenging and iron-reducing capacity | [62] |
| 6 | HEK-293 cells; C. elegans | Acidic polysaccharide OCP-3 | 50–800 μg/mL; 4–8 mg/mL | SOD ↑, CAT ↑, GSH-Px ↑, MDA ↓, PC ↓, radicals scavenging, DNA protection | [7] |
| 7 | Free radicals | Biogenic silver nanoparticles synthesized using aqueous extract of O. corniculata | 50–400 μg/mL | DPPH·/ABTS·scavenging | [31] |
| 8 | Free radicals, Fe2+, Fe3+, lipid | Ethanol extract | DPPH: 26.2 ± 2 µg/mL; Ferrous Ion: 74.3 ± 0.4 µg/mL; NO: 75.4 ± 7.3 µg/mL; ABTS: 59.9 ± 5.2 µg/mL; NBT: 118.2 ± 2.3 µg/mL; FRAP: 152.1 ± 9.5 µg/mL; LPO: 1.8 ± 0.4 µg/mL | The antioxidant effect was as demonstrated by IC50 values in seven assays. | [63] |
| 9 | Free radicals | TFO | 100–500 μg/mL | DPPH/OH/O2-scavenging | [10] |
| 10 | Pork lard | O. corniculata polyphenols | 0.02–0.04%, 50 °C, 21 days | Significantly inhibited lipid peroxidation | [64] |
| 11 | Fractured rats | O. corniculata (whole plant, 47.5% of mixture) aqueous extract | 150–600 mg/kg, percutaneous and p.o., 2 weeks | SOD ↑, CAT ↑, GSH ↑, enhances antioxidant enzyme activity and alleviates oxidative stress in bone tissue | [65] |
| 12 | AlCl3-induced AD rats | Methanol extract | 150 mg/kg, p.o., 5 weeks | TAC ↑, SOD ↑, MDA ↓; upregulates Nrf2/HO-1 pathway, enhances antioxidant defense, reduces oxidative damage in brain | [57] |
| 13 | SD rats (CCl4-induced acute liver injury) | Aqueous extract | 4–16 g/kg, p.o., 10 days | ALT ↓, AST ↓, MDA ↓, T-SOD ↑, GSH-Px ↑; downregulates TLR2/NF-κB pathway, inhibits oxidative stress | [66] |
| 14 | CCl4-induced nephrotoxic rats | Methanol extract | 100, 200 mg/kg, p.o., 7 days | CAT ↑, POD ↑, SOD ↑, GSH-Px ↑, GST ↑, GSR ↑, QR ↑, GSH ↑, MDA ↓, protein oxidation ↓, renal injury markers ↓, improved renal histology | [58] |
| 15 | CCl4-induced hepatotoxic rats | Methanol extract | 100, 200 mg/kg, p.o., 7 days | CAT ↑, POD ↑, SOD ↑, GSH-Px ↑, GST ↑, GSR ↑, QR ↑, GSH ↑, TBARS ↓, AST ↓, ALT ↓, ALP ↓, improved liver histology | [59] |
| 16 | Paracetamol-induced hepatotoxic rats | Ethanol extract | 100–500 mg/kg, p.o., 4 days | AST ↓, ALT ↓, ALP ↓, MDA ↓, improved liver histology; enhanced antioxidant defense and reduced oxidative stress | [67] |
| 17 | Streptozotocin (STZ)-induced diabetic rats | Ethanol extract | 100 and 300 mg/kg, p.o., 28 days | Reduced fasting glucose and MDA levels, enhanced SOD and GSH-Px activities, and improved pancreatic β-cell morphology. | [8] |
| 18 | Heat-stressed broilers | O. corniculata powder | 10 g/kg diet, 28–42 days | MDA ↓ in muscle, TAC ↑, improves lipid oxidative stability and mitigates oxidative damage | [60] |
| 19 | Heat-stressed broilers | O. corniculata powder + Chromium picolinate (CrPic) | 10 g O. corniculata powder + 0.2 mg CrPic/kg diet, 1–42 days. | Improvement of microbiota balance, enhancing gut health in heat-stressed broilers, with more significant effects in the early growth stage | [44] |
| 20 | Heat-stressed broilers | O. corniculata powder + CrPic | 10 g O. corniculata powder + 0.2 mg CrPic/kg diet, 28–42 days | Improves meat quality (crude protein ↑, fat in breast meat ↓) and enhances antioxidant defense against heat stress. | [3] |
5.2. Anti-Inflammatory Activity
Inflammation is an essential host defense mechanism, but chronic or excessive responses contribute to the pathogenesis of cancer, cardiovascular disorders, and diabetes [68,69]. Recent studies indicate that O. corniculata exhibits anti-inflammatory activity through multiple pathways involving its diverse phytochemicals, particularly flavonoids and phenolic acids [70]. These compounds act primarily by inhibiting nuclear factor kappa B (NF-κB)-mediated transcriptional activation, downregulating pro-inflammatory cytokines and enzymes such as cyclooxygenase-2 (COX-2), inducible nitric oxide synthase (iNOS), and phospholipase A2 (PLA2), and modulating oxidative stress-associated immune responses (Table 3).
In vitro, hot water extracts enriched in 3-O-caffeoylquinic acid and ellagic acid suppressed LPS-induced nitric oxide (NO) production in RAW264.7 cells [62], accompanied by downregulation of iNOS and COX-2 mRNA expression, and suppressed the protein levels of Interleukin-6 (IL-6) and tumor necrosis factor (TNF-α) [62]. Ethanol extracts inhibited NF-κB activation in PC-3 cells by downregulating phosphorylated p65 and IκBα, while upregulating IκBα expression [71]. In vivo, ethanol extracts significantly alleviated colonic inflammation in a rat model of acetic acid-induced inflammatory bowel disease (IBD) [72]. In a LPS-induced acute lung injury (ALI) rat model, the extracts also protected against lung damage by reducing inflammatory cell infiltration and tissue injury [73]. Serum metabolomics further identified flavonoids such as vicenin-3, isovitexin, and isoschaftoside as major absorbed constituents, which were proposed to act via suppression of IL-17/NF-κB signaling and concomitant antioxidant effects [73].
Among isolated compounds, diosmin demonstrated specific anti-inflammatory effects by inhibiting PLA2 activity [29]. Evidence from experimental studies suggests that this compound can attenuate venom-induced inflammation and protect against associated tissue injury, further supporting the role of O. corniculata flavonoids in modulating inflammatory pathways [29].

Table 3.
Summary of studies on anti-inflammatory, antipyretic and analgesic activity of O. corniculata.
Table 3.
Summary of studies on anti-inflammatory, antipyretic and analgesic activity of O. corniculata.
| No. | Testing Subjects | Application Part or Compounds | Doses/Duration | Effects | Ref. |
|---|---|---|---|---|---|
| 1 | RAW264.7 cells (LPS 2 μg/mL) | Hot water extract | 100–400 μg/mL, 24 h | NO ↓, iNOS ↓, COX-2 ↓, IL-6 ↓, TNF-α ↓ | [62] |
| 2 | RAW264.7 cells (LPS 0.25 μg/mL) | Ethanol extract | 10–50 μg/mL, 24 h | NO ↓, IL-17 ↓; NF-κB pathway ↓ | [73] |
| 3 | Human RBCs | Diosmin (isolated from O. corniculata) | 15–120 μM, 2 h | PLA2 inhibition ↑, anti-hemolysis, inflammation mediators ↓ | [29] |
| 4 | Human PC-3 prostate cancer cells | Ethanol extract | 50–200 μg/mL, 24 h | Cell proliferation ↓, migration, invasion; apoptosis ↑; NF-κB pathway activity ↓ (p-p65 ↓, p-IκBα ↓, IκBα ↑); anti-inflammatory mechanism via NF-κB inhibition | [71] |
| 5 | Swiss albino mice | Diosmin (for snake venom toxicity) | Diosmin (1:200 w/w) pre-incubated for 1 h (i.p. injection), observation for 5 h | Myotoxicity ↓, pulmonary hemorrhage ↓, CPK ↓, LDH ↓, tissue damage ↓ | [29] |
| 6 | Mice (acute peritonitis model) | Ethanol extract | 6% and 12% extract, p.o., 5 days | Abdominal writhing response ↓ (analgesic); abdominal capillary permeability ↓ (anti-inflammatory) | [74] |
| 7 | Swiss mice/Wistar rats | β-sitosterol (extract from leaves) | 5–20 mg/kg, i.p., single dose | Analgesia (hot plate latency ↑, writhing ↓), anti-inflammation (paw edema ↓); opioid receptor involvement, PGE inhibition | [70] |
| 8 | Rats (acetic acid-induced IBD) | Ethanol extract | 200–400 mg/kg, p.o., 7 days | Colon weight ↓, visible lesion score ↓, histopathological score ↓ | [72] |
| 9 | SD rats (LPS-induced ALI) | Ethanol extract | 0.8–3.2 g/kg, p.o., bid × 7 d | Lung edema ↓, inflammatory cell infiltration ↓; serum TNF-α ↓, IL-6 ↓, IL-1β ↓, IL-18 ↓ | [73] |
| 10 | Rats (pylorus ligation model, indomethacin-induced ulcer model) | Methanol extract | 125–500 mg/kg, p.o., single dose | Gastric secretion ↓, acidity ↓, ulcer index ↓; protection against NSAID-induced gastric injury; anti-inflammatory and gastroprotective effects | [75] |
| 11 | Rats (CNP model) | Compound prescription containing O. corniculata | 1 g/kg, p.o., 28 days | Inflammatory infiltration ↓, MCP-1 ↓, ROS ↓, GSH ↑, 4-HNE ↓, ALDH2 ↓, FGF2 ↓; improved tissue pathology; | [48] |
| 12 | Rats (CNP model) | Compound prescription containing O. corniculata | 9.37 g/kg, p.o., 49 days | Prostate index ↓, TNF-α ↓, IL-1β ↓, IL-6 ↓; improved histopathology; cGAS ↓, STING ↓, TRAF6 ↓, HSP70 ↑ | [76] |
5.3. Antibacterial Activity
O. corniculata extracts exhibit broad-spectrum antibacterial activity. Methanol extracts containing compounds such as rutin, p-hydroxybenzoic acid, and ferulic acid have been reported to contribute to both antimicrobial and antioxidant effects [61]. Recent findings suggest that the antibacterial effects of O. corniculata are associated with the inhibition of biofilm formation and interference with bacterial adhesion and colonization (Table 4) [77].
In vitro, ethanol and aqueous extracts inhibited the growth of both Gram-positive and Gram-negative bacteria, including S. aureus and E. coli, and were shown to suppress biofilm formation [77,78]. In vivo, methanol extracts reduced intestinal colonization by pathogenic Shigella strains in a suckling mouse diarrhea model, with stronger inhibition observed against S. dysenteriae type 1 compared to S. flexneri 2a [79]. High-performance liquid chromatography (HPLC) analysis suggested that polyphenols are the principal active constituents. The study further showed that efficacy depended on timing of administration, suggesting that the extract may interfere with bacterial adhesion or early colonization processes.
Recent advances in nanotechnology have expanded the antibacterial applications of O. corniculata (Table 5). Extracts have been utilized for the eco-friendly synthesis of silver nanoparticles (AgNPs), which display stronger and broader antibacterial activity compared with crude extracts [80]. Hybrid nanomaterials, including silver nanoparticles on graphene oxide (AgNPs@GO) [81] and AgNP-graphene nanocomposites, provide enhanced efficacy by synergistically disrupting bacterial membranes, inhibiting biofilm formation, and interfering with bacterial metabolism [82]. Furthermore, ZnO nanoflowers (ZnO NFs) synthesized using O. corniculata extracts damage bacterial membranes and intensify oxidative stress [83]. Flavonoids such as quercetin contribute both antibacterial effects and electron-donating capacity, thereby enhancing the overall activity of these nanostructures [83].

Table 4.
Summary of studies on the antimicrobial activity of O. corniculata.
Table 4.
Summary of studies on the antimicrobial activity of O. corniculata.
| No. | Testing Subjects | Application Part or Compounds | Methods | Effects | Ref. |
|---|---|---|---|---|---|
| 1 | S. aureus, E. coli | Aqueous extract | Disk diffusion | At 20% concentration, inhibition zones: S. aureus (21 mm), E. coli (19.33 mm) | [78] |
| 2 | E. coli, S. Typhi, MDR S. Typhi, K. pneumoniae, MDR C. koseri | Methanol extract | Agar well diffusion, MIC | At 50 mg/mL concentration, inhibition zones: E. coli (17 mm), S. Typhi (13 mm), MDR S. Typhi (16 mm), K. pneumoniae (11 mm), C. koseri (12 mm). MIC for E. coli, K. pneumoniae, C. koseri: 25 mg/mL; S. Typhi: 100 mg/mL; MDR S. Typhi: 50 mg/mL. | [84] |
| 3 | S. aureus | Ethanol extract | Microdilution, biofilm inhibition and eradication assays | At 1% (w/v), antibacterial activity against S. aureus was 76.23%; biofilm inhibition rate 71.32% (mid-phase), 69.33% (mature-phase); biofilm eradication rate 64.1%. | [77] |
| 4 | E. coli, S. dysenteriae, S. typhi, B. subtilis | 5-hydroxy-6,7,8,4′-tetramethoxyflavone and 5,7,4′-trihydroxy-6,8-dimethoxyflavone | Agar diffusion assay | The inhibition zones of these two flavonoids isolated from O. corniculata ranged from 10 to 16.5 mm. | [85] |
| 5 | Suckling mice infected with S. dysenteriae 1 (NT4907) and S. flexneri 2a (2457T) | Methanol extract | 20 mg/kg, single oral administration (simultaneous or 3 h post-infection) | Reduced intestinal colonization of Shigella strains. | [79] |
| 6 | Pomfret fish (food preservation model) | Methanol leaf extract (rutin, p-hydroxybenzoic acid, ferulic acid) | Food storage assay (bacterial growth and oxidative stability) | Significantly inhibited S. aureus growth during 10 °C storage (48 h) and reduced lipid oxidation, suggesting potential as a natural preservative. | [61] |

Table 5.
Antimicrobial activity of nanomaterials synthesized using O. corniculata.
Table 5.
Antimicrobial activity of nanomaterials synthesized using O. corniculata.
| No. | Nanomaterial Type | Biosynthesis Agent | Test Microorganisms | Methods | Effects | Ref. |
|---|---|---|---|---|---|---|
| 1 | Silver nanoparticles (AgNPs) | O. corniculata extract + AgNO3 | S. aureus, E. coli, B. subtilis, K. pneumoniae, S. typhi, S. pyogenes, P. aeruginosa, B. cereus, S. typhimurium, E. faecalis, A. baumannii, P. mirabilis | Disk diffusion, broth microdilution, biofilm inhibition assay | Inhibition zones 10–20 mm (25–50 μg/mL); MIC 0.11–11.5 μg/mL; disrupts membrane integrity, inhibits biofilm, induces ROS, interferes with bacterial metabolic pathways and interacts with intracellular targets | [31,80,86,87,88] |
| 2 | AgNPs@GO nanocomposite | O. corniculata extract + AgNO3 + GO | B. subtilis, E. coli | Agar well diffusion | Inhibition zones: B. subtilis 27 mm, E. coli 21 mm. Enhanced antibacterial activity due to synergistic interaction. | [81] |
| 3 | AgNPs-graphene nanocomposite | O. corniculata extract + AgNO3 + graphene | E. coli, S. aureus, B. cereus, S. typhimurium | MIC, MBC, inhibition zone | MIC and MBC both as low as 10 μg/mL for AgNPs-graphene composites; disrupts membranes and inhibits metabolism. | [82] |
| 4 | ZnO nanoflowers (ZnO NFs) | O. corniculata extract + Zn(NO3)2 | S. aureus, E. faecium, P. aeruginosa | Broth dilution | Dose-dependent antibacterial activity (40–120 μg/mL); Gram-positive bacteria more sensitive; induces membrane damage, ROS, and synergizes with flavonoids. | [83] |
5.4. Anticancer Activity
Research on the anticancer potential of O. corniculata has gained momentum in recent years, with investigations employing cell-based, animal, and molecular docking approaches to elucidate its activity across diverse cancer models (Table 6) [63]. Ethanol extracts have been shown to induce apoptosis in MCF-7 breast cancer cells by promoting oxidative stress and modulating apoptosis-related genes, including tumor protein p53 (p53), Fas cell surface death receptor (CD95), and B-cell lymphoma 2 (Bcl-2) [89]. Importantly, these extracts displayed low cytotoxicity toward normal cells, suggesting selective activity. Ethanol and ethyl acetate extracts also exhibited significant cytotoxicity against HepG2 cells, with IC50 values of 34.49 μg/mL and 30.25 μg/mL, respectively [63]. Molecular docking identified apigenin, a flavonoid constituent, as a potential inhibitor of epidermal growth factor receptor tyrosine kinase (EGFR-TK), with a binding energy of −7.90 kcal/mol, implicating interference with EGFR signaling [90]. In addition, corniculin, a newly identified lignan from O. corniculata, exhibited moderate cytotoxicity against SMMC-7721, MCF-7, HCT-15, and A549 cells, with potency comparable to cisplatin. In vivo, ethanol extracts inhibited tumor progression and prolonged survival in Ehrlich ascites carcinoma (EAC) mice, again without significant toxicity to normal tissues.
Advances in nanotechnology and drug delivery systems have further expanded its anticancer potential. Green-synthesized silver nanoparticles (O-AgNPs) using O. corniculata extract exhibited cytotoxicity in MCF-7 and AGS (human gastric carcinoma) cells by inducing apoptosis, while showing low toxicity toward normal cells [88]. Moreover, exosome-mediated delivery of O. corniculata polyphenols improved cellular uptake and cytotoxicity in HuH7 hepatocellular carcinoma cells [11]. This strategy promoted apoptosis by upregulating pro-apoptotic genes, including Bcl-2-associated X protein (Bax) and caspase-3, while downregulating the anti-apoptotic gene Bcl-2, thereby enhancing therapeutic efficacy and selectivity.
Antioxidant and anti-inflammatory properties of O. corniculata contribute to reducing the risk of cancer development. Selective induction of apoptosis in cancer cells is another important mechanism of its anticancer activity. Evidence suggests that the bioactive constituents of O. corniculata possess potential anticancer activity, and further elucidation of mitochondrial signaling, autophagy, and immune modulation may provide valuable insights into its underlying mechanisms.

Table 6.
Summary of studies on anticancer activity of O. corniculata.
Table 6.
Summary of studies on anticancer activity of O. corniculata.
| No. | Testing Subjects | Application Part or Compounds | Doses/Duration | Effects | Ref. |
|---|---|---|---|---|---|
| 1 | MCF-7 cell line | Ethanol extract | 31.25–2000 μg/mL, 24 and 72 h | p53 ↑, CD95 ↑, Bcl-2 ↓; selective cytotoxicity against cancer cells | [89] |
| 2 | HepG2 cell line | Ethanol extract and ethyl acetate fraction | 35–45 μg/mL, 48 h | Inhibited proliferation of HepG2 cells | [63] |
| 3 | SMMC-7721, MCF-7, HCT-15, A549 cancer cell lines | Corniculin | concentration range 0–100 μM, 48 h | Moderate cytotoxicity against multiple cell lines: IC50 for SMMC-7721, MCF-7, HCT-15, and A549 cells were 29.0, 35.6, 31.3, and 25.7 μM, respectively | [90] |
| 4 | EAC mice | Ethanol extract | 100 mg/kg and 400 mg/kg, p.o., 9 days | Inhibited tumor growth; prolonged survival; improved hematological and biochemical parameters; CAT ↑, GSH ↑, MDA ↓; no significant toxicity to normal cells | [91] |
| 5 | MCF-7 and AGS cell lines | O-AgNPs | 0.1–50 μg/mL, 24 h | Significant cytotoxicity and growth inhibition; induced apoptosis; low toxicity to normal cells | [88] |
| 6 | HuH7 cell line | Polyphenols from O. corniculata loaded in exosomes | 10–100 μg/mL, 24 h | Exosome delivery enhanced cytotoxicity and cellular uptake; promoted apoptosis via upregulation of Bax and caspase-3, downregulation of Bcl-2 | [11] |
5.5. Neuroprotective Activity
Neurodegenerative diseases, including Alzheimer’s disease, Parkinson’s disease, and depression, are commonly associated with oxidative stress and neuroinflammation. These processes drive protein aggregation, neuronal injury, and neurotransmitter imbalance, ultimately resulting in cognitive decline and motor dysfunction [92].
In an AlCl3-induced rat model of AD, methanol extracts of O. corniculata activated the Nrf2/HO-1 antioxidant pathway, elevated total antioxidant capacity (TAC) and SOD activity, and decreased MDA levels [57]. The extracts restored neurotransmitter balance by increasing dopamine (DA), norepinephrine (NE), and 5-hydroxytryptamine (5-HT), while inhibiting acetylcholinesterase (AChE). They also suppressed neuroinflammation by downregulating TLR4/NF-κB/NLRP3 signaling and reducing pro-inflammatory cytokines. Furthermore, the extracts attenuated endoplasmic reticulum stress by inhibiting PERK/CHOP-mediated apoptosis, upregulated anti-apoptotic Bcl-2 and autophagy-related Beclin-1, and activated Wnt3a/β-catenin signaling. These changes decreased GSK-3β, ApoE4, and AD-associated proteins (APP, Aβ, p-Tau), while enhancing low-density lipoprotein receptor-related protein 1 (LRP1)-mediated Aβ clearance. Phytochemical profiling indicated that the extract is rich in flavonoids, organic acids, terpenoids. Behavioral tests demonstrated that the extract significantly improved spatial learning and memory in AD rats, partly due to increased brain-derived neurotrophic factor (BDNF) levels [57].
O. corniculata extracts exhibit notable neuroprotective potential by mitigating oxidative stress, neuroinflammation, and neuronal apoptosis, while modulating autophagy and key intracellular signaling cascades. Evidence from multiple neurodegenerative models (Table 7) indicates that these effects are primarily mediated through activation of the Nrf2/HO-1 antioxidant pathway [93,94], inhibition of TLR4/NF-κB/NLRP3-driven inflammatory signaling, and regulation of apoptosis- and autophagy-associated proteins. Collectively, these integrated mechanisms contribute to the alleviation of oxidative stress, preservation of neuronal structure, and improvement of cognitive and motor functions.

Table 7.
Summary of neuroprotective studies of O. corniculata.
Table 7.
Summary of neuroprotective studies of O. corniculata.
| No. | Disease Model | Animal Subjects | Application Part or Compounds | Doses/Duration | Effects | Ref. |
|---|---|---|---|---|---|---|
| 1 | Parkinson’s disease (MPTP-induced) | C57 black male mice | Ethanol extract | 250, 500 mg/kg, p.o., co-administered with MPTP | Restored SOD, CAT, reduced LPO; attenuated oxidative stress; improved locomotor activity, muscle coordination, and cognitive performance | [93,94] |
| 2 | Parkinson’s disease (Rotenone-induced) | Swiss albino mice | Ethanol extract | 500 mg/kg, p.o., for 21 days | Improved motor performance; SOD ↑, CAT ↑, GSH ↑, DA ↑; MDA ↓, NO ↓, Glu ↓; reduced neuronal loss and neuroinflammation | [95] |
| 3 | Dementia | Albino mice | Methanol extract | 100, 200 mg/kg, p.o., for 21 days | Significantly improved learning and memory abilities | [96] |
| 4 | Epilepsy (MES/PTZ-induced) | Wistar rats | Methanol extract | 200, 400 mg/kg, i.p., single dose | Restored antioxidant enzymes (SOD, GPx, GR, CAT), reduced LPO; increased DA, NA, 5-HT, GABA; delayed seizures and reduced severity | [97] |
| 5 | Alzheimer’s disease (AlCl3-induced) | Male SD rats | Methanol extract | 150 mg/kg, p.o., weeks | Improved spatial learning and memory; TAC ↑, SOD ↑, MDA ↓; restored DA, NE, 5-HT levels, AChE ↓; upregulation of Nrf2/HO-1, Bcl-2, Beclin-1, Wnt3a, β-catenin, LRP1; downregulation of TLR4/NF-κB/NLRP3, PERK/CHOP, GSK-3β, ApoE4; reduced APP, BACE1, Aβ, p-Tau; alleviated neuronal damage and brain pathology. | [57] |
6. Safety Studies on O. corniculata
O. corniculata has a long history of medicinal use and is generally considered to have a good safety record. In modern clinical practice, several formulations derived from O. corniculata have received approval from the China National Medical Products Administration (NMPA). These include the prescription formulations Gu Kang Capsules, Mi Lin Granules, and Fu Yan Xiao Capsules, all of which are widely used in clinical practice, and no major adverse effects have been reported to date.
Evidence from experimental models further supports the potentially safety profile of O. corniculata. A brine shrimp lethality assay indicated low cytotoxicity, with an LC50 of 156 µg/mL [52]. In Swiss albino mice [75], oral administration of O. corniculata extract at doses up to 2000 mg/kg caused no observable behavioral abnormalities or toxic effects. Li et al. [12] reported that daily oral administration of 0.432 g/kg of crude herb in rats for seven consecutive days caused no observable toxic effects. In a rabbit fracture model, daily dosing of 0.224 g/kg for 30 days produced no adverse reactions [98].
Despite these findings, most studies to date have focused on acute or short-term exposure. Comprehensive data on long-term toxicity, genotoxicity, and reproductive safety remain limited. Moreover, because O. corniculata contains calcium oxalate [20], excessive or prolonged intake may pose a theoretical risk of oxalate accumulation, particularly in individuals susceptible to kidney stones [99]. Modern extraction and processing techniques may help to mitigate this risk, but further systematic and quantitative evaluation is necessary to confirm its safety under long-term or high-dose exposure conditions. Preliminary serum metabolomics studies have detected absorbed bioactive constituents after oral administration of O. corniculata extract, including flavonoids (e.g., vicenin-3, isovitexin, isoschaftoside) and phenolic acids (e.g., 3-O-, 4-O-, 5-O-p-coumaroylquinic acid) [73]. However, the interactions between different bioactive constituents and their potential effects when combined with other medications have not been systematically evaluated. Further investigation of toxicokinetics, tissue distribution, and maximum tolerated dose (MTD) would be meaningful for confirming safe dosage ranges and supporting its clinical applications.
7. Conclusions and Future Prospective
O. corniculata exhibits antioxidant, antimicrobial, and anti-inflammatory activities, and its wide distribution, ease of cultivation, and chemical diversity underscore its potential as a sustainable bioresource. Flavonoids are the principal absorbed constituents in vivo and likely mediate antioxidant and anti-inflammatory effects through multiple signaling pathways [73]. Recent studies have also identified structurally unique compounds, such as the alkaloid ATA, which represents a promising lead for α-glucosidase inhibition [39], and the lignan corniculin A, which demonstrates moderate anticancer activity comparable to cisplatin [100]. Advances in nanotechnology have further expanded its therapeutic potential. Green-synthesized nanomaterials derived from O. corniculata extracts exhibit enhanced antioxidants, antibacterial and anticancer activities. Exosome-mediated delivery systems for polyphenols also improve bioavailability, addressing limitations of conventional herbal extracts [11]. Collectively, these findings highlight the broad application potential of O. corniculata, as outlined below:
- (1)
- Dietary supplements and nutraceuticals: Flavonoids and polysaccharides enhance antioxidant defenses and help mitigate disorders associated with oxidative stress, supporting their potential development as dietary supplements and nutraceuticals [7].
- (2)
- Antimicrobial applications: Extracts exhibit antibacterial activity against S. aureus and E. coli, with topical formulations such as antibacterial creams showing efficacy [78]. Green-synthesized nanomaterials further enhance antibacterial activity, offering opportunities for wound-care and infection-control products.
- (3)
- Hypoglycemic potential: Ethanol extracts exhibit antihyperglycemic effects in STZ-induced diabetic rats, mainly through the enhancement of antioxidant defenses and protection of pancreatic β-cells [8]. The alkaloid ATA has been identified as one of the contributors to this activity [37,39]. These findings suggest that the plant’s bioactive constituents hold promise for the development of natural hypoglycemic agents.
- (4)
- Food preservation: Polyphenols delay lipid peroxidation in animal fats and extend shelf life. Oil-soluble extracts show stronger antioxidant effects than water-soluble forms, with optimal activity at 0.02–0.04% concentrations, comparable to butylated hydroxyanisole (BHA) and butylated hydroxytoluene (BHT) [77]. Methanol extracts of O. corniculata effectively inhibit bacterial growth and oxidative spoilage in fish meat, indicating its potential as a natural preservative [61].
- (5)
- Poultry production: Dietary supplementation with O. corniculata improves antioxidant stability of broiler meat and increases the levels of bioactive compounds such as lutein, zeaxanthin, and PUFAs [4]. It also optimizes the fatty acid profile, helps maintain gut microbiota balance, alleviates heat stress, and supports growth performance [44,60].
Future research should prioritize standardized cultivation and harvesting practices, as geographic origin, environmental conditions, and plant varieties strongly influence the accumulation and composition of bioactive compounds. Standardized extraction and quality-control protocols, particularly those based on antioxidant-rich components such as flavonoids and polyphenols, will be essential to ensure reproducibility. Mechanistic studies are also needed to clarify how these constituents exert their biological effects and to guide practical applications. Systematic evaluation of pharmacokinetics and long-term safety is also required to support clinical translation.
Considering its multi-target pharmacological properties, O. corniculata may have potential for synergistic or modulatory effects when combined with antibacterial, anticancer, or neuroprotective drugs. However, such interactions have not yet been systematically investigated and warrant further study. Moreover, mechanistic investigations focusing on the antioxidant, anti-inflammatory, and gut microbiota-modulating effects of O. corniculata in poultry production will be valuable to clarify its roles and optimize its practical application as a feed additive. With its low cost, broad availability, and multi-target bioactivities, O. corniculata holds considerable promise as a sustainable botanical resource for improving animal health, food preservation, and the development of novel antioxidant-based therapeutics.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/antiox14111352/s1. Figure S1: Structures of flavones (1–48) isolated from O. corniculata. Figure S2: Structures of other flavonoid subclasses isolated from O. corniculata, including flavonols (49–67), dihydroflavones (68–70), isoflavones (71–79), chalcone (80), dihydrochalcones (81–82), and flavanols (83–84). Figure S3: Structures of organic acids identified from O. corniculata. (A): Phenolic acids (85–107); (B): Other organic acids (108–135). Figure S4: Structures of terpenoids (136–155) identified from O. corniculata. Figure S5: Structures of alkaloids identified from O. corniculata. Table S1: Chemical compounds isolated from O. corniculata. References [19,20,21,22,23,27,29,31,35,37,39,42,57,61,62,63,68,70,83,85,90,100,101,102,103,104,105,106,107,108,109] are cited in the supplementary materials.
Author Contributions
T.Z.: conceptualization, literature search, software, figure preparation, validation and original draft preparation; J.K.: resources, supervision, project administration, funding acquisition, review and editing; C.W.: conceptualization, resources, supervision, project administration; H.Z.: validation and funding acquisition; J.H.: proofreading the manuscript and editing; C.T.: data curation and editing; W.Z.: software and data curation. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the 2115 Talent Development Program of China Agricultural University (NO. 00109015); Joint Project of the Scientific Research Innovation Fund for PhD Students (NO. HSPHDSRF-2024-05-013).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article; further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zhang, X.; Liu, Y.; Shen, Z.; Wang, S.; Wu, C.; Liu, D.; Tang, S.; Dai, C. Osthole ameliorates myonecrosis caused by Clostridium perfringens type A infection in mice. One Health Adv. 2023, 1, 27. [Google Scholar] [CrossRef]
- Gliozheni, E.; Salem, Y.; Cho, E.; Wahlstrom, S.; Olbrich, D.; Shams, B.; Alexander, M.; Ichii, H. Food-Derived Phytochemicals: Multicultural Approaches to Oxidative Stress and Immune Response. Int. J. Mol. Sci. 2025, 26, 7316. [Google Scholar] [CrossRef]
- Saracila, M.; Panaite, T.D.; Mironeasa, S.; Untea, A.E. Dietary Supplementation of Some Antioxidants as Attenuators of Heat Stress on Chicken Meat Characteristics. Agriculture 2021, 11, 638. [Google Scholar] [CrossRef]
- Saracila, M.; Untea, A.E.; Panaite, T.D.; Varzaru, I.; Oancea, A.; Turcu, R.P.; Vlaicu, P.A. Creeping wood sorrel and chromium picolinate effect on the nutritional composition and lipid oxidative stability of broiler meat. Antioxidants 2022, 11, 780. [Google Scholar] [CrossRef] [PubMed]
- Groom, Q.J.; Straeten JVder Hoste, I. The origin of Oxalis corniculata L. PeerJ 2019, 7, e6384. [Google Scholar] [CrossRef] [PubMed]
- Bharti, R.; Priyanka, P.; Bhargava, P.; Khatri, N. Ethnopharmacology and therapeutic potentials of Oxalis corniculata: An in-depth study. Beni-Suef Univ. J. Basic Appl. Sci. 2024, 13, 81. [Google Scholar] [CrossRef]
- Gao, T.; Hu, W.; Zhang, Z.; Tang, Z.; Chen, Y.; Zhang, Z.; Yuan, S.; Chen, T.; Huang, Y.; Feng, S.; et al. An acidic polysaccharide from Oxalis corniculata L. and the preliminary study on its antioxidant activity. J. Food Biochem. 2022, 46, e14235. [Google Scholar] [CrossRef]
- Ansari Khan, M.; Khan, N.; Ahmed, D.; Khan, M.; Lall, A. Exploring antihyperglycemic and histopathological analysis of Oxalis corniculata L. hydroethanolic extract in STZ induced diabetic rats with in silico α-amylase inhibitor assessment. Ann. Phytomedicine 2024, 13, 612–626. [Google Scholar] [CrossRef]
- Sarfraz, I.; Rasul, A.; Hussain, G.; Shah, M.A.; Nageen, B.; Jabeen, F.; Selamoğlu, Z.; Uçak, İ.; Asrar, M.; Adem, S. A review on phyto-pharmacology of Oxalis corniculata. Comb. Chem. High Throughput Screen. 2022, 25, 1181–1186. [Google Scholar] [CrossRef]
- Jiang, D.; Yu, D.; Zeng, M.; Liu, W.; Li, D.; Liu, K. Optimization of ultrasonic-assisted extraction of total flavonoids from Oxalis corniculata by a hybrid response surface methodology-artificial neural network-genetic algorithm (RSM-ANN-GA) approach, coupled with an assessment of antioxidant activities. RSC Adv. 2024, 14, 39069–39080. [Google Scholar] [CrossRef]
- Begum, S.; Mazumder, P.B.; Talukdar, A.D. In vitro evaluation of anticancer activity of Oxalis corniculata L., by harnessing exosome for effective drug delivery against hepatocellular carcinoma. Pharmacol. Res.-Nat. Prod. 2025, 8, 100298. [Google Scholar] [CrossRef]
- Li, J.; Yang, J.; Liu, T.; Li, Y.; Shen, Y.; Yang, C.; He, B.; Wang, A. Treatment of Soft Tissue Injury in Rats with Gukang Capsule, Oxalis Corniculata and Musa Basjoo Sieb. J. Guizhou Med. Univ. 2020, 45, 1015–1019. [Google Scholar]
- Shamso, E.; Draz, A.; Hosni, H.; Hussein, S. Taxonomic revision of genus Oxalis L. (Oxalidaceae) in the flora of Egypt. Taeckholmia 2022, 41, 56–69. [Google Scholar] [CrossRef]
- Yang, Y.; He, L.; Liu, Y.; Liu, Y.; Chen, J. Identification and Analysis of KNOX Gene Family Members Based on RNA-Seq in Oxalis corniculata. Mol. Plant Breed. 2022, 23, 4320. [Google Scholar]
- Loi, V.D.; Nguyet, L.T.; Duong, L.T.H.; Mai, N.T.; Huong, N.T.T.; Hai, P.T.M. Morphological and Microscopical Characteristics of Oxalis corniculata L. VNU J. Sci. Med. Pharm. Sci. 2021, 37, 1. [Google Scholar]
- Al Sheikh, B.; Gedeon, J. Newly Documented Invasive Alien Plant Species in the West Bank, Palestine. Feddes Repert. 2025, 136, 136–145. [Google Scholar] [CrossRef]
- Rokaya, M.B.; Münzbergová, Z.; Shrestha, M.R.; Timsina, B. Distribution pattern, ecological status and ethnomedicinal uses of medicinal plants along an altitudinal gradient among Jaunsar tribes of Western himalaya. J. Mt. Sci. 2025, 31, 1–25. [Google Scholar]
- Liu, Z.; Wang, L.; Ding, S.; Xiao, H. Enhancer assisted-phytoremediation of mercury-contaminated soils by Oxalis corniculata L., and rhizosphere microorganism distribution of Oxalis corniculata L. Ecotoxicol. Environ. Saf. 2018, 160, 171–177. [Google Scholar] [CrossRef]
- Imran, M.; Irfan, A.; Ibrahim, M.; Assiri, M.A.; Khalid, N.; Ullah, S.; Al-Sehemi, A.G. Carbonic anhydrase and cholinesterase inhibitory activities of isolated flavonoids from Oxalis corniculata L. and their first-principles investigations. Ind. Crops Prod. 2020, 148, 112285. [Google Scholar] [CrossRef]
- Badwaik, H.; Singh, M.; Thakur, D.; Giri, T.; Tripathi, D. The botany, chemistry, pharmacological and therapeutic application of Oxalis corniculata Linn-a review. Int. J. Phytomed. 2011, 3, 1. [Google Scholar]
- Zhang, B.; Kuang, W.; Liu, J.; Jiang, L.; Li, Y.; Ma, X. Chemical Constituents Analysis of Oxalis Corniculata L. Based on UPLC-Q-Exactive-Plus-Orbitrap-MS. Chin. J. Mod. Appl. Pharm. 2025, 42, 611–622. [Google Scholar]
- Zeb, A.; Imran, M. Carotenoids, pigments, phenolic composition and antioxidant activity of Oxalis corniculata leaves. Food Biosci. 2019, 32, 100472. [Google Scholar] [CrossRef]
- Zhang, S.; Dong, J.; Cheng, H. Essential oil composition of the leaves of Oxalis corniculata from China. Chem. Nat. Compd. 2018, 54, 380–381. [Google Scholar] [CrossRef]
- Al-Khayri, J.M.; Sahana, G.R.; Nagella, P.; Joseph, B.V.; Alessa, F.M. Flavonoids as potential anti-inflammatory molecules: A review. Molecules 2022, 27, 2901. [Google Scholar] [CrossRef]
- Dai, W.; Pan, M.; Peng, L.; Zhang, D.; Ma, Y.; Wang, M.; Wang, N. Integrated Transcriptome and Metabolome Analysis Reveals Insights into Flavone and Flavonol Biosynthesis in Salicylic Acid-Induced Citrus Huanglongbing Tolerance. J. Agric. Food Chem. 2024, 73, 919–937. [Google Scholar] [CrossRef]
- Tang, S.; Wang, B.; Liu, X.; Xi, W.; Yue, Y.; Tan, X.; Bai, J.; Huang, L. Structural insights and biological activities of flavonoids: Implications for novel applications. Food Front. 2025, 6, 218–247. [Google Scholar] [CrossRef]
- Yang, J.; Jiang, J.; Zong, D.; Peng, Y.; Chen, J.; Zhao, J. Chemical Composition from Oxalis corniculata L., Content of Total Flavonoids in Oxalis corniculata L. in Different Harvest Times. Guangzhou Chem. Ind. 2021, 49, 64–65. [Google Scholar]
- Zhang, B.; Sun, X.; Tang, W.; Jiang, L.; Liu, J.; Wang, Y.; Li, Y.; Li, Y.; Ma, X. An effective high-performance liquid chromatography method for fingerprint and multi-component quantification of Oxalis corniculata. Curr. Pharm. Anal. 2025, 21, 434–438. [Google Scholar] [CrossRef]
- Kiran, K.S.; Kameshwar, V.H.; Mudnakudu Nagaraju, K.K.; Nagalambika, P.; Varadaraju, K.R.; Karthik, N.A. Diosmin: A Daboia russelii venom PLA2s inhibitor-purified, and characterized from Oxalis corniculata L medicinal plant. J. Ethnopharmacol. 2024, 318, 116977. [Google Scholar] [CrossRef]
- Khan, F.; Bamunuarachchi, N.I.; Tabassum, N.; Kim, Y.M. Caffeic acid and its derivatives: Antimicrobial drugs toward microbial pathogens. J. Agric. Food Chem. 2021, 69, 2979–3004. [Google Scholar] [CrossRef]
- Karimzadeh, K.; Bakhshi, N.; Ramzanpoor, M. Biogenic silver nanoparticles using Oxalis corniculata characterization and their clinical implications. J. Drug Deliv. Sci. Technol. 2019, 54, 101263. [Google Scholar] [CrossRef]
- Vari, F.; Bisconti, E.; Serra, I.; Stanca, E.; Friuli, M.; Vergara, D.; Giudetti, A.M. Exploring the Role of Oleic Acid in Muscle Cell Differentiation: Mechanisms and Implications for Myogenesis and Metabolic Regulation in C2C12 Myoblasts. Biomedicines 2025, 13, 1568. [Google Scholar] [CrossRef]
- Peng, B.; Wei, S. Synthetic Engineering of Microbes for Production of Terpenoid Food Ingredients. J. Agric. Food Chem. 2025, 73, 10052–10068. [Google Scholar] [CrossRef]
- Jaramillo, S.P.; Calva, J.; Jiménez, A.; Armijos, C. Isolation of Geranyl Acetate and Chemical Analysis of the Essential Oil from Melaleuca armillaris (Sol. ex Gaertn.) Sm. Appl. Sci. 2024, 14, 1864. [Google Scholar] [CrossRef]
- Durgawale, P.P.; Hendre, A.S.; Phatak, R.S. GC/MS characterization, antioxidant and free radical scavenging capacities of methanolic extract of Oxalis corniculata LINN: An ayurvedic herb. Rasayan J. Chem. 2015, 8, 271–278. [Google Scholar]
- Ramakrishna, G.V.; Latif, Z.; Romiti, F. Enantioselective Total Syntheses of Vallesamidine and Schizozygane Alkaloids. J. Am. Chem. Soc. 2025, 147, 4613–4623. [Google Scholar] [CrossRef]
- Feng, Q.; Yang, W.; Peng, Z.; Wang, G. Utilizing bio-affinity ultrafiltration combined with UHPLC Q-Exactive Plus Orbitrap HRMS to detect potential α-glucosidase inhibitors in Oxalis corniculate L. Int. J. Biol. Macromol. 2023, 252, 126490. [Google Scholar] [CrossRef]
- Yang, Z.-J.; Huang, S.-Y.; Cheng, J.; Zeng, J.-W.; Wusiman, M.; Li, H.-B.; Zhu, H.-L. Betaine: A comprehensive review on dietary sources, health benefits, mechanisms of action, and application. Trends Food Sci. Technol. 2025, 159, 104993. [Google Scholar] [CrossRef]
- Feng, Q.; Yang, W.; Ma, X.; Peng, Z.; Wang, G. Investigation on the anti-α-glucosidase mechanism of aspergillus triazolate A from Oxalis corniculate L. Int. J. Biol. Macromol. 2024, 279, 135457. [Google Scholar] [CrossRef]
- Hu, W. Ultrasound-Enzyme Assisted Extraction, Physicochemical Properties and Antioxidant Activities of Polysaccharides from Oxalis corniculata L. Master’s Thesis, Sichuan Agricultural University, Ya’an, China, 2019. [Google Scholar]
- Aruna, K.; Devi, P.; Rajeswari, R.; Prabu, K.; Ramkumar, M.; Chidambaram, R.; Raja Sankar, S. Quantitative phytochemical analysis of Oxalis corniculata L. (Oxalidaceae). World J. Pharm. Sci 2014, 3, 711–716. [Google Scholar]
- Bordoloi, M.; Bordoloi, P.K.; Dutta, P.P.; Singh, V.; Nath, S.; Narzary, B.; Bhuyan, P.D.; Rao, P.G.; Barua, I.C. Studies on some edible herbs: Antioxidant activity, phenolic content, mineral content and antifungal properties. J. Funct. Foods 2016, 23, 220–229. [Google Scholar] [CrossRef]
- Chowdhury, M.M.K.; Islam, M.A.; Yasmin, F.; Ryhan, M.A. Biochemical potentials and stability of Oxalis corniculata L. leaf extracts. Dhaka Univ. J. Biol. Sci. 2025, 34, 75–83. [Google Scholar] [CrossRef]
- Saracila, M.; Panaite, T.D.; Tabuc, C.; Soica, C.; Untea, A.; Varzaru, I.; Wojdyło, A.; Criste, R.D. Maintaining intestinal microflora balance in heat-stressed broilers using dietary creeping wood sorrel (Oxalis corniculata) powder and chromium (chromium picolinate). Span. J. Agric. Res. 2020, 18, 19. [Google Scholar] [CrossRef]
- Jiang, X.; Zhao, R.; Zhou, X.; Fang, X.; Miao, M. Herbal research and analysis of ancient and modern clinical application of fresh Oxalis corniculata. China J. Tradit. Chin. Med. Pharm. 2024, 39, 3761–3767. [Google Scholar]
- Lou, Q.; Zhan, M.W.; Lai, Y.Q.; Zhan, X.X.; Xiao, Y.P.; Shang, X.J. Study on the treatment of chronic nonbacterial prostatitis caused by dampness-heat stasis with Oxalis Formula combined with transacupuncture. Natl. J. Androl. 2025, 31, 165–171. [Google Scholar]
- Zargar, S.A.; Ganie, A.H.; Reshi, Z.A.; Shah, M.A.; Sharma, N.; Khuroo, A.A. Oxalis corniculata L.(Oxalidaceae), an addition of an alien plant species to the flora of Ladakh, India. Vegetos 2024, 37, 373–378. [Google Scholar] [CrossRef]
- Lai, Y.; Yu, Y.; Liu, P.; Zhan, M.; Ma, M.; Wang, L.; Lou, Q.; Shang, X. Research on the mechanism of Miao ethnicity medicine formula of Oxalis corniculata against chronic non-bacterial prostatitis. Natl. J. Androl. 2023, 29, 783–789. [Google Scholar]
- He, L.; Sun, W.; Suo, X.; Shi, S.; Zhang, C.; Chen, Y. Herbal textual research of Oxalis corniculata L. Chin. J. Ethnomed. Ethnopharm. 2025, 34, 66–73. [Google Scholar]
- Kubade, M.; Shetti, P. Medicinal importance of traditional Indian herbal plant Oxalis corniculata: A review. Int. J. Pharm. Sci. Res. 2024, 15, 1015–1024. [Google Scholar]
- Noor, N.; Satapathy, K.B. Leafy vegetable diversity and their ethnomedicinal uses against gastrointestinal disorders in the Balasore district of Odisha, India. J. Appl. Biol. Biotechnol. 2023, 11, 259–267. [Google Scholar]
- Tibuhwa, D.D. Oxalis corniculata L. in Tanzania: Traditional use, cytotoxicity and antimicrobial activities. J. Appl. Biosci. 2016, 105, 10055–10063. [Google Scholar] [CrossRef][Green Version]
- Silalahi, M. Utilization of Oxalis corniculata Linn as a traditional medicine and its bioactivity. Magna Sci. Adv. Res. Rev. 2022, 5, 27–33. [Google Scholar] [CrossRef]
- Rahman, I.U.; Afzal, A.; Iqbal, Z.; Hart, R.; Abd_Allah, E.F.; Hashem, A.; Alsayed, M.F.; Ijaz, F.; Ali, N.; Shah, M.; et al. Herbal teas and drinks: Folk medicine of the Manoor valley, Lesser Himalaya, Pakistan. Plants 2019, 8, 581. [Google Scholar] [CrossRef]
- Jiang, S.; Li, H.; Zhang, L.; Mu, W.; Zhang, Y.; Chen, T.; Wu, J.; Tang, H.; Zheng, S.; Liu, Y.; et al. Generic Diagramming Platform (GDP): A comprehensive database of high-quality biomedical graphics. Nucleic Acids Res. 2025, 53, D1670–D1676. [Google Scholar] [CrossRef]
- Glorieux, C.; Liu, S.; Trachootham, D.; Huang, P. Targeting ROS in cancer: Rationale and strategies. Nat. Rev. Drug Discov. 2024, 23, 583–606. [Google Scholar] [CrossRef]
- Abu-Elfotuh, K.; Hamdan, A.M.E.; Mohamed, S.A.; Bakr, R.O.; Ahmed, A.H.; Atwa, A.M.; Hamdan, A.M.; Alanzai, A.G.; Alnahhas, R.K.; Gowifel, A.M.H.; et al. The potential anti-Alzheimer’s activity of Oxalis corniculata Linn. Methanolic extract in experimental rats: Role of APOE4/LRP1, TLR4/NF-κβ/NLRP3, Wnt 3/β-catenin/GSK-3β, autophagy and apoptotic cues. J. Ethnopharmacol. 2024, 324, 117731. [Google Scholar] [CrossRef]
- Khan, M.R.; Zehra, H. Amelioration of CCl4-induced nephrotoxicity by Oxalis corniculata in rat. Exp. Toxicol. Pathol. 2013, 65, 327–334. [Google Scholar] [CrossRef]
- Khan, M.R.; Marium, A.; Shabbir, M.; Saeed, N.; Bokhari, J. Antioxidant and hepatoprotective effects of Oxalis corniculata against carbon tetrachloride (CCl4) induced injuries in rat. Afr. J. Pharm. Pharmacol. 2012, 6, 2255–2267. [Google Scholar] [CrossRef]
- Saracila, M.; Tatiana, P.; Untea, A.; Varzaru, I. Effect of dietary supplementation of some antioxidant combinations on nutrient digestibility in heat-stressed broilers. Arch. Zootech. 2022, 25, 116–129. [Google Scholar] [CrossRef]
- Mukherjee, S.; Pal, S.; Chakrabarti, R.; Koley, H.; Dhar, P. Biochemical assessment of extract from Oxalis corniculata L.: Its role in food preservation, antimicrobial and antioxidative paradigms using in situ and in vitro models. Indian J. Exp. Biol. 2018, 56, 230–243. [Google Scholar]
- Kim, J.H.; Hong, M.; Han, J.H.; Lee, H.J.; Choi, D.H.; Hoang, K.; Van Dung, L.; Kwon, T.; Ahn, Y. Antioxidant and Anti-inflammatory Effects of Oxalis Corniculata Hot Water Extract. Korean J. Med. Crop Sci. 2022, 30, 419–429. [Google Scholar] [CrossRef]
- Gudasi, S.; Gharge, S.; Koli, R.; Patil, K. Antioxidant properties and cytotoxic effects of Oxalis corniculata on human Hepatocarcinoma (Hep-G2) cell line: An in vitro and in silico evaluation. Future J. Pharm. Sci. 2023, 9, 25. [Google Scholar] [CrossRef]
- Sun, X. Antioxidant Effect of Oxalis corniculata Polyphenols on Animal Fats. Heilongjiang Anim. Husb. Vet. Med. 2015, 129–130. [Google Scholar] [CrossRef]
- Ngueguim, F.; Jouonzo, J.; Donfack, J.; Gounoue, R.; Djientcheu Tientcheu, J.P.; Clarice, D.; Fifen, R.; Dzeufiet, P.; Théophile, D. The Mixture Aqueous Extracts from Oxalis corniculata L. and Acmella caulirhiza Delile Accelerates Bone Healing in Fractured Rats. J. Complement. Altern. Med. 2022, 17, 18–28. [Google Scholar] [CrossRef]
- Chen, C.; Chen, Y.; Cao, H.; Wang, G.; Guo, D.; Zhang, K. Protective effect and mechanism of Oxalidis Corniculatase Herba on acute hepatic injury induced by carbon tetrachloride in rats. Chin. J. Exp. Tradit. Med. Formulae 2018, 24, 141–145. [Google Scholar]
- Sreejith, G.; Jayasree, M.; Latha, P.G.; Suja, S.R.; Shyamal, S.; Shine, V.J.; Anuja, G.I.; Sini, S.; Shikha, P.; Krishnakumar, N.M.; et al. Hepatoprotective activity of Oxalis corniculata L. ethanolic extract against paracetamol induced hepatotoxicity in Wistar rats and its in vitro antioxidant effects. Indian J. Exp. Biol. 2014, 52, 147–152. [Google Scholar]
- Zhang, J.; Shen, W.; He, H. Exploring the action mechanism of Oxalis corniculata L. decoction in treating osteoarthritis utilizing liquid chromatography–mass spectrometry technology combined with network pharmacology. Medicine 2024, 103, e39515. [Google Scholar] [CrossRef]
- Ding, Q.; Huang, X.; Yang, X.; Zhang, M.; Qin, P.; Wu, H.; Wang, X. Study of the Mechanism of Oxalis corniculata (L.) in the Treatment of Hepatitis Based on Network Pharmacology; IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2021. [Google Scholar]
- Dighe, S.B.; Kuchekar, B.S.; Wankhede, S.B. Analgesic and anti-inflammatory activity of β-sitosterol isolated from leaves of Oxalis corniculata. Int. J. Pharmacol. Res. 2016, 6, 109–113. [Google Scholar]
- Zhang, G.W.; Zhan, M.W.; Zhan, X.X.; Yu, Y.; Liu, P.F.; Wang, L.; Wu, H.; He, C.Q.; Lou, Q.; Shang, X.J. Biological mechanisms of Oxalis corniculata regulating human prostate cancer PC-3 cells: An investigation based on the NF-κB pathway. Natl. J. Androl. 2023, 29, 202–209. [Google Scholar]
- Dutta, A.; Handique, C.; Lahkar, M. Evaluation of anti-inflammatory activity of Oxalis corniculata in experimentally induced inflammatory bowel disease in rats. Int. J. Basic Clin. Pharmacol. 2015, 4, 744–749. [Google Scholar] [CrossRef][Green Version]
- Ma, X.; Zhang, B.; Jiang, L.; Kuang, W.; Wang, M.; Wang, Y.; Sun, J.; Li, Y. The Activity, Composition, and Molecular Mechanism of Oxalis corniculata in Alleviating Acute Lung Injury. Nat. Prod. Commun. 2025, 20, 1934578X251361195. [Google Scholar] [CrossRef]
- Guo, M.; Wang, Y.; Shi, G.; Shen, L. Anti-inflammatory and analgesic effects of Oxalis corniculata L. on acute peritonitis in mice. J. Dali Univ. 2014, 13, 6–8. [Google Scholar]
- Sakat, S.S.; Tupe, P.; Juvekar, A. Gastroprotective effect of Oxalis corniculata (whole plant) on experimentally induced gastric ulceration in Wistar rats. Indian J. Pharm. Sci. 2012, 74, 48. [Google Scholar]
- Lou, Q.; Zhan, M.-W.; Lai, Y.-Q.; Zhan, X.-X.; Shang, X.-J. Mechanism of regulation of CNP rat model by Oxalis decoction via cGAS-STING signaling pathway. Natl. J. Androl. 2023, 29, 973–979. [Google Scholar]
- Hamzah, H.; Siregar, K.A.A.K.; Nurwijayanto, A.; Wahyuningrum, R.; Sari, S. Effectiveness of Oxalis corniculata L. ethanol extract against mono-species of biofilm staphylococcus aureus. Borneo J. Pharm. 2021, 4, 184–191. [Google Scholar] [CrossRef]
- Handali, S.; Hosseini, H.; Ameri, A. Formulation and evaluation of an antibacterial cream from Oxalis corniculata aqueous extract. Jundishapur J. Microbiol. 2011, 4, 255–260. [Google Scholar]
- Mukherjee, S.; Koley, H.; Barman, S.; Mitra, S.; Datta, S.; Ghosh, S.; Paul, D.; Dhar, P. Oxalis corniculata (Oxalidaceae) leaf extract exerts in vitro antimicrobial and in vivo anticolonizing activities against Shigella dysenteriae 1 (NT4907) and Shigella flexneri 2a (2457T) in induced diarrhea in suckling mice. J. Med. Food 2013, 16, 801–809. [Google Scholar] [CrossRef]
- Singh, B.; Kumar, D.; Soni, M.; Sinha, J.; Saroha, P.; Sharma, K.; Singh, K.; Gupta, S.K.; Singh, H. Larvicidal and anti-bacterial efficacy of silver nanoparticles derived from Oxalis corniculata. Biosci. Nanotechnol. 2025, 1, 2. [Google Scholar] [CrossRef]
- Perumal, S.; Atchudan, R.; Ramalingam, S.; Edison, T.N.J.I.; Lee, H.M.; Cheong, I.W.; Devarajan, N.; Lee, Y.R. Comparative investigation on antibacterial studies of Oxalis corniculata and silver nanoparticle stabilized graphene surface. J. Mater. Sci. 2022, 57, 11630–11648. [Google Scholar] [CrossRef]
- Jakhar, V.; Sharma, D.K. A sustainable approach for graphene–oxide surface decoration using Oxalis corniculata leaf extract–derived silver nanoparticles: Their antibacterial activities and electrochemical sensing. Dalton Trans. 2020, 49, 8625–8635. [Google Scholar] [CrossRef]
- Badgujar, H.F.; Bora, S.; Kumar, U. Eco-benevolent synthesis of ZnO nanoflowers using Oxalis corniculata leaf extract for potential antimicrobial application in agriculture and cosmeceutical. Biocatal. Agric. Biotechnol. 2021, 38, 102216. [Google Scholar] [CrossRef]
- Manandhar, S.; Luitel, S.; Dahal, R.K. In vitro antimicrobial activity of some medicinal plants against human pathogenic bacteria. J. Trop. Med. 2019, 2019, 1895340. [Google Scholar] [CrossRef]
- Rehman, A.; Rehman, A.; Ahmad, I. Antibacterial, antifungal, and insecticidal potentials of Oxalis corniculata and its isolated compounds. Int. J. Anal. Chem. 2015, 2015, 842468. [Google Scholar] [CrossRef]
- Sreya, K.R.; Uma, R.; Jayavardhanan, K.K. Antibacterial potential of silver nanoparticles synthesized using Oxalis corniculata leaf extract. Pharma Innov. J. 2022, 11, 1362–1366. [Google Scholar]
- Das Mahapatra, A.; Patra, C.; Pal, K.; Mondal, J.; Sinha, C.; Chattopadhyay, D. Green synthesis of AgNPs from aqueous extract of Oxalis corniculata and its antibiofilm and antimicrobial activity. J. Indian Chem. Soc. 2022, 99, 100529. [Google Scholar] [CrossRef]
- Ebrahimzadeh, M.A.; Alizadeh, S.R.; Hashemi, Z. Discovery of high antibacterial and antitumor effects against multi-drug resistant clinically isolated bacteria and MCF-7 and AGS cell lines by biosynthesized silver nanoparticles using Oxalis corniculata extract. Eur. J. Chem. 2023, 14, 202–210. [Google Scholar] [CrossRef]
- Gholipour, A.R.; Jafari, L.; Ramezanpour, M.; Evazalipour, M.; Chavoshi, M.; Yousefbeyk, F.; Kargar Moghaddam, S.J.; Yekta Kooshali, M.H.; Ramezanpour, N.; Daei, P.; et al. Apoptosis Effects of Oxalis corniculata L. Extract on Human MCF-7 Breast Cancer Cell Line. Galen Med. J. 2022, 11, e2484. [Google Scholar]
- Zhang, B.; Jiang, L.; Ma, X.; Wang, A.M.; Liu, T.; Zhou, M.; Liao, S.G.; Wang, Y.L.; Huang, Y.; Li, Y.J. A New arylnaphthalide Lignan from Oxalis corniculata. Nat. Prod. Commun. 2019, 14, 1934578X19875885. [Google Scholar] [CrossRef]
- Kathiriya, A.; Das, K.; Kumar, E.P.; Mathai, K.B. Evaluation of antitumor and antioxidant activity of Oxalis corniculata Linn. against Ehrlich ascites carcinoma on mice. Iran. J. Cancer Prev. 2010, 3, 157–165. [Google Scholar]
- Wilson, D.M.; Cookson, M.R.; Van Den Bosch, L.; Zetterberg, H.; Holtzman, D.M.; Dewachter, I. Hallmarks of neurodegenerative diseases. Cell 2023, 186, 693–714. [Google Scholar] [CrossRef]
- Aruna, K.; Rajeswari, P.D.R.; Sankar, S.R. The effects of Oxalis corniculata L. extract against MPTP induced oxidative stress in mouse model of Parkinson’s disease. J. Pharm. Sci. Res. 2016, 8, 1136. [Google Scholar]
- Aruna, K.; Rajeswari, P.D.R.; Sankar, S.R. The effect of Oxalis corniculata extract against the behavioral changes induced by 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine (MPTP) in mice. J. Appl. Pharm. Sci. 2017, 7, 148–153. [Google Scholar]
- Kumar, P.; Sachdeva, H.; Kalra, S.; Singh, B.; Gupta, R.; Singh, G. Oxalis corniculata and Ficus religiosa mitigates rotenone-induced Parkinson’s disease in Swiss Albino mice: Mechanistic insights and therapeutic potential. J. Appl. Pharm. Sci. 2024, 14, 253–262. [Google Scholar]
- Das, M.; Gohain, K. Evaluation of memory enhancing activity of methanolic Extract of Oxalis corniculata Linn on dementia in experimental animals. Int. J. Sci. Eng. Res. 2018, 9, 922–928. [Google Scholar]
- Al-Snafi, A.E. Medicinal plants with anticonvulsant activities with emphasis on their mechanisms of action. Int. J. Biol. Pharm. Sci. Arch. 2021, 1, 177–189. [Google Scholar] [CrossRef]
- Tang, Y.; Yang, J.; Li, J.; Wang, Y.; Li, Y.; Yang, C.; Sun, J.; Liu, C.; Liu, T. Effects of Oxalis corniculata and musa basjoo sieb of gukang capsule on fracture healing in rabbits and its mechanism. J. Guizhou Med. Univ. 2022, 47, 635–639. [Google Scholar]
- Wang, X.; Wang, Q. Current dietary and medical prevention of renal calcium oxalate stones. Int. J. Gen. Med. 2024, 17, 1635–1649. [Google Scholar] [CrossRef]
- Ibrahim, M.; Hussain, I.; Imran, M.; Hussain, N.; Hussain, A.; Mahboob, T. Corniculatin A, a new flavonoidal glucoside from Oxalis corniculata. Rev. Bras. Farmacogn. 2013, 23, 630–634. [Google Scholar] [CrossRef]
- Zhang, B.; Peng, X.; He, Y. Chemical constituents from Oxalis corniculata. J. Chin. Med. Mat. 2018, 41, 1883–1886. [Google Scholar]
- Prasad Pandey, B.; Prakash Pradhan, S.; Adhikari, K. LC-ESI-QTOF-MS for the profiling of the metabolites and in vitro enzymes inhibition activity of Bryophyllum pinnatum and Oxalis corniculata collected from Ramechhap District of Nepal. Chem. Biodivers. 2020, 17, e2000155. [Google Scholar] [CrossRef]
- Mondal, S.; Talukdar, P.; Mondal, T.K. Study of molecular docking to detect antihypertensive phytochemicals of Oxalis corniculata Linn. against angiotensin converting enzyme. World Sci. News 2018, 110, 42–55. [Google Scholar]
- Ahamad, T.; Khan, M.A.; Khan, M.F.; Ahmad, R.; Rahman, M.A.; Siddiqui, S. Oxalis corniculata-Derived Bioactive Compounds Target Hormone Receptors in Breast Cancer: HPLC-ESI-MS/MS Analysis, Cytotoxicity, and Computational Studies. ChemistrySelect 2025, 10, e202404547. [Google Scholar] [CrossRef]
- Jain, B. An evidence-based ethnomedicinal study on Oxalis corniculata: Review of decade study. Int. J. Green Pharm. 2023, 17, 3371. [Google Scholar]
- Duc, L.V.; Thi, M.N.; Le Hong, D.; Le Huong, G. Antioxidant Activity, Inhibition of No Production and Cytotoxicity of Chemical Compounds Isolated from Oxalis corniculata L. Pharm. Chem. J. 2023, 57, 388–394. [Google Scholar]
- Lei, Y.; Zhang, B.; Ma, X.; Zheng, L.; Gong, Z.P.; Wang, Y.L.; Li, Y.J. Chemical constituents from Oxalis corniculata L. Chin. Pharm. J. 2021, 56, 1378–1383. [Google Scholar]
- Loi, V.D.; Nga, D.T.Q.; Huong, D.T.M.; Huy, N.Q. Compounds Isolated from the Ethyl Acetate Fraction of the Aerial Parts of Oxalis corniculata L. VNU J. Sci. Med. Pharm. Sci. 2018, 34, 4112. [Google Scholar]
- Absar, K.M.B.; Rifat, H.B.S.M.; Das, S.; Eisha, J.A.; Das, T.R.; Dash, P.R. Phytochemical and Pharmacological Properties of Oxalis corniculata: A Review. Trop. J. Phytochem. Pharm. Sci. 2024, 3, 364–374. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).