Comparative Pharmacokinetics and Safety of a Micellar Chrysin–Quercetin–Rutin Formulation: A Randomized Crossover Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
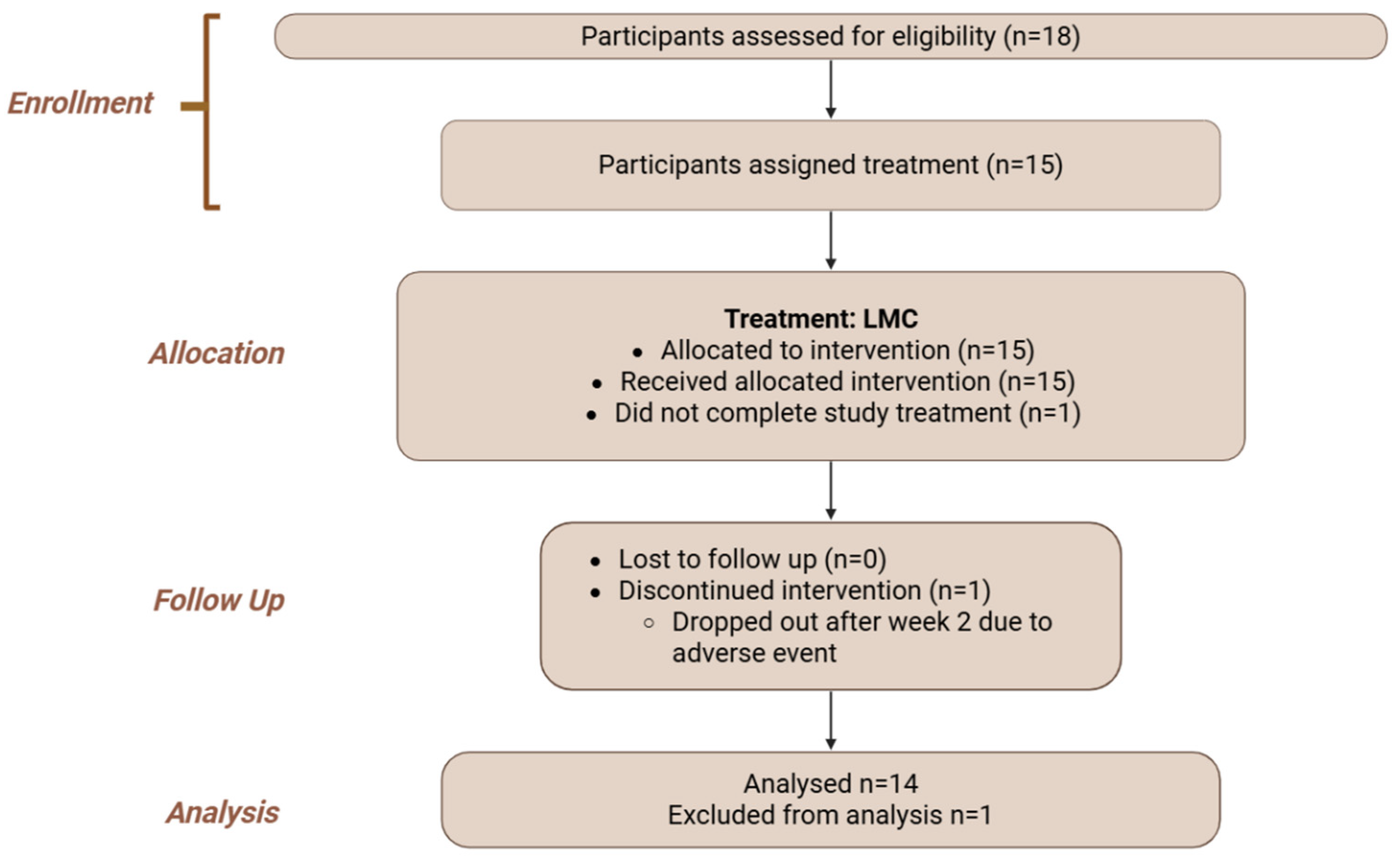
2.2. Participants
- Use of anti-inflammatory or non-steroidal anti-inflammatory (NSAIDs) medication.
- Presence of cardiovascular diseases and/or other acute or chronic diseases (e.g., liver, kidney or gastrointestinal).
- Use of cannabis, nicotine or tobacco.
- Excess drinking of alcohol (>20g/day).
- Those who were or planned to become pregnant, as well as breastfeeding mothers. Women of childbearing age not using birth control.
- Use of antioxidant supplements.
- Use of cholesterol-lowering agents.
- Concurrent participation in another investigational study.
2.3. Ethics and Regulatory Approvals
2.4. Formulations and Dosing
- Novel Micellar Chrysin: Chrysin, co-formulated with quercetin and rutin as a multi-flavonoid complex, encapsulated in a soft gel with a micelle-based delivery system (LipoMicel®; Natural Factors, BC, Canada). From here on written as LMC. More details regarding the LMC Development can be found in Supplementary Material S3.
- Non-micellar Chrysin: Chrysin co-formulated with quercetin and rutin in the same proportions as LMC but encapsulated in a standard hard gel capsule without micellar delivery technology and the required excipients for formulation purposes—enabling evaluation of the micellar system alone. From here on written as NMC.
- Unformulated/Standard Chrysin: Single ingredient chrysin powder without excipients, encapsulated in hard capsules; served as the baseline single-ingredient preparation, isolating the added effects of co-flavonoid synergy. From here on written as UFC.
2.5. In Vitro Caco-2 Permeability Assay
2.6. Bioavailability Study
2.7. Safety Study
2.8. Blood Sampling and LC-HRMS Analysis
2.9. Statistical Analysis
2.10. Sample Size and Power Analysis
2.11. Randomization and Blinding
2.12. Adverse Event Reporting
2.13. Formulation Characterization
3. Results
3.1. Demographic and Baseline Characteristics
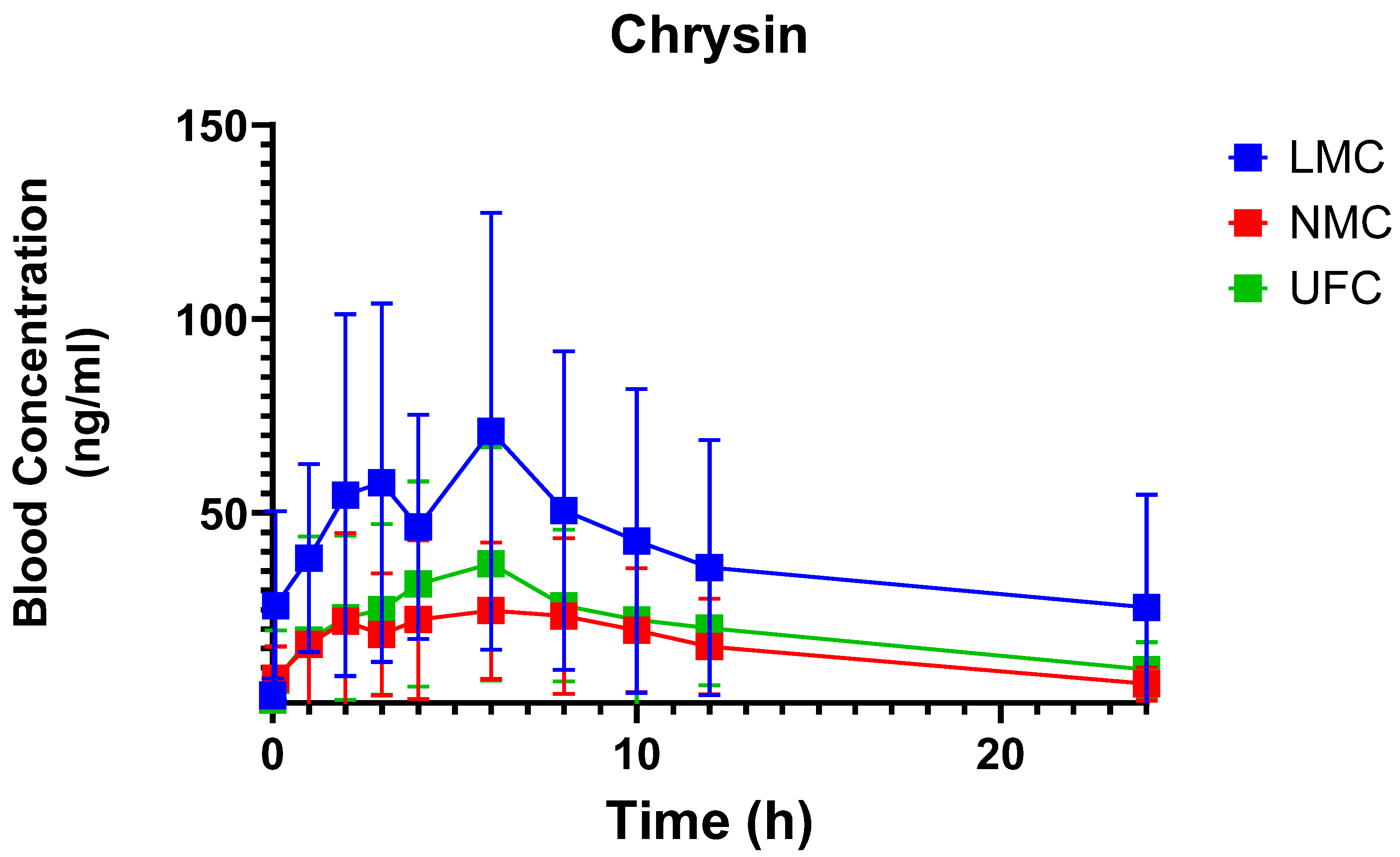
3.2. Pharmacokinetic Profiles
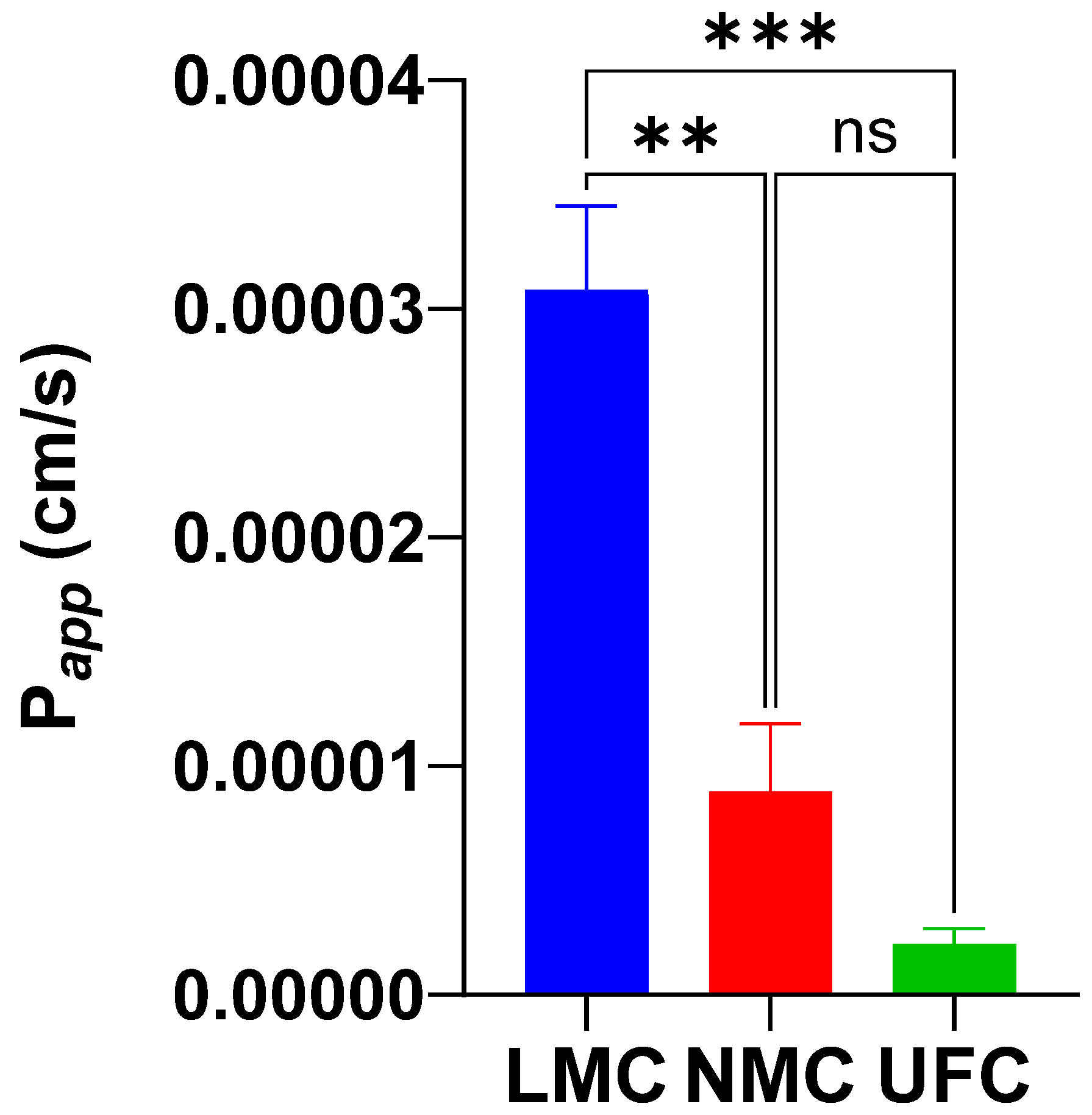
3.3. Caco-2 Permeability
3.4. 30-Day Safety Outcomes
3.5. Adverse Events Report
3.6. Micellar Formulation In-Vitro Characterization
4. Discussion
4.1. Enhanced Bioavailability Through Micellar Delivery
4.2. Caco-2 Permeability Results
4.3. Thirty-Day Safety Evaluation
4.4. Formulation Characterization and Stability
4.5. Overall Implications and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| UGT | UDP-glucuronosyltransferase |
| SULT | Sulfotransferase |
| LMC | Micellar chrysin |
| NMC | Non-micellar chrysin |
| UFC | Unformulated standard chrysin |
| Papp | Permeability coefficient |
| PK | Pharmacokinetic |
| AUC | Area under curve |
| Cmax | Maximum blood concentration |
| Tmax | Time to reach maximum blood concentration |
| T½ | Drug half-life time |
| MRTi | Mean residence time |
| ALT | Alanine aminotransferase |
| AST | Aspartate aminotransferase |
| eGFR | Estimated glomerular filtration rate |
| HDL | High density lipoprotein |
| LDL | Low density lipoprotein |
| UHPLC | Ultra high-performance liquid chromatography |
| AEs | Adverse events |
| MRP2 | Multidrug resistance-associated protein 2 |
| BCRP | Breast cancer resistance protein |
| AMPK | AMP-activated protein kinase |
References
- Lee, Y.; Byun, E.-B. Enhanced Bioaccessibility and Anti-Inflammatory Effect of Chrysin Nanoemulsion. J. Funct. Foods 2025, 128, 106820. [Google Scholar] [CrossRef]
- Moghadam, E.R.; Ang, H.L.; Asnaf, S.E.; Zabolian, A.; Saleki, H.; Yavari, M.; Esmaeili, H.; Zarrabi, A.; Ashrafizadeh, M.; Kumar, A.P. Broad-Spectrum Preclinical Antitumor Activity of Chrysin: Current Trends and Future Perspectives. Biomolecules 2020, 10, 1374. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, A.; Akhtar, J.; Uddin, M.S.S.; Khan, M.I.; Khalid, M.; Ahmad, M. A Naturally Occurring Flavone (Chrysin): Chemistry, Occurrence, Pharmacokinetic, Toxicity, Molecular Targets and Medicinal Properties. J. Biol. Act. Prod. Nat. 2018, 8, 208–227. [Google Scholar] [CrossRef]
- Majdi, A.; Hosseini, S.H.; Roozbeh, M.; Mohammadi, A. Antidepressant and Anxiolytic Effects of Geraniol in Mice: The Possible Role of Oxidative Stress and Apoptosis. Iran. Red Crescent Med. J. 2019, 21, e91593. [Google Scholar] [CrossRef]
- Cheraghi Abajlou, S.; Tofighi, A.; Tolouei Azar, J.; Khaki, A.A.; Razi, M. Combined Effects of Chrysin Supplementation and Exercise Training on Diabetes-Induced Oxidative Stress and Apoptosis in Rat Testicular Tissue. Int. J. Fertil. Steril. 2025, 19, 88–95. [Google Scholar] [CrossRef]
- Kim, K.-I.; An, S.-M.; Park, H.-G.; Lee, K.; Lee, W.-L.; Kim, K.-I.; An, S.-M.; Park, H.-G.; Lee, K.; Lee, W.-L. The Effect of Either Aerobic Exercise Training or Chrysin Supplementation on Mitochondrial Biogenesis in Skeletal Muscle of High Fat Diet-Induced Obese Mice. Exerc. Sci. 2019, 28, 365–372. [Google Scholar] [CrossRef]
- Yuce, M.; Ucar, S.; Yildiz, M.; Aydin, M.; Turan, M.; Ghosh, T.K.; Yildirim, E. Unraveling the Role of Chrysin in Mitigating Cadmium Toxicity in Pepper by Improving Antioxidant Defense, Phytohormone Biosynthesis and Photosystem II and Aquaporins Related Transcripts. Environ. Pollut. 2025, 381, 126627. [Google Scholar] [CrossRef]
- Balam, F.H.; Ahmadi, Z.S.; Ghorbani, A. Inhibitory Effect of Chrysin on Estrogen Biosynthesis by Suppression of Enzyme Aromatase (CYP19): A Systematic Review. Heliyon 2020, 6, e03557. [Google Scholar] [CrossRef]
- Talebi, M.; Talebi, M.; Farkhondeh, T.; Simal-Gandara, J.; Kopustinskiene, D.M.; Bernatoniene, J.; Samarghandian, S. Emerging Cellular and Molecular Mechanisms Underlying Anticancer Indications of Chrysin. Cancer Cell Int. 2021, 21, 214. [Google Scholar] [CrossRef]
- Faheem, M.A.; Akhtar, T.; Naseem, N.; Aftab, U.; Zafar, M.S.; Hussain, S.; Shahzad, M.; Gobe, G.C. Chrysin Is Immunomodulatory and Anti-Inflammatory against Complete Freund’s Adjuvant-Induced Arthritis in a Pre-Clinical Rodent Model. Pharmaceutics 2023, 15, 1225. [Google Scholar] [CrossRef]
- Xu, M.; Shi, H.; Liu, D. Chrysin Protects against Renal Ischemia Reperfusion Induced Tubular Cell Apoptosis and Inflammation in Mice. Exp. Ther. Med. 2019, 17, 2256–2262. [Google Scholar] [CrossRef] [PubMed]
- Mantawy, E.M.; Esmat, A.; El-Bakly, W.M.; Salah ElDin, R.A.; El-Demerdash, E. Mechanistic Clues to the Protective Effect of Chrysin against Doxorubicin-Induced Cardiomyopathy: Plausible Roles of P53, MAPK and AKT Pathways. Sci. Rep. 2017, 7, 4795. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.-K.; Park, S.-H.; Kim, Y.-H.; Lee, E.-J.; Antika, L.D.; Kim, D.Y.; Choi, Y.-J.; Kang, Y.-H. Chrysin Ameliorates Podocyte Injury and Slit Diaphragm Protein Loss via Inhibition of the PERK-eIF2α-ATF-CHOP Pathway in Diabetic Mice. Acta Pharmacol. Sin. 2017, 38, 1129–1140. [Google Scholar] [CrossRef] [PubMed]
- Shang, J.; Jiao, J.; Yan, M.; Wang, J.; Li, Q.; Shabuerjiang, L.; Lu, Y.; Song, Q.; Bi, L.; Huang, G.; et al. Chrysin Protects against Cerebral Ischemia-Reperfusion Injury in Hippocampus via Restraining Oxidative Stress and Transition Elements. Biomed. Pharmacother. 2023, 161, 114534. [Google Scholar] [CrossRef]
- Nabavi, S.F.; Braidy, N.; Habtemariam, S.; Orhan, I.E.; Daglia, M.; Manayi, A.; Gortzi, O.; Nabavi, S.M. Neuroprotective Effects of Chrysin: From Chemistry to Medicine. Neurochem. Int. 2015, 90, 224–231. [Google Scholar] [CrossRef]
- Gao, S.; Siddiqui, N.; Etim, I.; Du, T.; Zhang, Y.; Liang, D. Developing Nutritional Component Chrysin as a Therapeutic Agent: Bioavailability and Pharmacokinetics Consideration, and ADME Mechanisms. Biomed. Pharmacother. 2021, 142, 112080. [Google Scholar] [CrossRef]
- Feizi, S.; Jabbari, M.; Farajtabar, A. A Systematic Study on Solubility and Solvation of Bioactive Compound Chrysin in Some Water + Cosolvent Mixtures. J. Mol. Liq. 2016, 220, 478–483. [Google Scholar] [CrossRef]
- Adesina, A.F.; Adewuyi, A.; Otuechere, C.A. Exploratory Studies on Chrysin via Antioxidant, Antimicrobial, ADMET, PASS and Molecular Docking Evaluations. Pharmacol. Res.—Mod. Chin. Med. 2024, 11, 100413. [Google Scholar] [CrossRef]
- Kurkiewicz, M.; Moździerz, A.; Rzepecka-Stojko, A.; Stojko, J. Chrysin: A Comprehensive Review of Its Pharmacological Properties and Therapeutic Potential. Pharmaceuticals 2025, 18, 1162. [Google Scholar] [CrossRef]
- Castro, G.T.; Ferretti, F.H.; Blanco, S.E. Determination of the Overlapping pK(a) Values of Chrysin Using UV-Vis Spectroscopy and Ab Initio Methods. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2005, 62, 657–665. [Google Scholar] [CrossRef]
- Dong, X.; Cao, Y.; Wang, N.; Wang, P.; Li, M. Systematic Study on Solubility of Chrysin in Different Organic Solvents: The Synergistic Effect of Multiple Intermolecular Interactions on the Dissolution Process. J. Mol. Liq. 2021, 325, 115180. [Google Scholar] [CrossRef]
- Walle, T.; Otake, Y.; Brubaker, J.A.; Walle, U.K.; Halushka, P.V. Disposition and Metabolism of the Flavonoid Chrysin in Normal Volunteers. Brit. J. Clin. Pharma 2001, 51, 143–146. [Google Scholar] [CrossRef]
- Galijatovic, A.; Otake, Y.; Walle, U.K.; Walle, T. Extensive Metabolism of the Flavonoid Chrysin by Human Caco-2 and Hep G2 Cells. Xenobiotica 1999, 29, 1241–1256. [Google Scholar] [CrossRef] [PubMed]
- Ting, P.; Srinuanchai, W.; Suttisansanee, U.; Tuntipopipat, S.; Charoenkiatkul, S.; Praengam, K.; Chantong, B.; Temviriyanukul, P.; Nuchuchua, O. Development of Chrysin Loaded Oil-in-Water Nanoemulsion for Improving Bioaccessibility. Foods 2021, 10, 1912. [Google Scholar] [CrossRef] [PubMed]
- Kudatarkar, N.; Jalalpure, S.; Kurangi, B. Formulation and Characterization of Chrysin Loaded Phytosomes and Its Cytotoxic Effect against Colorectal Cancer Cells. Ind. J. Pharm. Edu. Res. 2022, 56, s407–s412. [Google Scholar] [CrossRef]
- Pandey, D.; Sharma, K.; Mehta, P.D. Formulation and Evaluation of Solid Dispersion of Chrysin for Improved Bioavailability. Curr. Res. Pharm. Sci. 2023, 13, 145–150. [Google Scholar] [CrossRef]
- Narayan, H.; Jangid, A.K.; Sharma, J.R.; Kishore, A.; Mahor, A.K.; Yadav, U.C.S.; Kulhari, H.; Singh, P.P. Effect of Amorphous Chrysin Loading in Hydrophobically Modified Pluronic F68 Nanomicelles on Its Anticancer Activity, Stability and Oral Bioavailability. RSC Pharm. 2024, 1, 716–726. [Google Scholar] [CrossRef]
- Lee, S.H.; Lee, Y.; Song, J.G.; Han, H.-K. Improved In Vivo Effect of Chrysin as an Absorption Enhancer Via the Preparation of Ternary Solid Dispersion with Brij®L4 and Aminoclay. Curr. Drug Deliv. 2019, 16, 86–92. [Google Scholar] [CrossRef]
- Kaur, H.; Malik, D.S.; Kaur, G. Enhanced Dissolution and Antioxidant Activity of Chrysin Nanoparticles Employing Co-Precipitation as a Technique. Pharm. Nanotechnol. 2015, 3, 205–218. [Google Scholar] [CrossRef]
- Wang, C.; Liu, X.; Zhao, R.; Yang, M.; Liu, W.; Dai, Q.; Bao, X.; Chen, Y.; Ma, J. The Amorphous Solid Dispersion of Chrysin in Plasdone® S630 Demonstrates Improved Oral Bioavailability and Antihyperlipidemic Performance in Rats. Pharmaceutics 2023, 15, 2378. [Google Scholar] [CrossRef]
- Xu, W.; Ling, P.; Zhang, T. Polymeric Micelles, a Promising Drug Delivery System to Enhance Bioavailability of Poorly Water-Soluble Drugs. J. Drug Deliv. 2013, 2013, 340315. [Google Scholar] [CrossRef]
- Slor, G.; Olea, A.R.; Pujals, S.; Tigrine, A.; De La Rosa, V.R.; Hoogenboom, R.; Albertazzi, L.; Amir, R.J. Judging Enzyme-Responsive Micelles by Their Covers: Direct Comparison of Dendritic Amphiphiles with Different Hydrophilic Blocks. Biomacromolecules 2021, 22, 1197–1210. [Google Scholar] [CrossRef] [PubMed]
- Mohos, V.; Fliszár-Nyúl, E.; Ungvári, O.; Kuffa, K.; Needs, P.W.; Kroon, P.A.; Telbisz, Á.; Özvegy-Laczka, C.; Poór, M. Inhibitory Effects of Quercetin and Its Main Methyl, Sulfate, and Glucuronic Acid Conjugates on Cytochrome P450 Enzymes, and on OATP, BCRP and MRP2 Transporters. Nutrients 2020, 12, 2306. [Google Scholar] [CrossRef] [PubMed]
- Boyle, S.P.; Dobson, V.L.; Duthie, S.J.; Hinselwood, D.C.; Kyle, J.A.M.; Collins, A.R. Bioavailability and Efficiency of Rutin as an Antioxidant: A Human Supplementation Study. Eur. J. Clin. Nutr. 2000, 54, 774–782. [Google Scholar] [CrossRef] [PubMed]
- Nakmode, D.; Bhavana, V.; Thakor, P.; Madan, J.; Singh, P.K.; Singh, S.B.; Rosenholm, J.M.; Bansal, K.K.; Mehra, N.K. Fundamental Aspects of Lipid-Based Excipients in Lipid-Based Product Development. Pharmaceutics 2022, 14, 831. [Google Scholar] [CrossRef]
- Teixé-Roig, J.; Oms-Oliu, G.; Artiga-Artigas, M.; Odriozola-Serrano, I.; Martín-Belloso, O. Enhanced in Vivo Absorption and Biodistribution of Curcumin Loaded into Emulsions with High Medium-Chain Triglyceride Content. Food Res. Int. 2023, 174, 113595. [Google Scholar] [CrossRef]
- Solnier, J.; Chang, C.; Roh, K.; Du, M.; Kuo, Y.C.; Hardy, M.; Lyon, M.; Gahler, R. Quercetin LipoMicel—A Novel Delivery System to Enhance Bioavailability of Quercetin. J. Nat. Health Prod. Res. 2021, 3, 1–8. [Google Scholar] [CrossRef]
- Solnier, J.; Zhang, Y.; Roh, K.; Kuo, Y.C.; Du, M.; Wood, S.; Hardy, M.; Gahler, R.J.; Chang, C. A Pharmacokinetic Study of Different Quercetin Formulations in Healthy Participants: A Diet-Controlled, Crossover, Single- and Multiple-Dose Pilot Study. Evid.-Based Complement. Altern. Med. 2023, 2023, 9727539. [Google Scholar] [CrossRef]
- Du, M.; Chang, C.; Zhang, X.; Zhang, Y.; Radford, M.J.; Gahler, R.J.; Kuo, Y.C.; Wood, S.; Solnier, J. Designing Vitamin D3 Formulations: An In Vitro Investigation Using a Novel Micellar Delivery System. Nutraceuticals 2023, 3, 290–305. [Google Scholar] [CrossRef]
- Ibi, A.; Chang, C.; Kuo, Y.C.; Zhang, Y.; Du, M.; Roh, Y.S.; Gahler, R.; Hardy, M.; Solnier, J. Evaluation of the Metabolite Profile of Fish Oil Omega-3 Fatty Acids (n-3 FAs) in Micellar and Enteric-Coated Forms—A Randomized, Cross-Over Human Study. Metabolites 2024, 14, 265. [Google Scholar] [CrossRef]
- Ibi, A.; Chang, C.; Kuo, Y.C.; Zhang, Y.; Du, M.; Roh, Y.S.; Gahler, R.; Hardy, M.; Solnier, J. A 30-Day Randomized Crossover Human Study on the Safety and Tolerability of a New Micellar Berberine Formulation with Improved Bioavailability. Metabolites 2025, 15, 240. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Zhang, Y.; Kuo, Y.C.; Du, M.; Roh, K.; Gahler, R.; Ibi, A.; Solnier, J. Novel Micellar Formulation of Silymarin (Milk Thistle) with Enhanced Bioavailability in a Double-Blind, Randomized, Crossover Human Trial. Pharmaceutics 2025, 17, 880. [Google Scholar] [CrossRef] [PubMed]
- Halevas, E.; Kokotidou, C.; Zaimai, E.; Moschona, A.; Lialiaris, E.; Mitraki, A.; Lialiaris, T.; Pantazaki, A. Evaluation of the Hemocompatibility and Anticancer Potential of Poly(ε-Caprolactone) and Poly(3-Hydroxybutyrate) Microcarriers with Encapsulated Chrysin. Pharmaceutics 2021, 13, 109. [Google Scholar] [CrossRef] [PubMed]
- Lea, T. Caco-2 Cell Line. In The Impact of Food Bioactives on Health: In Vitro and Ex Vivo Models; Verhoeckx, K., Cotter, P., López-Expósito, I., Kleiveland, C., Lea, T., Mackie, A., Requena, T., Swiatecka, D., Wichers, H., Eds.; Springer: Cham, Switzerland, 2015; ISBN 978-3-319-15791-7. [Google Scholar]
- Kus, M.; Ibragimow, I.; Piotrowska-Kempisty, H. Caco-2 Cell Line Standardization with Pharmaceutical Requirements and In Vitro Model Suitability for Permeability Assays. Pharmaceutics 2023, 15, 2523. [Google Scholar] [CrossRef]
- Lightbody, T.O. Capillary Blood Sampling: Are the Results Comparable to Venous Sampling in Health and Disease. Master’s Thesis, University of Birmingham, Birmingham, UK, 2017. [Google Scholar]
- Remanan, M.K.; Zhu, F. Encapsulation of Chrysin and Rutin Using Self-Assembled Nanoparticles of Debranched Quinoa, Maize, and Waxy Maize Starches. Carbohydr. Polym. 2024, 337, 122118. [Google Scholar] [CrossRef]
- Shahbaz, M.; Naeem, H.; Imran, M.; Ul Hassan, H.; Alsagaby, S.A.; Al Abdulmonem, W.; Waqar, A.B.; Ghorab, A.H.; Abdelgawad, M.A.; Ghoneim, M.M.; et al. Chrysin a Promising Anticancer Agent: Recent Perspectives. Int. J. Food Prop. 2023, 26, 2294–2337. [Google Scholar] [CrossRef]
- Stompor-Goracy, M.; Bajek-Bil, A.; Machaczka, M. Chrysin: Perspectives on Contemporary Status and Future Possibilities as Pro-Health Agent. Nutrients 2021, 13, 2038. [Google Scholar] [CrossRef]
- Ishii, Y.; Nurrochmad, A.; Yamada, H. Modulation of UDP-Glucuronosyltransferase Activity by Endogenous Compounds. Drug Metab. Pharmacokinet. 2010, 25, 134–148. [Google Scholar] [CrossRef]
- Fenyvesi, F.; Nguyen, T.L.P.; Haimhoffer, Á.; Rusznyák, Á.; Vasvári, G.; Bácskay, I.; Vecsernyés, M.; Ignat, S.-R.; Dinescu, S.; Costache, M.; et al. Cyclodextrin Complexation Improves the Solubility and Caco-2 Permeability of Chrysin. Materials 2020, 13, 3618. [Google Scholar] [CrossRef]
- van Zanden, J.J.; van der Woude, H.; Vaessen, J.; Usta, M.; Wortelboer, H.M.; Cnubben, N.H.P.; Rietjens, I.M.C.M. The Effect of Quercetin Phase II Metabolism on Its MRP1 and MRP2 Inhibiting Potential. Biochem. Pharmacol. 2007, 74, 345–351. [Google Scholar] [CrossRef]
- Fan, J.; Li, T.-J.; Zhao, X.-H. Barrier-Promoting Efficiency of Two Bioactive Flavonols Quercetin and Myricetin on Rat Intestinal Epithelial (IEC-6) Cells via Suppressing Rho Activation. RSC Adv. 2020, 10, 27249–27258. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, M.; Matsukawa, N.; Mineo, H.; Chiji, H.; Hara, H. A Soluble Flavonoid-Glycoside, αG-Rutin, Is Absorbed as Glycosides in the Isolated Gastric and Intestinal Mucosa. Biosci. Biotechnol. Biochem. 2004, 68, 1929–1934. [Google Scholar] [CrossRef] [PubMed]
- Ostadmohammadi, V.; Milajerdi, A.; Ayati, E.; Kolahdooz, F.; Asemi, Z. Effects of Quercetin Supplementation on Glycemic Control among Patients with Metabolic Syndrome and Related Disorders: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Phytother. Res. 2019, 33, 1330–1340. [Google Scholar] [CrossRef] [PubMed]
- Ansari, P.; Choudhury, S.T.; Seidel, V.; Rahman, A.B.; Aziz, M.A.; Richi, A.E.; Rahman, A.; Jafrin, U.H.; Hannan, J.M.A.; Abdel-Wahab, Y.H.A. Therapeutic Potential of Quercetin in the Management of Type-2 Diabetes Mellitus. Life 2022, 12, 1146. [Google Scholar] [CrossRef]
- Ghorbani, A. Mechanisms of Antidiabetic Effects of Flavonoid Rutin. Biomed. Pharmacother. 2017, 96, 305–312. [Google Scholar] [CrossRef]
- Kim, S.; Imm, J.-Y. The Effect of Chrysin-Loaded Phytosomes on Insulin Resistance and Blood Sugar Control in Type 2 Diabetic Db/Db Mice. Molecules 2020, 25, 5503. [Google Scholar] [CrossRef]
- Samarghandian, S.; Farkhondeh, T.; Azimi-Nezhad, M. Protective Effects of Chrysin Against Drugs and Toxic Agents. Dose-Response 2017, 15, 155932581771178. [Google Scholar] [CrossRef]

| Intervention | LMC | NMC | UFC |
|---|---|---|---|
| Dosage form | Soft-gelatin capsules | Hard-gelatin capsules | Hard-gelatin capsules |
| Co-formulation | Quercetin, Rutin | Quercetin, Rutin | None |
| Micellar | Yes | No | No |
| Number of capsules per dose | 1 | 1 | 1 |
| Physical form of capsule content | Liquid | Powder | Powder |
| Chrysin (mg/dose) | 469 | 412 | 558 |
| Quercetin (mg/dose) | 105 | 77.4 | 0 |
| Rutin (mg/dose) | 115 | 106.4 | 0 |
| Lead (mg/kg) | <0.03 | <0.03 | <0.03 |
| Mercury (mg/kg) | <0.02 | 0.029 | <0.02 |
| Cadmium (mg/kg) | <0.02 | <0.02 | <0.02 |
| Arsenic (mg/kg) | <0.2 | <0.2 | <0.2 |
| Excipients | Gelatin, glycerin, purified water, medium chain triglycerides, MSM, xylitol, lecithin, Stevia rebaudiana leaf extract, peppermint oil | Gelatin capsule | Cellulose capsule |
| Males | Females | Combined | |
|---|---|---|---|
| N | 7 | 11 | 18 |
| Age (years) | 39.4 ± 8.3 | 35.0 ± 12.1 | 37.2 ± 10.2 |
| Weight (kg) | 72.1 ± 7.4 | 58.8 ± 13.6 | 65.5 ± 10.5 |
| Height (cm) | 172.7 ± 5.1 | 162.8 ± 7.5 | 167.8 ± 6.3 |
| BMI | 24.2 ± 2.9 | 22.0 ± 3.5 | 23.1 ± 3.2 |
| LMC | NMC | UFC | p Value | |
|---|---|---|---|---|
| AUC0–24 (ng/mL·h) | 914.8 ± 697.5 | 345.7± 280.8 | 456.9 ± 356.4 | 0.0221 |
| Cmax (ng/mL) | 87.3 ± 59.4 | 32.9 ± 23.5 | 42.1 ± 28.4 | 0.0128 |
| T1/2 (h) | 25.3 ± 59.1 | 9.00 ± 3.3 | 11.0 ± 3.5 | 0.6963 |
| Tmax (h) | 4.8 ± 3.8 | 5.8 ± 3.9 | 4.2 ± 2.7 | 0.4903 |
| MRTi (h) | 38.4 ± 85.9 | 13.3 ± 4.2 | 17.2 ± 4.8 | 0.4207 |
| Male | Mean ± SD | p-Value | Normal Range | ||||
|---|---|---|---|---|---|---|---|
| Week 0 | Week 1 | Week 2 | Week 3 | 30 Days | |||
| Total Bilirubin (µmol/L) | 14.1 ± 6.2 | 15.0 ± 8.8 | 16.1 ± 3.9 | 16.2 ± 5.5 | 19.7 ± 9.0 | 0.4701 | 3.4–21.0 |
| AST (U/L) | 24 ± 3.5 | 27.6 ± 10.5 | 23.2 ± 3.1 | 21.6 ± 2.6 | 24.6 ± 8.0 | 0.4799 | 15.0–40.0 |
| ALT (U/L) | 35.4 ± 17.2 | 36.6 ± 20.9 | 33.8 ± 15.7 | 30.6 ± 11.2 | 31.8 ± 7.8 | 0.5766 | 9.0–50.0 |
| Creatinine (µmol/L) | 73.4 ± 11.5 | 75.9 ± 14.5 | 79.4 ± 8.5 | 72.3 ± 4.8 | 80.9 ± 11.2 | 0.5761 | 44.0–97.0 |
| eGFR (mL/min/1.73 m2) | 110.5 ± 7.7 | 106.6 ± 11.8 | 105.4 ± 7.9 | 112.0 ± 5.1 | 103.6 ± 13.5 | 0.5746 | >90 |
| Glucose (mmol/L) | 5.9 ± 0.4 | 5.9 ± 0.5 | 5.1 ± 0.3 | 5.3 ± 0.4 | 5.1 ± 0.7 | 0.0369 2 | 3.89–6.11 |
| HDL (mmol/L) | 1.4 ± 0.2 | 1.3 ± 0.1 | 1.5 ± 0.2 | 1.3 ± 0.1 | 1.5 ± 0.2 | 0.0944 | 1.16–1.42 |
| LDL (mmol/L) | 4.3 ± 1.2 | 4.4 ± 1.4 | 4.2 ± 1.0 | 4.2 ± 0.8 | 4.4 ± 1.3 | 0.8209 | 0.50–3.14 |
| TC (mmol/L) | 6.5 ± 1.4 | 6.5 ± 1.6 | 6.4 ± 1.2 | 6.2 ± 0.8 | 6.7 ± 1.1 | 0.4223 | 0–5.17 |
| TG (mmol/L) | 1.6 ± 0.6 | 1.7 ± 0.9 | 1.4 ± 0.6 | 1.4 ± 0.4 | 1.8 ± 0.8 | 0.3436 | 0–1.70 |
| HbA1c (mmol/L) | 4.4 ± 0.4 | 4.4 ± 0.3 | 4.5 ± 0.2 | 6.4 ± 3.6 | 4.7 ± 0.4 | 0.3001 | 3.89–6.11 |
| tCO2 (mmol/L) | 22.4 ± 0.7 | 22.2 ± 1.0 | 22.9 ± 1.2 | 23.1 ± 1.5 | 25.0 ± 1.2 | 0.0118 3 | 22.0–29.0 |
| Ca (mmol/L) | 2.6 ± 0.1 | 2.6 ± 0.06 | 2.6 ± 0.08 | 2.6 ± 0.06 | 2.5 ± 0.1 | 0.0865 | 2.00–2.58 |
| P (mmol/L) | 1.1 ± 0.1 | 1.0 ± 0.1 | 0.9 ± 0 | 1.1 ± 0.1 | 1.1 ± 0.1 | 0.0927 | 0.85–1.51 |
| Mg (mmol/L) | 0.9 ± 0.1 | 0.9 ± 0.1 | 0.9 ± 0.1 | 0.9 ± 0.05 | 0.9 ± 0.1 | 0.5788 | 0.65–1.25 |
| K (mmol/L) | 4.8 ± 0.3 | 4.8 ± 0.2 | 5.0 ± 0.2 | 4.6 ± 0.4 | 4.6 ± 0.3 | 0.3732 | 3.40–5.30 |
| Na (mmol/L) | 140.2 ± 1.9 | 140.1 ± 1.9 | 139.6 ± 3.1 | 138.5 ± 3.4 | 133.8 ± 0.7 | 0.0061 3 | 135.0–147.0 |
| Cl (mmol/L) | 105.0 ± 1.5 | 106.0 ± 2.3 | 104.5 ± 5.6 | 105.0 ± 1.0 | 103.3 ± 3.1 | 0.5246 | 99.0–122.0 |
| Female | Mean ± SD | p-Value | Normal Range | ||||
|---|---|---|---|---|---|---|---|
| Week 0 | Week 1 | Week 2 | Week 3 | 30 Days | |||
| Total Bilirubin (µmol/L) | 12.4 ± 8.9 | 10.7 ± 7.9 | 17.1 ± 7.2 | 11.0 ± 4.1 | 10.3 ± 5.7 | 0.2388 | 3.4–21.0 |
| AST (U/L) | 19.6 ± 5.7 | 20.1 ± 4.5 | 20.8 ± 5.2 | 19.3 ± 3.7 | 19.8 ± 3.6 | 0.8371 | 13.0–35.0 |
| ALT (U/L) | 18.8 ± 4.7 | 19.9 ± 6.1 | 20.6 ± 10.6 | 18.7 ± 4.4 | 19 ± 1.5 | 0.6655 | 7.0–40.0 |
| Creatinine (µmol/L) | 57.4 ± 8.5 | 55.2 ± 10.9 | 63.2 ± 9.6 | 64.8 ± 10.6 | 58.8 ± 8.9 | 0.116 | 35.0–80.0 |
| eGFR (mL/min/1.73 m2) | 115.6 ± 12.6 | 114.8 ± 12.4 | 109.1 ± 15.5 | 106.0 ± 15.7 | 114.1 ± 13.6 | 0.16 | >90 |
| Glucose (mmol/L) | 5.2 ± 0.6 | 5.3 ± 0.5 | 5.1 ± 0.8 | 4.9 ± 0.7 | 4.2 ± 0.5 | 0.0009 2 | 3.89–6.11 |
| HDL (mmol/L) | 1.6 ± 0.3 | 1.7 ± 0.3 | 1.6 ± 0.3 | 1.6 ± 0.3 | 1.6 ± 0.3 | 0.3011 | 1.29–1.55 |
| LDL (mmol/L) | 3.3 ± 1.0 | 3.5 ± 1.2 | 3.4 ± 1.1 | 3.4 ± 1.2 | 3.3 ± 1.0 | 0.5575 | 0.50–3.14 |
| TC (mmol/L) | 5.4 ± 1.1 | 5.6 ± 1.3 | 5.5 ± 1.2 | 5.4 ± 1.4 | 5.3 ± 1.2 | 0.3943 | 0–5.17 |
| TG (mmol/L) | 1.3 ± 0.5 | 1.0 ± 0.3 | 1.2 ± 0.7 | 1.1 ± 0.3 | 1.0 ± 0.3 | 0.3549 | 0–1.70 |
| HbA1c (mmol/L) | 4.4 ± 0.4 | 4.4 ± 0.5 | 4.4 ± 0.6 | 4.6 ± 0.5 | 4.3 ± 0.4 | 0.4551 | 3.89–6.11 |
| tCO2 (mmol/L) | 22.2 ± 0.6 | 23.2 ± 3.1 | 22.4 ± 1.1 | 23.1 ± 2.1 | 24.5 ± 2.0 | 0.1702 | 22.0–29.0 |
| Ca (mmol/L) | 2.4 ± 0.52 | 2.2 ± 0.59 | 2.5 ± 0.08 | 2.53 ± 0.08 | 2.4 ± 0.23 | 0.3504 | 2.00–2.58 |
| P (mmol/L) | 1.2 ± 0.1 | 1.3 ± 0.5 | 1.1 ± 0.09 | 1.2 ± 0.07 | 1.2 ± 0.2 | 0.4523 | 0.85–1.51 |
| Mg (mmol/L) | 0.9 ± 0.07 | 1.0 ± 0.4 | 0.9 ± 0.1 | 0.9 ± 0.03 | 0.9 ± 0.1 | 0.3011 | 0.65–1.25 |
| K (mmol/L) | 4.8 ± 0.2 | 4.6 ± 0.2 | 4.9 ± 0.2 | 4.5 ± 0.3 | 4.5 ± 0.6 | 0.115 | 3.40–5.30 |
| Na (mmol/L) | 140.6 ± 2.9 | 134.5 ± 15.4 | 139.7 ± 3.1 | 138.3 ± 2.8 | 133.4 ± 8.0 | 0.2726 | 135.0–147.0 |
| Cl (mmol/L) | 105.8 ± 3.9 | 103.8 ± 4.5 | 106.2 ± 3.2 | 105.8 ± 1.6 | 102.1 ± 7.0 | 0.2791 | 99.0–122.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibi, A.; Chang, C.; Kuo, Y.C.; Zhang, Y.; Do, P.; Du, M.; Roh, Y.S.; Gahler, R.; Hardy, M.; Solnier, J. Comparative Pharmacokinetics and Safety of a Micellar Chrysin–Quercetin–Rutin Formulation: A Randomized Crossover Trial. Antioxidants 2025, 14, 1313. https://doi.org/10.3390/antiox14111313
Ibi A, Chang C, Kuo YC, Zhang Y, Do P, Du M, Roh YS, Gahler R, Hardy M, Solnier J. Comparative Pharmacokinetics and Safety of a Micellar Chrysin–Quercetin–Rutin Formulation: A Randomized Crossover Trial. Antioxidants. 2025; 14(11):1313. https://doi.org/10.3390/antiox14111313
Chicago/Turabian StyleIbi, Afoke, Chuck Chang, Yun Chai Kuo, Yiming Zhang, Peony Do, Min Du, Yoon Seok Roh, Roland Gahler, Mary Hardy, and Julia Solnier. 2025. "Comparative Pharmacokinetics and Safety of a Micellar Chrysin–Quercetin–Rutin Formulation: A Randomized Crossover Trial" Antioxidants 14, no. 11: 1313. https://doi.org/10.3390/antiox14111313
APA StyleIbi, A., Chang, C., Kuo, Y. C., Zhang, Y., Do, P., Du, M., Roh, Y. S., Gahler, R., Hardy, M., & Solnier, J. (2025). Comparative Pharmacokinetics and Safety of a Micellar Chrysin–Quercetin–Rutin Formulation: A Randomized Crossover Trial. Antioxidants, 14(11), 1313. https://doi.org/10.3390/antiox14111313








