Lacticaseibacillus paracasei JY062 Postbiotic Alleviated 3% DSS-Induced Colitis in Mice via Integrated Antioxidant, Barrier Repair, Immunomodulatory and Microbiota Modulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Analysis of Antioxidant Capacity
2.2. Cell Culture
2.3. Reactive Oxygen Species Assay
2.4. Analysis of Bioactive Components in Pa JY062
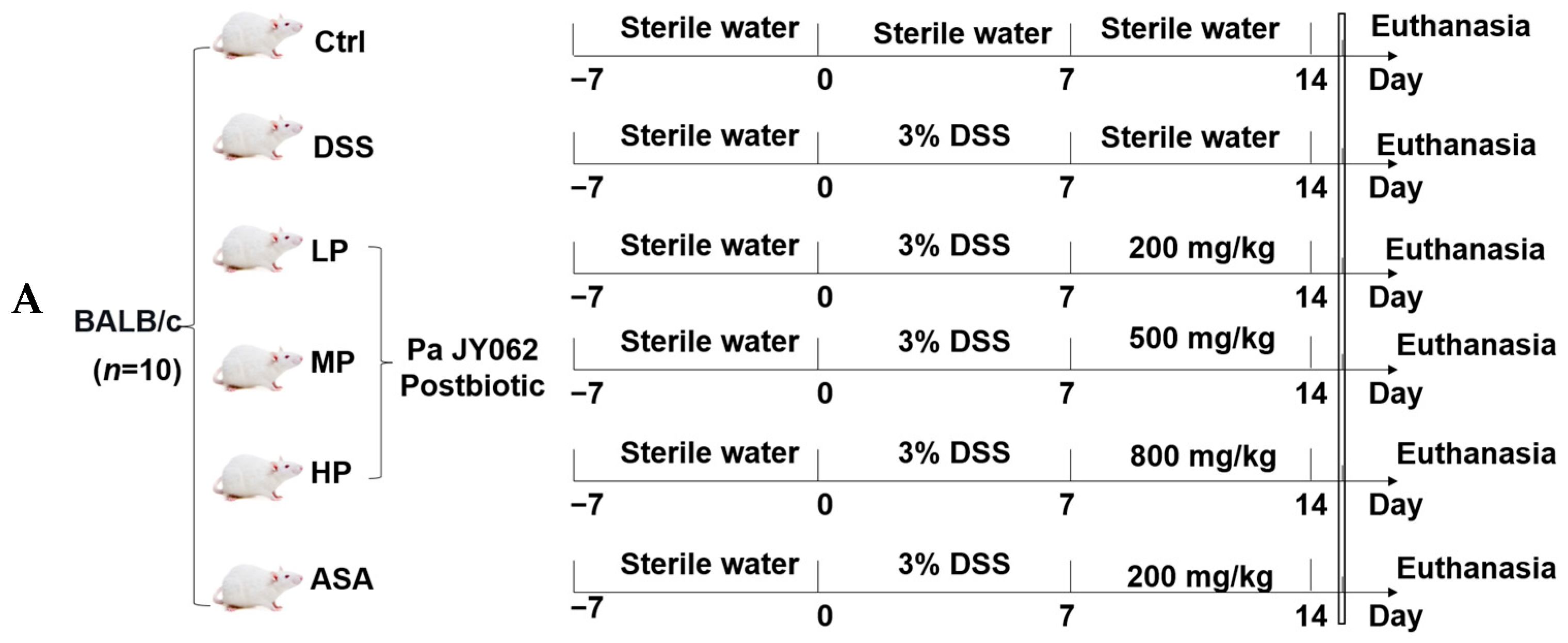
2.5. Animal Experimental Design
2.6. Disease Activity Index
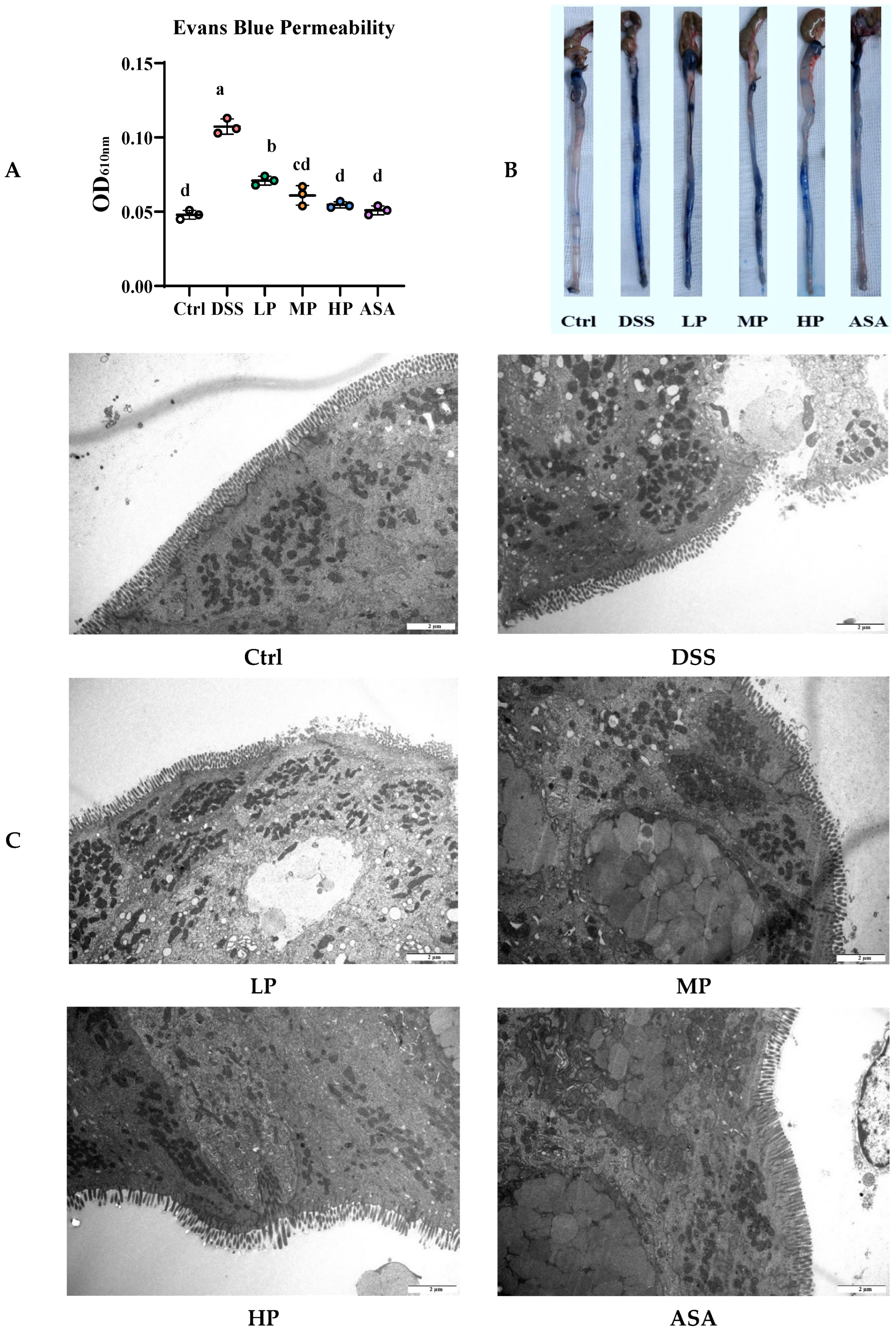
2.7. Evans Blue Permeability and Ultrastructural Analysis
2.8. Histopathological Staining
2.9. Real-Time Quantitative PCR
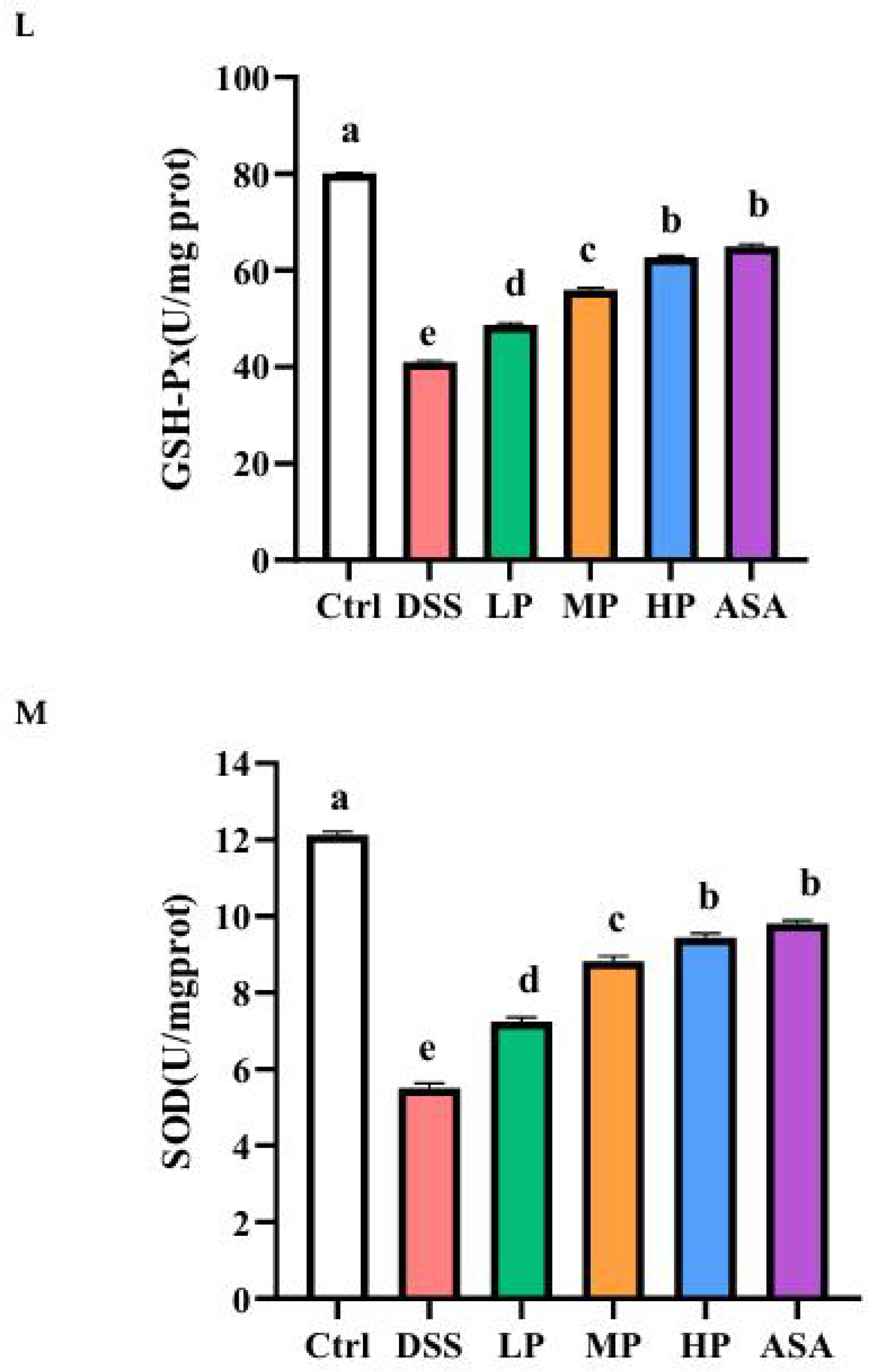
2.10. Evaluation of Oxidative Stress
2.11. Enzyme-Linked Immunosorbent Assay
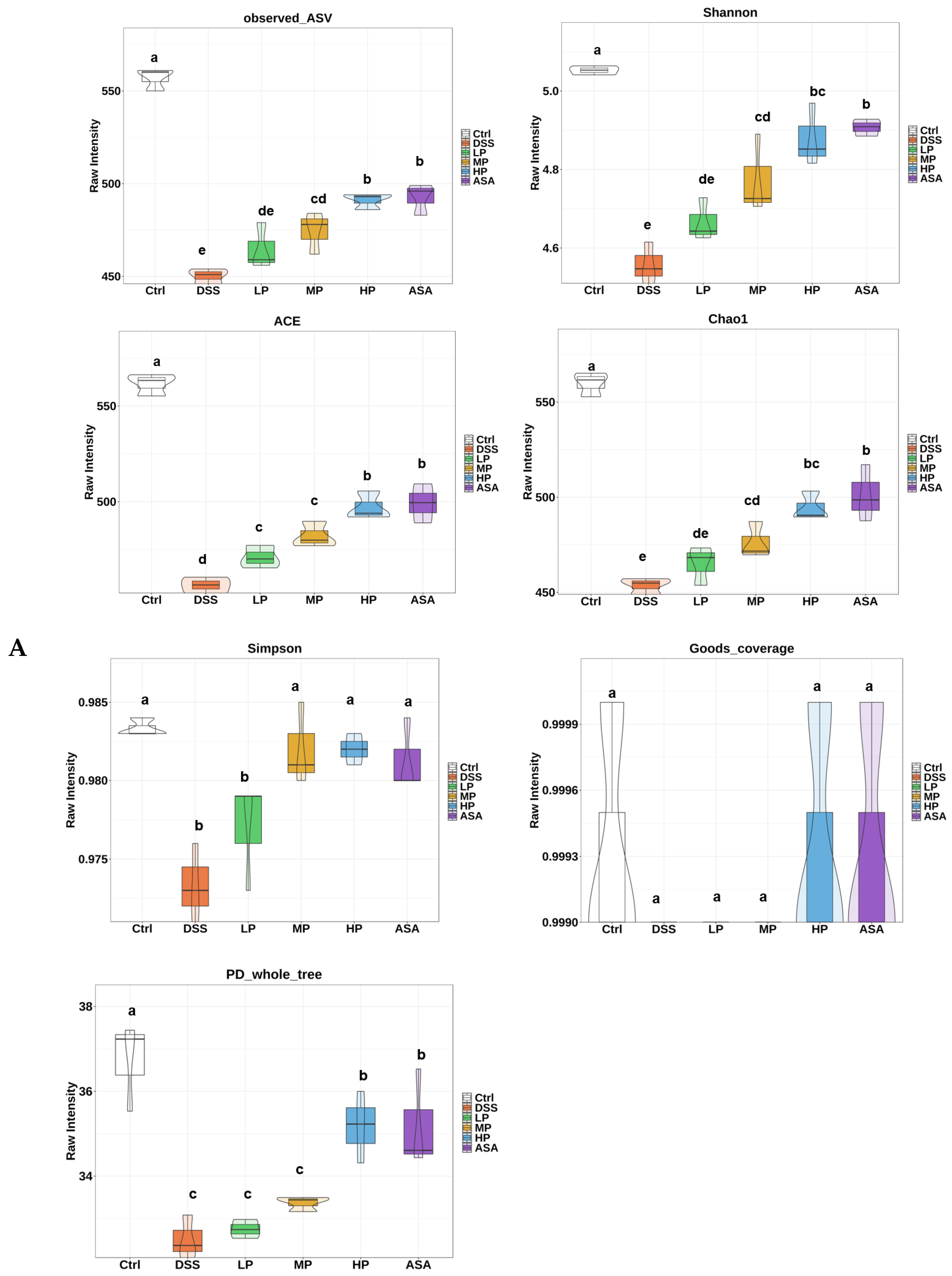
2.12. Gut Microbiota Analysis
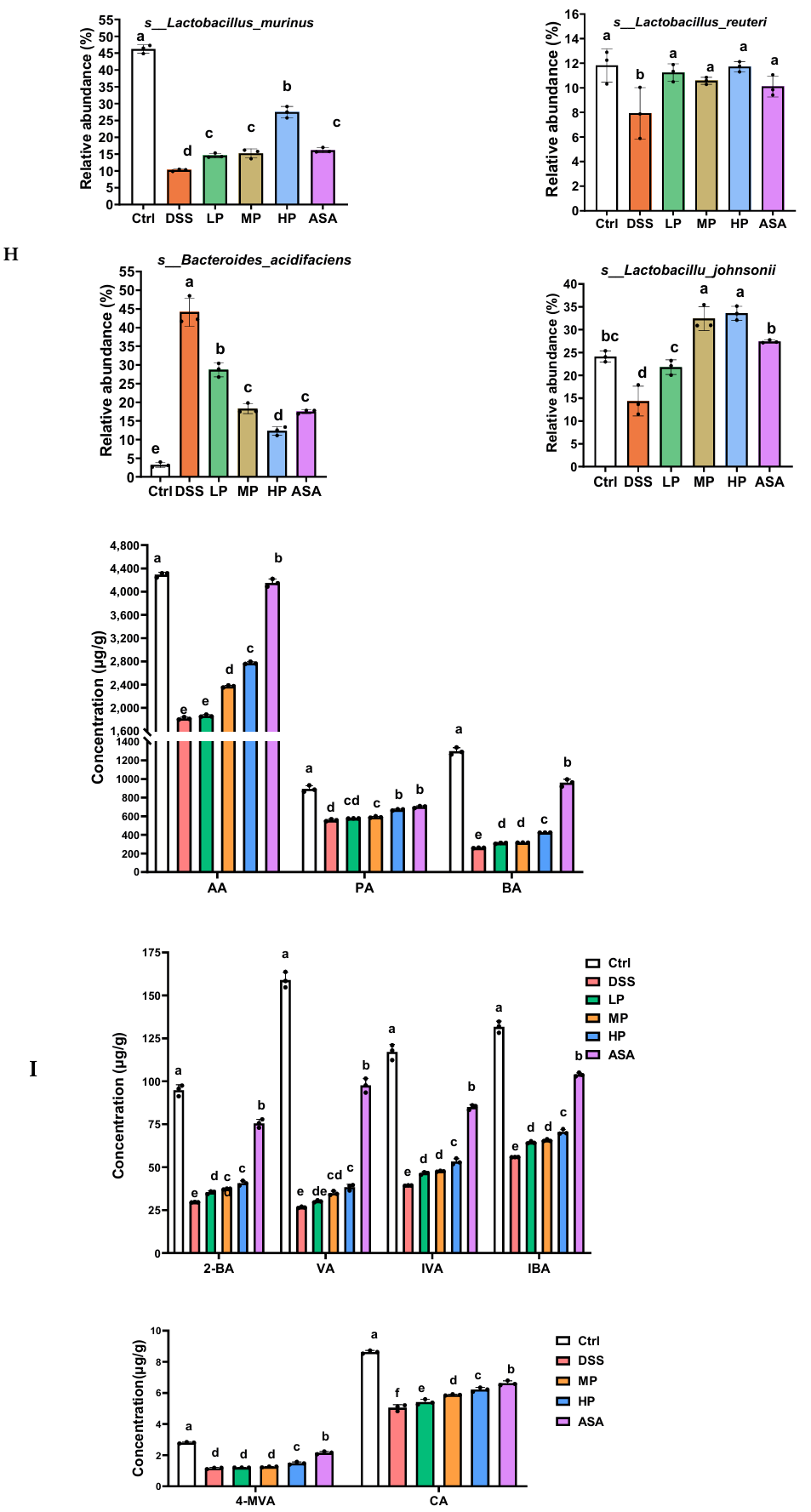
2.13. Detection of Short-Chain Fatty Acids
2.14. Statistical Analysis
3. Results
3.1. Antioxidant Capacity of Pa JY062 Postbiotics
3.2. The Influence of Pa JY062 on Reactive Oxygen Species
3.3. Bioactive Ingredients of Pa JY062
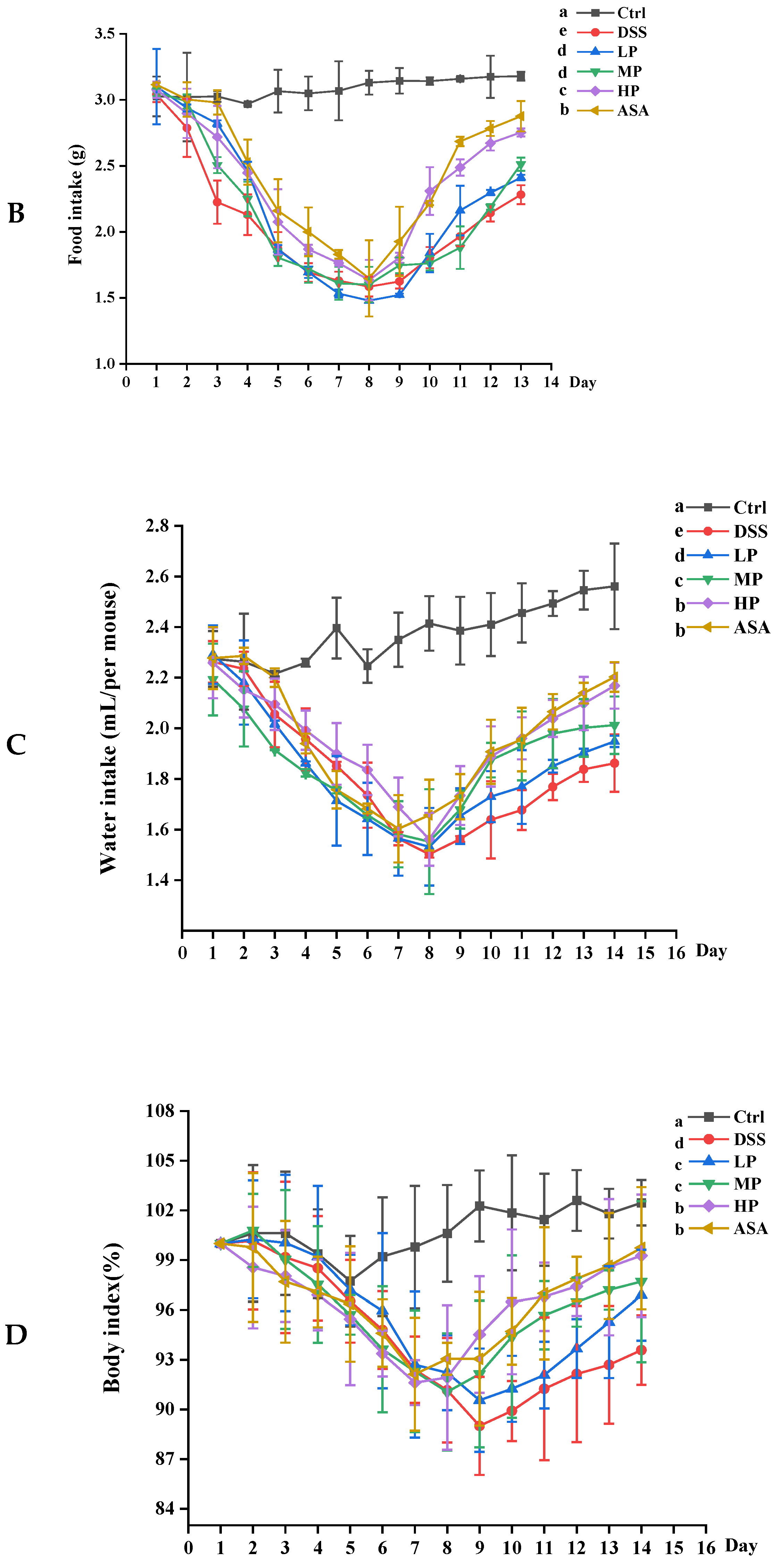
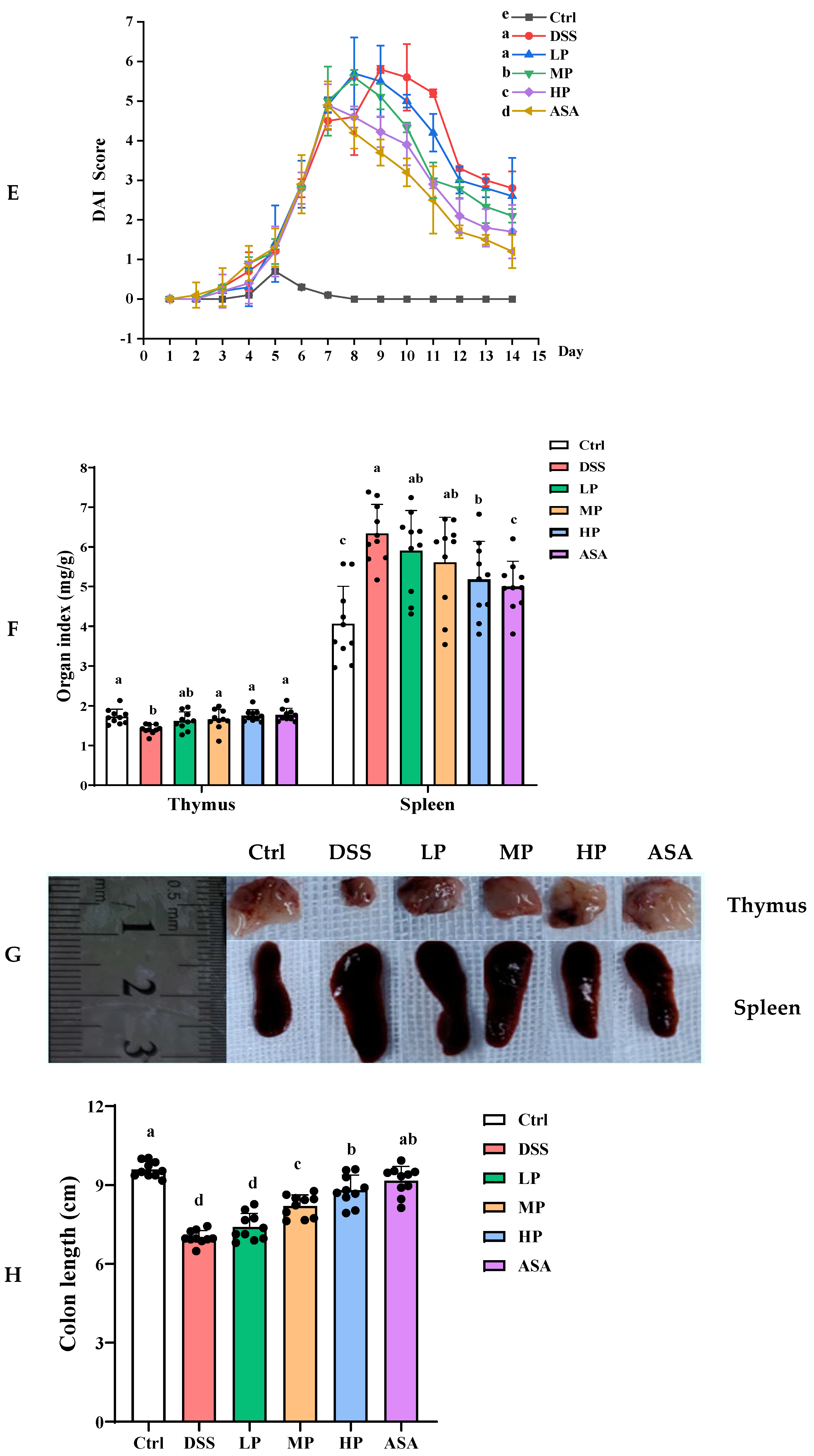
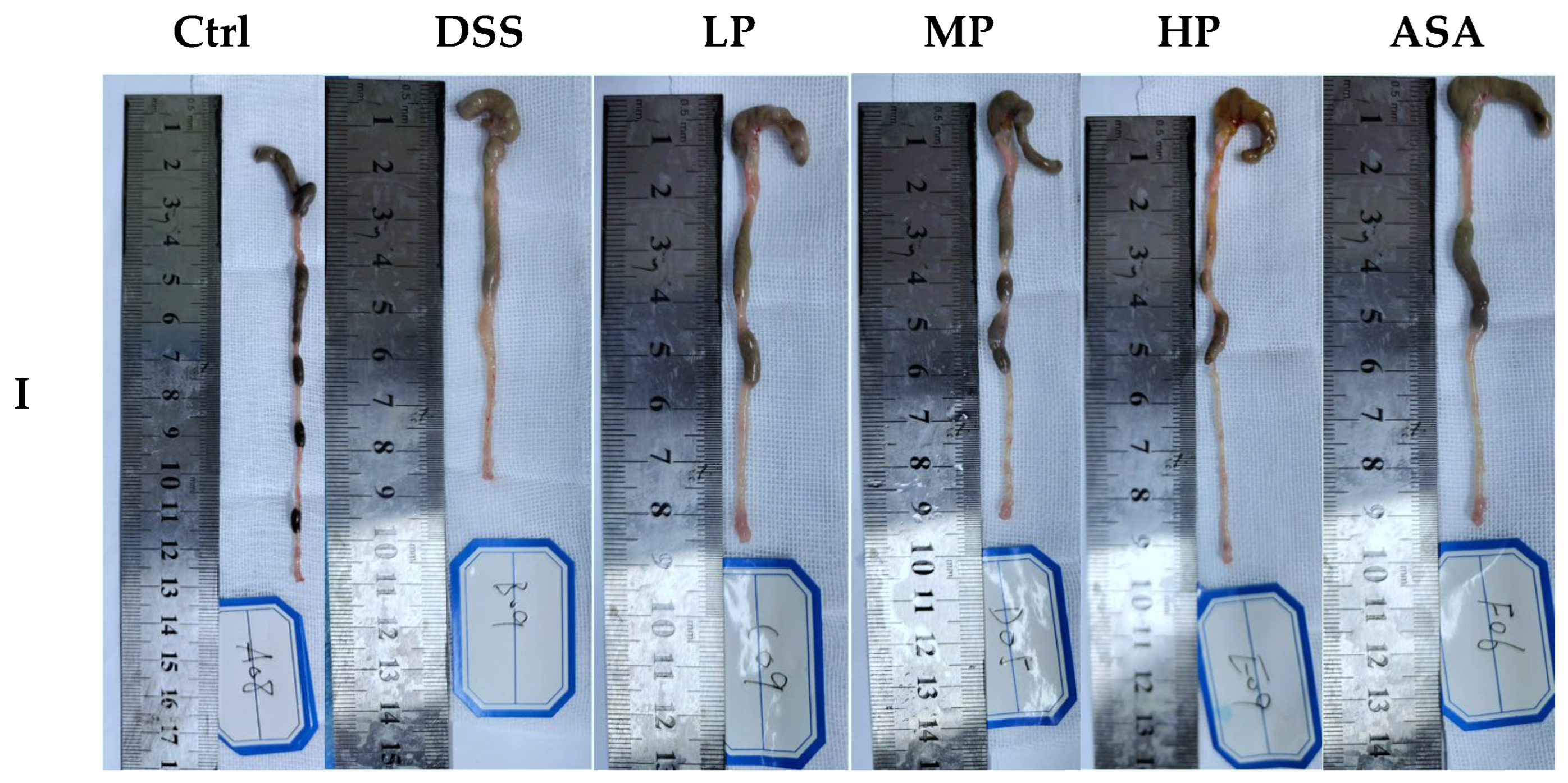
3.4. Pa JY062 Mitigated DSS-Induced Colitis Progression
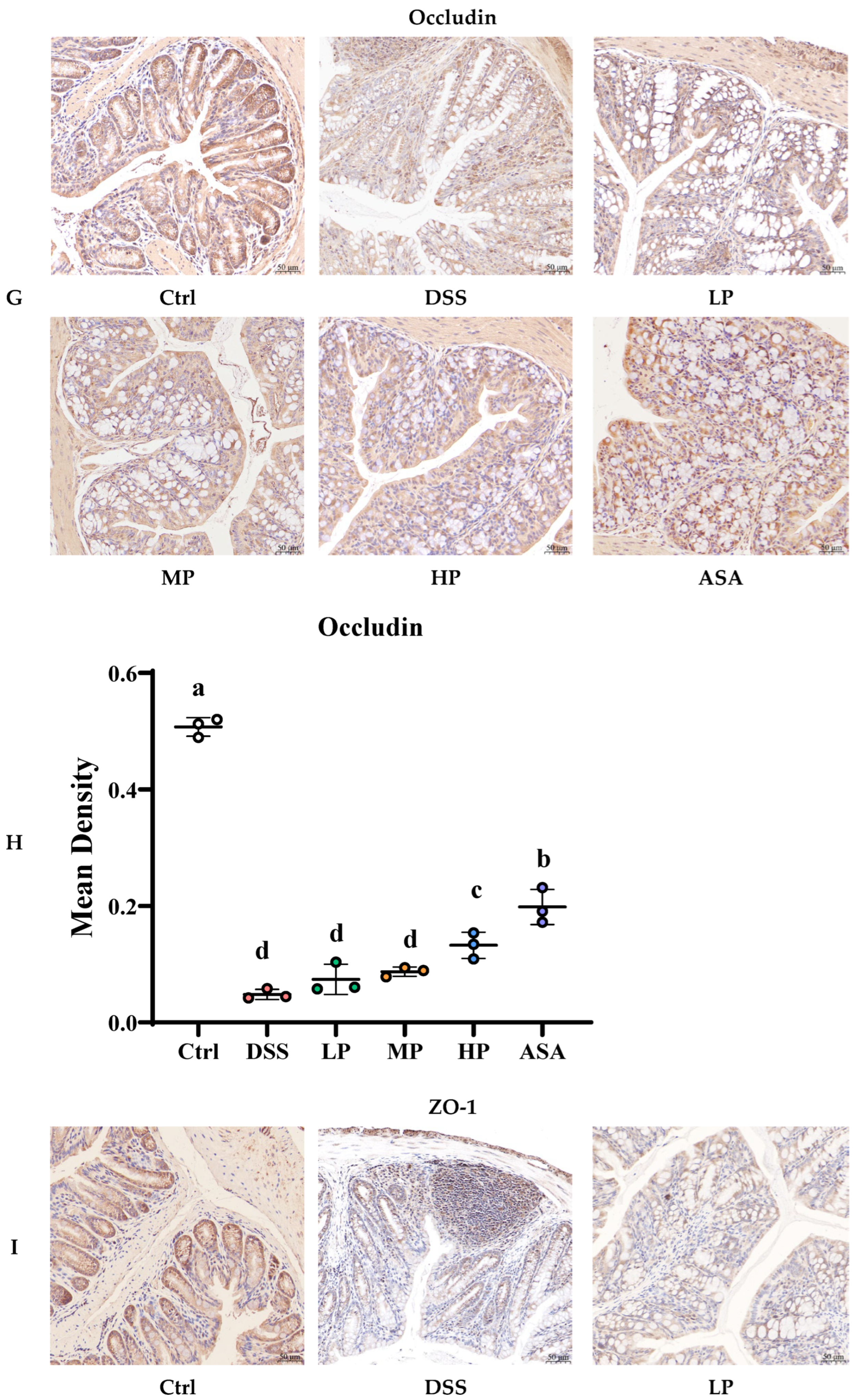
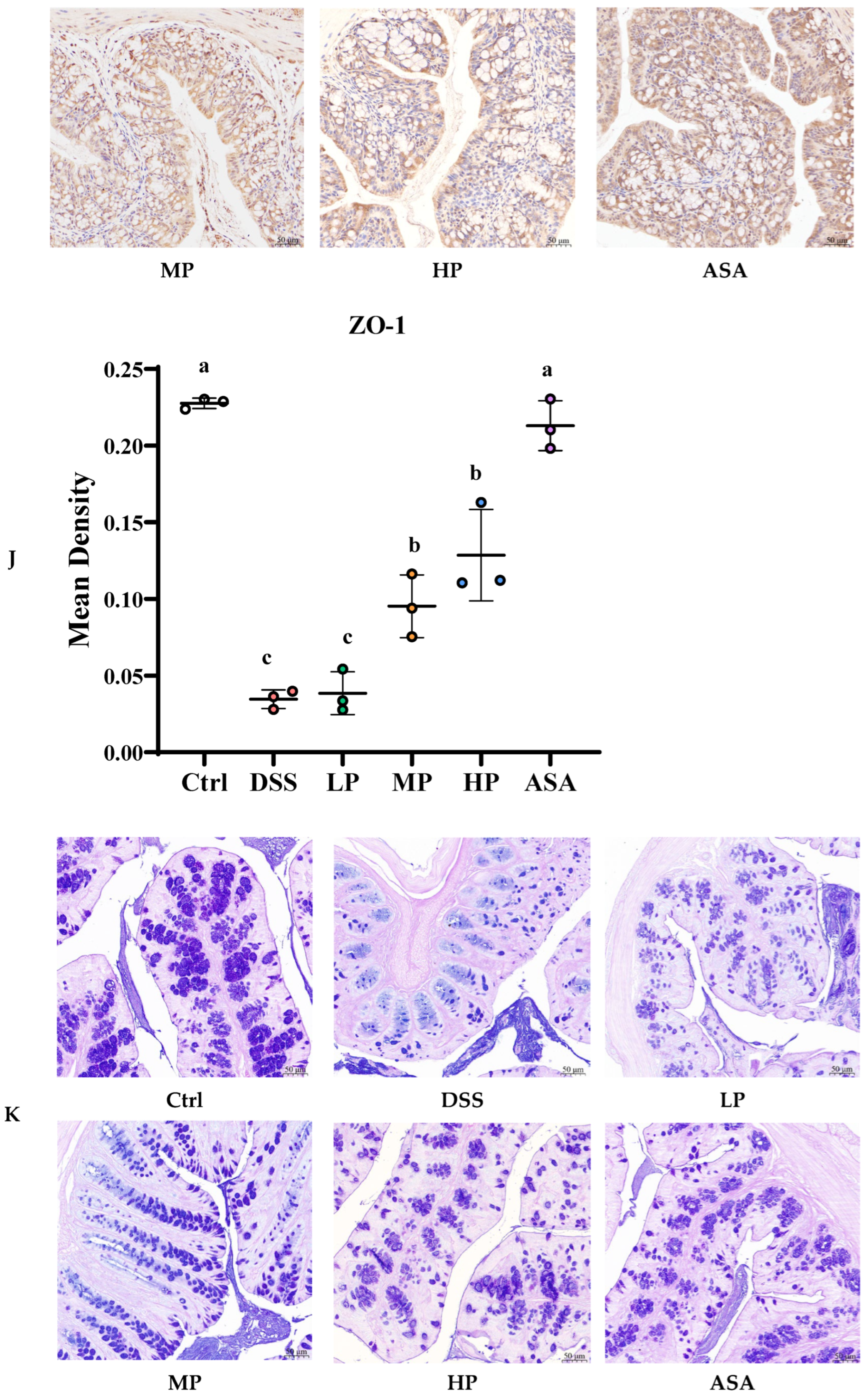
3.5. Pa JY062 Mitigated Damage to the Colonic Barrier in DSS Mice
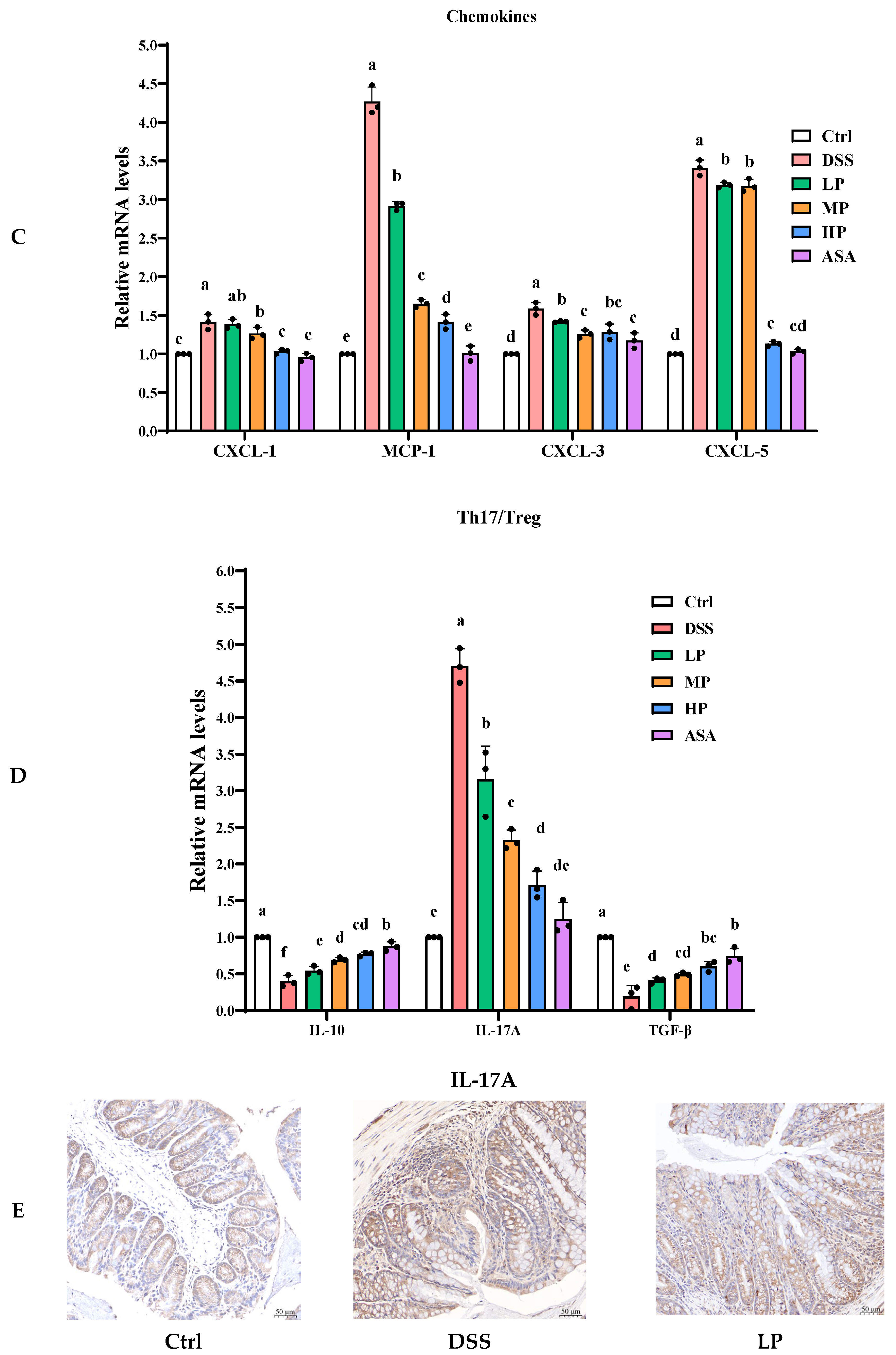
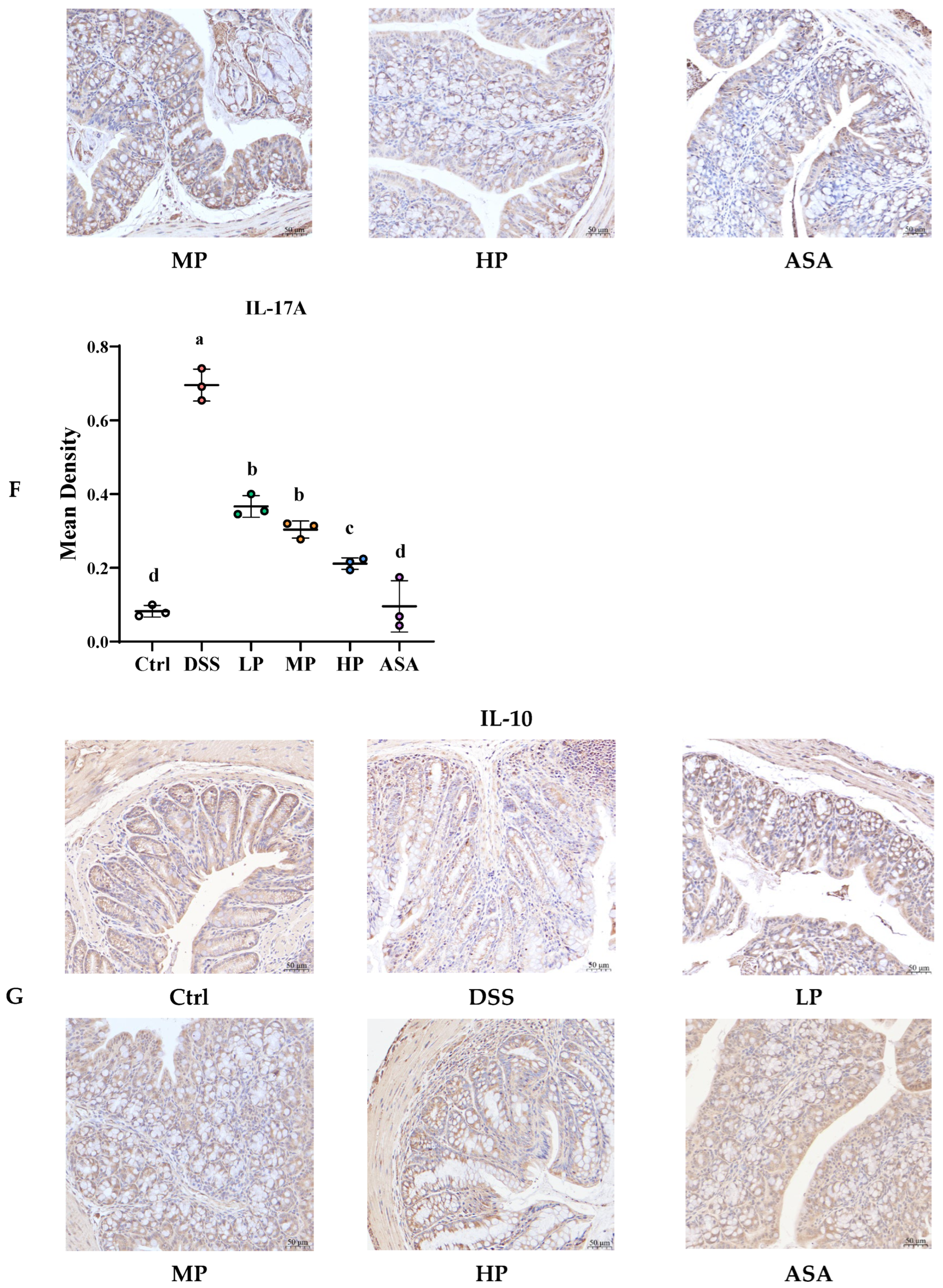
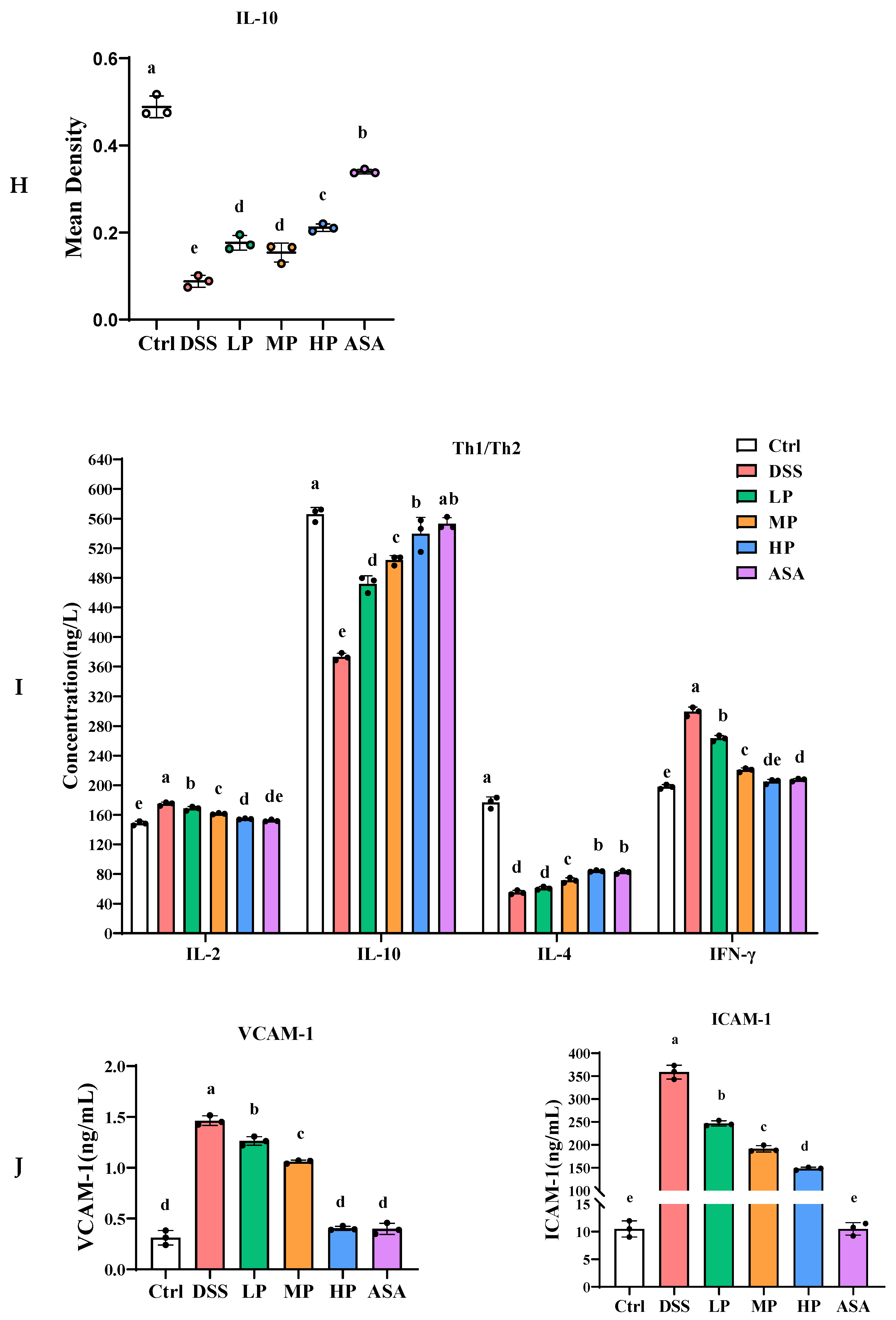
3.6. Pa JY062 Alleviated the Inflammatory Response Induced by DSS in Mice
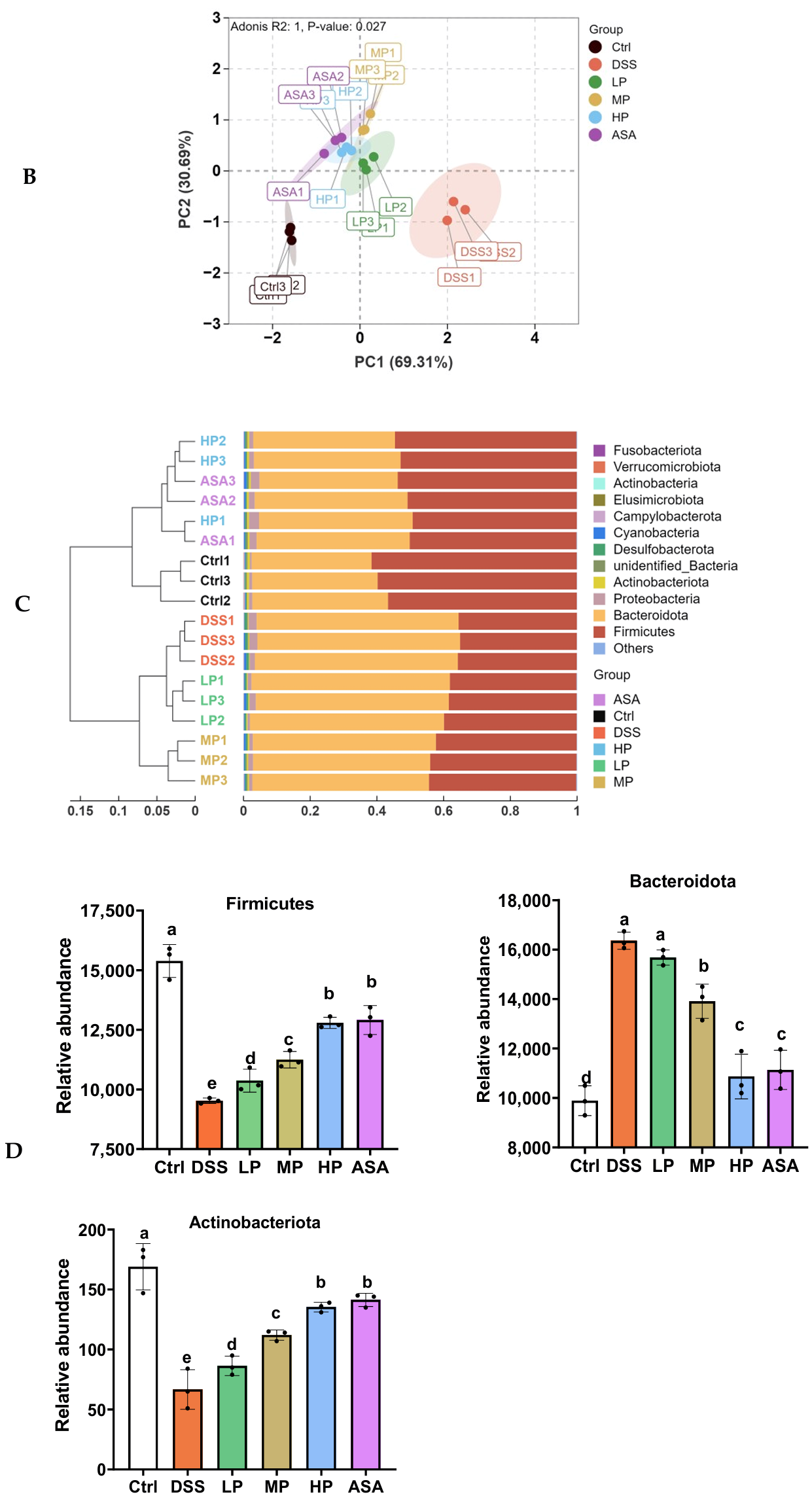
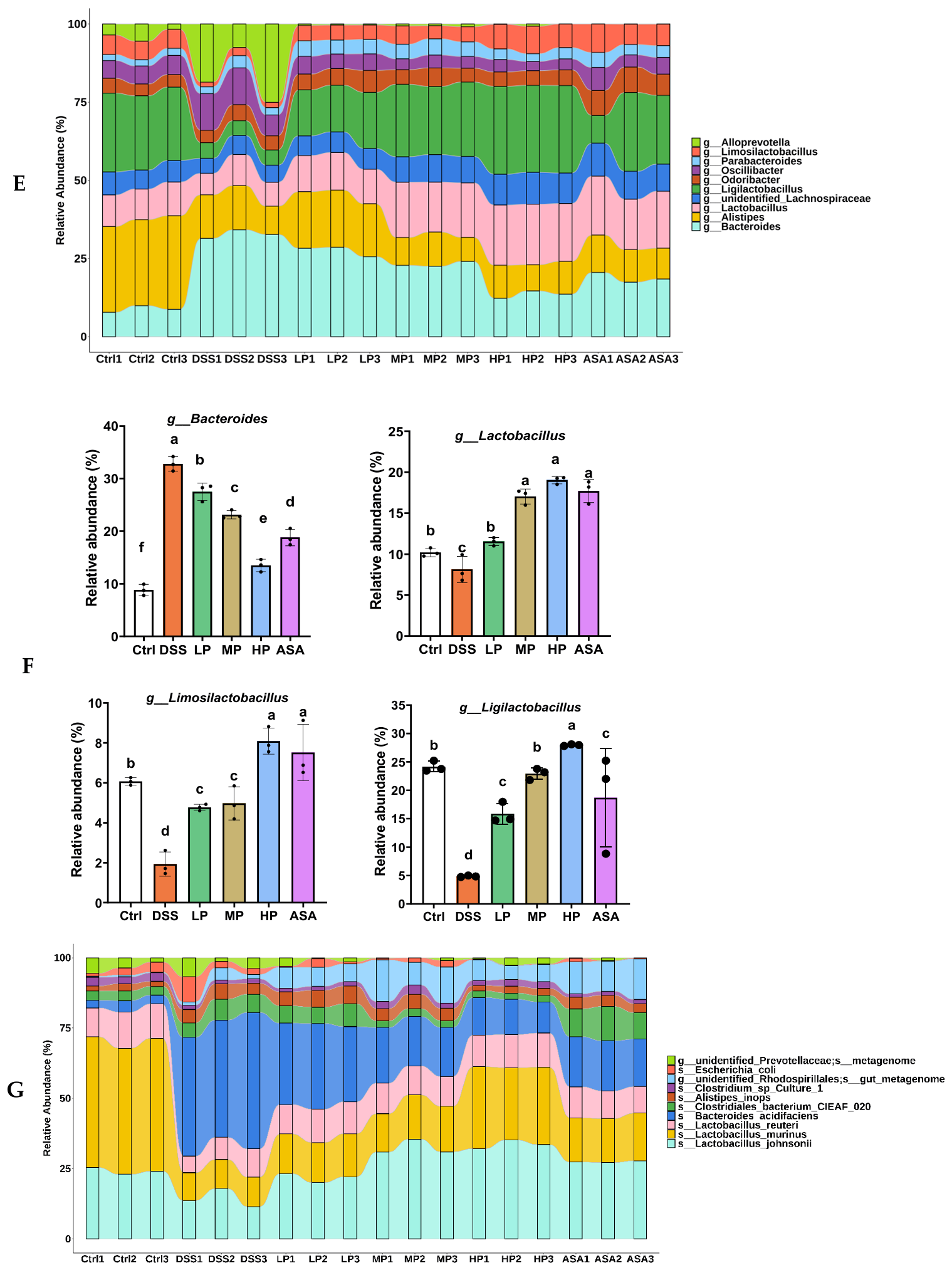
3.7. Pa JY062 Alleviated DSS-Induced Dysbiosis of the Intestinal Microbiota and Depletion of SCFAs in Mice
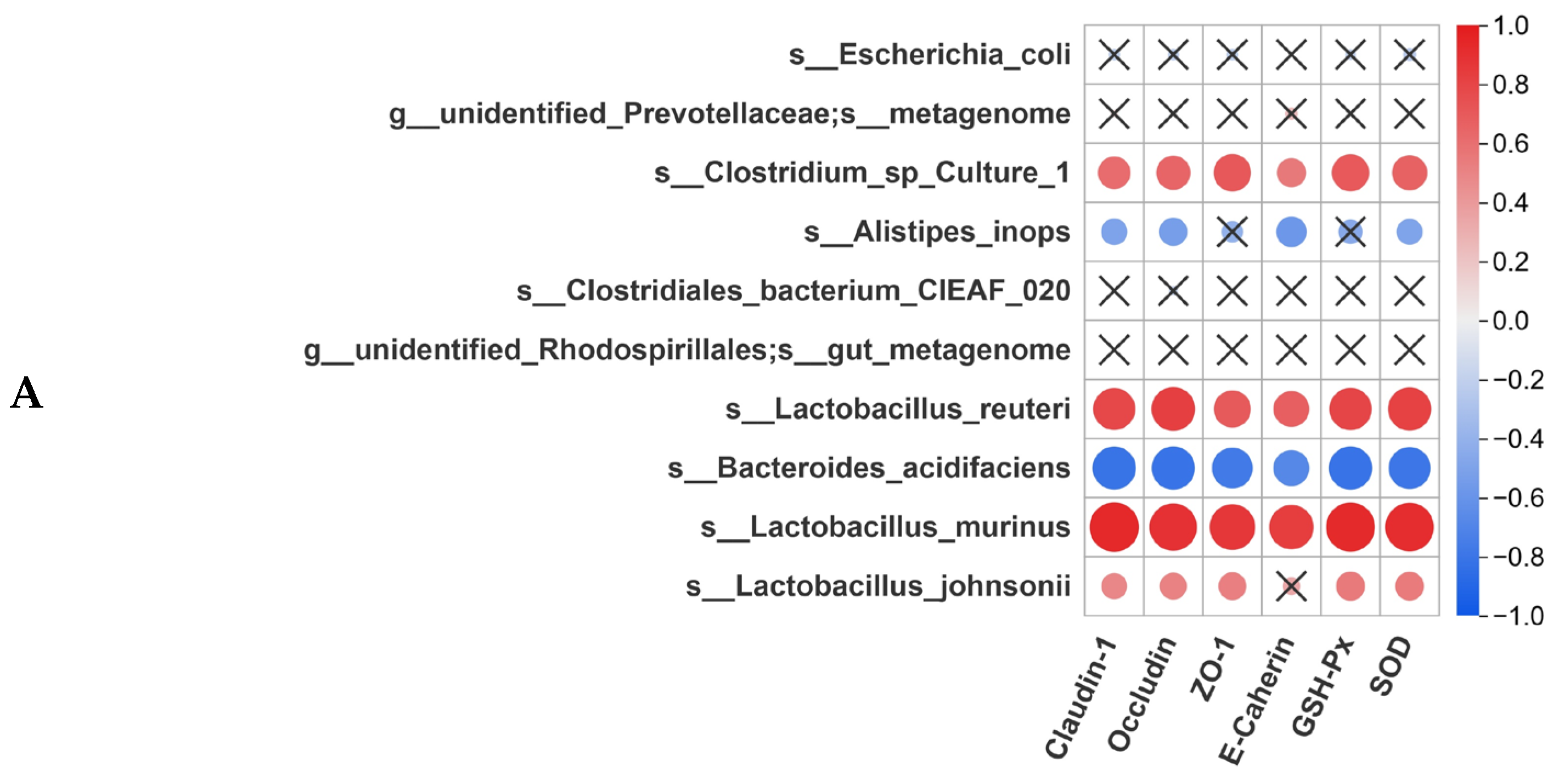
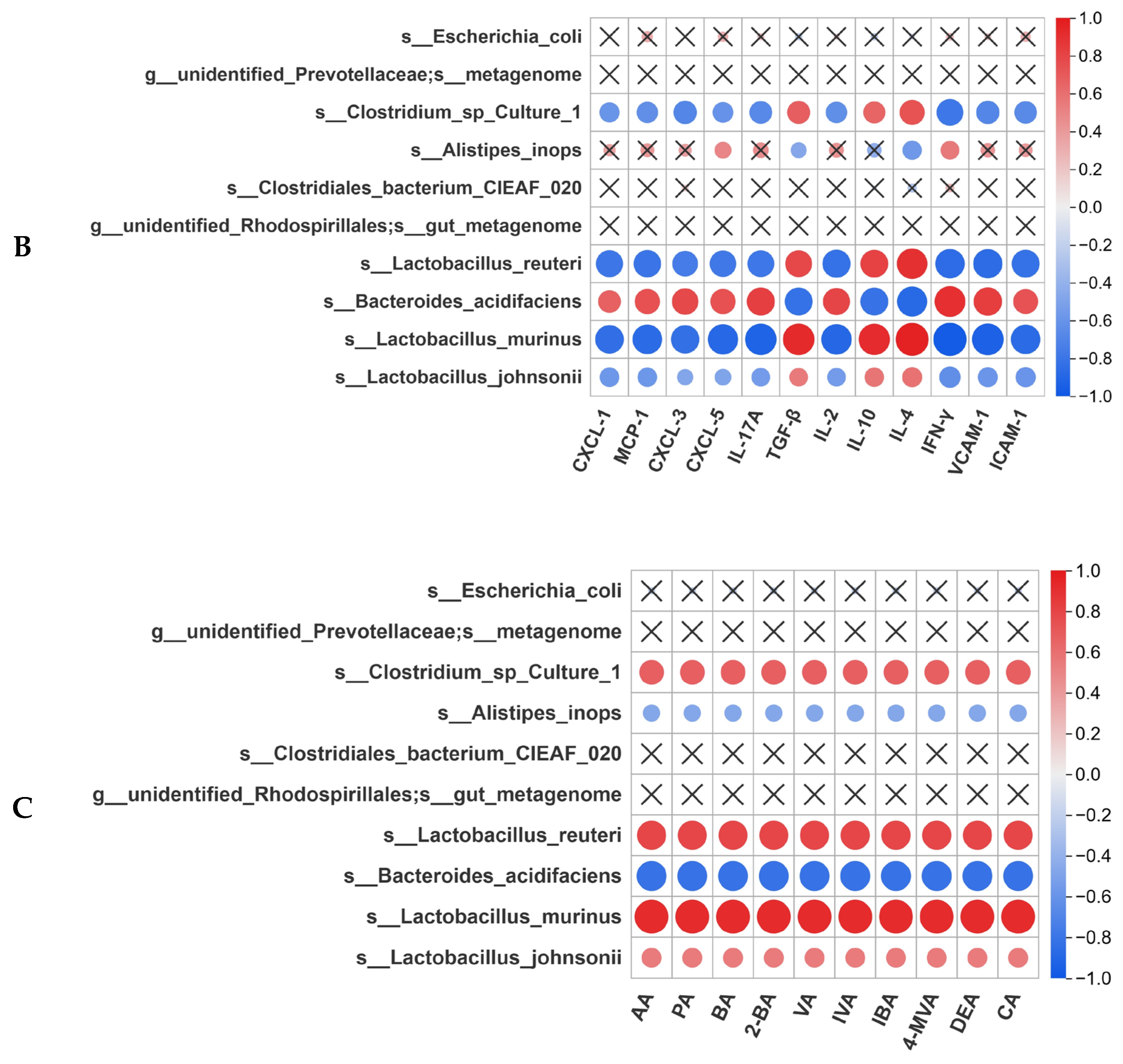
3.8. Correlation Analysis Between Gut Microbiota and Intestinal Homeostasis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| UC | Ulcerative colitis |
| IBD | Inflammatory bowel disease |
| Pa JY062 | Lacticaseibacillus paracasei JY062 postbiotic |
| ROS | Reactive oxygen species |
| Ctrl | Control |
| DSS | Dextran sulfate sodium |
| LP | Low-dose Pa JY062 |
| MP | Medium-dose Pa JY062 |
| HP | High-dose Pa JY062 |
| T-SOD | Total superoxide dismutase |
| GSH-Px | Glutathione peroxidase |
| Th1/Th2 | Helper T 1 cell/Helper T 2 cell |
| Th17/Treg | Helper T 17 cell/Regulatory T cells |
| ZO-1 | Zona occludens 1 |
| DAI | Disease activity index |
| SCFAs | Short-chain fatty acids |
| 5-ASA | 5-aminosalicylic acid |
| TNF-α | Tumor necrosis factor-α |
| IFN-γ | Interferon-γ |
| IL-2 | Interleukin 2 |
| IL-4 | Interleukin 4 |
| IL-10 | Interleukin 10 |
| IL-17A | Interleukin 17A |
| VCAM-1 | Vascular cell adhesion molecule 1 |
| ICAM-1 | Intercellular cell adhesion molecule-1 |
| LGG | Lacticaseibacillus rhamnosus GG |
| DPPH | 1,1-diphenyl-2-picryl-hydrazyl radical |
| OD | Optical density |
| T-AOC | Total Antioxidant Capacity |
| DMEM | Dulbecco’s modification of Eagle’s medium |
| DCFH-DA | 2′,7′-Dichlorodihydrofluorescein diacetate |
| PBS | Phosphate-buffer saline |
| H&E | Hematoxylin and eosin |
| AB-PAS | Alcian blue periodic acid–Schiff |
| IHC | Immunohistochemistry |
| RT-qPCR | Real-time quantitative polymerase chain reaction |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase |
| HRP | Horseradish peroxidase |
| CXCL-1/3/5 | C-X-C Motif Chemokine Ligand 1/3/5 |
| MCP-1 | Monocyte chemoattractant protein-1 |
| TGF-β | Transforming growth factor-β |
| ELISA | Enzyme-linked immunosorbent assay |
| PCA | Principal component analysis |
| IECs | Intestinal epithelial cells |
Appendix A
Appendix A.1. Evaluation of Oxidative Stress
Appendix A.1.1. Assay of Glutathione Peroxidase (GSH-PX)
Appendix A.1.2. Assay of Superoxide Dismutase (SOD)
Appendix A.2. 16S rRNA Analysis
Appendix A.2.1. Alpha Diversity
Appendix A.2.2. Beta Diversity
Appendix A.2.3. Analysis of the Relative Abundance
Appendix A.3. Detection of Short-Chain Fatty Acids
Appendix A.3.1. Chemicals and Reagents
Appendix A.3.2. Sample Preparation and Extraction
Appendix A.3.3. GC–MS Analysis
| Title 1 | Blank Well | Standard Well | Test Well |
|---|---|---|---|
| Sterile water (μL) | 10 | ||
| 0.1, 0.2, 0.4, 0.8, and 1.0 mM Trolox (μL) | 10 | ||
| Samples (μL) | 10 | ||
| Reagent IV application solution (μL) | 20 | 20 | 20 |
| ABTS working solution (μL) | 170 | 170 | 170 |
| Title 1 | Control Tube | Sample Tube | Blank Tube |
|---|---|---|---|
| Sample (μL) | 400 | 400 | |
| 80% methanol (μL) | 600 | 400 | |
| Working solution (μL) | 600 | 600 |
| Samples | RNA Concentration (ng/μL) |
|---|---|
| Ctrl 1 | 875 |
| Ctrl 2 | 937 |
| Ctrl 3 | 911 |
| LPS 1 | 1062 |
| LPS 2 | 1369 |
| LPS 3 | 1136 |
| LP 1 | 854 |
| LP 2 | 761 |
| LP3 | 1329 |
| MP 1 | 1291 |
| MP 2 | 1365 |
| MP 3 | 1555 |
| HP 1 | 955 |
| HP 2 | 1029 |
| HP 3 | 1422 |
| ASA-1 | 852 |
| ASA-2 | 1036 |
| ASA-3 | 1112 |
| Primer Name | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|
| Zo-1 | TGAGGCAGCTCACATAATGC | GGTCTCTGCTGGCTTGTTTC |
| Occludin | AAAGGGCATTGCTCATCCTGA | ACAATGGCAATGGCAATTCATC |
| Claudin-1 | CCAGTCAATGCCAGGTACGAAT | GGCCTTGGTGTTGGGTAAGA |
| E-cadherin | CCCAAACGTAACGAGGGTATC | GGCAGCTTGAAGTGGTAGAAGT |
| CXCL-1 | TGCACCCAAACCGAAGTCAT | ACTTGGGGACACCTTTTAGCAT |
| MCP-1 | CAGGTCCCTGTCATGCTTCT | CCCATTCCTTCTTGGGGTCA |
| CXCL-3 | TGAGGCAGTATTCCTTGGCTG | ACCGGCATGACCTTGTTTGT |
| CXCL-5 | TCCTCAGTCATAGCCGCAAC | TAGCTTTCTTTTTGTCACTGCCC |
| IL-10 | GGGTTGCCAAGCCTTGTCTGAG | CCTTGATGTCTGGGTCTTGGTTCTC |
| IL-17A | GTTAGGGTGCTTTAGGTCC | TAACAATGAGTTTCTGTACG |
| TGF-β | TACAGCAACAATTCCTGGCGATACC | CTCAACCACTGCCGCACAACTC |
| GAPDH | GAGAAGGCTGGGGCTCATTT | TAAGCAGTTGGTGGTGCAGG |
| Compounds | Function | References | |
|---|---|---|---|
| 1 | Allantoin | Antioxidant; anti-inflammatory | [70] |
| 2 | Trehalose | Antioxidant; anti-inflammatory; repair the intestinal barrier | [71] |
| 3 | Histidine | Antioxidant; anti-inflammatory; repair the intestinal barrier | [26,72] |
| 4 | L-Norvaline | Anti-inflammatory | [73] |
| 5 | Phenylalanine | Antioxidant; anti-inflammatory; repair the intestinal barrier | [74] |
| 6 | Glycine | Antioxidant; anti-inflammatory | [75,76] |
| 7 | Tryptophan | Anti-inflammatory | [77] |
| 8 | Betaine | Antioxidant; anti-inflammatory; repair the intestinal barrier | [78,79] |
| 9 | Citric acid | Antioxidant | [80] |
| 10 | 3-phenyllactic acid | Antioxidant | [81] |
| 11 | Threonine | Antioxidant; anti-inflammatory; repair the intestinal barrier | [82] |
| 12 | Benzoic acid | Repair the intestinal barrier | [83] |
| 13 | Biotin | Anti-inflammatory; repair the intestinal barrier | [84] |
| 14 | Serine | Anti-inflammatory; repair the intestinal barrier | [85] |
| 15 | Mannose | Antioxidant; anti-inflammatory; repair the intestinal barrier; modulate the gut microbiota | [86] |
| 16 | Oleamide | Anti-inflammatory | [87] |
| 17 | Glutamine | Anti-inflammatory; repair the intestinal barrier; modulate the gut microbiota | [88] |
| 18 | Taurine | Antioxidant; anti-inflammatory; repair the intestinal barrier | [89] |
| 19 | Salicylic acid | Anti-inflammatory; repair the intestinal barrier | [90] |
| 20 | Hexadecanamide | Anti-inflammatory | [91] |
| 21 | Adenosine | Antioxidant; anti-inflammatory; repair the intestinal barrier | [92] |
| 22 | Quinic acid | Antioxidant; anti-inflammatory; repair the intestinal barrier | [93] |
| 23 | Hypotaurine | Antioxidant | [94] |
| 24 | Stachydrine hydrochloride | Anti-inflammatory | [95] |
| 25 | Dimethylglycine | Anti-inflammatory; repair the intestinal barrier; modulate the gut microbiota | [96] |
| 26 | Trigonelline | Anti-inflammatory | [97] |
| 27 | Anserine | Antioxidant | [98] |
| 28 | Niacinamide | Antioxidant; anti-inflammatory | [99] |
| 29 | 4-hydroxybenzaldehyde | Antioxidant | [100] |
| 30 | 17α-estradiol | Anti-inflammatory | [101] |
| 31 | Palmitoyl ethanolamide | Anti-inflammatory | [102] |
Appendix B
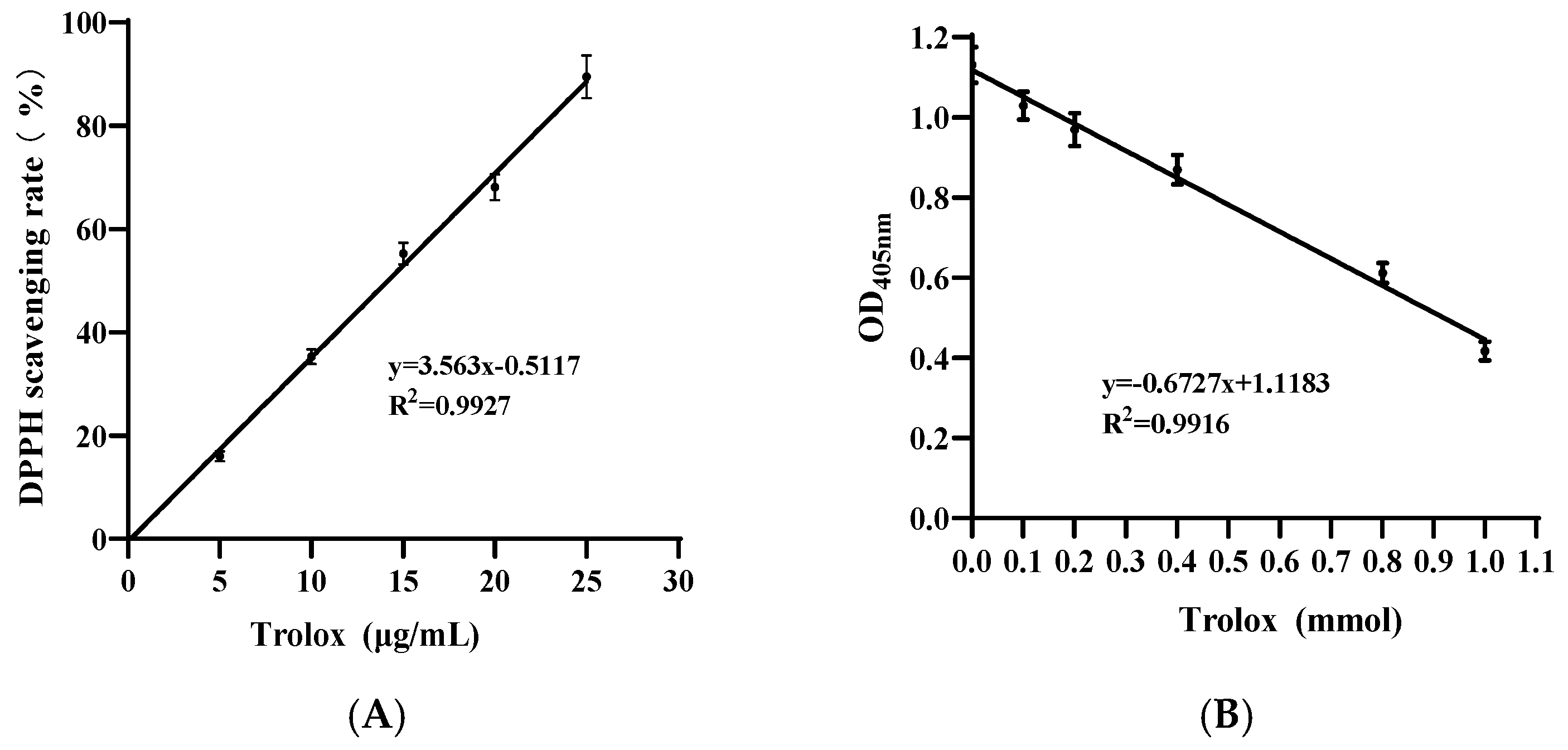
References
- Arnauts, K.; Sudhakar, P.; Verstockt, S.; Lapierre, C.; Potche, S.; Caenepeel, C.; Verstockt, B.; Raes, J.; Vermeire, S.; Sabino, J.; et al. Microbiota, not host origin drives ex vivo intestinal epithelial responses. Gut Microbes 2022, 14, 2089003. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Yu, M.; Ma, L.; Fu, J.; Guo, J.; Lei, J.; Fu, Z.; Fu, Y.; Zhang, Q.; Zhang, C.Y.; et al. In vivo self-assembled siRNA as a modality for combination therapy of ulcerative colitis. Nat. Commun. 2022, 13, 5700. [Google Scholar] [CrossRef]
- Wang, D.; Jiang, Q.; Shen, R.; Peng, L.; Zhou, W.; Meng, T.; Hu, F.; Wang, J.; Yuan, H. ROS-responsive nanoparticles targeting inflamed colon for synergistic therapy of inflammatory bowel disease via barrier repair and anti-inflammation. Nano Res. 2024, 17, 5409–5423. [Google Scholar] [CrossRef]
- Peluzio, M.d.C.G.; Martinez, J.A.; Milagro, F.I. Postbiotics: Metabolites and mechanisms involved in microbiota-host interactions. Trends Food Sci. Technol. 2021, 108, 11–26. [Google Scholar] [CrossRef]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667. [Google Scholar] [CrossRef]
- Pang, L.; Huang, Y.; Li, R.; Guo, L.; Man, C.; Yang, X.; Jiang, Y. Effects of postbiotics produced by Lactobacillus plantarum JM015 isolated from traditional fermented dairy products on Salmonella-induced intestinal inflammation: A preventive strategy. Food Chem. 2025, 469, 142549. [Google Scholar] [CrossRef]
- Nie, X.; Li, Q.; Ji, H.; Zhang, S.; Wang, Y.; Xie, J.; Nie, S. Bifidobacterium longum NSP001-derived extracellular vesicles ameliorate ulcerative colitis by modulating T cell responses in gut microbiota-(in)dependent manners. npj Biofilms Microbiomes 2025, 11, 27. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Yang, X.; Shang, L. Microfluidic-derived montmorillonite composite microparticles for oral codelivery of probiotic biofilm and postbiotics. Sci. Adv. 2025, 11, eadt2131. [Google Scholar] [CrossRef]
- Guo, J.; Zhao, Y.; Guo, W.; Sun, Y.; Zhang, W.; Zhao, Q.; Zhang, Y.; Jiang, Y. Effects of Lactobacillus paracei JY062 Postbiotic on Intestinal Barrier, Immunity, and Gut Microbiota. Nutrients 2025, 17, 1272. [Google Scholar] [CrossRef]
- Su, Y.; Cui, Z.; Yang, X.; Jiang, Y.; Zhang, W.; Zhang, Y.; Man, C. Lactobacillus paracasei JY062 and its exopolysaccharide enhance the intestinal barrier through macrophage polarization and Th17/Treg cell balance. Food Res. Int. 2024, 197, 115235. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Zhang, J.; Jiang, X.; Wang, W.; Chen, Y.Q. Free fatty acid receptor 4 deletion attenuates colitis by modulating Treg Cells via ZBED6-IL33 pathway. EBioMedicine 2022, 80, 104060. [Google Scholar] [CrossRef]
- Rizvi, Z.A.; Dalal, R.; Sadhu, S.; Kumar, Y.; Kumar, S.; Gupta, S.K.; Tripathy, M.R.; Rathore, D.K.; Awasthi, A. High-salt diet mediates interplay between NK cells and gut microbiota to induce potent tumor immunity. Sci. Adv. 2021, 7, eabg5016. [Google Scholar] [CrossRef]
- Wang, Y.; Lai, W.; Zheng, X.; Li, K.; Zhang, Y.; Pang, X.; Gao, J.; Lou, Z. Linderae Radix extract attenuates ulcerative colitis by inhibiting the JAK/STAT signaling pathway. Phytomedicine 2024, 132, 155868. [Google Scholar] [CrossRef]
- Shi, C.; Dawulieti, J.; Shi, F.; Yang, C.; Qin, Q.; Shi, T.; Wang, L.; Hu, H.; Sun, M.; Ren, L.; et al. A nanoparticulate dual scavenger for targeted therapy of inflammatory bowel disease. Sci. Adv. 2022, 8, eabj2372. [Google Scholar] [CrossRef] [PubMed]
- de Jesus, L.C.L.; Freitas, A.d.S.; Dutra, J.d.C.F.; Campos, G.M.; Américo, M.F.; Laguna, J.G.; Dornelas, E.G.; Carvalho, R.D.d.O.; Vital, K.D.; Fernandes, S.O.A.; et al. Lactobacillus delbrueckii CIDCA 133 fermented milk modulates inflammation and gut microbiota to alleviate acute colitis. Food Res. Int. 2024, 186, 114322. [Google Scholar] [CrossRef]
- Zhang, L.; Gui, S.; Xu, Y.; Zeng, J.; Wang, J.; Chen, Q.; Su, L.; Wang, Z.; Deng, R.; Chu, F.; et al. Colon tissue-accumulating mesoporous carbon nanoparticles loaded with Musca domestica cecropin for ulcerative colitis therapy. Theranostics 2021, 11, 3417–3438. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Y.; Liu, M.; Fan, C.; Feng, C.; Lu, Q.; Xiang, C.; Lu, H.; Yang, X.; Wu, B.; et al. Targeting PDE4 as a promising therapeutic strategy in chronic ulcerative colitis through modulating mucosal homeostasis. Acta Pharm. Sinica. B 2022, 12, 228–245. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Lee, S.J.; Jang, W.J.; Lee, E.W. Protective Effects of the Postbiotic Levilactobacillus brevis BK3 against H(2)O(2)-Induced Oxidative Damage in Skin Cells. J. Microbiol. Biotechnol. 2024, 34, 1401–1409. [Google Scholar] [CrossRef] [PubMed]
- Aydın, B.; Çiydem, T.; Kaya, E.; Açık, L. Evaluation of the antioxidant effects of postbiotics and paraprobiotics in lactic acid bacteria isolated from traditional fermented sausages. Avrupa Bilim Ve Teknol. Derg. 2021, 849–852. [Google Scholar] [CrossRef]
- Wang, J.; Li, Y.; Wang, Y.; Du, F.; Zhang, Y.; Yin, M.; Zhao, X.; Xu, J.; Yang, Y.; Wang, W.; et al. Transcriptome and Metabolome Analyses Reveal Complex Molecular Mechanisms Involved in the Salt Tolerance of Rice Induced by Exogenous Allantoin. Antioxidants 2022, 11, 2045. [Google Scholar] [CrossRef]
- Ryabova, A.; Cornette, R.; Cherkasov, A.; Watanabe, M.; Okuda, T.; Shagimardanova, E.; Kikawada, T.; Gusev, O. Combined metabolome and transcriptome analysis reveals key components of complete desiccation tolerance in an anhydrobiotic insect. Proc. Natl. Acad. Sci. USA 2020, 117, 19209–19220. [Google Scholar] [CrossRef]
- Martí-Quijal, F.J.; Tornos, A.; Príncep, A.; Luz, C.; Meca, G.; Tedeschi, P.; Ruiz, M.J.; Barba, F.J. Impact of Fermentation on the Recovery of Antioxidant Bioactive Compounds from Sea Bass Byproducts. Antioxidants 2020, 9, 239. [Google Scholar] [CrossRef]
- Mohd, S.; Qazi, F.; Christian Danve, M.C. Salicylic acid: A key regulator of redox signalling and plant immunity. Plant Physiol. Biochem. 2021, 168, 381–397. [Google Scholar] [CrossRef]
- El Din, R.H.; Thabit, S. Quinic acid protects against the development of Huntington’s disease in Caenorhabditis elegans model. BMC Complement. Med. Ther. 2024, 24, 377. [Google Scholar] [CrossRef]
- Fu, M.; Guo, S.; Yang, S.; Yang, K.; Li, R.; Shan, X.; Zhao, P.; Zhang, C.; Guo, W.; Xu, M.; et al. Stachydrine hydrochloride reduces NOX2 activity to suppress oxidative stress levels to improve cardiac insufficiency. Phytomedicine 2025, 140, 156621. [Google Scholar] [CrossRef]
- Zeng, W.; Wu, J.; Xie, H.; Xu, H.; Liang, D.; He, Q.; Yang, X.; Liu, C.; Gong, J.; Zhang, Q.; et al. Enteral nutrition promotes the remission of colitis by gut bacteria-mediated histidine biosynthesis. EBioMedicine 2024, 100, 104959. [Google Scholar] [CrossRef]
- Javrushyan, H.; Nadiryan, E.; Grigoryan, A.; Avtandilyan, N.; Maloyan, A. Antihyperglycemic activity of L-norvaline and L-arginine in high-fat diet and streptozotocin-treated male rats. Exp. Mol. Pathol. 2022, 126, 104763. [Google Scholar] [CrossRef]
- Kumar Patel, M.; Fanyuk, M.; Feyngenberg, O.; Maurer, D.; Sela, N.; Ovadia, R.; Oren-Shamir, M.; Alkan, N. Phenylalanine induces mango fruit resistance against chilling injuries during storage at suboptimal temperature. Food Chem. 2023, 405, 134909. [Google Scholar] [CrossRef]
- Rom, O.; Liu, Y.; Finney, A.C.; Ghrayeb, A.; Zhao, Y.; Shukha, Y.; Wang, L.; Rajanayake, K.K.; Das, S.; Rashdan, N.A.; et al. Induction of glutathione biosynthesis by glycine-based treatment mitigates atherosclerosis. Redox Biol. 2022, 52, 102313. [Google Scholar] [CrossRef]
- Ahmed, I.; Ahmad, I.; Malla, B.A. Effects of dietary tryptophan levels on growth performance, plasma profile, intestinal antioxidant capacity and growth related genes in rainbow trout (Oncorhynchus mykiss) fingerlings. Aquaculture 2024, 585, 740710. [Google Scholar] [CrossRef]
- Worlanyo, H.G.; Jiang, S.; Yu, Y.; Liu, B.; Zhou, Q.; Sun, C.; Miao, L.; Lin, Y.; Zheng, X.; Saidyleigh, M.; et al. Effects of dietary threonine on growth and immune response of oriental river prawn (Macrobrachium nipponense). Fish Shellfish Immunol. 2022, 128, 288–299. [Google Scholar] [CrossRef]
- Li, K.; Wu, J.L.; Qin, B.; Fan, Z.; Tang, Q.; Lu, W.; Zhang, H.; Xing, F.; Meng, M.; Zou, S.; et al. ILF3 is a substrate of SPOP for regulating serine biosynthesis in colorectal cancer. Cell Res. 2020, 30, 163–178. [Google Scholar] [CrossRef]
- Kempson, S.A.; Vovor-Dassu, K.; Day, C. Betaine Transport in Kidney and Liver: Use of Betaine in Liver Injury. Cell. Physiol. Biochem. 2013, 32, 32–40. [Google Scholar] [CrossRef]
- Go, E.K.; Jung, K.J.; Kim, J.Y.; Yu, B.P.; Chung, H.Y. Betaine suppresses proinflammatory signaling during aging: The involvement of nuclear factor-kappaB via nuclear factor-inducing kinase/IkappaB kinase and mitogen-activated protein kinases. J. gerontology. Ser. A Biol. Sci. Med. Sci. 2005, 60, 1252–1264. [Google Scholar] [CrossRef]
- Xin, Y.; Hu, C.; Li, Y.; Yang, Z.; Zhang, L.; Li, A.; Li, C.; Liu, L.; Du, P. Immunomodulatory potential of Lactobacillus helveticus KLDS 1.8701 postbiotics: By regulating the Th17/Treg balance. Food Biosci. 2024, 61, 104842. [Google Scholar] [CrossRef]
- Guo, S.; Ma, T.; Kwok, L.Y.; Quan, K.; Li, B.; Wang, H.; Zhang, H.; Menghe, B.; Chen, Y. Effects of postbiotics on chronic diarrhea in young adults: A randomized, double-blind, placebo-controlled crossover trial assessing clinical symptoms, gut microbiota, and metabolite profiles. Gut Microbes 2024, 16, 2395092. [Google Scholar] [CrossRef]
- Patankar, J.V.; Becker, C. Cell death in the gut epithelium and implications for chronic inflammation. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 543–556. [Google Scholar] [CrossRef]
- Postema, M.M.; Grega-Larson, N.E.; Neininger, A.C.; Tyska, M.J. IRTKS (BAIAP2L1) Elongates Epithelial Microvilli Using EPS8-Dependent and Independent Mechanisms. Curr. Biol. CB 2018, 28, 2876–2888.e2874. [Google Scholar] [CrossRef]
- Kwak, S.Y.; Jang, W.I.; Park, S.; Cho, S.S.; Lee, S.B.; Kim, M.J.; Park, S.; Shim, S.; Jang, H. Metallothionein 2 activation by pravastatin reinforces epithelial integrity and ameliorates radiation-induced enteropathy. EBioMedicine 2021, 73, 103641. [Google Scholar] [CrossRef]
- Zhou, Y.; Ji, H.; Xu, Q.; Zhang, X.; Cao, X.; Chen, Y.; Shao, M.; Wu, Z.; Zhang, J.; Lu, C.; et al. Congenital biliary atresia is correlated with disrupted cell junctions and polarity caused by Cdc42 insufficiency in the liver. Theranostics 2021, 11, 7262–7275. [Google Scholar] [CrossRef]
- Neurath, M.F.; Artis, D.; Becker, C. The intestinal barrier: A pivotal role in health, inflammation, and cancer. Lancet Gastroenterol. Hepatol. 2025, 10, 573–592. [Google Scholar] [CrossRef]
- Liu, X.; Ma, Y.; Guan, K.; Liu, R.; Mao, K.; Xu, X.; Li, Q.; Wang, R. Intestinal barrier, immunity and gut microbiota-based protective effects of Lactococcus lactis HF08 and its postbiotic derivative on aging and aging colitis mice. Food Res. Int. 2024, 197, 115164. [Google Scholar] [CrossRef]
- Xiao, L.; Zhang, C.; Zhang, X.; Zhao, X.; Chaeipeima Mahsa, G.; Ma, K.; Ji, F.; Azarpazhooh, E.; Ajami, M.; Rui, X.; et al. Effects of Lacticaseibacillus paracasei SNB-derived postbiotic components on intestinal barrier dysfunction and composition of gut microbiota. Food Res. Int. 2023, 175, 113773. [Google Scholar] [CrossRef]
- Lee, H.; Jung, K.B.; Kwon, O.; Son, Y.S.; Choi, E.; Yu, W.D.; Son, N.; Jeon, J.H.; Jo, H.; Yang, H.; et al. Limosilactobacillus reuteri DS0384 promotes intestinal epithelial maturation via the postbiotic effect in human intestinal organoids and infant mice. Gut Microbes 2022, 14, 2121580. [Google Scholar] [CrossRef]
- Herro, R.; Grimes, H.L. The diverse roles of neutrophils from protection to pathogenesis. Nat. Immunol. 2024, 25, 2209–2219. [Google Scholar] [CrossRef]
- Chen, S.; Saeed, A.F.U.H.; Liu, Q.; Jiang, Q.; Xu, H.; Xiao, G.G.; Rao, L.; Duo, Y. Macrophages in immunoregulation and therapeutics. Signal Transduct. Target. Ther. 2023, 8, 207. [Google Scholar] [CrossRef]
- Kimura, S.; Nakamura, Y.; Kobayashi, N.; Shiroguchi, K.; Kawakami, E.; Mutoh, M.; Takahashi-Iwanaga, H.; Yamada, T.; Hisamoto, M.; Nakamura, M.; et al. Osteoprotegerin-dependent M cell self-regulation balances gut infection and immunity. Nat. Commun. 2020, 11, 234. [Google Scholar] [CrossRef]
- Ciceroni, C.; Bonelli, M.; Mastrantoni, E.; Niccolini, C.; Laurenza, M.; Larocca, L.M.; Pallini, R.; Traficante, A.; Spinsanti, P.; Ricci-Vitiani, L.; et al. Type-3 metabotropic glutamate receptors regulate chemoresistance in glioma stem cells, and their levels are inversely related to survival in patients with malignant gliomas. Cell Death Differ. 2013, 20, 396–407. [Google Scholar] [CrossRef]
- Chu, S.; Sun, R.; Gu, X.; Chen, L.; Liu, M.; Guo, H.; Ju, S.; Vatsalya, V.; Feng, W.; McClain, C.J.; et al. Inhibition of Sphingosine-1-Phosphate-Induced Th17 Cells Ameliorates Alcohol-Associated Steatohepatitis in Mice. Hepatology 2021, 73, 952–967. [Google Scholar] [CrossRef]
- Wei, C.; Wang, J.Y.; Xiong, F.; Wu, B.H.; Luo, M.H.; Yu, Z.C.; Liu, T.T.; Li, D.F.; Tang, Q.; Li, Y.X.; et al. Curcumin ameliorates DSS-induced colitis in mice by regulating the Treg/Th17 signaling pathway. Mol. Med. Rep. 2021, 23, 34. [Google Scholar] [CrossRef]
- Sadeghi, N.; Mansoori, A.; Shayesteh, A.; Hashemi, S.J. The effect of curcumin supplementation on clinical outcomes and inflammatory markers in patients with ulcerative colitis. Phytother. Res. PTR 2020, 34, 1123–1133. [Google Scholar] [CrossRef]
- Uchiyama, K.; Takami, S.; Suzuki, H.; Umeki, K.; Mochizuki, S.; Kakinoki, N.; Iwamoto, J.; Hoshino, Y.; Omori, J.; Fujimori, S.; et al. Efficacy and safety of short-term therapy with indigo naturalis for ulcerative colitis: An investigator-initiated multicenter double-blind clinical trial. PLoS ONE 2020, 15, e0241337. [Google Scholar] [CrossRef]
- Xiao, H.-T.; Peng, J.; Hu, D.-D.; Lin, C.-Y.; Du, B.; Tsang, S.-W.; Lin, Z.-S.; Zhang, X.-J.; Lueng, F.-P.; Han, Q.-B.; et al. Qing-dai powder promotes recovery of colitis by inhibiting inflammatory responses of colonic macrophages in dextran sulfate sodium-treated mice. Chin. Med. 2015, 10, 29. [Google Scholar] [CrossRef]
- Chen, S.J.; Chen, L.H.; Yeh, Y.M.; Lin, C.K.; Lin, P.C.; Huang, H.W.; Shen, M.R.; Lin, B.W.; Lee, J.C.; Lee, C.C.; et al. Targeting lysosomal cysteine protease cathepsin S reveals immunomodulatory therapeutic strategy for oxaliplatin-induced peripheral neuropathy. Theranostics 2021, 11, 4672–4687. [Google Scholar] [CrossRef]
- Pickett, J.R.; Wu, Y.; Ta, H.T. VCAM-1 as a common biomarker in inflammatory bowel disease and colorectal cancer: Unveiling the dual anti-inflammatory and anti-cancer capacities of anti-VCAM-1 therapies. Cancer Metastasis Rev. 2025, 44, 40. [Google Scholar] [CrossRef]
- Xiao, Y.; Peng, X.; Peng, Y.; Zhang, C.; Liu, W.; Yang, W.; Dou, X.; Jiang, Y.; Wang, Y.; Yang, S.; et al. Macrophage-derived extracellular vesicles regulate follicular activation and improve ovarian function in old mice by modulating local environment. Clin. Transl. Med. 2022, 12, e1071. [Google Scholar] [CrossRef] [PubMed]
- Henn, M.R.; O’Brien, E.J.; Diao, L.; Feagan, B.G.; Sandborn, W.J.; Huttenhower, C.; Wortman, J.R.; McGovern, B.H.; Wang-Weigand, S.; Lichter, D.I.; et al. A Phase 1b Safety Study of SER-287, a Spore-Based Microbiome Therapeutic, for Active Mild to Moderate Ulcerative Colitis. Gastroenterology 2021, 160, 115–127.e130. [Google Scholar] [CrossRef]
- Schirmer, M.; Garner, A.; Vlamakis, H.; Xavier, R.J. Microbial genes and pathways in inflammatory bowel disease. Nat. Rev. Microbiol. 2019, 17, 497–511. [Google Scholar] [CrossRef]
- Yan, S.; Yu, L.; Tian, F.; Zhao, J.; Chen, W.; Chen, H.; Zhai, Q. Ligilactobacillus salivarius CCFM 1266 modulates gut microbiota and GPR109a-mediated immune suppression to attenuate immune checkpoint blockade-induced colitis. Food Funct. 2023, 14, 10549–10563. [Google Scholar] [CrossRef]
- Huang, Y.; Yang, C.; Fu, B.; Guo, H.; Chen, Y.; Xu, D. Impact of Ligilactobacillus salivarius Li01 on benzo[a]pyrene-induced colitis, based on host-microbiome interactions in Mongolian gerbils. Front. Nutr. 2025, 12, 1494525. [Google Scholar] [CrossRef]
- Shen, F.; Wang, Q.; Ullah, S.; Pan, Y.; Zhao, M.; Wang, J.; Chen, M.; Feng, F.; Zhong, H. Ligilactobacillus acidipiscis YJ5 modulates the gut microbiota and produces beneficial metabolites to relieve constipation by enhancing the mucosal barrier. Food Funct. 2024, 15, 310–325. [Google Scholar] [CrossRef]
- Jia, D.-J.-C.; Qi-Wen, W.; Ying-Ying, H.; Jia-Min, H.; Qi-Wei, G.; Ya-Dong, Q.; Lu-Yi, C.; Ying, Z.; Li-Na, F.; Yi-Feng, L.; et al. Lactobacillus johnsonii alleviates colitis by TLR1/2-STAT3 mediated CD206+ macrophagesIL-10 activation. Gut Microbes 2022, 14, 2145843. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Nie, Q.; Sun, Y.; Zuo, S.; Chen, C.; Li, S.; Yang, J.; Hu, J.; Zhou, X.; Yu, Y.; et al. Bacteroides uniformis degrades β-glucan to promote Lactobacillus johnsonii improving indole-3-lactic acid levels in alleviating colitis. Microbiome 2024, 12, 177. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.Y.; Giraud, A.; Seignez, C.; Ahl, D.; Guo, F.; Sedin, J.; Walden, T.; Oh, J.H.; van Pijkeren, J.P.; Holm, L.; et al. Distinct B cell subsets in Peyer’s patches convey probiotic effects by Limosilactobacillus reuteri. Microbiome 2021, 9, 198. [Google Scholar] [CrossRef]
- Yue, N.; Zhao, H.; Hu, P.; Zhang, Y.; Tian, C.; Kong, C.; Mai, Z.; Huang, L.; Luo, Q.; Wei, D.; et al. Real-world of Limosilactobacillus reuteri in mitigation of acute experimental colitis. J. Nanobiotechnol. 2025, 23, 65. [Google Scholar] [CrossRef]
- Si, W.; Liang, H.; Bugno, J.; Xu, Q.; Ding, X.; Yang, K.; Fu, Y.; Weichselbaum, R.R.; Zhao, X.; Wang, L. Lactobacillus rhamnosus GG induces cGAS/STING- dependent type I interferon and improves response to immune checkpoint blockade. Gut 2022, 71, 521–533. [Google Scholar] [CrossRef]
- Wang, R.X.; Lee, J.S.; Campbell, E.L.; Colgan, S.P. Microbiota-derived butyrate dynamically regulates intestinal homeostasis through regulation of actin-associated protein synaptopodin. Proc. Natl. Acad. Sci. USA 2020, 117, 11648–11657. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.M.; Desai, C.; Darby, T.M.; Luo, L.; Wolfarth, A.A.; Scharer, C.D.; Ardita, C.S.; Reedy, A.R.; Keebaugh, E.S.; Neish, A.S. Lactobacilli Modulate Epithelial Cytoprotection through the Nrf2 Pathway. Cell Rep. 2015, 12, 1217–1225. [Google Scholar] [CrossRef]
- Wu, L.; Xie, X.; Li, Y.; Liang, T.; Zhong, H.; Yang, L.; Xi, Y.; Zhang, J.; Ding, Y.; Wu, Q. Gut microbiota as an antioxidant system in centenarians associated with high antioxidant activities of gut-resident Lactobacillus. npj Biofilms Microbiomes 2022, 8, 102. [Google Scholar] [CrossRef] [PubMed]
- Dinica, R.M.; Sandu, C.; Dediu Botezatu, A.V.; Cazanevscaia Busuioc, A.; Balanescu, F.; Ionica Mihaila, M.D.; Dumitru, C.N.; Furdui, B.; Iancu, A.V. Allantoin from Valuable Romanian Animal and Plant Sources with Promising Anti-Inflammatory Activity as a Nutricosmetic Ingredient. Sustainability 2021, 13, 10170. [Google Scholar] [CrossRef]
- Mizunoe, Y.; Kobayashi, M.; Sudo, Y.; Watanabe, S.; Yasukawa, H.; Natori, D.; Hoshino, A.; Negishi, A.; Okita, N.; Komatsu, M.; et al. Trehalose protects against oxidative stress by regulating the Keap1-Nrf2 and autophagy pathways. Redox Biol. 2018, 15, 115–124. [Google Scholar] [CrossRef]
- Liang, H.; Xu, P.; Xu, G.; Zhang, L.; Huang, D.; Ren, M.; Zhang, L. Histidine Deficiency Inhibits Intestinal Antioxidant Capacity and Induces Intestinal Endoplasmic-Reticulum Stress, Inflammatory Response, Apoptosis, and Necroptosis in Largemouth Bass (Micropterus salmoides). Antioxidants 2022, 11, 2399. [Google Scholar] [CrossRef]
- Polis, B.; Srikanth, K.D.; Elliott, E.; Gil-Henn, H.; Samson, A.O. L-Norvaline Reverses Cognitive Decline and Synaptic Loss in a Murine Model of Alzheimer’s Disease. Neurotherapeutics 2018, 15, 1036–1054. [Google Scholar] [CrossRef]
- Feng, L.; Li, W.; Liu, Y.; Jiang, W.D.; Kuang, S.Y.; Jiang, J.; Tang, L.; Wu, P.; Tang, W.N.; Zhang, Y.A.; et al. Dietary phenylalanine-improved intestinal barrier health in young grass carp (Ctenopharyngodon idella) is associated with increased immune status and regulated gene expression of cytokines, tight junction proteins, antioxidant enzymes and related signalling molecules. Fish Shellfish Immunol. 2015, 45, 495–509. [Google Scholar] [CrossRef]
- Meyer, K.F.; Martins, J.L.; de Freitas Filho, L.G.; Oliva, M.L.; Patrício, F.R.; Macedo, M.; Wang, L. Glycine reduces tissue lipid peroxidation in hypoxia-reoxygenation-induced necrotizing enterocolitis in rats. Acta Cir. Bras. 2006, 21, 161–167. [Google Scholar] [CrossRef]
- Stoffels, B.; Türler, A.; Schmidt, J.; Nazir, A.; Tsukamoto, T.; Moore, B.A.; Schnurr, C.; Kalff, J.C.; Bauer, A.J. Anti-inflammatory role of glycine in reducing rodent postoperative inflammatory ileus. Neurogastroenterol. Motil. 2011, 23, 76–87. [Google Scholar] [CrossRef]
- Seo, S.K.; Kwon, B. Immune regulation through tryptophan metabolism. Exp. Mol. Med. 2023, 55, 1371–1379. [Google Scholar] [CrossRef]
- Mohamed, G.A.E.; Ali, N.I.; Torra, D.; Amen, O.A. The effect of betaine on broilers infected experimentally with Clostridium perfringens. SVU-Int. J. Vet. Sci. 2022, 5, 174–192. [Google Scholar] [CrossRef]
- Zhao, N.; Yang, Y.; Chen, C.; Jing, T.; Hu, Y.; Xu, H.; Wang, S.; He, Y.; Liu, E.; Cui, J. Betaine supplementation alleviates dextran sulfate sodium-induced colitis via regulating the inflammatory response, enhancing the intestinal barrier, and altering gut microbiota. Food Funct. 2022, 13, 12814–12826. [Google Scholar] [CrossRef]
- Singh, S.K.; Kaldate, R.; Bisht, A. Chapter4.5–Citric acid, antioxidant effects in health. In Antioxidants Effects in Health; Nabavi, S.M., Silva, A.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 309–322. [Google Scholar] [CrossRef]
- Kim, J.; Jo, Y.; Lim, G.; Ji, Y.; Roh, J.H.; Kim, W.G.; Yi, H.S.; Choi, D.W.; Cho, D.; Ryu, D. A microbiota-derived metabolite, 3-phenyllactic acid, prolongs healthspan by enhancing mitochondrial function and stress resilience via SKN-1/ATFS-1 in C. elegans. Nat. Commun. 2024, 15, 10773. [Google Scholar] [CrossRef]
- Wang, C.; Yang, Y.; Gao, N.; Lan, J.; Dou, X.; Li, J.; Shan, A. L-Threonine upregulates the expression of β-defensins by activating the NF-κB signaling pathway and suppressing SIRT1 expression in porcine intestinal epithelial cells. Food Funct. 2021, 12, 5821–5836. [Google Scholar] [CrossRef]
- Liu, R.; Tang, R.; Li, Y.; Zhong, Q.; Cao, Y.; Yang, Q. A novel function of benzoic acid to enhance intestinal barrier defense against PEDV infection in Piglets. Vet. Microbiol. 2024, 295, 110152. [Google Scholar] [CrossRef] [PubMed]
- Skupsky, J.; Sabui, S.; Hwang, M.; Nakasaki, M.; Cahalan, M.D.; Said, H.M. Biotin Supplementation Ameliorates Murine Colitis by Preventing NF-κB Activation. Cell. Mol. Gastroenterol. Hepatol. 2020, 9, 557–567. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhang, Y.; He, L.; Wan, D.; Liu, G.; Wu, X.; Yin, Y. Serine prevents LPS-induced intestinal inflammation and barrier damage via p53-dependent glutathione synthesis and AMPK activation. J. Funct. Foods 2017, 39, 225–232. [Google Scholar] [CrossRef]
- Dong, L.; Xie, J.; Wang, Y.; Jiang, H.; Chen, K.; Li, D.; Wang, J.; Liu, Y.; He, J.; Zhou, J.; et al. Mannose ameliorates experimental colitis by protecting intestinal barrier integrity. Nat. Commun. 2022, 13, 4804. [Google Scholar] [CrossRef]
- Moon, S.-M.; Lee, S.A.; Hong, J.H.; Kim, J.-S.; Kim, D.K.; Kim, C.S. Oleamide suppresses inflammatory responses in LPS-induced RAW264.7 murine macrophages and alleviates paw edema in a carrageenan-induced inflammatory rat model. Int. Immunopharmacol. 2018, 56, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Deters, B.J.; Saleem, M. The role of glutamine in supporting gut health and neuropsychiatric factors. Food Sci. Hum. Wellness 2021, 10, 149–154. [Google Scholar] [CrossRef]
- Zheng, J.; Zhang, J.; Zhou, Y.; Zhang, D.; Guo, H.; Li, B.; Cui, S. Taurine Alleviates Experimental Colitis by Enhancing Intestinal Barrier Function and Inhibiting Inflammatory Response through TLR4/NF-κB Signaling. J. Agric. Food Chem. 2024, 72, 12119–12129. [Google Scholar] [CrossRef]
- Xiang, S.; Xiao, J. Protective effects of syringic acid on inflammation, apoptosis and intestinal barrier function in Caco-2 cells following oxygen-glucose deprivation/reoxygenation-induced injury. Exp. Ther. Med. 2022, 23, 66. [Google Scholar] [CrossRef]
- Bao, L.; Sun, H.; Zhao, Y.; Feng, L.; Wu, K.; Shang, S.; Xu, J.; Shan, R.; Duan, S.; Qiu, M.; et al. Hexadecanamide alleviates Staphylococcus aureus-induced mastitis in mice by inhibiting inflammatory responses and restoring blood-milk barrier integrity. PLoS Pathog. 2023, 19, e1011764. [Google Scholar] [CrossRef]
- Ercan, G.; Yigitturk, G.; Erbas, O. Therapeutic effect of adenosine on experimentally induced acute ulcerative colitis model in rats. Acta Cir. Bras. 2020, 34, e201901204. [Google Scholar] [CrossRef]
- Ghasemi-Dehnoo, M.; Lorigooini, Z.; Amini-Khoei, H.; Sabzevary-Ghahfarokhi, M.; Rafieian-Kopaei, M. Quinic acid ameliorates ulcerative colitis in rats, through the inhibition of two TLR4-NF-κB and NF-κB-INOS-NO signaling pathways. Immun. Inflamm. Dis. 2023, 11, e926. [Google Scholar] [CrossRef]
- Wan, Q.L.; Fu, X.; Meng, X.; Luo, Z.; Dai, W.; Yang, J.; Wang, C.; Wang, H.; Zhou, Q. Hypotaurine promotes longevity and stress tolerance via the stress response factors DAF-16/FOXO and SKN-1/NRF2 in Caenorhabditis elegans. Food Funct. 2020, 11, 347–357. [Google Scholar] [CrossRef]
- Shang, Y.; Zhang, Z.; Tian, J.; Li, X. Anti-Inflammatory Effects of Natural Products on Cerebral Ischemia. Front. Pharmacol. 2022, 13, 914630. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Shao, D.; Wu, S.; Song, Z.; Shi, S. Heat stress-induced intestinal barrier damage and dimethylglycine alleviates via improving the metabolism function of microbiota gut brain axis. Ecotoxicol. Environ. Saf. 2022, 244, 114053. [Google Scholar] [CrossRef]
- Chowdhury, A.A.; Gawali, N.B.; Munshi, R.; Juvekar, A.R. Trigonelline insulates against oxidative stress, proinflammatory cytokines and restores BDNF levels in lipopolysaccharide induced cognitive impairment in adult mice. Metab. Brain Dis. 2018, 33, 681–691. [Google Scholar] [CrossRef] [PubMed]
- Kohen, R.; Yamamoto, Y.; Cundy, K.C.; Ames, B.N. Antioxidant activity of carnosine, homocarnosine, and anserine present in muscle and brain. Proc. Natl. Acad. Sci. USA 1988, 85, 3175–3179. [Google Scholar] [CrossRef] [PubMed]
- Madaan, P.; Sikka, P.; Malik, D.S. Cosmeceutical Aptitudes of Niacinamide: A Review. Recent Adv. Anti-Infect. Drug Discov. 2021, 16, 196–208. [Google Scholar] [CrossRef]
- Jadhav, R.V.; Redasani, V.; Kalbhare, S.B.; Yadav, K.; Langeh, A.; Tidke, A. Evaluation of anti-ulcer activity of 4-hydrooxy benzalydehide against NSAIDs induced ulcers in rats. Int. J. Res. Med. Sci. 2021, 9, 1412. [Google Scholar] [CrossRef]
- Bubak, M.P.; Mann, S.N.; Borowik, A.K.; Pranay, A.; Batushansky, A.; Vieira de Sousa Neto, I.; Mondal, S.A.; Doidge, S.M.; Davidyan, A.; Szczygiel, M.M.; et al. 17α-Estradiol alleviates high-fat diet-induced inflammatory and metabolic dysfunction in skeletal muscle of male and female mice. Am. J. physiology. Endocrinol. Metab. 2024, 326, E226–E244. [Google Scholar] [CrossRef]
- Gabrielsson, L.; Gouveia-Figueira, S.; Häggström, J.; Alhouayek, M.; Fowler, C.J. The anti-inflammatory compound palmitoylethanolamide inhibits prostaglandin and hydroxyeicosatetraenoic acid production by a macrophage cell line. Pharmacol. Res. Perspect. 2017, 5, e00300. [Google Scholar] [CrossRef] [PubMed]





| Compounds | Class | Chemical Formula | Content (ng/mL) | |
|---|---|---|---|---|
| 1 | 2-thiocytidine | Nucleotide and its metabolomics | C9H13N3O4S | 39,470.82 |
| 2 | Uridine | Nucleotide and its metabolomics | C9H12N2O6 | 11,549.76 |
| 3 | UMP | Nucleotide and its metabolomics | C9H13N2O9P | 4668.834 |
| 4 | Cytidine | Nucleotide and its metabolomics | C9H13N3O5 | 3045.108 |
| 5 | Uracil | Nucleotide and its metabolomics | C4H4N2O2 | 2854.398 |
| 6 | Guanosine | Nucleotide and its metabolomics | C10H13N5O5 | 2787.54 |
| 7 | Xanthosine | Nucleotide and its metabolomics | C10H12N4O6 | 2771.946 |
| 8 | Inosine | Nucleotide and its metabolomics | C10H12N4O5 | 2457.132 |
| 9 | 5-carboxycytidine | Nucleotide and its metabolomics | C10H13N3O7 | 2209.53 |
| 10 | 5′-deoxy-5′-methylthioadensine | Nucleotide and its metabolomics | C11H15N5O3S | 2207.274 |
| 11 | N4-acetyl-2′-O-methylcytidine | Nucleotide and its metabolomics | C12H17N3O6 | 1948.782 |
| 12 | N6-threonylcarbamoyladenosine | Nucleotide and its metabolomics | C15H20N6O8 | 1913.694 |
| 13 | 5′-cytidylic acid | Nucleotide and its metabolomics | C9H14N3O8P | 1047.882 |
| 14 | IMP | Nucleotide and its metabolomics | C10H13N4O8P | 1018.416 |
| 15 | Adenosine | Nucleotide and its metabolomics | C10H13N5O4 | 805.098 |
| 16 | AMP | Nucleotide and its metabolomics | C10H14N5O7P | 663.306 |
| 17 | Pseudouridine | Nucleotide and its metabolomics | C9H12N2O6 | 598.8162 |
| 18 | 2′-O-methylguanosine | Nucleotide and its metabolomics | C11H15N5O5 | 561.0024 |
| 19 | 2′-deoxycytidine | Nucleotide and its metabolomics | C9H13N3O4 | 318.0354 |
| 20 | dCMP | Nucleotide and its metabolomics | C9H14N3O7P | 301.2276 |
| 21 | Guanine | Nucleotide and its metabolomics | C5H5N5O | 299.766 |
| 22 | 2′-O-methyluridine | Nucleotide and its metabolomics | C10H14N2O6 | 254.6922 |
| 23 | N,N-dimethylguanosine | Nucleotide and its metabolomics | C12H17N5O5 | 150.2892 |
| 24 | 5-hydroxymethylcytidine | Nucleotide and its metabolomics | C10H15N3O6 | 131.3736 |
| 25 | Cytosine | Nucleotide and its metabolomics | C4H5N3O | 101.3124 |
| 26 | 1-methylhistamine | Nucleotide and its metabolomics | C6H13Cl2N3 | 79.2288 |
| 27 | N2,N2,7-trimethylguanosine | Nucleotide and its metabolomics | C13H19N5O5 | 75.4542 |
| 28 | 5-hydroxymethyluracil | Nucleotide and its metabolomics | C5H6N2O3 | 59.51946 |
| 29 | S-adenosyl-L-methioninate | Nucleotide and its metabolomics | C15H22N6O5S | 58.54818 |
| 30 | dTMP | Nucleotide and its metabolomics | C10H15N2O8P | 54.00894 |
| 31 | N6-methyladenosine | Nucleotide and its metabolomics | C11H15N5O4 | 49.44744 |
| 32 | 1-methyladenosine | Nucleotide and its metabolomics | C11H15N5O4 | 47.67612 |
| 33 | 7-methylguanosine | Nucleotide and its metabolomics | C11H15N5O5 | 30.29154 |
| 34 | 1-methylguanosine | Nucleotide and its metabolomics | C11H15N5O5 | 18.14586 |
| 35 | N6, N6-dimethyladenosine | Nucleotide and its metabolomics | C12H17N5O4 | 17.21268 |
| 36 | 1-methylinosine | Nucleotide and its metabolomics | C11H14N4O5 | 2.482842 |
| 37 | Adenine | Nucleotide and its metabolomics | C5H5N5 | 1.93587 |
| 38 | Histidine | Amino acids | C6H9N3O2 | 85,330.8 |
| 39 | L-norvaline | Amino acids | C5H11NO2 | 85,231.8 |
| 40 | Phenylalanine | Amino acids | C9H11NO2 | 81,640.2 |
| 41 | Sarcosine | Amino acids | C3H7NO2 | 77,844 |
| 42 | Tyrosine | Amino acids | C9H11NO3 | 77,773.2 |
| 43 | L-proline | Amino acids | C5H9NO2 | 73,215 |
| 44 | L-methionine | Amino acids | C5H11NO2S | 73,150.8 |
| 45 | Glycine | Amino acids | C2H5NO2 | 71,337.6 |
| 46 | Tryptophan | Amino acids | C11H12N2O2 | 61,059 |
| 47 | L-leucine | Amino acids | C6H13NO2 | 59,032.8 |
| 48 | L-isoleucine | Amino acids | C6H13NO2 | 49,271.58 |
| 49 | Arginine | Amino acids | C6H14N4O2 | 19,382.1 |
| 50 | Lysine | Amino acids | C6H14N2O2 | 13,724.82 |
| 51 | Threonine | Amino acids | C4H9NO3 | 11,059.86 |
| 52 | L-aspartate | Amino acids | C4H7NO4 | 10,520.7 |
| 53 | Serine | Amino acids | C3H7NO3 | 7868.04 |
| 54 | L-homocystine | Amino acids | C8H16N2O4S2 | 2382.066 |
| 55 | L-homocitrulline | Amino acids | C7H15N3O3 | 1522.74 |
| 56 | Homoserine | Amino acids | C4H9NO3 | 1503.816 |
| 57 | Ornithine | Amino acids | C5H12N2O2 | 1352.352 |
| 58 | L-asparagine | Amino acids | C4H8N2O3 | 1192.662 |
| 59 | L-citrulline | Amino acids | C6H13N3O3 | 1018.074 |
| 60 | H-Lys(Me)-OH chloride hydrochloride salt | Amino acids | C9H21ClN2O2 | 761.61 |
| 61 | Homo-L-arginine | Amino acids | C7H16N4O2 | 331.8738 |
| 62 | Allantoin | Organic acid and its derivatives | C4H6N4O3 | 99,550.2 |
| 63 | Creatinine | Organic acid and its derivatives | C4H7N3O | 61,650.6 |
| 64 | 4-aminobutyric acid | Organic acid and its derivatives | C4H9NO2 | 59,846.52 |
| 65 | Hydroxyphenyllactic acid | Organic acid and its derivatives | C9H10O4 | 33,299.94 |
| 66 | Creatine | Organic acid and its derivatives | C4H9N3O2 | 24,808.56 |
| 67 | 3-hydroxyisovaleric acid | Organic acid and its derivatives | C5H10O3 | 16,237.26 |
| 68 | N-formylkynurenine | Organic acid and its derivatives | C11H12N2O4 | 13,108.98 |
| 69 | Citric acid | Organic acid and its derivatives | C6H8O7 | 12,422.58 |
| 70 | 3-phenyllactic acid | Organic acid and its derivatives | C9H10O3 | 11,733.18 |
| 71 | 3-hydroxybutyric acid | Organic acid and its derivatives | C4H8O3 | 6365.04 |
| 72 | 2-hydroxyisovaleric acid | Organic acid and its derivatives | C5H10O3 | 6087.54 |
| 73 | Potassium phenyl sulfate | Organic acid and its derivatives | C6H5KO4S | 3206.172 |
| 74 | Sebacic acid | Organic acid and its derivatives | C10H18O4 | 2042.904 |
| 75 | Salicylic acid | Organic acid and its derivatives | C7H6O3 | 1362.336 |
| 76 | Urocanic acid | Organic acid and its derivatives | C6H6N2O2 | 1306.572 |
| 77 | Alpha-aminoadipic acid | Organic acid and its derivatives | C6H11NO4 | 848.568 |
| 78 | Quinic acid | Organic acid and its derivatives | C7H12O6 | 782.604 |
| 79 | Guanidinoacetic acid | Organic acid and its derivatives | C3H7N3O2 | 487.7532 |
| 80 | Stachydrine hydrochloride | Organic acid and its derivatives | C7H14NO2 | 287.0406 |
| 81 | 2,4-Hexadienoic acid | Organic acid and its derivatives | C6H8O2 | 157.9614 |
| 82 | Sumiki’s acid | Organic acid and its derivatives | C6H6O4 | 58.19304 |
| 83 | Acetoacetic acid sodium salt | Organic acid and its derivatives | C4H5NaO3 | 56.4012 |
| 84 | N-acetylputrescine | Organic acid and its derivatives | C6H14N2O | 7.49262 |
| 85 | Cyclamic acid | Organic acid and its derivatives | C6H13NO3S | 0.3217764 |
| 86 | Trans-4-hydroxy-L-proline | Amino acid derivatives | C5H9NO3 | 15,543.54 |
| 87 | Glutamine | Amino acid derivatives | C5H10N2O3 | 3402.09 |
| 88 | Aceglutamide | Amino acid derivatives | C7H12N2O4 | 2753.85 |
| 89 | 3-hydroxymethylglutaric acid | Amino acid derivatives | C6H10O5 | 1395.51 |
| 90 | Methionine sulfoxide | Amino acid derivatives | C5H11NO3S | 936.9 |
| 91 | 2-aminoisobutyric acid | Amino acid derivatives | C4H9NO2 | 569.7132 |
| 92 | Kynurenic acid | Amino acid derivatives | C10H7NO3 | 538.8558 |
| 93 | N-A-acetyl-L-arginine | Amino acid derivatives | C8H16N4O3 | 253.0788 |
| 94 | Dimethylglycine | Amino acid derivatives | C4H9NO2 | 196.1832 |
| 95 | N6-acetyl-L-lysine | Amino acid derivatives | C8H16N2O3 | 176.6814 |
| 96 | L-cystathionine | Amino acid derivatives | C7H14N2O4S | 137.0454 |
| 97 | N-acetyl-L-tyrosine | Amino acid derivatives | C11H13NO4 | 130.8588 |
| 98 | 3-NmMethyl-L-histidine | Amino acid derivatives | C7H11N3O2 | 60.3822 |
| 99 | S-(5-adenosy)-L-homocysteine | Amino acid derivatives | C14H20N6O5S | 43.86558 |
| 100 | 3-hydroxyhippuric acid | Amino acid derivatives | C9H9NO4 | 38.94438 |
| 101 | 5-hydroxylysine | Amino acid derivatives | C6H14N2O3 | 37.97568 |
| 102 | L-tyrosine methyl ester | Amino acid derivatives | C10H13NO3 | 9.99594 |
| 103 | Xanthurenic acid | Amino acid derivatives | C10H7NO4 | 0.859944 |
| 104 | Phe Met | Small peptide | C14H20N2O3S | 31,754.34 |
| 105 | Gamma-Glu-Met | Small peptide | C10H18N2O5S | 8757.42 |
| 106 | H-Glu(Leu-OH)-OH | Small peptide | C11H20N2O5 | 2768.154 |
| 107 | L-alpha-aspartyl-L-phenylalanine | Small peptide | C13H16N2O5 | 1252.206 |
| 108 | Gamma-glutamylalanine | Small peptide | C8H14N2O5 | 1129.818 |
| 109 | Prolylhydroxyproline | Small peptide | C10H16N2O4 | 704.256 |
| 110 | Glycyl-L-Proline | Small peptide | C7H12N2O3 | 647.298 |
| 111 | Gamma-Glu-Phe | Small peptide | C14H18N2O5 | 179.1786 |
| 112 | L-2-phenylglycine | Small peptide | C8H9NO2 | 120.2412 |
| 113 | D-alanyl-D-alanine | Small peptide | C6H12N2O3 | 66.7956 |
| 114 | Anserine | Small peptide | C10H16N4O3 | 59.87802 |
| 115 | Leu-Ala | Small peptide | C9H18N2O3 | 57.22938 |
| 116 | Phe Val | Small peptide | C14H20N2O3 | 1.918536 |
| 117 | Biotin | Coenzymes and vitamins | C10H16N2O3S | 10,261.5 |
| 118 | Nadide | Coenzymes and vitamins | C21H27N7O14P2 | 9476.7 |
| 119 | Pyridoxal hydrochloride | Coenzymes and vitamins | C8H10ClNO3 | 2699.274 |
| 120 | Riboflavin sodium phosphate | Coenzymes and vitamins | C17H20N4NaO9P | 389.1276 |
| 121 | vitamin B1 | Coenzymes and vitamins | C12H17ClN4OS | 362.4606 |
| 122 | Flavin mononucleotide | Coenzymes and vitamins | C17H21N4O9P | 230.5356 |
| 123 | Trigonelline | Coenzymes and vitamins | C7H7NO2 | 150.8532 |
| 124 | Dethiobiotin | Coenzymes and vitamins | C10H18N2O3 | 142.0878 |
| 125 | Pyridoxamine dichlorohydrate | Coenzymes and vitamins | C8H14Cl2N2O2 | 54.82812 |
| 126 | Pantothenol | Coenzymes and vitamins | C9H19NO4 | 49.37106 |
| 127 | Niacinamide | Coenzymes and vitamins | C6H6N2O | 28.4085 |
| 128 | Acetyl-carnitine | CAR | C9H17NO4 | 7657.56 |
| 129 | 2-methylbutyroylcarnitine | CAR | C12H23NO4 | 7291.62 |
| 130 | DL-carnitine | CAR | C7H15NO3 | 6279.66 |
| 131 | Isovaleryl-carnitine | CAR | C12H23NO4 | 5110.812 |
| 132 | Isobutyryl-L-carnitine | CAR | C11H21NO4 | 1932.33 |
| 133 | L-palmitoylcarnitine | CAR | C23H46NO4 | 8.33856 |
| 134 | Octanoyl-carnitine | CAR | C15H29NO4 | 4.018992 |
| 135 | Myristoyl-L-carnitine | CAR | C21H42ClNO4 | 1.626294 |
| 136 | Dodecanoylcarnitine | CAR | C19H38NO4 | 0.2799738 |
| 137 | Decanoyl-carnitine | CAR | C17H33NO4 | 0.135042 |
| 138 | Urea | Amines | CH4N2O | 44,732.22 |
| 139 | Trimethylamine N-oxide | Amines | C3H9NO | 6001.5 |
| 140 | Oleamide | Amines | C18H35NO | 4558.662 |
| 141 | Hexadecanamide | Amines | C16H33NO | 946.986 |
| 142 | 5-methoxytryptamine | Amines | C11H14N2O | 479.9064 |
| 143 | 3-methyl-1-butylamine | Amines | C5H13N | 51.13974 |
| 144 | Benzamide | Amines | C7H7NO | 7.99776 |
| 145 | N-cyclohexylformamide | Amines | C7H13NO | 2.7777 |
| 146 | 1-monopalmitoylglycerol | FFA | C19H38O4 | 51,437.4 |
| 147 | Apigenic acid | FFA | C18H34O2 | 8156.88 |
| 148 | Transvaccenic acid (C18:1T) | FFA | C18H34O2 | 3299.142 |
| 149 | Octadecanedioate (C18) | FFA | C18H34O4 | 338.8098 |
| 150 | 2-hydroxyhexadecanoic acid | FFA | C16H32O3 | 22.30626 |
| 151 | Benzoic acid | Benzene and substituted derivatives | C7H6O2 | 10,863.42 |
| 152 | Tyramine | Benzene and substituted derivatives | C8H11NO | 625.092 |
| 153 | 2-phenylacetamide | Benzene and substituted derivatives | C8H9NO | 25.30776 |
| 154 | Phenylethylamine | Benzene and substituted derivatives | C8H11N | 25.27692 |
| 155 | 4-hydroxybenzaldehyde | Benzene and substituted derivatives | C7H6O2 | 24.20568 |
| 156 | Cholic acid | Bile acids | C24H40O5 | 256.8258 |
| 157 | Taurocholic acid | Bile acids | C26H45NO7S | 1.507548 |
| 158 | Glycocholic acid | Bile acids | C26H43NO6 | 1.366428 |
| 159 | Lumichrome | Heterocyclic compounds | C12H10N4O2 | 293.6082 |
| 160 | 2-aminobenzoic acid | Phenolic acids | C7H7NO2 | 267.9906 |
| 162 | 2-piperidone | Heterocyclic compounds | C5H9NO | 247.3776 |
| 163 | Biopterin | Pteridines and derivatives | C9H11N5O3 | 59.94504 |
| 164 | 4-hydroxybenzoic acid | Phenolic acids | C7H6O3 | 58.10418 |
| 165 | Betaine aldehyde chloride | Others | C5H12ClNO | 45.2907 |
| 166 | Pyrrole-2-carboxylic acid | Heterocyclic compounds | C5H5NO2 | 20.29596 |
| 167 | Dihydrotestosterone | Hormones and hormone-related compounds | C19H30O2 | 19.09512 |
| 168 | Indole-3-carboxaldehyde | Indole and its derivatives | C9H7NO | 18.5718 |
| 169 | 17a-Estradiol | Hormones and hormone-related compounds | C18H24O2 | 7.79946 |
| 170 | Palmitoyl ethanolamide | LPE | C18H37NO2 | 6.8454 |
| 171 | 1-octadecylglycero-3-phosphocholine | Others | C26H56NO6P | 5.111292 |
| 172 | 1-palmitoyl-sn-glycero-3-phosphocholine | LPC | C24H50NO7P | 4.477656 |
| 173 | Quinoline-2-carboxylic acid | Pteridines and derivatives | C10H7NO2 | 1.756626 |
| 174 | Taurocholic acid | Bile acids | C26H45NO7S | 1.507548 |
| 175 | Glycocholic acid | Bile acids | C26H43NO6 | 1.366428 |
| 176 | 1-hexadecanoyl-sn-glycero-3-phosphoethanolamine | LPE | C21H44NO7P | 0.737412 |
| 177 | L-pipecolic Acid | Heterocyclic compounds | C6H11NO2 | 1895.538 |
| 178 | Acetamide | Carboximidic acids | CH3CONH2 | 41,799.18 |
| 179 | NADH | Dinucleotides | C21H29N7O14P2 | 22,004.58 |
| 180 | Oleoylethanolamide | Fatty acyl | C20H39NO2 | 0.2143722 |
| 181 | DL-mevalonic acid lactone | Esters | C6H10O3 | 1863.324 |
| 182 | Indolelactic acid | Indole and its derivatives | C11H11NO3 | 3846.162 |
| 183 | D-glucose 6-phosphate | Phosphate sugars | C6H13O9P | 3526.254 |
| 184 | Taurine | Sulfonic acids | C2H7NO3S | 2789.094 |
| 185 | 1,5-anhydro-D-glucitol | Sugar alcohols | C6H12O5 | 1732.818 |
| 186 | Tryptophol | Indole and its derivatives | C10H11NO | 438.7266 |
| 187 | 2-picolinic acid | Pteridines and derivatives | C6H5NO2 | 805.35 |
| 188 | Hypotaurine | Sulfonic acids | C2H7NO2S | 652.476 |
| 189 | Phosphorylethanolamine | LPE | C2H8NO4P | 543.3204 |
| 190 | dUMP | Nucleotide metabolomics | C9H13N2O8P | 807.042 |
| 191 | Glycerol 3-phosphate | Phosphoric acids | C3H9O6P | 87,501.6 |
| 192 | D-erythrose 4-phosphate | Phosphate sugars | C4H9O7P | 75,813 |
| 193 | Xyluose-5-phosphate | Phosphate sugars | C5H11O8P | 14,043.66 |
| 194 | Ethanolamine | Polyamines | C2H7NO | 11,848.26 |
| 195 | 2-methylbutanoic acid | Fatty acids | C5H10O2 | 11,774.04 |
| 196 | 2-(formylamino)benzoic acid | Phenolic acids | C8H7NO3 | 11,033.76 |
| 197 | Mannose | Sugars | C6H12O6 | 6619.68 |
| 198 | 2-amino-2-deoxymannose | Sugars | C6H13NO5 | 4629.444 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, J.; Sun, Y.; Gao, K.; Zhao, H.; Su, Y.; Zhao, Y.; Zhang, Y.; Jiang, Y. Lacticaseibacillus paracasei JY062 Postbiotic Alleviated 3% DSS-Induced Colitis in Mice via Integrated Antioxidant, Barrier Repair, Immunomodulatory and Microbiota Modulation. Antioxidants 2025, 14, 1256. https://doi.org/10.3390/antiox14101256
Guo J, Sun Y, Gao K, Zhao H, Su Y, Zhao Y, Zhang Y, Jiang Y. Lacticaseibacillus paracasei JY062 Postbiotic Alleviated 3% DSS-Induced Colitis in Mice via Integrated Antioxidant, Barrier Repair, Immunomodulatory and Microbiota Modulation. Antioxidants. 2025; 14(10):1256. https://doi.org/10.3390/antiox14101256
Chicago/Turabian StyleGuo, Jinfeng, Yilin Sun, Kaiqi Gao, Haijie Zhao, Yue Su, Ying Zhao, Yu Zhang, and Yujun Jiang. 2025. "Lacticaseibacillus paracasei JY062 Postbiotic Alleviated 3% DSS-Induced Colitis in Mice via Integrated Antioxidant, Barrier Repair, Immunomodulatory and Microbiota Modulation" Antioxidants 14, no. 10: 1256. https://doi.org/10.3390/antiox14101256
APA StyleGuo, J., Sun, Y., Gao, K., Zhao, H., Su, Y., Zhao, Y., Zhang, Y., & Jiang, Y. (2025). Lacticaseibacillus paracasei JY062 Postbiotic Alleviated 3% DSS-Induced Colitis in Mice via Integrated Antioxidant, Barrier Repair, Immunomodulatory and Microbiota Modulation. Antioxidants, 14(10), 1256. https://doi.org/10.3390/antiox14101256






