Spray-Dried Multiple Emulsions as Co-Delivery Systems for Chlorogenic Acid and Curcumin
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Multiple Emulsions (MEs)
2.3. Characterization of MEs
2.3.1. Oil Droplet Size and Size Distribution
2.3.2. Encapsulation Efficiency
2.3.3. Microstructure
2.4. Formulation of Microparticle Systems
2.5. Characterization of Microparticle Systems
2.5.1. Encapsulation Efficiency of Linseed Oil
2.5.2. Encapsulation Efficiency of Chlorogenic Acid and Curcumin
2.5.3. Oxidative Stability
2.5.4. Particle Size and Morphology
2.5.5. Moisture Content
2.5.6. Droplet Size and Size Distribution of Rehydrated Microparticles
2.6. Evaluation of Multiple Emulsions and Spray-Dried Multiple Emulsions Under Simulated Gastrointestinal Digestion Conditions
2.7. Statistical Analysis
3. Results
3.1. Microstructure, Droplet Size, and Encapsulation Efficiency of Multiple Emulsions
3.2. Formulation of Microparticle Systems
| Run | LO:Capsul® Ratio | Inlet Air Temperature (°C) | EE of LO (%) |
|---|---|---|---|
| 1 | 1:3.02 | 150 | 87.2 ± 0.38 |
| 2 | 1:6.28 | 150 | 93.6 ± 0.17 |
| 3 | 1:6 | 180 | 94.0 ± 0.80 |
| 4 | 1:3.3 | 180 | 91.8 ± 0.79 |
| 5 | 1:4.65 | 113.7 | 93.9 ± 0.77 |
| 6 | 1:3.3 | 120 | 86.6 ± 0.05 |
| 7 | 1:6 | 120 | 94.9 ± 0.92 |
| 8 | 1:4.65 | 186.3 | 95.0 ± 0.02 |
| 9 | 1:4.65 | 150 | 89.8 ± 0.32 |
| 10 | 1:4.65 | 150 | 89.3 ± 0.13 |
| 11 | 1:4.65 | 150 | 89.7 ± 0.66 |
| 12 | 1:4.65 | 150 | 91.2 ± 0.28 |
3.3. Characterization of Microparticle Systems
3.3.1. Encapsulation Efficiency of Linseed Oil
3.3.2. Oxidative Stability
3.3.3. Particle Size and Morphology
3.3.4. Moisture Content
3.3.5. Encapsulation Efficiency of Chlorogenic Acid and Curcumin
3.3.6. Droplet Size and Size Distribution of Rehydrated Microparticles
3.3.7. Evaluation of Multiple Emulsions and Spray-Dried Multiple Emulsions Under Simulated Gastrointestinal Digestion Conditions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| LO | Linseed Oil |
| ME | Multiple Emulsion |
| CGA | Chlorogenic Acid |
| CU | Curcumin |
| EE | Encapsulation Efficiency |
| OSA | Octenyl Succinic Anhydride |
| NaCas | Sodium Caseinate |
| PGPR | Polyglycerol Polyricinoleate |
| W1 | Internal Aqueous Phase (first water phase) |
| W2 | External Aqueous Phase (second water phase) |
| W/O/W | Water-in-Oil-in-Water Emulsion |
| O/W | Oil-in-Water Emulsion |
| ME-C | Multiple Emulsion without bioactives |
| ME-CGA | Multiple Emulsion with CGA in W1 |
| ME-CU | Multiple Emulsion with CU in the oil phase |
| ME-CGA/CU | Multiple Emulsion with CGA in W1 and CU in the oil phase |
| D4,3 | Volume-weighted Mean Diameter |
| UPLC | Ultra Performance Liquid Chromatography |
| UV/VIS | Ultraviolet/Visible Spectroscopy |
| CCD | Central Composite Design |
| RSM | Response Surface Methodology |
| ANOVA | Analysis of Variance |
| MP | Microparticle |
| MP-C | Microparticles without bioactives |
| MP-CGA | Microparticles with CGA in W1 |
| MP-CU | Microparticles with CU in the oil phase |
| MP-CGA/CU | Microparticles with CGA in W1 and CU in the oil phase |
| IP | Induction Period |
| SEM | Scanning Electron Microscopy |
| SSF | Simulated Salivary Fluid |
| SGF | Simulated Gastric Fluid |
References
- Huang, Y.; Chen, H.; Chen, W.; Chen, W.; Zhong, Q.; Pei, J.; Zhang, M.; Haenen, G.R.M.M. Development and Application of Polyphenols in Food: A Comprehensive Review. Food Front. 2025, 6, 1287–1302. [Google Scholar] [CrossRef]
- El-Saadony, M.T.; Yang, T.; Saad, A.M.; Alkafaas, S.S.; Elkafas, S.S.; Eldeeb, G.S.; Mohammed, D.M.; Salem, H.M.; Korma, S.A.; Loutfy, S.A.; et al. Polyphenols: Chemistry, Bioavailability, Bioactivity, Nutritional Aspects and Human Health Benefits: A Review. Int. J. Biol. Macromol. 2024, 277, 134223. [Google Scholar] [CrossRef]
- Wang, Z.; Zhou, D.; Liu, D.; Zhu, B. Food-Grade Encapsulated Polyphenols: Recent Advances as Novel Additives in Foodstuffs. Crit. Rev. Food Sci. Nutr. 2023, 63, 11545–11560. [Google Scholar] [CrossRef]
- Faridi Esfanjani, A.; Assadpour, E.; Jafari, S.M. Improving the Bioavailability of Phenolic Compounds by Loading Them within Lipid-Based Nanocarriers. Trends Food Sci. Technol. 2018, 76, 56–66. [Google Scholar] [CrossRef]
- Cao, H.; Saroglu, O.; Karadag, A.; Diaconeasa, Z.; Zoccatelli, G.; Conte-Junior, C.A.; Gonzalez-Aguilar, G.A.; Ou, J.; Bai, W.; Zamarioli, C.M.; et al. Available Technologies on Improving the Stability of Polyphenols in Food Processing. Food Front. 2021, 2, 109–139. [Google Scholar] [CrossRef]
- McClements, D.J.; Decker, E.A.; Park, Y.; Weiss, J. Structural Design Principles for Delivery of Bioactive Components in Nutraceuticals and Functional Foods. Crit. Rev. Food Sci. Nutr. 2009, 49, 577–606. [Google Scholar] [CrossRef]
- Aditya, N.P.; Aditya, S.; Yang, H.; Kim, H.W.; Park, S.O.; Ko, S. Co-Delivery of Hydrophobic Curcumin and Hydrophilic Catechin by a Water-in-Oil-in-Water Double Emulsion. Food Chem. 2015, 173, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, L.; Abdullah, N.; Tian, W.; Song, M.; Cao, Y.; Xiao, J. Co-Delivery of EGCG and Lycopene via a Pickering Double Emulsion Induced Synergistic Hypolipidemic Effect. Food Funct. 2022, 13, 3419–3430. [Google Scholar] [CrossRef]
- Wang, R.; Ma, C.; Yan, H.; Wang, P.; Yu, S.; Zhang, T.; Yin, Z. Preparation and Characterization of GX-50 and Vitamin C Co-Encapsulated Microcapsules by a Water-in-Oil-in-Water (W1/O/W2) Double Emulsion-Complex Coacervation Method. Langmuir 2023, 39, 13863–13875. [Google Scholar] [CrossRef]
- Zhang, P.; Sun, Y.; Hou, Y.; Wang, H.; Tan, M. Fabrication of Novel W/O/W Emulsion Gels Using Beeswax Stabilized W/O: Preparation, Characterization and Co-Delivery of Phycocyanin and Astaxanthin. Food Biosci. 2024, 57, 103536. [Google Scholar] [CrossRef]
- Zhao, S.; Deng, X.; Wang, Y.; Chen, S.; Liu, X.; Liu, F. Co-Delivery of Hydrophobic β-Carotene and Hydrophilic Riboflavin by Novel Water-in-Oleic Acid-in-Water (W/OA/W) Emulsions. Food Chem. 2024, 432, 137224. [Google Scholar] [CrossRef] [PubMed]
- Ramzan, R.; Muhammad, Z.; Khalid, N.; Xue, Z.; Shi, F.; Sameen, A.; Dong, Y.; Li, S.; Chen, A. Fabricating the Polysaccharides-Based Micro-Particles Co-Encapsulating the Hydrophilic-Hydrophobic Vitamins (B9 and D3) Using Spray-Dried Pickering Double Emulsions: Assessment of Multilayered Microcapsules Structure, Retention Stability, Encapsulation Efficiency, and in Vitro Release. J. Mol. Liq. 2025, 430, 127753. [Google Scholar] [CrossRef]
- He, L.; Hu, S.; Zhang, G.; Wang, X.; Zhao, Y.; Wang, Q.; Liu, M.; Wang, Z.; Sangeeta, P.; Ding, Z. Influence of Polysaccharide-Based Co-Encapsulants on Efficiency, Stability, and Release of Vitamins B12 and D3 in Multilayered Microcapsules. J. Food Eng. 2024, 365, 111817. [Google Scholar] [CrossRef]
- Kumar, A.; Kaur, R.; Kumar, V.; Kumar, S.; Gehlot, R.; Aggarwal, P. New Insights into Water-in-Oil-in-Water (W/O/W) Double Emulsions: Properties, Fabrication, Instability Mechanism, and Food Applications. Trends Food Sci. Technol. 2022, 128, 22–37. [Google Scholar] [CrossRef]
- Paredes-Toledo, J.; Herrera, J.; Díaz-Calderón, P.; Robert, P.; Giménez, B. In Vitro Gastrointestinal Release of Chlorogenic Acid and Curcumin Co-Encapsulated in Double Emulsions with the Outer Interface Stabilized by Cellulose Nanocrystals. Colloids Interfaces 2024, 8, 24. [Google Scholar] [CrossRef]
- Paredes-Toledo, J.; Herrera, J.; Morales, J.; Robert, P.; Oyarzun-Ampuero, F.; Giménez, B. Bioaccessibility of Chlorogenic Acid and Curcumin Co-Encapsulated in Double Emulsions with the Inner Interface Stabilized by Functionalized Silica Nanoparticles. Food Chem. 2024, 445, 138828. [Google Scholar] [CrossRef]
- Berendsen, R.; Güell, C.; Ferrando, M. Spray Dried Double Emulsions Containing Procyanidin-Rich Extracts Produced by Premix Membrane Emulsification: Effect of Interfacial Composition. Food Chem. 2015, 178, 251–258. [Google Scholar] [CrossRef]
- Toledo-Madrid, K.; Gallardo-Velázquez, T.; Osorio-Revilla, G. Microencapsulation of Purple Cactus Pear Fruit (Opuntia ficus indica) Extract by the Combined Method W/O/W Double Emulsion-Spray Drying and Conventional Spray Drying: A Comparative Study. Processes 2018, 6, 189. [Google Scholar] [CrossRef]
- Halahlah, A.; Piironen, V.; Mikkonen, K.S.; Ho, T.M. Polysaccharides as Wall Materials in Spray-Dried Microencapsulation of Bioactive Compounds: Physicochemical Properties and Characterization. Crit. Rev. Food Sci. Nutr. 2023, 63, 6983–7015. [Google Scholar] [CrossRef]
- Safaeian Laein, S.; Samborska, K.; Can Karaca, A.; Mostashari, P.; Akbarbaglu, Z.; Sarabandi, K.; Jafari, S.M. Strategies for Further Stabilization of Lipid-Based Delivery Systems with a Focus on Solidification by Spray-Drying. Trends Food Sci. Technol. 2024, 146, 104412. [Google Scholar] [CrossRef]
- Lee, Y.K.; Ganesan, P.; Baharin, B.S.; Kwak, H.S. Characteristics, Stability, and Release of Peanut Sprout Extracts in Powdered Microcapsules by Spray Drying. Dry. Technol. 2015, 33, 1991–2001. [Google Scholar] [CrossRef]
- Rodríguez-Huezo, M.E.; Pedroza-Islas, R.; Prado-Barragán, L.A.; Beristain, C.I.; Vernon-Carter, E.J. Microencapsulation by Spray Drying of Multiple Emulsions Containing Carotenoids. J. Food Sci. 2004, 69, 351–359. [Google Scholar] [CrossRef]
- Santos, M.G.; Carpinteiro, D.A.; Thomazini, M.; Rocha-Selmi, G.A.; da Cruz, A.G.; Rodrigues, C.E.C.; Favaro-Trindade, C.S. Coencapsulation of Xylitol and Menthol by Double Emulsion Followed by Complex Coacervation and Microcapsule Application in Chewing Gum. Food Res. Int. 2014, 66, 454–462. [Google Scholar] [CrossRef]
- Hu, S.; Ding, Z.; Zhang, G.; Wang, X.; Zhao, Y.; Fan, Z.; Liu, M.; Han, J.; Wang, Z. Fabrication and Spray-Drying Microencapsulation of Vitamin C-Loaded W1/O/W2 Emulsions: Influence of Gel Polymers in the Internal Water Phase on Encapsulation Efficiency, Reconstituted Stability, and Controlled Release Properties. LWT 2022, 170, 114113. [Google Scholar] [CrossRef]
- Nurhadi, B.; Wiraputra, D.; Wijaya, H.; Mahani, M.; Masruchin, N.; Saputra, R.A. Application of Pickering Emulsion with Microcrystalline Cellulose in Vitamin C Double Emulsion Process with Spray Drying Technology. CYTA J. Food 2023, 21, 542–553. [Google Scholar] [CrossRef]
- Silva, W.; Torres-Gatica, M.F.; Oyarzun-Ampuero, F.; Silva-Weiss, A.; Robert, P.; Cofrades, S.; Giménez, B. Double Emulsions as Potential Fat Replacers with Gallic Acid and Quercetin Nanoemulsions in the Aqueous Phases. Food Chem. 2018, 253, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Qi, W.; Zhao, T.; Yang, W.W.; Wang, G.H.; Yu, H.; Zhao, H.X.; Yang, C.; Sun, L.X. Comparative Pharmacokinetics of Chlorogenic Acid after Oral Administration in Rats. J. Pharm. Anal. 2011, 1, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Marczylo, T.H.; Steward, W.P.; Gescher, A.J. Rapid Analysis of Curcumin and Curcumin Metabolites in Rat Biomatrices Using a Novel Ultraperformance Liquid Chromatography (UPLC) Method. J. Agric. Food Chem. 2009, 57, 797–803. [Google Scholar] [CrossRef]
- Shamaei, S.; Seiiedlou, S.S.; Aghbashlo, M.; Tsotsas, E.; Kharaghani, A. Microencapsulation of Walnut Oil by Spray Drying: Effects of Wall Material and Drying Conditions on Physicochemical Properties of Microcapsules. Innov. Food Sci. Emerg. Technol. 2017, 39, 101–112. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST Static in Vitro Simulation of Gastrointestinal Food Digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- Ilyasoglu Buyukkestelli, H.; El, S.N. Enhancing Sweetness Using Double Emulsion Technology to Reduce Sugar Content in Food Formulations. Innov. Food Sci. Emerg. Technol. 2021, 74, 102809. [Google Scholar] [CrossRef]
- Nollet, M.; Laurichesse, E.; Besse, S.; Soubabère, O.; Schmitt, V. Determination of Formulation Conditions Allowing Double Emulsions Stabilized by PGPR and Sodium Caseinate to Be Used as Capsules. Langmuir 2018, 34, 2823–2833. [Google Scholar] [CrossRef]
- Dima, C.; Dima, S. Water-in-Oil-in-Water Double Emulsions Loaded with Chlorogenic Acid: Release Mechanisms and Oxidative Stability. J. Microencapsul. 2018, 35, 584–599. [Google Scholar] [CrossRef]
- Encina, C.; Vergara, C.; Giménez, B.; Oyarzún-Ampuero, F.; Robert, P. Conventional Spray-Drying and Future Trends for the Microencapsulation of Fish Oil. Trends Food Sci. Technol. 2016, 56, 46–60. [Google Scholar] [CrossRef]
- Geranpour, M.; Assadpour, E.; Jafari, S.M. Recent Advances in the Spray Drying Encapsulation of Essential Fatty Acids and Functional Oils. Trends Food Sci. Technol. 2020, 102, 71–90. [Google Scholar] [CrossRef]
- Gallardo, G.; Guida, L.; Martinez, V.; López, M.C.; Bernhardt, D.; Blasco, R.; Pedroza-Islas, R.; Hermida, L.G. Microencapsulation of Linseed Oil by Spray Drying for Functional Food Application. Food Res. Int. 2013, 52, 473–482. [Google Scholar] [CrossRef]
- Domian, E.; Cenkier, J.; Górska, A.; Brynda-Kopytowska, A. Effect of Oil Content and Drying Method on Bulk Properties and Stability of Powdered Emulsions with OSA Starch and Linseed Oil. LWT 2018, 88, 95–102. [Google Scholar] [CrossRef]
- Gomes, M.H.G.; Kurozawa, L.E. Improvement of the Functional and Antioxidant Properties of Rice Protein by Enzymatic Hydrolysis for the Microencapsulation of Linseed Oil. J. Food Eng. 2020, 267, 109761. [Google Scholar] [CrossRef]
- Goyal, A.; Sharma, V.; Sihag, M.K.; Tomar, S.K.; Arora, S.; Sabikhi, L.; Singh, A.K. Development and Physico-Chemical Characterization of Microencapsulated Flaxseed Oil Powder: A Functional Ingredient for Omega-3 Fortification. Powder Technol. 2015, 286, 527–537. [Google Scholar] [CrossRef]
- Golmakani, M.-T.; Soltani, A.; Sahraeian, S. Investigating the Impact of Palm Oil Refining on the Oxidative Stability of Its Blend with Linseed Oil: A Kinetic Approach. Grasas Aceites 2024, 75, 2212. [Google Scholar] [CrossRef]
- Cáceres, D.; Giménez, B.; Márquez-Ruiz, G.; Holgado, F.; Vergara, C.; Romero-Hasler, P.; Soto-Bustamante, E.; Robert, P. Influence of the Location of Ascorbic Acid in Walnut Oil Spray-Dried Microparticles with Outer Layer on the Physical Characteristics and Oxidative Stability. Antioxidants 2020, 9, 1272. [Google Scholar] [CrossRef]
- Cáceres, D.; Giménez, B.; Márquez-Ruiz, G.; Holgado, F.; Vergara, C.; Romero-Hasler, P.; Soto-Bustamante, E.; Robert, P. Incorporation of Hydroxytyrosol Alkyl Esters of Different Chain Length as Antioxidant Strategy in Walnut Oil Spray-Dried Microparticles with a Sodium Alginate Outer Layer. Food Chem. 2022, 395, 133595. [Google Scholar] [CrossRef]
- Tomac, I.; Šeruga, M.; Beinrohr, E. Characterization of Chlorogenic Acids in Coffee by Flow-Through Chronopotentiometry. Food Anal. Methods 2017, 10, 3924–3933. [Google Scholar] [CrossRef]
- Ziyatdinova, G.; Aytuganova, I.; Nizamova, A.; Budnikov, H. Differential Pulse Voltammetric Assay of Coffee Antioxidant Capacity with MWNT-Modified Electrode. Food Anal. Methods 2013, 6, 1629–1638. [Google Scholar] [CrossRef]
- Chiorcea-Paquim, A.M. Electrochemical Sensing of Curcumin: A Review. Antioxidants 2023, 12, 2029. [Google Scholar] [CrossRef]
- Jovanovic, S.V.; Boone, C.W.; Steenken, S.; Trinoga, M.; Kaskey, R.B. How Curcumin Works Preferentially with Water Soluble Antioxidants. J. Am. Chem. Soc. 2001, 123, 3064–3068. [Google Scholar] [CrossRef]
- Silva, E.K.; Azevedo, V.M.; Cunha, R.L.; Hubinger, M.D.; Meireles, M.A.A. Ultrasound-Assisted Encapsulation of Annatto Seed Oil: Whey Protein Isolate versus Modified Starch. Food Hydrocoll. 2016, 56, 71–83. [Google Scholar] [CrossRef]
- Botrel, D.A.; de Barros Fernandes, R.V.; Borges, S.V.; Yoshida, M.I. Influence of Wall Matrix Systems on the Properties of Spray-Dried Microparticles Containing Fish Oil. Food Res. Int. 2014, 62, 344–352. [Google Scholar] [CrossRef]
- Cosgun, G.; Gungor, K.K.; Balci-Torun, F.; Sahin, S.; Torun, M. Design of Encapsulation Method for Chlorogenic Acid and Caffeine in Coffee Waste By-Product. Phytochem. Anal. 2024, 35, 1720–1735. [Google Scholar] [CrossRef]
- Desai, N.M.; Gilbert Stanley, J.; Murthy, P.S. Green Coffee Nanoparticles: Optimisation, in Vitro Bioactivity and Bio-Release Property. J. Microencapsul. 2020, 37, 52–64. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Solis, S.E.; Perez-Perez, V.; Tapia-Maruri, D.; Villalobos-Castillejos, F.; Arenas-Ocampo, M.L.; Camacho-Diaz, B.H.; Alamilla-Beltran, L. Microencapsulation of the Green Coffee Waste Extract with High Antioxidant Activity by Spray-Drying. J. Food Process Preserv. 2022, 46, e16864. [Google Scholar] [CrossRef]
- Guo, J.; Li, P.; Kong, L.; Xu, B. Microencapsulation of Curcumin by Spray Drying and Freeze Drying. LWT 2020, 132, 109892. [Google Scholar] [CrossRef]
- Lin, Q.; Liang, R.; Zhong, F.; Ye, A.; Singh, H. Effect of Degree of Octenyl Succinic Anhydride (OSA) Substitution on the Digestion of Emulsions and the Bioaccessibility of β-Carotene in OSA-Modified-Starch-Stabilized-Emulsions. Food Hydrocoll. 2018, 84, 303–312. [Google Scholar] [CrossRef]
- Thaiwong, N.; Thaiudom, S. Stability of Oil-in-Water Emulsion Influenced by the Interaction of Modified Tapioca Starch and Milk Protein. Int. J. Dairy Technol. 2021, 74, 307–315. [Google Scholar] [CrossRef]
- Yang, L.; Qin, X.; Kan, J.; Liu, X.; Zhong, J. Improving the Physical and Oxidative Stability of Emulsions Using Mixed Emulsifiers: Casein-Octenyl Succinic Anhydride Modified Starch Combinations. Nanomaterials 2019, 9, 1018. [Google Scholar] [CrossRef]
- Mora, C.P.; Martinez-Alejo, J.M.; Roman, L.; Martinez, M.M.; Carvajal, T.; Pinal, R.; Mora-Huertas, C.E. Molecular and Physical Characterization of Octenyl Succinic Anhydride-Modified Starches with Potential Applications in Pharmaceutics. Int. J. Pharm. 2020, 579, 119163. [Google Scholar] [CrossRef]
- Paredes-Toledo, J.; Herrera, J.; Morales, J.; Robert, P.; Gómez-Estaca, J.; Giménez, B. Pickering Double Emulsions Stabilized with Chitin Nanocrystals and Myristic Acid-Functionalized Silica Nanoparticles for Curcumin and Chlorogenic Acid Co-Delivery. Pharmaceutics 2025, 17, 521. [Google Scholar] [CrossRef]
- Davidov-Pardo, G.; McClements, D.J. Resveratrol Encapsulation: Designing Delivery Systems to Overcome Solubility, Stability and Bioavailability Issues. Trends Food Sci. Technol. 2014, 38, 88–103. [Google Scholar] [CrossRef]
- Limwachiranon, J.; Huang, H.; Li, L.; Lin, X.; Zou, L.; Liu, J.; Zou, Y.; Aalim, H.; Duan, Z.; Luo, Z. Enhancing Stability and Bioaccessibility of Chlorogenic Acid Using Complexation with Amylopectin: A Comprehensive Evaluation of Complex Formation, Properties, and Characteristics. Food Chem. 2020, 311, 125879. [Google Scholar] [CrossRef]
- Narita, Y.; Inouye, K. Degradation Kinetics of Chlorogenic Acid at Various PH Values and Effects of Ascorbic Acid and Epigallocatechin Gallate on Its Stability under Alkaline Conditions. J. Agric. Food Chem. 2013, 61, 966–972. [Google Scholar] [CrossRef]
- Costa, S.; Afonso, C.; Cardoso, C.; Batista, I.; Chaveiro, N.; Nunes, M.L.; Bandarra, N.M. Fatty Acids, Mercury, and Methylmercury Bioaccessibility in Salmon (Salmo salar) Using an in Vitro Model: Effect of Culinary Treatment. Food Chem. 2015, 185, 268–276. [Google Scholar] [CrossRef] [PubMed]
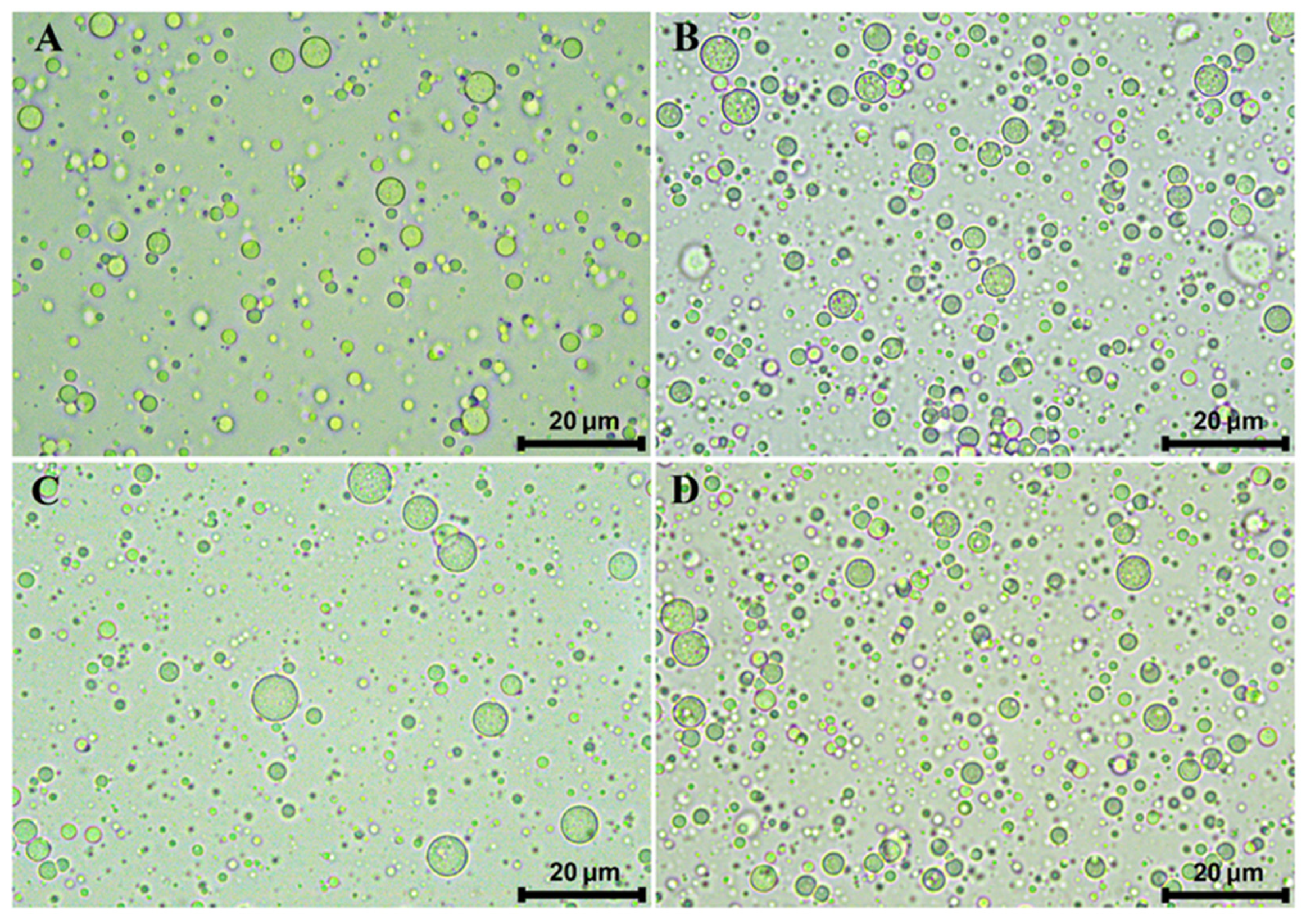

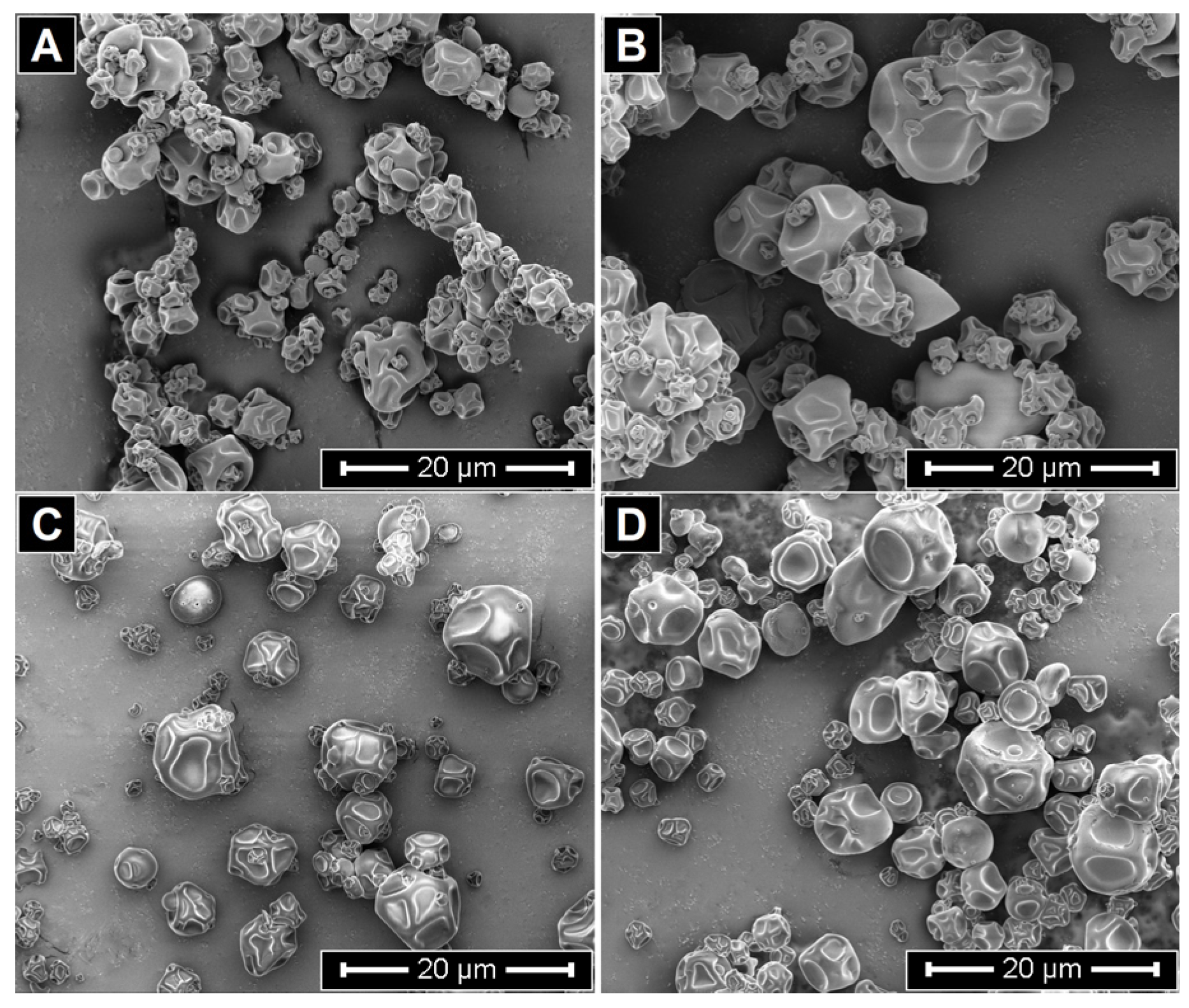
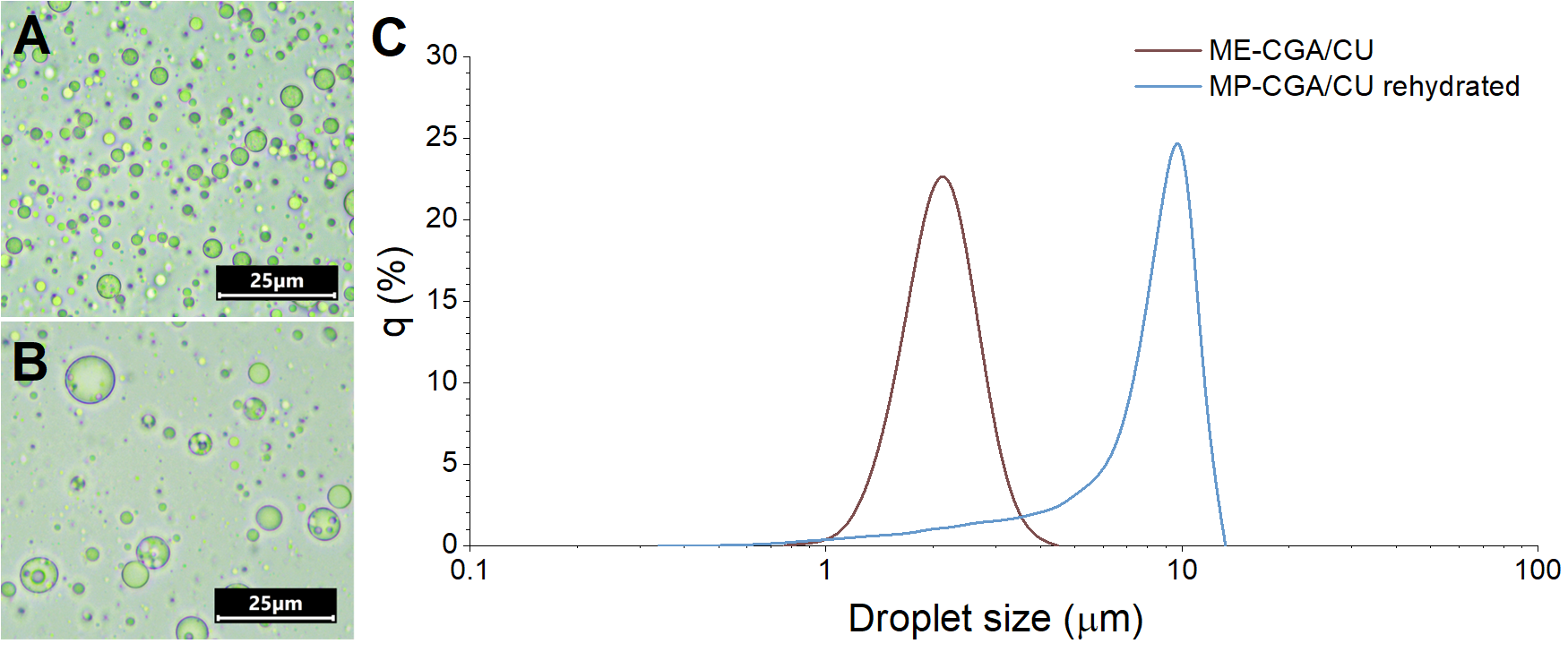
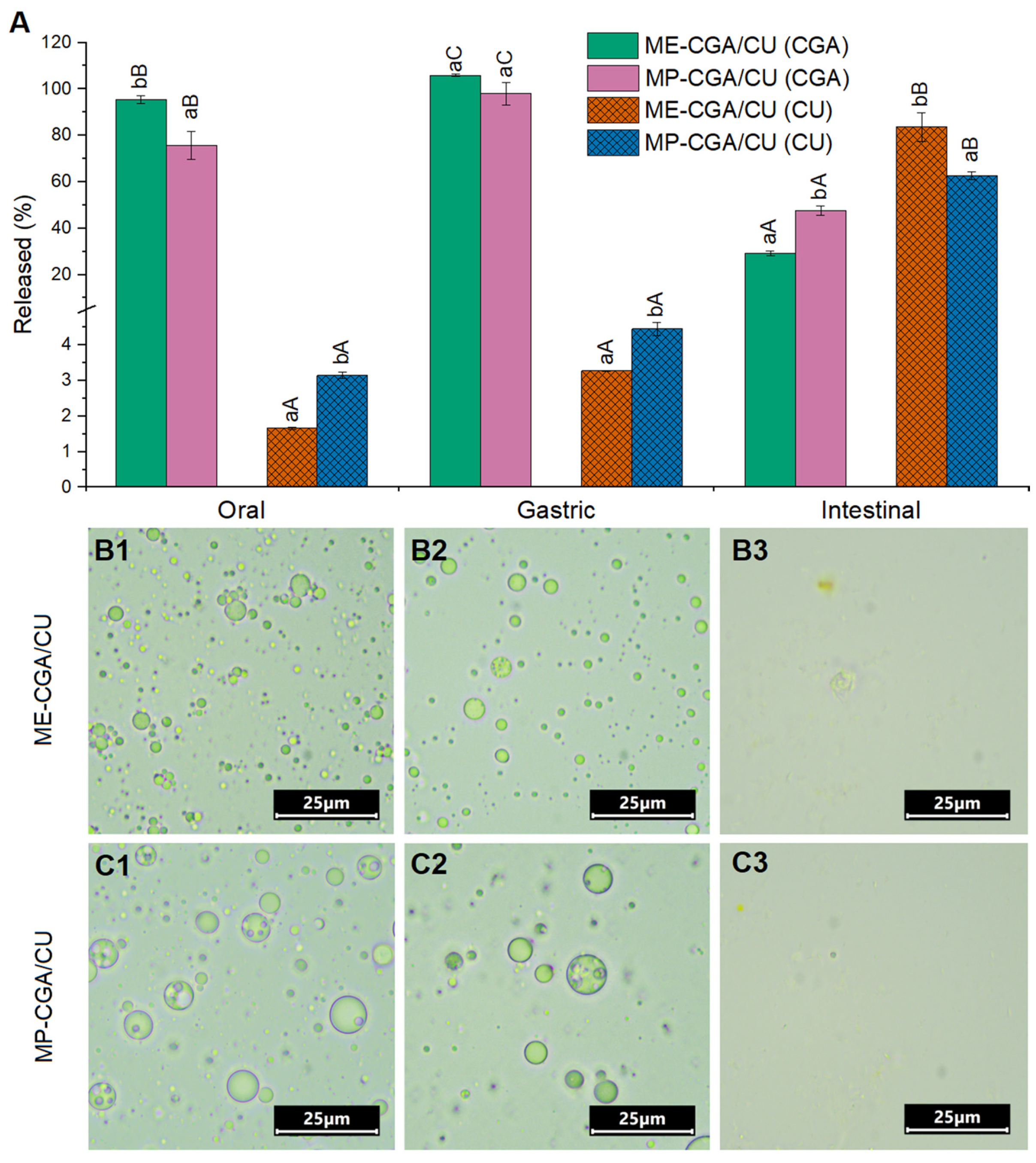

| ME-C | ME-CGA | ME-CU | ME-CGA/CU | |
|---|---|---|---|---|
| D4,3 (µm) | 2.07 ± 0.02 a | 2.02 ± 0.04 a | 2.06 ± 0.03 a | 2.01 ± 0.04 a |
| EE of CGA (%) | - | 59.0 ± 0.9 a | - | 60.1 ± 0.3 a |
| EE of CU (%) | - | - | 99.9 ± 0.0 a | 99.8 ± 0.1 a |
| Parameters | MP-C | MP-CGA | MP-CU | MP-CGA/CU |
|---|---|---|---|---|
| EE of LO (%) | 90.1 ± 1.0 a | 94.6 ± 3.4 a | 96.8 ± 1.2 a | 95.2 ± 2.7 a |
| Total LO (mg/g) | 136.6 ± 0.1 a | 136.2 ± 0.2 a | 136.3 ± 0.4 a | 137.0 ± 0.2 a |
| IP (h) | 10.2 ± 1.4 a | 20.1 ± 0.1 b | 24.3 ± 0.5 c | 23.4 ± 0.9 bc |
| Moisture content (%) | 3.7 ± 0.2 a | 4.4 ± 0.0 b | 4.3 ± 0.2 b | 4.1 ± 0.0 ab |
| D4,3 (µm) | 8.4 ± 0.4 b | 10.5 ± 0.2 c | 13.0 ± 0.2 d | 7.2 ± 0.2 a |
| EE of CGA | - | 89.1 ± 0.6 a | - | 87.8 ± 1.5 a |
| EE of CU | - | - | 90.8 ± 1.2 a | 88.9 ± 0.6 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paredes-Toledo, J.; Herrera, J.; González, E.; Robert, P.; Giménez, B. Spray-Dried Multiple Emulsions as Co-Delivery Systems for Chlorogenic Acid and Curcumin. Antioxidants 2025, 14, 1257. https://doi.org/10.3390/antiox14101257
Paredes-Toledo J, Herrera J, González E, Robert P, Giménez B. Spray-Dried Multiple Emulsions as Co-Delivery Systems for Chlorogenic Acid and Curcumin. Antioxidants. 2025; 14(10):1257. https://doi.org/10.3390/antiox14101257
Chicago/Turabian StyleParedes-Toledo, Javier, Javier Herrera, Estefanía González, Paz Robert, and Begoña Giménez. 2025. "Spray-Dried Multiple Emulsions as Co-Delivery Systems for Chlorogenic Acid and Curcumin" Antioxidants 14, no. 10: 1257. https://doi.org/10.3390/antiox14101257
APA StyleParedes-Toledo, J., Herrera, J., González, E., Robert, P., & Giménez, B. (2025). Spray-Dried Multiple Emulsions as Co-Delivery Systems for Chlorogenic Acid and Curcumin. Antioxidants, 14(10), 1257. https://doi.org/10.3390/antiox14101257







