Exercise-Induced FNDC5/Irisin Ameliorates Cognitive Impairment in Aged Mice, Associated with Antioxidant and Neurotrophic Responses
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Exercise Performance
2.3. Behavioral Test (Y-Maze)
2.4. Tissue Preparation
2.5. Immunohistochemistry and Immunofluorescence
2.6. Fluoro-Jade C Staining
2.7. Western Blotting
2.8. Statistical Analysis
3. Results
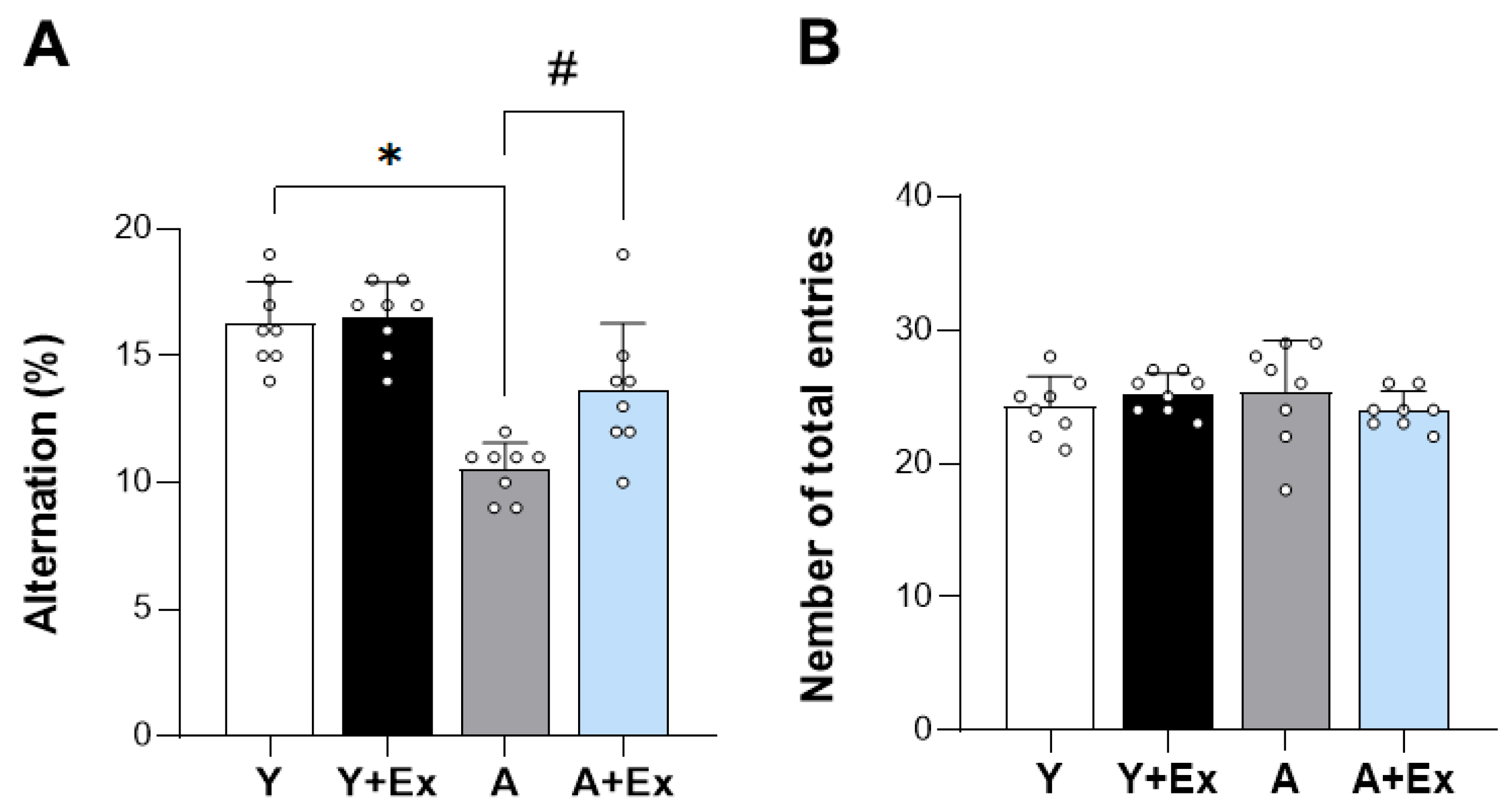
3.1. Exercise Alleviates Aging-Induced Cognitive Impiarment
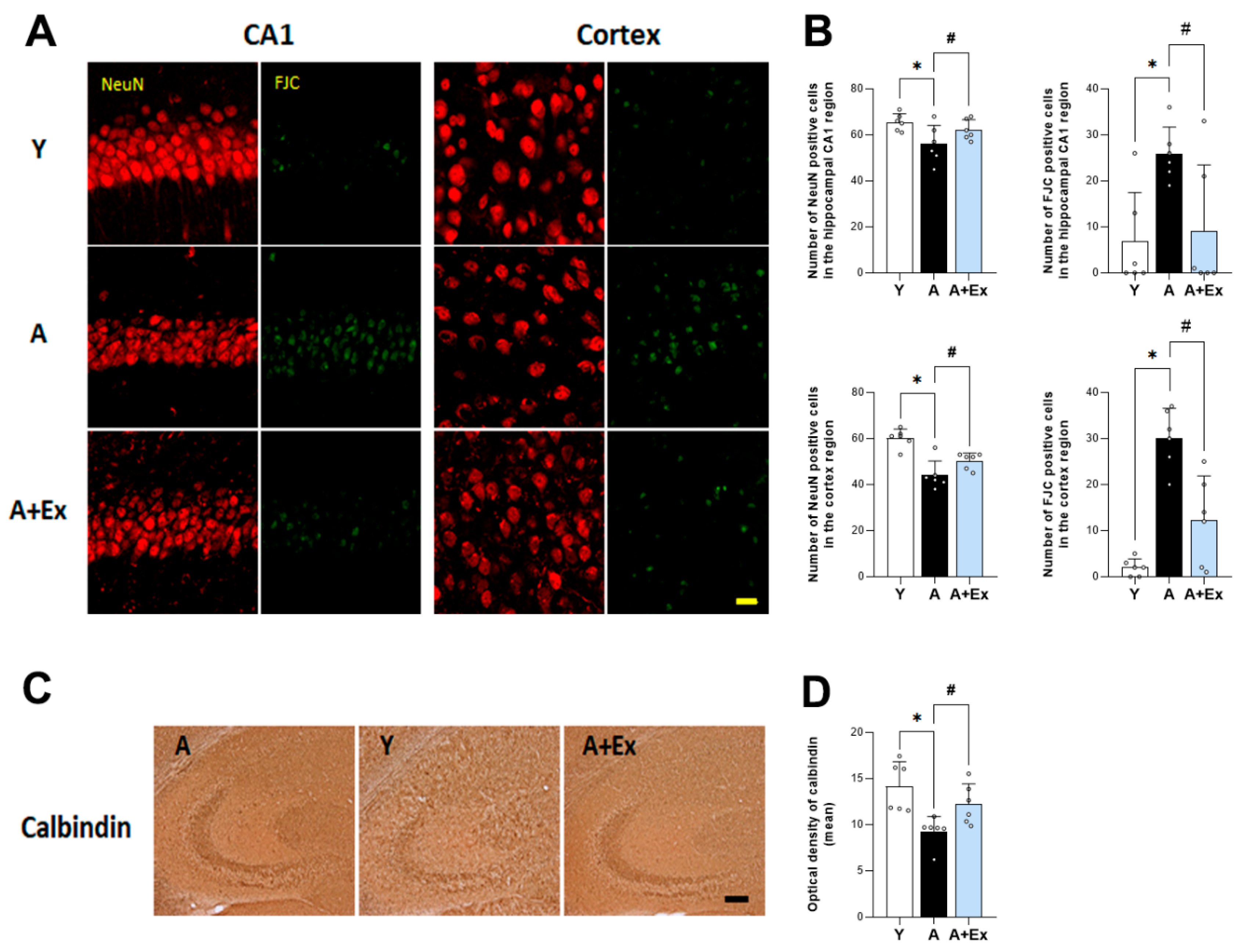
3.2. Exercise Inhibits Aging-Induced Neuronal Death in the Hippocampus and Cortex
3.3. Exercise Alleviates Activation of Microglia and Astrocyte in the Hippocampus
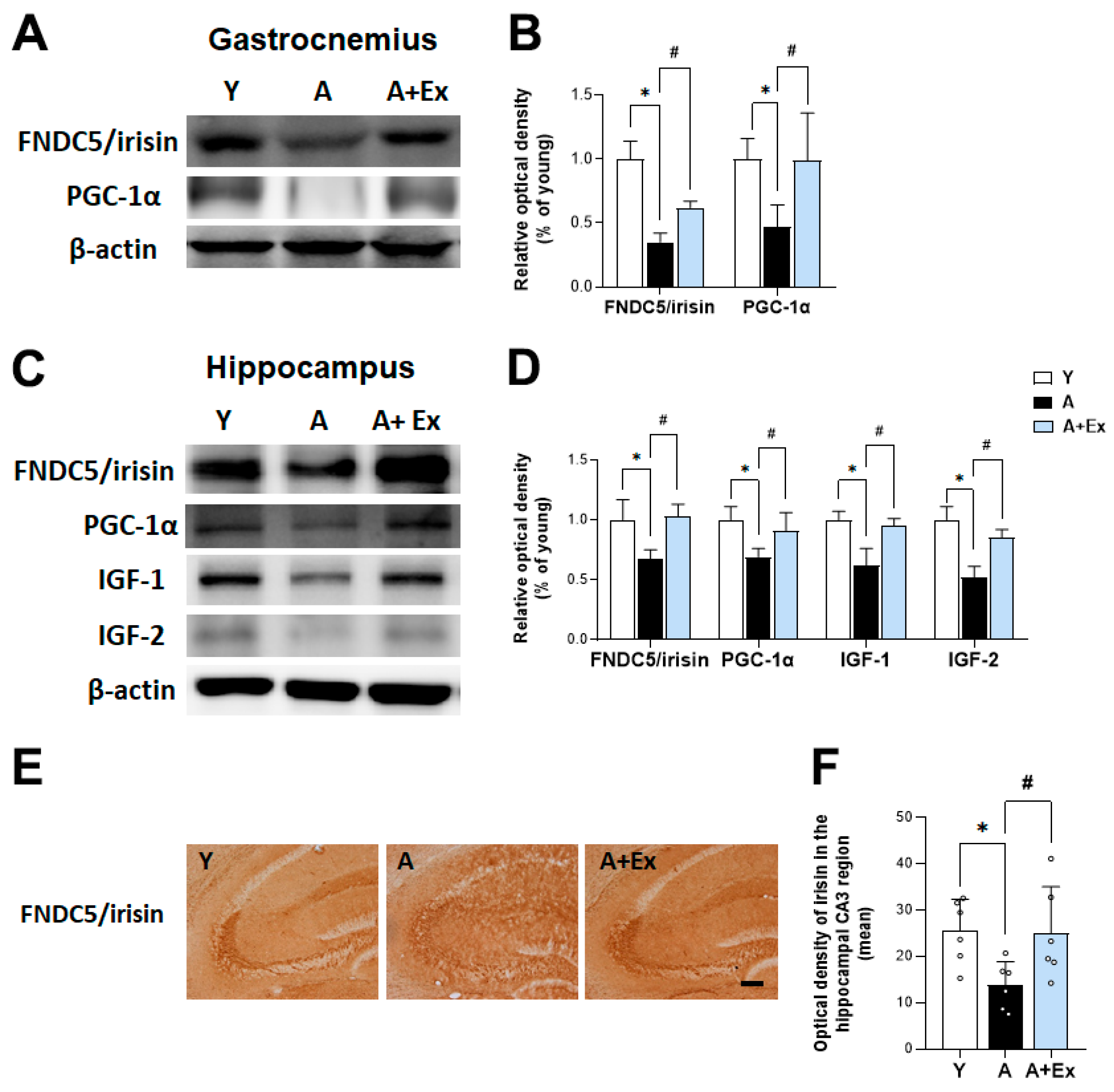
3.4. Effects of Treadmill Exercise on FNDC5/Irisin Expression in the Hippocampus and Gastrocnemius Muscle
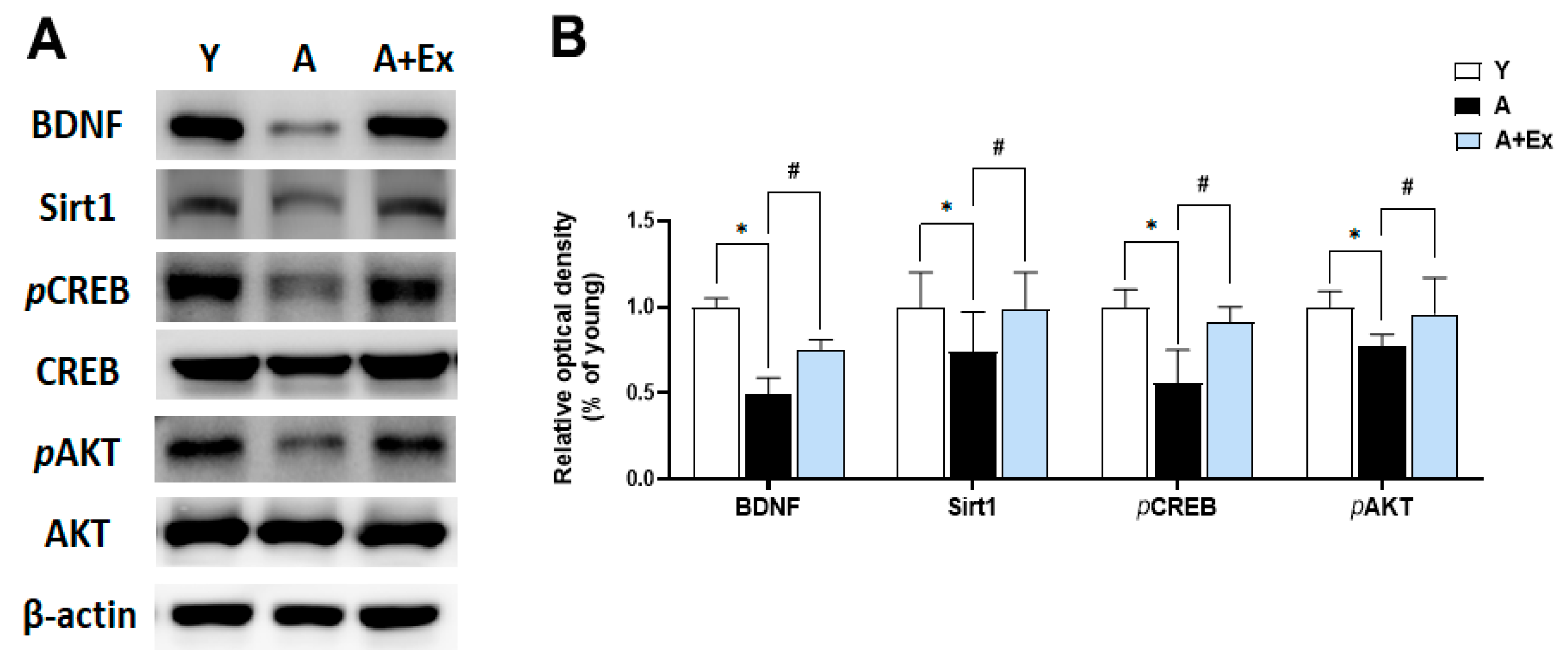
3.5. Exercise Improves Aging-Induced BDNF/Sirt1/CREB/AKT Signaling Pathway in the Hippocampus
3.6. Exercise Upregulates Nrf2 Expression in the Motor Cortex and Hippocampus
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guo, J.; Huang, X.; Dou, L.; Yan, M.; Shen, T.; Tang, W.; Li, J. Aging and Aging-Related Diseases: From Molecular Mechanisms to Interventions and Treatments. Signal Transduct. Target. Ther. 2022, 7, 391. [Google Scholar] [CrossRef]
- Scisciola, L.; Fontanella, R.A.; Surina; Cataldo, V.; Paolisso, G.; Barbieri, M. Sarcopenia and Cognitive Function: Role of Myokines in Muscle Brain Cross-Talk. Life 2021, 11, 173. [Google Scholar] [CrossRef]
- Barnes, J.N. Exercise, Cognitive Function, and Aging. Adv. Physiol. Educ. 2015, 39, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Kramer, A.F.; Erickson, K.I.; Colcombe, S.J. Exercise, Cognition, and the Aging Brain. J. Appl. Physiol. 2006, 101, 1237–1242. [Google Scholar] [CrossRef]
- Mee-inta, O.; Zhao, Z.-W.; Kuo, Y.-M. Physical Exercise Inhibits Inflammation and Microglial Activation. Cells 2019, 8, 691. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.K.; Åkerström, T.C.A.; Nielsen, A.R.; Fischer, C.P. Role of Myokines in Exercise and Metabolism. J. Appl. Physiol. 2007, 103, 1093–1098. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, Z.; Ren, Y.; Wang, Y.; Fang, J.; Yue, H.; Ma, S.; Guan, F. Aging and Age—related Diseases: From Mechanisms to Therapeutic Strategies. Biogerontology 2021, 22, 165–187. [Google Scholar] [CrossRef]
- Lourenco, M.V.; Frozza, R.L.; de Freitas, G.B.; Zhang, H.; Kincheski, G.C.; Ribeiro, F.C.; Gonçalves, R.A.; Clarke, J.R.; Beckman, D.; Staniszewski, A.; et al. Exercise-Linked FNDC5/Irisin Rescues Synaptic Plasticity and Memory Defects in Alzheimer’s Models. Nat. Med. 2019, 25, 165–175. [Google Scholar] [CrossRef]
- Lee, B.; Shin, M.; Park, Y.; Won, S.-Y.; Cho, K.S. Physical Exercise-Induced Myokines in Neurodegenerative Diseases. Int. J. Mol. Sci. 2021, 22, 5795. [Google Scholar] [CrossRef]
- Kwon, J.H.; Moon, K.M.; Min, K.-W. Exercise-Induced Myokines Can Explain the Importance of Physical Activity in the Elderly: An Overview. Healthcare 2020, 8, 378. [Google Scholar] [CrossRef]
- Eckardt, K.; Görgens, S.W.; Raschke, S.; Eckel, J. Myokines in Insulin Resistance and Type 2 Diabetes. Diabetologia 2014, 57, 1087–1099. [Google Scholar] [CrossRef] [PubMed]
- Laurens, C.; Bergouignan, A.; Moro, C. Exercise-Released Myokines in the Control of Energy Metabolism. Front. Physiol. 2020, 11, 91. [Google Scholar] [CrossRef] [PubMed]
- Jo, D.; Yoon, G.; Kim, O.Y.; Song, J. A New Paradigm in Sarcopenia: Cognitive Impairment Caused by Imbalanced Myokine Secretion and Vascular Dysfunction. Biomed. Pharmacother. 2022, 147, 112636. [Google Scholar] [CrossRef] [PubMed]
- Waseem, R.; Shamsi, A.; Mohammad, T.; Hassan, M.I.; Kazim, S.N.; Chaudhary, A.A.; Rudayni, H.A.; Al-Zharani, M.; Ahmad, F.; Islam, A. FNDC5/Irisin: Physiology and Pathophysiology. Molecules 2022, 27, 1118. [Google Scholar] [CrossRef]
- Wrann, C.D.; White, J.P.; Salogiannnis, J.; Laznik-Bogoslavski, D.; Wu, J.; Ma, D.; Lin, J.D.; Greenberg, M.E.; Spiegelman, B.M. Exercise Induces Hippocampal BDNF through a PGC-1α/FNDC5 Pathway. Cell Metab. 2013, 18, 649–659. [Google Scholar] [CrossRef]
- Leger, C.; Quirié, A.; Méloux, A.; Fontanier, E.; Chaney, R.; Basset, C.; Lemaire, S.; Garnier, P.; Prigent-Tessier, A. Impact of Exercise Intensity on Cerebral BDNF Levels: Role of FNDC5/Irisin. Int. J. Mol. Sci. 2024, 25, 1213. [Google Scholar] [CrossRef]
- Kim, J.; Cha, Y.-N.; Surh, Y.-J. A Protective Role of Nuclear Factor-Erythroid 2-Related Factor-2 (Nrf2) in Inflammatory Disorders. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2010, 690, 12–23. [Google Scholar] [CrossRef]
- Ma, Q. Role of Nrf2 in Oxidative Stress and Toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef]
- Saha, S.; Buttari, B.; Panieri, E.; Profumo, E.; Saso, L. An Overview of Nrf2 Signaling Pathway and Its Role in Inflammation. Molecules 2020, 25, 5474. [Google Scholar] [CrossRef]
- Johnson, D.A.; Johnson, J.A. Nrf2—A Therapeutic Target for the Treatment of Neurodegenerative Diseases. Free Radic. Biol. Med. 2015, 88, 253–267. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, Y.; Wang, Y.; Chao, Y.; Zhang, J.; Jia, Y.; Tie, J.; Hu, D. Regulation of SIRT1 and Its Roles in Inflammation. Front. Immunol. 2022, 13, 831168. [Google Scholar] [CrossRef]
- Cantó, C.; Auwerx, J. PGC-1alpha, SIRT1 and AMPK, an Energy Sensing Network That Controls Energy Expenditure. Curr. Opin. Lipidol. 2009, 20, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Bathina, S.; Das, U.N. Brain-Derived Neurotrophic Factor and Its Clinical Implications. Arch. Med. Sci. 2015, 11, 1164–1178. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.-C.; Guarente, L. SIRT1 and Other Sirtuins in Metabolism. Trends Endocrinol. Metab. 2014, 25, 138–145. [Google Scholar] [CrossRef]
- He, F.; Ru, X.; Wen, T. NRF2, a Transcription Factor for Stress Response and Beyond. Int. J. Mol. Sci. 2020, 21, 4777. [Google Scholar] [CrossRef]
- Li, M.; Su, S.; Cai, W.; Cao, J.; Miao, X.; Zang, W.; Gao, S.; Xu, Y.; Yang, J.; Tao, Y.-X.; et al. Differentially Expressed Genes in the Brain of Aging Mice with Cognitive Alteration and Depression- and Anxiety-Like Behaviors. Front. Cell Dev. Biol. 2020, 8, 814. [Google Scholar] [CrossRef]
- Drapeau, E.; Mayo, W.; Aurousseau, C.; Le Moal, M.; Piazza, P.-V.; Abrous, D.N. Spatial Memory Performances of Aged Rats in the Water Maze Predict Levels of Hippocampal Neurogenesis. Proc. Natl. Acad. Sci. USA 2003, 100, 14385–14390. [Google Scholar] [CrossRef]
- Kraeuter, A.-K.; Guest, P.C.; Sarnyai, Z. The Y-Maze for Assessment of Spatial Working and Reference Memory in Mice. In Pre-Clinical Models: Techniques and Protocols; Springer: New York, NY, USA, 2019; pp. 105–111. [Google Scholar]
- Mattson, M.P.; Cheng, B.; Baldwin, S.A.; Smith-Swintosky, V.L.; Keller, J.; Geddes, J.W.; Scheff, S.W.; Christakos, S. Brain Injury and Tumor Necrosis Factors Induce Calbindin D—28K in Astrocytes: Evidence for a Cytoprotective Response. J. Neurosci. Res. 1995, 42, 357–370. [Google Scholar] [CrossRef]
- Kook, S.-Y.; Jeong, H.; Kang, M.J.; Park, R.; Shin, H.J.; Han, S.-H.; Son, S.M.; Song, H.; Baik, S.H.; Moon, M.; et al. Crucial Role of Calbindin-D28k in the Pathogenesis of Alzheimer’s Disease Mouse Model. Cell Death Differ. 2014, 21, 1575–1587. [Google Scholar] [CrossRef]
- Soontornniyomkij, V.; Risbrough, V.B.; Young, J.W.; Soontornniyomkij, B.; Jeste, D.V.; Achim, C.L. Hippocampal Calbindin-1 Immunoreactivity Correlate of Recognition Memory Performance in Aged Mice. Neurosci. Lett. 2012, 516, 161–165. [Google Scholar] [CrossRef]
- Young, M.F.; Valaris, S.; Wrann, C.D. A Role for FNDC5/Irisin in the Beneficial Effects of Exercise on the Brain and in Neurodegenerative Diseases. Prog. Cardiovasc. Dis. 2019, 62, 172–178. [Google Scholar] [CrossRef]
- Chung, W.-S.; Allen, N.J.; Eroglu, C. Astrocytes Control Synapse Formation, Function, and Elimination. Cold Spring Harb. Perspect Biol. 2015, 7, a020370. [Google Scholar] [CrossRef]
- Harry, G.J. Microglia during Development and Aging. Pharmacol. Ther. 2013, 139, 313–326. [Google Scholar] [CrossRef] [PubMed]
- von Bernhardi, R.; Eugenín-von Bernhardi, L.; Eugenín, J. Microglial Cell Dysregulation in Brain Aging and Neurodegeneration. Front. Aging Neurosci. 2015, 7, 124. [Google Scholar] [CrossRef] [PubMed]
- Maak, S.; Norheim, F.; Drevon, C.A.; Erickson, H.P. Progress and Challenges in the Biology of FNDC5 and Irisin. Endocr. Rev. 2021, 42, 436–456. [Google Scholar] [CrossRef] [PubMed]
- Belviranlı, M.; Okudan, N. Exercise Training Protects Against Aging-Induced Cognitive Dysfunction via Activation of the Hippocampal PGC-1α/FNDC5/BDNF Pathway. Neuromol. Med. 2018, 20, 386–400. [Google Scholar] [CrossRef]
- Lin, J.-Y.; Kuo, W.-W.; Baskaran, R.; Kuo, C.-H.; Chen, Y.-A.; Chen, W.S.-T.; Ho, T.-J.; Day, C.H.; Mahalakshmi, B.; Huang, C.-Y. Swimming Exercise Stimulates IGF1/PI3K/Akt and AMPK/SIRT1/PGC1α Survival Signaling to Suppress Apoptosis and Inflammation in Aging Hippocampus. Aging 2020, 12, 6852–6864. [Google Scholar] [CrossRef]
- Cho, S.-H.; Chen, J.A.; Sayed, F.; Ward, M.E.; Gao, F.; Nguyen, T.A.; Krabbe, G.; Sohn, P.D.; Lo, I.; Minami, S.; et al. SIRT1 Deficiency in Microglia Contributes to Cognitive Decline in Aging and Neurodegeneration via Epigenetic Regulation of IL-1β. J. Neurosci. 2015, 35, 807–818. [Google Scholar] [CrossRef]
- Herskovits, A.Z.; Guarente, L. SIRT1 in Neurodevelopment and Brain Senescence. Neuron 2014, 81, 471–483. [Google Scholar] [CrossRef]
- Erickson, K.I.; Prakash, R.S.; Voss, M.W.; Chaddock, L.; Heo, S.; McLaren, M.; Pence, B.D.; Martin, S.A.; Vieira, V.J.; Woods, J.A.; et al. Brain-Derived Neurotrophic Factor Is Associated with Age-Related Decline in Hippocampal Volume. J. Neurosci. 2010, 30, 5368–5375. [Google Scholar] [CrossRef]
- Chen, Y.; Li, Y.; Hsieh, T.; Wang, C.; Cheng, K.; Wang, L.; Lin, T.; Cheung, C.H.A.; Wu, C.; Chiang, H. Aging—Induced Akt Activation Involves in Aging—Related Pathologies and Aβ—Induced Toxicity. Aging Cell 2019, 18, e12989. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Martínez, S. A New Perspective on the Role of the CREB Family of Transcription Factors in Memory Consolidation via Adult Hippocampal Neurogenesis. Front. Mol. Neurosci. 2015, 8, 46. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Yan, Z.; Zhou, T.; Wang, G. SIRT1 Regulates Cognitive Performance and Ability of Learning and Memory in Diabetic and Nondiabetic Models. J. Diabetes Res. 2017, 2017, 7121827. [Google Scholar] [CrossRef] [PubMed]
- Sonntag, W.E.; Deak, F.; Ashpole, N.; Toth, P.; Csiszar, A.; Freeman, W.; Ungvari, Z. Insulin-like Growth Factor-1 in CNS and Cerebrovascular Aging. Front. Aging Neurosci. 2013, 5, 27. [Google Scholar] [CrossRef]
- Yakar, S.; Adamo, M.L. Insulin-Like Growth Factor 1 Physiology. Endocrinol. Metab. Clin. N. Am. 2012, 41, 231–247. [Google Scholar] [CrossRef]
- Labandeira-Garcia, J.L.; Costa-Besada, M.A.; Labandeira, C.M.; Villar-Cheda, B.; Rodríguez-Perez, A.I. Insulin-Like Growth Factor-1 and Neuroinflammation. Front. Aging Neurosci. 2017, 9, 365. [Google Scholar] [CrossRef]
- Kandlur, A.; Satyamoorthy, K.; Gangadharan, G. Oxidative Stress in Cognitive and Epigenetic Aging: A Retrospective Glance. Front. Mol. Neurosci. 2020, 13, 41. [Google Scholar] [CrossRef]
- Nguyen, T.; Nioi, P.; Pickett, C.B. The Nrf2-Antioxidant Response Element Signaling Pathway and Its Activation by Oxidative Stress. J. Biol. Chem. 2009, 284, 13291–13295. [Google Scholar] [CrossRef]
- Zhang, H.; Davies, K.J.A.; Forman, H.J. Oxidative Stress Response and Nrf2 Signaling in Aging. Free Radic. Biol. Med. 2015, 88, 314–336. [Google Scholar] [CrossRef]
- Radák, Z.; Kaneko, T.; Tahara, S.; Nakamoto, H.; Pucsok, J.; Sasvári, M.; Nyakas, C.; Goto, S. Regular Exercise Improves Cognitive Function and Decreases Oxidative Damage in Rat Brain. Neurochem. Int. 2001, 38, 17–23. [Google Scholar] [CrossRef]
- Souza, J.; da Silva, R.A.; da Luz Scheffer, D.; Penteado, R.; Solano, A.; Barros, L.; Budde, H.; Trostchansky, A.; Latini, A. Physical-Exercise-Induced Antioxidant Effects on the Brain and Skeletal Muscle. Antioxidants 2022, 11, 826. [Google Scholar] [CrossRef]
- Ge, Y.; Wu, X.; Cai, Y.; Hu, Q.; Wang, J.; Zhang, S.; Zhao, B.; Cui, W.; Wu, Y.; Wang, Q.; et al. FNDC5 Prevents Oxidative Stress and Neuronal Apoptosis after Traumatic Brain Injury through SIRT3-Dependent Regulation of Mitochondrial Quality Control. Cell Death Dis. 2024, 15, 364. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.M.; Sim, T.H.; Kim, S.H.; Choi, Y.J.; Lee, J.H.; Yeo, S.G.; Kim, Y.-J. Exercise-Induced FNDC5/Irisin Ameliorates Cognitive Impairment in Aged Mice, Associated with Antioxidant and Neurotrophic Responses. Antioxidants 2025, 14, 1239. https://doi.org/10.3390/antiox14101239
Lee JM, Sim TH, Kim SH, Choi YJ, Lee JH, Yeo SG, Kim Y-J. Exercise-Induced FNDC5/Irisin Ameliorates Cognitive Impairment in Aged Mice, Associated with Antioxidant and Neurotrophic Responses. Antioxidants. 2025; 14(10):1239. https://doi.org/10.3390/antiox14101239
Chicago/Turabian StyleLee, Jae Min, Tae Hyeok Sim, So Hee Kim, You Jung Choi, Joo Hee Lee, Seung Geun Yeo, and Youn-Jung Kim. 2025. "Exercise-Induced FNDC5/Irisin Ameliorates Cognitive Impairment in Aged Mice, Associated with Antioxidant and Neurotrophic Responses" Antioxidants 14, no. 10: 1239. https://doi.org/10.3390/antiox14101239
APA StyleLee, J. M., Sim, T. H., Kim, S. H., Choi, Y. J., Lee, J. H., Yeo, S. G., & Kim, Y.-J. (2025). Exercise-Induced FNDC5/Irisin Ameliorates Cognitive Impairment in Aged Mice, Associated with Antioxidant and Neurotrophic Responses. Antioxidants, 14(10), 1239. https://doi.org/10.3390/antiox14101239






