The Role of Antioxidant Minerals in the Pathophysiology and Treatment of Endometriosis—Systematic Review
Abstract
1. Introduction
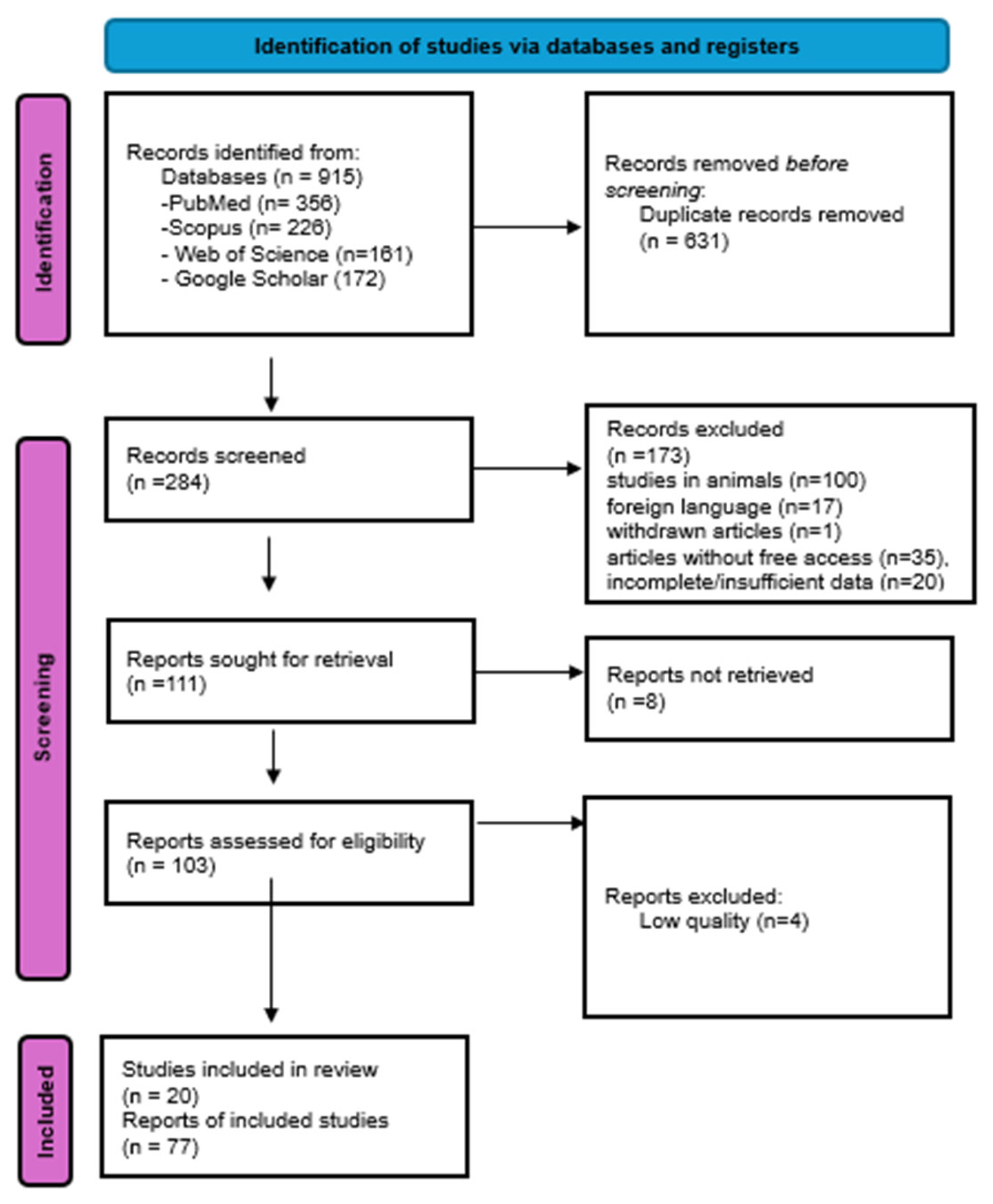
2. Materials and Methods
3. Results
3.1. The Mechanism of Endometriosis Development
- Increased production of pro-inflammatory cytokines: IL-6 (interleukin 6), TNF-α (tumor necrosis factor alpha), IL-1β (interleukin 1 beta) [16];
- Stimulation of VEGF (vascular endothelial growth factor) production, which stimulates angiogenesis [22];
- Stimulation of MAPK (mitogen-activated protein kinase) pathways [23];
- Activation of Notch pathways [24];
- Excessive formation of connective tissue (fibrosis)—adhesions and scars [18];
- Stimulation of adhesion processes [25];
- Activation of the transcription factor NF-κB (nuclear factor kappa-B) [16].
3.2. Minerals and Their Antioxidant Effects
3.2.1. Selenium (Se)
- Glutathione peroxidases (GPx1, GPx2, GPx3, GPx4, GPx6)—antioxidant properties—reduction of hydrogen peroxide, organic peroxides, including phospholipid peroxides
- Thioredoxin reductases (TrxR1, TrxR2, RGR)—antioxidant properties—reduction of thioredoxins and protein disulphides, participation in DNA synthesis and apoptosis processes
- Iodothyronine deiodinases (DIO1, DIO2, DIO3)—activate/deactivate thyroid hormones
3.2.2. Zinc (Zn)
- Is an important component of ZEB1 and ZEB2 molecules, which are involved in the epithelial–mesenchymal transition (EMT) process in EM and are associated with the severity of the disease. However, it has not been proven that zinc deficiency disrupts the expression of ZEB1 and ZEB2 [6].
3.2.3. Copper (Cu)
- Cu Zn superoxide dismutase (Cu Zn-SOD or SOD 1), which prevents damage to major biomolecules such as DNA, lipids and proteins by catalyzing the reduction of superoxide radicals to hydrogen peroxide [15].
- Cytochrome C oxidase (COX)—is an electron acceptor in the mitochondrial respiratory chain [48].
- Catechol oxidase is an enzyme involved in the metabolism of catecholamines (e.g., adrenaline, dopamine). However, during this process, free radicals may be produced, which may contribute to oxidative stress [51].
- It can interact with estrogen receptors, influencing the development and functioning of endometrial cells [15].
- Helps protect cells against damage caused by free radicals—presence in SOD [30].
3.2.4. Manganese (Mn)
3.2.5. Molybdenum (Mo)
- Sulfite oxidase (SUOX) found in mitochondria. It catalyzes the conversion of sulfite to sulfate and participates in the reduction of nitrates (III) to nitrogen oxide.
- Xanthine oxidase (XOR), which is involved in the breakdown of purines and the production of reactive oxygen species (ROS).
- Aldehyde oxidase (AOX), found primarily in the liver. It is involved in detoxification. particularly in the metabolism of alcohol and other compounds, and is important in drug metabolism.
3.3. Interactions Between Minerals
3.3.1. Cu/Zn
3.3.2. Se-Pb, Se-Cd, Se-As, Se-Hg
3.3.3. Mo-Cu, Mo-Fe
3.3.4. Mo-W, Mo-Pb, Mo-Na
3.3.5. Mn-Fe
4. Potential Clinical Applications
- Pharmacotherapy, mainly non-steroidal anti-inflammatory drugs (NSAIDs) and hormonal drugs [74].
- Surgical treatment.
Supplementation as a Treatment Method
5. Elements with Antioxidant Properties as Part of Endometriosis Treatment
6. Conclusions and Directions for Future Research
- Strengths of the review:
- Weakness:
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BMI | Body Mass Index |
| EM | Endometriosis |
| GPX | glutathione peroxidase |
| MAPK | mitogen-activated protein kinase |
| MMP | metalloproteinases |
| ROS | reactive oxygen species |
References
- Pavone, M.; Baroni, A.; Campolo, F.; Goglia, M.; Raimondo, D.; Carcagnì, A.; Akladios, C.; Marescaux, J.; Fanfani, F.; Scambia, G.; et al. Robotic assisted versus laparoscopic surgery for deep endometriosis: A meta-analysis of current evidence. J. Robot Surg. 2024, 18, 212. [Google Scholar] [CrossRef] [PubMed]
- Giampaolino, P.; Della Corte, L.; Foreste, V.; Barra, F.; Ferrero, S.; Bifulco, G. Dioxin and endometriosis: A new possible relation based on epigenetic theory. Gynecol. Endocrinol. Off. J. Int. Soc. Gynecol. Endocrinol. 2020, 36, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wei, Y.; Liang, F.; Huang, Y.; Huang, J.; Luo, X.; Xie, B. Exploring the link between dietary zinc intake and endometriosis risk: Insights from a cross-sectional analysis of American women. BMC Public Health 2024, 24, 2935. [Google Scholar] [CrossRef]
- Huang, L.; Shi, L.; Li, M.; Yin, X.; Ji, X. Oxidative stress in endometriosis: Sources, mechanisms and therapeutic potential of antioxidants. Int. J. Mol. Med. 2025, 55, 72. [Google Scholar] [CrossRef]
- Saunders, P.T.K.; Horne, A.W. Endometriosis: Etiology, pathobiology, and therapeutic prospects. Cell 2021, 184, 2807–2824. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Chen, Y.; Lin, R.; Mo, T.; Li, S.; Cao, Y.; Yin, T.; Diao, L.; Li, Y. Endometriosis: A new perspective on epigenetics and oxidative stress. J. Rep. Immun. 2025, 169, 104462. [Google Scholar] [CrossRef]
- WHO. 2025. Available online: https://www.who.int/news-room/fact-sheets/detail/endometriosis (accessed on 10 May 2025).
- Vannuccini, S.; Clemenza, S.; Rossi, M.; Petraglia, F. Hormonal treatments for endometriosis: The endocrine background. Rev. Endocr. Metab. Disord. 2022, 23, 333–355. [Google Scholar] [CrossRef]
- Imanaka, S.; Maruyama, S.; Kimura, M.; Nagayasu, M.; Kobayashi, H. Towards an understanding of the molecular mechanisms of endometriosis-associated symptoms. World Acad. Sci. J. 2020, 2, 12. [Google Scholar] [CrossRef]
- Smolarz, B.; Szyłło, K.; Romanowicz, H. Endometriosis: Epidemiology, Classification, Pathogenesis, Treatment and Genetics (Review of Literature). Int. J. Mol. Sci. 2021, 22, 10554. [Google Scholar] [CrossRef]
- Harder, C.; Velho, R.V.; Brandes, I.; Sehouli, J.; Mechsner, S. Assessing the true prevalence of endometriosis: A narrative review of literature data. Int. J. Gynecol. Obstet. 2024, 167, 883–900. [Google Scholar] [CrossRef]
- Nouri, B.; Naz Agili, S.; Arab, M. Endometriosis: A Six-Year Epidemiological Study. J. Obstet. Gynecol. Cancer Res. 2024, 9, 335–339. [Google Scholar] [CrossRef]
- Vallée, A.; Feki, A.; Josseran, L.; Ayoubi, J. Smoking and endometriosis: A narrative review. Tob. Induc. Dis. 2025, 23, 85. [Google Scholar] [CrossRef]
- Parazzini, F.; Chiaffarino, F.; Surace, M.; Chatenoud, L.; Cipriani, S.; Chiantera, V.; Benzi, G.; Fedele, L. Selected food intake and risk of endometriosis. Hum. Reprod. 2004, 19, 1755–1759. [Google Scholar] [CrossRef]
- Su, X.; Yue, X.; Zhang, Y.; Shen, L.; Zhang, H.; Wang, X.; Yin, T.; Zhang, H.; Peng, J.; Wang, X.; et al. Elevated levels of Zn, Cu and Co are associated with an increased risk of endometriosis: Results from a case control study. Ecotoxicol. Environ. Saf. 2024, 271, 115932. [Google Scholar] [CrossRef] [PubMed]
- Cano-Herrera, G.; Salmun Nehmad, S.; Ruiz de Chávez Gascón, J.; Méndez Vionet, A.; van Tienhoven, X.A.; Osorio Martínez, M.F.; Muleiro Alvarez, M.; Vasco Rivero, M.X.; López Torres, M.F.; Barroso Valverde, M.J.; et al. Endometriosis: A Comprehensive Analysis of the Pathophysiology, Treatment, and Nutritional Aspects, and Its Repercussions on the Quality of Life of Patients. Biomedicines 2024, 12, 1476. [Google Scholar] [CrossRef] [PubMed]
- Mazza, E.; Troiano, E.; Mazza, S.; Ferro, Y.; Abbinante, A.; Agneta, M.T.; Montalcini, T.; Pujia, A. The impact of endometriosis on dietary choices and activities of everyday life: A cross-sectional study. Front. Nutr. 2023, 10, 1273976. [Google Scholar] [CrossRef] [PubMed]
- Matek Sarić, M.; Sorić, T.; Sarić, A.; Marušić, E.; Čoklo, M.; Mavar, M.; Ljubičić, M.; Lisica Šikić, N. The Role of Plant-Based Diets and Personalized Nutrition in Endometriosis Management: A Review. Medicina 2025, 61, 1264. [Google Scholar] [CrossRef]
- Abramiuk, M.; Mertowska, P.; Frankowska, K.; Świechowska Starek, P.; Satora, M.; Polak, G.; Dymanowska-Dyjak, I.; Grywalska, E. How Can Selected Dietary Ingredients Influence the Development and Progression of Endometriosis? Nutrients 2024, 16, 154. [Google Scholar] [CrossRef]
- Zhou, L.; Liu, B.; Jian, X.; Jiang, L.; Liu, K. Effect of dietary patterns and nutritional supplementation in the management of endometriosis: A review. Front. Nutr. 2025, 12, 1539665. [Google Scholar] [CrossRef]
- Nirgianakis, K.; Egger, K.; Kalaitzopoulos, D.R.; Lanz, S.; Bally, L.; Mueller, M.D. Effectiveness of dietary interventions in the treatment of endometriosis: A systematic review. Reprod. Sci. 2022, 29, 26–42. [Google Scholar] [CrossRef]
- Camajani, E.; Feraco, A.; Verde, L.; Moriconi, E.; Marchetti, M.; Colao, A.; Caprio, M.; Muscogiuri, G.; Barrea, L. Ketogenic diet as a possible non-pharmacological therapy in main endocrine diseases of the female reproductive system: A practical guide for nutritionists. Curr. Obes. Rep. 2023, 12, 231–249. [Google Scholar] [CrossRef]
- Muscogiuri, G.; Verde, L.; Frias-Toral, E.; Reytor-González, C.; Annunziata, G.; Proganò, M.; Savastano, S.; Simancas-Racines, D.; Colao, A.; Barrea, L. Weight loss, changes in body composition and inflammatory status after a very low-energy ketogenic therapy (VLEKT): Does gender matter? J. Transl. Med. 2024, 22, 949. [Google Scholar] [CrossRef] [PubMed]
- Dymanowska-Dyjak, I.; Frankowska, K.; Abramiuk, M.; Polak, G. Oxidative Imbalance in Endometriosis-Related Infertility—The Therapeutic Role of Antioxidants. Int. J. Mol. Sci. 2024, 25, 6298. [Google Scholar] [CrossRef] [PubMed]
- Barnard, N.D.; Holtz, D.N.; Schmidt, N.; Kolipaka, S.; Hata, E.; Sutton, M.; Znayenko-Miller, T.; Hazen, N.D.; Cobb, C.; Kahleova, H. Nutrition in the Prevention and Treatment of Endometriosis: A Review. Front. Nutr. 2023, 10, 1089891. [Google Scholar] [CrossRef] [PubMed]
- Türkoğlu, İ.; Sacinti, K.G.; Panattoni, A.; Namazov, A.; Sanlier, N.T.; Sanlier, N.; Cela, V. Eating for Optimization: Unraveling the Dietary Patterns and Nutritional Strategies in Endometriosis Management. Nutr. Rev. 2025, 83, 869–879. [Google Scholar] [CrossRef]
- Akgun, N.; Sofiyeva, N.; Yalcın, P.B.; Laganà, A.S.; Oral, E. Role of macronutrients, dairy products, fruits and vegetables in occurrence and progression of endometriosis: A summary of current evidence in a systematic review. Facts Views Vis. ObGyn 2024, 16, 409–428. [Google Scholar] [CrossRef]
- Lai, G.L.; Yeh, C.C.; Yeh, C.Y.; Chen, R.Y.; Fu, C.L.; Chen, C.H.; Tzeng, C.R. Decreased zinc and increased lead blood levels are associated with endometriosis in Asian Women. Reprod. Toxicol. 2017, 74, 77–84. [Google Scholar] [CrossRef]
- Li, D.; Jiang, T.; Wang, X.; Yin, T.; Shen, L.; Zhang, Z.; Zou, W.; Liu, Y.; Zong, K.; Liang, D.; et al. Serum Essential Trace Element Status in Women and the Risk of Endometrial Diseases: A Case-Control Study: Serum Essential Trace Element Status in Women and the Risk of Endometrial Diseases: A Case-Control Study. Biol. Trace Elem. Res. 2023, 201, 2151–2161. [Google Scholar] [CrossRef]
- Liu, Y.; Cheng, G.; Li, H.; Meng, Q. Serum copper to zinc ratio and risk of endometriosis: Insights from a case-control study in infertile patients. Reprod. Med. Biol. 2025, 24, e12644. [Google Scholar] [CrossRef]
- Pollack, A.Z.; Louis, G.M.; Chen, Z.; Peterson, C.M.; Sundaram, R.; Croughan, M.S.; Sun, L.; Hediger, M.L.; Stanford, J.B.; Varner, M.W.; et al. Trace elements and endometriosis: The ENDO study. Reprod. Toxicol. 2013, 42, 41–48. [Google Scholar] [CrossRef]
- Messalli, E.M.; Schettino, M.T.; Mainini, G.; Ercolano, S.; Fuschillo, G.; Falcone, F.; Esposito, E.; Di Donna, M.C.; De Franciscis, P.; Torella, M. The possible role of zinc in the etiopathogenesis of endometriosis. Clin. Exp. Obstet. Gynecol. 2014, 41, 541–546. [Google Scholar] [CrossRef]
- Turgut, A.; Ozler, A.; Goruk, N.Y.; Tunc, S.Y.; Evliyaoglu, O.; Gul, T. Copper, ceruloplasmin and oxidative stress in patients with advanced- stage endometriosis. Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 1472–1478. [Google Scholar] [PubMed]
- Haddaway, N.R.; Page, M.J.; Pritchard, C.C.; McGuinness, L.A. PRISMA2020: An R package and Shiny app for producing PRISMA 2020-compliant flow diagrams, with interactivity for optimised digital transparency and Open Synthesis. Campbell Syst. Rev. 2022, 18, e1230. [Google Scholar] [CrossRef] [PubMed]
- Hariharan, S.; Dharmaraj, S. Selenium and Selenoproteins: It’s role in regulation of inflammation. Inflammopharmacology 2020, 28, 667–695. [Google Scholar] [CrossRef] [PubMed]
- Klecha, B.; Bukowska, B. 2016. Selenium in human organism—Characteristic of the element and its potential therapeutic application. [Selen w organizmie człowieka—Charakterystyka pierwiastka i potencjalne zastosowanie terapeutyczne]. Bromat. Chem. Toksykol. XLIX 2016, 4, 818–829. (In polish) [Google Scholar]
- Janowska, M.; Potocka, N.; Paszek, S.; Skrzypa, M.; Wróbel, A.; Kluz, M.; Baszuk, P.; Marciniak, W.; Gronwald, J.; Lubiński, J.; et al. An Assessment of Serum Selenium Concentration in Women with Endometrial Cancer. Nutrients 2022, 14, 958. [Google Scholar] [CrossRef]
- Guerrero, H.C.A.; Bujalil Montenegro, L.; De la Jara, D.J.; Mier Cabrera, J.; Bouchán, V.P. Endometriosis and deficient intake of antioxidants molecules related to peripheral and peritoneal oxidative stress. Ginecol. Obstet. Mex. 2006, 74, 20–28. [Google Scholar]
- Singh, A.K.; Chattopadhyay, R.; Chakravarty, B.; Chaudhury, K. Markers of oxidative stress in follicular fluid of women with endometriosis and tubal infertility undergoing Ivf. Reprod. Toxicol. 2013, 42, 116–124. [Google Scholar] [CrossRef]
- Pei, J.; Pan, X.; Wei, G.; Hua, Y. Research progress of glutathione peroxidase family (GPX) in redoxidation. Front. Pharmacol. 2023, 14, 1147414. [Google Scholar] [CrossRef]
- Hou, W.; Xu, H. Incorporating selenium into heterocycles and natural products–From chemical properties to pharmacological activities. J. Med. Chem. 2022, 65, 4436–4456. [Google Scholar] [CrossRef]
- Chasapis, C.T.; Ntoupa, P.S.A.; Spiliopoulou, C.A.; Stefanidou, M.E. Recent aspects of the effects of zinc on human health. Arch. Toxicol. 2020, 94, 1443–1460. [Google Scholar] [CrossRef]
- Nasiadek, M.; Stragierowicz, J.; Klimczak, M.; Kilanowicz, A. The Role of Zinc in Selected Female Reproductive System Disorders. Nutrients 2020, 12, 2464. [Google Scholar] [CrossRef] [PubMed]
- Verit, F.F.; Erel, O.; Celik, N. Serum paraoxonase-1 activity in women with endometriosis and its relationship with the stage of the disease. Hum. Reprod. 2007, 23, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Prieto, L.; Quesada, J.F.; Cambero, O.; Pacheco, A.; Pellicer, A.; Codoceo, R.; Garcia-Velasco, J.A. Analysis of follicular fluid and serum markers of oxidative stress in women with infertility related to endometriosis. Fertil. Steril. 2012, 98, 126–130. [Google Scholar] [CrossRef]
- Vickram, S.; Rohini, K.; Srinivasan, S.; Nancy Veenakumari, D.; Archana, K.; Anbarasu, K.; Jeyanthi, P.; Thanigaivel, S.; Gulothungan, G.; Rajendiran, N.; et al. Role of Zinc (Zn) in Human- Reproduction: A Journey from Initial Spermatogenesis to Childbirth. Int. J. Mol. Sci. 2021, 22, 2188. [Google Scholar] [CrossRef]
- Kluza, K.; Zawlik, I.; Janowska, M.; Kmiéc, A.; Paszek, S.; Potocka, N.; Skrzypa, M.; Zuchowska, A.; Kluz, M.; Wróbel, A.; et al. Study of Serum Copper and Zinc Levels and Serum Cu/Zn Ratio among Polish Women with Endometrial Cancer. Nutrients 2024, 16, 144. [Google Scholar] [CrossRef]
- Stachowicz, K. Regulation of COX-2 expression by selected trace elements and heavy metals: Health implications, and changes in neuronal plasticity. A review. J. Trace Elem. Med. Biol. Organ Soc. Miner. Trace Elem. 2024, 79, 127226. [Google Scholar] [CrossRef]
- Schieven, G.L. Tyrosine Phosphorylation in Oxidative Stress. In Oxidative Stress and Signal Transduction; Forman, H.J., Cadenas, E., Eds.; Springer: Boston, MA, USA, 1997. [Google Scholar] [CrossRef]
- Ipson, B.R.; Green, R.A.; Wilson, J.T.; Watson, J.N.; Faull, K.F.; Fisher, A.L. Tyrosine aminotransferase is involved in the oxidative stress response by metabolizing meta-tyrosine in Caenorhabditis elegans. J. Biol. Chem. 2019, 294, 9536–9554. [Google Scholar] [CrossRef]
- Jomova, K.; Makova, M.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Rhodes, C.J.; Valko, M. Essential metals in health and disease. Chem. Biol. Interact. 2022, 367, 110173. [Google Scholar] [CrossRef]
- Lai, Z.Z.; Yang, H.L.; Ha, S.Y.; Chang, K.K.; Mei, J.; Zhou, W.J.; Qiu, X.M.; Wang, X.Q.; Zhu, R.; Li, D.J.; et al. Cyclooxygenase-2 in Endometriosis. Int. J. Biol. Sci. 2019, 15, 2783–2797. [Google Scholar] [CrossRef]
- Lin, Y.; Yuan, M.; Wang, G. Copper homeostasis and cuproptosis in gynecological disorders: Pathogenic insights and therapeutic implications. J. Trace Elem. Med. Biol. Organ Soc. Miner. Trace Elem. 2024, 84, 127436. [Google Scholar] [CrossRef]
- Marinov, B.; Tsachev, K.; Doganov, N.; Dzherov, L.; Atanasova, B.; Markova, M. The copper concentration in the blood serum of women with ovarian tumors (a preliminary report). Akusherstvo Ginekol. 2000, 39, 36–37. [Google Scholar]
- Kippler, M.; Oskarsson, A. Manganese—A scoping review for Nordic Nutrition Recommendations 2023. Food Nutr. Res. 2024, 68, 10-29219. [Google Scholar] [CrossRef] [PubMed]
- Winarto, H.; Tan, M.; Sadikin, M.; Wanandi, S. Expression Is Down-Regulated by Oxidative Stress in Endometriosis and Endometriosis-Associated Ovarian Cancer. Transl. Oncogenom. 2017, 9, 1177272716689818. [Google Scholar] [CrossRef] [PubMed]
- Kaleler, İ.; Acikgoz, A.S.; Gezer, A.; Uslu, E. A potential role of Sirtuin3 and its target enzyme activities in patients with ovarian endometrioma. Gynecol. Endocrinol. Off. J. Int. Soc. Gynecol. Endocrinol. 2021, 37, 1035–1040. [Google Scholar] [CrossRef]
- Ota, H.; Igarashi, S.; Tanaka, T. Xanthine oxidase in eutopic and ectopic endometrium in endometriosis and adenomyosis. Fertil. Steril. 2001, 75, 785–790. [Google Scholar] [CrossRef]
- Struwe, M.A.; Scheidig, A.J.; Clement, B. The mitochondrial amidoxime reducing component-from prodrug-activation mechanism to drug-metabolizing enzyme and onward to drug target. J. Biol. Chem. 2023, 299, 105306. [Google Scholar] [CrossRef]
- Trapero, C.; Vidal, A.; Fernández-Montolí, M.E.; Coroleu, B.; Tresserra, F.; Barri, P.; De Aranda, I.G.; Sévigny, J.; Ponce, J.; Matias-Guiu, X.; et al. Impaired Expression of Ectonucleotidases in Ectopic and Eutopic Endometrial Tissue Is in Favor of ATP Accumulation in the Tissue Microenvironment in Endometriosis. Int. J. Mol. Sci. 2019, 20, 5532. [Google Scholar] [CrossRef]
- Alotaibi, F.T. Pathophysiology of Endometriosis: Insights from Immunohistochemical Analysis of Ectopic and Eutopic Tissues. Int. J. Mol. Sci. 2025, 26, 5998. [Google Scholar] [CrossRef]
- Ivanova, I.D.; Pal, A.; Simonelli, I.; Atanasova, B.; Ventriglia, M.; Rongioletti, M.; Squitti, R. Evaluation of zinc, copper, and Cu: Zn ratio in serum, and their implications in the course of COVID-19. J. Trace Elem. Med. Biol. 2022, 71, 126944. [Google Scholar] [CrossRef]
- Mravunac, M.; Szymlek- Gay, E.A.; Daly, R.M.; Roberts, B.R.; Formica, M.; Gianoudis, J.; O’Connell, S.L.; Nowson, C.A.; Cardoso, B.R. Greater circulating copper concentrations and cop per/zinc ratios are associated with lower psychological distress, but not cognitive performance, in a sample of Australian older adults. Nutrients 2019, 11, 2503. [Google Scholar] [CrossRef]
- Tolunay, H.E.; Şükür, Y.E.; Ozkavukcu, S.; Seval, M.M.; Ateş, C.; Türksoy, V.A.; Ecemiş, T.; Atabekoğlu, C.S.; Özmen, B.; Berker, B.; et al. Heavy metal and trace element concentrations in blood and follicular fluid affect ART outcome. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016, 198, 73–77. [Google Scholar] [CrossRef]
- Zakerkish, F.; Brännström, M.; Carlsohn, E.; Sihlbom, C.; van der Post, S.; Thoroddsen, A. Proteomic analysis of follicular fluid during human ovulation. Acta Obstet. Gynecol. Scand. 2020, 99, 917–924. [Google Scholar] [CrossRef]
- Schmalbrock, L.J.; Weiss, G.; Rijntjes, E.; Reinschissler, N.; Sun, Q.; Schenk, M.; Schomburg, L. Pronounced Trace Element Variation in Follicular Fluids of Subfertile Women Undergoing Assisted Reproduction. Nutrients 2021, 13, 4134. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on Dietary Reference Values for zinc. EFSA J. 2014, 12, 3844. [Google Scholar] [CrossRef]
- Ramírez-Acosta, S.; Uhlírová, R.; Navarro, F.; Gómez-Ariza, J.; García-Barrera, T. Antagonistic Interaction of Selenium and Cadmium in Human Hepatic Cells Through Selenoproteins. Front. Chem. 2022, 1, 891933. [Google Scholar] [CrossRef] [PubMed]
- Osuchowska-Grochowska, I.; Blicharska, E.; Gogacz, M.; Nogalska, A.; Winkler, I.; Szopa, A.; Ekiert, H.; Tymczyna-Borowicz, B.; Rahnama-Hezavah, M.; Grochowski, C. Brief Review of Endometriosis and the Role of Trace Elements. Int. J. Mol. Sci. 2021, 22, 11098. [Google Scholar] [CrossRef]
- Gao, X.Y.; Zhang, Y.; Zhao, W.P.; Tian, E.J.; Ommati, M.M.; Wang, J.C.; Wang, H.W.; Zhou, B.H. Molybdenum interferes with MMPs/TIMPs expression to reduce the receptivity of porcine endometrial epithelial cells. Chem. Biol. Interact. 2025, 405, 111304. [Google Scholar] [CrossRef]
- López-Botella, A.; Romero, A.; Sánchez, R.; Todolí-Torró, J.L.; Velasco, I.; Acién, M.; Gómez-Torres, M.J. Unexpected high levels of iron and rubidium in the peritoneal fluid of endometriosis patients. Sci. Total Environ. 2025, 998, 180270. [Google Scholar] [CrossRef]
- Kek, T.; Geršak, K.; Karas Kuželički, N.; Celar Šturm, D.; Mazej, D.; Snoj Tratnik, J.; Falnoga, I.; Horvat, M.; Virant-Klun, I. Associations of Essential and Non-Essential Trace Elements’ Levels in the Blood, Serum, and Urine in Women with Premature Ovarian Insufficiency. Biol. Trace Elem. Res. 2025, 203, 4439–4456. [Google Scholar] [CrossRef]
- Polak, G.; Barczyński, B.; Wertel, I.; Kwaśniewski, W.; Bednarek, W.; Derewianka-Polak, M.; Frąszczak, K.; Olajossy, M.; Kotarski, J. Disrupted iron metabolism in peritoneal fluid may induce oxidative stress in the peritoneal cavity of women with endometriosis. Ann. Agric. Environ. Med. 2018, 25, 587–592. [Google Scholar] [CrossRef]
- Biasioli, A.; Xholli, A.; Previtera, F.; Balzano, A.; Capodicasa, V.; Tassi, A.; Londero, A.P.; Cagnacci, A. Systemic Oxidative Stress in Women with Ovarian and Pelvic Endometriosis: Role of Hormonal Therapy. J. Clin. Med. 2022, 11, 7460. [Google Scholar] [CrossRef]
- Gu, X.; Zhou, H.; Miao, M.; Hu, D.; Wang, X.; Zhou, J.; Teichmann, A.T.; Yang, Y.; Wang, C. Therapeutic Potential of Natural Resources Against Endometriosis: Current Advances and Future Perspectives. Drug Des. Dev. Ther. 2024, 18, 3667–3696. [Google Scholar]
- Habib, N.; Buzzaccarini, G.; Centini, G.; Moawad, G.N.; Ceccaldi, P.F.; Gitas, G.; Alkatout, I.; Gullo, G.; Terzic, S.; Sleiman, Z. Impact of lifestyle and diet on endometriosis: A fresh look to a busy corner. Menopause Rev. 2022, 212, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Yavuz, S.; Aydin, N.E.; Celik, O.; Yilmaz, E.; Ozerol, E.; Tanbek, K. Resveratrol successfully treats experimental endometriosis through modulation of oxidative stress and lipid peroxidation. J. Cancer Res. Ther. 2014, 1, 324–329. [Google Scholar] [CrossRef]
- Mier-Cabrera, J.; Aburto-Soto, T.; Burrola-Méndez, S.; Jiménez-Zamudio, L.; Tolentino, M.C.; Casanueva, E.; Hernández-Guerrero, C. Women with endometriosis improved their peripheral antioxidant markers after the application of a high antioxidant diet. Reprod. Biol. Endocrinol. 2009, 7, 54. [Google Scholar] [CrossRef] [PubMed]
- Bayu, P.; Wibisono, J.J. Vitamin C and E antioxidant supplementation may significantly reduce pain symptoms in endometriosis: A systematic review and meta-analysis of randomized controlled trials. PLoS ONE 2024, 19, e0301867. [Google Scholar] [CrossRef]
- Ibrahim Abd El-Fadil Sehsah, F.; Taha Abd El-Fattah, A.; Mohammed Saeed, A. The Role of Antioxidant Supplementation in Reducing the Endometriosis Related Chronic Pelvic Pain in Women. Al-Azhar Med. J. 2022, 51, 121–134. [Google Scholar] [CrossRef]
- Socha, K.; Kochanowicz, J.; Karpińska, E.; Soroczyńska, J.; Jakoniuk, M.; Mariak, Z.; Borawska, M.H. Dietary habits and selenium, glutathione peroxidase and total antioxidant status in the serum of patients with relapsing-remitting multiple sclerosis. Nutr. J. 2014, 13, 62. [Google Scholar] [CrossRef]
- Hedera, P.; Peltier, A.; Fink, J.K.; Wilcock, S.; London, Z.; Brewer, G.J. Myelopolyneuropathy and pancytopenia due to copper deficiency and high zinc levels of unknown origin II. The denture cream is a primary source of excessive zinc. Neurotox 2009, 30, 996–999. [Google Scholar] [CrossRef]
- Oberweis, D.; Madelenat, P.; Nisolle, M.; Demanet, E. A pilot double-blind, randomized, placebo-controlled trial of the efficacy of trace elements in the treatment of endometriosis-related pain: Study design and methodology. Nutr. Diet. Suppl. 2016, 8, 1. [Google Scholar] [CrossRef]
- Michalczyk, K.; Cymbaluk-Płoska, A. The Role of Zinc and Copper in Gynecological Malignancies. Nutrients 2020, 12, 3732. [Google Scholar] [CrossRef]
- Arab, A.; Karimi, E.; Vingrys, K.; Kelishadi, M.R.; Mehrabani, S.; Askari, G. Food groups and nutrients consumption and risk of endometriosis: A systematic review and meta-analysis of observational studies. Nutr. J. 2022, 21, 58. [Google Scholar] [CrossRef]
- Martire, F.G.; Costantini, E.; d’Abate, C.; Capria, G.; Piccione, E.; Andreoli, A. Endometriosis and Nutrition: Therapeutic Perspectives. J. Clin. Med. 2025, 14, 3987. [Google Scholar] [CrossRef] [PubMed]
- Afrin, S.; AlAshqar, A.; El Sabeh, M.; Miyashita-Ishiwata, M.; Reschke, L.; Brennan, J.T.; Fader, A.; Borahay, M.A. Diet and nutrition in gynecological disorders: A focus on clinical studies. Nutrients 2021, 1, 1747. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, N.R.M.; Afeiche, M.C.; Terry, K.L.; Farland, L.V.; Chavarro, J.E.; Missmer, S.A.; Harris, H.R. Glycemic index, glycemic load, fiber, and gluten intake and risk of laparoscopically confirmed endometriosis in premenopausal women. J. Nutr. 2022, 1, 2088–2096. [Google Scholar] [CrossRef]
- Meneghetti, J.K.; Pedrotti, M.T.; Coimbra, I.M.; da Cunha-Filho, J.S.L. Effect of dietary interventions on endometriosis: A systematic review and meta- analysis of randomized controlled trials. Reprod. Sci. 2024, 31, 3613–3623. [Google Scholar] [CrossRef]
- Salmeri, N.; Ragusi, A.; Buffo, C.; Somigliana, E.; Viganò, P.; Vercellini, P. Dietary Supplements for Endometriosis-Associated Pain: A Systematic Review and Meta-Analysis of Randomized Placebo-Controlled Trials. Gynecol. Obstet. Investig. [CrossRef]
- Román, G.C.; Jackson, R.E.; Gadhia, R.; Román, A.N.; Reis, J. Mediterranean diet: The role of long-chain omega-3 fatty acids in fish; polyphenols in fruits, vegetables, cereals, coffee, tea, cacao and wine; probiotics and vitamins in prevention of stroke, age-related cognitive decline, and Alzheimer disease. Rev. Neurol. 2019, 1, 724–741. [Google Scholar] [CrossRef]
- Dring, J.C.; Forma, A.; Chilimoniuk, Z.; Dobosz, M.; Teresiński, G.; Buszewicz, G.; Flieger, J.; Cywka, T.; Januszewski, J.; Baj, J. Essentiality of Trace Elements in Pregnancy, Fertility, and Gynecologic Cancers-A State-of-the-Art Review. Nutrients 2021, 14, 185. [Google Scholar] [CrossRef]
- Bost, M.; Houdart, S.; Oberli, M.; Kalonji, E.; Huneau, J.F.; Margaritis, I. Dietary copper and human health: Current evidence and unresolved issues. J. Trace Elem. Med. Biol. Organ Soc. Miner. Trace Elem. GMS 2016, 35, 107–115. [Google Scholar] [CrossRef]
- Blomhoff, R.; Andersen, R.; Arnesen, E.K.; Christensen, J.J.; Eneroth, H.; Erkkola, M.; Gudanaviciene, I.; Halldórsson, Þ.; Ingi, H.-L.; Anne, L.; et al. Nordic Nutrition Recommendations 2023; Nordic Council of Ministers: Copenhagen, Denmark, 2023. [Google Scholar]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific opinion on the tolerable upper intake level for manganese. EFSA J. 2023, 21, e8413. [Google Scholar]
- Hattori, H.; Ashida, A.; Itô, C.; Yoshida, M. Determination of molybdenum in foods and human milk, and an estimate of average molybdenum intake in the Japanese population. Nutr. Sci. Vitaminol. 2004, 50, 404–409. [Google Scholar] [CrossRef] [PubMed][Green Version]
- EFSA Panel on Dietetic Products, Nutrition, and Allergies (NDA). Scientific opinion on dietary reference values for molybdenum. EFSA J. 2013, 11, 3333. [Google Scholar] [CrossRef]
| Element | Body Fluids | Number of Samples | Women with Endometriosis | Control Group | Observations | References |
|---|---|---|---|---|---|---|
| Se | blood | 217 cases/234 controls | 151.15 μg/L | 131.63 μg/L | Significantly increased Sn levels in EM group | [15] |
| Se | serum | 302 cases/ 302 controls | 94.71 μg/L | 97.71 μg/L | Significantly decreased Se levels in EM group | [29] |
| Se | follicular fluid | 182 cases/203 controls | 52.65 μg/L | 40.51 μg/L | Significantly increased Se levels in EM group | [15] |
| Zn | blood | 68 cases/122 controls | 6.72 mg/L | 11.86 mg/L | Significantly decreased Zn levels in EM group | [28] |
| Zn | serum | 302 cases/302 controls | 921.68 μg/L | 945.98 μg/L | Significantly decreased Zn levels in EM group | [29] |
| Zn | serum | 42 cases/44 controls | 1.01 ± 59.2 μg/L | 1.29 ± 62.22 μg/L | Significantly decreased Zn levels in EM group | [32] |
| Zn | blood | 217 cases/234 controls | 8666.4 μg/L | 4847.88 μg/L | Significantly increased Zn levels in EM group | [15] |
| Zn | serum | 568 cases/819 controls | 14.6 μmol/L | 15.1 μmol/L | Significantly decreased Zn levels in EM group | [30] |
| Zn | follicular fluid | 182 cases/203 controls | 475.91 μg/L | 290.11 μg/L | Significantly increased Zn levels in EM group | [15] |
| Zn | urine | 190 cases/283 controls | 265.64 μg/L | 283.96 μg/L | No significant differences | [31] |
| Cu | serum | 302 cases/302 controls | 1528.25 μg/L | 1465.56 μg/L | Significantly increased Cu levels in EM group | [29] |
| Cu | serum | 31 cases/41 controls | 1088 ± 273.58 μg/mL | 811.20 ± 265.77 μg/mL | Significantly increased Cu levels in EM group | [33] |
| Cu | blood | 68 cases/122 controls | 0.39 mg/L | 0.48 mg/L | No significant differences | [28] |
| Cu | serum | 568 cases/819 controls | 15.77 μmol/L | 15.6 μmol/L | No significant differences | [30] |
| Cu | blood | 217 cases/234 controls | 963.05 μg/L | 991.18 μg/L | No significant differences | [15] |
| Cu | follicular fluid | 182 cases/203 controls | 759.43 μg/L | 479.51 μg/L | Significantly increased Cu levels in EM group | [15] |
| Cu | urine | 190 cases/283 controls | 10.64 μg/L | 10.33 μg/L | No significant differences | [31] |
| Mo | blood | 217 cases/234 controls | 1.19 μg/L | 1.37 μg/L | No significant differences | [15] |
| Mo | follicular fluid | 182 cases/203 controls | 0.99 μg/L | 0.56 μg/L | Significantly increased Mo levels in EM group | [15] |
| Mo | serum | 302 cases/302 controls | 1.23 μg/L | 1.13 μg/L | Significantly increased Mo levels in EM group | [29] |
| Mo | urine | 190 cases/283 controls | 44.49 μg/L | 44.56 μg/L | No significant differences | [31] |
| Mn | urine | 190 cases/283 controls | 1.41 μg/L | 1.39 μg/L | No significant differences | [31] |
| Mn | blood | 68 cases/122 controls | 0.72 μg/L | 0.65 μg/L | No significant differences | [28] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pokorska-Niewiada, K.; Ziętek, M.; Szydłowska, I.; Ryterska, K.; Szczuko, M. The Role of Antioxidant Minerals in the Pathophysiology and Treatment of Endometriosis—Systematic Review. Antioxidants 2025, 14, 1238. https://doi.org/10.3390/antiox14101238
Pokorska-Niewiada K, Ziętek M, Szydłowska I, Ryterska K, Szczuko M. The Role of Antioxidant Minerals in the Pathophysiology and Treatment of Endometriosis—Systematic Review. Antioxidants. 2025; 14(10):1238. https://doi.org/10.3390/antiox14101238
Chicago/Turabian StylePokorska-Niewiada, Kamila, Maciej Ziętek, Iwona Szydłowska, Karina Ryterska, and Małgorzata Szczuko. 2025. "The Role of Antioxidant Minerals in the Pathophysiology and Treatment of Endometriosis—Systematic Review" Antioxidants 14, no. 10: 1238. https://doi.org/10.3390/antiox14101238
APA StylePokorska-Niewiada, K., Ziętek, M., Szydłowska, I., Ryterska, K., & Szczuko, M. (2025). The Role of Antioxidant Minerals in the Pathophysiology and Treatment of Endometriosis—Systematic Review. Antioxidants, 14(10), 1238. https://doi.org/10.3390/antiox14101238








