Does Gut Microbial Methylglyoxal Metabolism Impact Human Physiology?
Abstract
1. Introduction
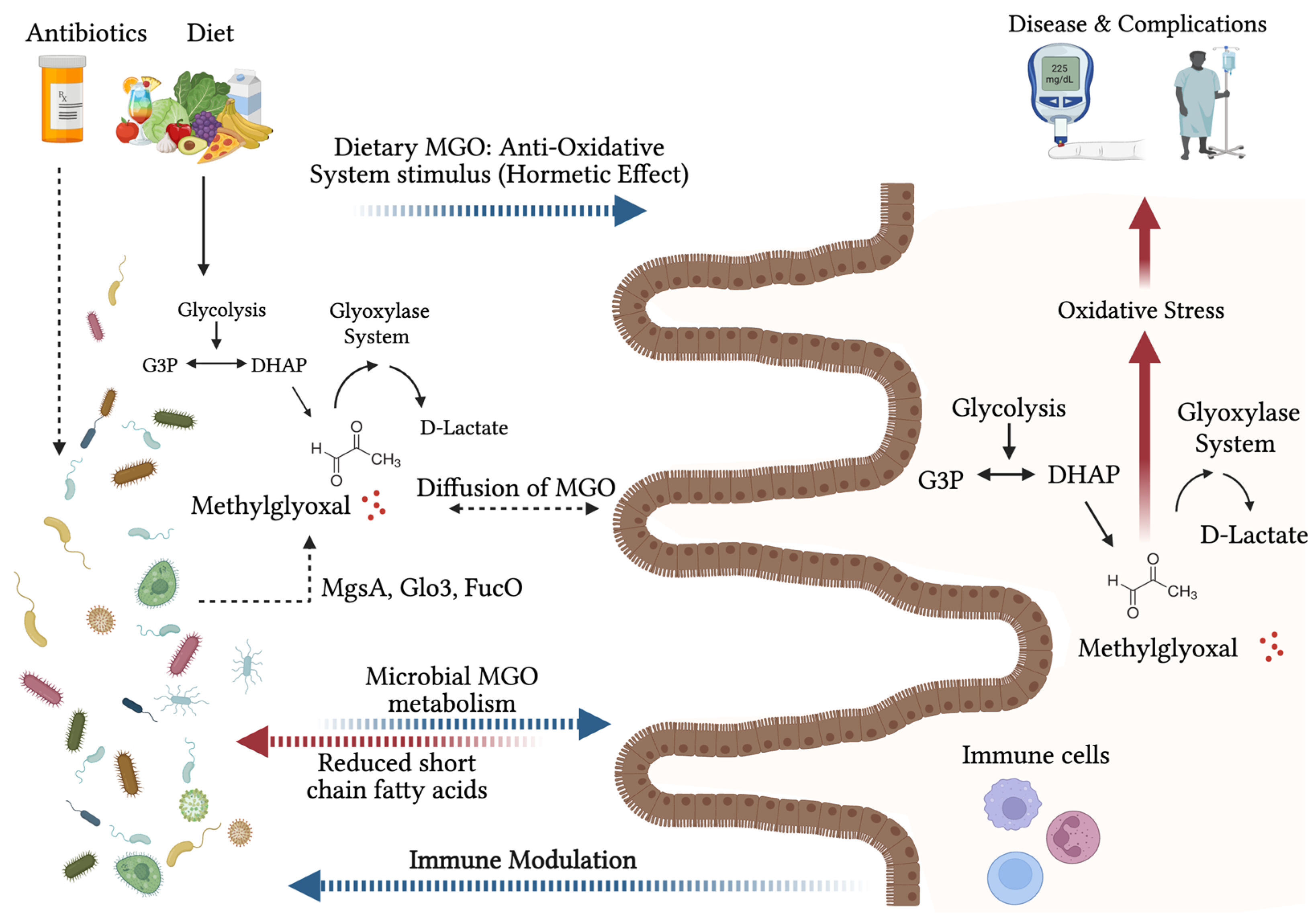
2. Host Production of Methylglyoxal: Linking Transient Increases in Glycolysis to Long-Term Cellular Dysfunction
2.1. Infections—An Emerging Inducer of Methylglyoxal Stress
2.2. How Essential Is Glyoxalase 1 for Maintaining Methylglyoxal Levels?
3. Methylglyoxal and the Microbiome
3.1. Theories on Why Microorganisms Produce Methylglyoxal
3.2. Microbial Detoxification of Methylglyoxal
3.3. The Microbiome as a Potential Therapeutic Target to Lower Methylglyoxal Stress in the Host
4. Dietary Methylglyoxal
4.1. Methylglyoxal in Manuka Honey
4.2. Metabolism of Methylglyoxal in the Gut
4.3. Consequences of Dietary Methylglyoxal
5. Consequences of Methylglyoxal Stress
5.1. Methylglyoxal and “Classic” Microvascular Diabetic Complications—Its Mechanisms of Action
5.2. Methylglyoxal as an Inducer of Cerebral Ageing and Cellular Senescence
5.3. Methylglyoxal and Cardiovascular Disease
5.4. Methylglyoxal and Cancer
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AGEs | Advanced glycation end products |
| DHAP | Dihydroxyacetone phosphate |
| FFQs | Food Frequency Questionnaires |
| G3P | Glyceraldehyde-3-phosphate |
| GLO1 | Glyoxalase 1 |
| GLO2 | Glyoxalase 2 |
| GSH | Glutathione |
| MGO | Methylglyoxal |
| SCFA | Short-chain fatty acids |
References
- Schalkwijk, C.G.; Stehouwer, C.D.A. Methylglyoxal, a Highly Reactive Dicarbonyl Compound, in Diabetes, Its Vascular Complications, and Other Age-Related Diseases. Physiol. Rev. 2020, 100, 407–461. [Google Scholar] [CrossRef] [PubMed]
- Inoue, Y.; Kimura, A. Methylglyoxal and regulation of its metabolism in microorganisms. Adv. Microb. Physiol. 1995, 37, 177–227. [Google Scholar] [PubMed]
- Ranganathan, S.; Walsh, E.S.; Godwin, A.K.; Tew, K.D. Cloning and characterization of human colon glyoxalase-I. J. Biol. Chem. 1993, 268, 5661–5667. [Google Scholar] [CrossRef]
- Berends, E.; van Oostenbrugge, R.J.; Foulquier, S.; Schalkwijk, C.G. Methylglyoxal, a highly reactive dicarbonyl compound, as a threat for blood brain barrier integrity. Fluids Barriers CNS 2023, 20, 75. [Google Scholar] [CrossRef] [PubMed]
- Baars, D.P.; Fondevila, M.F.; Meijnikman, A.S.; Nieuwdorp, M. The central role of the gut microbiota in the pathophysiology and management of type 2 diabetes. Cell Host Microbe 2024, 32, 1280–1300. [Google Scholar] [CrossRef]
- Fromentin, S.; Forslund, S.K.; Chechi, K.; Aron-Wisnewsky, J.; Chakaroun, R.; Nielsen, T.; Tremaroli, V.; Ji, B.; Prifti, E.; Myridakis, A.; et al. Microbiome and metabolome features of the cardiometabolic disease spectrum. Nat. Med. 2022, 28, 303–314. [Google Scholar] [CrossRef]
- Suez, J.; Korem, T.; Zeevi, D.; Zilberman-Schapira, G.; Thaiss, C.A.; Maza, O.; Israeli, D.; Zmora, N.; Gilad, S.; Weinberger, A.; et al. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature 2014, 514, 181–186. [Google Scholar] [CrossRef]
- Vrieze, A.; Van Nood, E.; Holleman, F.; Salojärvi, J.; Kootte, R.S.; Bartelsman, J.F.W.M.; Dallinga-Thie, G.M.; Ackermans, M.T.; Serlie, M.J.; Oozeer, R.; et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology 2012, 143, 913–916.e7. [Google Scholar] [CrossRef]
- Gandhi, N.N.; Cobra, P.F.; Steele, J.L.; Markley, J.L.; Rankin, S.A. Lactobacillus demonstrate thiol-independent metabolism of methylglyoxal: Implications toward browning prevention in Parmesan cheese. J. Dairy Sci. 2018, 101, 968–978. [Google Scholar] [CrossRef]
- Huang, K.; Rudolph, F.B.; Bennett, G.N. Characterization of methylglyoxal synthase from Clostridium acetobutylicum ATCC 824 and its use in the formation of 1,2-propanediol. Appl. Environ. Microbiol. 1999, 65, 3244–3247. [Google Scholar] [CrossRef]
- Saxena, R.K.; Anand, P.; Saran, S.; Isar, J.; Agarwal, L. Microbial production and applications of 1,2-propanediol. Indian J. Microbiol. 2010, 50, 2–11. [Google Scholar] [CrossRef]
- Sun, S.; Shu, L.; Lu, X.; Wang, Q.; Tišma, M.; Zhu, C.; Shi, J.; Baganz, F.; Lye, G.J.; Hao, J. 1,2-Propanediol production from glycerol via an endogenous pathway of Klebsiella pneumoniae. Appl. Microbiol. Biotechnol. 2021, 105, 9003–9016. [Google Scholar] [CrossRef]
- Bennett, G.N.; San, K.Y. Microbial formation, biotechnological production and applications of 1,2-propanediol. Appl. Microbiol. Biotechnol. 2001, 55, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Baskaran, S.; Prasanna Rajan, D.; Balasubramanian, K.A. Formation of methylglyoxal by bacteria isolated from human faeces. J. Med. Microbiol. 1989, 28, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Degen, J.; Hellwig, M.; Henle, T. 1,2-dicarbonyl compounds in commonly consumed foods. J. Agric. Food Chem. 2012, 60, 7071–7079. [Google Scholar] [CrossRef] [PubMed]
- Vašková, J.; Kováčová, G.; Pudelský, J.; Palenčár, D.; Mičková, H. Methylglyoxal Formation—Metabolic Routes and Consequences. Antioxidants 2025, 14, 212. [Google Scholar] [CrossRef]
- Thornalley, P.J.; Langborg, A.; Minhas, H.S. Formation of glyoxal, methylglyoxal and 3-deoxyglucosone in the glycation of proteins by glucose. Biochem. J. 1999, 344 Pt 1, 109–116. [Google Scholar] [CrossRef]
- Rabbani, N.; Thornalley, P.J. Dicarbonyl proteome and genome damage in metabolic and vascular disease. Biochem. Soc. Trans. 2014, 42, 425–432. [Google Scholar] [CrossRef]
- Thornalley, P.J. Modification of the glyoxalase system in human red blood cells by glucose in vitro. Biochem. J. 1988, 254, 751–755. [Google Scholar] [CrossRef]
- Borysiuk, K.; Ostaszewska-Bugajska, M.; Vaultier, M.N.; Hasenfratz-Sauder, M.P.; Szal, B. Enhanced Formation of Methylglyoxal-Derived Advanced Glycation End Products in Arabidopsis Under Ammonium Nutrition. Front. Plant Sci. 2018, 9, 667. [Google Scholar] [CrossRef]
- Allaman, I.; Bélanger, M.; Magistretti, P.J. Methylglyoxal, the dark side of glycolysis. Front Neurosci. 2015, 9, 23. [Google Scholar] [CrossRef] [PubMed]
- Kalapos, M.P. Methylglyoxal and glucose metabolism: A historical perspective and future avenues for research. Drug Metabol. Drug Interact 2008, 23, 69–91. [Google Scholar] [CrossRef] [PubMed]
- Lai, S.W.T.; Lopez Gonzalez, E.D.J.; Zoukari, T.; Ki, P.; Shuck, S.C. Methylglyoxal and Its Adducts: Induction, Repair, and Association with Disease. Chem. Res. Toxicol. 2022, 35, 1720–1746. [Google Scholar] [CrossRef]
- Rabbani, N.; Thornalley, P.J. Measurement of methylglyoxal by stable isotopic dilution analysis LC-MS/MS with corroborative prediction in physiological samples. Nat. Protoc. 2014, 9, 1969–1979. [Google Scholar] [CrossRef]
- Morgenstern, J.; Campos Campos, M.; Nawroth, P.; Fleming, T. The Glyoxalase System—New Insights into an Ancient Metabolism. Antioxidants 2020, 9, 939. [Google Scholar] [CrossRef] [PubMed]
- Synold, T.; Xi, B.; Wuenschell, G.E.; Tamae, D.; Figarola, J.L.; Rahbar, S.; Termini, J. Advanced Glycation End Products of DNA: Quantification of N2-(1-carboxyethyl)-2’-deoxyguanosine (CEdG) in Biological Samples by LC-ESI-MS/MS. Chem. Res. Toxicol. 2008, 21, 2148–2155. [Google Scholar] [CrossRef]
- Galligan, J.J.; Wepy, J.A.; Streeter, M.D.; Kingsley, P.J.; Mitchener, M.M.; Wauchope, O.R.; Beavers, W.N.; Rose, K.L.; Wang, T.; Spiegel, D.A.; et al. Methylglyoxal-derived posttranslational arginine modifications are abundant histone marks. Proc. Natl. Acad. Sci. USA 2018, 115, 9228–9233. [Google Scholar] [CrossRef]
- El-Osta, A.; Brasacchio, D.; Yao, D.; Pocai, A.; Jones, P.L.; Roeder, R.G.; Cooper, M.E.; Brownlee, M. Transient high glucose causes persistent epigenetic changes and altered gene expression during subsequent normoglycemia. J. Exp. Med. 2008, 205, 2409–2417. [Google Scholar] [CrossRef]
- Chan, C.; Huang, D.; Huang, Y.; Hsu, S.; Kang, L.; Shen, C.; Lin, W. Methylglyoxal induces cell death through endoplasmic reticulum stress-associated ROS production and mitochondrial dysfunction. J. Cell Mol. Med. 2016, 20, 1749–1760. [Google Scholar] [CrossRef]
- Brouwers, O.; Niessen, P.M.G.; Miyata, T.; Østergaard, J.A.; Flyvbjerg, A.; Peutz-Kootstra, C.J.; Sieber, J.; Mundel, P.H.; Brownlee, M.; Janssen, B.J.A.; et al. Glyoxalase-1 overexpression reduces endothelial dysfunction and attenuates early renal impairment in a rat model of diabetes. Diabetologia 2014, 57, 224–235. [Google Scholar] [CrossRef]
- Queisser, M.A.; Yao, D.; Geisler, S.; Hammes, H.P.; Lochnit, G.; Schleicher, E.D.; Brownlee, M.; Preissner, K.T. Hyperglycemia impairs proteasome function by methylglyoxal. Diabetes 2010, 59, 670–678. [Google Scholar] [CrossRef] [PubMed]
- Shinohara, M.; Thornalley, P.J.; Giardino, I.; Beisswenger, P.; Thorpe, S.R.; Onorato, J.; Brownlee, M. Overexpression of glyoxalase-I in bovine endothelial cells inhibits intracellular advanced glycation endproduct formation and prevents hyperglycemia-induced increases in macromolecular endocytosis. J. Clin. Investig. 1998, 101, 1142–1147. [Google Scholar] [CrossRef]
- Nokin, M.J.; Durieux, F.; Bellier, J.; Peulen, O.; Uchida, K.; Spiegel, D.A.; Cochrane, J.R.; Hutton, C.A.; Castronovo, V.; Bellahcène, A. Hormetic potential of methylglyoxal, a side-product of glycolysis, in switching tumours from growth to death. Sci. Rep. 2017, 7, 11722. [Google Scholar] [CrossRef]
- Bollong, M.J.; Lee, G.; Coukos, J.S.; Yun, H.; Zambaldo, C.; Chang, J.W.; Chin, E.N.; Ahmad, I.; Chatterjee, A.K.; Lairson, L.L.; et al. A metabolite-derived protein modification integrates glycolysis with KEAP1-NRF2 signaling. Nature 2018, 562, 600–604. [Google Scholar] [CrossRef] [PubMed]
- Hanssen, N.M.J.; Wouters, K.; Huijberts, M.S.; Gijbels, M.J.; Sluimer, J.C.; Scheijen, J.L.J.M.; Heeneman, S.; Biessen, E.A.L.; Daemen, M.J.A.P.; Brownlee, M.; et al. Higher levels of advanced glycation endproducts in human carotid atherosclerotic plaques are associated with a rupture-prone phenotype. Eur. Heart J. 2014, 35, 1137–1146. [Google Scholar] [CrossRef]
- Van Herreweghe, F.; Mao, J.; Chaplen, F.W.R.; Grooten, J.; Gevaert, K.; Vandekerckhove, J.; Vancompernolle, K. Tumor necrosis factor-induced modulation of glyoxalase I activities through phosphorylation by PKA results in cell death and is accompanied by the formation of a specific methylglyoxal-derived AGE. Proc. Natl. Acad. Sci. USA 2002, 99, 949–954. [Google Scholar] [CrossRef]
- Zhang, X.; Schalkwijk, C.G.; Wouters, K. Immunometabolism and the modulation of immune responses and host defense: A role for methylglyoxal? Biochim. Biophys. Acta BBA Mol. Basis Dis. 2022, 1868, 166425. [Google Scholar] [CrossRef] [PubMed]
- Rachman, H.; Kim, N.; Ulrichs, T.; Baumann, S.; Pradl, L.; Nasser Eddine, A.; Bild, M.; Rother, M.; Kuban, R.J.; Lee, J.S.; et al. Critical role of methylglyoxal and AGE in mycobacteria-induced macrophage apoptosis and activation. PLoS ONE 2006, 1, e29. [Google Scholar] [CrossRef]
- Brenner, T.; Fleming, T.; Uhle, F.; Silaff, S.; Schmitt, F.; Salgado, E.; Ulrich, A.; Zimmermann, S.; Bruckner, T.; Martin, E.; et al. Methylglyoxal as a new biomarker in patients with septic shock: An observational clinical study. Crit. Care 2014, 18, 683. [Google Scholar] [CrossRef]
- Schmoch, T.; Uhle, F.; Siegler, B.H.; Fleming, T.; Morgenstern, J.; Nawroth, P.P.; Weigand, M.A.; Brenner, T. The Glyoxalase System and Methylglyoxal-Derived Carbonyl Stress in Sepsis: Glycotoxic Aspects of Sepsis Pathophysiology. Int. J. Mol. Sci. 2017, 18, 657. [Google Scholar] [CrossRef]
- Zhang, M.M.; Ong, C.L.Y.; Walker, M.J.; McEwan, A.G. Defence against methylglyoxal in Group A Streptococcus: A role for Glyoxylase I in bacterial virulence and survival in neutrophils? Pathog. Dis. 2016, 74, ftv122. [Google Scholar] [CrossRef]
- Chakraborty, S.; Chaudhuri, D.; Balakrishnan, A.; Chakravortty, D. Salmonella methylglyoxal detoxification by STM3117-encoded lactoylglutathione lyase affects virulence in coordination with Salmonella pathogenicity island 2 and phagosomal acidification. Microbiol. Read. Engl. 2014, 160 Pt 9, 1999–2017. [Google Scholar] [CrossRef] [PubMed]
- Anaya-Sanchez, A.; Feng, Y.; Berude, J.C.; Portnoy, D.A. Detoxification of methylglyoxal by the glyoxalase system is required for glutathione availability and virulence activation in Listeria monocytogenes. PLoS Pathog. 2021, 17, e1009819. [Google Scholar] [CrossRef] [PubMed]
- Moraru, A.; Wiederstein, J.; Pfaff, D.; Fleming, T.; Miller, A.K.; Nawroth, P.; Teleman, A.A. Elevated Levels of the Reactive Metabolite Methylglyoxal Recapitulate Progression of Type 2 Diabetes. Cell Metab. 2018, 27, 926–934.e8. [Google Scholar] [CrossRef] [PubMed]
- Lodd, E.; Wiggenhauser, L.M.; Morgenstern, J.; Fleming, T.H.; Poschet, G.; Büttner, M.; Tabler, C.T.; Wohlfart, D.P.; Nawroth, P.P.; Kroll, J. The combination of loss of glyoxalase1 and obesity results in hyperglycemia. JCI Insight 2019, 4, e126154. [Google Scholar] [CrossRef]
- Morgenstern, J.; Fleming, T.; Schumacher, D.; Eckstein, V.; Freichel, M.; Herzig, S.; Nawroth, P. Loss of Glyoxalase 1 Induces Compensatory Mechanism to Achieve Dicarbonyl Detoxification in Mammalian Schwann Cells. J. Biol. Chem. 2017, 292, 3224–3238. [Google Scholar] [CrossRef]
- Dobariya, P.; Xie, W.; Rao, S.P.; Xie, J.; Seelig, D.M.; Vince, R.; Lee, M.K.; More, S.S. Deletion of Glyoxalase 1 Exacerbates Acetaminophen-Induced Hepatotoxicity in Mice. Antioxidants 2024, 13, 648. [Google Scholar] [CrossRef]
- Giacco, F.; Du, X.; D’Agati, V.D.; Milne, R.; Sui, G.; Geoffrion, M.; Brownlee, M. Knockdown of Glyoxalase 1 Mimics Diabetic Nephropathy in Nondiabetic Mice. Diabetes 2014, 63, 291–299. [Google Scholar] [CrossRef]
- Geoffrion, M.; Du, X.; Irshad, Z.; Vanderhyden, B.C.; Courville, K.; Sui, G.; D’Agati, V.D.; Ott-Braschi, S.; Rabbani, N.; Thornalley, P.J.; et al. Differential effects of glyoxalase 1 overexpression on diabetic atherosclerosis and renal dysfunction in streptozotocin-treated, apolipoprotein E-deficient mice. Physiol. Rep. 2014, 2, e12043. [Google Scholar] [CrossRef]
- Spadoni, I.; Zagato, E.; Bertocchi, A.; Paolinelli, R.; Hot, E.; Di Sabatino, A.; Caprioli, F.; Bottiglieri, L.; Oldani, A.; Viale, G.; et al. A gut-vascular barrier controls the systemic dissemination of bacteria. Science 2015, 350, 830–834. [Google Scholar] [CrossRef]
- Min, S.; Than, N.; Shin, Y.C.; Hu, G.; Shin, W.; Ambrosini, Y.M.; Kim, H.J. Live probiotic bacteria administered in a pathomimetic Leaky Gut Chip ameliorate impaired epithelial barrier and mucosal inflammation. Sci. Rep. 2022, 12, 22641. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, H.; Liu, W.; Yang, X.; Zhu, L.; Wu, G.; Zhang, H. Methylglyoxal scavenging capacity of fiber-bound polyphenols from highland barley during colonic fermentation and its modulation on methylglyoxal-interfered gut microbiota. Food Chem. 2024, 434, 137409. [Google Scholar] [CrossRef] [PubMed]
- Brighina, S.; Poveda Turrado, C.; Restuccia, C.; Walton, G.; Fallico, B.; Oruna-Concha, M.J.; Arena, E. Detrimental effect on the gut microbiota of 1,2-dicarbonyl compounds after in vitro gastro-intestinal and fermentative digestion. Food Chem. 2021, 341 Pt 1, 128237. [Google Scholar] [CrossRef]
- Cooper, R.A. Metabolism of methylglyoxal in microorganisms. Annu Rev. Microbiol. 1984, 38, 49–68. [Google Scholar] [CrossRef]
- Willetts, A.J.; Turner, J.M. Threonine metabolism in a strain of Bacillus subtilis: Enzymes acting on methylglyoxal. Biochim. Biophys. Acta BBA -Gen. Subj. 1970, 222, 668–670. [Google Scholar] [CrossRef]
- Elliott, W.H. Methylglyoxal Formation from Aminoacetone by Ox Plasma. Nature 1960, 185, 467–468. [Google Scholar] [CrossRef]
- Green, M.L.; Lewis, J.B. The oxidation of aminoacetone by a species of Arthrobacter. Biochem. J. 1968, 106, 267–270. [Google Scholar] [CrossRef] [PubMed]
- McLeod, A.; Snipen, L.; Naterstad, K.; Axelsson, L. Global transcriptome response in Lactobacillus sakei during growth on ribose. BMC Microbiol. 2011, 11, 145. [Google Scholar] [CrossRef]
- Galimberti, S.; Rocchetti, G.; Di Rico, F.; Rossetti, C.; Fontana, A.; Lucini, L.; Callegari, M.L. Untargeted metabolomics provide new insights into the implication of Lactobacillus helveticus strains isolated from natural whey starter in methylglyoxal-mediated browning. Food Res. Int. 2023, 174, 113644. [Google Scholar] [CrossRef]
- Zhao, Q.; Su, Y.; Wang, Z.; Chen, C.; Wu, T.; Huang, Y. Identification of glutathione (GSH)-independent glyoxalase III from Schizosaccharomyces pombe. BMC Evol. Biol. 2014, 14, 86. [Google Scholar] [CrossRef]
- Scheckhuber, C.Q. Penicillium chrysogenum as a model system for studying cellular effects of methylglyoxal. BMC Microbiol. 2015, 15, 138. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.M.; Cordeiro, C.A.; Ponces Freire, A.M. In situ analysis of methylglyoxal metabolism in Saccharomyces cerevisiae. FEBS Lett. 2001, 499, 41–44. [Google Scholar] [CrossRef]
- Ozyamak, E.; Black, S.S.; Walker, C.A.; Maclean, M.J.; Bartlett, W.; Miller, S.; Booth, I.R. The critical role of S-lactoylglutathione formation during methylglyoxal detoxification in Escherichia coli. Mol. Microbiol. 2010, 78, 1577–1590. [Google Scholar] [CrossRef] [PubMed]
- Darling, T.N.; Blum, J.J. D-lactate production by Leishmania braziliensis through the glyoxalase pathway. Mol. Biochem. Parasitol. 1988, 28, 121–127. [Google Scholar] [CrossRef]
- Tirelli, E.; Pucci, M.; Squillario, M.; Bignotti, G.; Messali, S.; Zini, S.; Bugatti, M.; Cadei, M.; Memo, M.; Caruso, A.; et al. Effects of methylglyoxal on intestine and microbiome composition in aged mice. Food Chem. Toxicol. 2025, 197, 115276. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, L.; Lin, Z.; Jiang, C.; Chen, X.; Wang, K.; Liu, L.; Shao, L.; Pan, J.; Li, J.; et al. Methylglyoxal from gut microbes boosts radiosensitivity and radioimmunotherapy in rectal cancer by triggering endoplasmic reticulum stress and cGAS-STING activation. J. Immunother. Cancer 2023, 11, e007840. [Google Scholar] [CrossRef]
- Maiden, M.F.J.; Pham, C.; Kashket, S. Glucose toxicity effect and accumulation of methylglyoxal by the periodontal anaerobe Bacteroides forsythus. Anaerobe 2004, 10, 27–32. [Google Scholar] [CrossRef]
- Kashket, S.; Maiden, M.F.J.; Haffajee, A.D.; Kashket, E.R. Accumulation of methylglyoxal in the gingival crevicular fluid of chronic periodontitis patients. J. Clin. Periodontol. 2003, 30, 364–367. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, G.P.; Tötemeyer, S.; MacLean, M.J.; Booth, I.R. Methylglyoxal production in bacteria: Suicide or survival? Arch. Microbiol. 1998, 170, 209–218. [Google Scholar] [CrossRef]
- Chakraborty, S.; Karmakar, K.; Chakravortty, D. Cells producing their own nemesis: Understanding methylglyoxal metabolism. IUBMB Life 2014, 66, 667–678. [Google Scholar] [CrossRef]
- Landmann, J.J.; Busse, R.A.; Latz, J.H.; Singh, K.D.; Stülke, J.; Görke, B. Crh, the paralogue of the phosphocarrier protein HPr, controls the methylglyoxal bypass of glycolysis in Bacillus subtilis. Mol. Microbiol. 2011, 82, 770–787. [Google Scholar] [CrossRef]
- Culp, E.J.; Goodman, A.L. Cross-feeding in the gut microbiome: Ecology and mechanisms. Cell Host Microbe 2023, 31, 485–499. [Google Scholar] [CrossRef]
- Weber, J.; Kayser, A.; Rinas, U. Metabolic flux analysis of Escherichia coli in glucose-limited continuous culture. II. Dynamic response to famine and feast, activation of the methylglyoxal pathway and oscillatory behaviour. Microbiology 2005, 151, 707–716. [Google Scholar] [CrossRef]
- Tötemeyer, S.; Booth, N.A.; Nichols, W.W.; Dunbar, B.; Booth, I.R. From famine to feast: The role of methylglyoxal production in Escherichia coli. Mol. Microbiol. 1998, 27, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Misra, K.; Banerjee, A.B.; Ray, S.; Ray, M. Glyoxalase III from Escherichia coli: A single novel enzyme for the conversion of methylglyoxal into D-lactate without reduced glutathione. Biochem. J. 1995, 305 Pt 3, 999–1003. [Google Scholar] [CrossRef]
- Lee, C.; Park, C. Bacterial Responses to Glyoxal and Methylglyoxal: Reactive Electrophilic Species. Int. J. Mol. Sci. 2017, 18, 169. [Google Scholar] [CrossRef] [PubMed]
- Subedi, K.P.; Choi, D.; Kim, I.; Min, B.; Park, C. Hsp31 of Escherichia coli K-12 is glyoxalase III. Mol. Microbiol. 2011, 81, 926–936. [Google Scholar] [CrossRef]
- Hasim, S.; Hussin, N.A.; Alomar, F.; Bidasee, K.R.; Nickerson, K.W.; Wilson, M.A. A Glutathione-independent Glyoxalase of the DJ-1 Superfamily Plays an Important Role in Managing Metabolically Generated Methylglyoxal in Candida albicans. J. Biol. Chem. 2014, 289, 1662–1674. [Google Scholar] [CrossRef] [PubMed]
- Farrera, D.O.; Galligan, J.J. The human glyoxalase gene family in health and disease. Chem. Res. Toxicol. 2022, 35, 1766–1776. [Google Scholar] [CrossRef]
- Misra, K.; Banerjee, A.B.; Ray, S.; Ray, M. Reduction of methylglyoxal in Escherichia coli K12 by an aldehyde reductase and alcohol dehydrogenase. Mol. Cell Biochem. 1996, 156, 117–124. [Google Scholar] [CrossRef]
- Sánchez-Riera, F.; Cameron, D.C.; Cooney, C.L. Influence of environmental factors in the production of R(−)-1,2-propanediol by clostridium thermosaccharolyticum. Biotechnol. Lett. 1987, 9, 449–454. [Google Scholar] [CrossRef]
- Tao, Y.M.; Bu, C.Y.; Zou, L.H.; Hu, Y.L.; Zheng, Z.J.; Ouyang, J. A comprehensive review on microbial production of 1,2-propanediol: Micro-organisms, metabolic pathways, and metabolic engineering. Biotechnol. Biofuels 2021, 14, 216. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; and Onishi, H. Aerobic Dissimilation of l-Rhamnose and the Production of l-Rhamnonic Acid and 1,2-Propanediol by Yeasts. Agric. Biol. Chem. 1968, 32, 888–893. [Google Scholar]
- Cameron, D.C.; Cooney, C.L. A Novel Fermentation: The Production of R(–)–1,2–Propanediol and Acetol by Clostridium thermosaccharolyticum. Bio/Technology 1986, 4, 651–654. [Google Scholar] [CrossRef]
- Tran-Din, K.; Gottschalk, G. Formation of d(-)-1,2-propanediol and d(-)-lactate from glucose by Clostridium sphenoides under phosphate limitation. Arch. Microbiol. 1985, 142, 87–92. [Google Scholar] [CrossRef]
- Douglas, R.M.; Roberts, J.A.; Munro, A.W.; Ritchie, G.Y.; Lamb, A.J.; Booth, I.R. The distribution of homologues of the Escherichia coli KefC K+-efflux system in other bactèrial species. Microbiology 1991, 137, 1999–2005. [Google Scholar] [CrossRef]
- Elmore, M.J.; Lamb, A.J.; Ritchie, G.Y.; Douglas, R.M.; Munro, A.; Gajewska, A.; Booth, I.R. Activation of potassium efflux from Escherichia coli by glutathione metabolites. Mol. Microbiol. 1990, 4, 405–412. [Google Scholar] [CrossRef]
- Ferguson, G.P.; Munro, A.W.; Douglas, R.M.; McLaggan, D.; Booth, I.R. Activation of potassium channels during metabolite detoxification in Escherichia coli. Mol. Microbiol. 1993, 9, 1297–1303. [Google Scholar] [CrossRef]
- Ferguson, G.P.; Nikolaev, Y.; McLaggan, D.; Maclean, M.; Booth, I.R. Survival during exposure to the electrophilic reagent N-ethylmaleimide in Escherichia coli: Role of KefB and KefC potassium channels. J. Bacteriol. 1997, 179, 1007–1012. [Google Scholar] [CrossRef]
- Ferguson, G.P.; McLaggan, D.; Booth, I.R. Potassium channel activation by glutathione-S-conjugates in Escherichia coli: Protection against methylglyoxal is mediated by cytoplasmic acidification. Mol. Microbiol. 1995, 17, 1025–1033. [Google Scholar] [CrossRef]
- Cox, T.O.; Lundgren, P.; Nath, K.; Thaiss, C.A. Metabolic control by the microbiome. Genome Med. 2022, 14, 80. [Google Scholar] [CrossRef] [PubMed]
- Mei, Z.; Wang, F.; Bhosle, A.; Dong, D.; Mehta, R.; Ghazi, A.; Zhang, Y.; Liu, Y.; Rinott, E.; Ma, S.; et al. Strain-specific gut microbial signatures in type 2 diabetes identified in a cross-cohort analysis of 8117 metagenomes. Nat. Med. 2024, 30, 2265–2276. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef] [PubMed]
- Hadadi, N.; Berweiler, V.; Wang, H.; Trajkovski, M. Intestinal microbiota as a route for micronutrient bioavailability. Curr. Opin. Endocr. Metab. Res. 2021, 20, 100285. [Google Scholar] [CrossRef]
- Fetissov, S.O. Role of the gut microbiota in host appetite control: Bacterial growth to animal feeding behaviour. Nat. Rev. Endocrinol. 2017, 13, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Hou, K.; Chen, Z.S. Gut microbes regulate the feeding center: A new discovery of Gut Brain Axis. Signal Transduct. Target. Ther. 2022, 7, 1–2. [Google Scholar] [CrossRef]
- Blok, L.; Hanssen, N.; Nieuwdorp, M.; Rampanelli, E. From Microbes to Metabolites: Advances in Gut Microbiome Research in Type 1 Diabetes. Metabolites 2025, 15, 138. [Google Scholar] [CrossRef]
- Nemet, I.; Li, X.S.; Haghikia, A.; Li, L.; Wilcox, J.; Romano, K.A.; Buffa, J.A.; Witkowski, M.; Demuth, I.; König, M.; et al. Atlas of gut microbe-derived products from aromatic amino acids and risk of cardiovascular morbidity and mortality. Eur. Heart J. 2023, 44, 3085–3096. [Google Scholar] [CrossRef]
- Dekkers, K.F.; Sayols-Baixeras, S.; Baldanzi, G.; Nowak, C.; Hammar, U.; Nguyen, D.; Varotsis, G.; Brunkwall, L.; Nielsen, N.; Eklund, A.C.; et al. An online atlas of human plasma metabolite signatures of gut microbiome composition. Nat. Commun. 2022, 13, 5370. [Google Scholar] [CrossRef]
- Pérez-Reytor, D.; Puebla, C.; Karahanian, E.; García, K. Use of Short-Chain Fatty Acids for the Recovery of the Intestinal Epithelial Barrier Affected by Bacterial Toxins. Front Physiol. 2021, 12, 650313. [Google Scholar] [CrossRef]
- Puddu, A.; Sanguineti, R.; Montecucco, F.; Viviani, G.L. Evidence for the gut microbiota short-chain fatty acids as key pathophysiological molecules improving diabetes. Mediat. Inflamm. 2014, 2014, 162021. [Google Scholar] [CrossRef] [PubMed]
- Mandaliya, D.K.; Seshadri, S. Short Chain Fatty Acids, pancreatic dysfunction and type 2 diabetes. Pancreatology 2019, 19, 280–284. [Google Scholar]
- Hernández, M.A.G.; Canfora, E.E.; Jocken, J.W.E.; Blaak, E.E. The Short-Chain Fatty Acid Acetate in Body Weight Control and Insulin Sensitivity. Nutrients 2019, 11, 1943. [Google Scholar] [CrossRef] [PubMed]
- Canfora, E.E.; Jocken, J.W.; Blaak, E.E. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat. Rev. Endocrinol. 2015, 11, 577–591. [Google Scholar] [CrossRef]
- Shah, A.B.; Baiseitova, A.; Zahoor, M.; Ahmad, I.; Ikram, M.; Bakhsh, A.; Shah, M.A.; Ali, I.; Idress, M.; Ullah, R.; et al. Probiotic significance of Lactobacillus strains: A comprehensive review on health impacts, research gaps, and future prospects. Gut Microbes 2024, 16, 2431643. [Google Scholar] [CrossRef] [PubMed]
- Saeedi, B.J.; Liu, K.H.; Owens, J.A.; Hunter-Chang, S.; Camacho, M.C.; Eboka, R.U.; Chandrasekharan, B.; Baker, N.F.; Darby, T.M.; Robinson, B.S.; et al. Gut-Resident Lactobacilli Activate Hepatic Nrf2 and Protect Against Oxidative Liver Injury. Cell Metab. 2020, 31, 956–968.e5. [Google Scholar] [CrossRef]
- Nishimoto, S.; Koike, S.; Inoue, N.; Suzuki, T.; Ogasawara, Y. Activation of Nrf2 attenuates carbonyl stress induced by methylglyoxal in human neuroblastoma cells: Increase in GSH levels is a critical event for the detoxification mechanism. Biochem. Biophys. Res. Commun. 2017, 483, 874–879. [Google Scholar] [CrossRef]
- Shin, M.G.; Lee, J.W.; Han, J.S.; Lee, B.; Jeong, J.H.; Park, S.H.; Kim, J.H.; Jang, S.; Park, M.; Kim, S.Y.; et al. Bacteria-derived metabolite, methylglyoxal, modulates the longevity of C. elegans through TORC2/SGK-1/DAF-16 signaling. Proc. Natl. Acad. Sci. USA 2020, 117, 17142–17150. [Google Scholar] [CrossRef]
- Hellwig, M.; Gensberger-Reigl, S.; Henle, T.; Pischetsrieder, M. Food-derived 1,2-dicarbonyl compounds and their role in diseases. Semin. Cancer Biol. 2018, 49, 1–8. [Google Scholar] [CrossRef]
- Maasen, K.; Scheijen, J.L.J.M.; Opperhuizen, A.; Stehouwer, C.D.A.; Van Greevenbroek, M.M.; Schalkwijk, C.G. Quantification of dicarbonyl compounds in commonly consumed foods and drinks; presentation of a food composition database for dicarbonyls. Food Chem. 2021, 339, 128063. [Google Scholar] [CrossRef]
- Amoroso, A.; Maga, G.; Daglia, M. Cytotoxicity of α-dicarbonyl compounds submitted to in vitro simulated digestion process. Food Chem. 2013, 140, 654–659. [Google Scholar] [CrossRef]
- Treibmann, S.; Venema, K.; Henle, T. Glycation reactions of methylglyoxal during digestion in a dynamic, in vitro model of the upper gastrointestinal tract (TIM-1). Food Sci. Nutr. 2024, 12, 4702–4712. [Google Scholar] [CrossRef]
- Hamzalıoğlu, A.; Gökmen, V. Investigations on the reactions of α-dicarbonyl compounds with amino acids and proteins during in vitro digestion of biscuits. Food Funct. 2016, 7, 2544–2550. [Google Scholar] [CrossRef]
- Degen, J.; Vogel, M.; Richter, D.; Hellwig, M.; Henle, T. Metabolic transit of dietary methylglyoxal. J. Agric. Food Chem. 2013, 61, 10253–10260. [Google Scholar] [CrossRef]
- Maasen, K.; Eussen, S.J.P.M.; Dagnelie, P.C.; Houben, A.J.H.M.; Webers, C.A.B.; Schram, M.T.; Berendschot, T.T.J.M.; Stehouwer, C.D.A.; Opperhuizen, A.; van Greevenbroek, M.M.J.; et al. Habitual intake of dietary methylglyoxal is associated with less low-grade inflammation: The Maastricht Study. Am. J. Clin. Nutr. 2022, 116, 1715–1728. [Google Scholar] [CrossRef] [PubMed]
- Kaźmierczak-Barańska, J.; Karwowski, B.T. The Antioxidant Potential of Commercial Manuka Honey from New Zealand—Biochemical and Cellular Studies. Curr. Issues Mol. Biol. 2024, 46, 6366–6376. [Google Scholar] [CrossRef] [PubMed]
- Niaz, K.; Maqbool, F.; Bahadar, H.; Abdollahi, M. Health Benefits of Manuka Honey as an Essential Constituent for Tissue Regeneration. Curr. Drug Metab. 2017, 18, 881–892. [Google Scholar] [CrossRef] [PubMed]
- Mavric, E.; Wittmann, S.; Barth, G.; Henle, T. Identification and quantification of methylglyoxal as the dominant antibacterial constituent of Manuka (Leptospermum scoparium) honeys from New Zealand. Mol. Nutr. Food Res. 2008, 52, 483–489. [Google Scholar] [CrossRef]
- Kilty, S.J.; Duval, M.; Chan, F.T.; Ferris, W.; Slinger, R. Methylglyoxal: (active agent of manuka honey) in vitro activity against bacterial biofilms. Int. Forum Allergy Rhinol. 2011, 1, 348–350. [Google Scholar] [CrossRef]
- Lu, J.; Carter, D.A.; Turnbull, L.; Rosendale, D.; Hedderley, D.; Stephens, J.; Gannabathula, S.; Steinhorn, G.; Schlothauer, R.C.; Whitchurch, C.B.; et al. The Effect of New Zealand Kanuka, Manuka and Clover Honeys on Bacterial Growth Dynamics and Cellular Morphology Varies According to the Species. PLoS ONE 2013, 8, e55898. [Google Scholar] [CrossRef]
- Maddocks, S.E.; Lopez, M.S.; Rowlands, R.S.; Cooper, R.A. Manuka honey inhibits the development of Streptococcus pyogenes biofilms and causes reduced expression of two fibronectin binding proteins. Microbiology 2012, 158, 781–790. [Google Scholar] [CrossRef]
- Lu, J.; Turnbull, L.; Burke, C.M.; Liu, M.; Carter, D.A.; Schlothauer, R.C.; Whitchurch, C.B.; Harry, E.J. Manuka-type honeys can eradicate biofilms produced by Staphylococcus aureus strains with different biofilm-forming abilities. PeerJ 2014, 2, e326. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.S.; Compton, B.J.; Marshall, A.; Anderson, R.; Li, Y.; van der Woude, H.; Hermans, I.F.; Painter, G.F.; Gasser, O. Mānuka honey-derived methylglyoxal enhances microbial sensing by mucosal-associated invariant T cells. Food Funct. 2020, 11, 5782–5787. [Google Scholar] [CrossRef]
- Rabie, E.; Serem, J.C.; Oberholzer, H.M.; Gaspar, A.R.M.; Bester, M.J. How methylglyoxal kills bacteria: An ultrastructural study. Ultrastruct. Pathol. 2016, 40, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Brighina, S.; Restuccia, C.; Arena, E.; Palmeri, R.; Fallico, B. Antibacterial activity of 1,2-dicarbonyl compounds and the influence of the in vitro assay system. Food Chem. 2020, 311, 125905. [Google Scholar] [CrossRef]
- Hayashi, K.; Fukushima, A.; Hayashi-Nishino, M.; Nishino, K. Effect of methylglyoxal on multidrug-resistant Pseudomonas aeruginosa. Front Microbiol. 2014, 5, 180. [Google Scholar] [CrossRef] [PubMed]
- Hayes, G.; Wright, N.; Gardner, S.L.; Telzrow, C.L.; Wommack, A.J.; Vigueira, P.A. Manuka honey and methylglyoxal increase the sensitivity of Staphylococcus aureus to linezolid. Lett. Appl. Microbiol. 2018, 66, 491–495. [Google Scholar] [CrossRef]
- Alluhaim, W.; Alkhulaifi, M.M.; Alzahrani, R.R.; Alrfaei, B.M.; Yassin, A.E.B.; Alghoribi, M.F.; Alsaadi, A.M.; Al-Asmari, A.I.; Al-Fahad, A.J.; Ali, R.; et al. Effectiveness of a Novel Liposomal Methylglyoxal–Tobramycin Formulation in Reducing Biofilm Formation and Bacterial Adhesion. Antibiotics 2025, 14, 3. [Google Scholar] [CrossRef]
- Rosendale, D.; Butts, C.A.; de Guzman, C.E.; Maddox, I.S.; Martell, S.; McIntyre, L.; Skinner, M.A.; Dinnan, H.; Ansell, J. Consumption of antimicrobial manuka honey does not significantly perturb the microbiota in the hind gut of mice. PeerJ 2016, 4, e2787. [Google Scholar] [CrossRef][Green Version]
- Zunkel, K.; Simm, A.; Bartling, B. Long-term intake of the reactive metabolite methylglyoxal is not toxic in mice. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2020, 141, 111333. [Google Scholar] [CrossRef]
- Sena, C.M.; Matafome, P.; Crisóstomo, J.; Rodrigues, L.; Fernandes, R.; Pereira, P.; Seiça, R.M. Methylglyoxal promotes oxidative stress and endothelial dysfunction. Pharmacol. Res. 2012, 65, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Okada, K.; Fukabori, R.; Hayashi, Y.; Asahi, K.; Terawaki, H.; Kobayashi, K.; Watanabe, T.; Nakayama, M. Methylglyoxal (MG) and Cerebro-Renal Interaction: Does Long-Term Orally Administered MG Cause Cognitive Impairment in Normal Sprague-Dawley Rats? Toxins 2014, 6, 254–269. [Google Scholar] [CrossRef]
- Maasen, K.; Eussen, S.J.P.M.; Scheijen, J.L.J.M.; van der Kallen, C.J.H.; Dagnelie, P.C.; Opperhuizen, A.; Stehouwer, C.D.A.; van Greevenbroek, M.M.J.; Schalkwijk, C.G. Higher habitual intake of dietary dicarbonyls is associated with higher corresponding plasma dicarbonyl concentrations and skin autofluorescence: The Maastricht Study. Am. J. Clin. Nutr. 2022, 115, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Daglia, M.; Ferrari, D.; Collina, S.; Curti, V. Influence of in vitro simulated gastroduodenal digestion on methylglyoxal concentration of Manuka (Lectospermum scoparium) honey. J. Agric. Food Chem. 2013, 61, 2140–2145. [Google Scholar] [CrossRef]
- Hellwig, M.; Geissler, S.; Matthes, R.; Peto, A.; Silow, C.; Brandsch, M.; Henle, T. Transport of free and peptide-bound glycated amino acids: Synthesis, transepithelial flux at Caco-2 cell monolayers, and interaction with apical membrane transport proteins. Chembiochem Eur. J. Chem. Biol. 2011, 12, 1270–1279. [Google Scholar] [CrossRef]
- Kellow, N.J.; Coughlan, M.T. Effect of diet-derived advanced glycation end products on inflammation. Nutr. Rev. 2015, 73, 737–759. [Google Scholar] [CrossRef]
- Snelson, M.; Coughlan, M.T. Dietary Advanced Glycation End Products: Digestion, Metabolism and Modulation of Gut Microbial Ecology. Nutrients 2019, 11, 215. [Google Scholar] [CrossRef]
- Kuntz, S.; Kunz, C.; Rudloff, S. Carbonyl compounds methylglyoxal and glyoxal affect interleukin-8 secretion in intestinal cells by superoxide anion generation and activation of MAPK p38. Mol. Nutr. Food Res. 2010, 54, 1458–1467. [Google Scholar] [CrossRef] [PubMed]
- Kuntz, S.; Rudloff, S.; Ehl, J.; Bretzel, R.G.; Kunz, C. Food derived carbonyl compounds affect basal and stimulated secretion of interleukin-6 and -8 in Caco-2 cells. Eur. J. Nutr. 2009, 48, 499–503. [Google Scholar] [CrossRef]
- Papetti, A.; Mascherpa, D.; Gazzani, G. Free α-dicarbonyl compounds in coffee, barley coffee and soy sauce and effects of in vitro digestion. Food Chem. 2014, 164, 259–265. [Google Scholar] [CrossRef]
- Papetti, A.; Mascherpa, D.; Marrubini, G.; Gazzani, G. Effect of in vitro digestion on free α-dicarbonyl compounds in balsamic vinegars. J. Food Sci. 2013, 78, C514–C519. [Google Scholar] [CrossRef]
- Treibmann, S.; Spengler, F.; Degen, J.; Löbner, J.; Henle, T. Studies on the Formation of 3-Deoxyglucosone- and Methylglyoxal-Derived Hydroimidazolones of Creatine during Heat Treatment of Meat. J. Agric. Food Chem. 2019, 67, 5874–5881. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, H.; Troise, A.D.; Fogliano, V. Melanoidins from Coffee, Cocoa, and Bread Are Able to Scavenge α-Dicarbonyl Compounds under Simulated Physiological Conditions. J. Agric. Food Chem. 2019, 67, 10921–10929. [Google Scholar] [CrossRef]
- Tikellis, C.; Pickering, R.J.; Tsorotes, D.; Huet, O.; Cooper, M.E.; Jandeleit-Dahm, K.; Thomas, M.C. Dicarbonyl stress in the absence of hyperglycemia increases endothelial inflammation and atherogenesis similar to that observed in diabetes. Diabetes 2014, 63, 3915–3925. [Google Scholar] [CrossRef] [PubMed]
- Berlanga, J.; Cibrian, D.; Guillén, I.; Freyre, F.; Alba, J.S.; Lopez-Saura, P.; Merino, N.; Aldama, A.; Quintela, A.M.; Triana, M.E.; et al. Methylglyoxal administration induces diabetes-like microvascular changes and perturbs the healing process of cutaneous wounds. Clin. Sci. Lond. Engl. 2005, 109, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Golej, J.; Hoeger, H.; Radner, W.; Unfried, G.; Lubec, G. Oral administration of methylglyoxal leads to kidney collagen accumulation in the mouse. Life Sci. 1998, 63, 801–807. [Google Scholar] [CrossRef]
- Schlotterer, A.; Kolibabka, M.; Lin, J.; Acunman, K.; Dietrich, N.; Sticht, C.; Fleming, T.; Nawroth, P.; Hammes, H.P. Methylglyoxal induces retinopathy-type lesions in the absence of hyperglycemia: Studies in a rat model. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2019, 33, 4141–4153. [Google Scholar] [CrossRef]
- Guo, Q.; Mori, T.; Jiang, Y.; Hu, C.; Osaki, Y.; Yoneki, Y.; Sun, Y.; Hosoya, T.; Kawamata, A.; Ogawa, S.; et al. Methylglyoxal contributes to the development of insulin resistance and salt sensitivity in Sprague-Dawley rats. J. Hypertens. 2009, 27, 1664–1671. [Google Scholar] [CrossRef]
- Nigro, C.; Raciti, G.A.; Leone, A.; Fleming, T.H.; Longo, M.; Prevenzano, I.; Fiory, F.; Mirra, P.; D’Esposito, V.; Ulianich, L.; et al. Methylglyoxal impairs endothelial insulin sensitivity both in vitro and in vivo. Diabetologia 2014, 57, 1485–1494. [Google Scholar] [CrossRef]
- Dhar, A.; Dhar, I.; Jiang, B.; Desai, K.M.; Wu, L. Chronic methylglyoxal infusion by minipump causes pancreatic beta-cell dysfunction and induces type 2 diabetes in Sprague-Dawley rats. Diabetes 2011, 60, 899–908. [Google Scholar] [CrossRef]
- Zemva, J.; Fink, C.A.; Fleming, T.H.; Schmidt, L.; Loft, A.; Herzig, S.; Knieß, R.A.; Mayer, M.; Bukau, B.; Nawroth, P.P.; et al. Hormesis enables cells to handle accumulating toxic metabolites during increased energy flux. Redox Biol. 2017, 13, 674–686. [Google Scholar] [CrossRef] [PubMed]
- Dafre, A.L.; Goldberg, J.; Wang, T.; Spiegel, D.A.; Maher, P. Methylglyoxal, the foe and friend of glyoxalase and Trx/TrxR systems in HT22 nerve cells. Free Radic. Biol. Med. 2015, 89, 8–19. [Google Scholar] [CrossRef]
- Maasen, K.; Eussen, S.J.; Dagnelie, P.C.; Stehouwer, C.D.; Opperhuizen, A.; van Greevenbroek, M.M.; Schalkwijk, C.G. Habitual Intake of Dietary Dicarbonyls is Associated with Greater Insulin Sensitivity and Lower Prevalence of Type 2 Diabetes: The Maastricht Study. Am. J. Clin. Nutr. 2023, 118, 151–161. [Google Scholar] [CrossRef]
- Berner, A.K.; Brouwers, O.; Pringle, R.; Klaassen, I.; Colhoun, L.; McVicar, C.; Brockbank, S.; Curry, J.W.; Miyata, T.; Brownlee, M.; et al. Protection against methylglyoxal-derived AGEs by regulation of glyoxalase 1 prevents retinal neuroglial and vasodegenerative pathology. Diabetologia 2012, 55, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Sachdeva, R.; Schlotterer, A.; Schumacher, D.; Matka, C.; Mathar, I.; Dietrich, N.; Medert, R.; Kriebs, U.; Lin, J.; Nawroth, P.; et al. TRPC proteins contribute to development of diabetic retinopathy and regulate glyoxalase 1 activity and methylglyoxal accumulation. Mol. Metab. 2018, 9, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Rowan, S.; Jiang, S.; Korem, T.; Szymanski, J.; Chang, M.L.; Szelog, J.; Cassalman, C.; Dasuri, K.; McGuire, C.; Nagai, R.; et al. Involvement of a gut-retina axis in protection against dietary glycemia-induced age-related macular degeneration. Proc. Natl. Acad. Sci. USA 2017, 114, E4472–E4481. [Google Scholar] [CrossRef]
- Bierhaus, A.; Fleming, T.; Stoyanov, S.; Leffler, A.; Babes, A.; Neacsu, C.; Sauer, S.K.; Eberhardt, M.; Schnölzer, M.; Lasitschka, F.; et al. Methylglyoxal modification of Nav1.8 facilitates nociceptive neuron firing and causes hyperalgesia in diabetic neuropathy. Nat. Med. 2012, 18, 926–933. [Google Scholar] [CrossRef]
- Braun, J.D.; Pastene, D.O.; Breedijk, A.; Rodriguez, A.; Hofmann, B.B.; Sticht, C.; von Ochsenstein, E.; Allgayer, H.; van den Born, J.; Bakker, S.; et al. Methylglyoxal down-regulates the expression of cell cycle associated genes and activates the p53 pathway in human umbilical vein endothelial cells. Sci. Rep. 2019, 9, 1152. [Google Scholar] [CrossRef]
- Li, H.; Zheng, L.; Chen, C.; Liu, X.; Zhang, W. Brain Senescence Caused by Elevated Levels of Reactive Metabolite Methylglyoxal on D-Galactose-Induced Aging Mice. Front. Neurosci. 2019, 13, 1004. [Google Scholar] [CrossRef]
- Takeda, A.; Yasuda, T.; Miyata, T.; Goto, Y.; Wakai, M.; Watanabe, M.; Yasuda, Y.; Horie, K.; Inagaki, T.; Doyu, M.; et al. Advanced glycation end products co-localized with astrocytes and microglial cells in Alzheimer’s disease brain. Acta Neuropathol. 1998, 95, 555–558. [Google Scholar] [CrossRef]
- de Almeida, G.R.L.; Szczepanik, J.C.; Selhorst, I.; Cunha, M.P.; Dafre, A.L. The expanding impact of methylglyoxal on behavior-related disorders. Prog. Neuropsychopharmacol. Biol. Psychiatry 2023, 120, 110635. [Google Scholar] [CrossRef]
- Berends, E.; Pencheva, M.G.; van de Waarenburg, M.P.H.; Scheijen, J.L.J.M.; Hermes, D.J.H.P.; Wouters, K.; van Oostenbrugge, R.J.; Foulquier, S.; Schalkwijk, C.G. Glyoxalase 1 overexpression improves neurovascular coupling and limits development of mild cognitive impairment in a mouse model of type 1 diabetes. J. Physiol. 2024, 602, 6209–6223. [Google Scholar] [CrossRef] [PubMed]
- Hanssen, N.M.J.; Scheijen, J.L.J.M.; Jorsal, A.; Parving, H.H.; Tarnow, L.; Rossing, P.; Stehouwer, C.D.A.; Schalkwijk, C.G. Higher Plasma Methylglyoxal Levels Are Associated with Incident Cardiovascular Disease in Individuals with Type 1 Diabetes: A 12-Year Follow-up Study. Diabetes 2017, 66, 2278–2283. [Google Scholar] [CrossRef] [PubMed]
- Hanssen, N.M.; Teraa, M.; Scheijen, J.L.; Van de Waarenburg, M.; Gremmels, H.; Stehouwer, C.D.; Verhaar, M.C.; Schalkwijk, C.G. Plasma Methylglyoxal Levels Are Associated with Amputations and Mortality in Severe Limb Ischemia Patients with and Without Diabetes. Diabetes Care 2021, 44, 157–163. [Google Scholar] [CrossRef]
- Koren, O.; Spor, A.; Felin, J.; Fåk, F.; Stombaugh, J.; Tremaroli, V.; Behre, C.J.; Knight, R.; Fagerberg, B.; Ley, R.E.; et al. Human oral, gut, and plaque microbiota in patients with atherosclerosis. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. S1), 4592–4598. [Google Scholar] [CrossRef]
- Chhibber-Goel, J.; Singhal, V.; Bhowmik, D.; Vivek, R.; Parakh, N.; Bhargava, B.; Sharma, A. Linkages between oral commensal bacteria and atherosclerotic plaques in coronary artery disease patients. Npj Biofilms Microbiomes 2016, 2, 7. [Google Scholar] [CrossRef]
- Lanter, B.B.; Sauer, K.; Davies, D.G. Bacteria Present in Carotid Arterial Plaques Are Found as Biofilm Deposits Which May Contribute to Enhanced Risk of Plaque Rupture. MBio 2014, 5, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Hanssen, N.M.; Brouwers, O.; Gijbels, M.J.; Wouters, K.; Wijnands, E.; Cleutjens, J.P.; De Mey, J.G.; Miyata, T.; Biessen, E.A.; Stehouwer, C.D.; et al. Glyoxalase 1 overexpression does not affect atherosclerotic lesion size and severity in ApoE−/− mice with or without diabetes. Cardiovasc. Res. 2014, 104, 160–170. [Google Scholar] [CrossRef]
- Vulesevic, B.; McNeill, B.; Giacco, F.; Maeda, K.; Blackburn, N.J.R.; Brownlee, M.; Milne, R.W.; Suuronen, E.J. Methylglyoxal-Induced Endothelial Cell Loss and Inflammation Contribute to the Development of Diabetic Cardiomyopathy. Diabetes 2016, 65, 1699–1713. [Google Scholar] [CrossRef]
- Dube, G.; Tiamiou, A.; Bizet, M.; Boumahd, Y.; Gasmi, I.; Crake, R.; Bellier, J.; Nokin, M.J.; Calonne, E.; Deplus, R.; et al. Methylglyoxal: A novel upstream regulator of DNA methylation. J. Exp. Clin. Cancer Res. 2023, 42, 78. [Google Scholar] [CrossRef]
- Kong, L.R.; Gupta, K.; Wu, A.J.; Perera, D.; Ivanyi-Nagy, R.; Ahmed, S.M.; Tan, T.Z.; Tan, S.L.W.; Fuddin, A.; Sundaramoorthy, E.; et al. A glycolytic metabolite bypasses “two-hit” tumor suppression by BRCA2. Cell 2024, 187, 2269–2287.e16. [Google Scholar] [CrossRef] [PubMed]
- Bellier, J.; Nokin, M.J.; Caprasse, M.; Tiamiou, A.; Blomme, A.; Scheijen, J.L.; Koopmansch, B.; MacKay, G.M.; Chiavarina, B.; Costanza, B.; et al. Methylglyoxal Scavengers Resensitize KRAS-Mutated Colorectal Tumors to Cetuximab. Cell Rep. 2020, 30, 1400–1416.e6. [Google Scholar] [CrossRef] [PubMed]
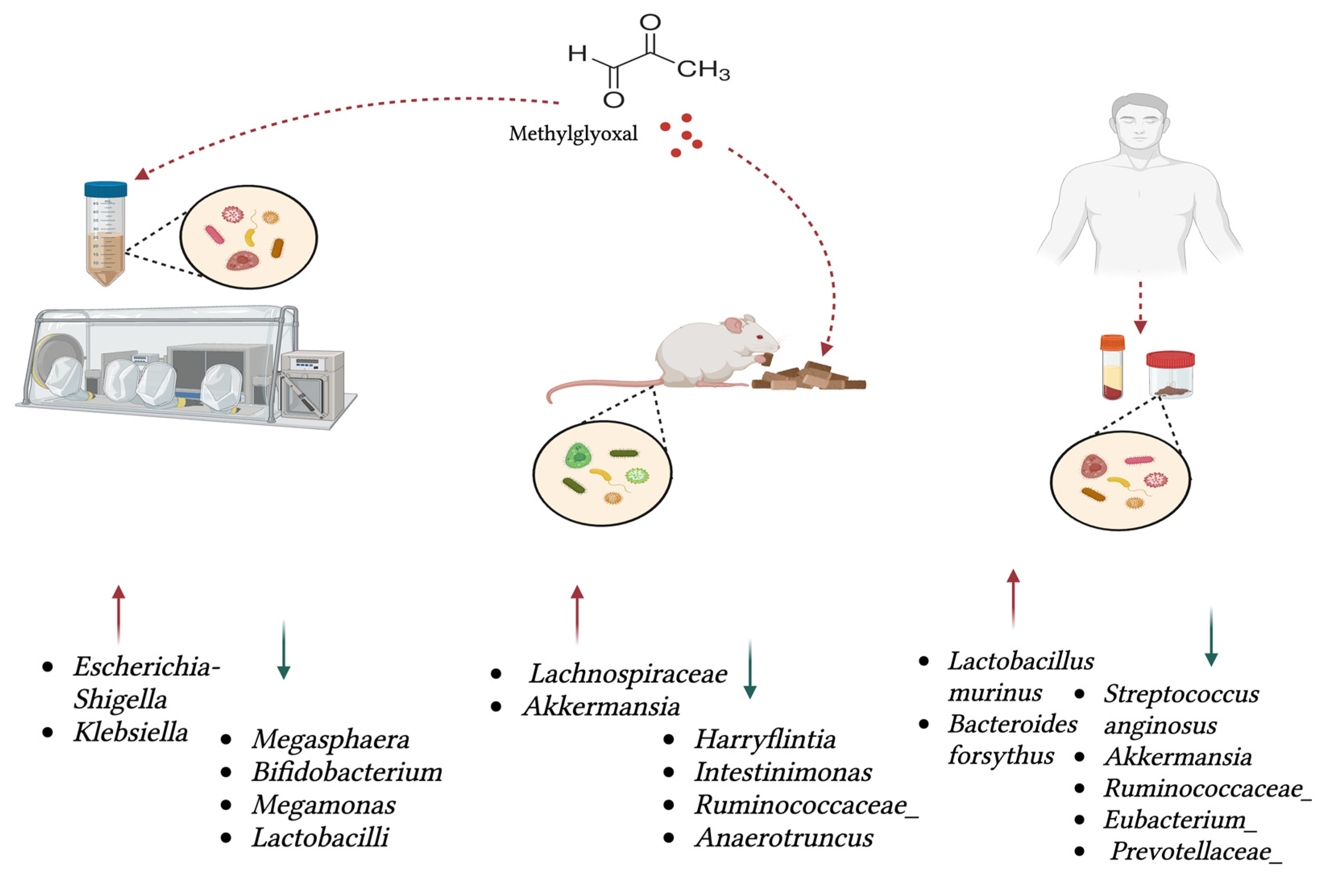
| Study Summary | Positively Associated with MGO | Negatively Associated with MGO | Reference |
|---|---|---|---|
| Oral MGO administration in aged mice caused shifts in specific taxa and metabolic pathways, with no change in alpha or beta diversity; mice were given MGO (100 mg/kg/day) for 4 weeks. | Lachnospiraceae, Akkermansia | Harryflintia, Intestinimonas, Ruminococcaceae Family, Anaerotruncus | [65] |
| Faecal and serum samples from 26 patients with locally advanced rectal cancer showed that MGO predicts treatment response and correlates with specific microbes. | Lactobacillus murinus | Streptococcus anginosus, Akkermansia, Ruminococcaceae, Eubacterium, Prevotellaceae | [66] |
| In vitro fermentation of human faecal samples with MGO was analysed using 16S rRNA gene sequencing. Principal coordinate analysis (PCoA) of the microbiome shows separation between the blank group and the MGO group. | Escherichia-Shigella and Klebsiella | Megasphaera, Bifidobacterium, Megamonas | [52] |
| Simulated digestion of dicarbonyls and in vitro fermentation with 3 human faecal samples showed that MGO and other 1,2-dicarbonyls reduced gut microbial abundance and activity. Low MGO level used—6.0 mg/kg; high MGO level used—219.6 mg/kg. | Lactobacilli | [53] | |
| In 14 patients with chronic periodontitis, MGO accumulated in gingival crevicular fluid at high levels compared to the reference group. | Bacteroides forsythus | [67,68] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akinrimisi, O.I.; Maasen, K.; Scheijen, J.L.J.M.; Nemet, I.; Nieuwdorp, M.; Schalkwijk, C.G.; Hanssen, N.M.J. Does Gut Microbial Methylglyoxal Metabolism Impact Human Physiology? Antioxidants 2025, 14, 763. https://doi.org/10.3390/antiox14070763
Akinrimisi OI, Maasen K, Scheijen JLJM, Nemet I, Nieuwdorp M, Schalkwijk CG, Hanssen NMJ. Does Gut Microbial Methylglyoxal Metabolism Impact Human Physiology? Antioxidants. 2025; 14(7):763. https://doi.org/10.3390/antiox14070763
Chicago/Turabian StyleAkinrimisi, Oluwatomisono I., Kim Maasen, Jean L. J. M. Scheijen, Ina Nemet, Max Nieuwdorp, Casper G. Schalkwijk, and Nordin M. J. Hanssen. 2025. "Does Gut Microbial Methylglyoxal Metabolism Impact Human Physiology?" Antioxidants 14, no. 7: 763. https://doi.org/10.3390/antiox14070763
APA StyleAkinrimisi, O. I., Maasen, K., Scheijen, J. L. J. M., Nemet, I., Nieuwdorp, M., Schalkwijk, C. G., & Hanssen, N. M. J. (2025). Does Gut Microbial Methylglyoxal Metabolism Impact Human Physiology? Antioxidants, 14(7), 763. https://doi.org/10.3390/antiox14070763







