The Heme Oxygenase/Biliverdin Reductase System as a Therapeutic Target to Counteract Cellular Senescence in Alzheimer’s Disease
Abstract
1. Introduction
| SASP Factor(s) |
|---|
| IL-1α, IL-1β |
| IL-6 |
| IL-8 |
| TNF-α |
| TGF-β |
| MMP-3 |
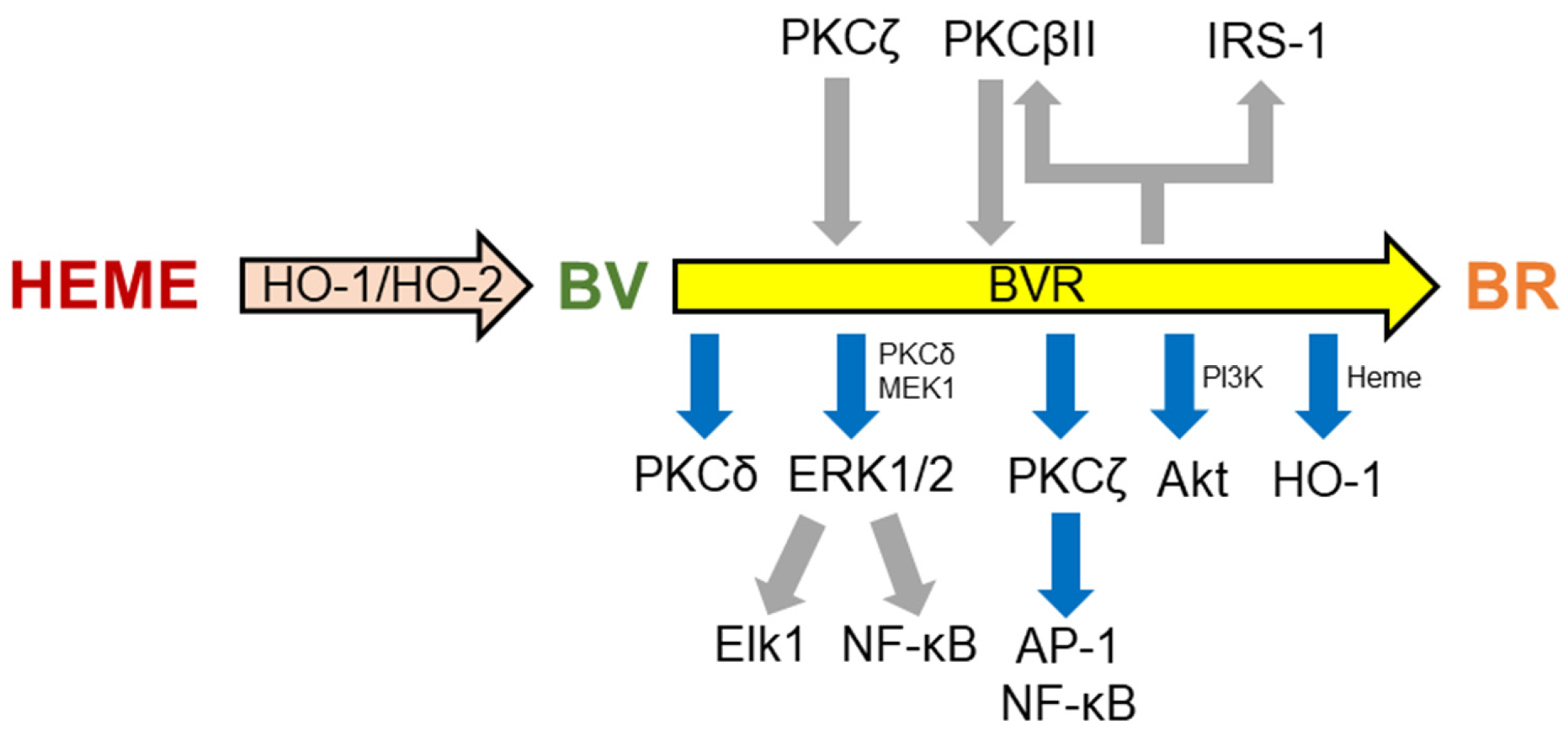
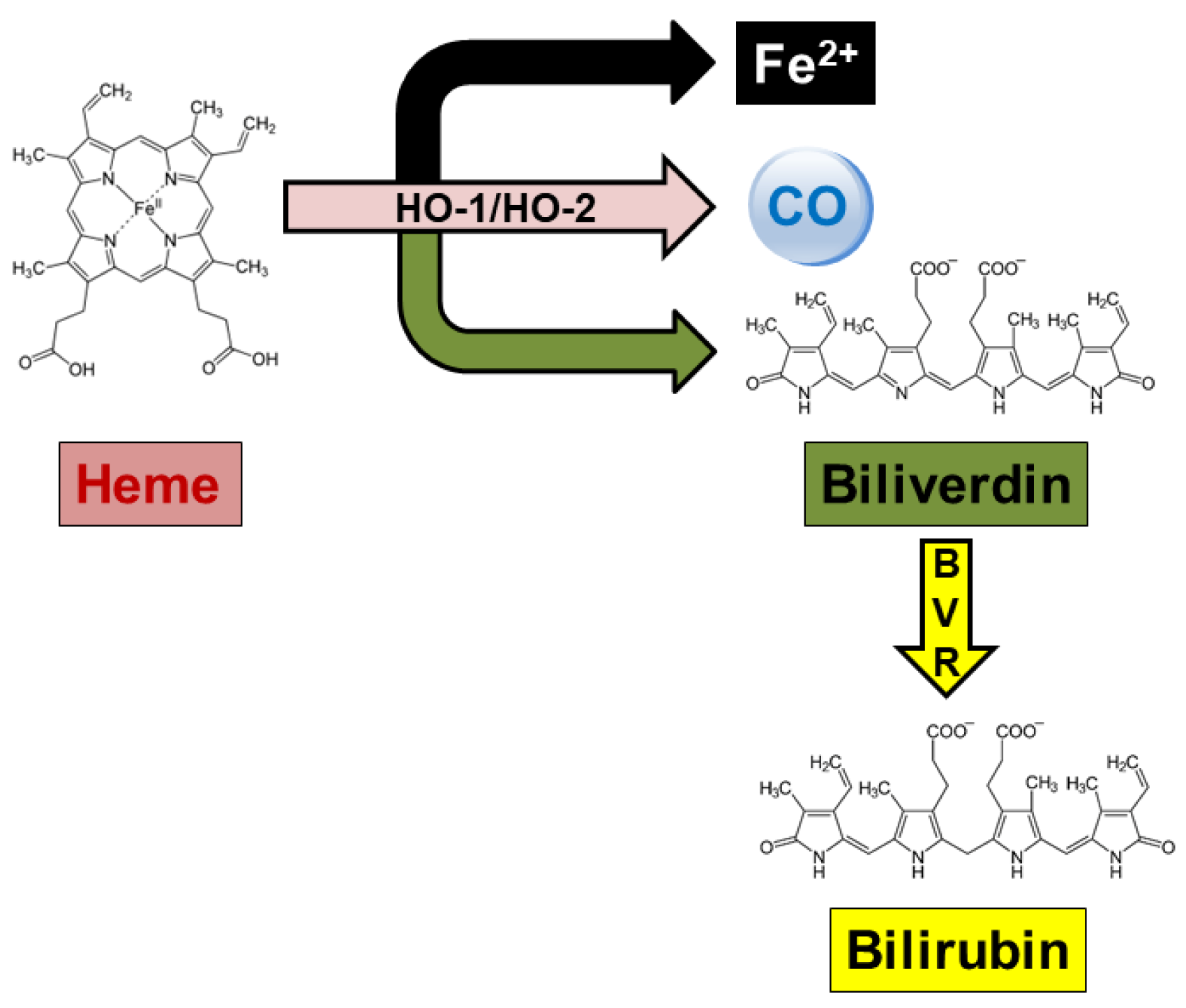
2. Cellular Senescence and Alzheimer’s Disease
3. Alzheimer’s Disease and the Heme Oxygenase/Biliverdin Reductase System
4. The HO/BVR System and Cellular Senescence
5. HO/BVR Modulation and Cellular Senescence in AD: A Significant Field for Future Research
5.1. Acetylcholinesterase Inhibitors
5.2. Hydroxy-Methylglutaryl-Coenzyme a Inhibitors (Statins)
5.3. Non-Steroidal Anti-Inflammatory Drugs
5.4. Proliferation Signal Inhibitors
5.5. Ferulic Acid
6. Conclusions and Future Perspectives
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gianakos, D. Cicero and healthy aging. Am. J. Med. 2007, 120, 1097. [Google Scholar] [CrossRef]
- Caterini, M.; Gallo, M. The elderly and prison in Italy: A proposal to overcome discrimination. In Elderly People and Discrimination: Prevention and Reaction; Stevanović, I., Beljanski, V., Eds.; Institute of Criminological and Sociological Research, Vojvodina Bar Association: Belgrade, Serbia, 2023; pp. 181–195. [Google Scholar]
- Della Vedova, L.; Baron, G.; Morazzoni, P.; Aldini, G.; Gado, F. The Potential of Polyphenols in Modulating the Cellular Senescence Process: Implications and Mechanism of Action. Pharmaceuticals 2025, 18, 138. [Google Scholar] [CrossRef] [PubMed]
- Riessland, M.; Ximerakis, M.; Jarjour, A.A.; Zhang, B.; Orr, M.E. Therapeutic targeting of senescent cells in the CNS. Nat. Rev. Drug Discov. 2024, 23, 817–837. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Wu, C.; Yang, L. Cellular senescence in Alzheimer’s disease: From physiology to pathology. Transl. Neurodegener. 2024, 13, 55. [Google Scholar] [CrossRef]
- Wang, B.; Han, J.; Elisseeff, J.H.; Demaria, M. The senescence-associated secretory phenotype and its physiological and pathological implications. Nat. Rev. Mol. Cell Biol. 2024, 25, 958–978. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Kuca, K.; You, L.; Nepovimova, E.; Heger, Z.; Valko, M.; Adam, V.; Wu, Q.; Jomova, K. The role of cellular senescence in neurodegenerative diseases. Arch. Toxicol. 2024, 98, 2393–2408. [Google Scholar] [CrossRef]
- Coppé, J.P.; Desprez, P.Y.; Krtolica, A.; Campisi, J. The senescence-associated secretory phenotype: The dark side of tumor suppression. Annu. Rev. Pathol. 2010, 5, 99–118. [Google Scholar] [CrossRef]
- Ma, Y.; Erb, M.L.; Moore, D.J. Aging, cellular senescence and Parkinson’s disease. J. Parkinsons Dis. 2025, 15, 239–254. [Google Scholar] [CrossRef]
- Dai, L.; Gao, F.; Wang, Q.; Lv, X.; Cheng, Z.; Wu, Y.; Chai, X.; Zetterberg, H.; Blennow, K.; Levey, A.I.; et al. Molecules of senescent glial cells differentiate Alzheimer’s disease from ageing. J. Neurol. Neurosurg. Psychiatry 2023, 94, 550–559. [Google Scholar] [CrossRef]
- Mancuso, C. Biliverdin as a disease-modifying agent: An integrated viewpoint. Free Radic. Biol. Med. 2023, 207, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, C. The impact of heme oxygenase-2 on pharmacological research: A bibliometric analysis and beyond. Front. Pharmacol. 2023, 14, 1156333. [Google Scholar] [CrossRef]
- Mancuso, C. Biliverdin reductase as a target in drug research and development: Facts and hypotheses. Free Radic. Biol. Med. 2021, 172, 521–529. [Google Scholar] [CrossRef]
- Maines, M.D. The heme oxygenase system: A regulator of second messenger gases. Annu. Rev. Pharmacol. Toxicol. 1997, 37, 517–554. [Google Scholar] [CrossRef]
- Mancuso, C. The Heme Oxygenase/Biliverdin Reductase System and Its Genetic Variants in Physiology and Diseases. Antioxidants 2025, 14, 187. [Google Scholar] [CrossRef] [PubMed]
- O’Rourke, S.A.; Shanley, L.C.; Dunne, A. The Nrf2-HO-1 system and inflammaging. Front. Immunol. 2024, 15, 1457010. [Google Scholar] [CrossRef]
- Wu, J.; Li, S.; Li, C.; Cui, L.; Ma, J.; Hui, Y. The non-canonical effects of heme oxygenase-1, a classical fighter against oxidative stress. Redox Biol. 2021, 47, 102170. [Google Scholar] [CrossRef]
- Mancuso, C. The brain heme oxygenase/biliverdin reductase system as a target in drug research and development. Expert Opin. Ther. Targets 2022, 26, 361–374. [Google Scholar] [CrossRef]
- Ryter, S.W. Heme Oxygenase-1: An Anti-Inflammatory Effector in Cardiovascular, Lung, and Related Metabolic Disorders. Antioxidants 2022, 11, 555. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Sánchez, J.; Chánez-Cárdenas, M.E. A review on hemeoxygenase-2: Focus on cellular protection and oxygen response. Oxid. Med. Cell Longev. 2014, 2014, 604981. [Google Scholar] [CrossRef] [PubMed]
- Paul, B.D.; Pieper, A.A. Neuroprotective Roles of the Biliverdin Reductase-A/Bilirubin Axis in the Brain. Biomolecules 2024, 14, 155. [Google Scholar] [CrossRef]
- Mancuso, C. Bilirubin and brain: A pharmacological approach. Neuropharmacology 2017, 118, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Barone, E.; Di Domenico, F.; Sultana, R.; Coccia, R.; Mancuso, C.; Perluigi, M.; Butterfield, D.A. Heme oxygenase-1 posttranslational modifications in the brain of subjects with Alzheimer disease and mild cognitive impairment. Free Radic. Biol. Med. 2012, 52, 2292–2301. [Google Scholar] [CrossRef]
- Barone, E.; Di Domenico, F.; Cenini, G.; Sultana, R.; Cini, C.; Preziosi, P.; Perluigi, M.; Mancuso, C.; Butterfield, D.A. Biliverdin reductase-A protein levels and activity in the brains of subjects with Alzheimer disease and mild cognitive impairment. Biochim. Biophys. Acta 2011, 1812, 480–487. [Google Scholar] [CrossRef]
- Barone, E.; Di Domenico, F.; Cenini, G.; Sultana, R.; Coccia, R.; Preziosi, P.; Perluigi, M.; Mancuso, C.; Butterfield, D.A. Oxidative and nitrosative modifications of biliverdin reductase-A in the brain of subjects with Alzheimer’s disease and amnestic mild cognitive impairment. J. Alzheimers Dis. 2011, 25, 623–633. [Google Scholar] [CrossRef]
- Mhillaj, E.; Cuomo, V.; Trabace, L.; Mancuso, C. The Heme Oxygenase/Biliverdin Reductase System as Effector of the Neuroprotective Outcomes of Herb-Based Nutritional Supplements. Front. Pharmacol. 2019, 10, 1298. [Google Scholar] [CrossRef]
- Mhillaj, E.; Catino, S.; Miceli, F.M.; Santangelo, R.; Trabace, L.; Cuomo, V.; Mancuso, C. Ferulic Acid Improves Cognitive Skills Through the Activation of the Heme Oxygenase System in the Rat. Mol. Neurobiol. 2018, 55, 905–916. [Google Scholar] [CrossRef] [PubMed]
- Catino, S.; Paciello, F.; Miceli, F.; Rolesi, R.; Troiani, D.; Calabrese, V.; Santangelo, R.; Mancuso, C. Ferulic Acid Regulates the Nrf2/Heme Oxygenase-1 System and Counteracts Trimethyltin-Induced Neuronal Damage in the Human Neuroblastoma Cell Line SH-SY5Y. Front. Pharmacol. 2016, 6, 305. [Google Scholar] [CrossRef] [PubMed]
- Barone, E.; Mancuso, C.; Di Domenico, F.; Sultana, R.; Murphy, M.P.; Head, E.; Butterfield, D.A. Biliverdin reductase-A: A novel drug target for atorvastatin in a dog pre-clinical model of Alzheimer disease. J. Neurochem. 2012, 120, 135–146. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Barone, E.; Mancuso, C. Cholesterol-independent neuroprotective and neurotoxic activities of statins: Perspectives for statin use in Alzheimer disease and other age-related neurodegenerative disorders. Pharmacol. Res. 2011, 64, 180–186. [Google Scholar] [CrossRef]
- Si, Z.; Wang, X. The Neuroprotective and Neurodegeneration Effects of Heme Oxygenase-1 in Alzheimer’s Disease. J. Alzheimers Dis. 2020, 78, 1259–1272. [Google Scholar] [CrossRef]
- Hardy, J.A.; Higgins, G.A. Alzheimer’s disease: The amyloid cascade hypothesis. Science 1992, 256, 184–185. [Google Scholar] [CrossRef] [PubMed]
- Karran, E.; De Strooper, B. The amyloid cascade hypothesis: Are we poised for success or failure? J. Neurochem. 2016, 139, 237–252. [Google Scholar] [CrossRef] [PubMed]
- Karran, E.; Mercken, M.; De Strooper, B. The amyloid cascade hypothesis for Alzheimer’s disease: An appraisal for the development of therapeutics. Nat. Rev. Drug Discov. 2011, 10, 698–712. [Google Scholar] [CrossRef]
- Haass, C.; Selkoe, D.J. Soluble protein oligomers in neurodegeneration: Lessons from the Alzheimer’s amyloid beta-peptide. Nat. Rev. Mol. Cell Biol. 2007, 8, 101–112. [Google Scholar] [CrossRef]
- Jiang, S.; Srikanth, M.; Serpe, R.; Yavari, S.; Gaur, P.; Collins, G.A.; Soni, R.; Menon, V.; Myeku, N. Early proteasome downregulation and dysfunction drive proteostasis failure in Alzheimer’s disease. Brain 2025, awaf222. [Google Scholar] [CrossRef] [PubMed]
- Cornejo, V.H.; Hetz, C. The unfolded protein response in Alzheimer’s disease. Semin. Immunopathol. 2013, 35, 277–292. [Google Scholar] [CrossRef]
- Imaizumi, K.; Miyoshi, K.; Katayama, T.; Yoneda, T.; Taniguchi, M.; Kudo, T.; Tohyama, M. The unfolded protein response and Alzheimer’s disease. Biochim. Biophys. Acta 2001, 1536, 85–96. [Google Scholar] [CrossRef]
- Rekha, A.; Afzal, M.; Babu, M.A.; Menon, S.V.; Nathiya, D.; Supriya, S.; Mishra, S.B.; Gupta, S.; Goyal, K.; Rana, M.; et al. GSK-3β dysregulation in aging: Implications for tau pathology and Alzheimer’s disease progression. Mol. Cell Neurosci. 2025, 133, 104005. [Google Scholar] [CrossRef]
- Garemilla, S.; Kumari, R.; Kumar, R. CDK5 as a therapeutic tool for the treatment of Alzheimer’s disease: A review. Eur. J. Pharmacol. 2024, 978, 176760. [Google Scholar] [CrossRef]
- Gehlot, P.; Pathak, R.; Kumar, S.; Choudhary, N.K.; Vyas, V.K. A review on synthetic inhibitors of dual-specific tyrosine phosphorylation-regulated kinase 1A (DYRK1A) for the treatment of Alzheimer’s disease (AD). Bioorg. Med. Chem. 2024, 113, 117925. [Google Scholar] [CrossRef]
- Mancuso, C.; Siciliano, R.; Barone, E.; Butterfield, D.A.; Preziosi, P. Pharmacologists and Alzheimer disease therapy: To boldly go where no scientist has gone before. Expert Opin. Investig. Drugs 2011, 20, 1243–1261. [Google Scholar] [CrossRef]
- Poppek, D.; Keck, S.; Ermak, G.; Jung, T.; Stolzing, A.; Ullrich, O.; Davies, K.J.; Grune, T. Phosphorylation inhibits turnover of the tau protein by the proteasome: Influence of RCAN1 and oxidative stress. Biochem. J. 2006, 400, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wu, Z.; Si, X.; Li, J.; Wu, G.; Wang, M. The role of mitochondrial dysfunction in the pathogenesis of Alzheimer’s disease and future strategies for targeted therapy. Eur. J. Med. Res. 2025, 30, 434. [Google Scholar] [CrossRef] [PubMed]
- Kamila, P.; Kar, K.; Chowdhury, S.; Chakraborty, P.; Dutta, R.S.; Sowmiya, S.; Singh, S.A.; Prajapati, B.G. Effect of neuroinflammation on the progression of Alzheimer’s disease and its significant ramifications for novel anti-inflammatory treatments. IBRO Neurosci. Rep. 2025, 18, 771–782. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Dhapola, R.; Reddy, D.H. Apoptosis in Alzheimer’s disease: Insight into the signaling pathways and therapeutic avenues. Apoptosis 2023, 28, 943–957. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Boyd-Kimball, D. Mitochondrial Oxidative and Nitrosative Stress and Alzheimer Disease. Antioxidants 2020, 9, 818. [Google Scholar] [CrossRef]
- Mao, P.; Reddy, P.H. Aging and amyloid beta-induced oxidative DNA damage and mitochondrial dysfunction in Alzheimer’s disease: Implications for early intervention and therapeutics. Biochim. Biophys. Acta 2011, 1812, 1359–1370. [Google Scholar] [CrossRef]
- Calabrese, V.; Sultana, R.; Scapagnini, G.; Guagliano, E.; Sapienza, M.; Bella, R.; Kanski, J.; Pennisi, G.; Mancuso, C.; Stella, A.M.; et al. Nitrosative stress, cellular stress response, and thiol homeostasis in patients with Alzheimer’s disease. Antioxid. Redox Signal. 2006, 8, 1975–1986. [Google Scholar] [CrossRef]
- Quintanilla, R.A.; von Bernhardi, R.; Godoy, J.A.; Inestrosa, N.C.; Johnson, G.V. Phosphorylated tau potentiates Aβ-induced mitochondrial damage in mature neurons. Neurobiol. Dis. 2014, 71, 260–269. [Google Scholar] [CrossRef]
- Ma, T.; Hoeffer, C.A.; Wong, H.; Massaad, C.A.; Zhou, P.; Iadecola, C.; Murphy, M.P.; Pautler, R.G.; Klann, E. Amyloid β-induced impairments in hippocampal synaptic plasticity are rescued by decreasing mitochondrial superoxide. J. Neurosci. 2011, 31, 5589–5595. [Google Scholar] [CrossRef]
- Huang, Z.; Yan, Q.; Wang, Y.; Zou, Q.; Li, J.; Liu, Z.; Cai, Z. Role of Mitochondrial Dysfunction in the Pathology of Amyloid-β. J. Alzheimers Dis. 2020, 78, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Mutisya, E.M.; Bowling, A.C.; Beal, M.F. Cortical cytochrome oxidase activity is reduced in Alzheimer’s disease. J. Neurochem. 1994, 63, 2179–2184. [Google Scholar] [CrossRef]
- Du, H.; Yan, S.S. Mitochondrial permeability transition pore in Alzheimer’s disease: Cyclophilin D and amyloid beta. Biochim. Biophys. Acta 2010, 1802, 198–204. [Google Scholar] [CrossRef]
- Harada, J.; Sugimoto, M. Activation of caspase-3 in beta-amyloid-induced apoptosis of cultured rat cortical neurons. Brain Res. 1999, 842, 311–323. [Google Scholar] [CrossRef]
- Koppal, T.; Drake, J.; Yatin, S.; Jordan, B.; Varadarajan, S.; Bettenhausen, L.; Butterfield, D.A. Peroxynitrite-induced alterations in synaptosomal membrane proteins: Insight into oxidative stress in Alzheimer’s disease. J. Neurochem. 1999, 72, 310–317. [Google Scholar] [CrossRef]
- Sultana, R.; Poon, H.F.; Cai, J.; Pierce, W.M.; Merchant, M.; Klein, J.B.; Markesbery, W.R.; Butterfield, D.A. Identification of nitrated proteins in Alzheimer’s disease brain using a redox proteomics approach. Neurobiol. Dis. 2006, 22, 76–87. [Google Scholar] [CrossRef]
- Sultana, R.; Perluigi, M.; Butterfield, D.A. Protein oxidation and lipid peroxidation in brain of subjects with Alzheimer’s disease: Insights into mechanism of neurodegeneration from redox proteomics. Antioxid. Redox Signal 2006, 8, 2021–2037. [Google Scholar] [CrossRef]
- Youssef, P.; Chami, B.; Lim, J.; Middleton, T.; Sutherland, G.T.; Witting, P.K. Evidence supporting oxidative stress in a moderately affected area of the brain in Alzheimer’s disease. Sci. Rep. 2018, 8, 11553. [Google Scholar] [CrossRef] [PubMed]
- Gsell, W.; Conrad, R.; Hickethier, M.; Sofic, E.; Frölich, L.; Wichart, I.; Jellinger, K.; Moll, G.; Ransmayr, G.; Beckmann, H.; et al. Decreased catalase activity but unchanged superoxide dismutase activity in brains of patients with dementia of Alzheimer type. J. Neurochem. 1995, 64, 1216–1223. [Google Scholar] [CrossRef]
- Thapa, R.; Ahmad Bhat, A.; Shahwan, M.; Ali, H.; PadmaPriya, G.; Bansal, P.; Rajotiya, S.; Barwal, A.; Siva Prasad, G.V.; Pramanik, A.; et al. Proteostasis disruption and senescence in Alzheimer’s disease pathways to neurodegeneration. Brain Res. 2024, 1845, 149202. [Google Scholar] [CrossRef] [PubMed]
- Yu, N.; Pasha, M.; Chua, J.J.E. Redox changes and cellular senescence in Alzheimer’s disease. Redox Biol. 2024, 70, 103048. [Google Scholar] [CrossRef]
- Nelke, C.; Schroeter, C.B.; Pawlitzki, M.; Meuth, S.G.; Ruck, T. Cellular senescence in neuroinflammatory disease: New therapies for old cells? Trends Mol. Med. 2022, 28, 850–863. [Google Scholar] [CrossRef]
- Carusillo, A.; Mussolino, C. DNA Damage: From Threat to Treatment. Cells 2020, 9, 1665. [Google Scholar] [CrossRef]
- Schmitt, C.A.; Wang, B.; Demaria, M. Senescence and cancer-role and therapeutic opportunities. Nat. Rev. Clin. Oncol. 2022, 19, 619–636. [Google Scholar] [CrossRef]
- D’Arcangelo, D.; Tinaburri, L.; Dellambra, E. The Role of p16INK4a Pathway in Human Epidermal Stem Cell Self-Renewal, Aging and Cancer. Int. J. Mol. Sci. 2017, 18, 1591. [Google Scholar] [CrossRef] [PubMed]
- Rayess, H.; Wang, M.B.; Srivatsan, E.S. Cellular senescence and tumor suppressor gene p16. Int. J. Cancer 2012, 130, 1715–1725. [Google Scholar] [CrossRef] [PubMed]
- Anzola, M.; Cuevas, N.; Lopez-Martinez, M.; Martinez de Pancorbo, M.; Burgos, J.J. p16INK4A gene alterations are not a prognostic indicator for survival in patients with hepatocellular carcinoma undergoing curative hepatectomy. J. Gastroenterol. Hepatol. 2004, 19, 397–405. [Google Scholar] [CrossRef]
- Zhang, P.; Kishimoto, Y.; Grammatikakis, I.; Gottimukkala, K.; Cutler, R.G.; Zhang, S.; Abdelmohsen, K.; Bohr, V.A.; Misra Sen, J.; Gorospe, M.; et al. Senolytic therapy alleviates Aβ-associated oligodendrocyte progenitor cell senescence and cognitive deficits in an Alzheimer’s disease model. Nat. Neurosci. 2019, 22, 719–728. [Google Scholar] [CrossRef]
- Musi, N.; Valentine, J.M.; Sickora, K.R.; Baeuerle, E.; Thompson, C.S.; Shen, Q.; Orr, M.E. Tau protein aggregation is associated with cellular senescence in the brain. Aging Cell 2018, 17, e12840. [Google Scholar] [CrossRef]
- Weichhart, T. mTOR as Regulator of Lifespan, Aging, and Cellular Senescence: A Mini-Review. Gerontology 2018, 64, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Di Domenico, F.; Coccia, R.; Cocciolo, A.; Murphy, M.P.; Cenini, G.; Head, E.; Butterfield, D.A.; Giorgi, A.; Schinina, M.E.; Mancuso, C.; et al. Impairment of proteostasis network in Down syndrome prior to the development of Alzheimer’s disease neuropathology: Redox proteomics analysis of human brain. Biochim. Biophys. Acta 2013, 1832, 1249–1259. [Google Scholar] [CrossRef]
- Premkumar, D.R.; Smith, M.A.; Richey, P.L.; Petersen, R.B.; Castellani, R.; Kutty, R.K.; Wiggert, B.; Perry, G.; Kalaria, R.N. Induction of heme oxygenase-1 mRNA and protein in neocortex and cerebral vessels in Alzheimer’s disease. J. Neurochem. 1995, 65, 1399–1402. [Google Scholar] [CrossRef]
- Schipper, H.M.; Cissé, S.; Stopa, E.G. Expression of heme oxygenase-1 in the senescent and Alzheimer-diseased brain. Ann. Neurol. 1995, 37, 758–768. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.A.; Kutty, R.K.; Richey, P.L.; Yan, S.D.; Stern, D.; Chader, G.J.; Wiggert, B.; Petersen, R.B.; Perry, G. Heme oxygenase-1 is associated with the neurofibrillary pathology of Alzheimer’s disease. Am. J. Pathol. 1994, 145, 42–47. [Google Scholar] [PubMed]
- Takahashi, M.; Doré, S.; Ferris, C.D.; Tomita, T.; Sawa, A.; Wolosker, H.; Borchelt, D.R.; Iwatsubo, T.; Kim, S.H.; Thinakaran, G.; et al. Amyloid precursor proteins inhibit heme oxygenase activity and augment neurotoxicity in Alzheimer’s disease. Neuron 2000, 28, 461–473. [Google Scholar] [CrossRef]
- Takeda, A.; Perry, G.; Abraham, N.G.; Dwyer, B.E.; Kutty, R.K.; Laitinen, J.T.; Petersen, R.B.; Smith, M.A. Overexpression of heme oxygenase in neuronal cells, the possible interaction with Tau. J. Biol. Chem. 2000, 275, 5395–5399. [Google Scholar] [CrossRef]
- Triani, F.; Tramutola, A.; Di Domenico, F.; Sharma, N.; Butterfield, D.A.; Head, E.; Perluigi, M.; Barone, E. Biliverdin reductase-A impairment links brain insulin resistance with increased Aβ production in an animal model of aging: Implications for Alzheimer disease. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 3181–3194. [Google Scholar] [CrossRef]
- Zhuo, M.; Laitinen, J.T.; Li, X.C.; Hawkins, R.D. On the respective roles of nitric oxide and carbon monoxide in long-term potentiation in the hippocampus. Learn. Mem. 1999, 6, 63–76. [Google Scholar] [CrossRef] [PubMed]
- Bernabeu, R.; Princ, F.; de Stein, M.L.; Fin, C.; Juknat, A.A.; Batile, A.; Izquierdo, I.; Medina, J.H. Evidence for the involvement of hippocampal CO production in the acquisition and consolidation of inhibitory avoidance learning. Neuroreport 1995, 6, 516–518. [Google Scholar] [CrossRef]
- Verma, A.; Hirsch, D.J.; Glatt, C.E.; Ronnett, G.V.; Snyder, S.H. Carbon monoxide: A putative neural messenger. Science 1993, 259, 381–384. [Google Scholar] [CrossRef]
- Stocker, R.; Yamamoto, Y.; McDonagh, A.F.; Glazer, A.N.; Ames, B.N. Bilirubin is an antioxidant of possible physiological importance. Science 1987, 235, 1043–1046. [Google Scholar] [CrossRef]
- Doré, S.; Takahashi, M.; Ferris, C.D.; Zakhary, R.; Hester, L.D.; Guastella, D.; Snyder, S.H. Bilirubin, formed by activation of heme oxygenase-2, protects neurons against oxidative stress injury. Proc. Natl. Acad. Sci. USA 1999, 96, 2445–2450. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, C.; Bonsignore, A.; Di Stasio, E.; Mordente, A.; Motterlini, R. Bilirubin and S-nitrosothiols interaction: Evidence for a possible role of bilirubin as a scavenger of nitric oxide. Biochem. Pharmacol. 2003, 66, 2355–2363. [Google Scholar] [CrossRef] [PubMed]
- Barone, E.; Trombino, S.; Cassano, R.; Sgambato, A.; De Paola, B.; Di Stasio, E.; Picci, N.; Preziosi, P.; Mancuso, C. Characterization of the S-denitrosylating activity of bilirubin. J. Cell Mol. Med. 2009, 13, 2365–2375. [Google Scholar] [CrossRef] [PubMed]
- Ham, D.; Schipper, H.M. Heme oxygenase-1 induction and mitochondrial iron sequestration in astroglia exposed to amyloid peptides. Cell Mol. Biol. (Noisy-le-Grand) 2000, 46, 587–596. [Google Scholar]
- Schipper, H.M.; Bernier, L.; Mehindate, K.; Frankel, D. Mitochondrial iron sequestration in dopamine-challenged astroglia: Role of heme oxygenase-1 and the permeability transition pore. J. Neurochem. 1999, 72, 1802–1811. [Google Scholar] [CrossRef]
- Gupta, A.; Lacoste, B.; Pistell, P.J.; Ingram, D.K.; Hamel, E.; Alaoui-Jamali, M.A.; Szarek, W.A.; Vlahakis, J.Z.; Jie, S.; Song, W.; et al. Neurotherapeutic effects of novel HO-1 inhibitors in vitro and in a transgenic mouse model of Alzheimer’s disease. J. Neurochem. 2014, 131, 778–790. [Google Scholar] [CrossRef]
- Malojirao, V.H.; Vasquez, V.; Kodavati, M.; Mitra, J.; Provasek, V.; Voh, A.T.T.; Liopo, A.V.; Derry, P.J.; Mikheev, A.M.; Rostomily, R.C.; et al. Hemin-induced transient senescence via DNA damage response: A neuroprotective mechanism against ferroptosis in intracerebral hemorrhage. Commun. Biol. 2025, 8, 622. [Google Scholar] [CrossRef]
- Brisson, L.; Henrique Geraldo, L.; Bikfalvi, A.; Mathivet, T. The strange Microenvironment of Glioblastoma. Rev. Neurol. 2023, 179, 490–501. [Google Scholar] [CrossRef]
- Ordónez-Rubiano, E.G.; Cómbita, A.; Baldoncini, M.; Payán-Gómez, C.; Gómez-Amarillo, D.F.; Hakim, F.; Camargo, J.; Zorro-Sepúlveda, V.; Luzzi, S.; Zorro, O.; et al. Cellular Senescence in Diffuse Gliomas: From Physiopathology to Possible Treatments. World Neurosurg. 2024, 191, 138–148. [Google Scholar] [CrossRef]
- Jagadeesh, A.S.V.; Fang, X.; Kim, S.H.; Guillen-Quispe, Y.N.; Zheng, J.; Surh, Y.J.; Kim, S.J. Non-canonical vs. Canonical Functions of Heme Oxygenase-1 in Cancer. J. Cancer Prev. 2022, 27, 7–15. [Google Scholar] [CrossRef]
- Zhou, Y.; Zeng, L.; Cai, L.; Zheng, W.; Liu, X.; Xiao, Y.; Jin, X.; Bai, Y.; Lai, M.; Li, H.; et al. Cellular senescence-associated gene IFI16 promotes HMOX1-dependent evasion of ferroptosis and radioresistance in glioblastoma. Nat. Commun. 2025, 16, 1212. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, H.; Zheng, W.; Chen, Q.; Hu, S.; Pan, Y.; Bai, Y.; Zhang, J.; Shao, C. MMP14 Contributes to HDAC Inhibition-Induced Radiosensitization of Glioblastoma. Int. J. Mol. Sci. 2021, 22, 10403. [Google Scholar] [CrossRef]
- Padda, I.; Sethi, Y.; Das, M.; Fabian, D.; Ralhan, T.; Aziz, D.; Sexton, J.; Johal, G. Heme Oxygenase-1, Cardiac Senescence, and Myocardial Infarction: A Critical Review of the Triptych. Cardiovasc. Drugs Ther. 2024. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Shan, H.; Li, T.; Zhang, L.; Yang, R.; Li, Y.; Zhang, M.; Dong, Y.; Zhou, Y.; Xu, C.; Yang, B.; et al. Heme oxygenase-1 prevents heart against myocardial infarction by attenuating ischemic injury-induced cardiomyocytes senescence. EBioMedicine 2019, 39, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Shi, M.; Zhou, J.; Wang, W.; Zhang, Y.; Li, Y. Circulating Exosomal miR-181b-5p Promoted Cell Senescence and Inhibited Angiogenesis to Impair Diabetic Foot Ulcer via the Nuclear Factor Erythroid 2-Related Factor 2/Heme Oxygenase-1 Pathway. Front. Cardiovasc. Med. 2022, 9, 844047. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Wang, Y.; Yang, H.; Dai, C.; Hong, H.; Li, J.; Liu, Z.; Guo, Z.; Chen, X.; He, P.; et al. Heme oxygenase-1 ameliorates oxidative stress-induced endothelial senescence via regulating endothelial nitric oxide synthase activation and coupling. Aging 2018, 10, 1722–1744. [Google Scholar] [CrossRef]
- Wang, Q.; Li, A.; Li, Q.; Li, J.; Wang, Q.; Wu, S.; Meng, J.; Liu, C.; Wang, D.; Chen, Y. Carbon monoxide attenuates cellular senescence-mediated pulmonary fibrosis via modulating p53/PAI-1 pathway. Eur. J. Pharmacol. 2024, 980, 176843. [Google Scholar] [CrossRef]
- Kim, S.Y.; Kang, H.T.; Choi, H.R.; Park, S.C. Biliverdin reductase A in the prevention of cellular senescence against oxidative stress. Exp. Mol. Med. 2011, 43, 15–23. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, Y.; Yu, S.; Li, W.; Li, J.; Zhao, B.; Hu, X.; Jin, H. Biliverdin Reductase A Protects Lens Epithelial Cells against Oxidative Damage and Cellular Senescence in Age-Related Cataract. Oxid. Med. Cell Longev. 2022, 2022, 5628946. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, L.; Wu, Z.; Chen, X.; Ma, T. Galantamine alleviates senescence of U87 cells induced by beta-amyloid through decreasing ROS production. Neurosci. Lett. 2017, 653, 183–188. [Google Scholar] [CrossRef]
- Ota, H.; Ogawa, S.; Ouchi, Y.; Akishita, M. Protective effects of NMDA receptor antagonist, memantine, against senescence of PC12 cells: A possible role of nNOS and combined effects with donepezil. Exp. Gerontol. 2015, 72, 109–116. [Google Scholar] [CrossRef]
- Zhang, T.; Tian, F.; Wang, J.; Zhou, S.; Dong, X.; Guo, K.; Jing, J.; Zhou, Y.; Chen, Y. Donepezil attenuates high glucose-accelerated senescence in human umbilical vein endothelial cells through SIRT1 activation. Cell Stress Chaperones 2015, 20, 787–792. [Google Scholar] [CrossRef]
- Nakao, A.; Kaczorowski, D.J.; Zuckerbraun, B.S.; Lei, J.; Faleo, G.; Deguchi, K.; McCurry, K.R.; Billiar, T.R.; Kanno, S. Galantamine and carbon monoxide protect brain microvascular endothelial cells by heme oxygenase-1 induction. Biochem. Biophys. Res. Commun. 2008, 367, 674–679. [Google Scholar] [CrossRef]
- Shitara, Y.; Sugiyama, Y. Pharmacokinetic and pharmacodynamic alterations of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors: Drug-drug interactions and interindividual differences in transporter and metabolic enzyme functions. Pharmacol. Ther. 2006, 112, 71–105. [Google Scholar] [CrossRef] [PubMed]
- Bersot, T.P. Drug therapy for hypercholesterolemia and dyslipidemia. In Goodman and Gilman’s, The Pharmacological Basis of Therapeutics; Laurence, L.B., Ed.; McGraw-Hill: New York, NY, USA, 2011; pp. 877–908. [Google Scholar]
- Wang, C.Y.; Liu, P.Y.; Liao, J.K. Pleiotropic effects of statin therapy: Molecular mechanisms and clinical results. Trends Mol. Med. 2008, 14, 37–44. [Google Scholar] [CrossRef]
- McTaggart, S.J. Isoprenylated proteins. Cell Mol. Life Sci. 2006, 63, 255–267. [Google Scholar] [CrossRef]
- Farah, S.; Agazie, Y.; Ohan, N.; Ngsee, J.K.; Liu, X.J. A rho-associated protein kinase, ROKalpha, binds insulin receptor substrate-1 and modulates insulin signaling. J. Biol. Chem. 1998, 273, 4740–4746. [Google Scholar] [CrossRef] [PubMed]
- Sundaresan, M.; Yu, Z.X.; Ferrans, V.J.; Sulciner, D.J.; Gutkind, J.S.; Irani, K.; Goldschmidt-Clermont, P.J.; Finkel, T. Regulation of reactive-oxygen-species generation in fibroblasts by Rac1. Biochem. J. 1996, 318, 379–382. [Google Scholar] [CrossRef] [PubMed]
- Assmus, B.; Urbich, C.; Aicher, A.; Hofmann, W.K.; Haendeler, J.; Rössig, L.; Spyridopoulos, I.; Zeiher, A.M.; Dimmeler, S. HMG-CoA reductase inhibitors reduce senescence and increase proliferation of endothelial progenitor cells via regulation of cell cycle regulatory genes. Circ. Res. 2003, 92, 1049–1055. [Google Scholar] [CrossRef]
- Ota, H.; Eto, M.; Kano, M.R.; Kahyo, T.; Setou, M.; Ogawa, S.; Iijima, K.; Akishita, M.; Ouchi, Y. Induction of endothelial nitric oxide synthase, SIRT1, and catalase by statins inhibits endothelial senescence through the Akt pathway. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 2205–2211. [Google Scholar] [CrossRef]
- Du, Y.; Yu, Z.; Li, C.; Zhang, Y.; Xu, B. The role of statins in dementia or Alzheimer’s disease incidence: A systematic review and meta-analysis of cohort studies. Front. Pharmacol. 2025, 16, 1473796. [Google Scholar] [CrossRef]
- Saeedi Saravi, S.S.; Saeedi Saravi, S.S.; Arefidoust, A.; Dehpour, A.R. The beneficial effects of HMG-CoA reductase inhibitors in the processes of neurodegeneration. Metab. Brain Dis. 2017, 32, 949–965. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Yan, J.; Chen, X.; Li, J.; Yang, Y.; Weng, J.; Deng, C.; Yenari, M.A. Statins: Multiple neuroprotective mechanisms in neurodegenerative diseases. Exp. Neurol. 2011, 230, 27–34. [Google Scholar] [CrossRef]
- Johnstone, E.M.; Chaney, M.O.; Norris, F.H.; Pascual, R.; Little, S.P. Conservation of the sequence of the Alzheimer’s disease amyloid peptide in dog, polar bear and five other mammals by cross-species polymerase chain reaction analysis. Brain Res. Mol. Brain Res. 1991, 10, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Butterfield, D.A.; Barone, E.; Di Domenico, F.; Cenini, G.; Sultana, R.; Murphy, M.P.; Mancuso, C.; Head, E. Atorvastatin treatment in a dog preclinical model of Alzheimer’s disease leads to up-regulation of haem oxygenase-1 and is associated with reduced oxidative stress in brain. Int. J. Neuropsychopharmacol. 2012, 15, 981–987. [Google Scholar] [CrossRef]
- Mancuso, C.; Perluigi, M.; Cini, C.; De Marco, C.; Giuffrida Stella, A.M.; Calabrese, V. Heme oxygenase and cyclooxygenase in the central nervous system: A functional interplay. J. Neurosci. Res. 2006, 84, 1385–1391. [Google Scholar] [CrossRef]
- Yamagata, K.; Andreasson, K.I.; Kaufmann, W.E.; Barnes, C.A.; Worley, P.F. Expression of a mitogen-inducible cyclooxygenase in brain neurons: Regulation by synaptic activity and glucocorticoids. Neuron 1993, 11, 371–386. [Google Scholar] [CrossRef]
- Rouzer, C.A.; Marnett, L.J. Mechanism of free radical oxygenation of polyunsaturated fatty acids by cyclooxygenases. Chem. Rev. 2003, 103, 2239–2304. [Google Scholar] [CrossRef] [PubMed]
- Hwa, J.; Sessa, W.C.; Martin, K. The eicosanoids: Prostaglandins, thromboxanes, leukotrienes, & related compounds. In Basic & Clinical Pharmacology, 15th ed.; Katzung, B.G., Vanderah, T.W., Eds.; McGraw Hill: New York, NY, USA, 2021; pp. 335–352. [Google Scholar]
- Moussa, N.; Dayoub, N. Exploring the role of COX-2 in Alzheimer’s disease: Potential therapeutic implications of COX-2 inhibitors. Saudi Pharm J. 2023, 31, 101729. [Google Scholar] [CrossRef]
- Ghazanfari, N.; van Waarde, A.; Dierckx, R.A.J.O.; Doorduin, J.; de Vries, E.F.J. Is cyclooxygenase-1 involved in neuroinflammation? J. Neurosci. Res. 2021, 99, 2976–2998. [Google Scholar] [CrossRef]
- López, D.E.; Ballaz, S.J. The Role of Brain Cyclooxygenase-2 (Cox-2) Beyond Neuroinflammation: Neuronal Homeostasis in Memory and Anxiety. Mol. Neurobiol. 2020, 57, 5167–5176. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, S.; Yin, K.; Ito, Y.; Chan, A.; Olan, I.; Gough, S.; Cassidy, L.; Serrao, E.; Smith, S.; Young, A.; et al. COX2 regulates senescence secretome composition and senescence surveillance through PGE2. Cell Rep. 2021, 34, 108860. [Google Scholar] [CrossRef]
- Cormenier, J.; Martin, N.; Deslé, J.; Salazar-Cardozo, C.; Pourtier, A.; Abbadie, C.; Pluquet, O. The ATF6α arm of the Unfolded Protein Response mediates replicative senescence in human fibroblasts through a COX2/prostaglandin E2 intracrine pathway. Mech. Ageing Dev. 2018, 170, 82–91. [Google Scholar] [CrossRef]
- Martien, S.; Pluquet, O.; Vercamer, C.; Malaquin, N.; Martin, N.; Gosselin, K.; Pourtier, A.; Abbadie, C. Cellular senescence involves an intracrine prostaglandin E2 pathway in human fibroblasts. Biochim. Biophys. Acta 2013, 1831, 1217–1227. [Google Scholar] [CrossRef]
- Zdanov, S.; Bernard, D.; Debacq-Chainiaux, F.; Martien, S.; Gosselin, K.; Vercamer, C.; Chelli, F.; Toussaint, O.; Abbadie, C. Normal or stress-induced fibroblast senescence involves COX-2 activity. Exp. Cell Res. 2007, 313, 3046–3056. [Google Scholar] [CrossRef]
- Wang, P.; Wang, P.; Luan, H.; Wu, Y.; Chen, Y. Midazolam alleviates cellular senescence in SH-SY5Y neuronal cells in Alzheimer’s disease. Brain Behav. 2023, 13, e2822. [Google Scholar] [CrossRef]
- Feng, M.; Kim, J.; Field, K.; Reid, C.; Chatzistamou, I.; Shim, M. Aspirin ameliorates the long-term adverse effects of doxorubicin through suppression of cellular senescence. FASEB Bioadv 2019, 1, 579–590. [Google Scholar] [CrossRef]
- Bode-Böger, S.M.; Martens-Lobenhoffer, J.; Täger, M.; Schröder, H.; Scalera, F. Aspirin reduces endothelial cell senescence. Biochem. Biophys. Res. Commun. 2005, 334, 1226–1232. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.R.; Kim, E.J.; Choi, H.J.; Park, J.J.; Kim, H.S.; Lee, Y.J.; Park, M.J.; Lee, M. Aspirin Targets SIRT1 and AMPK to Induce Senescence of Colorectal Carcinoma Cells. Mol. Pharmacol. 2015, 88, 708–719. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Chen, Q.; Sui, J.; Tu, Y.; Guo, X.; Li, F. Celecoxib prevents tumor necrosis factor-α (TNF-α)-induced cellular senescence in human chondrocytes. Bioengineered 2021, 12, 12812–12820. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Zhang, Y.; Liu, S.; Liu, X. Celecoxib, Beyond Anti-inflammation, Alleviates Tendon-Derived Stem Cell Senescence in Degenerative Rotator Cuff Tendinopathy. Am. J. Sports Med. 2022, 50, 2488–2496. [Google Scholar] [CrossRef] [PubMed]
- Hoozemans, J.J.; Rozemuller, A.J.; Janssen, I.; De Groot, C.J.; Veerhuis, R.; Eikelenboom, P. Cyclooxygenase expression in microglia and neurons in Alzheimer’s disease and control brain. Acta Neuropathol. 2001, 101, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Yermakova, A.V.; Rollins, J.; Callahan, L.M.; Rogers, J.; O’Banion, M.K. Cyclooxygenase-1 in human Alzheimer and control brain: Quantitative analysis of expression by microglia and CA3 hippocampal neurons. J. Neuropathol. Exp. Neurol. 1999, 58, 1135–1146. [Google Scholar] [CrossRef] [PubMed]
- Tyrrell, A.D.; Cisbani, G.; Smith, M.E.; Chen, C.T.; Chen, Y.T.; Chouinard-Watkins, R.; Hopperton, K.E.; Taha, A.Y.; Bazinet, R.P. Lipid mediators in post-mortem brain samples from patients with Alzheimer’s disease: A systematic review. Brain Behav. Immun. Health 2024, 43, 100938. [Google Scholar] [CrossRef]
- Ho, L.; Pieroni, C.; Winger, D.; Purohit, D.P.; Aisen, P.S.; Pasinetti, G.M. Regional distribution of cyclooxygenase-2 in the hippocampal formation in Alzheimer’s disease. J. Neurosci. Res. 1999, 57, 295–303. [Google Scholar] [CrossRef]
- Kitamura, Y.; Shimohama, S.; Koike, H.; Kakimura, J.I.; Matsuoka, Y.; Nomura, Y.; Gebicke-Haerter, P.J.; Taniguchi, T. Increased expression of cyclooxygenases and peroxisome proliferator-activated receptor-gamma in Alzheimer’s disease brains. Biochem. Biophys. Res. Commun. 1999, 254, 582–586. [Google Scholar] [CrossRef]
- Yasojima, K.; Schwab, C.; McGeer, E.G.; McGeer, P.L. Distribution of cyclooxygenase-1 and cyclooxygenase-2 mRNAs and proteins in human brain and peripheral organs. Brain Res. 1999, 830, 226–236. [Google Scholar] [CrossRef]
- Pasinetti, G.; Aisen, P.S. Cyclooxygenase-2 expression is increased in frontal cortex of Alzheimer’s disease brain. Neuroscience 1998, 87, 319–324. [Google Scholar] [CrossRef]
- Kumari, S.; Kaur, P.; Singh, A.K.; Ashar, M.S.; Pradhan, R.; Rao, A.; Haldar, P.; Chakrawarty, A.; Chatterjee, P.; Dey, S. Quantification of COX-2 Level in Alzheimer’s Disease Patients to Develop Potential Blood-Based Biomarker for Early Diagnosis and Therapeutic Target. J. Alzheimers Dis. 2024, 98, 699–713. [Google Scholar] [CrossRef]
- Ahmad, S.; Yang, W.; Orellana, A.; Frölich, L.; de Rojas, I.; Cano, A.; Boada, M.; Hernández, I.; Hausner, L.; Harms, A.C.; et al. Association of oxidative stress and inflammatory metabolites with Alzheimer’s disease cerebrospinal fluid biomarkers in mild cognitive impairment. Alzheimers Res. Ther. 2024, 16, 171. [Google Scholar] [CrossRef] [PubMed]
- Do, K.V.; Hjorth, E.; Wang, Y.; Jun, B.; Kautzmann, M.I.; Ohshima, M.; Eriksdotter, M.; Schultzberg, M.; Bazan, N.G. Cerebrospinal Fluid Profile of Lipid Mediators in Alzheimer’s Disease. Cell Mol. Neurobiol. 2023, 43, 797–811. [Google Scholar] [CrossRef]
- Cao, L.L.; Guan, P.P.; Liang, Y.Y.; Huang, X.S.; Wang, P. Cyclooxygenase-2 is Essential for Mediating the Effects of Calcium Ions on Stimulating Phosphorylation of Tau at the Sites of Ser 396 and Ser 404. J. Alzheimers Dis. 2019, 68, 1095–1111. [Google Scholar] [CrossRef] [PubMed]
- Kulmacz, R.J.; Lands, W.E. Prostaglandin H synthase. Stoichiometry of heme cofactor. J. Biol. Chem. 1984, 259, 6358–6363. [Google Scholar] [CrossRef]
- Mancuso, C.; Pistritto, G.; Tringali, G.; Grossman, A.B.; Preziosi, P.; Navarra, P. Evidence that carbon monoxide stimulates prostaglandin endoperoxide synthase activity in rat hypothalamic explants and in primary cultures of rat hypothalamic astrocytes. Brain Res. Mol. Brain Res. 1997, 45, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, C.; Tringali, G.; Grossman, A.; Preziosi, P.; Navarra, P. The generation of nitric oxide and carbon monoxide produces opposite effects on the release of immunoreactive interleukin-1beta from the rat hypothalamus in vitro: Evidence for the involvement of different signaling pathways. Endocrinology 1998, 139, 1031–1037. [Google Scholar] [CrossRef][Green Version]
- Mhillaj, E.; Papi, M.; Paciello, F.; Silvestrini, A.; Rolesi, R.; Palmieri, V.; Perini, G.; Fetoni, A.R.; Trabace, L.; Mancuso, C. Celecoxib Exerts Neuroprotective Effects in β-Amyloid-Treated SH-SY5Y Cells Through the Regulation of Heme Oxygenase-1: Novel Insights for an Old Drug. Front. Cell Dev. Biol. 2020, 8, 561179. [Google Scholar] [CrossRef]
- Jordan, F.; Quinn, T.J.; McGuinness, B.; Passmore, P.; Kelly, J.P.; Tudur Smith, C.; Murphy, K.; Devane, D. Aspirin and other non-steroidal anti-inflammatory drugs for the prevention of dementia. Cochrane Database Syst. Rev. 2020, 4, CD011459. [Google Scholar] [CrossRef]
- Lake, D.F.; Briggs, A.D. Immunopharmacology. In Basic & Clinical Pharmacology, 15th ed.; Katzung, B.G., Vanderah, T.W., Eds.; McGraw Hill: New York, NY, USA, 2021; pp. 1015–1042. [Google Scholar]
- Lai, C.; Chen, Z.; Ding, Y.; Chen, Q.; Su, S.; Liu, H.; Ni, R.; Tang, Z. Rapamycin Attenuated Zinc-Induced Tau Phosphorylation and Oxidative Stress in Rats: Involvement of Dual mTOR/p70S6K and Nrf2/HO-1 Pathways. Front. Immunol. 2022, 13, 782434. [Google Scholar] [CrossRef]
- Islam, M.T. Oxidative stress and mitochondrial dysfunction-linked neurodegenerative disorders. Neurol. Res. 2017, 39, 73–82. [Google Scholar] [CrossRef]
- Sun, X.Y.; Wei, Y.P.; Xiong, Y.; Wang, X.C.; Xie, A.J.; Wang, X.L.; Yang, Y.; Wang, Q.; Lu, Y.M.; Liu, R.; et al. Synaptic released zinc promotes tau hyperphosphorylation by inhibition of protein phosphatase 2A (PP2A). J. Biol. Chem. 2012, 287, 11174–11182. [Google Scholar] [CrossRef]
- Besser, L.; Chorin, E.; Sekler, I.; Silverman, W.F.; Atkin, S.; Russell, J.T.; Hershfinkel, M. Synaptically released zinc triggers metabotropic signaling via a zinc-sensing receptor in the hippocampus. J. Neurosci. 2009, 29, 2890–2901. [Google Scholar] [CrossRef] [PubMed]
- Barone, E.; Calabrese, V.; Mancuso, C. Ferulic acid and its therapeutic potential as a hormetin for age-related diseases. Biogerontology 2009, 10, 97–108. [Google Scholar] [CrossRef]
- Mancuso, C.; Santangelo, R. Ferulic acid: Pharmacological and toxicological aspects. Food Chem. Toxicol. 2014, 65, 185–195. [Google Scholar] [CrossRef]
- Hahn, H.J.; Kim, K.B.; Bae, S.; Choi, B.G.; An, S.; Ahn, K.J.; Kim, S.Y. Pretreatment of Ferulic Acid Protects Human Dermal Fibroblasts against Ultraviolet A Irradiation. Ann. Dermatol. 2016, 28, 740–748. [Google Scholar] [CrossRef] [PubMed]
- DiNicolantonio, J.J.; McCarty, M.F.; Assanga, S.I.; Lujan, L.L.; O’Keefe, J.H. Ferulic acid and berberine, via Sirt1 and AMPK, may act as cell cleansing promoters of healthy longevity. Open Heart 2022, 9, e001801. [Google Scholar] [CrossRef] [PubMed]
- Min, X.L.; Shi, Y.; Xu, Y.; Li, Y.Y.; Dong, Y.K.; Chen, F.X.; Chen, Q.M.; Sun, Y.L.; Liao, R.; Wang, J.P. Mechanism of Sirtuin1-Mediated Deacetylation of p65-Mediated Ferroptosis of Hippocampal Neurons in Cerebral Injury after Cardiopulmonary Resuscitation in Rats. Neurochem. Res. 2025, 50, 66. [Google Scholar] [CrossRef]
- Li, B.S.; Jin, A.L.; Zhou, Z.; Seo, J.H.; Choi, B.M. DRG2 Accelerates Senescence via Negative Regulation of SIRT1 in Human Diploid Fibroblasts. Oxid. Med. Cell Longev. 2021, 2021, 7301373. [Google Scholar] [CrossRef]
- Yang, H.; Hu, J.; Chen, Y.J.; Ge, B. Role of Sirt1 in innate immune mechanisms against Mycobacterium tuberculosis via the inhibition of TAK1 activation. Arch. Biochem. Biophys. 2019, 667, 49–58. [Google Scholar] [CrossRef]
- Ding, Y.W.; Zhao, G.J.; Li, X.L.; Hong, G.L.; Li, M.F.; Qiu, Q.M.; Wu, B.; Lu, Z.Q. SIRT1 exerts protective effects against paraquat-induced injury in mouse type II alveolar epithelial cells by deacetylating NRF2 in vitro. Int. J. Mol. Med. 2016, 37, 1049–1058. [Google Scholar] [CrossRef]
- Kanski, J.; Aksenova, M.; Stoyanova, A.; Butterfield, D.A. Ferulic acid antioxidant protection against hydroxyl and peroxyl radical oxidation in synaptosomal and neuronal cell culture systems in vitro: Structure-activity studies. J. Nutr. Biochem. 2002, 13, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Joshi, G.; Perluigi, M.; Sultana, R.; Ravagna, A.; Calabrese, V.; Butterfield, D.A. In vivo protection of synaptosomes by ferulic acid ethyl ester (FAEE) from oxidative stress mediated by 2,2-azobis(2-amidino-propane)dihydrochloride (AAPH) or Fe(2+)/H(2)O(2): Insight into mechanisms of neuroprotection and relevance to oxidative stress-related neurodegenerative disorders. Neurochem. Int. 2006, 48, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Scapagnini, G.; Butterfield, D.A.; Colombrita, C.; Sultana, R.; Pascale, A.; Calabrese, V. Ethyl ferulate, a lipophilic polyphenol, induces HO-1 and protects rat neurons against oxidative stress. Antioxid. Redox Signal 2004, 6, 811–818. [Google Scholar] [CrossRef] [PubMed]
- Fetoni, A.R.; Mancuso, C.; Eramo, S.L.; Ralli, M.; Piacentini, R.; Barone, E.; Paludetti, G.; Troiani, D. In vivo protective effect of ferulic acid against noise-induced hearing loss in the guinea-pig. Neuroscience 2010, 169, 1575–1588. [Google Scholar] [CrossRef]
- Perluigi, M.; Joshi, G.; Sultana, R.; Calabrese, V.; De Marco, C.; Coccia, R.; Cini, C.; Butterfield, D.A. In vivo protective effects of ferulic acid ethyl ester against amyloid-beta peptide 1-42-induced oxidative stress. J. Neurosci. Res. 2006, 84, 418–426. [Google Scholar] [CrossRef]
- Sultana, R.; Ravagna, A.; Mohmmad-Abdul, H.; Calabrese, V.; Butterfield, D.A. Ferulic acid ethyl ester protects neurons against amyloid beta- peptide(1-42)-induced oxidative stress and neurotoxicity: Relationship to antioxidant activity. J. Neurochem. 2005, 92, 749–758. [Google Scholar] [CrossRef]
- Greenberg, E.F.; Voorbach, M.J.; Smith, A.; Reuter, D.R.; Zhuang, Y.; Wang, J.Q.; Wooten, D.W.; Asque, E.; Hu, M.; Hoft, C.; et al. Navitoclax safety, tolerability, and effect on biomarkers of senescence and neurodegeneration in aged nonhuman primates. Heliyon 2024, 10, e36483. [Google Scholar] [CrossRef]
- Fatt, M.P.; Tran, L.M.; Vetere, G.; Storer, M.A.; Simonetta, J.V.; Miller, F.D.; Frankland, P.W.; Kaplan, D.R. Restoration of hippocampal neural precursor function by ablation of senescent cells in the aging stem cell niche. Stem Cell Reports 2022, 17, 259–275. [Google Scholar] [CrossRef]
- Nguyen, L.X.T.; Troadec, E.; Kalvala, A.; Kumar, B.; Hoang, D.H.; Viola, D.; Zhang, B.; Nguyen, D.Q.; Aldoss, I.; Ghoda, L.; et al. The Bcl-2 inhibitor venetoclax inhibits Nrf2 antioxidant pathway activation induced by hypomethylating agents in AML. J. Cell Physiol. 2019, 234, 14040–14049. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, S.; Zhang, X.; Liu, F.; Yang, K.; Du, G.; Rui, X. Dasatinib protects against acute respiratory distress syndrome via Nrf2-regulated M2 macrophages polarization. Drug Dev. Res. 2021, 82, 1247–1257. [Google Scholar] [CrossRef]
- Gonzales, M.M.; Garbarino, V.R.; Kautz, T.F.; Palavicini, J.P.; Lopez-Cruzan, M.; Dehkordi, S.K.; Mathews, J.J.; Zare, H.; Xu, P.; Zhang, B.; et al. Senolytic therapy in mild Alzheimer’s disease: A phase 1 feasibility trial. Nat. Med. 2023, 29, 2481–2488. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, M.M.; Garbarino, V.R.; Marques Zilli, E.; Petersen, R.C.; Kirkland, J.L.; Tchkonia, T.; Musi, N.; Seshadri, S.; Craft, S.; Orr, M.E. Senolytic Therapy to Modulate the Progression of Alzheimer’s Disease (SToMP-AD): A Pilot Clinical Trial. J. Prev. Alzheimers Dis. 2022, 9, 22–29. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mancuso, C. The Heme Oxygenase/Biliverdin Reductase System as a Therapeutic Target to Counteract Cellular Senescence in Alzheimer’s Disease. Antioxidants 2025, 14, 1237. https://doi.org/10.3390/antiox14101237
Mancuso C. The Heme Oxygenase/Biliverdin Reductase System as a Therapeutic Target to Counteract Cellular Senescence in Alzheimer’s Disease. Antioxidants. 2025; 14(10):1237. https://doi.org/10.3390/antiox14101237
Chicago/Turabian StyleMancuso, Cesare. 2025. "The Heme Oxygenase/Biliverdin Reductase System as a Therapeutic Target to Counteract Cellular Senescence in Alzheimer’s Disease" Antioxidants 14, no. 10: 1237. https://doi.org/10.3390/antiox14101237
APA StyleMancuso, C. (2025). The Heme Oxygenase/Biliverdin Reductase System as a Therapeutic Target to Counteract Cellular Senescence in Alzheimer’s Disease. Antioxidants, 14(10), 1237. https://doi.org/10.3390/antiox14101237






