Role of Cell Oxidant Status and Redox State in Controlling Cell Proliferation and Apoptosis in Two Models of Wallerian Degeneration of Rat Sciatic Nerve
Abstract
1. Introduction
2. Material and Methods
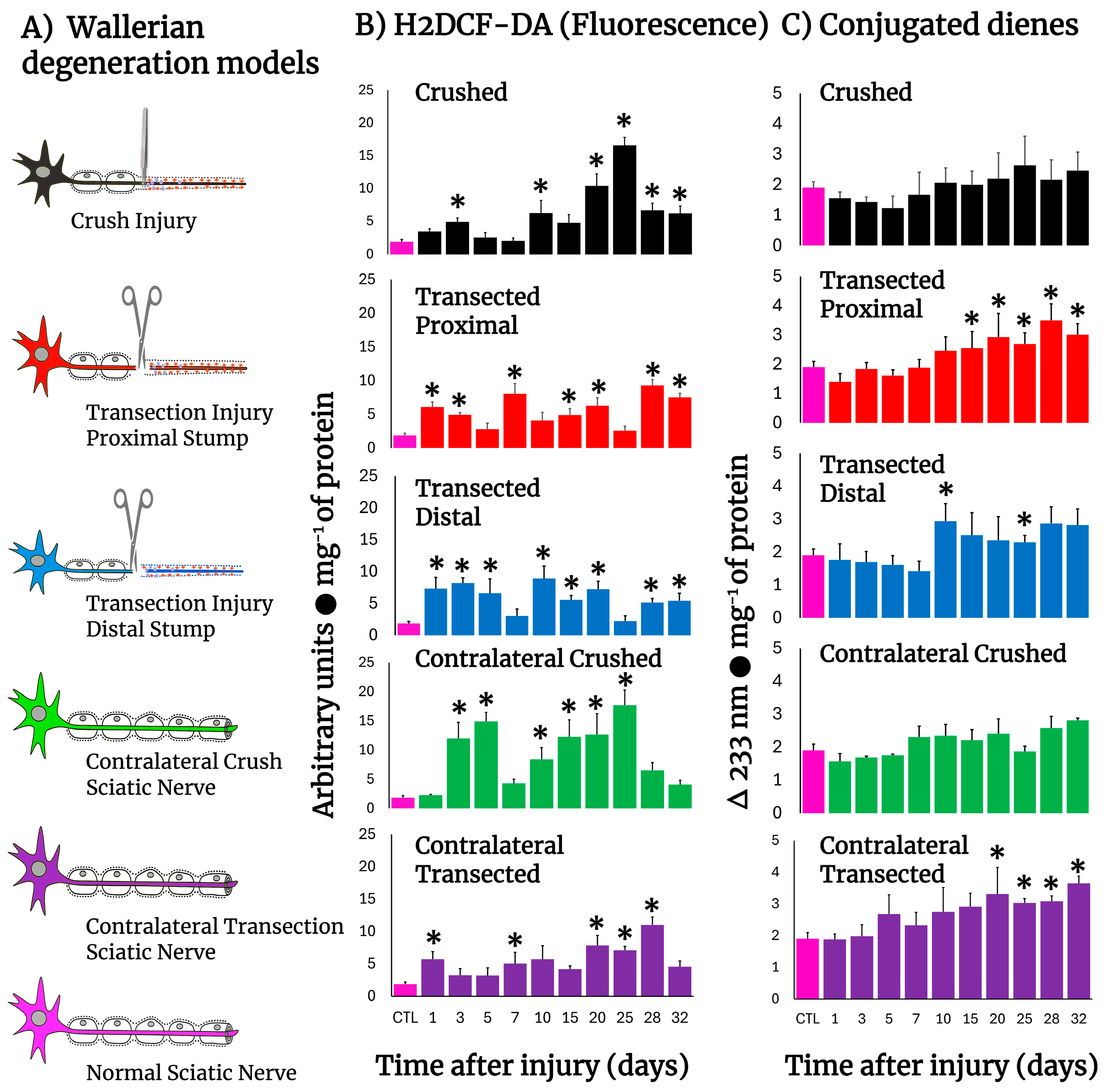
2.1. Experimental Models
2.2. Obtaining Nerve Samples
2.3. Sampling Other Organs
2.4. Assays for LP and Protein Carbonyl Groups
2.5. Estimation of DNA Synthesis and Compensatory Cell Proliferation During Wallerian Degeneration of the Sciatic Nerve
2.6. Assessment of Apoptosis
2.7. Determination of Redox-Pair Cytosolic Metabolites, NO Metabolism, and Activity of Cytochrome Oxidase
2.8. Calculations and Statistics
3. Results
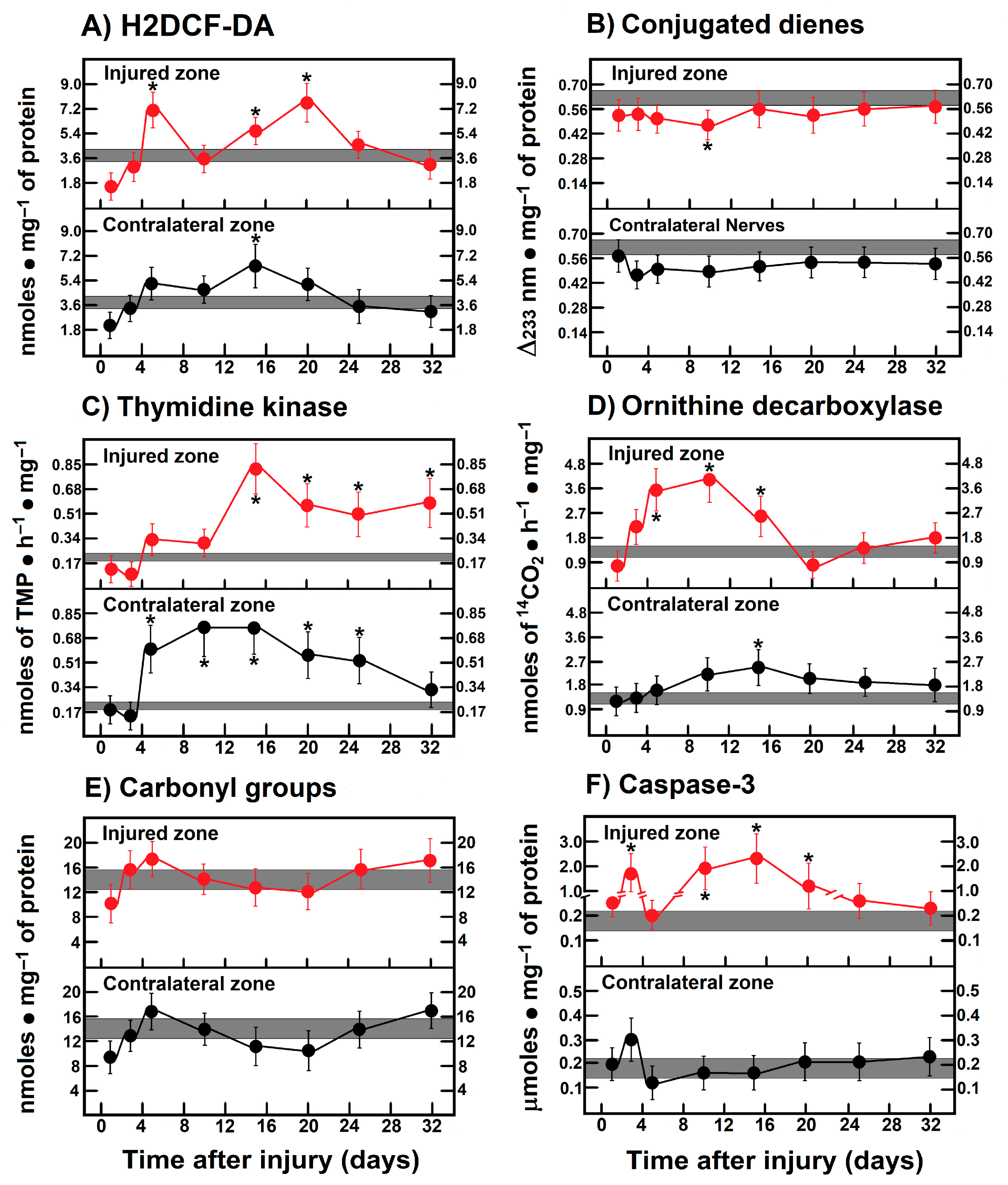
3.1. Production of ROS By-Products and Conjugated Dienes in the Injured Sciatic Nerve After Crushing or Transection
3.2. H2DCF-DA Reacting By-Product Content in Other Tissues During Wallerian Degeneration of Crushed Nerves
3.3. Production of Free Radicals Detected by Chemiluminescence in Crushed or Transected Sciatic Nerves
3.4. Rate of Protein Oxidation (Carbonyl Groups) in Crushed or Transected Nerves
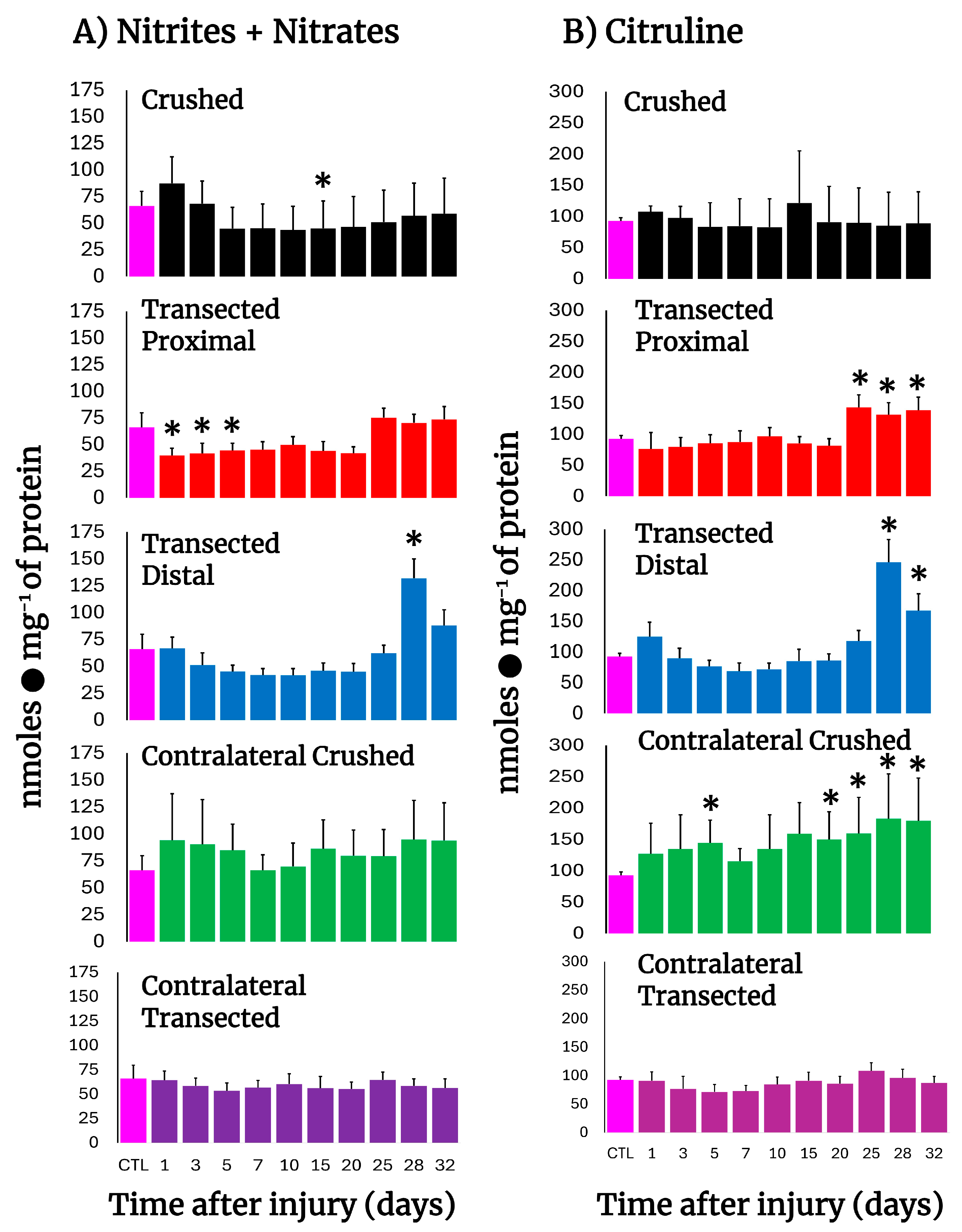
3.5. NO Metabolism in Crushed and Transected Sciatic Nerves
3.6. Parameters Indicative of Cell Proliferation in Crushed and Transected Sciatic Nerves
3.7. Activity of Caspase-3 in the Injured Sciatic Nerve After Crushing or Transection
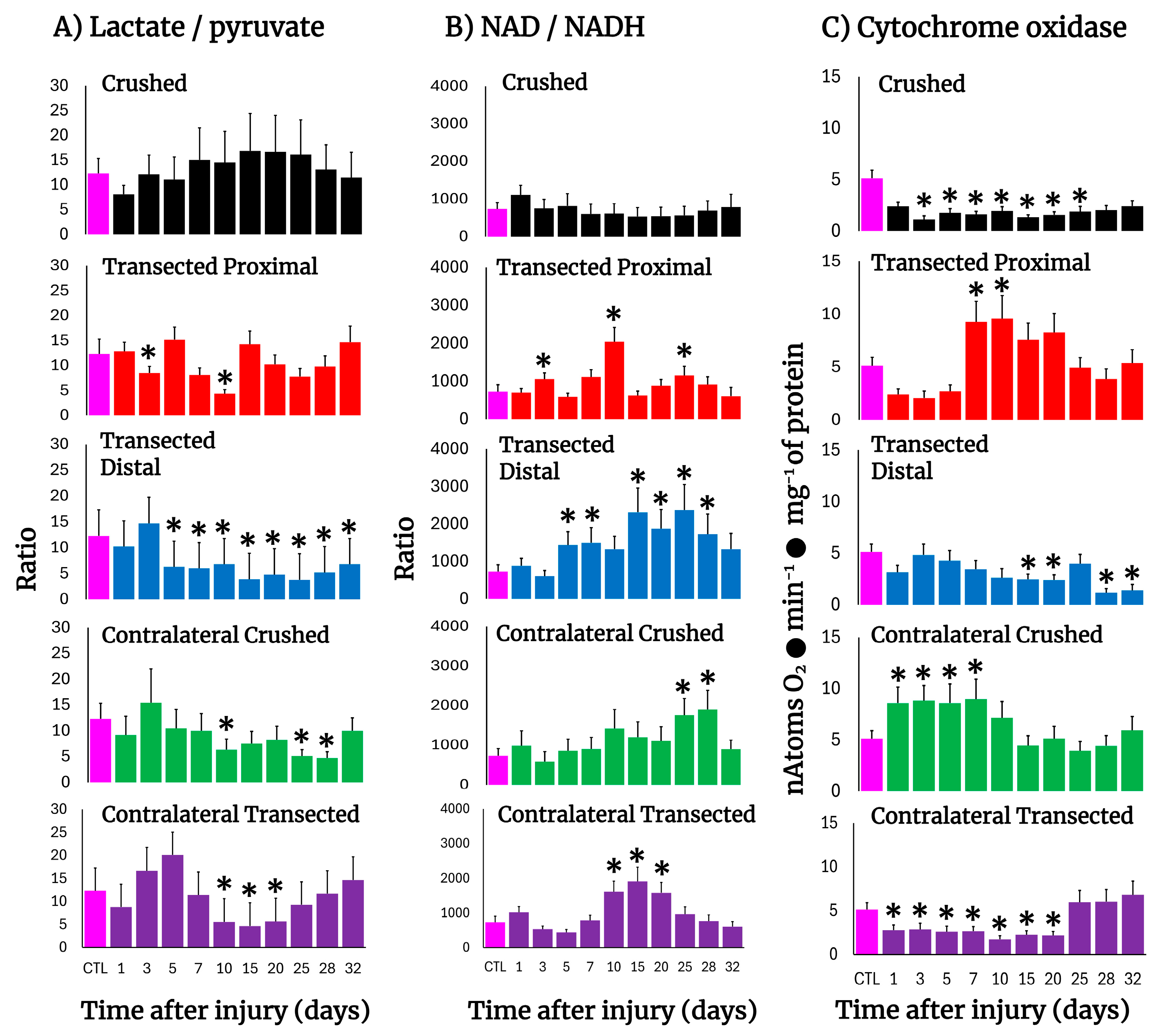
3.8. Changes in the Cell Redox State (Cytoplasmic) in the Injured Sciatic Nerve After Crushing or Transection
3.9. Mitochondrial Cytochrome Oxidase Activity in the Injured Sciatic Nerve After Crushing or Transection
3.10. Parameters Are Indicative of Oxidant Stress, Proliferation, and Apoptosis in Leg Muscles After Crushing the Right Sciatic Nerve
3.11. Correlations Among Parameters Indicative of Oxidant Stress, Cell Proliferation, Apoptosis, and Onset of Mitochondrial Biogenesis in Crushed and Transected Sciatic Nerves with Their Respective Contralateral Nerves
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jessen, K.R.; Mirsky, R. The repair Schwann cell and its function in regenerating nerves. J. Physiol. 2016, 594, 3521–3531. [Google Scholar] [CrossRef] [PubMed]
- Jessen, K.R.; Mirsky, R. The success and failure of the Schwann cell response to nerve injury. Front. Cell. Neurosci. 2019, 13, 33. [Google Scholar] [CrossRef] [PubMed]
- Forese, M.G.; Pellegatta, M.; Canevazzi, P.; Gullotta, G.S.; Podini, P.; Rivellini, C.; Previtali, S.C.; Bacigaluppi, M.; Quattrini, A.; Taveggia, C. Prostaglandin D2 synthase modulates macrophage activity and accumulation in injured peripheral nerves. Glia 2020, 68, 95–110. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Piao, X.; Bonaldo, P. Role of macrophages in Wallerian degeneration and axonal regeneration after peripheral nerve injury. Acta Neuropathol. 2015, 130, 605–618. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Wang, Y.; Wu, S.; Zhao, M.Y. Review: Myelin clearance is critical for regeneration after peripheral nerve injury. Front. Neurol. 2022, 13, 908148. [Google Scholar] [CrossRef] [PubMed]
- Ramón y Cajal, S. Degeneration & Regeneration of the Nervous System. In Cajal’s Degeneration & Regeneration of the Nervous System; De Felipe, J., Jones, E.G., Eds.; Oxford University Press: Oxford, UK; New York, NY, USA, 1928; pp. 55–60. [Google Scholar]
- Jessen, K.R.; Mirsky, R.; Lloyd, A.C. Schwann cells: Development and role in nerve repair. Cold Spring Harb. Perspect. Biol. 2015, 7, a020487. [Google Scholar] [CrossRef] [PubMed]
- Cuppini, R.; Cecchini, T.; Ciaroni, S.; Ambrogini, P.; Del Grande, P. Nodal and terminal sprouting by regenerating nerve in vitamin E-deficient rats. J. Neurol. Sci. 1993, 117, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Enrione, E.B.; Weeks, O.I.; Kranz, S.; Shen, J. A vitamin E-deficient diet affects nerve regeneration in rats. Nutrition 1999, 15, 140–144. [Google Scholar] [CrossRef] [PubMed]
- Sayan, H.; Ugurlu, B.; Babül, A.; Take, G.; Erdogan, D. Effects of L-arginine and NG-nitro L-arginine methyl ester on lipid peroxide, superoxide dismutase and nitrate levels after experimental sciatic nerve ischemia-reperfusion in rats. Int. J. Neurosci. 2004, 114, 349–364. [Google Scholar] [CrossRef] [PubMed]
- Boucher, Y.; Moreau, N.; Mauborgne, A.; Dieb, W. Lipopolysaccharide-mediated inflammatory priming potentiates painful post-traumatic trigeminal neuropathy. Physiol. Behav. 2018, 194, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Panthi, S.; Gautam, K. Roles of nitric oxide and ethyl pyruvate after peripheral nerve injury. Inflamm. Regen. 2017, 2, 20, Erratum in Inflamm. Regen. 2019, 39, 1.. [Google Scholar] [CrossRef] [PubMed]
- Moreno-López, B. Local isoform-specific NOS inhibition: A promising approach to promote motor function recovery after nerve injury. J. Neurosci. Res. 2010, 88, 1846–1857. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.J.; Kapoor, R.; Felts, P.A. Demyelination: The role of reactive oxygen and nitrogen species. Brain Pathol. 1999, 9, 69–92. [Google Scholar] [CrossRef] [PubMed]
- Tonev, D.; Momchilova, A. Oxidative stress and the Nuclear Factor Erythroid 2-Related Factor 2 (Nrf2) pathway in multiple sclerosis: Focus on certain exogenous and endogenous Nrf2 activators and therapeutic plasma exchange modulation. Int. J. Mol. Sci. 2023, 24, 17223. [Google Scholar] [CrossRef] [PubMed]
- Rabinovich, D.; Yaniv, S.P.; Alyagor, I.; Schuldiner, O. Nitric Oxide as a switching mechanism between axon degeneration and regrowth during developmental remodeling. Cell 2016, 164, 170–182. [Google Scholar] [CrossRef] [PubMed]
- Barrera, G.; Pizzimenti, S.; Dianzani, M.U. Lipid peroxidation: Control of cell proliferation, cell differentiation and cell death. Mol. Asp. Med. 2008, 29, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Segura-Anaya, E.; Martínez-Gómez, A.; Dent, M.A.R. Differences in the localization of AQP1 and expression patterns of AQP isoforms in rat and mouse sciatic nerve and changes in rat AQPs expression after nerve crush injury. IBRO Neurosci. Rep. 2022, 12, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Viarengo, A.; Burlando, B.; Cavaletto, M.; Marchi, B.; Ponzano, E.; Blasco, J. Role of metallothionein against oxidative stress in the mussel Mytilus galloprovincialis. Am. J. Physiol. 1999, 277, R1612–R1619. [Google Scholar] [PubMed]
- Hernández-Muñoz, R.; Montiel-Ruíz, C.; Vázquez-Martínez, O. Gastric mucosal cell proliferation in ethanol-induced chronic mucosal injury is related to oxidative stress and lipid peroxidation in rats. Lab. Investig. 2000, 80, 1161–1169. [Google Scholar] [CrossRef] [PubMed]
- Cadenas, E.; Sies, H. Low-level chemiluminescence as an indicator of singlet molecular oxygen in biological systems. Methods Enzymol. 1984, 5, 221–231. [Google Scholar] [PubMed]
- Levine, R.L.; Garland, D.; Oliver, C.N.; Amici, A.; Climent, I.; Lenz, A.G.; Ahn, B.W.; Shaltiel, S.; Stadtman, E.R. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 1990, 186, 464–478. [Google Scholar] [PubMed]
- Sauer, H.; Wilmanns, H. Thymidine kinase. In Methods of Enzymatic Analysis; Bermeyer, H.U., Ed.; Deerfield Beach; Wiley-VCH: Weinheim, Germany, 1985; Volume 3, pp. 468–473. [Google Scholar]
- Diehl, A.M.; Wells, M.; Brown, N.D.; Thorgeirsson, S.S.; Steer, C.J. Effect of ethanol on polyamine synthesis during liver regeneration in rats. J. Clin. Investig. 1990, 85, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Olguín-Martínez, M.; Hernández-Espinosa, D.R.; Hernández-Muñoz, R. α-Tocopherol administration blocks adaptive changes in cell NADH/NAD+ redox state and mitochondrial function leading to inhibition of gastric mucosa cell proliferation in rats. Free Radic. Biol. Med. 2013, 65, 1090–1100. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, H.M.; Harris, J.L.; Carl, S.M.; Lezi, E.; Lu, J.; Eva Selfridge, J.; Roy, N.; Hutfles, L.; Koppel, S.; Morris, J.; et al. Oxaloacetate activates brain mitochondrial biogenesis, enhances the insulin pathway, reduces inflammation and stimulates neurogenesis. Hum. Mol. Genet. 2014, 23, 6528–6541. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Zamora, S.; Méndez-Rodríguez, M.L.; Olguín-Martínez, M.; Sánchez-Sevilla, L.; Quintana-Quintana, M.; García-García, N.; Hernández-Muñoz, R. Increased erythrocytes by-products of arginine catabolism are associated with hyperglycemia and could be involved in the pathogenesis of type 2 diabetes mellitus. PLoS ONE 2013, 8, e66823. [Google Scholar] [CrossRef] [PubMed]
- Stubbs, M.; Veech, R.L.; Krebs, H.A. Control of the redox state of the nicotinamide-adenine dinucleotide couple in rat liver cytoplasm. Biochem. J. 1972, 126, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.-L.; Yu, W.-M.; Strickland, S. Peripheral regeneration. Annu. Rev. Neurosci. 2007, 30, 209–233. [Google Scholar] [CrossRef] [PubMed]
- Menorca, R.M.G.; Fussell, T.S.; Elfar, J.C. Peripheral Nerve Trauma: Mechanisms of injury and recovery. Hand. Clin. 2013, 29, 317–330. [Google Scholar] [CrossRef] [PubMed]
- Cheeseman, K.H.; Collins, M.; Maddix, S.; Milia, A.; Proudfoot, K.; Slater, T.F.; Burton, G.W.; Webb, A.; Ingold, K.U. Lipid peroxidation in regenerating rat liver. FEBS Lett. 1986, 209, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Alison, M.R. Regulation of hepatic growth. Physiol. Rev. 1986, 66, 499–541. [Google Scholar] [CrossRef] [PubMed]
- Dubinina, E.E.; Shchedrina, L.V.; Gomzyakova, N.A. The role of the redox signaling system (H2O2 and the thiol system) in the regulation of the functional activity of nervous tissue in health and disease. Biomed. Khim. 2025, 71, 243–255. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Delfín, I.; López-Barrera, F.; Hernández-Muñoz, R. Selective enhancement of lipid peroxidation in plasma membrane in two experimental models of liver regeneration: Partial hepatectomy and acute CC14 administration. Hepatology 1996, 24, 657–662. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M.; Matsuoka, I.; Wetmore, C.; Olson, L.; Thoenen, H. Enhanced synthesis of brain-derived neurotrophic factor in the lesioned peripheral nerve: Different mechanisms are responsible for the regulation of BDNF and NGF mRNA. J. Cell Biol. 1992, 119, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Gilad, G.M.; Gilad, V.H. Overview of the brain polyamine-stress-response: Regulation, development, and modulation by lithium and role in cell survival. Cell. Mol. Neurobiol. 2003, 23, 637–649. [Google Scholar] [CrossRef] [PubMed]
- Paulraj, R.; Behari, J. Biochemical changes in rat brain exposed to low intensity 9.9 GHz microwave radiation. Cell Biochem. Biophys. 2012, 63, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Saito, K.; Packianathan, S.; Longo, L.D. Free radical-induced elevation of ornithine decarboxylase activity in developing rat brain slices. Brain Res. 1997, 763, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.K.; MacLean, H.E. Polyamines, androgens, and skeletal muscle hypertrophy. J. Cell Physiol. 2011, 226, 1453–1460. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Sevilla, L.; Mendieta-Condado, E.; Hernández-Muñoz, R. Putrescine treatment reverses α-tocopherol-induced desynchronization of polyamine and retinoid metabolism during rat liver regeneration. J. Transl. Med. 2016, 14, 307. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Sevilla, L.; Mendieta-Condado, E.; Hernández-Muñoz, R. High dosing of α-tocopherol inhibits rat liver regeneration by modifying signal transducer and activator of transcription protein expression and its correlation with cell redox state and retinoid metabolism. Exp. Biol. Med. 2012, 237, 811–821. [Google Scholar] [CrossRef] [PubMed]
- Zochodne, D.W.; Levy, D. Nitric oxide in damage, disease and repair of the peripheral nervous system. Cell. Mol. Biol. 2005, 51, 255–267. [Google Scholar] [PubMed]
- Lehmann, H.C.; Köhne, A.; Meyer zu Hörste, G.; Dehmel, T.; Kiehl, O.; Hartung, H.P.; Kastenbauer, S.; Kieseier, B.C. Role of nitric oxide as mediator of nerve injury in inflammatory neuropathies. J. Neuropathol. Exp. Neurol. 2007, 66, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Khalili-Tanha, G.; Radisky, E.S.; Radisky, D.C.; Shoari, A. Matrix metalloproteinase-driven epithelial-mesenchymal transition: Implications in health and disease. J. Transl. Med. 2025, 23, 436. [Google Scholar] [CrossRef] [PubMed]
- Conti, G.; Rostami, A.; Scarpini, E.; Baron, P.; Galimberti, D.; Bresolin, N.; Contri, M.; Palumbo, C.; De Pol, A. Inducible nitric oxide synthase (iNOS) in immune-mediated demyelination and Wallerian degeneration of the rat peripheral nervous system. Exp. Neurol. 2004, 187, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Shin, T. Expression of constitutive endothelial and inducible nitric oxide synthase in the sciatic nerve of Lewis rats with experimental autoimmune neuritis. J. Neuroimmunol. 2002, 126, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Yogathasan, V.; Wischmann, P.; Solga, I.; Jäger, L.; Becher, S.; Cortese-Krott, M.M.; Gerdes, N.; Kelm, M.; Jung, C.; Chennupati, R. Divergent roles of endothelial and red blood cell nitric oxide synthase in regulating cardiovascular function during anemia. Nitric Oxide 2025, 159, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Jaerve, A.; Müller, H.W. Chemokines in CNS injury and repair. Cell Tissue Res. 2012, 349, 229–248. [Google Scholar] [CrossRef] [PubMed]
- Lara-Ramírez, R.; Segura-Anaya, E.; Martínez-Gómez, A.; Dent, M.A.R. Expression of interleukin-6 receptor alpha in normal and injured rat sciatic nerve. Neuroscience 2008, 152, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Navarro, X.; Vivó, M.; Valero-Cabré, A. Neural plasticity after peripheral nerve injury and regeneration. Prog. Neurobiol. 2007, 82, 163–201. [Google Scholar] [CrossRef] [PubMed]
- Stoll, G.; Müller, H.W. Nerve injury, axonal degeneration and neural regeneration: Basic insights. Brain Pathol. 1999, 9, 313–325. [Google Scholar] [CrossRef] [PubMed]
- Hirata, H.; Hibasami, H.; Hineno, T.; Shi, D.; Morita, A.; Inada, H.; Fujisawa, K.; Nakashima, K.; Ogihara, Y. Role of ornithine decarboxylase in proliferation of Schwann cells during Wallerian degeneration and its enhancement by nerve expansion. Muscle Nerve 1995, 18, 1341–1343. [Google Scholar] [CrossRef] [PubMed]
- Lane, A.N.; Fan, T.W. Regulation of mammalian nucleotide metabolism and biosynthesis. Nucleic Acids Res. 2015, 43, 2466–2485. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Chen, X.; Kang, S.; Wang, T.; Gnanaprakasam, J.R.; Yao, Y.; Liu, L.; Fan, G.; Burns, M.R.; Wang, R. De novo synthesis and salvage pathway coordinately regulate polyamine homeostasis and determine T cell proliferation and function. Sci. Adv. 2020, 6, eabc4275. [Google Scholar] [CrossRef] [PubMed]
- Tran, D.H.; Kim, D.; Kesavan, R.; Brown, H.; Dey, T.; Soflaee, M.H.; Vu, H.S.; Tasdogan, A.; Guo, J.; Bezwada, D.; et al. De novo and salvage purine synthesis pathways across tissues and tumors. Cell 2024, 187, 3602–3618. [Google Scholar] [CrossRef] [PubMed]
- Hirata, H.; Hibasami, H.; Yoshida, T.; Morita, A.; Ohkaya, S.; Matsumoto, M.; Sasaki, H.; Uchida, A. Differentiation and apoptosis without DNA fragmentation in cultured Schwann cells derived from Wallerian-degenerated nerve. Apoptosis 1998, 3, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Raff, M.C.; Whitmore, A.V.; Finn, J.T. Axonal self-destruction and neurodegeneration. Science 2002, 296, 868–871. [Google Scholar] [CrossRef] [PubMed]
- Koltzenburg, M.; Wall, P.D.; McMahon, S.B. Does the right side know what the left is doing? Trends Neurosci. 1999, 22, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Oaklander, A.L.; Brown, J.M. Unilateral nerve injury produces bilateral loss of distal innervation. Ann. Neurol. 2004, 55, 639–644. [Google Scholar] [CrossRef] [PubMed]
- Dubový, P.; Tucková, L.; Jancálek, R.; Svízenská, I.; Klusáková, I. Increased invasion of ED-1 positive macrophages in both ipsi- and contralateral dorsal root ganglia following unilateral nerve injuries. Neurosci. Lett. 2007, 427, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Kozin, F.; Genant, H.K.; Bekerman, C.; McCarty, D.J. The reflex sympathetic dystrophy syndrome. II. Roentgenographic and scintigraphic evidence of bilaterality and of periarticular accentuation. Am. J. Med. 1976, 60, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Lucas-Ruiz, F.; Galindo-Romero, C.; Albaladejo-García, V.; Vidal-Sanz, M.; Agudo-Barriuso, M. Mechanisms implicated in the contralateral effect in the central nervous system after unilateral injury: Focus on the visual system. Neural Regen. Res. 2021, 16, 2125–2131. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.S.; Xie, K.Q.; Zhang, C.L.; Zhu, Y.J.; Zhang, L.P.; Guo, X.; Yu, S.F. Allyl chloride-induced time dependent changes of lipid peroxidation in rat nerve tissue. Neurochem. Res. 2005, 30, 1387–1395. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Kaundal, R.K.; Iyer, S.; Sharma, S.S. Effects of resveratrol on nerve functions, oxidative stress and DNA fragmentation in experimental diabetic neuropathy. Life Sci. 2007, 80, 1236–1244. [Google Scholar] [CrossRef] [PubMed]
- Araki, T.; Sasaki, Y.; Milbrandt, J. Increased nuclear NAD biosynthesis and SIRT1 activation prevent axonal degeneration. Science 2004, 305, 1010–1013. [Google Scholar] [CrossRef]
- Mack, T.G.; Reiner, M.; Beirowski, B.; Mi, W.; Emanuelli, M.; Wagner, D.; Thomson, D.; Gillingwater, T.; Court, F.; Conforti, L.; et al. Wallerian degeneration of injured axons and synapses is delayed by a Ube4b/Nmnat chimeric gene. Nat. Neurosci. 2001, 4, 1199–1206. [Google Scholar] [PubMed]
- Rone, M.B.; Cui, Q.L.; Fang, J.; Wang, L.C.; Zhang, J.; Khan, D.; Bedard, M.; Almazan, G.; Ludwin, S.K.; Jones, R.; et al. Oligodendrogliopathy in Multiple Sclerosis: Low Glycolytic Metabolic Rate Promotes Oligodendrocyte Survival. J. Neurosci. 2016, 36, 4698–4707. [Google Scholar] [CrossRef] [PubMed]
- Glass, J.D.; Griffin, J.W. Retrograde transport of radiolabeled cytoskeletal proteins in transected nerves. J. Neurosci. 1994, 14, 3915–3921. [Google Scholar] [CrossRef] [PubMed]
- Williams, I.R.; Gilliatt, R.W. Regeneration distal to a prolonged conduction block. J. Neurol. Sci. 1977, 33, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Wee, A.S. Effects of acute and chronic denervation on human myotonia. Electromyogr. Clin. Neurophysiol. 2004, 44, 443–446. [Google Scholar] [PubMed]
- Gomez-Nicola, D.; Perry, V.H. Microglial dynamics and role in the healthy and diseased brain: A paradigm of functional plasticity. Neuroscientist 2015, 21, 169–184. [Google Scholar] [CrossRef] [PubMed]
- Beirowski, B.; Adalbert, R.; Wagner, D.; Grumme, D.S.; Addicks, K.; Ribchester, R.R.; Coleman, M.P. The progressive nature of Wallerian degeneration in wild-type and slow Wallerian degeneration (WldS) nerves. BMC Neurosci. 2005, 6, 6. [Google Scholar] [CrossRef] [PubMed]



| Parameter | Lipid Peroxidation (Arbitrary Units • 102 • mg−1 of Protein) | |||
|---|---|---|---|---|
| Organ | Brain Cortex | Brachial Nerve | Liver | Muscle |
| Controls | 5.8 ± 1.6 | 2.7 ± 0.7 | 1.3 ± 0.4 | 1.4 ± 0.5 |
| Time after Surgery | ||||
| Day 1 | 6.1 ± 1.8 | 2.3 ± 0.7 | 1.2 ± 0.4 | 3.0 ± 0.7 * |
| Day 3 | 6.2 ± 2.0 | 1.7 ± 0.4 * | 1.3 ± 0.4 | 4.1 ± 0.4 * |
| Day 5 | 6.1 ± 4.0 | 1.3 ± 0.4 * | 1.2 ± 0.2 | 1.4 ± 0.4 |
| Day 7 | 6.0 ± 4.0 | 2.0 ± 0.7 | 3.3 ± 1.3 * | 1.6 ± 0.4 |
| Day 10 | 5.9 ± 4.0 | 2.7 ± 1.1 | 2.0 ± 0.7 | 1.7 ± 0.5 |
| Day 15 | 6.0 ± 1.8 | 1.8 ± 0.7 | 1.5 ± 0.4 | 2.4 ± 0.7 * |
| Day 20 | 5.7 ± 1.8 | 1.6 ± 0.7 | 1.4 ± 0.4 | 3.0 ± 0.7 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dent, M.A.R.; Martínez-Gómez, A.; Hernández-Muñoz, R. Role of Cell Oxidant Status and Redox State in Controlling Cell Proliferation and Apoptosis in Two Models of Wallerian Degeneration of Rat Sciatic Nerve. Antioxidants 2025, 14, 1236. https://doi.org/10.3390/antiox14101236
Dent MAR, Martínez-Gómez A, Hernández-Muñoz R. Role of Cell Oxidant Status and Redox State in Controlling Cell Proliferation and Apoptosis in Two Models of Wallerian Degeneration of Rat Sciatic Nerve. Antioxidants. 2025; 14(10):1236. https://doi.org/10.3390/antiox14101236
Chicago/Turabian StyleDent, Myrna Alexandra Roberta, Alejandro Martínez-Gómez, and Rolando Hernández-Muñoz. 2025. "Role of Cell Oxidant Status and Redox State in Controlling Cell Proliferation and Apoptosis in Two Models of Wallerian Degeneration of Rat Sciatic Nerve" Antioxidants 14, no. 10: 1236. https://doi.org/10.3390/antiox14101236
APA StyleDent, M. A. R., Martínez-Gómez, A., & Hernández-Muñoz, R. (2025). Role of Cell Oxidant Status and Redox State in Controlling Cell Proliferation and Apoptosis in Two Models of Wallerian Degeneration of Rat Sciatic Nerve. Antioxidants, 14(10), 1236. https://doi.org/10.3390/antiox14101236






