Natural Deep Eutectic Solvent (NaDES) Extraction, HPLC-DAD Analysis, and Antioxidant Activity of Chilean Ugni molinae Turcz. Fruits
Abstract
1. Introduction
2. Materials and Methods
2.1. Solvent and Reagents
2.2. Plant Material
2.3. Extraction Process
2.3.1. NaDES Extraction
2.3.2. Methanolic Extraction
2.4. Spectrophotometric Analysis
2.4.1. Total Phenolic Content (TPC)
2.4.2. Total Flavonoid Content (TFC)
2.4.3. Antioxidant Activity
2.5. Chromatographic Profile Using HPLC–DAD
2.5.1. HPLC-DAD Analysis
2.5.2. Extraction Performance Analysis
2.6. Multivariate Statistical Analyses
3. Results and Discussion
3.1. Total Phenolics, Total Flavonoids, and Antioxidant Activity
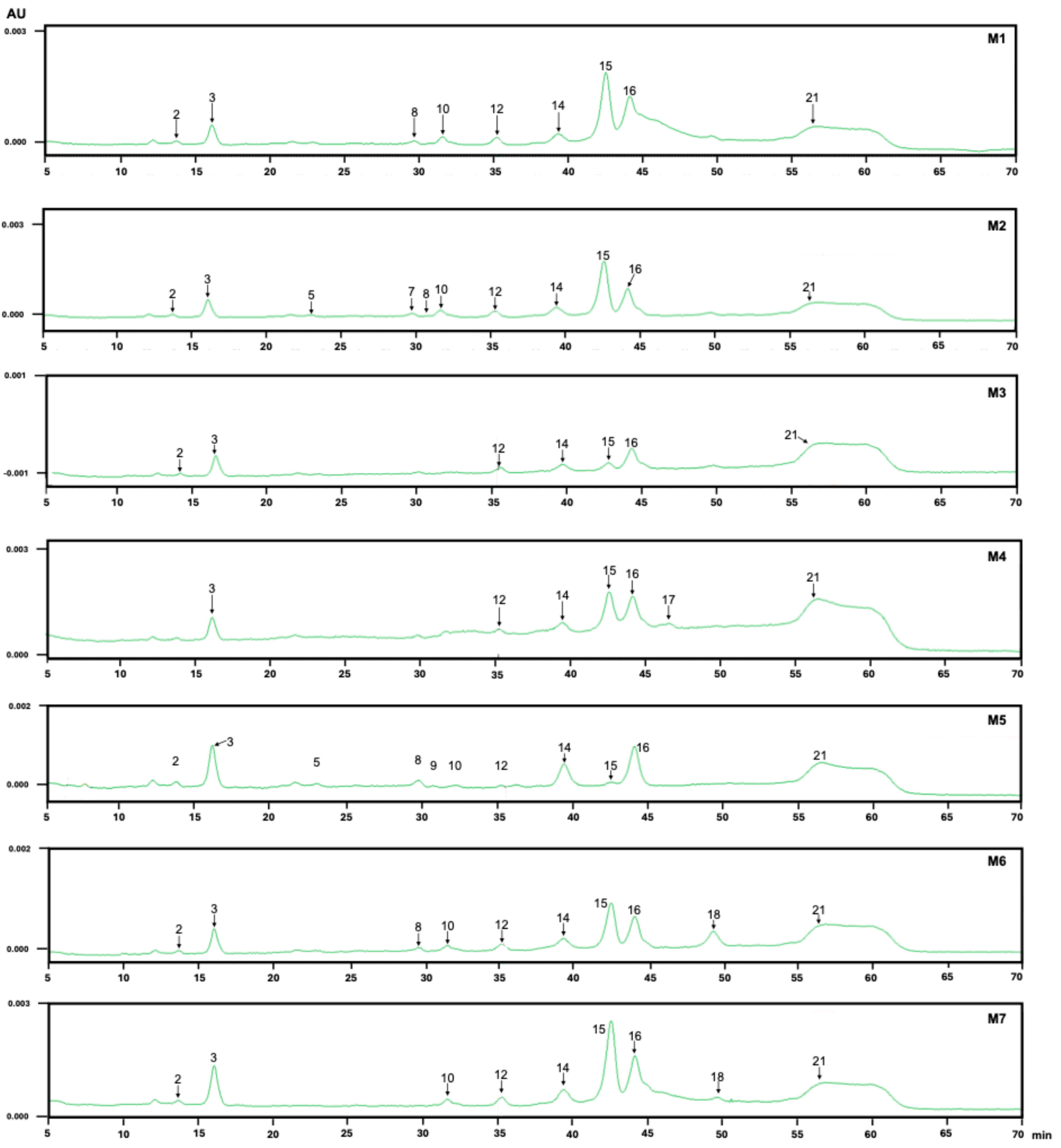
3.2. HPLC-DAD
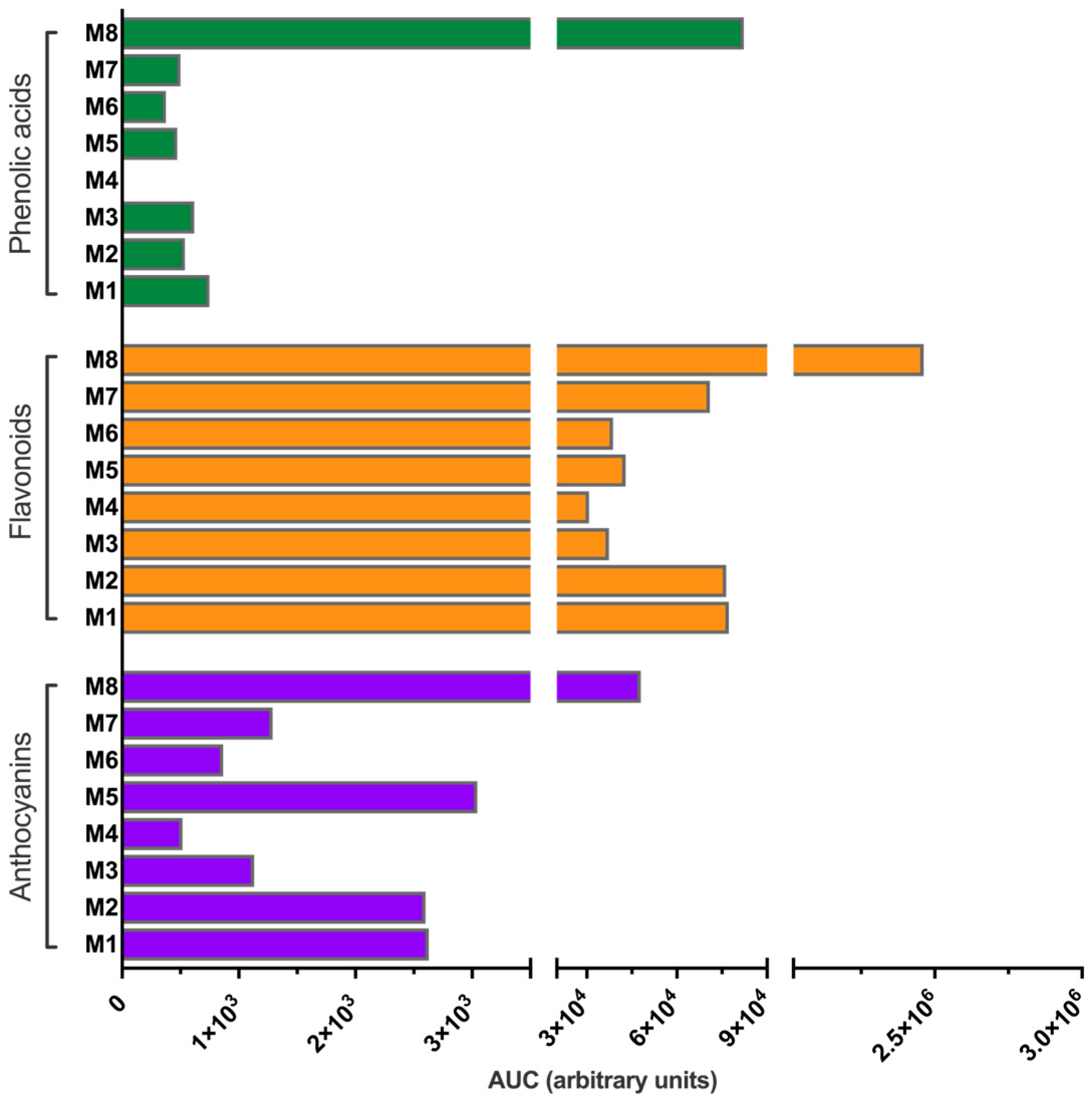
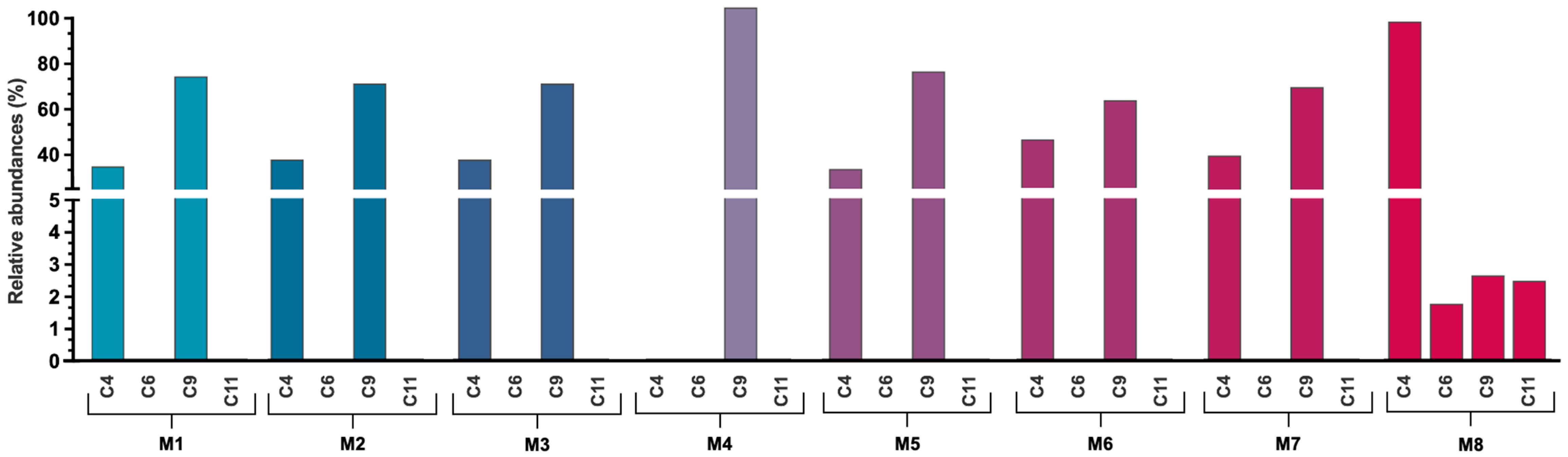
3.3. Extraction Performance Analysis
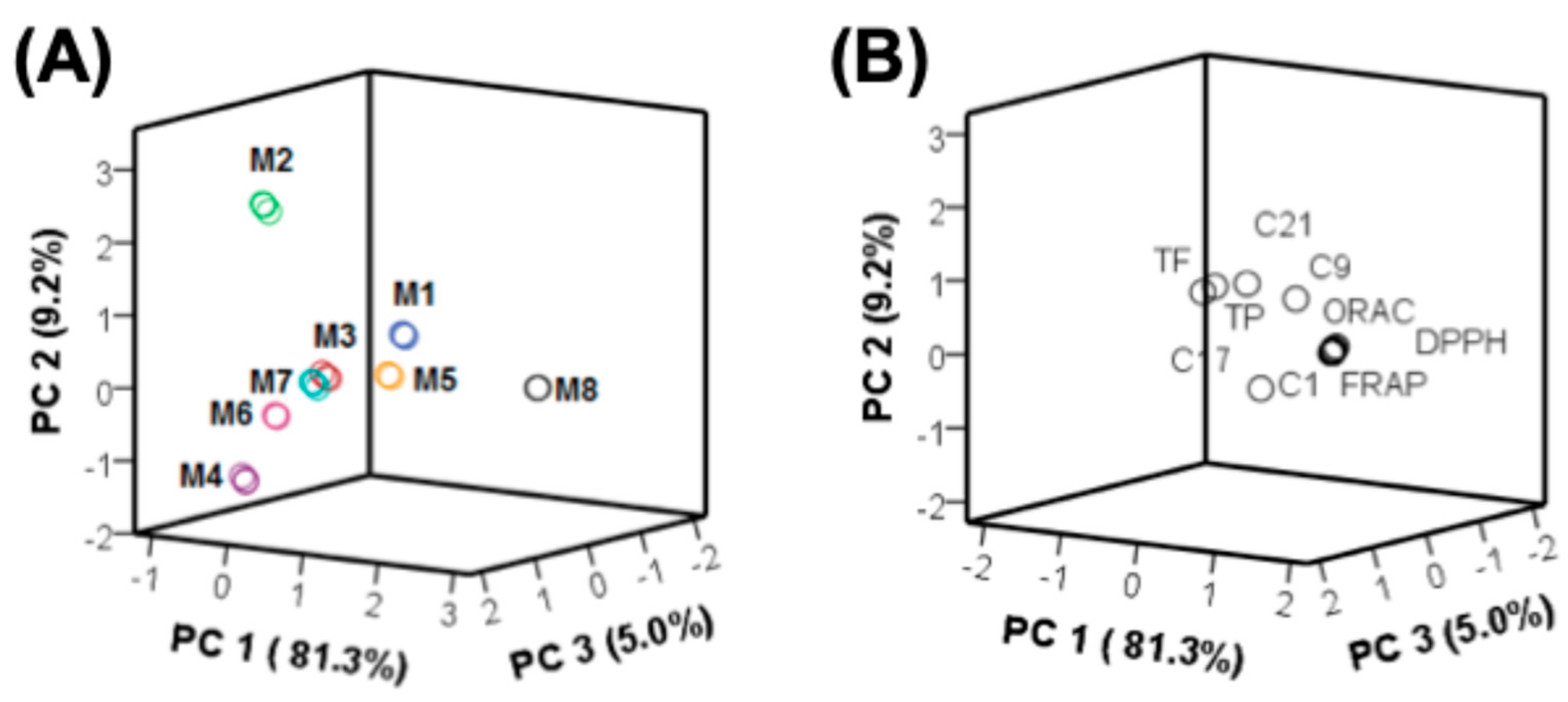
3.4. Multivariate Statistical Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, Y.; Zhang, J.J.; Xu, D.P.; Zhou, T.; Zhou, Y.; Li, S.; Li, H. Bin Bioactivities and Health Benefits of Wild Fruits. Int. J. Mol. Sci. 2016, 17, 1258. [Google Scholar] [CrossRef]
- Nile, S.H.; Park, S.W. Edible berries: Bioactive components and their effect on human health. Nutrition 2014, 30, 134–144. [Google Scholar] [CrossRef]
- Luo, J.; Si, H.; Jia, Z.; Liu, D. Dietary anti-aging polyphenols and potential mechanisms. Antioxidants 2021, 10, 283. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H. Targeting oxidative stress in disease: Promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709. [Google Scholar]
- Montenegro, G. Montenegro: Chile Nuestra Flora Útil; Ediciones Universidad Católica de Chile: Santiago, Chile, 2000. [Google Scholar]
- Rubilar, M.; Pinelo, M.; Ihl, M.; Scheuermann, E.; Sineiro, J.; Nuñez, M.J. Murta leaves (Ugni molinae Turcz) as a source of antioxidant polyphenols. J. Agric. Food Chem. 2006, 54, 59–64. [Google Scholar] [CrossRef]
- Cabrera-Barjas, G.; Quezada, A.; Bernardo, Y.; Moncada, M.; Zúñiga, E.; Wilkens, M.; Giordano, A.; Nesic, A.; Delgado, N. Chemical composition and antibacterial activity of red murta (Ugni molinae Turcz.) seeds: An undervalued Chilean resource. J. Food Meas. Charact. 2020, 14, 1810–1821. [Google Scholar] [CrossRef]
- López, J.; Vega-Gálvez, A.; Ah-Hen, K.S.; Rodríguez, A.; Quispe-Fuentes, I.; Delporte, C.; Valenzuela-Barra, G.; Arancibia, Y.; Zambrano, A. Evaluation of the antioxidant, anti-inflammatory, and anti-tumoral properties of bioactive compounds extracted from murta berries (Ugni molinae T.) dried by different methods. Front. Plant Sci. 2023, 14, 1095179. [Google Scholar] [CrossRef]
- Fredes, C.; Parada, A.; Salinas, J.; Robert, P. Phytochemicals and Traditional Use of Two Southernmost Chilean Berry Fruits: Murta (Ugni molinae Turcz) and Calafate (Berberis buxifolia Lam.). Foods 2020, 9, 54. [Google Scholar] [CrossRef] [PubMed]
- de Dicastillo, C.L.; Bustos, F.; Valenzuela, X.; López-Carballo, G.; Vilariño, J.M.; Galotto, M.J. Chilean berry Ugni molinae Turcz. fruit and leaves extracts with interesting antioxidant, antimicrobial and tyrosinase inhibitory properties. Food Res. Int. 2017, 102, 119–128. [Google Scholar] [CrossRef]
- Vega-Galvez, A.; Rodríguez, A.; Stucken, K. Antioxidant, functional properties and health-promoting potential of native South American berries: A review. J. Sci. Food Agric. 2021, 101, 364–378. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Pérez, L.S.; Moraga, N.; Ah-Hen, K.S.; Rodríguez, A.; Vega-Gálvez, A. Dietary fibre in processed murta ( Ugni molinae Turcz) berries: Bioactive components and antioxidant capacity. J. Food Sci. Technol. 2022, 59, 3093–3101. [Google Scholar] [CrossRef]
- Suwalsky, M.; Orellana, P.; Avello, M.; Villena, F. Protective effect of Ugni molinae Turcz against oxidative damage of human erythrocytes. Food Chem. Toxicol. 2007, 45, 130–135. [Google Scholar] [CrossRef]
- Albertini, B.; Bertoni, S.; Sangiorgi, S.; Nucci, G.; Passerini, N.; Mezzina, E. NaDES as a green technological approach for the solubility improvement of BCS class II APIs: An insight into the molecular interactions. Int. J. Pharm. 2023, 634, 122696. [Google Scholar] [CrossRef]
- Martinović, M.; Krgović, N.; Nešić, I.; Žugić, A.; Tadić, V.M. Conventional vs. Green Extraction Using Natural Deep Eutectic Solvents—Differences in the Composition of Soluble Unbound Phenolic Compounds and Antioxidant Activity. Antioxidants 2022, 11, 2295. [Google Scholar] [CrossRef]
- da Silva, D.T.; Rodrigues, R.F.; Machado, N.M.; Maurer, L.H.; Ferreira, L.F.; Somacal, S.; da Veiga, M.L.; Rocha, M.I.U.M.D.; Vizzotto, M.; Rodrigues, E.; et al. Natural deep eutectic solvent (NADES)-based blueberry extracts protect against ethanol-induced gastric ulcer in rats. Food Res. Int. 2020, 138, 109718. [Google Scholar] [CrossRef] [PubMed]
- Lanari, D.; Zadra, C.; Negro, F.; Njem, R.; Marcotullio, M.C. Influence of choline chloride-based NADES on the composition of Myristica fragrans Houtt. essential oil. Heliyon 2022, 8, e09531. [Google Scholar] [CrossRef] [PubMed]
- Picot-Allain, C.; Mahomoodally, M.F.; Ak, G.; Zengin, G. Conventional versus green extraction techniques—A comparative perspective. Curr. Opin. Food Sci. 2021, 40, 144–156. [Google Scholar] [CrossRef]
- de Oliveira, I.L.; Domínguez-Rodríguez, G.; Montero, L.; Viganó, J.; Cifuentes, A.; Rostagno, M.A.; Ibáñez, E. Advanced Extraction Techniques Combined with Natural Deep Eutectic Solvents for Extracting Phenolic Compounds from Pomegranate (Punica granatum L.) Peels. Int. J. Mol. Sci. 2024, 25, 9992. [Google Scholar] [CrossRef]
- Jovanović, M.S.; Krgović, N.; Radan, M.; Ćujić-Nikolić, N.; Mudrić, J.; Lazarević, Z.; Šavikin, K. Natural deep eutectic solvents combined with cyclodextrins: A novel strategy for chokeberry anthocyanins extraction. Food Chem. 2023, 405, 134816. [Google Scholar] [CrossRef]
- de Oliveira, J.A.R.; de Paula Menezes Barbosa, P.; Macêdo, G.A. High Concentrate Flavonoids Extract from Citrus Pomace Using Enzymatic and Deep Eutectic Solvents Extraction. Foods 2022, 11, 3205. [Google Scholar] [CrossRef]
- Santos-Martín, M.; Cubero-Cardoso, J.; González-Domínguez, R.; Cortés-Triviño, E.; Sayago, A.; Urbano, J.; Fernández-Recamales, Á. Ultrasound-assisted extraction of phenolic compounds from blueberry leaves using natural deep eutectic solvents (NADES) for the valorization of agrifood wastes. Biomass Bioenergy 2023, 175, 106882. [Google Scholar] [CrossRef]
- Pereira, T.C.; Souza, V.P.; Padilha, A.P.F.; Duarte, F.A.; Flores, E.M. Trends and perspectives on the ultrasound-assisted extraction of bioactive compounds using natural deep eutectic solvents. Curr. Opin. Chem. Eng. 2025, 47, 101088. [Google Scholar] [CrossRef]
- Fernández-Galleguillos, C.; Quesada-Romero, L.; Puerta, A.; Padrón, J.M.; Souza, E.; Romero-Parra, J.; Simirgiotis, M.J. UHPLC-MS Chemical Fingerprinting and Antioxidant, Antiproliferative, and Enzyme Inhibition Potential of Gaultheria pumila Berries. Metabolites 2021, 11, 523. [Google Scholar] [CrossRef]
- Burgos-Edwards, A.; Jiménez-Aspee, F.; Thomas-Valdés, S.; Schmeda-Hirschmann, G.; Theoduloz, C. Qualitative and quantitative changes in polyphenol composition and bioactivity of Ribes magellanicum and R. punctatum after in vitro gastrointestinal digestion. Food Chem. 2017, 237, 1073–1082. [Google Scholar] [CrossRef] [PubMed]
- Fuentes-Jorquera, N.; Canales, R.I.; Pérez-Correa, J.R.; Pérez-Jiménez, J.; Mariotti-Celis, M.S. Differential Extraction and Preliminary Identification of Polyphenols from Ugni candollei (White Murta) Berries. Antioxidants 2024, 13, 623. [Google Scholar] [CrossRef]
- Bashir, I.; Dar, A.H.; Dash, K.K.; Pandey, V.K.; Fayaz, U.; Shams, R.; Srivastava, S.; Singh, R. Deep eutectic solvents for extraction of functional components from plant-based products: A promising approach. Sustain. Chem. Pharm. 2023, 33, 101102. [Google Scholar] [CrossRef]
- Alsaud, N.; Shahbaz, K.; Farid, M. Application of deep eutectic solvents in the extraction of polyphenolic antioxidants from New Zealand Manuka leaves (Leptospermum Scoparium): Optimization and antioxidant activity. J. Mol. Liq. 2021, 337, 116385. [Google Scholar] [CrossRef]
- Peña-Cerda, M.; Arancibia-Radich, J.; Valenzuela-Bustamante, P.; Pérez-Arancibia, R.; Barriga, A.; Seguel, I.; García, L.; Delporte, C. Phenolic composition and antioxidant capacity of Ugni molinae Turcz. leaves of different genotypes. Food Chem. 2017, 215, 219–227. [Google Scholar] [CrossRef]
- Junqueira-Gonçalves, M.P.; Yáñez, L.; Morales, C.; Navarro, M.; Contreras, R.A.; Zúñiga, G.E. Isolation and Characterization of Phenolic Compounds and Anthocyanins from Murta (Ugni molinae Turcz.) Fruits. Assessment of Antioxidant and Antibacterial Activity. Molecules 2015, 20, 5698–5713. [Google Scholar] [CrossRef] [PubMed]
- Dudonné, S.; Vitrac, X.; Coutiére, P.; Woillez, M.; Mérillon, J.M. Comparative study of antioxidant properties and total phenolic content of 30 plant extracts of industrial interest using DPPH, ABTS, FRAP, SOD, and ORAC assays. J. Agric. Food Chem. 2009, 57, 1768–1774. [Google Scholar] [CrossRef]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Hawkins Byrne, D. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compos. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Suwalsky, M.; Avello, M. Antioxidant capacity of Ugni molinae fruit extract on human erythrocytes: An in vitro study. J. Membr. Biol. 2014, 247, 703–712. [Google Scholar] [CrossRef]
- Pérez-Arancibia, R.; Ordoñez, J.L.; Rivas, A.; Pihán, P.; Sagredo, A.; Ahumada, U.; Barriga, A.; Seguel, I.; Cárdenas, C.; Vidal, R.L.; et al. A phenolic-rich extract from Ugni molinae berries reduces abnormal protein aggregation in a cellular model of Huntington’s disease. PLoS ONE 2021, 16, e0254834. [Google Scholar]
- Ordóñez, J.L.; Pérez, R.; Barriga, A.; Seguel, I.; Guzman, P.; Zúñiga, M.C.; Delporte, C. Comparative study of antioxidant and inhibitory activity on α-glucosidase and glycogen phosphorylase A of berry extracts from Ugni molinae genotypes. J. Berry Res. 2022, 12, 279–296. [Google Scholar] [CrossRef]
- Mieres-Castro, D.; Schmeda-Hirschmann, G.; Theoduloz, C.; Rojas, A.; Piderit, D.; Jiménez-Aspee, F. Isolation and characterization of secondary metabolites from Gaultheria tenuifolia berries. J. Food Sci. 2020, 85, 2792–2802. [Google Scholar] [CrossRef]
- Mieres-Castro, D.; Schmeda-Hirschmann, G.; Theoduloz, C.; Gómez-Alonso, S.; Pérez-Navarro, J.; Márquez, K.; Jiménez-Aspee, F. Antioxidant activity and the isolation of polyphenols and new iridoids from Chilean Gaultheria phillyreifolia and G. poeppigii berries. Food Chem. 2019, 291, 167–179. [Google Scholar] [CrossRef]
- Mabry, T.J.; Markham, K.R.; Thomas, M.B. The Systematic Identification of Flavonoids; Springer: Berlin/Heidelberg, Germany, 1970. [Google Scholar]
- Wolfender, J.L.; Marti, G.; Thomas, A.; Bertrand, S. Current approaches and challenges for the metabolite profiling of complex natural extracts. J. Chromatogr. A 2015, 1382, 136–164. [Google Scholar] [CrossRef]
- Simirgiotis, M.; Silva, M.; Becerra, J.; Schmeda-Hirschmann, G. Direct characterisation of phenolic antioxidants in infusions from four Mapuche medicinal plants by liquid chromatography with diode array detection (HPLC-DAD) and electrospray ionisation tandem mass spectrometry (HPLC-ESI–MS). Food Chem. 2012, 131, 318–327. [Google Scholar] [CrossRef]
- De Villiers, A.; Venter, P.; Pasch, H. Recent advances and trends in the liquid-chromatography–mass spectrometry analysis of flavonoids. J. Chromatogr. A 2016, 1430, 16–78. [Google Scholar] [CrossRef]
- Haminiuk, C.W.I.; Maciel, G.M.; Plata-Oviedo, M.S.V.; Peralta, R.M. Phenolic compounds in fruits—An overview. Int. J. Food Sci. Technol. 2012, 47, 2023–2044. [Google Scholar] [CrossRef]
- Ferreira, C.; Sarraguça, M.A.; Ferreira, C.; Sarraguça, M. A Comprehensive Review on Deep Eutectic Solvents and Its Use to Extract Bioactive Compounds of Pharmaceutical Interest. Pharmaceuticals 2024, 17, 124. [Google Scholar] [CrossRef]
- Chemat, F.; Vian, M.A.; Cravotto, G. Green Extraction of Natural Products: Concept and Principles. Int. J. Mol. Sci. 2012, 13, 8615–8627. [Google Scholar] [CrossRef] [PubMed]
- Jovanović, M.S.; Krgović, N.; Živković, J.; Stević, T.; Zdunić, G.; Bigović, D.; Šavikin, K. Ultrasound-Assisted Natural Deep Eutectic Solvents Extraction of Bilberry Anthocyanins: Optimization, Bioactivities, and Storage Stability. Plants 2022, 11, 2680. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; De Oliveira Vigier, K.; Royer, S.; Jérôme, F. Deep eutectic solvents: Syntheses, properties and applications. Chem. Soc. Rev. 2012, 41, 7108–7146. [Google Scholar] [CrossRef] [PubMed]


| Mixtures | Hydrogen Bond Acceptor | Hydrogen Bond Donor | Molar Ratio |
|---|---|---|---|
| M1 | Choline chloride | Glycerol | 1:2 |
| M2 | Choline chloride | 1,2-Propanediol | 1:2 |
| M3 | Choline chloride | D-glucose | 1:2 |
| M4 | Choline chloride | Citric acid | 1:2 |
| M5 | Choline chloride | Oxalic acid | 1:1 |
| M6 | Choline chloride | Lactic acid | 1:2 |
| M7 | Choline chloride | Urea | 1:2 |
| M8 (control) | Methanol: Formic Acid | 95:5 | |
| Treatment | TPC (mg GAE/g ext) | TFC (mg QE/g ext) | DPPH (IC50) (µg ext/mL) | FRAP (µmol TE/g ext) | ORAC (µmol TE/g ext) |
|---|---|---|---|---|---|
| M1 | 3.19 ± 0.12 a | 3.16 ± 0.76 b | 2.15 ± 0.04 g | 2.45 ± 0.11 a | 39,073 ± 3 e |
| M2 | 64.87 ± 4.85 c | 35.38 ± 0.76 e | 1.05 ± 0.03 d | 3.12 ± 0.15 a | 40,291 ± 4 g |
| M3 | 7.43 ± 1.73 a | 7.81 ± 0.76 c | 1.42 ± 0.04 f | 2.39 ± 0.05 a | 38,428 ± 6 b |
| M4 | 6.32 ± 3.68 a | 8.52 ± 1.38 cd | 1.20 ± 0.02 e | 3.05 ± 0.06 a | 38,356 ± 3 a |
| M5 | 8.00 ± 0.5 a | 0.94 ± 0.61 a | 0.94 ± 0.03 c | 3.50 ± 0.15 a | 38,948 ± 9 d |
| M6 | 19.58 ± 1.38 b | 10.64 ± 0.30 d | 0.78 ± 0.04 b | 3.52 ± 0.11 a | 39,168 ± 3 f |
| M7 | 8.78 ± 2.67 a | 8.92 ± 0.76 cd | 0.68 ± 0.01 a | 4.91 ± 0.21 a | 38,920 ± 6 c |
| M8 | 4.10 ± 0.36 a | 1.37 ± 0.03 ab | 3.62 ± 0.02 h | 45.91 ± 6.87 b | 568,851 ± 4 h |
| Peak | UV-Vis Spectra | λmax (nm) | λused (nm) | tR (min) | M8 | M1 | M2 | M3 | M4 | M5 | M6 | M7 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 244, 276 | 276 | 280 | 10.967 | x | - | - | - | - | - | - | - |
| 2 | 244, 278 | 278 | 280 | 13.833 | x | x | x | x | - | x | x | x |
| 3 | 244, 276, 352 | 352 | 354 | 16.087 | x | x | x | x | x | x | x | x |
| 4 | 244, 276, 364, 513 | 513 | 520 | 20.773 | x | x | x | x | - | x | x | x |
| 5 | 244, 278, 352, 370 sh | 352 | 354 | 22.720 | x | - | - | - | - | x | - | - |
| 6 | 244, 280, 359, 518 | 518 | 520 | 26.807 | x | - | - | - | - | - | - | - |
| 7 | 244, 276, 356 | 356 | 354 | 27.713 | x | - | - | - | - | - | - | - |
| 8 | 244, 276, 356 | 356 | 354 | 29.720 | x | - | - | - | - | x | x | - |
| 9 | 244, 266, 300, 520 | 520 | 520 | 30.767 | x | x | x | x | x | x | x | x |
| 10 | 244, 272, 354 | 354 | 354 | 31.620 | x | x | x | - | x | x | x | x |
| 11 | 244, 352, 520 | 520 | 520 | 32.313 | x | - | - | - | - | - | - | - |
| 12 | 244, 272, 354 | 354 | 354 | 35.267 | x | x | x | x | x | x | x | x |
| 13 | 244, 272, 354 | 354 | 354 | 37.687 | x | - | - | - | - | - | - | - |
| 14 | 245, 304, 363 | 363 | 354 | 39.367 | x | x | x | x | x | x | x | x |
| 15 | 244, 268 sh, 352 | 352 | 354 | 42.527 | x | x | x | x | x | x | x | x |
| 16 | 244, 270 sh, 351 | 351 | 354 | 44.107 | x | x | x | x | x | x | x | x |
| 17 | 244, 270 sh, 351 | 351 | 354 | 45.953 | x | - | - | - | x | - | - | |
| 18 | 244, 270 sh, 352 | 352 | 354 | 49.653 | x | - | - | - | - | - | x | x |
| 19 | 244, 270 sh, 352 | 352 | 354 | 52.940 | x | - | - | - | - | - | - | - |
| 20 | 244, 270 sh, 352 | 352 | 354 | 54.527 | x | - | - | - | - | - | - | - |
| 21 | 244, 270 sh, 352 | 352 | 354 | 55.353 | x | x | x | x | x | x | x | x |
| Family | M1 | M2 | M3 | M4 | M5 | M6 | M7 | M8 |
|---|---|---|---|---|---|---|---|---|
| Phenolic acids | 0.93 | 0.68 | 1.58 | 0.00 | 1.02 | 0.93 | 0.69 | 3.17 |
| Flavonoids | 95.81 | 96.06 | 95.54 | 98.35 | 92.42 | 96.90 | 97.54 | 94.98 |
| Anthocyanins | 3.26 | 3.27 | 2.89 | 1.65 | 6.56 | 2.17 | 1.77 | 1.85 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antileo-Laurie, J.; Olate-Olave, V.; Fehrmann-Riquelme, V.; Anabalón-Alvarez, C.; Cid-Carrillo, L.; Campanini-Salinas, J.; Fernández-Galleguillos, C.; Quesada-Romero, L. Natural Deep Eutectic Solvent (NaDES) Extraction, HPLC-DAD Analysis, and Antioxidant Activity of Chilean Ugni molinae Turcz. Fruits. Antioxidants 2025, 14, 1234. https://doi.org/10.3390/antiox14101234
Antileo-Laurie J, Olate-Olave V, Fehrmann-Riquelme V, Anabalón-Alvarez C, Cid-Carrillo L, Campanini-Salinas J, Fernández-Galleguillos C, Quesada-Romero L. Natural Deep Eutectic Solvent (NaDES) Extraction, HPLC-DAD Analysis, and Antioxidant Activity of Chilean Ugni molinae Turcz. Fruits. Antioxidants. 2025; 14(10):1234. https://doi.org/10.3390/antiox14101234
Chicago/Turabian StyleAntileo-Laurie, Javier, Verónica Olate-Olave, Valentina Fehrmann-Riquelme, Camila Anabalón-Alvarez, Luis Cid-Carrillo, Javier Campanini-Salinas, Carlos Fernández-Galleguillos, and Luisa Quesada-Romero. 2025. "Natural Deep Eutectic Solvent (NaDES) Extraction, HPLC-DAD Analysis, and Antioxidant Activity of Chilean Ugni molinae Turcz. Fruits" Antioxidants 14, no. 10: 1234. https://doi.org/10.3390/antiox14101234
APA StyleAntileo-Laurie, J., Olate-Olave, V., Fehrmann-Riquelme, V., Anabalón-Alvarez, C., Cid-Carrillo, L., Campanini-Salinas, J., Fernández-Galleguillos, C., & Quesada-Romero, L. (2025). Natural Deep Eutectic Solvent (NaDES) Extraction, HPLC-DAD Analysis, and Antioxidant Activity of Chilean Ugni molinae Turcz. Fruits. Antioxidants, 14(10), 1234. https://doi.org/10.3390/antiox14101234








