Androgen-Induced Lactic Acid Accumulation Contributes to the Apoptosis of Ovarian Granulosa Cells in Polycystic Ovary Syndrome Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Treatments
2.2. Estrous Cycle Determination
2.3. Cell Culture
2.4. TUNEL Staining
2.5. Measurement of Intracellular pH (pHi)
2.6. Determination of Extracellular pH Values (pHe)
2.7. Analysis of Extracellular Acidification Rate
2.8. Analysis of Oxygen Consumption Rate Assay
2.9. Measurement of Lactic Acid Contents and the Activity of Lactic Acid Dehydrogenase (LDH)
2.10. Detection of Cell Apoptosis by Flow Cytometry
2.11. Detection of Reactive Oxygen Species (ROS) by DCFH-DA
2.12. Determination of ATP Content
2.13. Measurement of Mitochondrial DNA (mtDNA) Copy Number
2.14. The Comet Assay
2.15. Western Blot Analysis
2.16. Statistical Analysis
3. Result
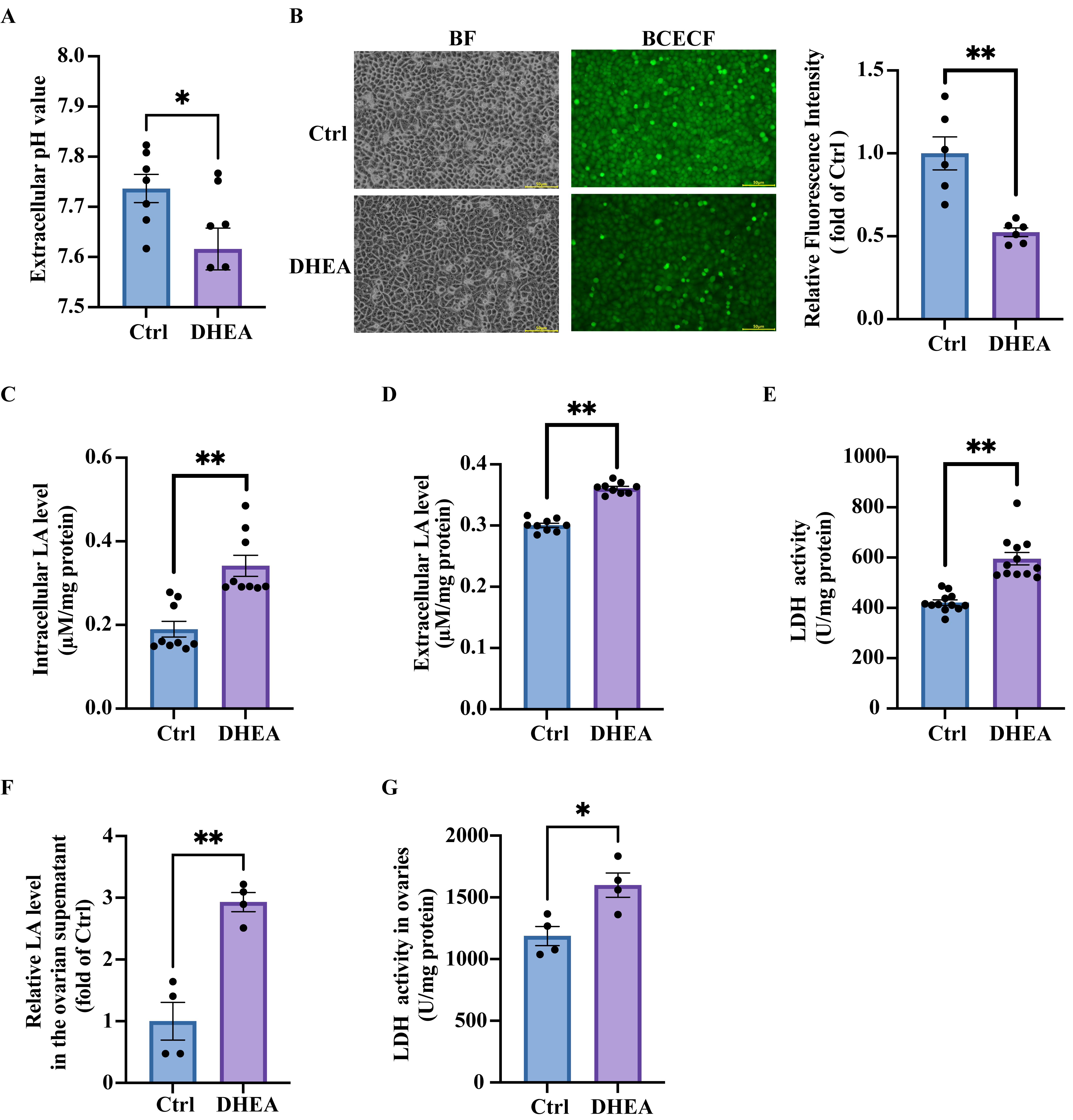
3.1. High Concentrations of Androgen Reduce Extracellular pH by Increasing Lactic Acid Production in Granulosa Cells
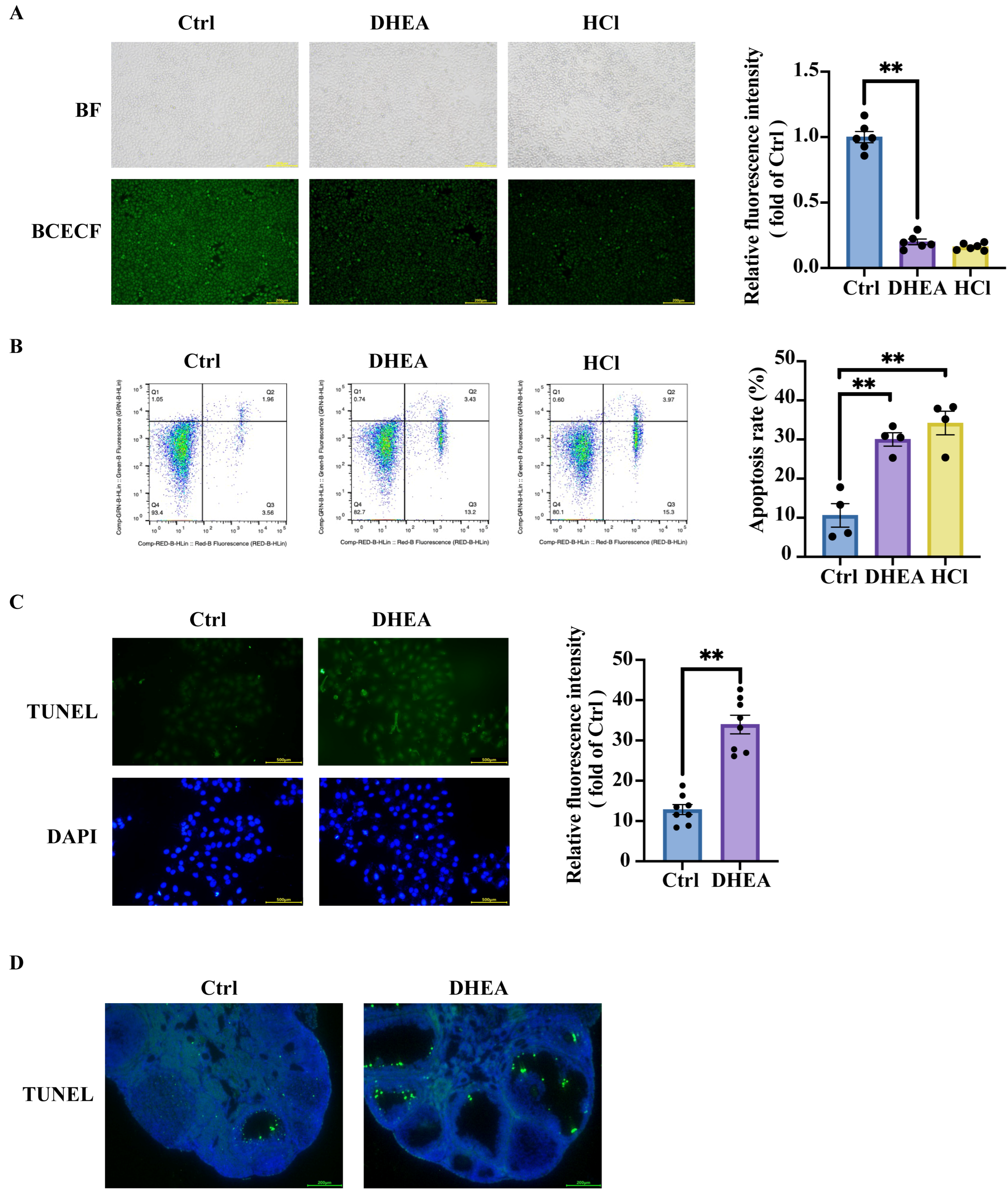
3.2. Intracellular Acidification Promotes Granulosa Cell Apoptosis and Contributes to Ovarian Impairment in PCOS
3.3. Hyperandrogenemia Contributes to Mitochondria Damage and Promotes Anaerobic Glycolysis, Leading to Lactic Acid Accumulation in KGN Cells
3.4. Mitochondria Damage Also Triggers Granulosa Cell Apoptosis Through Acidification—Independent Pathways
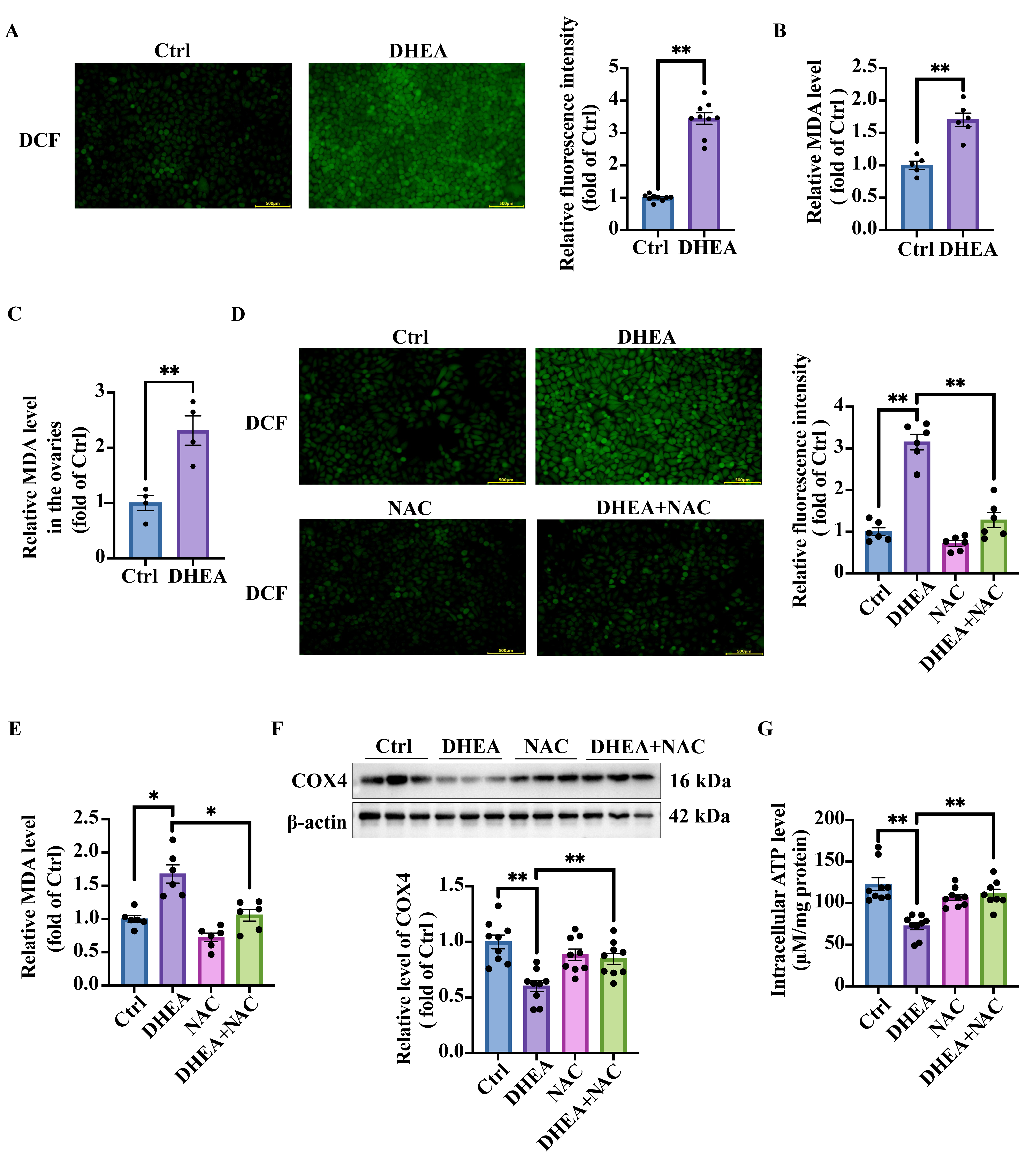
3.5. Oxidative Stress in DHEA-Treated KGN Cells and Mouse Ovaries
3.6. NAC Targets Oxidative Stress and Ameliorates Androgen Excess-Induced Mitochondrial Damage in KGN Cells
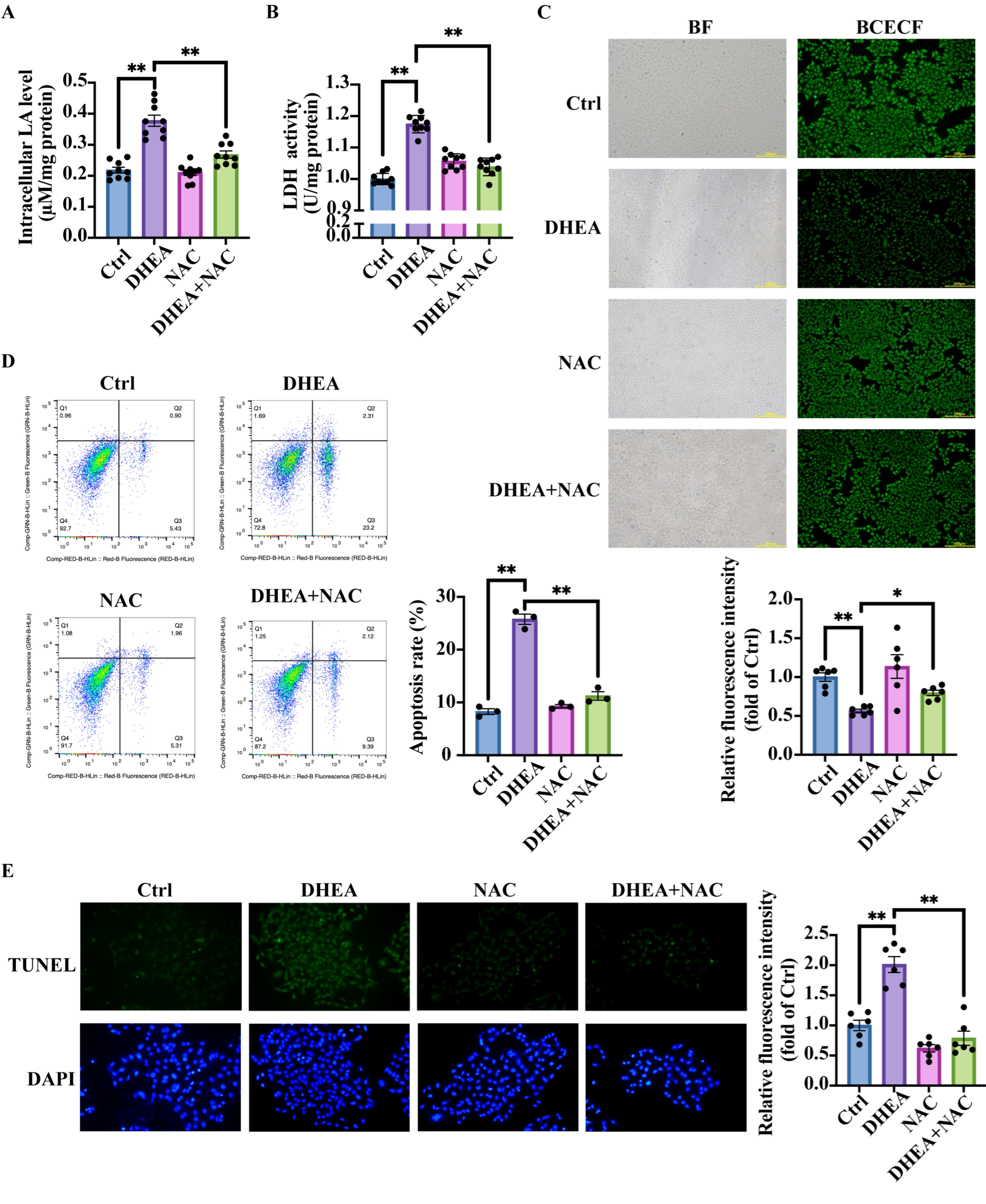
3.7. NAC Reduces Lactic Acid Accumulation, Ameliorates Intracellular pH, and Decreases Cell Apoptosis
3.8. NAC Mitigates Lactic Acid Accumulation and Restores Ovarian Morphology in PCOS Mice
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Eriksson, G.; Li, C.; Sparovec, T.G.; Dekanski, A.; Torstensson, S.; Risal, S.; Ohlsson, C.; Hirschberg, A.L.; Petropoulos, S.; Deng, Q.; et al. Single-Cell Profiling of the Human Endometrium in Polycystic Ovary Syndrome. Nat. Med. 2025, 31, 1925–1938. [Google Scholar] [CrossRef]
- Stener-Victorin, E.; Teede, H.; Norman, R.J.; Legro, R.; Goodarzi, M.O.; Dokras, A.; Laven, J.; Hoeger, K.; Piltonen, T.T. Polycystic Ovary Syndrome. Nat. Rev. Dis. Primers 2024, 10, 27. [Google Scholar] [CrossRef]
- Gong, Y.; Luo, S.; Fan, P.; Zhu, H.; Li, Y.; Huang, W. Growth Hormone Activates PI3K/Akt Signaling and Inhibits ROS Accumulation and Apoptosis in Granulosa Cells of Patients with Polycystic Ovary Syndrome. Reprod. Biol. Endocrinol. 2020, 18, 121. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.-H.; Zhang, F.-F.; Zhang, Z.-L.; Fang, K.-F.; Sun, W.-X.; Kong, N.; Wu, M.; Liu, H.-O.; Liu, Y.; Li, Z.; et al. Follicle Stimulating Hormone Controls Granulosa Cell Glutamine Synthesis to Regulate Ovulation. Protein Cell 2024, 15, 512–529. [Google Scholar] [CrossRef] [PubMed]
- Xing, J.; Qiao, G.; Luo, X.; Liu, S.; Chen, S.; Ye, G.; Zhang, C.; Yi, J. Ferredoxin 1 Regulates Granulosa Cell Apoptosis and Autophagy in Polycystic Ovary Syndrome. Clin. Sci. 2023, 137, 453–468. [Google Scholar] [CrossRef] [PubMed]
- Tong, C.; Wu, Y.; Zhang, L.; Yu, Y. Insulin Resistance, Autophagy and Apoptosis in Patients with Polycystic Ovary Syndrome: Association with PI3K Signaling Pathway. Front. Endocrinol. 2022, 13, 1091147. [Google Scholar] [CrossRef]
- Zhang, W.; Wu, F. Linoleic Acid Induces Human Ovarian Granulosa Cell Inflammation and Apoptosis through the ER-FOXO1-ROS-NFκB Pathway. Sci. Rep. 2024, 14, 6392. [Google Scholar] [CrossRef]
- Ma, Y.; Zheng, L.; Wang, Y.; Gao, Y.; Xu, Y. Arachidonic Acid in Follicular Fluid of PCOS Induces Oxidative Stress in a Human Ovarian Granulosa Tumor Cell Line (KGN) and Upregulates GDF15 Expression as a Response. Front. Endocrinol. 2022, 13, 865748. [Google Scholar] [CrossRef]
- Tan, W.; Dai, F.; Yang, D.; Deng, Z.; Gu, R.; Zhao, X.; Cheng, Y. MiR-93-5p Promotes Granulosa Cell Apoptosis and Ferroptosis by the NF-kB Signaling Pathway in Polycystic Ovary Syndrome. Front. Immunol. 2022, 13, 967151. [Google Scholar] [CrossRef]
- Ye, W.; Xia, S.; Xie, T.; Ye, H.; Yang, Y.; Sun, Y.; Cai, J.; Luo, X.; Zhou, L.; Song, Y. Klotho Accelerates the Progression of Polycystic Ovary Syndrome through Promoting Granulosa Cell Apoptosis and Inflammation†. Biol. Reprod. 2024, 111, 625–639. [Google Scholar] [CrossRef]
- Huang, J.-C.; Duan, C.-C.; Jin, S.; Sheng, C.-B.; Wang, Y.-S.; Yue, Z.-P.; Guo, B. HB-EGF Induces Mitochondrial Dysfunction via Estrogen Hypersecretion in Granulosa Cells Dependent on cAMP-PKA-JNK/ERK-Ca2+-FOXO1 Pathway. Int. J. Biol. Sci. 2022, 18, 2047–2059. [Google Scholar] [CrossRef]
- Xiang, Y.; Wang, H.; Ding, H.; Xu, T.; Liu, X.; Huang, Z.; Wu, H.; Ge, H. Hyperandrogenism Drives Ovarian Inflammation and Pyroptosis: A Possible Pathogenesis of PCOS Follicular Dysplasia. Int. Immunopharmacol. 2023, 125, 111141. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Weng, Y.; Zhang, Y.; Wang, R.; Wang, T.; Zhou, J.; Shen, S.; Wang, H.; Wang, Y. Exposure to Hyperandrogen Drives Ovarian Dysfunction and Fibrosis by Activating the NLRP3 Inflammasome in Mice. Sci. Total Environ. 2020, 745, 141049. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Wu, H.; Yao, Q.; Bai, W.; Kang, J. A Ketogenic Diet Alleviates the Apoptosis of Granulosa Cells by Inhibiting the Activation of cGAS-STING Signaling Pathway in PCOS Mice. Cell Commun. Signal. 2024, 22, 568. [Google Scholar] [CrossRef] [PubMed]
- Yao, Q.; Zou, X.; Liu, S.; Wu, H.; Shen, Q.; Kang, J. Oxidative Stress as a Contributor to Insulin Resistance in the Skeletal Muscles of Mice with Polycystic Ovary Syndrome. Int. J. Mol. Sci. 2022, 23, 11384. [Google Scholar] [CrossRef]
- Yan, H.; Wang, L.; Zhang, G.; Li, N.; Zhao, Y.; Liu, J.; Jiang, M.; Du, X.; Zeng, Q.; Xiong, D.; et al. Oxidative Stress and Energy Metabolism Abnormalities in Polycystic Ovary Syndrome: From Mechanisms to Therapeutic Strategies. Reprod. Biol. Endocrinol. 2024, 22, 159. [Google Scholar] [CrossRef]
- Buszewska-Forajta, M.; Rachoń, D.; Stefaniak, A.; Wawrzyniak, R.; Konieczna, A.; Kowalewska, A.; Markuszewski, M.J. Identification of the Metabolic Fingerprints in Women with Polycystic Ovary Syndrome Using the Multiplatform Metabolomics Technique. J. Steroid Biochem. Mol. Biol. 2019, 186, 176–184. [Google Scholar] [CrossRef]
- Sun, L.; Hu, W.; Liu, Q.; Hao, Q.; Sun, B.; Zhang, Q.; Mao, S.; Qiao, J.; Yan, X. Metabonomics Reveals Plasma Metabolic Changes and Inflammatory Marker in Polycystic Ovary Syndrome Patients. J. Proteome Res. 2012, 11, 2937–2946. [Google Scholar] [CrossRef]
- Miyauchi, K.; Marin, M.; Melikyan, G.B. Visualization of Retrovirus Uptake and Delivery into Acidic Endosomes. Biochem. J. 2011, 434, 559–569. [Google Scholar] [CrossRef]
- Fang, Y.; Liu, Z.; Chen, Z.; Xu, X.; Xiao, M.; Yu, Y.; Zhang, Y.; Zhang, X.; Du, Y.; Jiang, C.; et al. Smad5 Acts as an Intracellular pH Messenger and Maintains Bioenergetic Homeostasis. Cell Res. 2017, 27, 1083–1099. [Google Scholar] [CrossRef]
- Song, X.; Shen, Q.; Fan, L.; Yu, Q.; Jia, X.; Sun, Y.; Bai, W.; Kang, J. Dehydroepiandrosterone-Induced Activation of mTORC1 and Inhibition of Autophagy Contribute to Skeletal Muscle Insulin Resistance in a Mouse Model of Polycystic Ovary Syndrome. Oncotarget 2018, 9, 11905–11921. [Google Scholar] [CrossRef] [PubMed]
- Casey, J.R.; Grinstein, S.; Orlowski, J. Sensors and Regulators of Intracellular pH. Nat. Rev. Mol. Cell Biol. 2010, 11, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Levin, L.R.; Buck, J. Physiological Roles of Acid-Base Sensors. Annu. Rev. Physiol. 2015, 77, 347–362. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yang, Y.; Zhang, B.; Lin, X.; Fu, X.; An, Y.; Zou, Y.; Wang, J.-X.; Wang, Z.; Yu, T. Lactate Metabolism in Human Health and Disease. Signal Transduct. Target. Ther. 2022, 7, 305. [Google Scholar] [CrossRef]
- Cozzolino, M.; Herraiz, S.; Cakiroglu, Y.; Garcia-Velasco, J.A.; Tiras, B.; Pacheco, A.; Rabadan, S.; Kohls, G.; Barrio, A.I.; Pellicer, A.; et al. Distress Response in Granulosa Cells of Women Affected by PCOS with or without Insulin Resistance. Endocrine 2023, 79, 200–207. [Google Scholar] [CrossRef]
- Cong, P.; Shang, B.; Zhang, L.; Wu, Z.; Wang, Y.; Li, J.; Zhang, L. New Insights into the Treatment of Polycystic Ovary Syndrome: HKDC1 Promotes the Growth of Ovarian Granulocyte Cells by Regulating Mitochondrial Function and Glycolysis. J. Mol. Histol. 2024, 55, 187–199. [Google Scholar] [CrossRef]
- Maruthini, D.; Harris, S.E.; Barth, J.H.; Balen, A.H.; Campbell, B.K.; Picton, H.M. The Effect of Metformin Treatment in Vivo on Acute and Long-Term Energy Metabolism and Progesterone Production in Vitro by Granulosa Cells from Women with Polycystic Ovary Syndrome. Hum. Reprod. 2014, 29, 2302–2316. [Google Scholar] [CrossRef]
- Liu, K.; Wei, H.; Nong, W.; Peng, H.; Li, Y.; Lei, X.; Zhang, S. Nampt/SIRT2/LDHA Pathway-Mediated Lactate Production Regulates Follicular Dysplasia in Polycystic Ovary Syndrome. Free Radic. Biol. Med. 2024, 225, 776–793. [Google Scholar] [CrossRef]
- Rice, S.; Christoforidis, N.; Gadd, C.; Nikolaou, D.; Seyani, L.; Donaldson, A.; Margara, R.; Hardy, K.; Franks, S. Impaired Insulin-Dependent Glucose Metabolism in Granulosa-Lutein Cells from Anovulatory Women with Polycystic Ovaries. Hum. Reprod. 2005, 20, 373–381. [Google Scholar] [CrossRef]
- Hillier, S.G.; Purohit, A.; Reichert, L.E. Control of Granulosa Cell Lactate Production by Follicle-Stimulating Hormone and Androgen. Endocrinology 1985, 116, 1163–1167. [Google Scholar] [CrossRef]
- Zhao, S.; Xu, H.; Cui, Y.; Wang, W.; Qin, Y.; You, L.; Chan, W.-Y.; Sun, Y.; Chen, Z.-J. Metabolic Actions of Insulin in Ovarian Granulosa Cells Were Unaffected by Hyperandrogenism. Endocrine 2016, 53, 823–830. [Google Scholar] [CrossRef]
- Vincent, J.-L.; e Silva, A.Q.; Couto, L.; Taccone, F.S. The Value of Blood Lactate Kinetics in Critically Ill Patients: A Systematic Review. Crit. Care 2016, 20, 257. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Yan, J.; Ji, K.; Guo, Y.; Xu, S.; Shen, D.; Li, C.; Gao, H.; Zhao, L. Fibroblast Growth Factor 21 Enhances Learning and Memory Performance in Mice by Regulating Hippocampal L-Lactate Homeostasis. Int. J. Biol. Macromol. 2024, 271, 132667. [Google Scholar] [CrossRef] [PubMed]
- Xia, Q.; Lan, J.; Pan, Y.; Wang, Y.; Song, T.; Yang, Y.; Tian, X.; Chen, L.; Gu, Z.; Ding, Y.-Y. Effects of Dityrosine on Lactic Acid Metabolism in Mice Gastrocnemius Muscle During Endurance Exercise via the Oxidative Stress-Induced Mitochondria Damage. J. Agric. Food Chem. 2024, 72, 5269–5282. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-Y.; Zhou, B.; Sun, R.-Y.; Ai, Y.-L.; Cheng, K.; Li, F.-N.; Wang, B.-R.; Liu, F.-J.; Jiang, Z.-H.; Wang, W.-J.; et al. The Metabolite α-KG Induces GSDMC-Dependent Pyroptosis through Death Receptor 6-Activated Caspase-8. Cell Res. 2021, 31, 980–997. [Google Scholar] [CrossRef]
- Lu, H.; Xu, L.; Steriopoulos, J.; McLeod, P.; Huang, X.; Min, J.; Peng, T.; Jevnikar, A.M.; Zhang, Z.-X. An Acidic pH Environment Converts Necroptosis to Apoptosis. Biochem. Biophys. Res. Commun. 2024, 725, 150215. [Google Scholar] [CrossRef]
- Awasthi, D.; Nagarkoti, S.; Sadaf, S.; Chandra, T.; Kumar, S.; Dikshit, M. Glycolysis Dependent Lactate Formation in Neutrophils: A Metabolic Link between NOX-Dependent and Independent NETosis. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 165542. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, F.; Wang, H.; Tong, Y.; Fu, Y.; Wu, K.; Li, J.; Wang, C.; Wang, Z.; Jia, Y.; et al. NEDD4 Lactylation Promotes APAP Induced Liver Injury through Caspase11 Dependent Non-Canonical Pyroptosis. Int. J. Biol. Sci. 2024, 20, 1413–1435. [Google Scholar] [CrossRef]
- Sun, Z.; He, W.; Meng, H.; Ji, Z.; Qu, J.; Yu, G. Lactate Activates ER Stress to Promote Alveolar Epithelial Cells Apoptosis in Pulmonary Fibrosis. Respir. Res. 2024, 25, 401. [Google Scholar] [CrossRef]
- Xie, C.; Lu, H.; Zhang, X.; An, Z.; Chen, T.; Yu, W.; Wang, S.; Shang, D.; Wang, X. Mitochondrial Abnormality in Ovarian Granulosa Cells of Patients with Polycystic Ovary Syndrome. Mol. Med. Rep. 2024, 29, 27. [Google Scholar] [CrossRef]
- Yang, H.; Xie, Y.; Yang, D.; Ren, D. Oxidative Stress-Induced Apoptosis in Granulosa Cells Involves JNK, P53 and Puma. Oncotarget 2017, 8, 25310–25322. [Google Scholar] [CrossRef]
- Fang, Y.-Q.; Ding, H.; Li, T.; Zhao, X.-J.; Luo, D.; Liu, Y.; Li, Y. N-Acetylcysteine Supplementation Improves Endocrine-Metabolism Profiles and Ovulation Induction Efficacy in Polycystic Ovary Syndrome. J. Ovarian Res. 2024, 17, 205. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, B.; Fan, L.; Liu, M.; Wu, H.; Zhang, Y.; Shen, Q.; Kang, J. Androgen-Induced Lactic Acid Accumulation Contributes to the Apoptosis of Ovarian Granulosa Cells in Polycystic Ovary Syndrome Mice. Antioxidants 2025, 14, 1235. https://doi.org/10.3390/antiox14101235
Zhao B, Fan L, Liu M, Wu H, Zhang Y, Shen Q, Kang J. Androgen-Induced Lactic Acid Accumulation Contributes to the Apoptosis of Ovarian Granulosa Cells in Polycystic Ovary Syndrome Mice. Antioxidants. 2025; 14(10):1235. https://doi.org/10.3390/antiox14101235
Chicago/Turabian StyleZhao, Bining, Liting Fan, Mengfei Liu, Haowen Wu, Youyou Zhang, Qiyang Shen, and Jihong Kang. 2025. "Androgen-Induced Lactic Acid Accumulation Contributes to the Apoptosis of Ovarian Granulosa Cells in Polycystic Ovary Syndrome Mice" Antioxidants 14, no. 10: 1235. https://doi.org/10.3390/antiox14101235
APA StyleZhao, B., Fan, L., Liu, M., Wu, H., Zhang, Y., Shen, Q., & Kang, J. (2025). Androgen-Induced Lactic Acid Accumulation Contributes to the Apoptosis of Ovarian Granulosa Cells in Polycystic Ovary Syndrome Mice. Antioxidants, 14(10), 1235. https://doi.org/10.3390/antiox14101235





