Comparative Analysis of the Tolerance of Young and Old Kidneys to Injury in a Rat Model of Reversible Ureteral Obstruction
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Experimental Design
2.3. Serum Analysis
2.4. Western Blotting
2.5. Zymography
2.6. RT-PCR
2.7. Histochemical Analysis
2.8. Statistical Analysis
3. Results
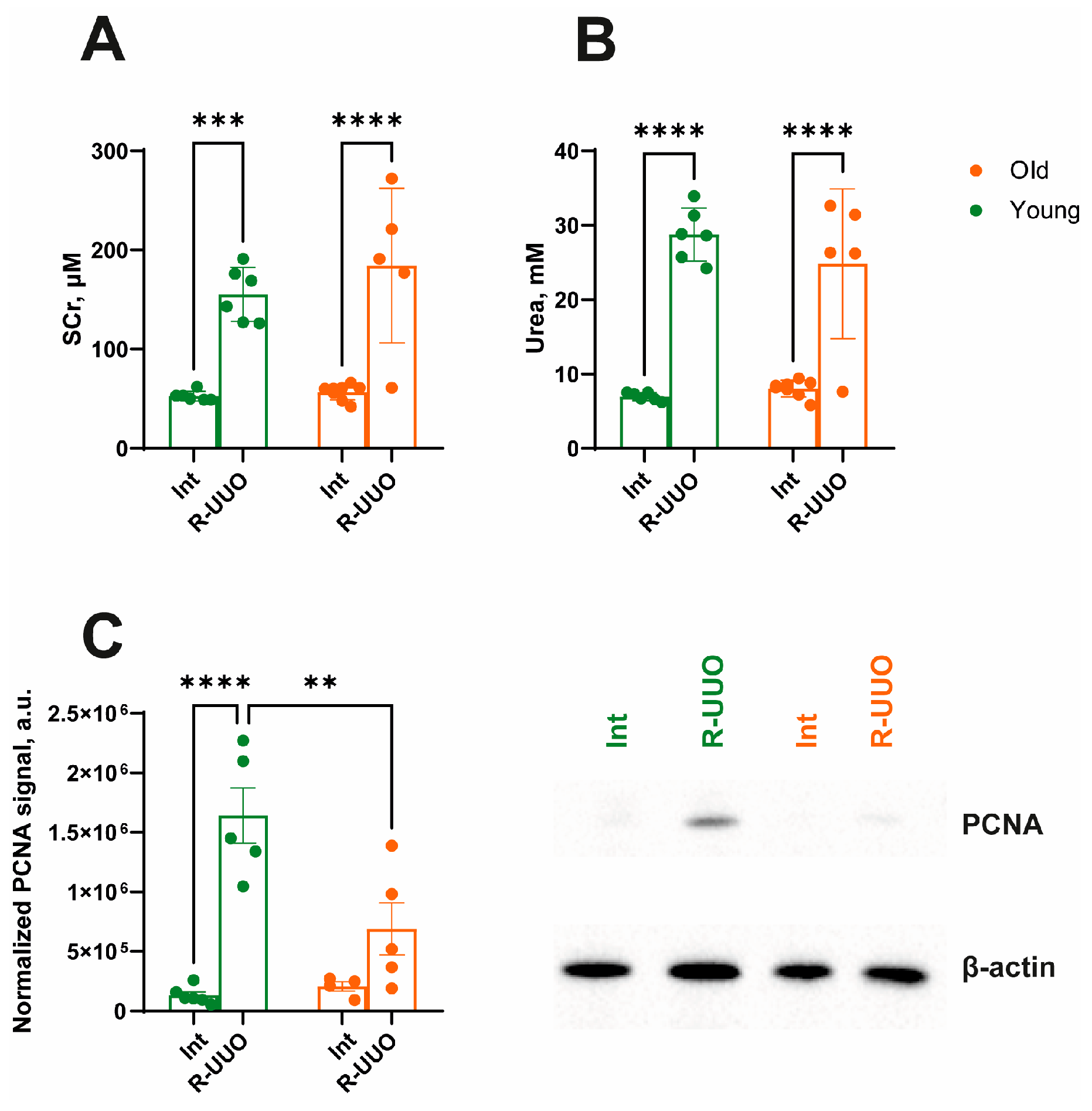
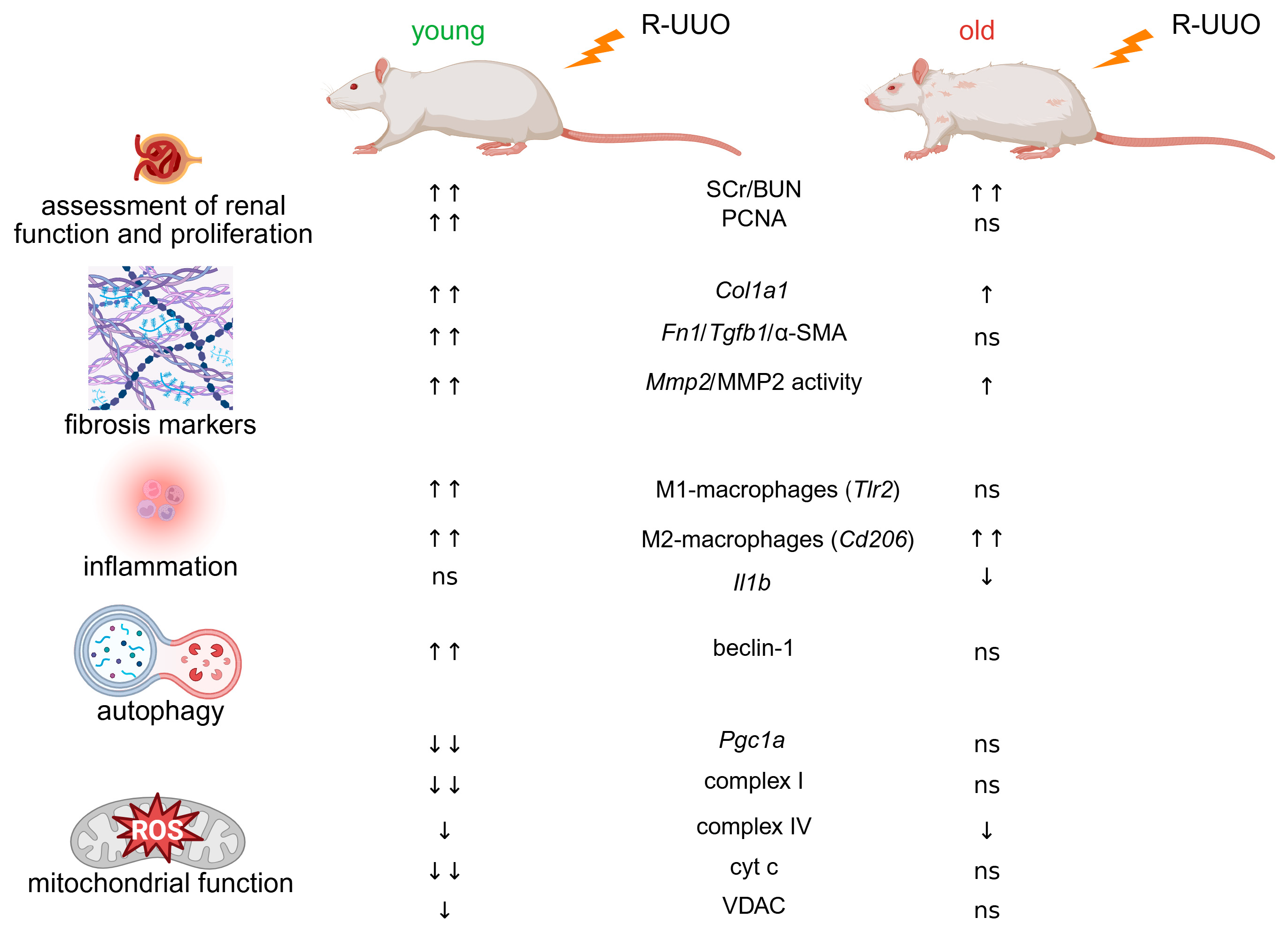
3.1. The Severity of Renal Injury Induced by R-UUO
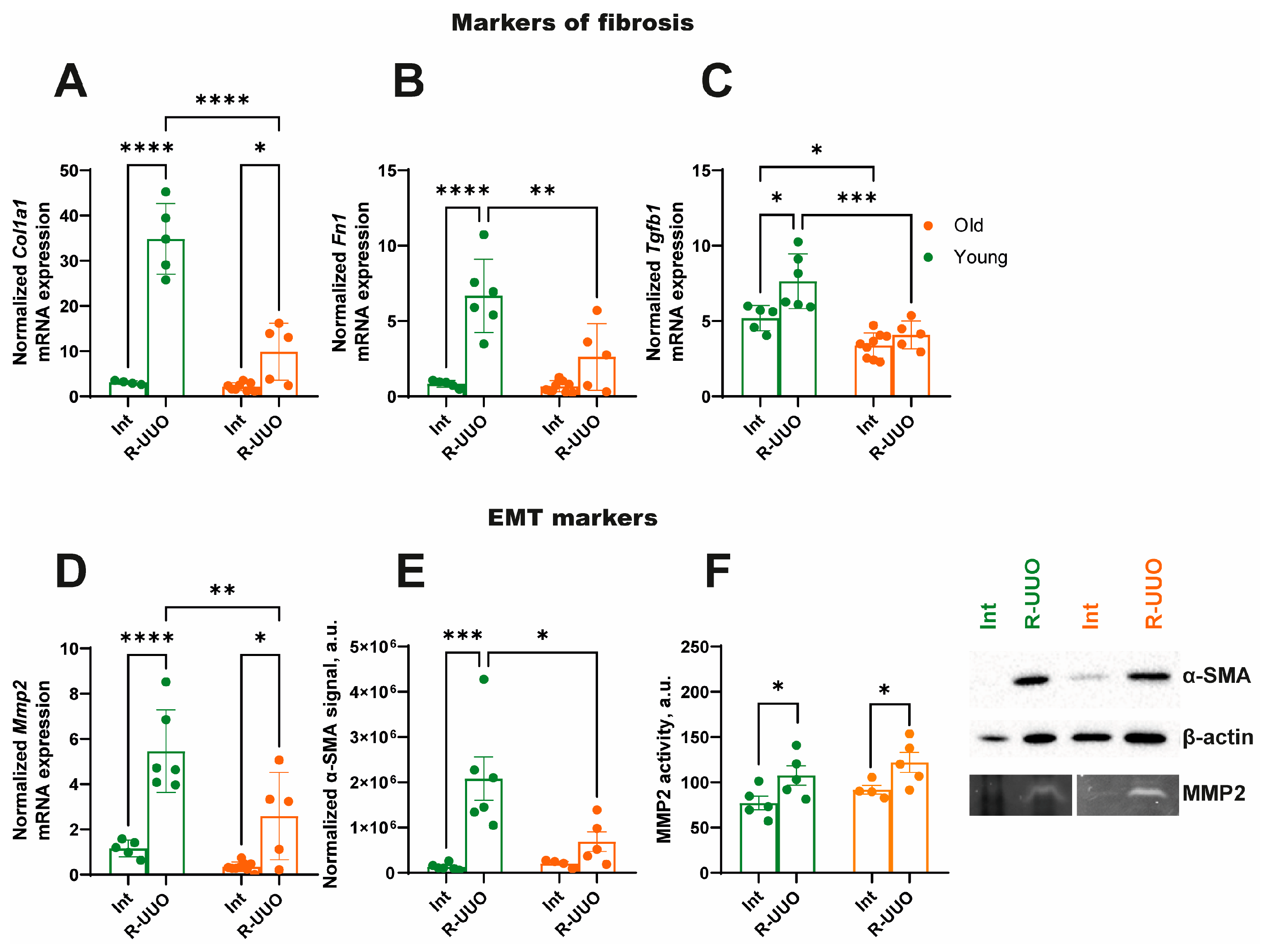
3.2. R-UUO Induces More Pronounced Fibrosis Marker Expression in Young Rats Compared to Old
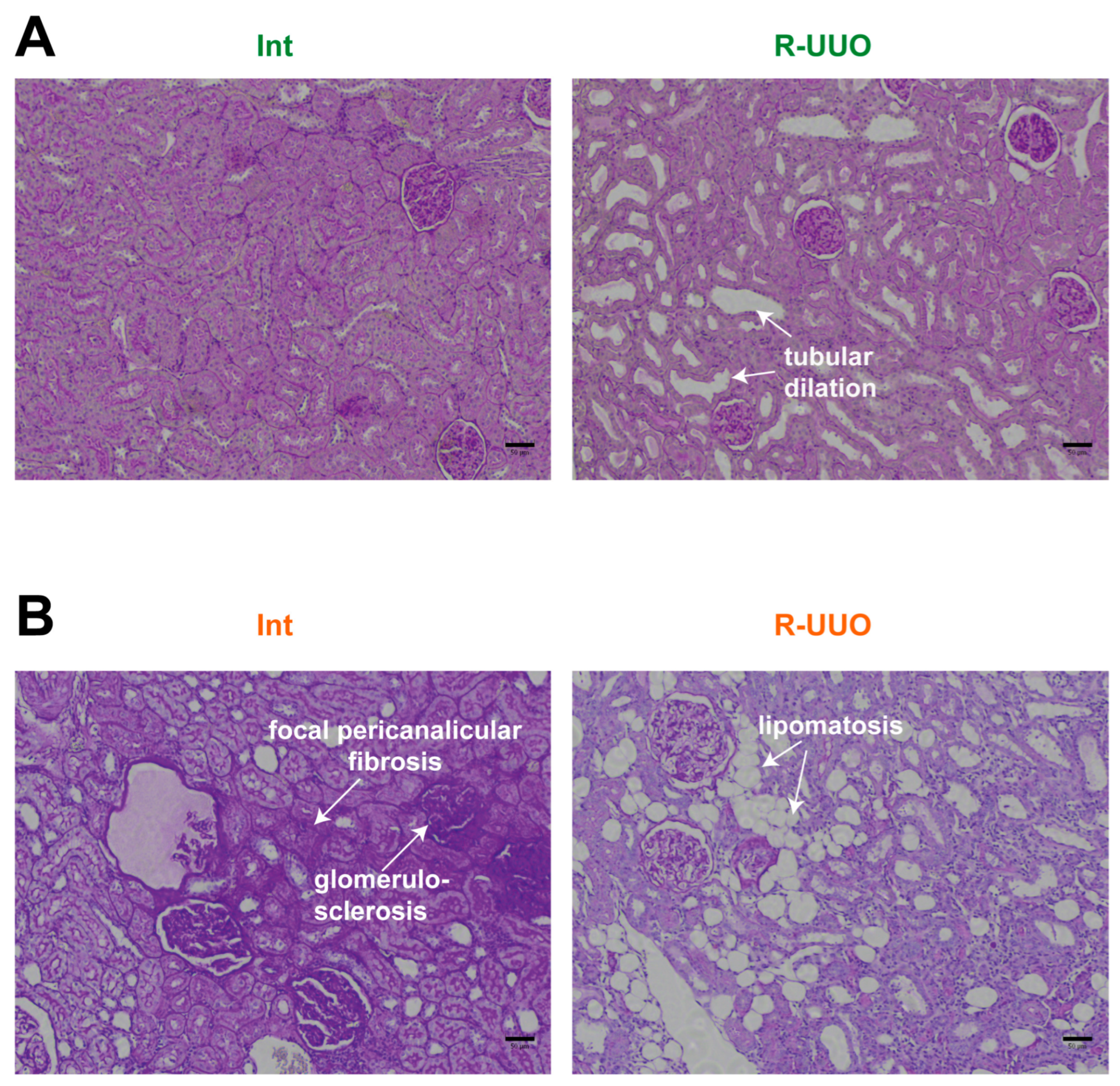
3.3. R-UUO-Induced Histopathological Changes in the Kidney
3.4. Age-Related Differences in Immune Cell and Cytokine Marker Expression
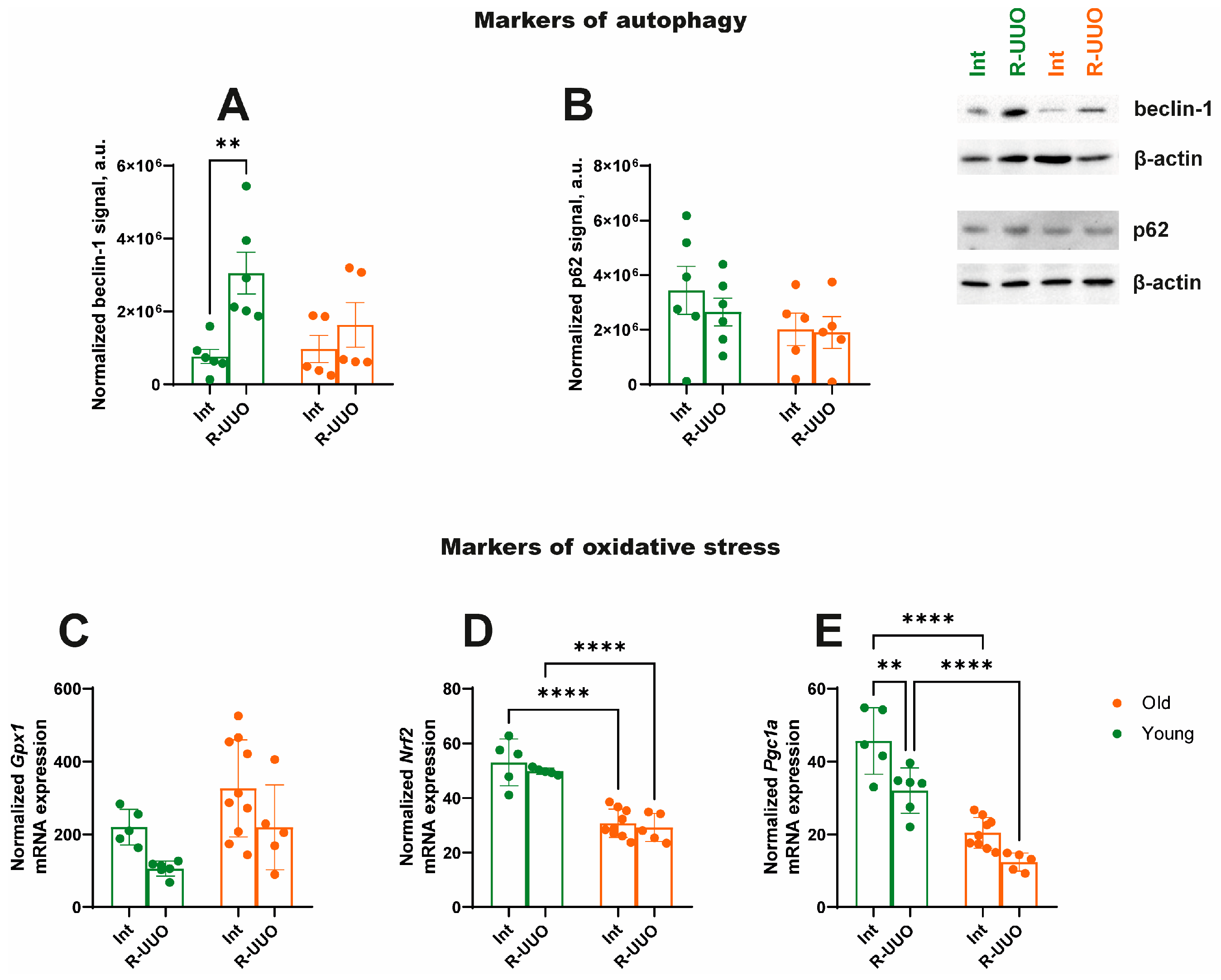
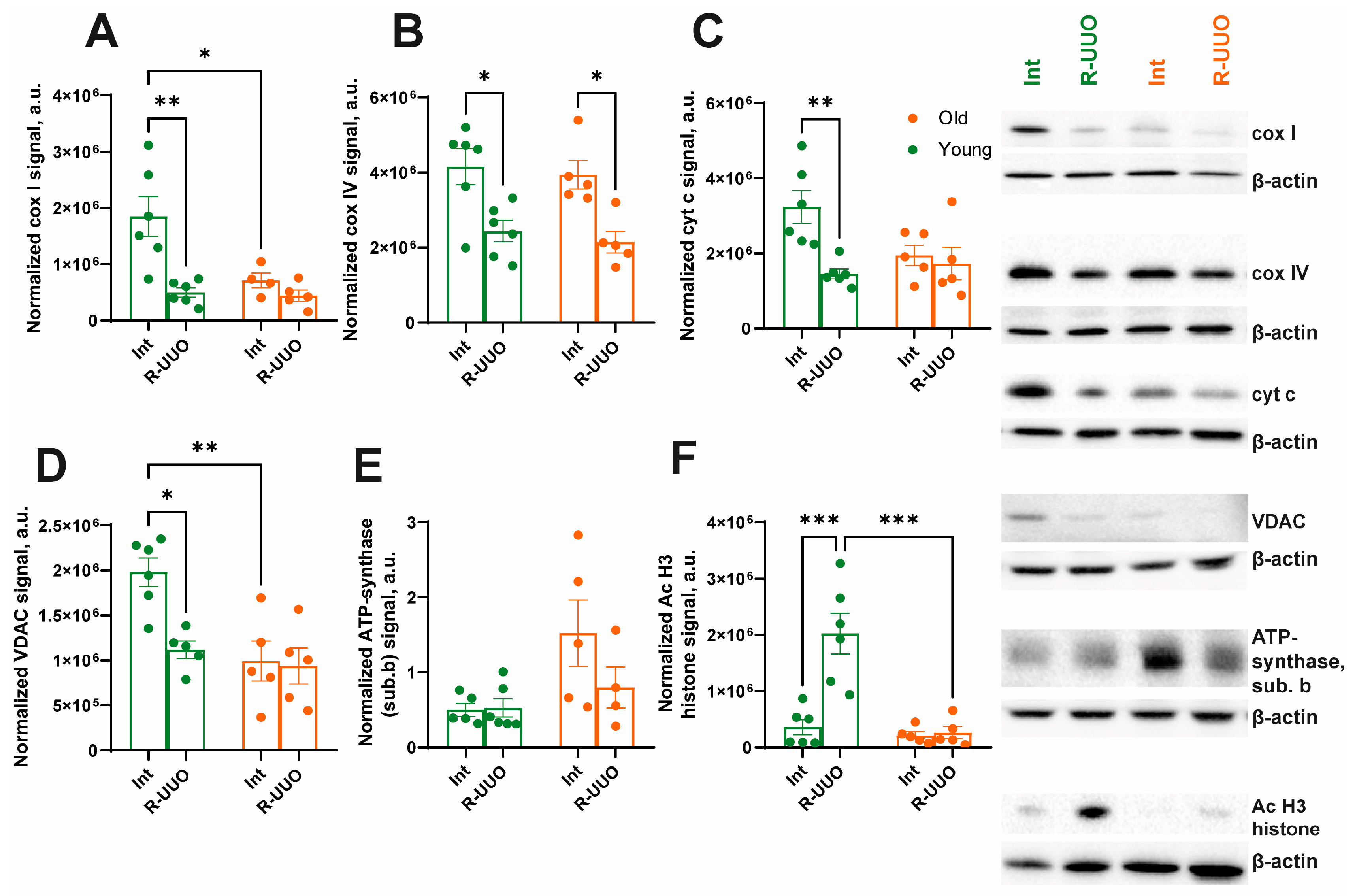
3.5. Markers of Autophagy, Oxidative Stress and Mitochondrial Biogenesis
3.6. Age-Dependent Mitochondrial Dysfunction Following R-UUO
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AKI | Acute kidney injury |
| BUN | Blood urea nitrogen |
| CKD | Chronic kidney disease |
| Col1a1 | Collagen type I α1 chain |
| CXCL | C-X-C motif chemokine ligand |
| cyt c | Cytochrome c |
| ECM | Extracellular matrix |
| IL1β | Interleukin 1 beta |
| GFR | Glomerular filtration rate |
| Gpx1 | Glutathione peroxidase 1 |
| MMP | Matrix metalloproteinase |
| NRF2 | Nuclear respiratory factor 2 |
| OXPHOS | Oxidative phosphorylation |
| PAS | Periodic acid–Schiff staining |
| PCNA | Proliferating cell nuclear antigen |
| PGC-1α | Proliferator-activated receptor γ co-activator-1 alpha |
| p62 | Sequestosome 1 |
| SCr | Serum creatinine |
| TGFβ1 | Transforming growth factor β1 |
| TLR2 | Toll-like receptor 2 |
| TNFα | Tumor necrosis factor alpha |
| R-UUO | Reversible unilateral ureteral obstruction |
| VDAC | Voltage-dependent anion-selective channel |
| α-SMA | Alpha-smooth actin |
References
- Ronco, C.; Bellomo, R.; Kellum, J.A. Acute Kidney Injury. Lancet 2019, 394, 1949–1964. [Google Scholar] [CrossRef] [PubMed]
- Kellum, J.A.; Romagnani, P.; Ashuntantang, G.; Ronco, C.; Zarbock, A.; Anders, H.-J. Acute Kidney Injury. Nat. Rev. Dis. Primers 2021, 7, 52. [Google Scholar] [CrossRef] [PubMed]
- Gembillo, G.; Spadaro, G.; Santoro, D. Link between Obstructive Uropathy and Acute Kidney Injury. World J. Nephrol. 2025, 14, 99120. [Google Scholar] [CrossRef] [PubMed]
- Chaabane, W.; Praddaude, F.; Buleon, M.; Jaafar, A.; Vallet, M.; Rischmann, P.; Galarreta, C.I.; Chevalier, R.L.; Tack, I. Renal Functional Decline and Glomerulotubular Injury Are Arrested but Not Restored by Release of Unilateral Ureteral Obstruction (UUO). Am. J. Physiol. Ren. Physiol. 2013, 304, F432–F439. [Google Scholar] [CrossRef]
- Song, J.; Liu, J.; Luo, J.; Zhang, Q.; Xia, Y.; Shao, Q.; Sun, C.; Jiang, C.; Zhang, M.; Zhu, W. A Modified Relief of Unilateral Ureteral Obstruction Model. Ren. Fail. 2019, 41, 497–506. [Google Scholar] [CrossRef]
- Baumgartner, A.; Reichelt-Wurm, S.; Gronwald, W.; Samol, C.; Schröder, J.A.; Fellner, C.; Holler, K.; Steege, A.; Putz, F.J.; Oefner, P.J.; et al. Assessment of Physiological Rat Kidney Ageing-Implications for the Evaluation of Allograft Quality prior to Renal Transplantation. Metabolites 2022, 12, 162. [Google Scholar] [CrossRef]
- Wrońska, A.; Kieżun, J.; Kmieć, Z. High-Dose Fenofibrate Stimulates Multiple Cellular Stress Pathways in the Kidney of Old Rats. Int. J. Mol. Sci. 2024, 25, 3038. [Google Scholar] [CrossRef]
- Andrianova, N.V.; Zorova, L.D.; Pevzner, I.B.; Kolosova, N.G.; Plotnikov, E.Y.; Zorov, D.B. Calorie Restriction Provides Kidney Ischemic Tolerance in Senescence-Accelerated OXYS Rats. Int. J. Mol. Sci. 2022, 23, 15224. [Google Scholar] [CrossRef]
- Zhang, M.; Ni, H.; Lin, Y.; Wang, K.; He, T.; Yuan, L.; Han, Z.; Zuo, X. Renal Aging and Its Consequences: Navigating the Challenges of an Aging Population. Front. Pharmacol. 2025, 16, 1615681. [Google Scholar] [CrossRef]
- Klahr, S. The Geriatric Patient with Obstructive Uropathy. In Nephrology and Geriatrics Integrated; Springer: Dordrecht, The Netherlands, 2000; pp. 167–177. ISBN 9789401057950. [Google Scholar]
- Yaxley, J.; Yaxley, W. Obstructive Uropathy—Acute and Chronic Medical Management. World J. Nephrol. 2023, 12, 1. [Google Scholar] [CrossRef]
- Jankauskas, S.S.; Pevzner, I.B.; Andrianova, N.V.; Zorova, L.D.; Popkov, V.A.; Silachev, D.N.; Kolosova, N.G.; Plotnikov, E.Y.; Zorov, D.B. The Age-Associated Loss of Ischemic Preconditioning in the Kidney Is Accompanied by Mitochondrial Dysfunction, Increased Protein Acetylation and Decreased Autophagy. Sci. Rep. 2017, 7, 44430. [Google Scholar] [CrossRef]
- Chen, H.; Xing, B.; Wang, L.; Weng, X.; Chen, Z.; Liu, X. Aged Kidneys Are Refractory to Ischemic Postconditioning in a Rat Model. Ren. Fail. 2014, 36, 1575–1580. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Klimova, E.; Aparicio-Trejo, O.E.; Tapia, E.; Pedraza-Chaverri, J. Unilateral Ureteral Obstruction as a Model to Investigate Fibrosis-Attenuating Treatments. Biomolecules 2019, 9, 141. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, R.L.; Forbes, M.S.; Thornhill, B.A. Ureteral Obstruction as a Model of Renal Interstitial Fibrosis and Obstructive Nephropathy. Kidney Int. 2009, 75, 1145–1152. [Google Scholar] [CrossRef] [PubMed]
- Brissette, M.-J.; Laplante, P.; Qi, S.; Latour, M.; Cailhier, J.-F. Milk Fat Globule Epidermal Growth Factor-8 Limits Tissue Damage through Inflammasome Modulation during Renal Injury. J. Leukoc. Biol. 2016, 100, 1135–1146. [Google Scholar] [CrossRef]
- Li, Z.I.; Chung, A.C.K.; Zhou, L.; Huang, X.R.; Liu, F.; Fu, P.; Fan, J.M.; Szalai, A.J.; Lan, H.Y. C-Reactive Protein Promotes Acute Renal Inflammation and Fibrosis in Unilateral Ureteral Obstructive Nephropathy in Mice. Lab. Investig. 2011, 91, 837–851. [Google Scholar] [CrossRef]
- Edelstein, C.L. Biomarkers of Acute Kidney Injury. Adv. Chronic Kidney Dis. 2008, 15, 222–234. [Google Scholar] [CrossRef]
- Waikar, S.S.; Bonventre, J.V. Biomarkers for the Diagnosis of Acute Kidney Injury. Nephron Clin. Pract. 2008, 109, c192–c197. [Google Scholar] [CrossRef]
- McNair, E.D.; Bezaire, J.; Moser, M.; Mondal, P.; Conacher, J.; Franczak, A.; Sawicki, G.; Reid, D.; Khani-Hanjani, A. The Association of Matrix Metalloproteinases with Acute Kidney Injury Following CPB-Supported Cardiac Surgery. Can. J. Kidney Health Dis. 2021, 8, 20543581211019640. [Google Scholar] [CrossRef]
- Wolosowicz, M.; Prokopiuk, S.; Kaminski, T.W. The Complex Role of Matrix Metalloproteinase-2 (MMP-2) in Health and Disease. Int. J. Mol. Sci. 2024, 25, 13691. [Google Scholar] [CrossRef]
- Choh, N.A.; Jehangir, M.; Choh, S.A. Renal Replacement Lipomatosis: A Rare Type of Renal Pseudotumor. Indian J. Nephrol. 2010, 20, 92–93. [Google Scholar] [CrossRef]
- Kumar, A.V.; Mills, J.; Lapierre, L.R. Selective Autophagy Receptor p62/SQSTM1, a Pivotal Player in Stress and Aging. Front. Cell Dev. Biol. 2022, 10, 793328. [Google Scholar] [CrossRef] [PubMed]
- Tan, R.-Z.; Jia, J.; Li, T.; Wang, L.; Kantawong, F. A Systematic Review of Epigenetic Interplay in Kidney Diseases: Crosstalk between Long Noncoding RNAs and Methylation, Acetylation of Chromatin and Histone. Biomed. Pharmacother. 2024, 176, 116922. [Google Scholar] [CrossRef] [PubMed]
- Bracken, O.V.; De Maeyer, R.P.H.; Akbar, A.N. Enhancing Immunity during Ageing by Targeting Interactions within the Tissue Environment. Nat. Rev. Drug Discov. 2025, 24, 300–315. [Google Scholar] [CrossRef] [PubMed]
- Kusaka, J.; Koga, H.; Hagiwara, S.; Hasegawa, A.; Kudo, K.; Noguchi, T. Age-Dependent Responses to Renal Ischemia-Reperfusion Injury. J. Surg. Res. 2012, 172, 153–158. [Google Scholar] [CrossRef]
- Doi, K.; Leelahavanichkul, A.; ST Yuen, P.; Star, R.A. Animal Models of Sepsis and Sepsis-Induced Kidney Injury. J. Clin. Investig. 2009, 119, 2868. [Google Scholar] [CrossRef]
- Chi, M.; Tian, Z.; Ma, K.; Li, Y.; Wang, L.; Nasser, M.I.; Liu, C. The Diseased Kidney: Aging and Senescent Immunology. Immun. Ageing 2022, 19, 58. [Google Scholar] [CrossRef]
- Schmitt, R.; Marlier, A.; Cantley, L.G. Zag Expression during Aging Suppresses Proliferation after Kidney Injury. J. Am. Soc. Nephrol. JASN 2008, 19, 2375. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, X.; Lin, S.; King, L.; Liu, L. The Potential Role of Advanced Glycation End Products in the Development of Kidney Disease. Nutrients 2025, 17, 758. [Google Scholar] [CrossRef]
- Reiss, A.B.; Jacob, B.; Zubair, A.; Srivastava, A.; Johnson, M.; De Leon, J. Fibrosis in Chronic Kidney Disease: Pathophysiology and Therapeutic Targets. J. Clin. Med. 2024, 13, 1881. [Google Scholar] [CrossRef]
- Misseri, R.; Meldrum, D.R.; Dagher, P.; Hile, K.; Rink, R.C.; Meldrum, K.K. Unilateral Ureteral Obstruction Induces Renal Tubular Cell Production of Tumor Necrosis Factor-Alpha Independent of Inflammatory Cell Infiltration. J. Urol. 2004, 172, 1595–1599; discussion 1599. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Chen, J.; Yan, J.; Zhang, L.; Chen, G.; He, L.; Wang, Y. Effect of Interleukin 6 Deficiency on Renal Interstitial Fibrosis. PLoS ONE 2012, 7, e52415. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Yuan, H.; Cao, W.; Wang, T.; Chen, W.; Yu, H.; Fu, Y.; Jiang, B.; Zhou, H.; Guo, H.; et al. Blocking Interleukin-6 Trans-Signaling Protects against Renal Fibrosis by Suppressing STAT3 Activation. Theranostics 2019, 9, 3980–3991. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ren, X.; Lu, H.; Guo, Z.; Li, X.; Tian, Y.; Yin, Y.; Qin, Z.; Yun, K.; Wu, M.; et al. Restoration of SIRT3 Expression in Aged Mice Alleviates UUO-Induced Renal Fibrosis by Reducing GSK-3β Hyperacetylation. Adv. Sci. 2025, e17248. [Google Scholar] [CrossRef]
- Sun, M.; Brady, R.D.; Casillas-Espinosa, P.M.; Wright, D.K.; Semple, B.D.; Kim, H.A.; Mychasiuk, R.; Sobey, C.G.; O’Brien, T.J.; Vinh, A.; et al. Aged Rats Have an Altered Immune Response and Worse Outcomes after Traumatic Brain Injury. Brain Behav. Immun. 2019, 80, 536–550. [Google Scholar] [CrossRef]
- Hooshmand, M.J.; Galvan, M.D.; Partida, E.; Anderson, A.J. Characterization of Recovery, Repair, and Inflammatory Processes Following Contusion Spinal Cord Injury in Old Female Rats: Is Age a Limitation? Immun. Ageing 2014, 11, 15. [Google Scholar] [CrossRef]
- Simonetti, O.; Lucarini, G.; Cirioni, O.; Zizzi, A.; Orlando, F.; Provinciali, M.; Di Primio, R.; Giacometti, A.; Offidani, A. Delayed Wound Healing in Aged Skin Rat Models after Thermal Injury Is Associated with an Increased MMP-9, K6 and CD44 Expression. Burns 2013, 39, 776–787. [Google Scholar] [CrossRef]
- Gupta, S.; Mandal, S.; Banerjee, K.; Almarshood, H.; Pushpakumar, S.B.; Sen, U. Complex Pathophysiology of Acute Kidney Injury (AKI) in Aging: Epigenetic Regulation, Matrix Remodeling, and the Healing Effects of HS. Biomolecules 2024, 14, 1165. [Google Scholar] [CrossRef]
- Hao, J.; Qiang, P.; Fan, L.; Xiong, Y.; Chang, Y.; Yang, F.; Wang, X.; Shimosawa, T.; Mu, S.; Xu, Q. Eplerenone Reduces Lymphangiogenesis in the Contralateral Kidneys of UUO Rats. Sci. Rep. 2024, 14, 9976. [Google Scholar] [CrossRef]
- Xiong, Y.; Chang, Y.; Hao, J.; Zhang, C.; Yang, F.; Wang, Z.; Liu, Y.; Wang, X.; Mu, S.; Xu, Q. Eplerenone Attenuates Fibrosis in the Contralateral Kidney of UUO Rats by Preventing Macrophage-to-Myofibroblast Transition. Front. Pharmacol. 2021, 12, 620433. [Google Scholar] [CrossRef]
- Hammad, F.T.; Al Salam, S.; Nemmar, A.; Ali, M.; Lubbad, L. The Effect of Arabic Gum on Renal Function in Reversible Unilateral Ureteric Obstruction. Biomolecules 2019, 9, 25. [Google Scholar] [CrossRef]
- Hesketh, E.E.; Vernon, M.A.; Ding, P.; Clay, S.; Borthwick, G.; Conway, B.; Hughes, J. A Murine Model of Irreversible and Reversible Unilateral Ureteric Obstruction. J. Vis. Exp. JoVE 2014, 94, 52559. [Google Scholar] [CrossRef]
- Chevalier, R.L.; Kim, A.; Thornhill, B.A.; Wolstenholme, J.T. Recovery Following Relief of Unilateral Ureteral Obstruction in the Neonatal Rat. Kidney Int. 1999, 55, 793–807. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Irazabal, M.V.; Eirin, A. Role of Renal Sinus Adipose Tissue in Obesity-Induced Renal Injury. eBioMedicine 2016, 13, 21. [Google Scholar] [CrossRef] [PubMed]
- Miricescu, D.; Balan, D.G.; Tulin, A.; Stiru, O.; Vacaroiu, I.A.; Mihai, D.A.; Popa, C.C.; Enyedi, M.; Nedelea, A.S.; Nica, A.E.; et al. Impact of Adipose Tissue in Chronic Kidney Disease Development (Review). Exp. Ther. Med. 2021, 21, 539. [Google Scholar] [CrossRef]
- Mahmoud, N.N.; Hamad, K.; Al Shibitini, A.; Juma, S.; Sharifi, S.; Gould, L.; Mahmoudi, M. Investigating Inflammatory Markers in Wound Healing: Understanding Implications and Identifying Artifacts. ACS Pharmacol. Transl. Sci. 2024, 7, 18. [Google Scholar] [CrossRef]
- Hu, Z.; Kopparapu, P.K.; Deshmukh, M.; Jarneborn, A.; Gupta, P.; Ali, A.; Fei, Y.; Engdahl, C.; Pullerits, R.; Mohammad, M.; et al. The Impact of Aging and Toll-like Receptor 2 Deficiency on the Clinical Outcomes of Staphylococcus aureus Bacteremia. J. Infect. Dis. 2023, 228, 332–342. [Google Scholar] [CrossRef]
- Jackaman, C.; Radley-Crabb, H.G.; Soffe, Z.; Shavlakadze, T.; Grounds; Nelson, D.J. Targeting Macrophages Rescues Age-Related Immune Deficiencies in C57BL/6J Geriatric Mice. Aging Cell 2013, 12, 345–357. [Google Scholar] [CrossRef]
- Sharma, S.; Dominguez, A.L.; Lustgarten, J. High Accumulation of T Regulatory Cells Prevents the Activation of Immune Responses in Aged Animals. J. Immunol. 2006, 177, 8348–8355. [Google Scholar] [CrossRef]
- Wang, Y.; Wehling-Henricks, M.; Samengo, G.; Tidball, J.G. Increases of M2a Macrophages and Fibrosis in Aging Muscle Are Influenced by Bone Marrow Aging and Negatively Regulated by Muscle-Derived Nitric Oxide. Aging Cell 2015, 14, 678–688. [Google Scholar] [CrossRef]
- Kelly, J.; Khan, A.A.; Yin, J.; Ferguson, T.A.; Apte, R.S. Senescence Regulates Macrophage Activation and Angiogenic Fate at Sites of Tissue Injury in Mice. J. Clin. Investig. 2007, 117, 3421–3426. [Google Scholar] [CrossRef] [PubMed]
- Sato-Yamada, Y.; Surboyo, M.D.C.; Rosenkranz, A.L.; Ujita, T.; Fang, M.; EI Fadhlallah, P.M.; Maekawa, T.; Sato, Y.; Yoshiba, N.; Maeda, T. Macrophage Induces Angiogenesis via HIF Signaling in Denervated Muscle Following Nerve Injury. Sci. Rep. 2025, 15, 26239. [Google Scholar] [CrossRef] [PubMed]
- Becker, L.; Nguyen, L.; Gill, J.; Kulkarni, S.; Pasricha, P.J.; Habtezion, A. Age-Dependent Shift in Macrophage Polarization Causes Inflammation Mediated Degeneration of Enteric Nervous System. Gut 2017, 67, 827. [Google Scholar] [CrossRef] [PubMed]
- Moss, C.E.; Phipps, H.; Wilson, H.L.; Kiss-Toth, E. Markers of the Ageing Macrophage: A Systematic Review and Meta-Analysis. Front. Immunol. 2023, 14, 1222308. [Google Scholar] [CrossRef]
- Aprahamian, T.; Takemura, Y.; Goukassian, D.; Walsh, K. Ageing Is Associated with Diminished Apoptotic Cell Clearance in Vivo. Clin. Exp. Immunol. 2008, 152, 448–455. [Google Scholar] [CrossRef]
- Metcalf, T.U.; Cubas, R.A.; Ghneim, K.; Cartwright, M.J.; Van Grevenynghe, J.; Richner, J.M.; Olagnier, D.P.; Wilkinson, P.A.; Cameron, M.J.; Park, B.S.; et al. Global Analyses Revealed Age-Related Alterations in Innate Immune Responses after Stimulation of Pathogen Recognition Receptors. Aging Cell 2015, 14, 421–432. [Google Scholar] [CrossRef]
- Metcalf, T.U.; Wilkinson, P.A.; Cameron, M.J.; Ghneim, K.; Chiang, C.; Wertheimer, A.M.; Hiscott, J.B.; Nikolich-Zugich, J.; Haddad, E.K. Human Monocyte Subsets Are Transcriptionally and Functionally Altered in Aging in Response to Pattern Recognition Receptor Agonists. J. Immunol. 2017, 199, 1405–1417. [Google Scholar] [CrossRef]
- van Duin, D.; Mohanty, S.; Thomas, V.; Ginter, S.; Montgomery, R.R.; Fikrig, E.; Allore, H.G.; Medzhitov, R.; Shaw, A.C. Age-Associated Defect in Human TLR-1/2 Function1. J. Immunol. 2007, 178, 970–975. [Google Scholar] [CrossRef]
- Bailey, K.L.; Smith, L.M.; Heires, A.J.; Katafiasz, D.M.; Romberger, D.J.; LeVan, T.D. Aging Leads to Dysfunctional Innate Immune Responses to TLR2 and TLR4 Agonists. Aging Clin. Exp. Res. 2018, 31, 1185. [Google Scholar] [CrossRef]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The Hallmarks of Aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef]
- Nikolich-Žugich, J. The Twilight of Immunity: Emerging Concepts in Aging of the Immune System. Nat. Immunol. 2017, 19, 10–19. [Google Scholar] [CrossRef]
- Tu, W.C.; He, Y.K.; Wang, D.W.; Ming, S.X.; Zhao, Y. Progranulin Enhances M2 Macrophage Polarization and Renal Fibrosis by Modulating Autophagy in Chronic Kidney Disease. Cell. Mol. Life Sci. 2025, 82, 186. [Google Scholar] [CrossRef]
- Chen, M.; Menon, M.C.; Wang, W.; Fu, J.; Yi, Z.; Sun, Z.; Liu, J.; Li, Z.; Mou, L.; Banu, K.; et al. HCK Induces Macrophage Activation to Promote Renal Inflammation and Fibrosis via Suppression of Autophagy. Nat. Commun. 2023, 14, 4297. [Google Scholar] [CrossRef]
- Wen, J.-H.; Li, D.-Y.; Liang, S.; Yang, C.; Tang, J.-X.; Liu, H.-F. Macrophage Autophagy in Macrophage Polarization, Chronic Inflammation and Organ Fibrosis. Front. Immunol. 2022, 13, 946832. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Jin, Y.; Sun, T.; Zhu, P.; Li, J.; Zhang, Q.; Wang, X.; Jiang, J.; Chen, G.; Zhao, X. p62/SQSTM1 Accumulation due to Degradation Inhibition and Transcriptional Activation Plays a Critical Role in Silica Nanoparticle-Induced Airway Inflammation via NF-κB Activation. J. Nanobiotechnol. 2020, 18, 77. [Google Scholar] [CrossRef]
- Bian, A.; Shi, M.; Flores, B.; Gillings, N.; Li, P.; Yan, S.X.; Levine, B.; Xing, C.; Hu, M.C. Downregulation of Autophagy Is Associated with Severe Ischemia-Reperfusion-Induced Acute Kidney Injury in Overexpressing C-Reactive Protein Mice. PLoS ONE 2017, 12, e0181848. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Takabatake, Y.; Takahashi, A.; Kimura, T.; Namba, T.; Matsuda, J.; Minami, S.; Kaimori, J.-Y.; Matsui, I.; Matsusaka, T.; et al. High-Fat Diet-Induced Lysosomal Dysfunction and Impaired Autophagic Flux Contribute to Lipotoxicity in the Kidney. J. Am. Soc. Nephrol. 2017, 28, 1534–1551. [Google Scholar] [CrossRef] [PubMed]
- Sureshbabu, A.; Ryter, S.W.; Choi, M.E. Oxidative Stress and Autophagy: Crucial Modulators of Kidney Injury. Redox Biol. 2015, 4, 208–214. [Google Scholar] [CrossRef]
- Yamamoto, T.; Takabatake, Y.; Kimura, T.; Takahashi, A.; Namba, T.; Matsuda, J.; Minami, S.; Kaimori, J.Y.; Matsui, I.; Kitamura, H.; et al. Time-Dependent Dysregulation of Autophagy: Implications in Aging and Mitochondrial Homeostasis in the Kidney Proximal Tubule. Autophagy 2016, 12, 801–813. [Google Scholar] [CrossRef]
- Zhang, H.; Davies, K.J.A.; Forman, H.J. Oxidative Stress Response and Nrf2 Signaling in Aging. Free Radic. Biol. Med. 2015, 88, 314. [Google Scholar] [CrossRef]
- Mohammad, R.S.; Lokhandwala, M.F.; Banday, A.A. Age-Related Mitochondrial Impairment and Renal Injury Is Ameliorated by Sulforaphane via Activation of Transcription Factor NRF2. Antioxidants 2022, 11, 156. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, X.; Hu, Y.; Sun, H.; He, Z.; Yuan, J.; Cai, H.; Sun, Y.; Huang, X.; Kong, W.; et al. Age-Associated Decline in Nrf2 Signaling and Associated mtDNA Damage May Be Involved in the Degeneration of the Auditory Cortex: Implications for Central Presbycusis. Int. J. Mol. Med. 2018, 42, 3371. [Google Scholar] [CrossRef]
- Jo, M.J.; Kim, J.E.; Bae, S.Y.; Cho, E.; Ahn, S.Y.; Kwon, Y.J.; Ko, G.-J. Impaired NRF2 Inhibits Recovery from Ischemic Reperfusion Injury in the Aging Kidney. Antioxidants 2023, 12, 1440. [Google Scholar] [CrossRef]
- Zhao, H.; Liu, Y.-J.; Liu, Z.-R.; Tang, D.-D.; Chen, X.-W.; Chen, Y.-H.; Zhou, R.-N.; Chen, S.-Q.; Niu, H.-X. Role of Mitochondrial Dysfunction in Renal Fibrosis Promoted by Hypochlorite-Modified Albumin in a Remnant Kidney Model and Protective Effects of Antioxidant Peptide SS-31. Eur. J. Pharmacol. 2017, 804, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Chen, Y.; Zhang, P.; Huang, S.; Zhu, C.; Ding, G.; Liu, B.; Yang, T.; Zhang, A. Mitochondrial Dysfunction Accounts for Aldosterone-Induced Epithelial-to-Mesenchymal Transition of Renal Proximal Tubular Epithelial Cells. Free Radic. Biol. Med. 2012, 53, 30–43. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Uribe, A.P.; Bellido, B.; Aparicio-Trejo, O.E.; Tapia, E.; Sánchez-Lozada, L.G.; Hernández-Santos, J.A.; Fernández-Valverde, F.; Hernández-Cruz, E.Y.; Orozco-Ibarra, M.; Pedraza-Chaverri, J. Temporal Characterization of Mitochondrial Impairment in the Unilateral Ureteral Obstruction Model in Rats. Free Radic. Biol. Med. 2021, 172, 358–371. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.M.; Ahn, S.H.; Choi, P.; Ko, Y.-A.; Han, S.H.; Chinga, F.; Park, A.S.D.; Tao, J.; Sharma, K.; Pullman, J.; et al. Defective Fatty Acid Oxidation in Renal Tubular Epithelial Cells Has a Key Role in Kidney Fibrosis Development. Nat. Med. 2015, 21, 37–46. [Google Scholar] [CrossRef]
- Martínez-Klimova, E.; Aparicio-Trejo, O.E.; Gómez-Sierra, T.; Jiménez-Uribe, A.P.; Bellido, B.; Pedraza-Chaverri, J. Mitochondrial Dysfunction and Endoplasmic Reticulum Stress in the Promotion of Fibrosis in Obstructive Nephropathy Induced by Unilateral Ureteral Obstruction. Biofactors 2020, 46, 716–733. [Google Scholar] [CrossRef]
- Yakupova, E.I.; Zorov, D.B.; Plotnikov, E.Y. Bioenergetics of the Fibrosis. Biochemistry 2021, 86, 1599–1606. [Google Scholar] [CrossRef]
- Feng, L.; Chen, X.; Huang, Y.; Zhang, X.; Zheng, S.; Xie, N. Immunometabolism Changes in Fibrosis: From Mechanisms to Therapeutic Strategies. Front. Pharmacol. 2023, 14, 1243675. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, J.; Zhang, Y.; Zhou, J. Focus on Mitochondrial Respiratory Chain: Potential Therapeutic Target for Chronic Renal Failure. Int. J. Mol. Sci. 2024, 25, 949. [Google Scholar] [CrossRef] [PubMed]
- Özsoy, M.; Zimmermann, F.A.; Feichtinger, R.G.; Mayr, J.A.; Kofler, B.; Neureiter, D.; Klieser, E.; Schütz, S.; Weghuber, D.; Schneider, A.M. Changes in the Expression of Oxidative Phosphorylation Complexes in the Aging Intestinal Mucosa. Exp. Gerontol. 2020, 135, 110924. [Google Scholar] [CrossRef]
- Guo, Y.; Guan, T.; Shafiq, K.; Yu, Q.; Jiao, X.; Na, D.; Li, M.; Zhang, G.; Kong, J. Mitochondrial Dysfunction in Aging. Ageing Res. Rev. 2023, 88, 101955. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Gong, A.Y.; Haller, S.T.; Dworkin, L.D.; Liu, Z.; Gong, R. The Ageing Kidney: Molecular Mechanisms and Clinical Implications. Ageing Res. Rev. 2020, 63, 101151. [Google Scholar] [CrossRef] [PubMed]
- Buyan, M.I.; Pevzner, I.B.; Buyan, A.I.; Zorova, L.D.; Zorov, D.B.; Andrianova, N.V.; Plotnikov, E.Y. Deacetylase Inhibitor Trichostatin A Promotes the Proliferation of Epithelial Cells and Suppresses Glycolytic Activity of Fibroblasts in the Kidney. IUBMB Life 2025, 77, e70044. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhang, T.-N.; Hao, P.-H.; Yang, N.; Du, Y. Histone Deacetylases and Their Inhibitors in Kidney Diseases. Mol. Ther. 2025, 33, 3485–3527. [Google Scholar] [CrossRef]
- Shen, F.; Zhuang, S. Histone Acetylation and Modifiers in Renal Fibrosis. Front. Pharmacol. 2022, 13, 760308. [Google Scholar] [CrossRef]
- Chung, S.; Kim, S.; Son, M.; Kim, M.; Koh, E.S.; Shin, S.J.; Park, C.W.; Kim, H.-S. Inhibition of p300/CBP-Associated Factor Attenuates Renal Tubulointerstitial Fibrosis through Modulation of NF-kB and Nrf2. Int. J. Mol. Sci. 2019, 20, 1554. [Google Scholar] [CrossRef]
- Chouliaras, L.; van den Hove, D.L.A.; Kenis, G.; van Draanen, M.; Hof, P.R.; van Os, J.; Steinbusch, H.W.M.; Schmitz, C.; Rutten, B.P.F. Histone Deacetylase 2 in the Mouse Hippocampus: Attenuation of Age-Related Increase by Caloric Restriction. Curr. Alzheimer Res. 2013, 10, 868–876. [Google Scholar] [CrossRef][Green Version]
- Levine, D.C.; Kuo, H.-Y.; Hong, H.-K.; Cedernaes, J.; Hepler, C.; Wright, A.G.; Sommars, M.A.; Kobayashi, Y.; Marcheva, B.; Gao, P.; et al. NADH Inhibition of SIRT1 Links Energy State to Transcription during Time-Restricted Feeding. Nat. Metab. 2021, 3, 1621–1632. [Google Scholar] [CrossRef]
- Camacho-Pereira, J.; Tarragó, M.G.; Chini, C.C.S.; Nin, V.; Escande, C.; Warner, G.M.; Puranik, A.S.; Schoon, R.A.; Reid, J.M.; Galina, A.; et al. CD38 Dictates Age-Related NAD Decline and Mitochondrial Dysfunction through an SIRT3-Dependent Mechanism. Cell Metab. 2016, 23, 1127–1139. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abramicheva, P.A.; Sokolov, I.A.; Manskikh, V.N.; Andrianova, N.V.; Semenovich, D.S.; Zorova, L.D.; Pevzner, I.B.; Plotnikov, E.Y. Comparative Analysis of the Tolerance of Young and Old Kidneys to Injury in a Rat Model of Reversible Ureteral Obstruction. Antioxidants 2025, 14, 1219. https://doi.org/10.3390/antiox14101219
Abramicheva PA, Sokolov IA, Manskikh VN, Andrianova NV, Semenovich DS, Zorova LD, Pevzner IB, Plotnikov EY. Comparative Analysis of the Tolerance of Young and Old Kidneys to Injury in a Rat Model of Reversible Ureteral Obstruction. Antioxidants. 2025; 14(10):1219. https://doi.org/10.3390/antiox14101219
Chicago/Turabian StyleAbramicheva, Polina A., Ilya A. Sokolov, Vasily N. Manskikh, Nadezda V. Andrianova, Dmitry S. Semenovich, Ljubava D. Zorova, Irina B. Pevzner, and Egor Y. Plotnikov. 2025. "Comparative Analysis of the Tolerance of Young and Old Kidneys to Injury in a Rat Model of Reversible Ureteral Obstruction" Antioxidants 14, no. 10: 1219. https://doi.org/10.3390/antiox14101219
APA StyleAbramicheva, P. A., Sokolov, I. A., Manskikh, V. N., Andrianova, N. V., Semenovich, D. S., Zorova, L. D., Pevzner, I. B., & Plotnikov, E. Y. (2025). Comparative Analysis of the Tolerance of Young and Old Kidneys to Injury in a Rat Model of Reversible Ureteral Obstruction. Antioxidants, 14(10), 1219. https://doi.org/10.3390/antiox14101219






