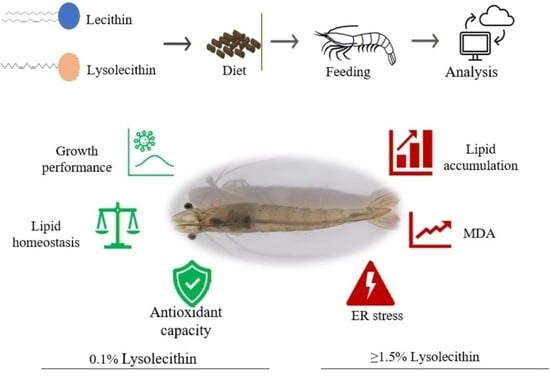
Effects of Lysolecithin on Growth Performance, Antioxidant Capacity, and Lipid Metabolism of Litopenaeus vannamei
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Experimental Animals and Husbandry Management
2.3. Sample Collection and Storage
2.4. Growth Parameters Analysis
2.5. Nutritional Composition Analysis
2.6. Antioxidant Enzyme Activity Analysis
2.7. Triglyceride and Fatty Acid Profile Analysis
2.8. Lipid Metabolism-Related Enzyme Activity Analysis
2.9. Antioxidant and Lipid Metabolism Gene Expression Analysis
2.10. Hepatopancreatic Lipidomics Analysis
2.11. Histological Morphological Observation
2.12. Statistical Analysis
3. Results
3.1. Growth Performance of the Shrimp
3.2. Nutritional Composition of the Whole Shrimp, Muscle, and Hepatopancreas
3.3. Changes in the Antioxidant Function of the Hepatopancreas
3.3.1. Antioxidant Enzyme Activity in the Hepatopancreas
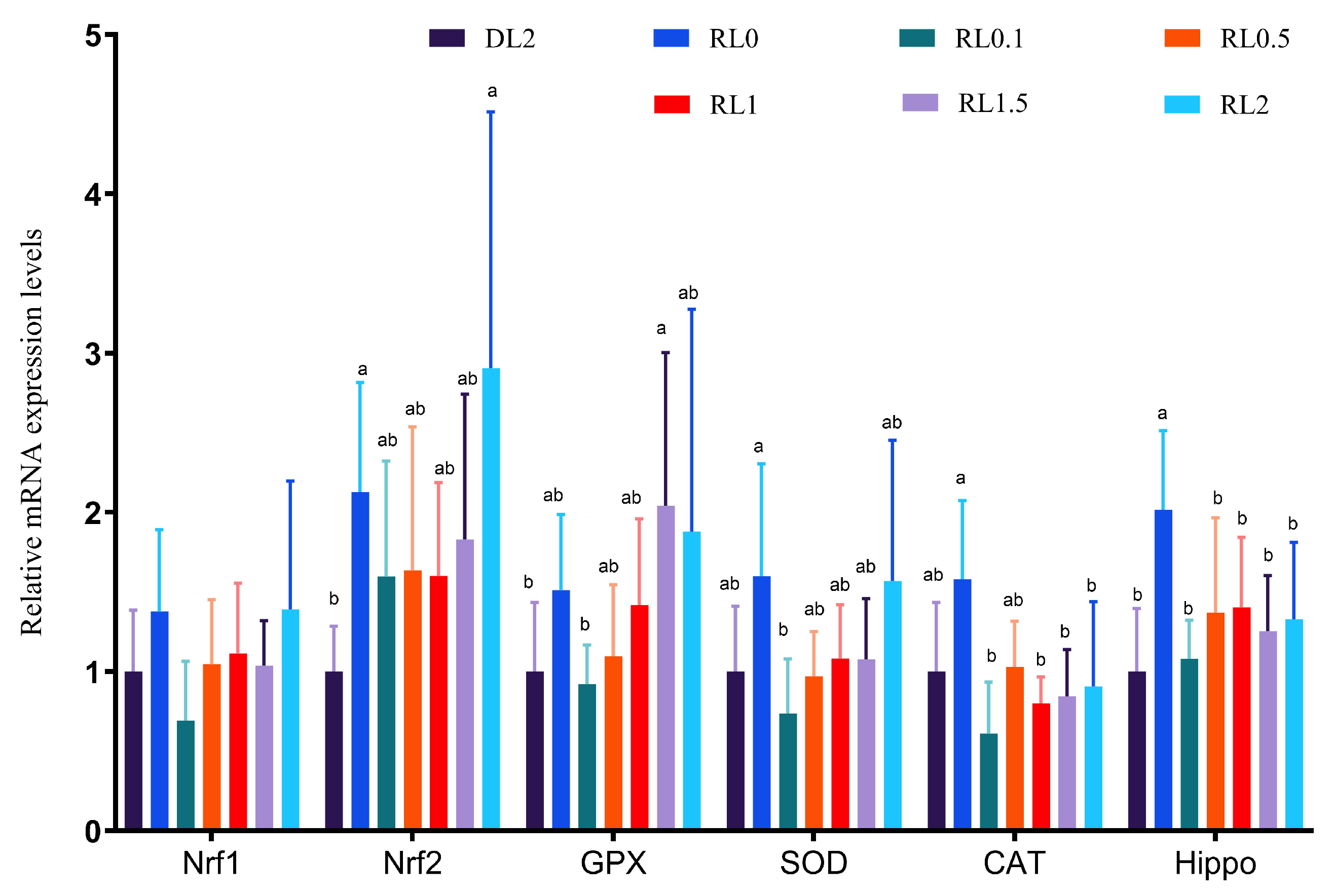
3.3.2. Relative Expression Levels of Antioxidant Genes in the Hepatopancreas
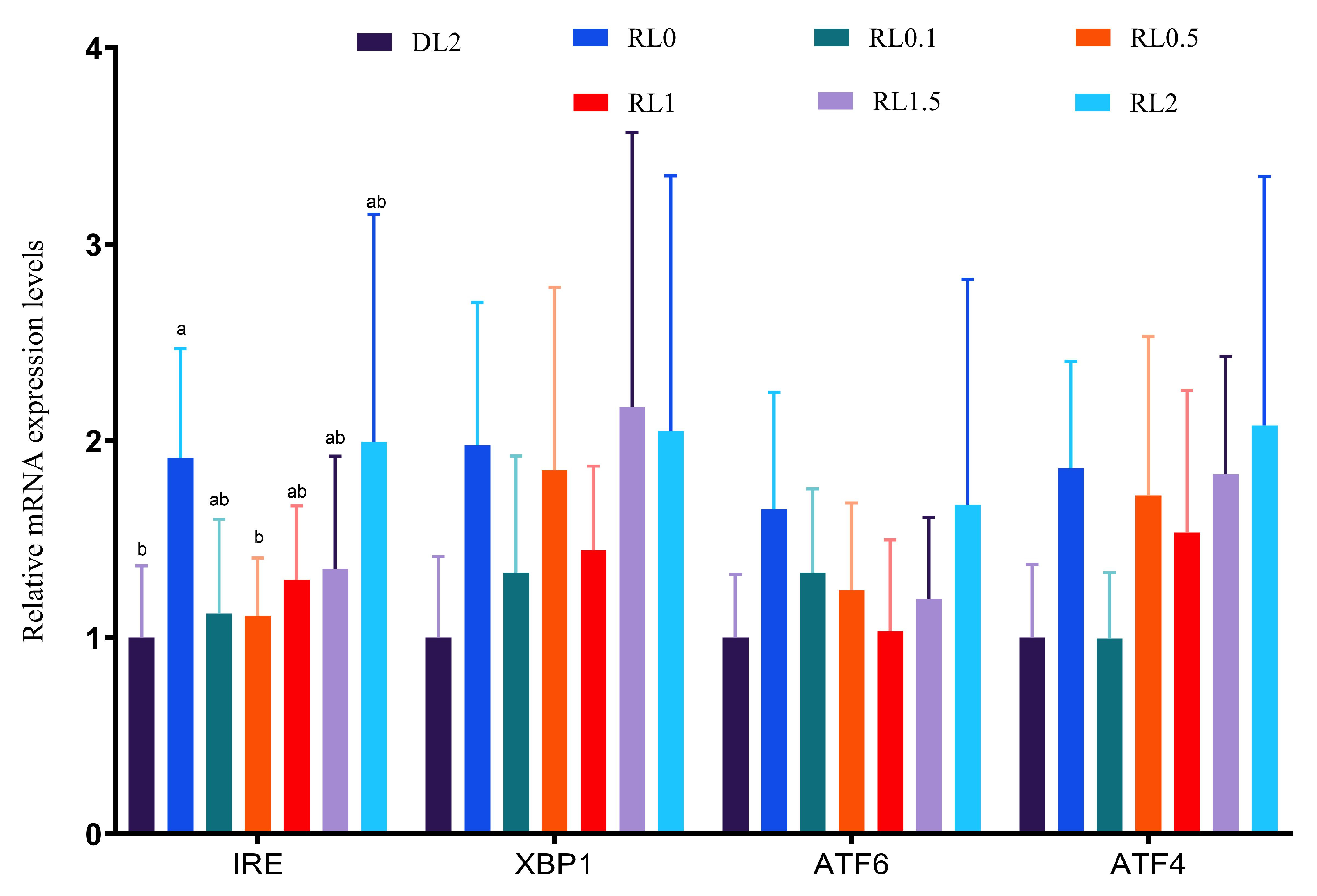
3.3.3. Endoplasmic Reticulum (ER) Stress Genes in the Hepatopancreas
3.4. Changes in the Lipid Metabolism of the Shrimp
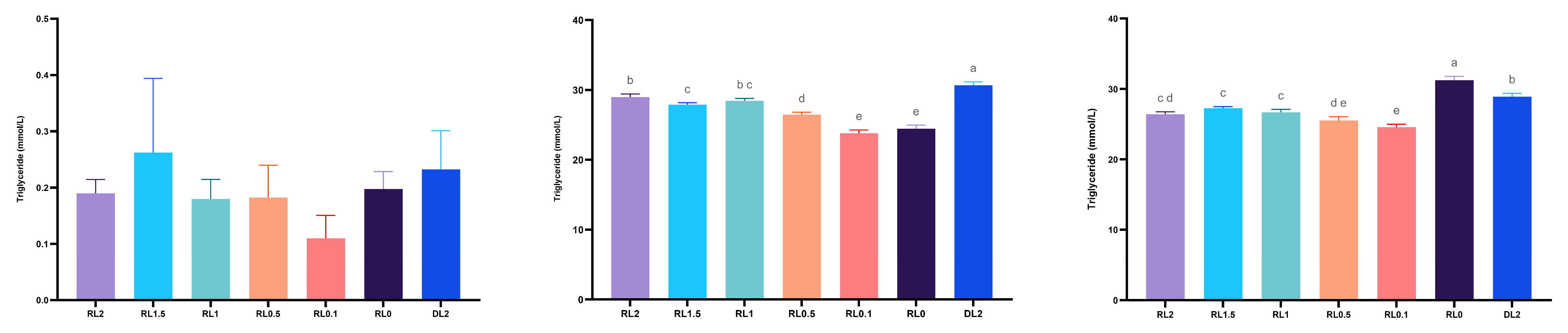
3.4.1. Triglyceride Content
3.4.2. Fatty Acid Profile in the Hepatopancreas and Muscle
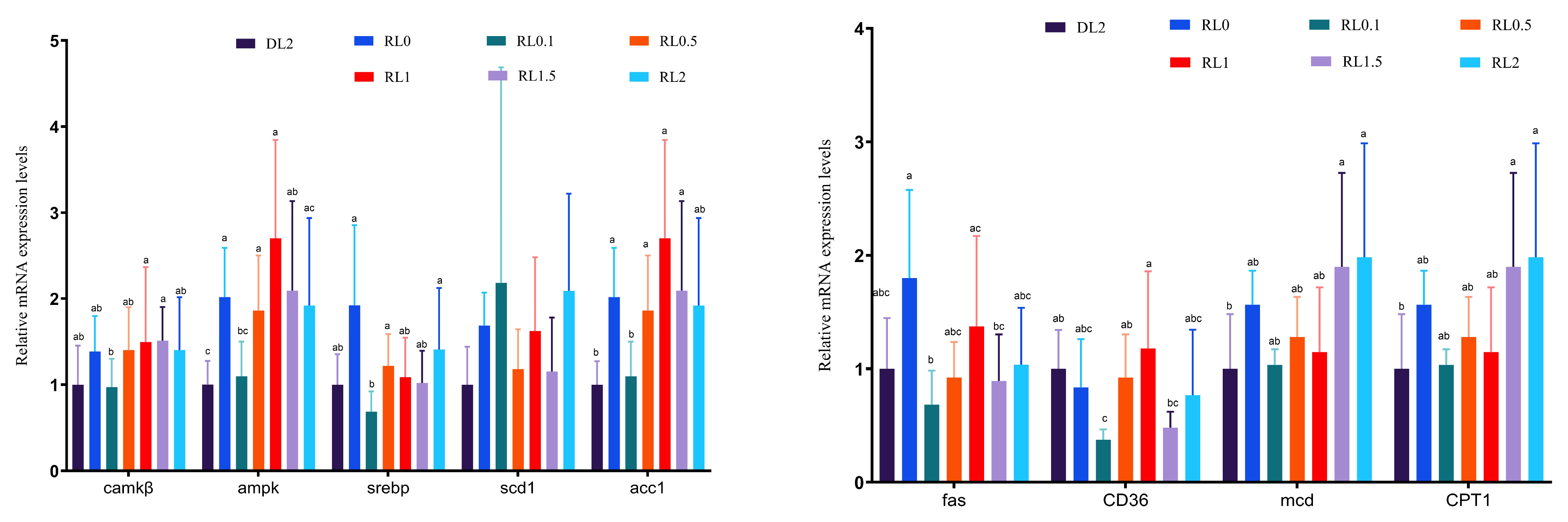
3.4.3. Lipid Metabolism Enzymes in the Hepatopancreas
3.4.4. Expression of Lipid Metabolism Genes in the Hepatopancreas
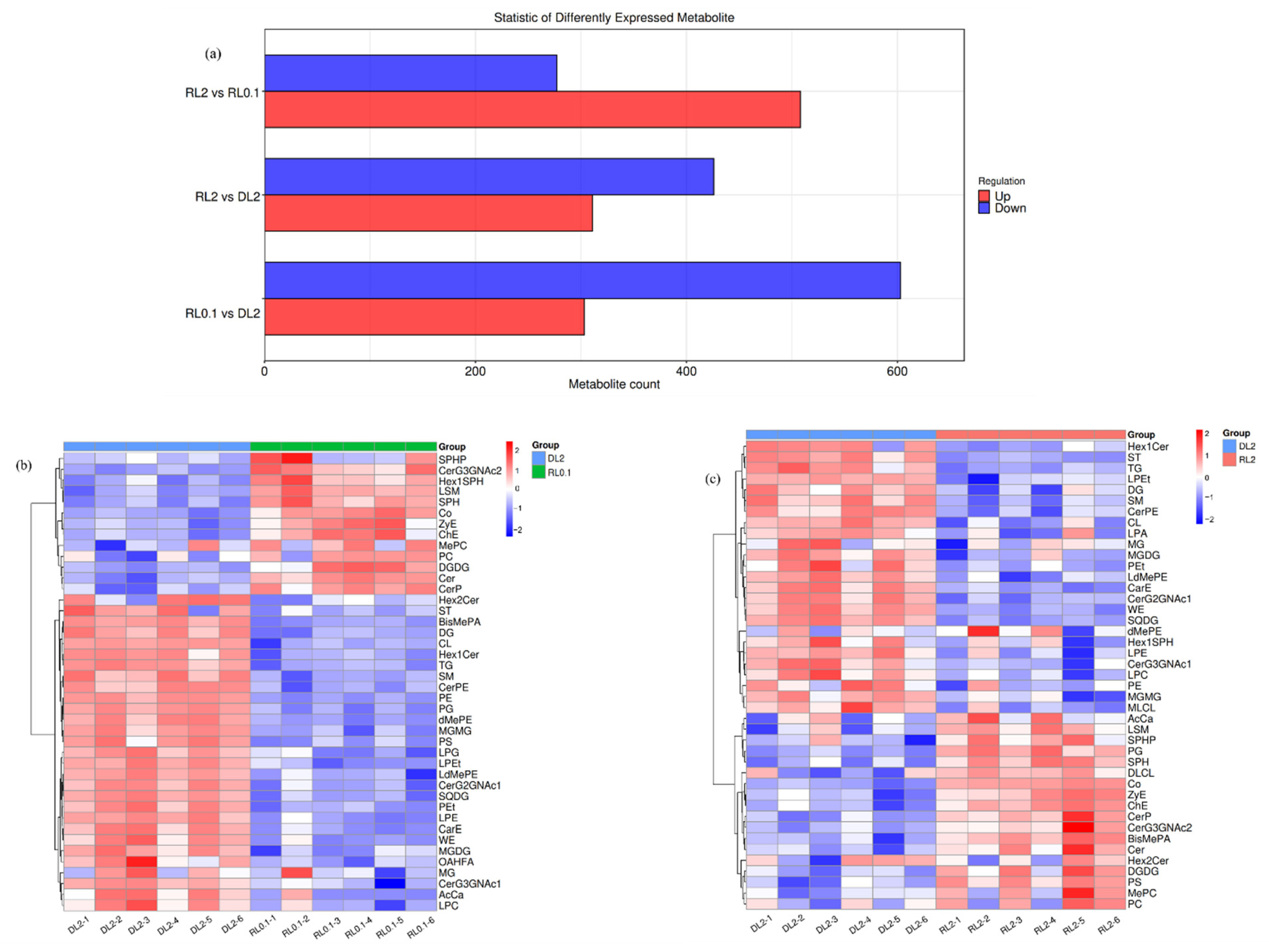
3.4.5. Hepatopancreas Lipidomics Changes
Lipid Composition
Lipid Identification and Verification
Differential Lipid Analysis
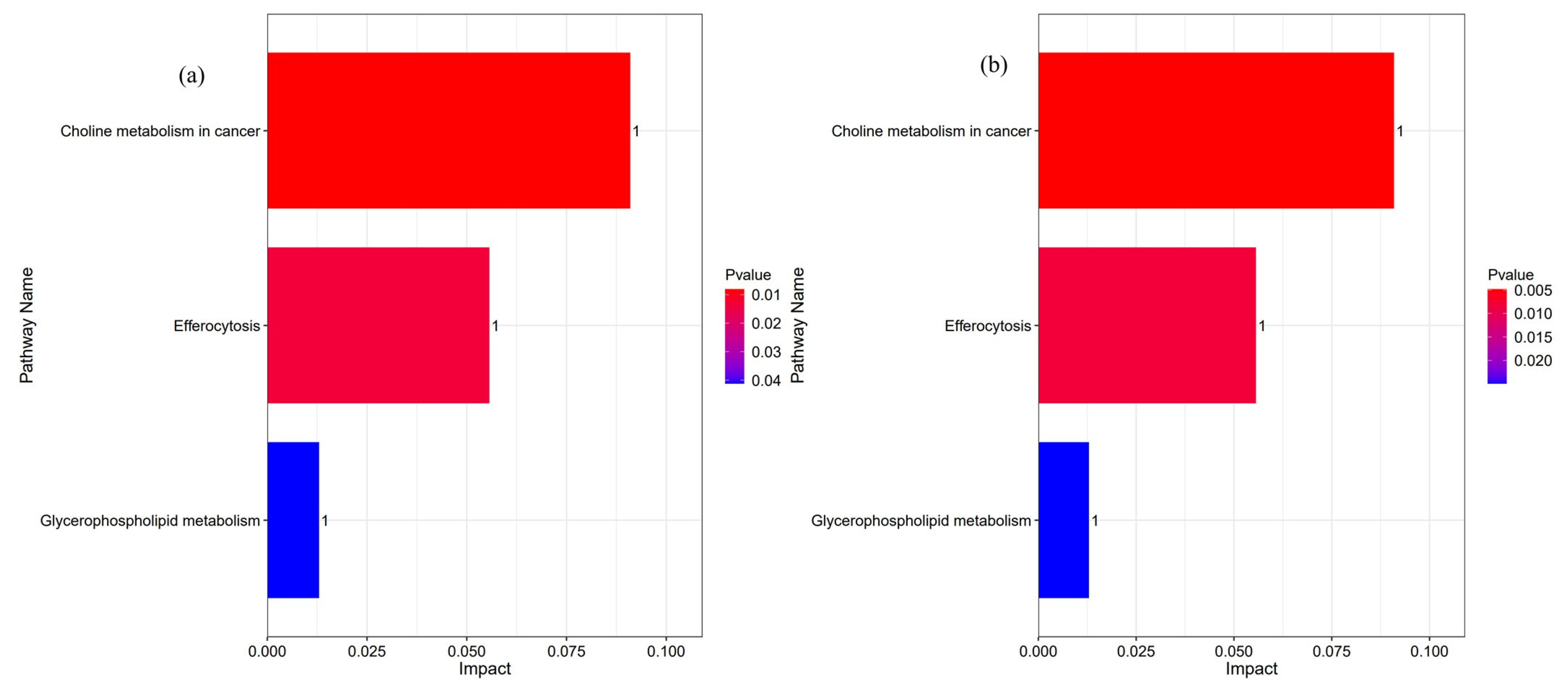
Analysis of Lipid Structural Characteristics and Functional Enrichment
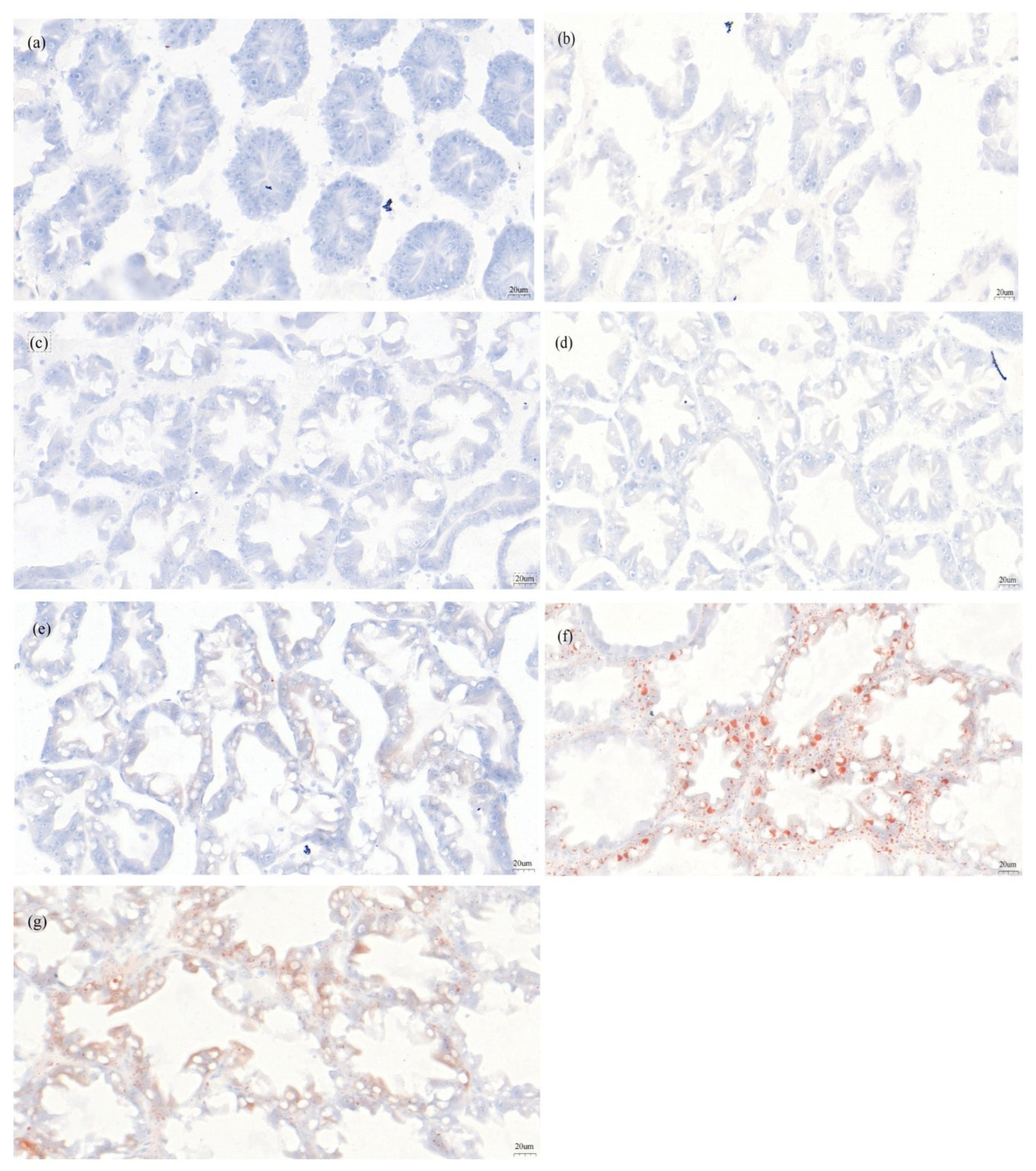
3.5. Histological Changes of the Hepatopancreas
3.5.1. HE-Stained Sections of the Hepatopancreas
3.5.2. Oil Red O Sections of the Hepatopancreas
4. Discussion
4.1. Effects of Dietary Growth Performance of the Shrimp
4.2. Effects of Dietary Lysolecithins on Nutritional Composition of Shrimp
4.3. Effects of Dietary Lysolecithins on Antioxidant Capacity of Shrimp
4.4. Effects of Dietary Lysolecithins on the Lipid Metabolism of Shrimp
4.5. Effects of Dietary Lysolecithins on the Hepatopancreas Lipidomics of Shrimp
4.6. Limitations of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Holmstrom, K.M.; Finkel, T. Cellular mechanisms and physiological consequences of redox-dependent signalling. Nat. Rev. Mol. Cell Biol. 2014, 15, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Shi, X.; Guo, J.; Mao, X.; Fan, B. Acute stress response in hepatopancreas of Pacific white shrimp Litopenaeus vannamei to high alkalinity. Aquacul. Rep. 2024, 35, 101981. [Google Scholar] [CrossRef]
- Feng, W.; Su, S.; Song, C.; Yu, F.; Zhou, J.; Li, J.; Jia, R.; Xu, P.; Tang, Y. Effects of copper exposure on oxidative stress, apoptosis, endoplasmic reticulum stress, autophagy and immune response in different tissues of Chinese Mitten Crab (Eriocheir sinensis). Antioxidants 2022, 11, 2029. [Google Scholar] [CrossRef]
- Lin, Y.; Hang, J.J.; Dahums, H.U.; Zhen, J.J.; Ying, X.P. Cell damage and apoptosis in the hepatopancreas of Eriocheir sinensis induced by cadmium. Aquat. Toxicol. 2017, 190, 190–198. [Google Scholar] [CrossRef]
- Wu, H.; Xuan, R.J.; Li, Y.J.; Zhang, X.M.; Jing, W.X.; Wang, L. Biochemical, histological and ultrastructural alterations of the alimentary system in the freshwater crab Sinopotamon henanense subchronically exposed to cadmium. Ecotoxicology 2014, 23, 65–75. [Google Scholar] [CrossRef]
- Hao, Y.; Guo, M.; Feng, Y.; Dong, Q.; Cui, M. Lysophospholipids and their G-coupled protein signaling in Alzheimer’s disease: From physiological performance to pathological impairment. Front. Mol. Neurosci. 2020, 13, 58. [Google Scholar] [CrossRef]
- Heringdorf, D.M.z. Lysophospholipids. In Encyclopedia of Molecular Pharmacology; Offermanns, S., Rosenthal, W., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 710–716. ISBN 978-3-540-38918-7. [Google Scholar]
- Grove, S.S.; Dall, J.; Madsen, J.G. The effect of lysophospholipids and sex on growth performance and small intestine morphology in weanling pigs, 7–30 kg. Animals 2024, 14, 1213. [Google Scholar] [CrossRef]
- Ghazalah, A.; Abd-Elsamee, M.; Ibrahim, M.; Abdelgayed, S.S.; Abdelkader, M.; Gonzalez-Sanchez, D.; Wealleans, A. Effects of a combination of lysolecithin, synthetic emulsifier, and monoglycerides on growth performance, intestinal morphology, and selected carcass traits in broilers fed low-energy diets. Animals 2021, 11, 3037. [Google Scholar] [CrossRef]
- Li, B.; Li, Z.; Sun, Y.; Wang, S.; Huang, B.; Wang, J. Effects of dietary lysolecithin (LPC) on growth, apparent digestibility of nutrients, and lipid metabolism in juvenile turbot Scophthalmus maximus L. Aquac. Fish. 2019, 4, 61–66. [Google Scholar] [CrossRef]
- Liu, G.; Ma, S.; Chen, F.; Gao, W.; Zhang, W.; Mai, K. Effects of dietary lysolecithin on growth performance, feed utilization, intestinal morphology, and metabolic responses of channel catfish (Ictalurus punctatus). Aquacult. Nutr. 2020, 26, 456–465. [Google Scholar] [CrossRef]
- Weng, M.; Zhang, W.; Zhang, Z.; Tang, Y.; Lai, W.; Dan, Z.; Liu, Y.; Zheng, J.; Gao, S.; Mai, K.; et al. Effects of dietary lysolecithin on growth performance, serum biochemical indexes, antioxidant capacity, lipid metabolism, and inflammation-related genes expression of juvenile large yellow croaker (Larimichthys crocea). Fish Shellfish Immun. 2022, 128, 50–59. [Google Scholar] [CrossRef]
- Taghavizadeh, M.; Hosseini Shekarabi, S.P.; Mehrgan, M.S.; Islami, H.R. Efficacy of dietary lysophospholipids (LipidolTM) on growth performance, serum immuno-biochemical parameters, and the expression of immune and antioxidant-related genes in rainbow trout (Oncorhynchus mykiss). Aquaculture 2020, 525, 735315. [Google Scholar] [CrossRef]
- Bousquet, D.; Guillot, N. Laminar shear stress promotes accumulation of polyunsaturated fatty acid in aortic endothelial cells through upregulation of LPCAT3 enzyme activity. BBA-Mol. Cell Biol. Lipids 2025, 1870, 159638. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wu, A.; Mo, R.; Zhou, Q.; Song, L.; Li, Z.; Zhao, H.; Fang, Z.; Lin, Y.; Xu, S.; et al. Dietary lysolecithin supplementation improves growth performance of weaned piglets via improving nutrients absorption, lipid metabolism, and redox status. J. Anim. Sci. 2023, 101, skad293. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Wang, B.; Li, Q.L.; Zhao, T.; Xu, P.C.; Song, Y.F.; Luo, Z. Dietary lecithin attenuates adverse effects of high fat diet on growth performance, lipid metabolism, endoplasmic reticulum stress and antioxidant capacity in the intestine of largemouth bass (Micropterus salmoides). Aquaculture 2025, 595, 741688. [Google Scholar] [CrossRef]
- Zhang, R.Q.; Zhang, X.Y.; Guo, J.T.; Shi, X. Gene expression response patterns in the heptaopnacreas of Litopenaeus vannamei under combined high pH and alkalinity stress. Aquacult. Int. 2025, 33, 366. [Google Scholar] [CrossRef]
- Amaya, E.; Davis, D.A.; Rouse, D.B. Alternative diets for the Pacific white shrimp Litopenaeus vannamei. Aquaculture 2007, 262, 419–425. [Google Scholar] [CrossRef]
- Duan, Y.; Xiao, M.; Wang, Y.; Huang, J.; Yang, Y.; Li, H. The high temperature stress responses in the hepatopancreas of Litopenaeus vannamei: From immune dysfunction to metabolic remodeling cascade. Front. Immunol. 2025, 16, 1631655. [Google Scholar] [CrossRef]
- Jamal, M.R.; Kristiansen, P.; Kabir, M.J.; de Bruyn, L.L. Risks and adaptation dynamics in shrimp and prawn-based farming systems in southwest coastal Bangladesh. Aquaculture 2023, 562, 738819. [Google Scholar] [CrossRef]
- Yan, H.; Wang, Y.; Liang, H.; Duan, Y.F.; Wang, J.; Zhou, C.; Huang, Z. Effects of lysophospholipids on the antioxidant capacity, digestive performance, and intestinal microbiota of Litopenaeus vannamei. Biology 2025, 14, 90. [Google Scholar] [CrossRef]
- Baur, F.J.; Ensminger, L.G. The Association of Official Analytical Chemists (AOAC). J. Am. Oil Chem. Soc. 1977, 54, 171–172. [Google Scholar] [CrossRef]
- Xia, Y.M.; Zhu, L.Z. Detection method of glutathione peroxidase in blood and tissue. J. Hyg. Res. 1987, 16, 29–32. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- GB 5009.168-2016; National Food Safety Standard Determination of Fatty Acids in Foods. National Standard of the People’s Republic of China: Beijing, China, 2016.
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−∆∆ct method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Narváez-Rivas, M.; Zhang, Q. Comprehensive untargeted lipidomic analysis using core–shell C30 particle column and high field orbitrap mass spectrometer. J. Chromatogr. A 2016, 1440, 123–134. [Google Scholar] [CrossRef]
- Dalli, J.; Colas, R.A.; Walker, M.E.; Serhan, C.N. Lipid mediator metabolomics via LC-MS/MS profiling and analysis. In Clinical Metabolomics; Methods in Molecular Biology; Giera, M., Ed.; Springer: New York, NY, USA, 2018; Volume 1730, pp. 59–72. ISBN 978-1-4939-7591-4. [Google Scholar]
- Brunson, J.F.; Romaire, R.P.; Reigh, R.C. Apparent digestibility of selected ingredients in diets for white shrimp Penaeus setiferus L. Aquacult. Nutr. 1997, 3, 9–16. [Google Scholar] [CrossRef]
- Deng, L. Current progress in the utilization of soy-based emulsifiers in food applications—A Review. Foods 2021, 10, 1354. [Google Scholar] [CrossRef]
- Law, S.H.; Chan, M.L.; Marathe, G.K.; Parveen, F.; Chen, C.H.; Ke, L.Y. An updated review of lysophosphatidylcholine metabolism in human diseases. Int. J. Mol. Sci. 2019, 20, 1149. [Google Scholar] [CrossRef]
- Zhao, P.Y.; Li, H.L.; Hossain, M.M.; Kim, I.H. Effect of emulsifier (lysophospholipids) on growth performance, nutrient digestibility, and blood profile in weanling pigs. Anim. Feed Sci. Technol. 2015, 207, 190–195. [Google Scholar] [CrossRef]
- Moolenaar, W.H. Lysophosphatidic acid, a multifunctional phospholipid messenger. J. Biol. Chem. 1995, 270, 12949–12952. [Google Scholar] [CrossRef]
- Xu, H.G.; Luo, X.; Bi, Q.Z.; Wang, Z.D.; Meng, X.X.; Liu, J.S.; Duan, M.; Wei, Y.L.; Liang, M.Q. Effects of dietary lysophosphatidylcholine on growth performance and lipid metabolism of juvenile turbot. Aquacult. Nutr. 2022, 2022, 3515101. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, P.; Tan, P.; Xu, D.D.; Wang, L.G.; Ding, Z.L.; Shao, Q.J. Yarrowia lipolytica as a promising protein source for Pacific white shrimp (Litopenaeus vannamei) diet: Impact on growth performance, metabolism, antioxidant capacity, and apparent digestibility. Front. Mar. Sci. 2024, 11, 1370371. [Google Scholar] [CrossRef]
- Qiao, X.L.; Liang, Q.J.; Liu, Y.; Wang, W.N. A novel Kelch-like-1 is involved in antioxidant response by regulating antioxidant enzyme system in Penaeus vannamei. Genes 2020, 11, 1077. [Google Scholar] [CrossRef] [PubMed]
- Ishii, T.; Itoh, K.; Takahashi, S.; Sato, H.; Yanagawa, T.; Katoh, Y.; Bannai, S.; Yamamoto, M. Transcription factor Nrf2 coordinately regulates a group of oxidative stress-inducible genes in macrophages. J. Biol. Chem. 2000, 275, 16023–16029. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.C.; Zhu, B.H.; Yun, J.T.; Yang, Y.; Tian, J.T.; Xu, W.Y.; Du, X.L.; Zhao, Y.L.; Li, Y.M. Comparison of antioxidant capacity and immune response between low salinity tolerant hybrid and normal variety of Pacific white shrimp (Litopenaeus vannamei). Aquacult. Int. 2024, 32, 1879–1894. [Google Scholar] [CrossRef]
- Zhu, J.Q.; Shi, W.J.; Zhao, R.; Gu, C.; Li, H.; Wang, L.B.; Wan, X.H. Effects of cold stress on the hemolymph of the Pacific white shrimp Penaeus vannamei. Fishes 2024, 9, 36. [Google Scholar] [CrossRef]
- de Souza, D.M.; Kutter, M.T.; Furtado, P.S.; Romano, L.A.; Wasielesky Junior, W.; Monserrat, J.M.; Garcia, L. Growth, antioxidant system, and immunological status of shrimp in bioflocs and clear water culture systems. Pesq. Agropec. Bras. 2019, 54, e00363. [Google Scholar] [CrossRef]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxidative Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef]
- Ponnampalam, E.N.; Kiani, A.; Santhiravel, S.; Holman, B.W.B.; Lauridsen, C.; Dunshea, F.R. The importance of dietary antioxidants on oxidative stress, meat and milk production, and their preservative aspects in farm animals: Antioxidant action, animal health, and product quality—Invited review. Animals 2022, 12, 3279. [Google Scholar] [CrossRef]
- Xu, H.Y.; Liu, T.; Feng, W.P.; He, J.L.; Han, T.; Wang, J.T.; Wang, C.L. Dietary phosphatidylcholine improved the survival, growth performance, antioxidant, and osmoregulation ability of early juvenile mud crab Scylla paramamosain. Aquaculture 2023, 563, 738899. [Google Scholar] [CrossRef]
- Chen, F.L.; Xiao, M.; Hu, S.F.; Wang, M. Keap1-Nrf2 Pathway: A key mechanism in the occurrence and development of cancer. Front. Oncol. 2024, 14, 1381467. [Google Scholar] [CrossRef]
- Ibrahim, L.; Mesgarzadeh, J.; Xu, I.; Powers, E.T.; Wiseman, R.L.; Bollong, M.J. Defining the functional targets of Cap‘n’collar transcription factors NRF1, NRF2, and NRF3. Antioxidants 2020, 9, 1025. [Google Scholar] [CrossRef]
- Sykiotis, G.P. Keap1/Nrf2 signaling pathway. Antioxidants 2021, 10, 828. [Google Scholar] [CrossRef] [PubMed]
- Li, S.L.; Li, H.R.; Xu, X.D.; Saw, P.E.; Zhang, L. Nanocarrier-mediated antioxidant delivery for liver diseases. Theranostics 2020, 10, 1262–1280. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Gao, J.; Huang, S.Q. Effects of different dietary phospholipid levels on growth performance, fatty acid composition, PPAR gene expressions and antioxidant responses of blunt snout bream Megalobrama amblycephala fingerlings. Fish Physiol. Biochem. 2015, 41, 423–436. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.Z.; Zhao, T.F.; Li, X.Y.; Zhang, X.C.; Chen, X.; Li, H.M.; Tan, B.P.; Xie, S.W. The impacts of a low fishmeal diet on the antioxidant capacity, endoplasmic reticulum stress, apoptosis, and intestinal health of Litopenaeus vannamei during the feeding trial. Anim. Feed Sci. Technol. 2024, 311, 115948. [Google Scholar] [CrossRef]
- Wang, Z.L.; Liao, G.W.; Chen, B.; Fan, L.F. Impacts of acute cold-stress in Pacific white shrimp Litopenaeus vannamei: Investigating the tissue-specific stress resistance response. Aquacult. Int. 2023, 31, 2649–2663. [Google Scholar] [CrossRef]
- Raghunath, A.; Panneerselvam, L.; Sundarraj, K.; Perumal, E. Heat Shock Proteins and Endoplasmic Reticulum Stress. In Heat Shock Proteins and Stress; Heat Shock Proteins; Asea, A.A.A., Kaur, P., Eds.; Springer International Publishing: Cham, Switzerland, 2018; Volume 15, pp. 39–78. ISBN 978-3-319-90724-6. [Google Scholar]
- Wang, Y.; Zeng, D.; Wei, L.; Chen, J.; Li, H.; Wen, L.; Huang, G.; Dai, Z.; Luo, J.; Sun, J.; et al. Effects of emulsifiers on lipid metabolism and performance of yellow-feathered broilers. BMC Vet. Res. 2024, 20, 246. [Google Scholar] [CrossRef]
- Ibrahim, M.S.; Mohammady, E.Y.; EL-Erian, M.A.; Ragaza, J.A.; El-Haroun, E.R.; Hassaan, M.S. Dietary zinc oxide for growth and immune stimulation of aquatic animal species: A review. Proc. Zool. Soc. 2023, 76, 59–72. [Google Scholar] [CrossRef]
- Zhang, P.; Liu, N.C.; Xue, M.Y.; Zhang, M.J.; Xiao, Z.D.; Xu, C.; Fan, Y.D.; Qiu, J.Q.; Zhang, Q.H.; Zhou, Y. β-sitosterol reduces the content of triglyceride and cholesterol in a high-fat diet-induced non-alcoholic fatty liver disease Zebrafish (Danio rerio) model. Animals 2024, 14, 1289. [Google Scholar] [CrossRef]
- Tocher, D.R. Omega-3 long-chain polyunsaturated fatty acids and aquaculture in perspective. Aquaculture 2015, 449, 94–107. [Google Scholar] [CrossRef]
- Nicoll, A.; Lewis, B. Evaluation of the roles of lipoprotein lipase and hepatic lipase in lipoprotein metabolism: In vivo and in vitro studies in man. Eur. J. Clin. Investig. 1980, 10, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Song, T.; Ke, L.; Sun, Y.; Ye, J. Dietary high levels of coconut oil replacing fish oil did not affect growth, but promoted liver lipid deposition of orange-spotted groupers (Epinephelus coioides). Animals 2024, 14, 1534. [Google Scholar] [CrossRef] [PubMed]
- Tokumitsu, H.; Sakagami, H. Molecular Mechanisms Underlying Ca2+/Calmodulin-Dependent Protein Kinase Kinase Signal Transduction. Int. J. Mol. Sci. 2022, 23, 11025. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Ou, H.; Ren, F.; Huan, Y.; Zhong, T.; Gao, M.; Cai, H. Propofol inhibited autophagy through Ca2+/CaMKKβ/AMPK/mTOR pathway in OGD/R-induced neuron injury. Mol. Med. 2018, 24, 58. [Google Scholar] [CrossRef]
- Saikia, R.; Joseph, J. AMPK: A key regulator of energy stress and calcium-induced autophagy. J. Mol. Med. 2021, 99, 1539–1551. [Google Scholar] [CrossRef]
- Samovski, D.; Sun, J.; Pietka, T.; Gross, R.W.; Eckel, R.H.; Su, X.; Stahl, P.D.; Abumrad, N.A. Regulation of AMPK activation by CD36 links fatty acid uptake to β-Oxidation. Diabetes 2015, 64, 353–359. [Google Scholar] [CrossRef]
- Li, X.N.; Song, J.; Zhang, L.; LeMaire, S.A.; Hou, X.Y.; Zhang, C.; Coselli, J.S.; Chen, L.; Wang, X.L.; Zhang, Y.; et al. Activation of the AMPK-FOXO3 pathway reduces fatty acid–induced increase in intracellular reactive oxygen species by upregulating thioredoxin. Diabetes 2009, 58, 2246–2257. [Google Scholar] [CrossRef]
- Shi, B.; Jin, M.; Jiao, L.F.; Betancor, M.B.; Tocher, D.R.; Zhou, Q.C. Effects of dietary Zn Level on growth performance, lipolysis and expression of genes involved in the Ca2+/CaMKKβ/AMPK pathway in juvenile Pacific white shrimp. Br. J. Nutr. 2020, 124, 773–784. [Google Scholar] [CrossRef]
- Chen, K.; Li, E.; Xu, Z.; Li, T.; Xu, C.; Qin, J.G.; Chen, L. Comparative transcriptome analysis in the hepatopancreas tissue of Pacific white shrimp Litopenaeus vannamei fed different lipid sources at low salinity. PLoS ONE 2015, 10, e0144889. [Google Scholar] [CrossRef]
- Guo, A.; Yang, Y.; Wu, J.; Qin, N.; Hou, F.; Gao, Y.; Li, K.; Xing, G.; Li, S. Lipidomic and transcriptomic profiles of glycerophospholipid metabolism during Hemerocallis citrina Baroni flowering. BMC Plant Biol. 2023, 23, 50. [Google Scholar] [CrossRef]
- Schultheis, N.; Becker, R.; Berhanu, G.; Kapral, A.; Roseman, M.; Shah, S.; Connell, A.; Selleck, S. Regulation of autophagy, lipid metabolism, and neurodegenerative pathology by heparan sulfate proteoglycans. Front. Genet. 2023, 13, 1012706. [Google Scholar] [CrossRef]
- Prado, L.G.; Camara, N.O.S.; Barbosa, A.S. Cell lipid biology in infections: An overview. Front. Cell. Infect. Microbiol. 2023, 13, 1148383. [Google Scholar] [CrossRef]
- Ye, C.; Shen, Z.; Greenberg, M.L. Cardiolipin remodeling: A regulatory hub for modulating cardiolipin metabolism and function. J. Bioenerg. Biomembr. 2016, 48, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Tian, Q.; Mitchell, B.A.; Zampino, M.; Ferrucci, L. Longitudinal associations between blood lysophosphatidylcholines and skeletal muscle mitochondrial function. Geroscience 2022, 44, 2213–2221. [Google Scholar] [CrossRef] [PubMed]
- Omi, J.; Kano, K.; Aoki, J. Current knowledge on the biology of lysophosphatidylserine as an emerging bioactive lipid. Cell Biochem. Biophys. 2021, 79, 497–508. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Li, X.; Wang, Y.; Zhang, X.; Gong, W. Structural basis for ligand recognition and signaling of the lysophosphatidylserine receptors GPR34 and GPR174. PLoS Biol. 2023, 21, e3002387. [Google Scholar] [CrossRef]
- Kameoka, S.; Adachi, Y.; Okamoto, K.; Iijima, M.; Sesaki, H. Phosphatidic acid and cardiolipin coordinate mitochondrial dynamics. Trends Cell Biol. 2018, 28, 67–76. [Google Scholar] [CrossRef]
- Furse, S. Is Phosphatidylglycerol Essential for Terrestrial Life? J. Chem. Biol. 2017, 10, 1–9. [Google Scholar] [CrossRef]
- Pan, J.; Heberle, F.A.; Tristram-Nagle, S.; Szymanski, M.; Koepfinger, M.; Katsaras, J.; Kučerka, N. Molecular structures of fluid phase phosphatidylglycerol bilayers as determined by small-angle neutron and X-Ray scattering. BBA-Biomembr. 2012, 1818, 2135–2148. [Google Scholar] [CrossRef]
- Sugawara, T. Sphingolipids as Functional Food Components: Benefits in Skin Improvement and Disease Prevention. J. Agric. Food Chem. 2022, 70, 9597–9609. [Google Scholar] [CrossRef] [PubMed]
- Saville, J.T.; Fuller, M. Sphingolipid dyshomeostasis in the brain of the mouse model of mucopolysaccharidosis type IIIA. Mol. Genet. Metab. 2020, 129, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Olešová, D.; Majerová, P.; Hájek, R.; Piešťanský, J.; Brumarová, R.; Michalicová, A.; Jurkanin, B.; Friedecký, D.; Kováč, A. GM3 ganglioside linked to neurofibrillary pathology in a transgenic rat model for tauopathy. Int. J. Mol. Sci. 2021, 22, 12581. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, L.; Zhang, W.; Wan, Z. The role of sphingomyelin metabolism in the protection of rat brain microvascular endothelial cells by mild hypothermia. Neurocrit. Care 2022, 36, 546–559. [Google Scholar] [CrossRef]
- Chaurasia, B.; Summers, S.A. Ceramides–lipotoxic inducers of metabolic disorders. Trends Endocrin. Met. 2015, 26, 538–550. [Google Scholar] [CrossRef]
- Kleuser, B.; Bäumer, W. Sphingosine 1-phosphate as essential signaling molecule in inflammatory skin diseases. Int. J. Mol. Sci. 2023, 24, 1456. [Google Scholar] [CrossRef]
- McCluskey, G.; Donaghy, C.; Morrison, K.E.; McConville, J.; Duddy, W.; Duguez, S. The Role of Sphingomyelin and Ceramide in Motor Neuron Diseases. J. Pers. Med. 2022, 12, 1418. [Google Scholar] [CrossRef]
- Yunoki, K.; Sato, M.; Seki, K.; Ohkubo, T.; Tanaka, Y.; Ohnishi, M. Simultaneous quantification of plant glyceroglycolipids including sulfoquinovosyldiacylglycerol by HPLC–ELSD with binary gradient elution. Lipids 2009, 44, 77–83. [Google Scholar] [CrossRef]
- Kalisch, B.; Dörmann, P.; Hölzl, G. DGDG and Glycolipids in Plants and Algae. In Lipids in Plant and Algae Development; Subcellular Biochemistry; Nakamura, Y., Li-Beisson, Y., Eds.; Springer International Publishing: Cham, Switzerland, 2016; Volume 86, pp. 51–83. ISBN 978-3-319-25977-2. [Google Scholar]


| Ingredients % a | DL2 | RL0 | RL0.1 | RL0.5 | RL1 | RL1.5 | RL2 |
|---|---|---|---|---|---|---|---|
| Fish meal | 25.00 | 25.00 | 25.00 | 25.00 | 25.00 | 25.00 | 25.00 |
| Soybean meal | 18.00 | 18.00 | 18.00 | 18.00 | 18.00 | 18.00 | 18.00 |
| Wheat flour | 21.99 | 23.99 | 23.89 | 23.49 | 22.99 | 22.49 | 21.99 |
| Krill meal | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 |
| Peanut meal | 16.40 | 16.40 | 16.40 | 16.40 | 16.40 | 16.40 | 16.40 |
| Brewer’s yeast | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 |
| Fish oil | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Soy oil | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Mineral premix b | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Vitamin premix c | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Dicalcium phosphate | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Choline chloride | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 |
| Soy lecithin | 2.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Lysolecithin Soy lysolecithin | 0.00 | 0.00 | 0.10 | 0.50 | 1.00 | 1.50 | 2.00 |
| Vitamin C phosphate | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 |
| Sodium alginate | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Y2O3 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| Total | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 |
| Calculated composition d | |||||||
| Crude fat, % | 7.81 | 5.85 | 5.95 | 6.34 | 6.83 | 7.32 | 7.81 |
| Crude protein, % | 40.69 | 40.93 | 40.92 | 40.87 | 40.81 | 40.75 | 40.69 |
| Crude ash, % | 8.55 | 8.58 | 8.57 | 8.57 | 8.56 | 8.55 | 8.55 |
| Items | DL2 | RL0 | RL0.1 | RL0.5 | RL1 | RL1.5 | RL2 |
|---|---|---|---|---|---|---|---|
| Survival, % | 97.50 ± 3.20 | 95.85 ± 4.20 | 100.00 ± 0.00 | 95.83 ± 5.00 | 99.18 ± 1.66 | 100.00 ± 0.00 | 97.50 ± 5.00 |
| IBW 1 | 2.21 ± 0.06 | 2.18 ± 0.06 | 2.23 ± 0.05 | 2.21 ± 0.04 | 2.21 ± 0.04 | 2.16 ± 0.04 | 2.22 ± 0.09 |
| FBW 2 | 10.70 ± 0.37 | 10.69 ± 0.28 | 11.25 ± 0.20 | 10.76 ± 0.52 | 10.80 ± 0.63 | 10.49 ± 0.56 | 10.68 ± 0.40 |
| WG, % | 385.70 ± 25.73 | 390.25 ± 1.10 | 404.51 ± 9.54 | 388.26 ± 30.30 | 387.82 ± 22.52 | 386.47 ± 25.89 | 381.45 ± 30.16 |
| SGR | 2.83 ± 0.08 | 2.82 ± 0.09 | 2.89 ± 0.04 | 2.83 ± 0.11 | 2.83 ± 0.08 | 2.82 ± 0.10 | 2.81 ± 0.11 |
| FCR | 1.46 ± 0.08 | 1.47 ± 0.03 | 1.46 ± 0.02 | 1.43 ± 0.06 | 1.44 ± 0.09 | 1.41 ± 0.03 | 1.44 ± 0.08 |
| PR, % | 125.55 ± 6.21 | 123.26 ± 4.41 | 125.69 ± 1.94 | 124.73 ± 6.10 | 125.81 ± 8.18 | 129.81 ± 2.19 | 129.94 ± 9.18 |
| LR, % | 50.07 ± 6.07 b | 56.01 ± 6.19 ab | 58.28 ± 2.16 ab | 62.83 ± 3.01 ab | 62.98 ± 3.21 ab | 67.24 ± 5.99 a | 66.69 ± 3.27 a |
| Items | DL2 | RL0 | RL0.1 | RL0.5 | RL1 | RL1.5 | RL2 |
|---|---|---|---|---|---|---|---|
| Crude protein | 72.13 ± 2.65 | 74.48 ± 0.19 | 71.59 ± 1.90 | 72.41 ± 1.87 | 74.36 ± 0.57 | 71.36 ± 1.03 | 70.76 ± 1.22 |
| Crude fat | 72.30 ± 1.36 d | 75.95 ± 1.77 bc | 76.32 ± 1.83 abc | 79.32 ± 0.07 a | 78.79 ± 0.21 ab | 76.07 ± 0.87 bc | 75.50 ± 0.47 cd |
| Items | DL2 | RL0 | RL0.1 | RL0.5 | RL1 | RL1.5 | RL2 |
|---|---|---|---|---|---|---|---|
| Composition of Whole Shrimp | |||||||
| Moisture | 77.53 ± 0.62 | 77.44 ± 0.53 | 77.59 ± 0.52 | 76.64 ± 0.55 | 77.15 ± 0.61 | 77.35 ± 0.30 | 77.41 ± 0.45 |
| Crude Fat | 4.43 ± 0.33 d | 4.62 ± 0.36 d | 5.00 ± 0.20 cd | 5.83 ± 0.31 bc | 6.24 ± 0.49 ab | 6.79 ± 0.58 ab | 6.97 ± 0.54 a |
| Crude Protein | 78.73 ± 0.84 | 78.12 ± 1.13 | 78.24 ± 0.18 | 77.33 ± 1.01 | 78.07 ± 0.75 | 78.05 ± 0.70 | 77.64 ± 1.08 |
| Ash Content | 12.70 ± 0.62 | 12.94 ± 1.17 | 12.22 ± 0.56 | 12.26 ± 0.86 | 12.21 ± 0.55 | 11.85 ± 0.73 | 12.75 ± 0.20 |
| Muscle | |||||||
| Moisture | 75.91 ± 0.43 | 75.96 ± 0.21 | 76.42 ± 0.44 | 76.11 ± 0.56 | 76.2 ± 0.31 | 76.45 ± 0.32 | 75.99 ± 0.23 |
| Crude Fat | 3.43 ± 0.24 c | 3.49 ± 0.17 c | 3.72 ± 0.32 bc | 3.93 ± 0.23 abc | 4.09 ± 0.28 ab | 4.17 ± 0.04 ab | 4.29 ± 0.23 a |
| Crude Protein | 92.93 ± 0.26 a | 92.41 ± 0.24 a | 92.03 ± 0.45 ab | 91.82 ± 0.37 ab | 91.94 ± 0.42 ab | 91.95 ± 0.54 ab | 91.19 ± 0.56 b |
| Ash Content | 5.71 ± 0.51 | 5.52 ± 0.08 | 5.65 ± 0.27 | 5.37 ± 0.27 | 5.66 ± 0.24 | 5.69 ± 0.24 | 5.49 ± 0.17 |
| Hepatopancreas | |||||||
| Moisture | 80.30 ± 0.82 | 81.96 ± 1.39 | 82.62 ± 2.14 | 81.05 ± 1.59 | 80.51 ± 0.43 | 81.81 ± 1.50 | 79.40 ± 0.89 |
| Crude Fat | 11.04 ± 1.99 d | 11.40 ± 0.46 cd | 10.38 ± 0.35 d | 12.17 ± 0.26 bcd | 15.03 ± 0.53 ab | 14.17 ± 0.18 abc | 15.59 ± 1.68 a |
| Items | DL2 | RL0 | RL0.1 | RL0.5 | RL1 | RL1.5 | RL2 |
|---|---|---|---|---|---|---|---|
| T-AOC (U/mg protein) | 3.73 ± 0.79 | 3.56 ± 0.38 | 3.10 ± 0.38 | 2.91 ± 0.71 | 3.66 ± 0.88 | 3.50 ± 0.53 | 2.55 ± 0.50 |
| SOD (U/mg protein) | 5.08 ± 0.13 | 5.01 ± 1.04 | 3.68 ± 0.43 | 5.54 ± 0.34 | 4.36 ± 2.01 | 5.38 ± 0.36 | 3.11 ± 0.98 |
| GPx (U/mg protein) | 255.70 ± 41.71 | 185.39 ± 36.25 | 195.39 ± 7.12 | 221.93 ± 23.53 | 244.82 ± 68.41 | 238.44 ± 36.36 | 240.53 ± 42.85 |
| MDA (nmol/mg protein) | 1.76 ± 0.52 | 1.19 ± 0.46 | 0.80 ± 0.30 | 1.65 ± 0.87 | 1.45 ± 0.40 | 2.44 ± 0.67 | 2.15 ± 1.01 |
| Items | DL2 | RL0 | RL0.1 | RL0.5 | RL1 | RL1.5 | RL2 |
|---|---|---|---|---|---|---|---|
| TL (U/mg protein) | 11.20 ± 1.42 a | 8.72 ± 1.44 a | 9.83 ± 0.44 a | 11.57 ± 1.94 a | 12.38 ± 3.41 a | 18.54 ± 3.52 b | 16.88 ± 2.68 b |
| HL (U/mg protein) | 5.13 ± 0.77 a | 3.65 ± 0.77 a | 4.65 ± 0.36 a | 5.33 ± 1.15 a | 5.77 ± 1.90 a | 8.15 ± 2.02 b | 7.81 ± 1.71 b |
| LPL (U/mg protein) | 6.06 ± 0.73 a | 5.07 ± 0.97 a | 5.18 ± 0.51 a | 6.24 ± 0.92 a | 6.60 ± 1.57 a | 10.39 ± 1.68 b | 9.07 ± 1.16 b |
| Lipase (U/mg protein) | 0.52 ± 0.09 | 0.70 ± 0.13 | 0.53 ± 0.11 | 0.65 ± 0.12 | 0.66 ± 0.12 | 0.66 ± 0.13 | 0.72 ± 0.19 |
| ACC (U/L) | 10.4 ± 0.18 a | 10.78 ± 0.19 ab | 10.98 ± 0.08 ab | 11.24 ± 0.32 b | 11.12 ± 0.01 b | 11.31 ± 0.63 b | 11.34 ± 0.46 b |
| FAS (U/mL) | 377.32 ± 26.74 a | 401.61 ± 37.92 ab | 420.73 ± 37.57 ab | 415.55 ± 26.13 ab | 399.58 ± 13.56 ab | 445.75 ± 34.45 b | 422.36 ± 19.01 ab |
| CPT-1 (U/L) | 78.23 ± 6.04 | 74.25 ± 8.15 | 78.56 ± 3.18 | 81.06 ± 0.85 | 80.42 ± 9.81 | 88.36 ± 8.70 | 84.11 ± 4.94 |
| ATGL (U/mL) | 72.64 ± 7.77 | 69.94 ± 5.82 | 70.08 ± 6.68 | 83.80 ± 22.26 | 83.37 ± 8.48 | 86.00 ± 21.13 | 81.9 ± 9.21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Yan, H.; Liang, H.; Duan, Y.; Wang, J.; Zhou, C.; Huang, Z. Effects of Lysolecithin on Growth Performance, Antioxidant Capacity, and Lipid Metabolism of Litopenaeus vannamei. Antioxidants 2025, 14, 1209. https://doi.org/10.3390/antiox14101209
Wang Y, Yan H, Liang H, Duan Y, Wang J, Zhou C, Huang Z. Effects of Lysolecithin on Growth Performance, Antioxidant Capacity, and Lipid Metabolism of Litopenaeus vannamei. Antioxidants. 2025; 14(10):1209. https://doi.org/10.3390/antiox14101209
Chicago/Turabian StyleWang, Yun, Hailiang Yan, Hong Liang, Yafei Duan, Jun Wang, Chuanpeng Zhou, and Zhong Huang. 2025. "Effects of Lysolecithin on Growth Performance, Antioxidant Capacity, and Lipid Metabolism of Litopenaeus vannamei" Antioxidants 14, no. 10: 1209. https://doi.org/10.3390/antiox14101209
APA StyleWang, Y., Yan, H., Liang, H., Duan, Y., Wang, J., Zhou, C., & Huang, Z. (2025). Effects of Lysolecithin on Growth Performance, Antioxidant Capacity, and Lipid Metabolism of Litopenaeus vannamei. Antioxidants, 14(10), 1209. https://doi.org/10.3390/antiox14101209