Modulation of Egg Elemental Metabolomics by Dietary Supplementation with Flavonoids and Orange Pulp (Citrus sinensis)
Abstract
1. Introduction
2. Materials and Methods
2.1. Trial Design and Experimental Procedures
2.2. Determination of Selected Elements in Eggs
2.3. Statistical Analysis
3. Results
3.1. Elemental Content
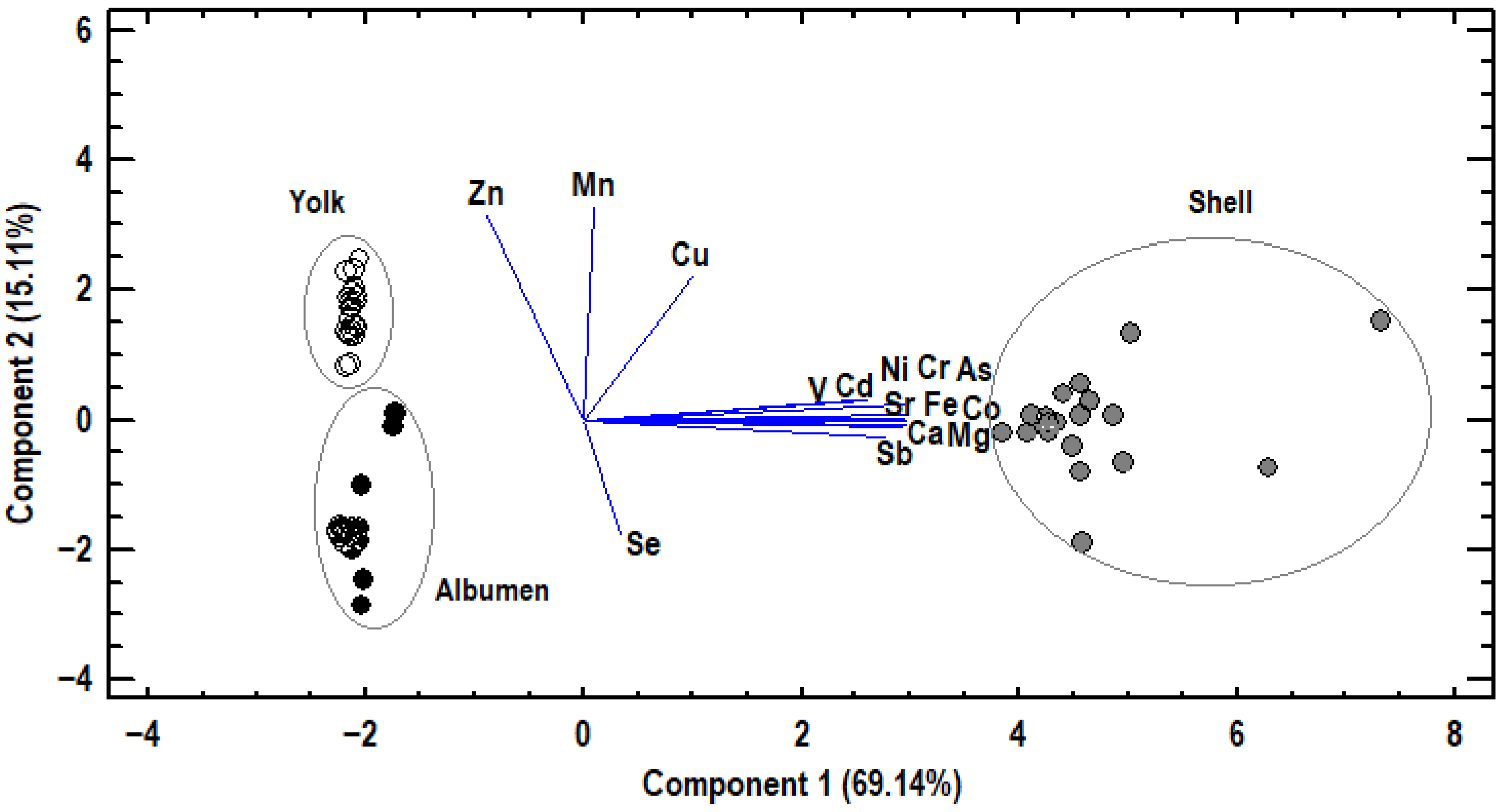
3.2. Principal Component Analysis
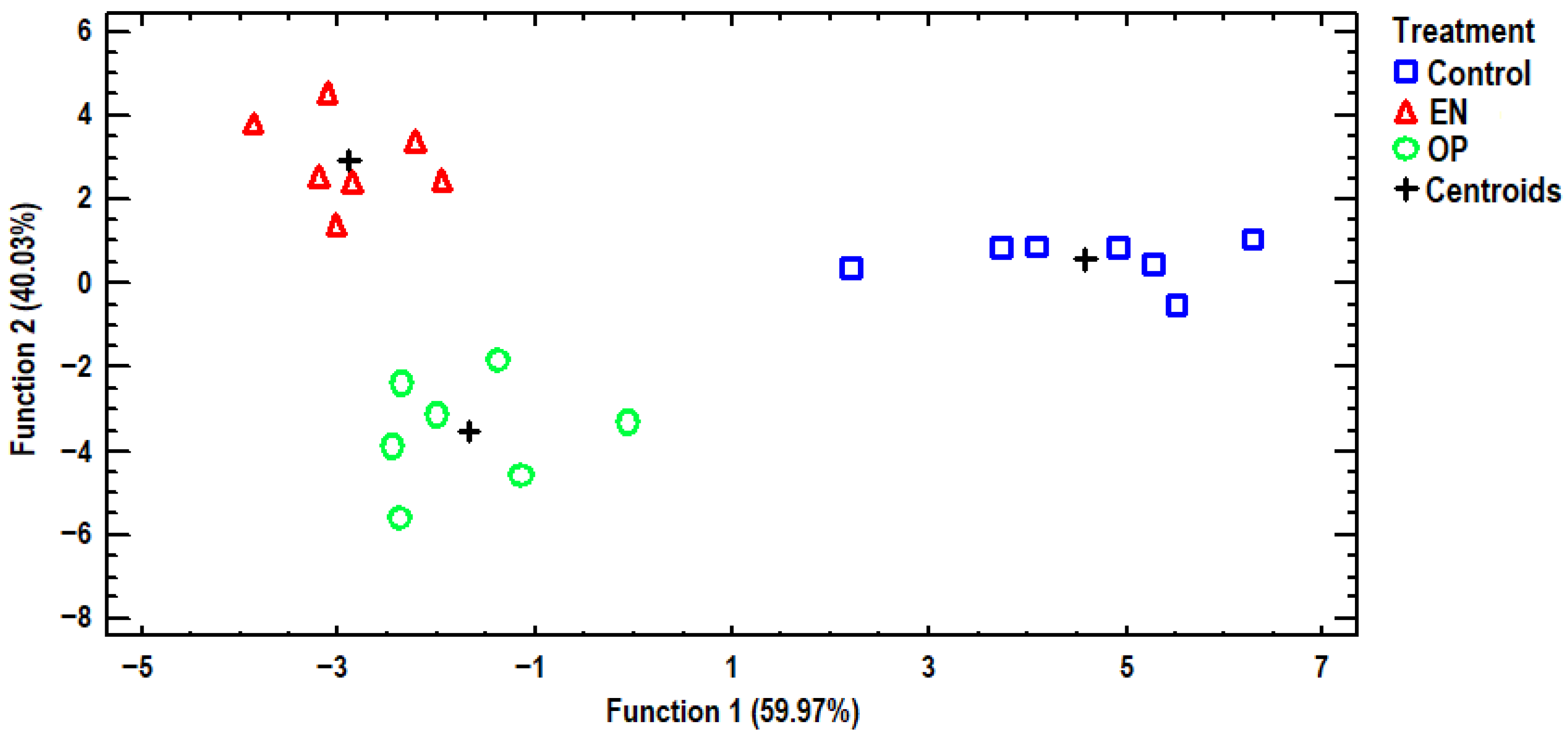
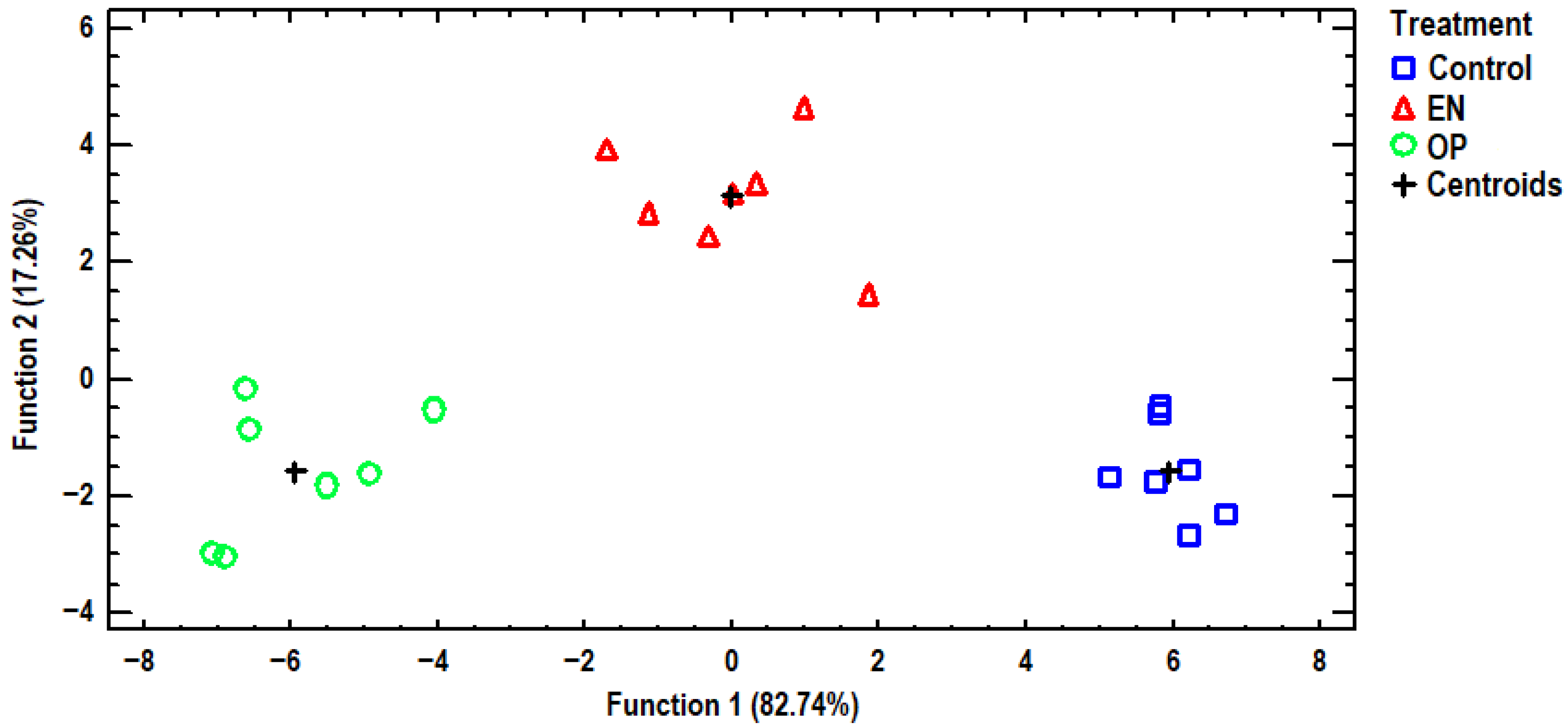
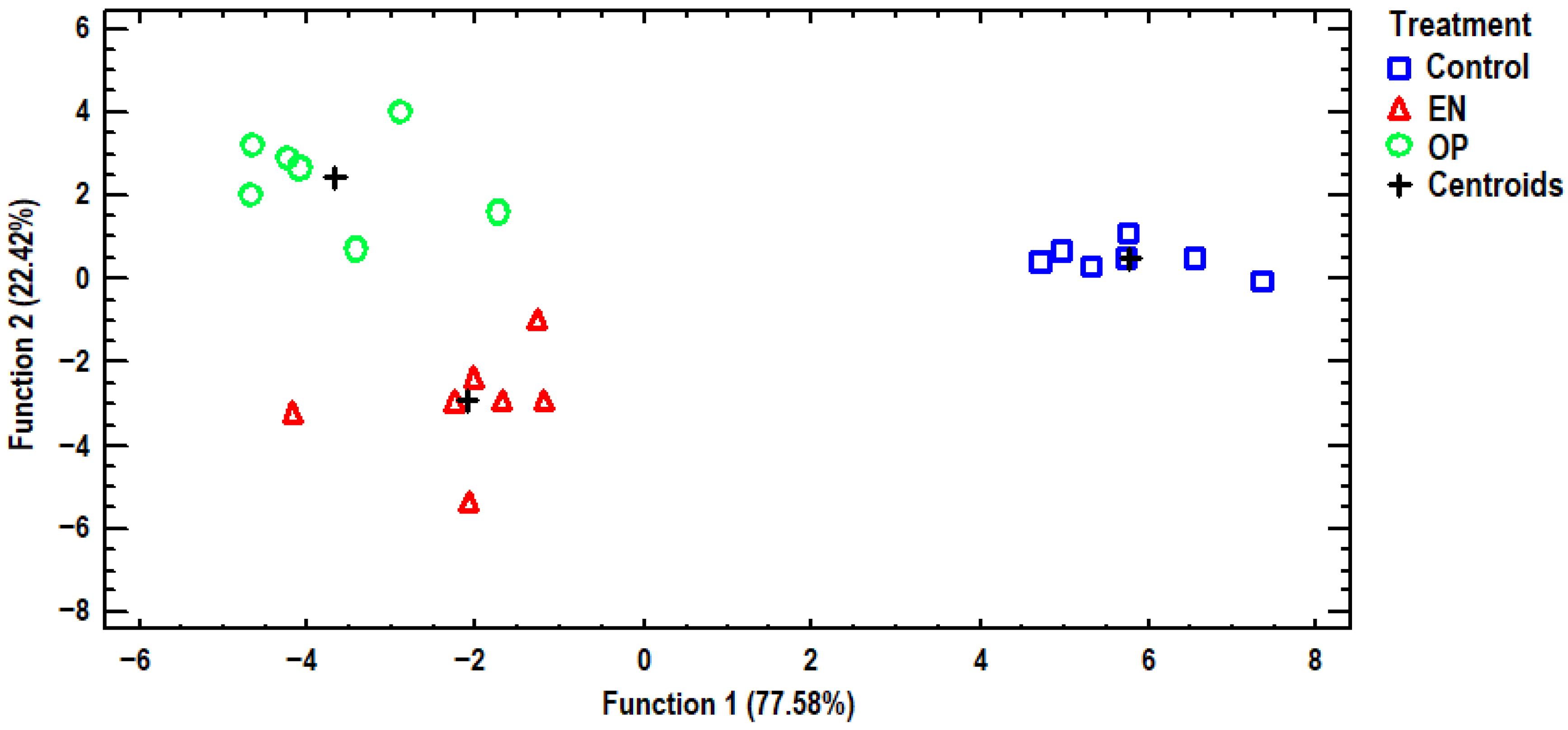
3.3. Discriminant Analysis
4. Discussion
4.1. Antioxidant Substances in Animals’ Nutrition
4.2. “Metallophenolomics” and Alteration of Egg Metallome
5. Conclusions
Author Contributions
Funding

Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| C | control group; |
| EN | hesperidin plus naringin group fed with 0.767 g hesperidin plus 0.002 g naringin per kg feed; |
| OP | orange pulp group fed with 90 g dried orange pulp per kg feed; |
| ICP-MS | inductively coupled plasma-mass spectrometry. |
References
- Silver, S. BioMetals: A historical and personal perspective. Biometals 2011, 24, 379–390. [Google Scholar] [CrossRef]
- Mounicou, S.; Szpunar, J.; Lobinski, R. Metallomics: The concept and methodology. Chem. Soc. Rev. 2009, 38, 1119–1138. [Google Scholar] [CrossRef]
- Wishart, D. Metabolomics: Applications to food science and nutrition research. Trends Food Sci. Technol. 2008, 19, 482–493. [Google Scholar] [CrossRef]
- Wu, W.; Zhang, L.; Zheng, X.; Huang, Q.; Farag, M.A.; Zhu, R.; Zhao, C. Emerging applications of metabolomics in food science and future trends. Food Chem. X 2022, 16, 100500. [Google Scholar] [CrossRef]
- Alagawany, M.; Farag, M.R.; Dhama, K.; Patra, A. Nutritional significance and health benefits of designer eggs. World’s Poult. Sci. J. 2018, 74, 317–330. [Google Scholar] [CrossRef]
- Réhault-Godbert, S.; Guyot, N.; Nys, Y. The Golden Egg: Nutritional Value, Bioactivities, and Emerging Benefits for Human Health. Nutrients 2019, 11, 684. [Google Scholar] [CrossRef]
- Pappas, A.C.; Zoidis, E.; Goliomytis, M.; Simitzis, P.E.; Sotirakoglou, K.; Charismiadou, M.A.; Nikitas, C.; Danezis, G.; Deligeorgis, S.G.; Georgiou, C.A. Elemental Metabolomics: Modulation of Egg Metallome with Flavonoids, an Exploratory Study. Antioxidants 2019, 8, 361. [Google Scholar] [CrossRef]
- Zoidis, E.; Pappas, A.C.; Goliomytis, M.; Simitzis, P.E.; Sotirakoglou, K.; Tavrizelou, S.; Danezis, G.; Georgiou, C.A. Quercetin and Egg Metallome. Antioxidants 2021, 10, 80. [Google Scholar] [CrossRef] [PubMed]
- Filipiak-Florkiewicz, A.; Dymińska-Czyż, M.; Szymczyk, B.; Franczyk-Żarów, M.; Kostogrys, R.; Florkiewicz, A.; Lukasiewicz, M. Design of Physicochemical Properties of Eggs as a Result of Modification of the Fat Fraction of Laying Feed. Molecules 2024, 29, 1242. [Google Scholar] [CrossRef] [PubMed]
- Usturoi, M.G.; Rațu, R.N.; Crivei, I.C.; Veleșcu, I.D.; Usturoi, A.; Stoica, F.; Radu Rusu, R.-M. Unlocking the Power of Eggs: Nutritional Insights, Bioactive Compounds, and the Advantages of Omega-3 and Omega-6 Enriched Varieties. Agriculture 2025, 15, 242. [Google Scholar] [CrossRef]
- Roy, A.; Khan, A.; Ahmad, I.; Alghamdi, S.; Rajab, B.S.; Babalghith, A.O.; Alshahrani, M.Y.; Islam, S.; Islam, M.R. Flavonoids a Bioactive Compound from Medicinal Plants and Its Therapeutic Applications. BioMed Res. Int. 2022, 2022, 5445291. [Google Scholar] [CrossRef]
- Hilal, B.; Khan, M.M.; Fariduddin, Q. Recent advancements in deciphering the therapeutic properties of plant secondary metabolites: Phenolics, terpenes, and alkaloids. Plant Physiol. Biochem. 2024, 211, 108674. [Google Scholar] [CrossRef]
- Bolat, E.; Sarıtaş, S.; Duman, H.; Eker, F.; Akdaşçi, E.; Karav, S.; Witkowska, A.M. Polyphenols: Secondary Metabolites with a Biological Impression. Nutrients 2024, 16, 2550. [Google Scholar] [CrossRef]
- Vasta, V.; Luciano, G. The effects of dietary consumption of plants secondary compounds on small ruminants’ products quality. Small Rumin. Res. 2011, 101, 150–159. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur. J. Med. Chem. 2015, 97, 55–74. [Google Scholar] [CrossRef]
- Embuscado, M.E. Spices and herbs: Natural sources of antioxidants—A mini review. J. Funct. Foods 2015, 18, 811–819. [Google Scholar] [CrossRef]
- Eliopoulos, C.; Markou, G.; Langousi, I.; Arapoglou, D. Reintegration of Food Industry By-Products: Potential Applications. Foods 2022, 11, 3743. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, H.; Seidavi, A.; Liu, W.; Asadpour, L. Investigation on the effect of different levels of dried sweet orange (Citrus sinensis) pulp on performance, carcass characteristics and physiological and biochemical parameters in broiler chicken. Saudi J. Biol. Sci. 2015, 22, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.R.; Zhang, H.J.; Wang, J.; Wu, S.G.; Yue, H.Y.; Lü, H.Y.; Cui, H.; Bontempo, V.; Qi, G.H. Effect of dried tangerine peel extract supplementation on the growth performance and antioxidant status of broiler chicks. Ital. J. Anim. Sci. 2016, 15, 642–648. [Google Scholar] [CrossRef]
- Mourão, J.L.; Pinheiro, V.M.; Prates, J.A.M.; Bessa, R.J.B.; Ferreira, L.M.A.; Fontes, C.M.G.A.; Ponte, P.I.P. Effect of dietary dehydrated pasture and citrus pulp on the performance and meat quality of broiler chickens. Poult. Sci. 2008, 87, 733–743. [Google Scholar] [CrossRef]
- Mahfuz, S.; Shang, Q.; Piao, X. Phenolic compounds as natural feed additives in poultry and swine diets: A review. J. Anim. Sci. Biotechnol. 2021, 12, 48. [Google Scholar] [CrossRef]
- Pitino, R.; De Marchi, M.; Manuelian, C.L.; Johnson, M.; Simoni, M.; Righi, F.; Tsiplakou, E. Plant Feed Additives as Natural Alternatives to the Use of Synthetic Antioxidant Vitamins on Yield, Quality, and Oxidative Status of Poultry Products: A Review of the Literature of the Last 20 Years. Antioxidants 2021, 10, 757. [Google Scholar] [CrossRef]
- Li, Y.; An, M.; Wan, S.; Li, Y.; Du, Y.; Zhao, Y.; Li, H.; Zhong, Q.; Sun, Z. Hesperidin enhances broiler growth performance by augmenting gastric acid secretion via the proton pump pathway. Poult. Sci. 2025, 104, 104781. [Google Scholar] [CrossRef]
- Kamboh, A.A.; Zhu, W.Y. Individual and combined effects of genistein and hesperidin supplementation on meat quality in meat-type broiler chickens. J. Sci. Food Agric. 2013, 93, 3362–3367. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Hou, Y.; Chen, J.; Wu, H.; Huang, L.; Hu, J.; Zhang, Z.; Lu, Y.; Liu, X. Dietary naringin supplementation on laying performance and antioxidant capacity of Three-Yellow breeder hens during the late laying period. Poult. Sci. 2022, 101, 102023. [Google Scholar] [CrossRef] [PubMed]
- Goliomytis, M.; Kostaki, A.; Avgoulas, G.; Lantzouraki, D.Z.; Siapi, E.; Zoumpoulakis, P.; Simitzis, P.; Deligeorgis, S.G. Dietary supplementation with orange pulp (Citrus sinensis) improves egg yolk oxidative stability in laying hens. Anim. Feed. Sci. Technol. 2018, 244, 28–35. [Google Scholar] [CrossRef]
- Simitzis, P.E.; Goliomytis, M.; Papalexi, K.; Veneti, N.; Charismiadou, M.A.; Kominakis, A.; Deligeorgis, S.G. Effects of flavonoids dietary supplementation on egg yolk antioxidant capacity and cholesterol level. In Proceedings of the 65th Annual Meeting of the European Association for Animal Production, Copenhagen, Denmark, 25–29 August 2014; p. 109. [Google Scholar]
- Baira, E.; Dagla, I.; Siapi, E.; Zoumpoulakis, P.; Simitzis, P.; Goliomytis, M.; Deligeorgis, S.G.; Skaltsounis, A.L.; Gikas, E. UHPLC–HRMS-based tissue untargeted metabolomics study of naringin and hesperidin after dietary supplementation in chickens. Food Chem. 2018, 269, 276–285. [Google Scholar] [CrossRef]
- Baira, E.; Dagla, I.; Siapi, E.; Zoumpoulakis, P.; Tsarbopoulos, A.; Simitzis, P.; Goliomytis, M.; Deligeorgis, S.G.; Skaltsounis, A.L.; Gikas, E. Development of a Validated UHPLC-ESI(-)-HRMS Methodology for the Simultaneous Quantitative Determination of Hesperidin, Hesperetin, Naringin, and Naringenin in Chicken Plasma. Food Anal. Methods 2019, 12, 1187–1196. [Google Scholar] [CrossRef]
- Baira, E.; Dagla, I.; Siapi, E.; Zoumpoulakis, P.; Tsarbopoulos, A.; Simitzis, P.; Goliomytis, M.; Deligeorgis, S.G.; Skaltsounis, A.L.; Gikas, E. Development and Validation of a UPLC-ESI(-)-MS/MS Methodology for the Simultaneous Quantification of Hesperidin, Naringin, and their Aglycones in Chicken Tissue Samples. J. AOAC Int. 2020, 103, 83–88. [Google Scholar] [CrossRef]
- Lohmann Breeders, G.H. Lohmann Brown-Classic Layers Management Guide, Cage Housing. Available online: https://lohmann-breeders.com/files/downloads/MG/Cage/LB_MG_Cage_LB-Classic_EN.pdf (accessed on 28 August 2025).
- Hussein, E.; Alhotan, R.A.; Ebrahim, A.; Selim, S. Unraveling the Potential of Orange Pulp for Improving Laying Rate, Egg Quality, Oxidative Stability, Fatty Acids Composition, and Reproductive Tract Morphology of Laying Hens. Animals 2023, 13, 2199. [Google Scholar] [CrossRef] [PubMed]
- Abbate, J.M.; Macrì, F.; Capparucci, F.; Iaria, C.; Briguglio, G.; Cicero, L.; Salvo, A.; Arfuso, F.; Ieni, A.; Piccione, G.; et al. Administration of Protein Hydrolysates from Anchovy (Engraulis encrasicolus) Waste for Twelve Weeks Decreases Metabolic Dysfunction-Associated Fatty Liver Disease Severity in ApoE−/−Mice. Animals 2020, 10, 2303. [Google Scholar] [CrossRef]
- Barbosa, C.H.; Andrade, M.A.; Séndon, R.; Silva, A.S.; Ramos, F.; Vilarinho, F.; Khwaldia, K.; Barbosa-Pereira, L. Industrial fruits by-products and their antioxidant profile: Can they be exploited for industrial food applications? Foods 2021, 10, 272. [Google Scholar] [CrossRef]
- Chen, J.; Xia, P. Health effects of synthetic additives and the substitution potential of plant-based additives. Food Res. Int. 2024, 197, 115177. [Google Scholar] [CrossRef]
- Zuñiga-Martínez, B.S.; Domínguez-Avila, J.A.; Robles-Sánchez, R.M.; Ayala-Zavala, J.F.; Villegas-Ochoa, M.A.; González-Aguilar, G.A. Agro-Industrial Fruit Byproducts as Health-Promoting Ingredients Used to Supplement Baked Food Products. Foods 2022, 11, 3181. [Google Scholar] [CrossRef] [PubMed]
- Cuchillo-Hilario, M.; Fournier-Ramírez, M.-I.; Díaz Martínez, M.; Montaño Benavides, S.; Calvo-Carrillo, M.-C.; Carrillo Domínguez, S.; Carranco-Jáuregui, M.-E.; Hernández-Rodríguez, E.; Mora-Pérez, P.; Cruz-Martínez, Y.R.; et al. Animal Food Products to Support Human Nutrition and to Boost Human Health: The Potential of Feedstuffs Resources and Their Metabolites as Health-Promoters. Metabolites 2024, 14, 496. [Google Scholar] [CrossRef]
- Siwach, A.; Saini, S.; Giri, A.; Khatri, P.; Kuhad, R.C.; Kumar, A. Sustainable poultry feed formulations from fruit and vegetable residues for advancing animal health. Bioresour. Technol. Rep. 2025, 30, 102159. [Google Scholar] [CrossRef]
- Chen, X.M.; Tait, A.R.; Kitts, D.D. Flavonoid composition of orange peel and its association with antioxidant and anti-inflammatory activities. Food Chem. 2017, 218, 15–21. [Google Scholar] [CrossRef]
- Vlaicu, P.A.; Untea, A.E.; Panaite, T.D.; Turcu, R.P. Effect of dietary orange and grapefruit peel on growth performance, health status, meat quality and intestinal microflora of broiler chickens. Italalian J. Anim. Sci. 2020, 19, 1394–1405. [Google Scholar] [CrossRef]
- Zoidis, E.; Simitzis, P.; Kampantais, D.; Katsoulas, P.; Pappas, A.C.; Papadomichelakis, G.; Goliomytis, M. Dietary orange pulp and organic selenium effects on growth performance, meat quality, fatty acid profile, and oxidative stability parameters of broiler chickens. Sustainability 2022, 14, 1534. [Google Scholar] [CrossRef]
- Nazok, A.; Rezaei, M.; Sayyahzadeh, H. Effect of different levels of dried citrus pulp on performance, egg quality, and blood parameters of laying hens in early phase of production. Trop. Anim. Health Prod. 2010, 42, 737–742. [Google Scholar] [CrossRef] [PubMed]
- Ferrali, M.; Signorini, C.; Caciotti, B.; Sugherini, L.; Ciccoli, L.; Giachetti, D.; Comporti, M. Protection against oxidative damage of erythrocyte membrane by the flavonoid quercetin and its relation to iron chelating activity. FEBS Lett. 1997, 416, 123–129. [Google Scholar] [CrossRef]
- Kuşi, M.; Becer, E.; Vatansever, H.S. Basic approach on the protective effects of hesperidin and naringin in Alzheimer’s disease. Nutr. Neurosci. 2024, 28, 550–562. [Google Scholar] [CrossRef]
- Siddique, S.; Firdous, S.; Durrani, A.I.; Khan, S.J.; Saeed, A. Hesperidin, a citrus flavonoid, increases the bioavailability of micronutrients of Gallus domesticus (chicken) eggshell: In Vitro study. Chem. Speciat. Bioavailab. 2016, 28, 88–94. [Google Scholar] [CrossRef]
- Goff, J.P. Mineral absorption mechanisms, mineral interactions that affect acid–base and antioxidant status, and diet considerations to improve mineral status. J. Dairy Sci. 2018, 101, 2763–2813. [Google Scholar] [CrossRef]
- Li, J.; Lu, H.; Liu, J.; Hong, H.; Yan, C. The influence of flavonoid amendment on the absorption of cadmium in Avicennia marina roots. Ecotoxicol. Environ. Saf. 2015, 120, 1–6. [Google Scholar] [CrossRef]
- Xiao, L.; Luo, G.; Tang, Y.; Yao, P. Quercetin and iron metabolism: What we know and what we need to know. Food Chem. Toxicol. 2018, 114, 190–203. [Google Scholar] [CrossRef] [PubMed]
- Jabeen, E.; Janjua, N.K.; Ahmed, S.; Murtaza, I.; Ali, T.; Hameed, S. Radical scavenging propensity of Cu2+, Fe3+ complexes of flavonoids and in-vivo radical scavenging by Fe3+-primuletin. Spectrochim. Acta Part A 2017, 171, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Jaccob, A.A.; Hussain, S.A.; Hussain, S.A. Effects of long-term use of flavonoids on the absorption and tissue distribution of orally administered doses of trace elements in rats. Pharmacol. Pharm. 2012, 3, 474–480. [Google Scholar] [CrossRef]
- Surai, P.F. Polyphenol compounds in the chicken/animal diet: From the past to the future. J. Anim. Physiol. Animal Nutr. 2014, 98, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Fedenko, V.S.; Landi, M.; Shemet, S.A. Metallophenolomics: A Novel Integrated Approach to Study Complexation of Plant Phenolics with Metal/Metalloid Ions. Int. J. Mol. Sci. 2022, 23, 11370. [Google Scholar] [CrossRef]
| Treatment | |||||
|---|---|---|---|---|---|
| Elements (μg/kg) | C | EN | OP | SEM | p-Value |
| As | 5.04 | 4.79 | 4.64 | 0.147 | 0.178 |
| Ca | 6.78 × 104 | 7.32 × 104 | 4.99 × 104 | 918 × 10 | 0.199 |
| Cd | 4.21 | 3.21 | 4.06 | 0.801 | 0.644 |
| Co | 2.49 b | 1.89 a | 1.77 a | 0.158 | 0.011 |
| Cr | 95.0 b | 85.1 ab | 80.9 a | 3.90 | 0.049 |
| Cu | 733 b | 168 × 10 b | 353 a | 317 | 0.001 |
| Fe | 584 × 10 b | 525 × 10 ab | 499 × 10 a | 206 | 0.025 |
| Mg | 1.10 × 105 | 1.10 × 105 | 9.53 × 104 | 831 × 10 | 0.369 |
| Mn | 65.9 | 59.3 | 54.7 | 6.53 | 0.492 |
| Ni | 67.2 b | 38.1 a | 44.8 ab | 8.68 | 0.012 |
| Sb | 1.63 a | 2.61 b | 1.49 a | 0.229 | 0.005 |
| Se | 240 × 10 b | 354 × 10 b | 891 a | 774 | 0.014 |
| Sr | 158 | 142 | 150 | 11.5 | 0.628 |
| V | 64.9 | 66.8 | 66.2 | 1.08 | 0.463 |
| Zn | 113 × 10 | 130 × 10 | 107 × 10 | 92.6 | 0.225 |
| Treatment | |||||
|---|---|---|---|---|---|
| Elements (μg/kg) | C | EN | OP | SEM | p-Value |
| As | 4.24 b | 3.60 a | 3.94 ab | 0.199 | 0.048 |
| Ca | 1.07 × 106 | 1.05 × 106 | 9.59 × 105 | 4.70 × 104 | 0.227 |
| Cd | 4.86 | 4.43 | 4.51 | 0.242 | 0.434 |
| Co | 3.96 b | 3.43 a | 3.21 a | 0.118 | <0.001 |
| Cr | 145 | 141 | 151 | 3.22 | 0.138 |
| Cu | 173 × 10 | 168 × 10 | 160 × 10 | 94.1 | 0.639 |
| Fe | 737 × 102 b | 676 × 102 b | 594 × 102 a | 212 × 10 | <0.001 |
| Mg | 1.39 × 105 | 1.41 × 105 | 1.51 × 105 | 541 × 10 | 0.299 |
| Mn | 125 × 10 b | 115 × 10 b | 748 a | 78.2 | <0.001 |
| Ni | 74.9 b | 67.5 ab | 65.8 a | 2.85 | 0.046 |
| Sb | 1.41 | 1.43 | 1.20 | 0.128 | 0.387 |
| Se | 1286 b | 637 a | 536 a | 201.6 | 0.035 |
| Sr | 699 | 684 | 637 | 39.8 | 0.531 |
| V | 80.7 a | 84.7 ab | 90.3 b | 1.89 | 0.008 |
| Zn | 432 × 102 | 420 × 102 | 443 × 102 | 154 × 10 | 0.594 |
| Treatment | |||||
|---|---|---|---|---|---|
| Elements (μg/kg) | C | EN | OP | SEM | p-Value |
| As | 82.5 b | 40.8 ab | 23.1 a | 17.1 | 0.008 |
| Ca | 2.49 × 108 b | 2.37 × 108 ab | 2.31 × 108 a | 5.58 × 106 | 0.049 |
| Cd | 342 b | 115 ab | 68.5 a | 88.2 | 0.041 |
| Co | 361 | 283 | 273 | 33.7 | 0.160 |
| Cr | 840 b | 719 a | 744 a | 22.5 | 0.003 |
| Cu | 640 × 10 b | 169 × 10 a | 149 × 10 a | 165 × 10 | 0.043 |
| Fe | 1.96 × 106 | 1.86 × 106 | 1.85 × 106 | 5.45 × 104 | 0.307 |
| Mg | 3.56 × 106 b | 3.06 × 106 a | 3.43 × 106 ab | 1.32 × 105 | 0.038 |
| Mn | 939 | 600 | 535 | 137 | 0.245 |
| Ni | 913 × 10 | 879 × 10 | 928 × 10 | 359 | 0.618 |
| Sb | 67.0 | 48.4 | 64.4 | 7.71 | 0.211 |
| Se | 358 × 10 b | 362 a | 192 × 10 ab | 899 | 0.002 |
| Sr | 1.07 × 105 ab | 9.77 × 104 a | 1.14 × 105 b | 406 × 10 | 0.031 |
| V | 107 × 10 b | 794 a | 702 a | 74.8 | 0.007 |
| Zn | 11 8× 102 | 107 × 102 | 99 × 102 | 109 × 10 | 0.491 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zoidis, E.; Pappas, A.C.; Goliomytis, M.; Simitzis, P.E.; Sotirakoglou, K.; Tavrizelou, S.; Danezis, G.P.; Georgiou, C.A. Modulation of Egg Elemental Metabolomics by Dietary Supplementation with Flavonoids and Orange Pulp (Citrus sinensis). Antioxidants 2025, 14, 1179. https://doi.org/10.3390/antiox14101179
Zoidis E, Pappas AC, Goliomytis M, Simitzis PE, Sotirakoglou K, Tavrizelou S, Danezis GP, Georgiou CA. Modulation of Egg Elemental Metabolomics by Dietary Supplementation with Flavonoids and Orange Pulp (Citrus sinensis). Antioxidants. 2025; 14(10):1179. https://doi.org/10.3390/antiox14101179
Chicago/Turabian StyleZoidis, Evangelos, Athanasios C. Pappas, Michael Goliomytis, Panagiotis E. Simitzis, Kyriaki Sotirakoglou, Savvina Tavrizelou, George P. Danezis, and Constantinos A. Georgiou. 2025. "Modulation of Egg Elemental Metabolomics by Dietary Supplementation with Flavonoids and Orange Pulp (Citrus sinensis)" Antioxidants 14, no. 10: 1179. https://doi.org/10.3390/antiox14101179
APA StyleZoidis, E., Pappas, A. C., Goliomytis, M., Simitzis, P. E., Sotirakoglou, K., Tavrizelou, S., Danezis, G. P., & Georgiou, C. A. (2025). Modulation of Egg Elemental Metabolomics by Dietary Supplementation with Flavonoids and Orange Pulp (Citrus sinensis). Antioxidants, 14(10), 1179. https://doi.org/10.3390/antiox14101179









