Antioxidant, Anti-Melanogenic, and Anti-Aging Activities of the Aqueous–Ethanolic Dry Extract of Rosa lucieae with Phytochemical Profiling
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Plant Material and Extraction
2.3. Total Polyphenol and Flavonoid Content
2.4. Liquid Chromatography with Tandem Mass Spectrometry (LC-MS/MS) Analysis of the 70% EtOH Extract from R. lucieae
2.5. Antioxidant Activities
2.6. Tyrosinase and DOPA Oxidation Inhibition
2.7. Anti-Aging Enzyme Inhibition Assays
2.8. Statistical Analysis
3. Results
3.1. Determination of Polyphenol and Flavonoid Content and UPLC–QTOF–MS Analysis of Rosa lucieae Extract
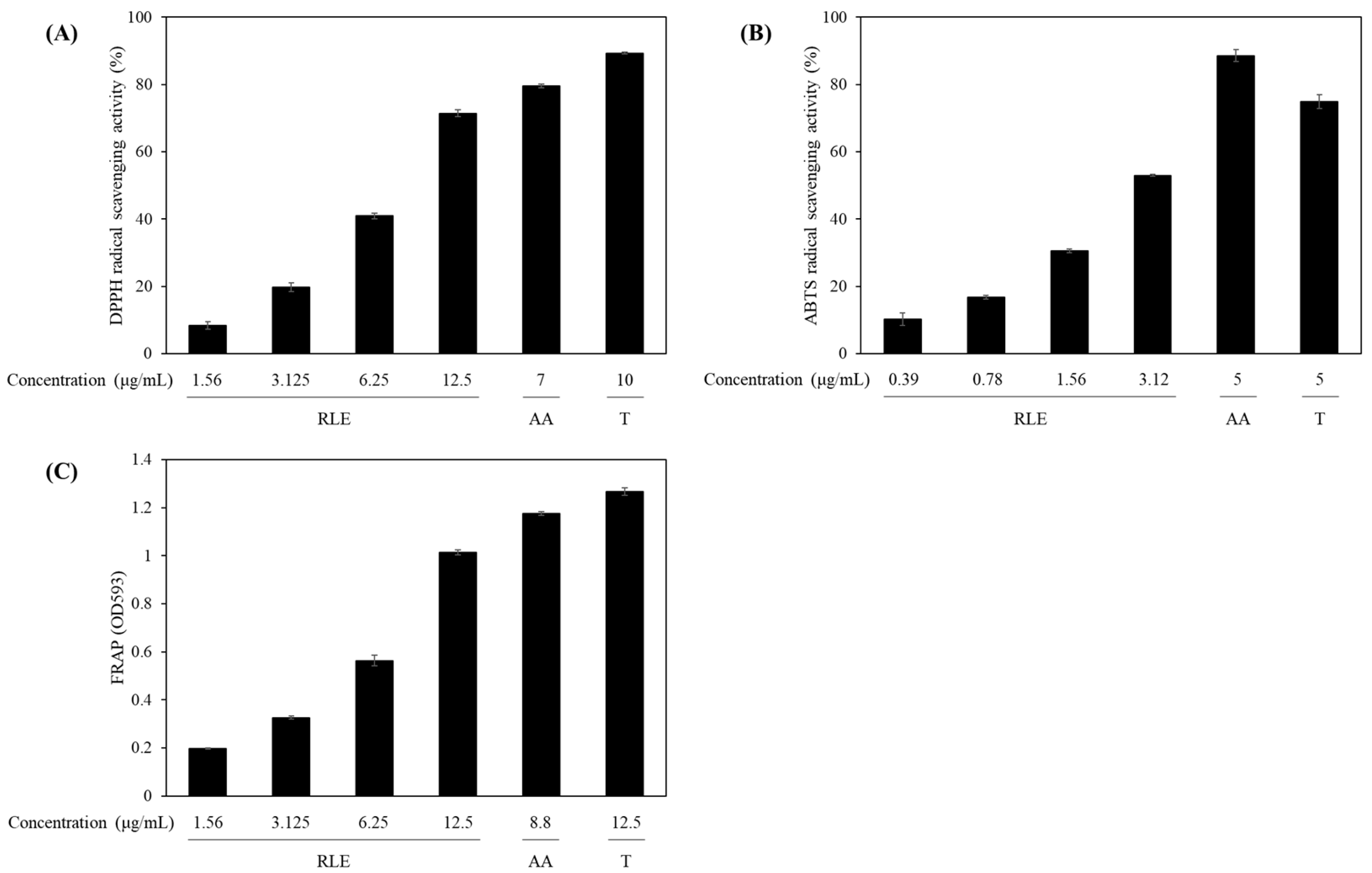
3.2. Antioxidant Properties of R. lucieae Extracts
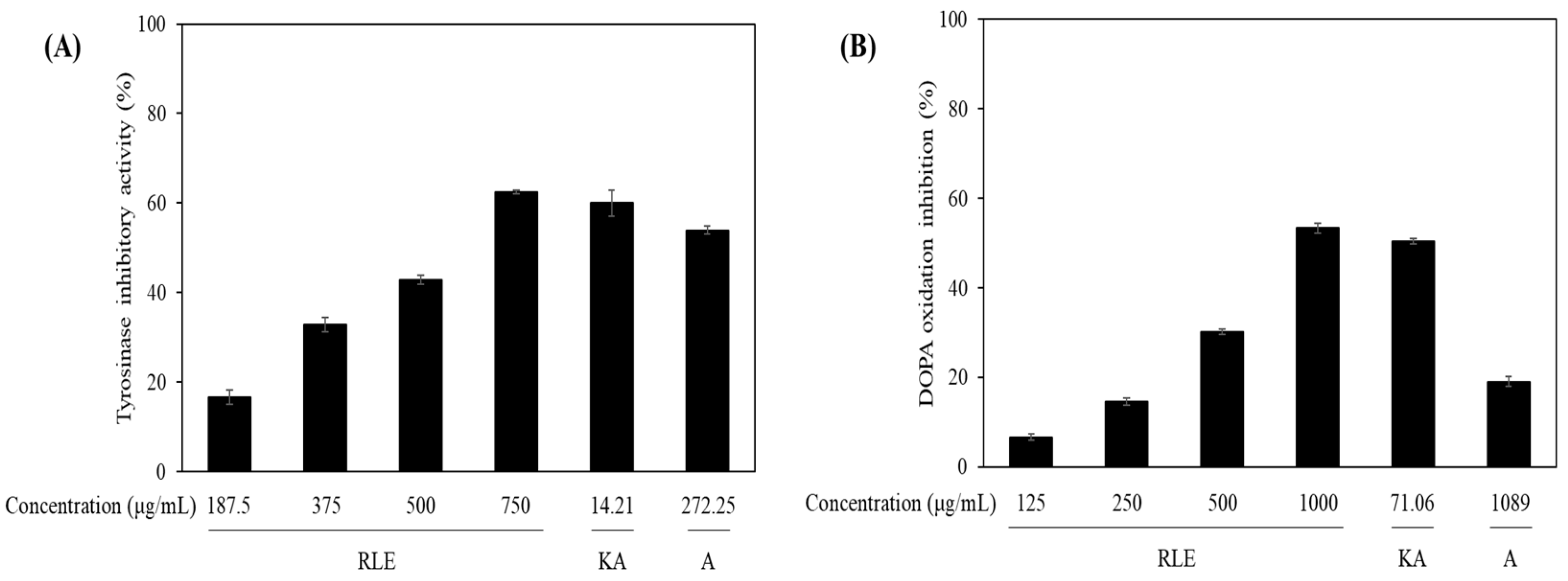
3.3. Anti-Melanogenic Activity of R. lucieae Extracts
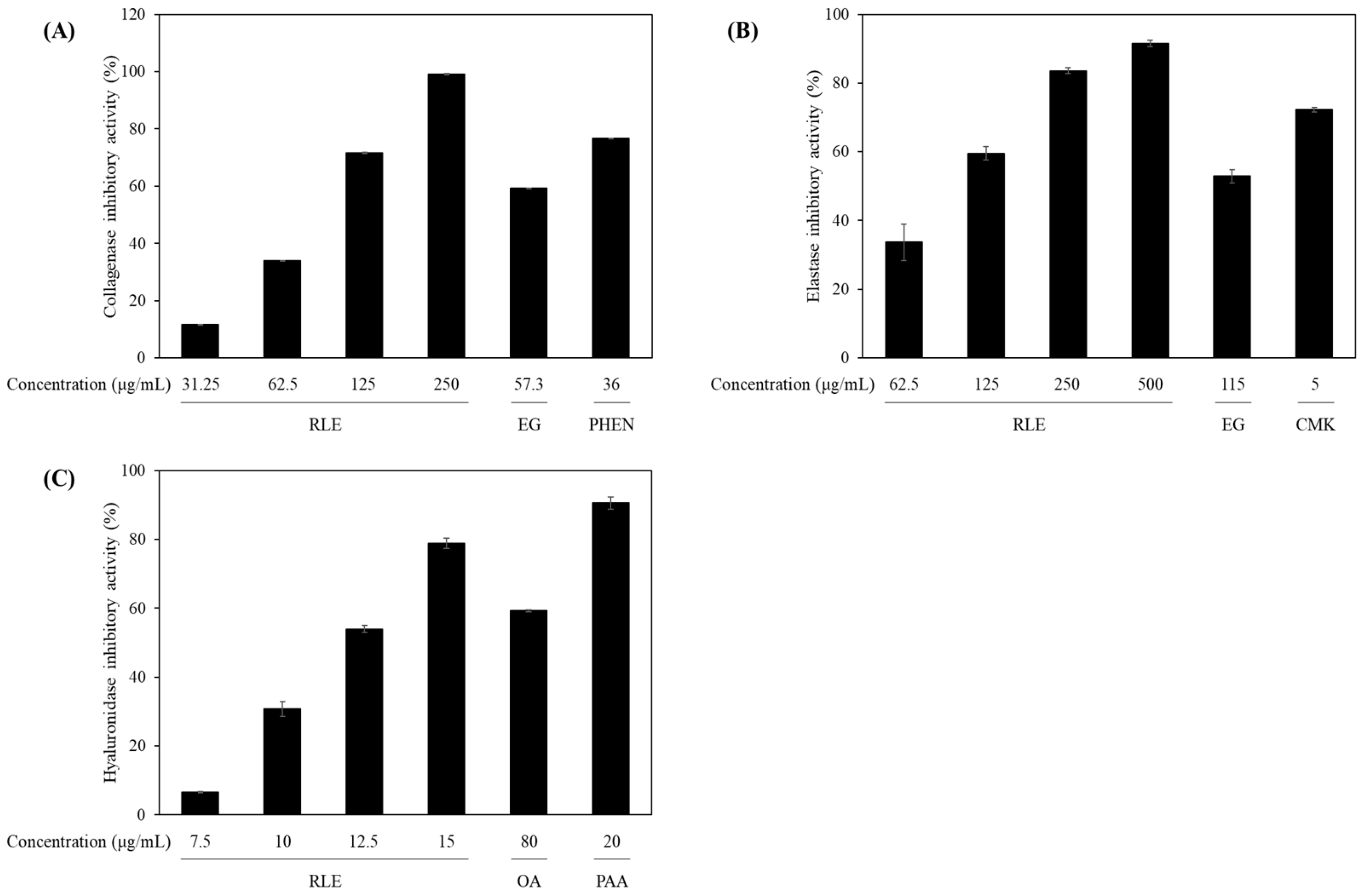
3.4. Anti-Aging Enzyme Inhibition of the R. lucieae Extract
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| UV | Ultraviolet |
| ROS | Reactive Oxygen Species |
| ECM | Extracellular Matrix |
| MMP | Metalloproteinases |
| UPL | Ultra-High-Performance Liquid Chromatography |
| Q-TOF/MS | Quadrupole Time-of-Flight Mass Spectrometry |
| AlCl3 | Aluminum Chloride |
| L-DOPA | L-3,4-Dihydroxyphenylalanine |
| EGCG | Epigallocatechin Gallate |
| ABTS | 2,2′-Azino-Bis(3-ethylbenzothiazoline-6-sulfonic acid) |
| EtOH | Ethanol |
| MeOH | Methanol |
| DMSO | Dimethyl Sulfoxide |
| DW | Distilled Water |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl |
| Na2CO3 | Sodium Carbonate |
| FRAP | Ferric Reducing Ability |
| IDA | Information Dependent Acquisition |
| SD | Standard Deviation |
| Fe3+ | Ferric |
| Fe2+ | Ferrous |
References
- Potter, D.; Eriksson, T.; Evans, R.C.; Oh, S.; Smedmark, J.E.; Morgan, D.R.; Kerr, M.; Robertson, K.R.; Arsenault, M.; Dickinson, T.A.; et al. Phylogeny and classification of Rosaceae. Plant Syst. Evol. 2007, 266, 5–43. [Google Scholar] [CrossRef]
- Shulaev, V.; Korban, S.S.; Sosinski, B.; Abbott, A.G.; Aldwinckle, H.S.; Folta, K.M.; Iezzoni, A.; Main, D.; Arús, P.; Dandekar, A.M.; et al. Multiple models for Rosaceae genomics. Plant Physiol. 2008, 147, 985–1003. [Google Scholar] [CrossRef]
- Butkevičiūtė, A.; Urbštaitė, R.; Liaudanskas, M.; Obelevičius, K.; Janulis, V. Phenolic Content and Antioxidant Activity in Fruit of the Genus Rosa L. Antioxidants 2022, 11, 912. [Google Scholar] [CrossRef]
- Oszmianski, J.; Wojdylo, A.; Lamer-Zarawska, E.; Swiader, K. Antioxidant tannins from Rosaceae plant roots. Food Chem. 2007, 100, 579–583. [Google Scholar] [CrossRef]
- Hummer, K.E.; Janick, J. Rosaceae: Taxonomy, economic importance, genomics. In Genetics and Genomics of Rosaceae; Springer: New York, NY, USA, 2009; pp. 1–17. [Google Scholar]
- Shen, W.; Dong, Z.; Zhao, W.; Ma, L.; Wang, F.; Li, W.; Xin, P. Complete chloroplast genome sequence of Rosa lucieae and its characteristics. Horticulturae 2022, 8, 788. [Google Scholar] [CrossRef]
- Serviss, B.E.; Tumlison, R. Guide to the naturalized, escaped, and adventive woody flora of Arkansas. Phytoneuron 2021, 29, 1–193. [Google Scholar]
- Jeon, J.H.; Shin, Y.; Kim, S.C. Genomic insights into the evolution and adaptation of the memorial roses (Rosa lucieae; Rosaceae) in saline environments. bioRxiv 2024. [Google Scholar] [CrossRef]
- Kouassi, M.C.; Grisel, M.; Gore, E. Multifunctional active ingredient-based delivery systems for skincare formulations: A review. Colloids Surf. B Biointerfaces 2022, 217, 112676. [Google Scholar] [CrossRef] [PubMed]
- Nisa, R.U.; Nisa, A.U.; Tantray, A.Y.; Shah, A.H.; Jan, A.T.; Shah, A.A.; Wani, I.A. Plant phenolics with promising therapeutic applications against skin disorders: A mechanistic review. J. Agric. Food Res. 2024, 16, 101090. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, C.; Li, J.; Xiong, J.; Xiao, B.L. Inhibitory mechanism on tyrosinase activity of flavonoids from flower buds of Sophora japonica L. Heliyon 2024, 10, e38252. [Google Scholar] [CrossRef]
- Chowdhury, A.; Nosoudi, N.; Karamched, S.; Parasaram, V.; Vyavahare, N. Polyphenol treatments increase elastin and collagen deposition by human dermal fibroblasts; Implications to improve skin health. J. Dermatol. Sci. 2021, 102, 94–100. [Google Scholar] [CrossRef]
- Pillai, S.; Oresajo, C.; Hayward, J. Ultraviolet radiation and skin aging: Roles of reactive oxygen species, inflammation and protease activation, and strategies for prevention of inflammation-induced matrix degradation—A review. Int. J. Cosmet. Sci. 2005, 27, 17–34. [Google Scholar] [CrossRef] [PubMed]
- Papaccio, F.; Caputo, S.; Bellei, B. Focus on the contribution of oxidative stress in skin aging. Antioxidants 2022, 11, 1121. [Google Scholar] [CrossRef] [PubMed]
- Zolghadri, S.; Bahrami, A.; Hassan Khan, M.T.; Munoz-Munoz, J.; Garcia-Molina, F.; Garcia-Canovas, F.; Akbar Saboury, A. A comprehensive review on tyrosinase inhibitors. J. Enzyme Inhib. Med. Chem. 2019, 34, 279–309. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Gao, X.; Guo, Y.; Xie, W. Research progress on bioactive factors against skin aging. Int. J. Mol. Sci. 2024, 25, 3797. [Google Scholar] [CrossRef]
- Borzabadi-Farahani, A.; Mosahebi, A.; Zargaran, D. A scoping review of hyaluronidase use in managing the complications of aesthetic interventions. Aesthet. Plast. Surg. 2024, 48, 1193–1209. [Google Scholar] [CrossRef]
- Al-Yafeai, A.; Bellstedt, P.; Böhm, V. Bioactive compounds and antioxidant capacity of Rosa rugosa depending on degree of ripeness. Antioxidants 2018, 7, 134. [Google Scholar] [CrossRef]
- Ercisli, S. Chemical composition of fruits in some rose (Rosa spp.) species. Food Chem. 2007, 104, 1379–1384. [Google Scholar] [CrossRef]
- Li, J.; Li, J.; Yang, H.; Ma, Y.; Huo, Z.; Wang, S.; Lin, Y.; Zhang, C. Enhancing Antioxidant Bioaccessibility in Rosa rugosa through Lactobacillus plantarum Fermentation. Fermentation 2024, 10, 368. [Google Scholar] [CrossRef]
- Ren, H.; Yang, W.; Jing, W.; Shahid, M.O.; Liu, Y.; Qiu, X.; Choisy, P.; Xu, T.; Ma, N.; Gao, J.; et al. Multi-omics analysis reveals key regulatory defense pathways and genes involved in salt tolerance of rose plants. Hortic. Res. 2024, 11, uhae068. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, J.; Shen, J.; Silva, A.; Dennis, D.A.; Barrow, C.J. A simple 96-well microplate method for estimation of total polyphenol content in seaweeds. J. Appl. Phycol. 2006, 18, 445–450. [Google Scholar] [CrossRef]
- Curcic, M.G.; Stankovic, M.S.; Radojevic, I.D.; Stefanovic, O.D.; Comic, L.R.; Topuzovic, M.D.; Djacic, D.S.; Markovic, S.D. Biological effects, total phenolic content and flavonoid concentrations of fragrant yellow onion (Allium flavum L.). Med. Chem. 2012, 8, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.; Veiga, F.; Cardoso, C.; Dias, F.; Cerqueira, F.; Medeiros, R.; Paiva-Santos, A.C. A rapid and simplified DPPH assay for analysis of antioxidant interactions in binary combinations. Microchem. J. 2024, 202, 110801. [Google Scholar] [CrossRef]
- Zheng, L.; Zhao, M.; Xiao, C.; Zhao, Q.; Su, G. Practical problems when using ABTS assay to assess the radical-scavenging activity of peptides: Importance of controlling reaction pH and time. Food Chem. 2016, 192, 288–294. [Google Scholar] [CrossRef]
- Kim, S.S.; Hyun, C.G.; Choi, Y.H.; Lee, N.H. Tyrosinase inhibitory activities of the compounds isolated from Neolitsea aciculata (Blume) Koidz. J. Enzyme Inhib. Med. Chem. 2013, 28, 685–689. [Google Scholar] [CrossRef] [PubMed]
- Thitilertdecha, N. Storage effect on phenolic compounds and antioxidant activity of Nephelium lappaceum L. extract. Cosmetics 2022, 9, 33. [Google Scholar] [CrossRef]
- Losada-Barreiro, S.; Bravo-Diaz, C. Free radicals and polyphenols: The redox chemistry of neurodegenerative diseases. Eur. J. Med. Chem. 2017, 133, 379–402. [Google Scholar] [CrossRef]
- Kostikova, V.A.; Zarubaev, V.V.; Esaulkova, I.L.; Sinegubova, E.O.; Kadyrova, R.A.; Shaldaeva, T.M.; Veklich, T.N.; Kuzentsov, A.A. The antiviral, antiradical, and phytochemical potential of dry extracts from Spiraea hypericifolia, S. media, and S. salicifolia (Rosaceae). S. Afr. J. Bot. 2022, 147, 215–222. [Google Scholar] [CrossRef]
- Veličković, I.; Živković, J.; Stojković, D.; Sokovic, M.D.; Marin, P.D.; Grujić, S. Evaluation of antioxidant, antimicrobial and potential food preserving properties of Rubus discolor (Rosaceae) fruit extracts. Nat. Prod. Commun. 2021, 16, 1934578X211009692. [Google Scholar] [CrossRef]
- Chansriniyom, C.; Nooin, R.; Nuengchamnong, N.; Wongwanakul, R.; Petpiroon, N.; Srinuanchai, W.; Chantarasuwan, B.; Pitchakarn, P.; Temviriyanukul, P.; Nuchuchua, O. Tandem mass spectrometry of aqueous extract from Ficus dubia sap and its cell-based assessments for use as a skin antioxidant. Sci. Rep. 2021, 11, 16899. [Google Scholar] [CrossRef]
- Ousji, O.; Sleno, L. Structural elucidation of novel stable and reactive metabolites of green tea catechins and alkyl gallates by LC-MS/MS. Antioxidants 2022, 11, 1635. [Google Scholar] [CrossRef]
- Świątek, Ł.; Sieniawska, E.; Sinan, K.I.; Maciejewska-Turska, M.; Boguszewska, A.; Polz-Dacewicz, M.; Senkardes, I.; Guler, G.O.; Sadeer, N.B.; Mahomoodally, M.F.; et al. LC-ESI-QTOF-MS/MS analysis, cytotoxic, antiviral, antioxidant, and enzyme inhibitory properties of four extracts of Geranium pyrenaicum Burm. f.: A good gift from the natural treasure. Int. J. Mol. Sci. 2021, 22, 7621. [Google Scholar] [CrossRef] [PubMed]
- Lijia, X.; Guo, J.; Chen, Q.; Baoping, J.; Zhang, W. Quantitation of phlorizin and phloretin using an ultra high performance liquid chromatography–electrospray ionization tandem mass spectrometric method. J. Chromatogr. B 2014, 960, 67–72. [Google Scholar] [CrossRef]
- Ma, F.Y.; Huang, T.C.; Nayi, P.; Chen, H.H. Combined effects of sunlight and tempering treatment on the oligomeric procyanidin formation in dried ume (Prunus mume Sieb. et Zucc.). Dry. Technol. 2022, 40, 3273–3284. [Google Scholar] [CrossRef]
- Yuan, W.; Wang, J.; An, X.; Dai, M.; Jiang, Z.; Zhang, L.; Yu, S.; Huang, X. UPLC-MS/MS method for the determination of hyperoside and application to pharmacokinetics study in rat after different administration routes. Chromatographia 2021, 84, 249–256. [Google Scholar] [CrossRef]
- Mustafa, A.M.; Abouelenein, D.; Angeloni, S.; Maggi, F.; Navarini, L.; Sagratini, G.; Santanatoglia, A.; Torregiani, E.; Vittori, S.; Caprioli, G. A new HPLC-MS/MS method for the simultaneous determination of quercetin and its derivatives in green coffee beans. Foods 2022, 11, 3033. [Google Scholar] [CrossRef]
- Zeng, X.; Su, W.; Zheng, Y.; Liu, H.; Li, P.; Zhang, W.; Liang, Y.; Bai, Y.; Peng, W.; Yao, H. Uflc-q-tof-ms/ms-based screening and identification of flavonoids and derived metabolites in human urine after oral administration of exocarpium citri grandis extract. Molecules 2018, 23, 895. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Wang, Y.; Luo, G. Determination of scutellarin in Erigeron breviscapus extract by liquid chromatography–tandem mass spectrometry. J. Chromatogr. A 2001, 919, 437–441. [Google Scholar] [CrossRef]
- Jang, G.H.; Kim, H.W.; Lee, M.K.; Jeong, S.Y.; Bak, A.R.; Lee, D.J.; Kim, J.B. Characterization and quantification of flavonoid glycosides in the Prunus genus by UPLC-DAD-QTOF/MS. Saudi J. Biol. Sci. 2018, 25, 1622–1631. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, D.; Zhou, E.; Wang, W.; Wang, H.; Li, Q. Hepatoprotective effect of tiliroside and characterization of its metabolites in human hepatocytes by ultra-high performance liquid chromatography-high resolution mass spectrometry. J. Funct. Foods 2023, 107, 105675. [Google Scholar] [CrossRef]
- Korać, R.R.; Khambholja, K.M. Potential of herbs in skin protection from ultraviolet radiation. Pharmacogn. Rev. 2011, 5, 164. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Pulido, R.; Bravo, L.; Saura-Calixto, F. Antioxidant activity of dietary polyphenols as determined by a modified ferric reducing/antioxidant power assay. J. Agric. Food Chem. 2000, 48, 3396–3402. [Google Scholar] [CrossRef] [PubMed]
- Kejík, Z.; Kaplánek, R.; Masařík, M.; Babula, P.; Matkowski, A.; Filipenský, P.; Veselá, K.; Gburek, J.; Sýkora, D.; Martásek, P.; et al. Iron complexes of flavonoids-antioxidant capacity and beyond. Int. J. Mol. Sci. 2021, 22, 646. [Google Scholar] [CrossRef]
- Masum, M.N.; Yamauchi, K.; Mitsunaga, T. Tyrosinase inhibitors from natural and synthetic sources as skin-lightening agents. Rev. Agric. Sci. 2019, 7, 41–58. [Google Scholar] [CrossRef]
- Soares, A.R.; Marchiosi, R.; Siqueira-Soares, R.d.C.; Barbosa de Lima, R.; Dantas dos Santos, W.; Ferrarese-Filho, O. The role of L-DOPA in plants. Plant Signal. Behav. 2014, 9, e28275. [Google Scholar] [CrossRef]
- Ndlovu, G.; Fouche, G.; Tselanyane, M.; Cordier, W.; Steenkamp, V. In vitro determination of the anti-aging potential of four southern African medicinal plants. BMC Complement. Altern. Med. 2013, 13, 304. [Google Scholar] [CrossRef]
- Rocchetti, G.; Zhang, L.; Bocchi, S.; Giuberti, G.; Ak, G.; Elbasan, F.; Yildiztugay, E.; Ceylan, R.; Picot-Allain, M.C.N.; Mahomoodally, M.F.; et al. The functional potential of nine Allium species related to their untargeted phytochemical characterization, antioxidant capacity and enzyme inhibitory ability. Food Chem. 2022, 368, 130782. [Google Scholar] [CrossRef]
- Jafri, S.A.A.; Khalid, Z.M.; Khan, M.Z.; Jogezai, N. Evaluation of phytochemical and antioxidant potential of various extracts from traditionally used medicinal plants of Pakistan. Open Chem. 2022, 20, 1337–1356. [Google Scholar] [CrossRef]
- Muflihah, Y.M.; Gollavelli, G.; Ling, Y.C. Correlation study of antioxidant activity with phenolic and flavonoid compounds in 12 Indonesian indigenous herbs. Antioxidants 2021, 10, 1530. [Google Scholar] [CrossRef]
- Khiya, Z.; Oualcadi, Y.; Gamar, A.; Berrekhis, F.; Zair, T.; Hilali, F.E. Correlation of total polyphenolic content with antioxidant activity of hydromethanolic extract and their fractions of the Salvia officinalis leaves from different regions of Morocco. J. Chem. 2021, 2021, 8585313. [Google Scholar] [CrossRef]
- Arora, N.; Amin, S. Analyzing Global Interest in Skin Whitening by Geographic Region. Bayl. Univ. Med. Cent. Proc. 2024, 37, 505–507. [Google Scholar] [CrossRef]
- Phasha, V.; Senabe, J.; Ndzotoyi, P.; Okole, B.; Fouche, G.; Chuturgoon, A. Review on the use of kojic acid—A skin-lightening ingredient. Cosmetics 2022, 9, 64. [Google Scholar] [CrossRef]
- Migas, P.; Krauze-Baranowska, M. The significance of arbutin and its derivatives in therapy and cosmetics. Phytochem. Lett. 2015, 13, 35–40. [Google Scholar] [CrossRef]
- Obaid, R.J.; Mughal, E.U.; Naeem, N.; Sadiq, A.; Alsantali, R.I.; Jassas, R.S.; Moussa, Z.; Ahmed, S.A. Natural and synthetic flavonoid derivatives as new potential tyrosinase inhibitors: A systematic review. RSC Adv. 2021, 11, 22159–22198. [Google Scholar] [CrossRef]
- Kamiński, K.; Kazimierczak, U.; Kolenda, T. Oxidative stress in melanogenesis and melanoma development. Contemp. Oncol. 2022, 26, 1–7. [Google Scholar] [CrossRef]
- Younis, M.M.; Ayoub, I.M.; Mostafa, N.M.; El Hassab, M.A.; Eldehna, W.M.; Al-Rashood, S.T.; Eldahshan, O.A. GC/MS profiling, anti-collagenase, anti-elastase, anti-tyrosinase and anti-hyaluronidase activities of a Stenocarpus sinuatus leaves extract. Plants 2022, 11, 918. [Google Scholar] [CrossRef] [PubMed]
- Szewczyk, K.; Pietrzak, W.; Klimek, K.; Miazga-Karska, M.; Firlej, A.; Flisiński, M.; Grzywa-Cellńska, A. Flavonoid and phenolic acids content and in vitro study of the potential anti-aging properties of Eutrema japonicum (Miq.) Koidz cultivated in Wasabi Farm Poland. Int. J. Mol. Sci. 2021, 22, 6219. [Google Scholar] [CrossRef] [PubMed]
- Bravo, B.; Correia, P.; Gonçalves Junior, J.E.; Sant’Anna, B.; Kerob, D. Benefits of topical hyaluronic acid for skin quality and signs of skin aging: From literature review to clinical evidence. Dermatol. Ther. 2022, 35, e15903. [Google Scholar] [CrossRef]
- Kim, Y.J. Antimelanogenic and antioxidant properties of gallic acid. Biol. Pharm. Bull. 2007, 30, 1052–1055. [Google Scholar] [CrossRef]
- Yang, H.L.; Lin, C.P.; Gowrisankar, Y.V.; Huang, P.J.; Chang, W.L.; Shrestha, S.; Hseu, Y.C. The anti-melanogenic effects of ellagic acid through induction of autophagy in melanocytes and suppression of UVA-activated α-MSH pathways via Nrf2 activation in keratinocytes. Biochem. Pharmacol. 2021, 185, 114454. [Google Scholar] [CrossRef]
- Sartor, L.; Pezzato, E.; Garbisa, S. (−) Epigallocatechin-3-gallate inhibits leukocyte elastase: Potential of the phyto-factor in hindering inflammation, emphysema, and invasion. J. Leukoc. Biol. 2002, 71, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.Y.; Choi, J.S.; Choi, Y.J.; Shin, S.Y.; Kang, S.W.; Han, S.J.; Kang, Y.H. (−) Epigallocatechin gallate hampers collagen destruction and collagenase activation in ultraviolet-B-irradiated human dermal fibroblasts: Involvement of mitogen-activated protein kinase. Food Chem. Toxicol. 2008, 46, 1298–1307. [Google Scholar] [CrossRef]
- Kim, Y.J.; Uyama, H.; Kobayashi, S. Inhibition effects of (+)-catechin–aldehyde polycondensates on proteinases causing proteolytic degradation of extracellular matrix. Biochem. Biophys. Res. Commun. 2004, 320, 256–261. [Google Scholar] [CrossRef]
- Lee, S.J.; Koh, J.S.; Ha, B.J.; Boo, Y.C. Quercus glauca extract and rutin inhibit the UVB-induced expression of matrix metalloproteinase-1 in human dermal fibroblasts. J. Korean Soc. Appl. Biol. Chem. 2010, 53, 677–684. [Google Scholar] [CrossRef]
- Vicentini, F.T.; He, T.; Shao, Y.; Fonseca, M.J.; Verri, W.A., Jr.; Fisher, G.J.; Xu, Y. Quercetin inhibits UV irradiation-induced inflammatory cytokine production in primary human keratinocytes by suppressing NF-κB pathway. J. Dermatol. Sci. 2011, 61, 162–168. [Google Scholar] [CrossRef]
- Shin, E.J.; Lee, J.S.; Hong, S.; Lim, T.G.; Byun, S. Quercetin directly targets JAK2 and PKCδ and prevents UV-induced photoaging in human skin. Int. J. Mol. Sci. 2019, 20, 5262. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Kum, H.; Ryu, D.; Kim, M.; Jung, E.; Park, D. Protective effects of a new phloretin derivative against UVB-induced damage in skin cell model and human volunteers. Int. J. Mol. Sci. 2014, 15, 18919–18940. [Google Scholar] [CrossRef] [PubMed]
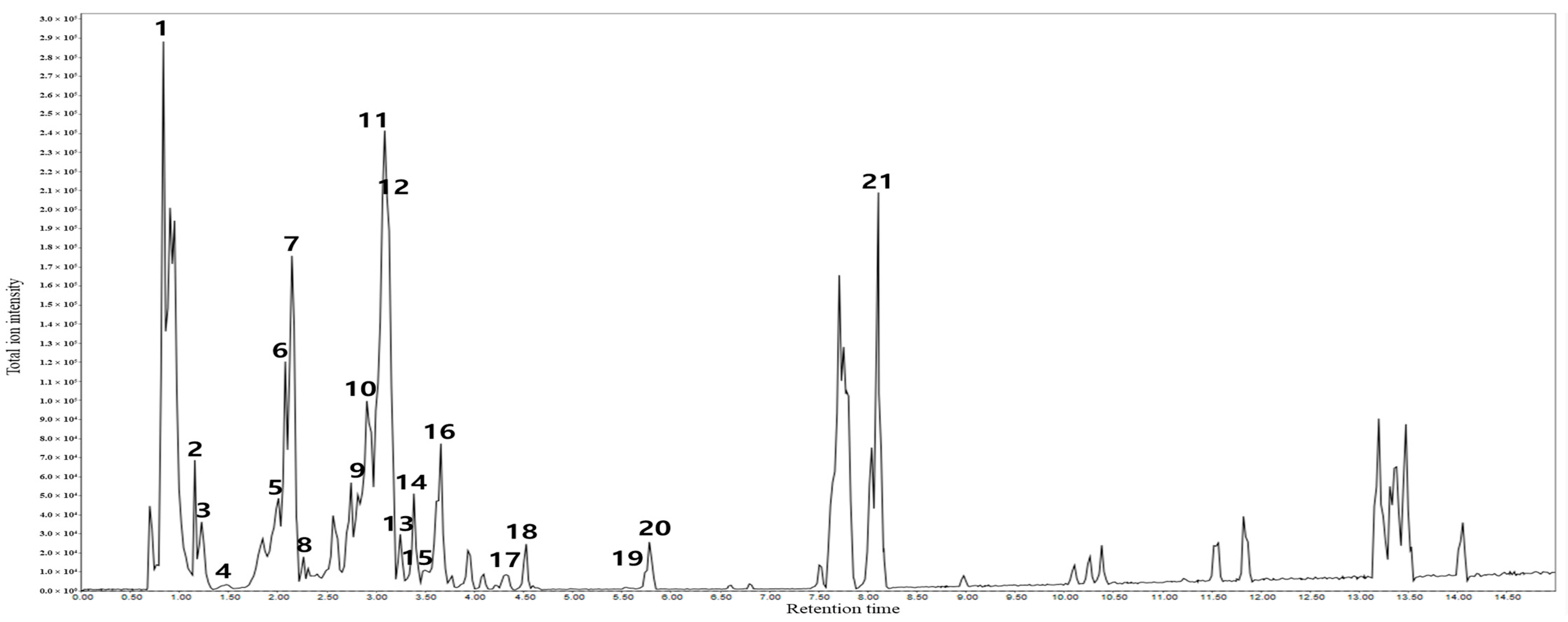
| Peaks | Retention Time | Molecular Formula | Detected Ion [M − H] | Calculated Ion [M − H] | ppm (Error) | Tentative Identification |
|---|---|---|---|---|---|---|
| 1 | 0.83 | C7H12O6 | 191.0548 | 191.0556 | −4.1 | Quinic acid |
| 2 | 1.15 | C6H8O7 | 191.0175 | 191.0192 | −8.8 | Citric acid |
| 3 | 1.22 | C7H6O5 | 169.0132 | 169.0137 | −2.9 | Gallic acid |
| 4 | 1.46 | C15H14O7 | 305.0667 | 305.0661 | 1.9 | Gallocatechin |
| 5 | 1.95 | C30H26O12 | 577.1331 | 577.1346 | −2.5 | Procyanidin B2 |
| 6 | 2.06 | C27H22O18 | 633.0727 | 633.0728 | −0.1 | Corilagin |
| 7 | 2.13 | C15H14O6 | 289.0701 | 289.0712 | −3.8 | Catechin |
| 8 | 2.39 | C45H37O18 | 865.1962 | 865.1980 | −2.0 | Procyanidin C1 |
| 9 | 2.89 | C27H30O16 | 609.1449 | 609.1456 | −1.1 | Rutin |
| 10 | 2.92 | C14H6O8 | 300.9976 | 300.9984 | −2.6 | Ellagic acid |
| 11 | 3.04 | C21H20O12 | 463.0863 | 463.0877 | −3.0 | Hyperoside |
| 12 | 3.10 | C21H18O13 | 477.0656 | 477.0669 | −2.7 | Miquelianin |
| 13 | 3.24 | C9H10O5 | 197.0450 | 197.0450 | 0 | Ethyl gallate |
| 14 | 3.40 | C23H22O13 | 505.0978 | 505.0982 | −0.7 | 6″-O-acetylisoquercitrin |
| 15 | 3.47 | C21H20O11 | 447.0904 | 447.0928 | −5.3 | Quercitrin |
| 16 | 3.65 | C21H18O12 | 461.0704 | 461.0720 | −3.4 | Scutellarin |
| 17 | 4.03 | C21H22O10 | 433.1129 | 433.1135 | −1.3 | Prunin |
| 18 | 4.53 | C21H24O10 | 435.1291 | 435.1287 | 0.9 | Phlorizin |
| 19 | 5.77 | C15H10O7 | 301.0332 | 301.0348 | −5.3 | Quercetin |
| 20 | 5.78 | C30H26O13 | 593.1284 | 593.1295 | −1.8 | Tiliroside |
| 21 | 8.10 | C36H58O10 | 695.3992 [M + FA]- | 695.4007 [M + FA]- | −2.1 | Rosamultin |
| Tyrosinase | DOPA Oxidation | Collagenase | Elastase | Hyaluronidase | |
|---|---|---|---|---|---|
| IC50 (μg/mL) | 918.02 ± 11.42 | 591.45 ± 3.46 | 80.46 ± 0.21 | 96.94 ± 11.81 | 12.03 ± 0.17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, Y.G.; Kim, J.-Y.; Ko, S.-C.; Kim, K.W.; Yang, D.; Jo, D.-M.; Lee, H.-G.; Lee, J.M.; Yim, M.-J.; Kim, C.H.; et al. Antioxidant, Anti-Melanogenic, and Anti-Aging Activities of the Aqueous–Ethanolic Dry Extract of Rosa lucieae with Phytochemical Profiling. Antioxidants 2025, 14, 1177. https://doi.org/10.3390/antiox14101177
Park YG, Kim J-Y, Ko S-C, Kim KW, Yang D, Jo D-M, Lee H-G, Lee JM, Yim M-J, Kim CH, et al. Antioxidant, Anti-Melanogenic, and Anti-Aging Activities of the Aqueous–Ethanolic Dry Extract of Rosa lucieae with Phytochemical Profiling. Antioxidants. 2025; 14(10):1177. https://doi.org/10.3390/antiox14101177
Chicago/Turabian StylePark, Yun Gyeong, Ji-Yul Kim, Seok-Chun Ko, Kyung Woo Kim, Dongwoo Yang, Du-Min Jo, Hyo-Geun Lee, Jeong Min Lee, Mi-Jin Yim, Chul Hwan Kim, and et al. 2025. "Antioxidant, Anti-Melanogenic, and Anti-Aging Activities of the Aqueous–Ethanolic Dry Extract of Rosa lucieae with Phytochemical Profiling" Antioxidants 14, no. 10: 1177. https://doi.org/10.3390/antiox14101177
APA StylePark, Y. G., Kim, J.-Y., Ko, S.-C., Kim, K. W., Yang, D., Jo, D.-M., Lee, H.-G., Lee, J. M., Yim, M.-J., Kim, C. H., Lee, D.-S., Kim, H.-S., & Oh, G.-W. (2025). Antioxidant, Anti-Melanogenic, and Anti-Aging Activities of the Aqueous–Ethanolic Dry Extract of Rosa lucieae with Phytochemical Profiling. Antioxidants, 14(10), 1177. https://doi.org/10.3390/antiox14101177






