Pequi (Caryocar brasiliense, Camb) Bark Extract Reduces ROS Production in Diabetic Human Coronary Endothelial Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Pequi Extract Preparation
2.2. Cell Culture Procedure
2.3. Experimental Groups and Proliferation Assay in D-HCAECs
2.4. Stress Induction in D-HCAECs
2.5. Determination of Cytosolic ROS Production in D-HCAECs
2.6. Mitochondrial ROS Measurement in D-HCAECs
2.7. Western Blotting
2.8. Statistical Analysis
3. Results
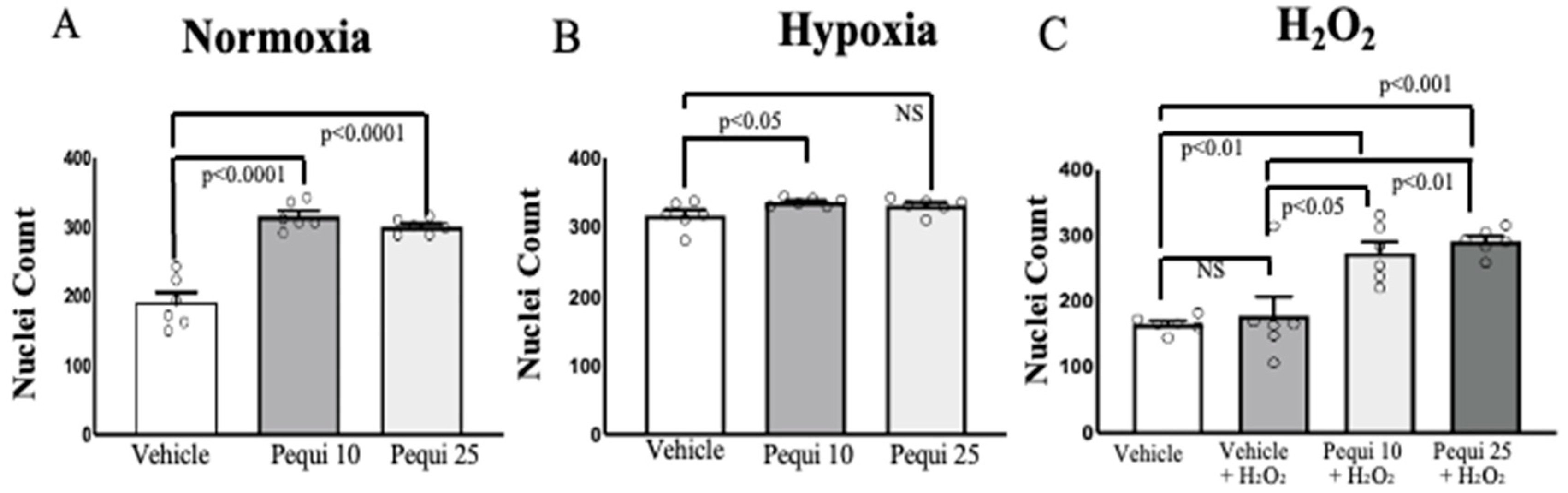
3.1. Pequi Extract Enhances Proliferation in D-HCAECs Under Stress Conditions
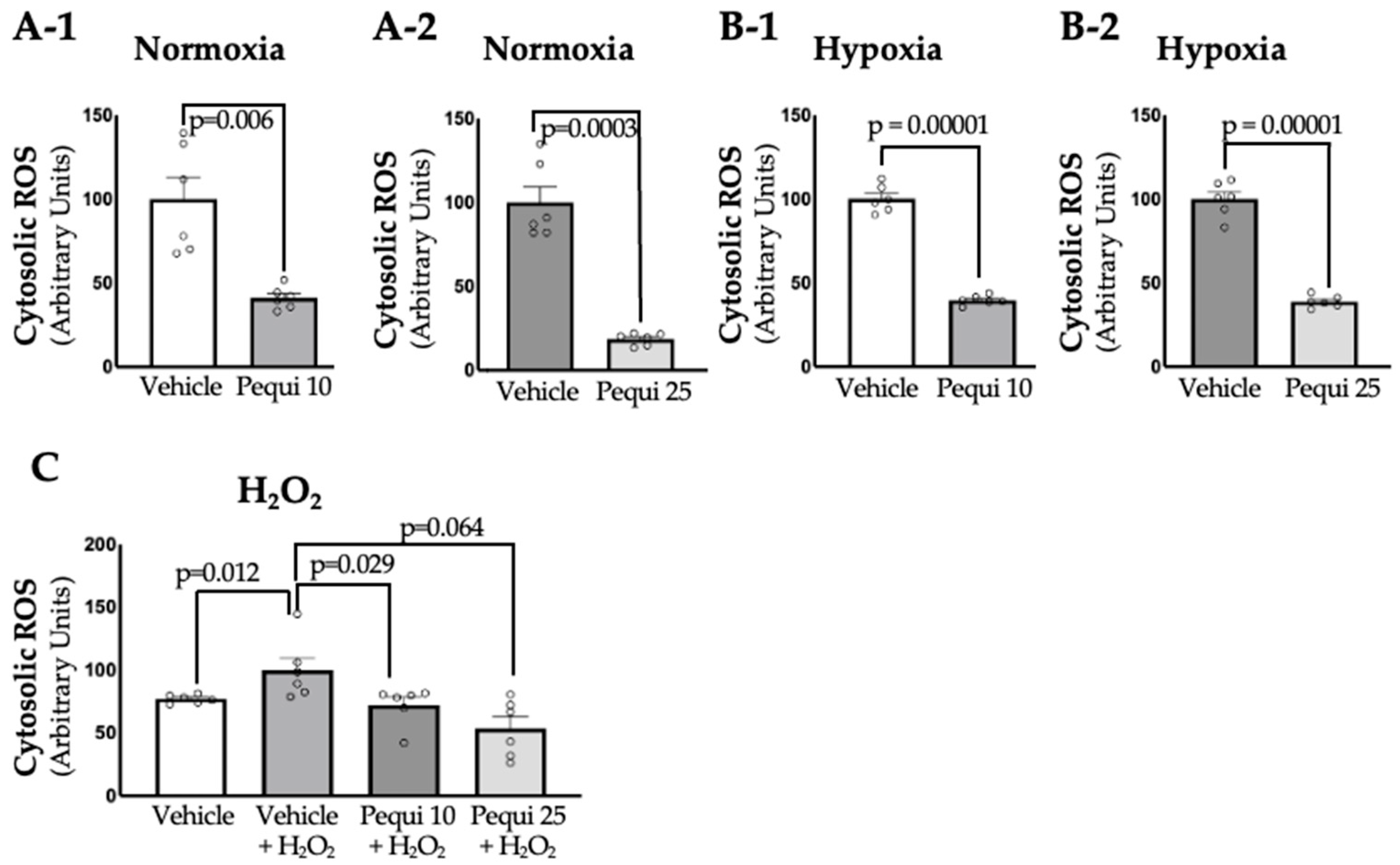
3.2. Treatment with Pequi Extract Reduces Cytosolic ROS Levels in D-HCAECs
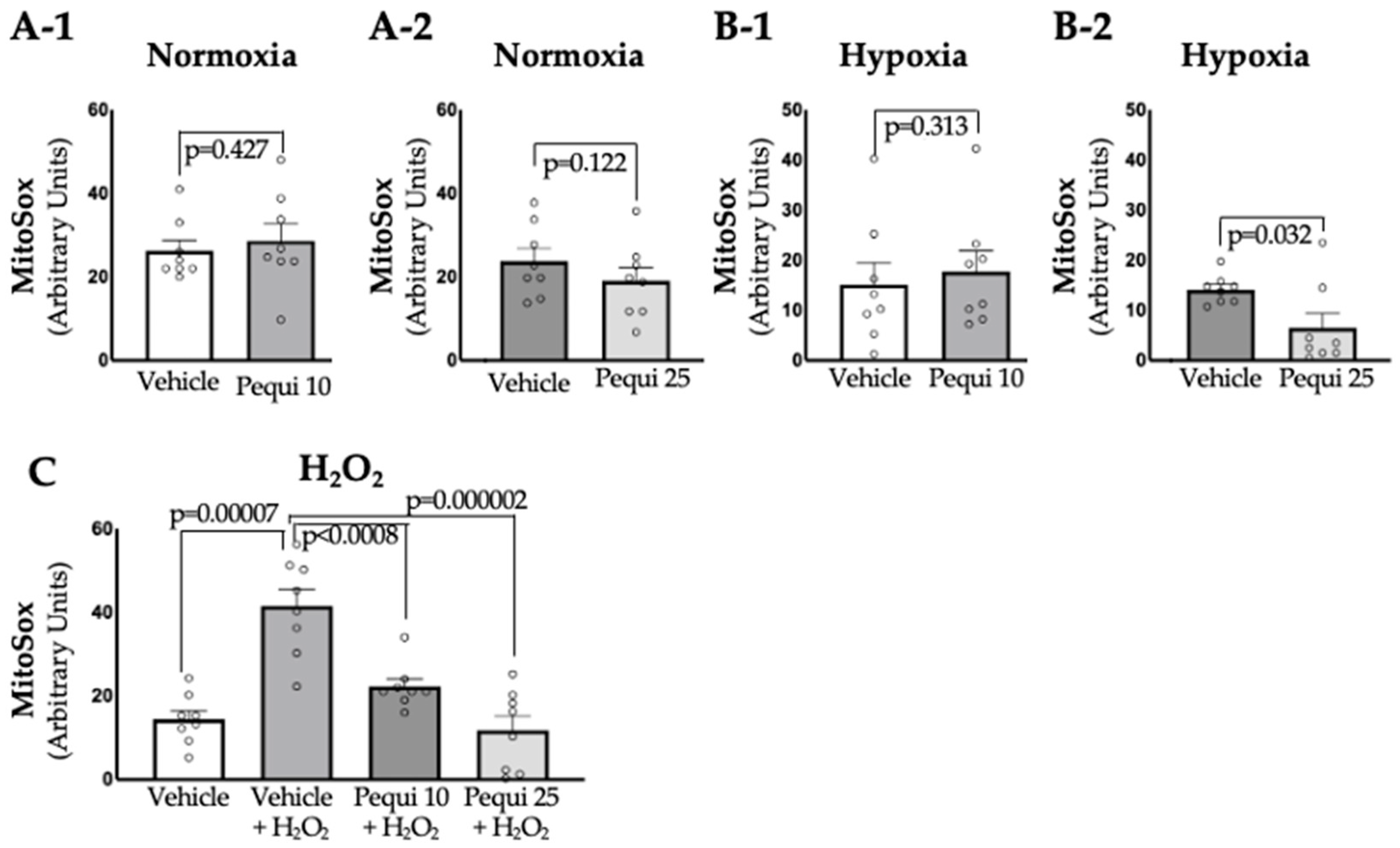
3.3. Pequi Extract Also Reduces Mitochondrial ROS Levels in D-HCAECs
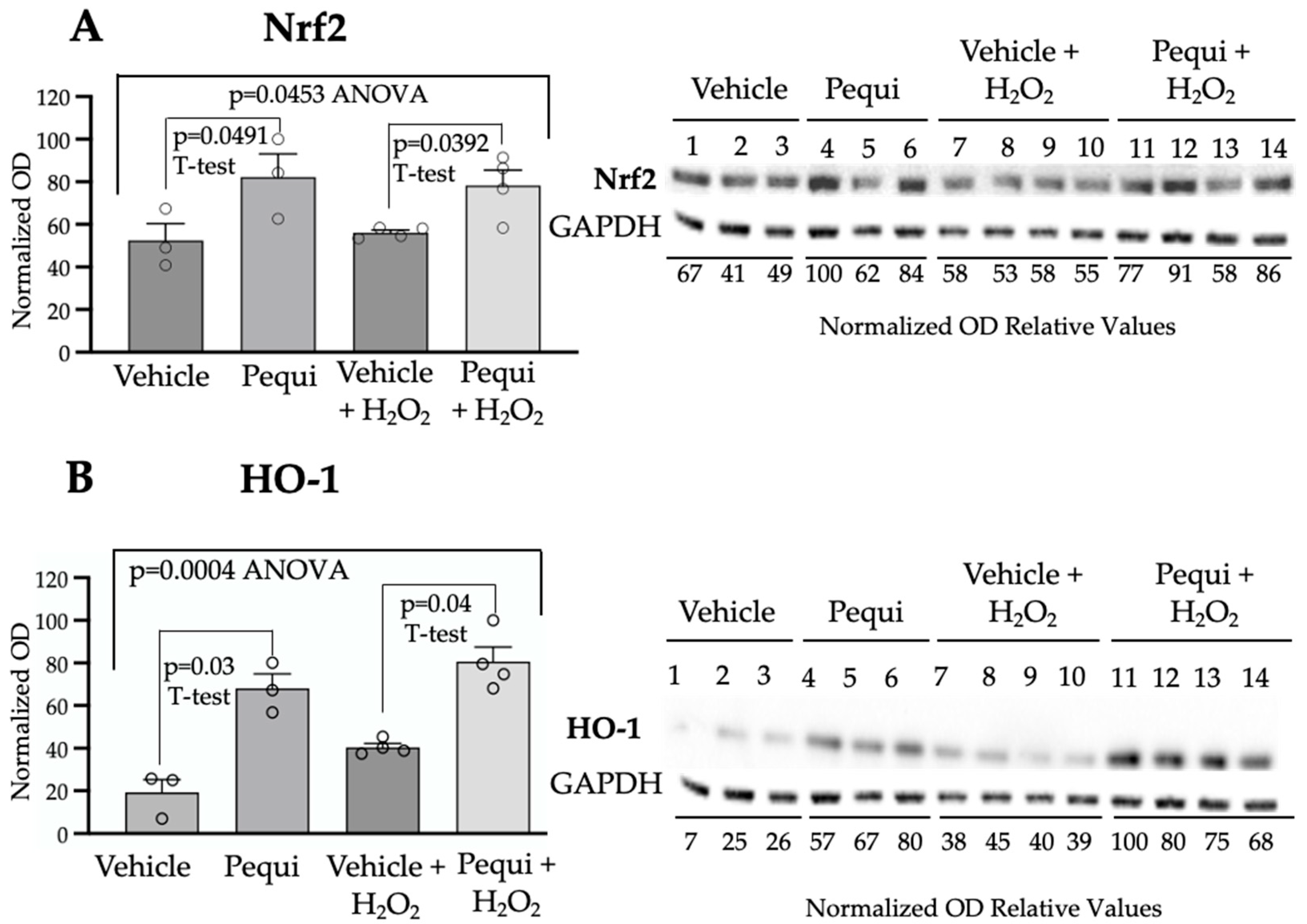
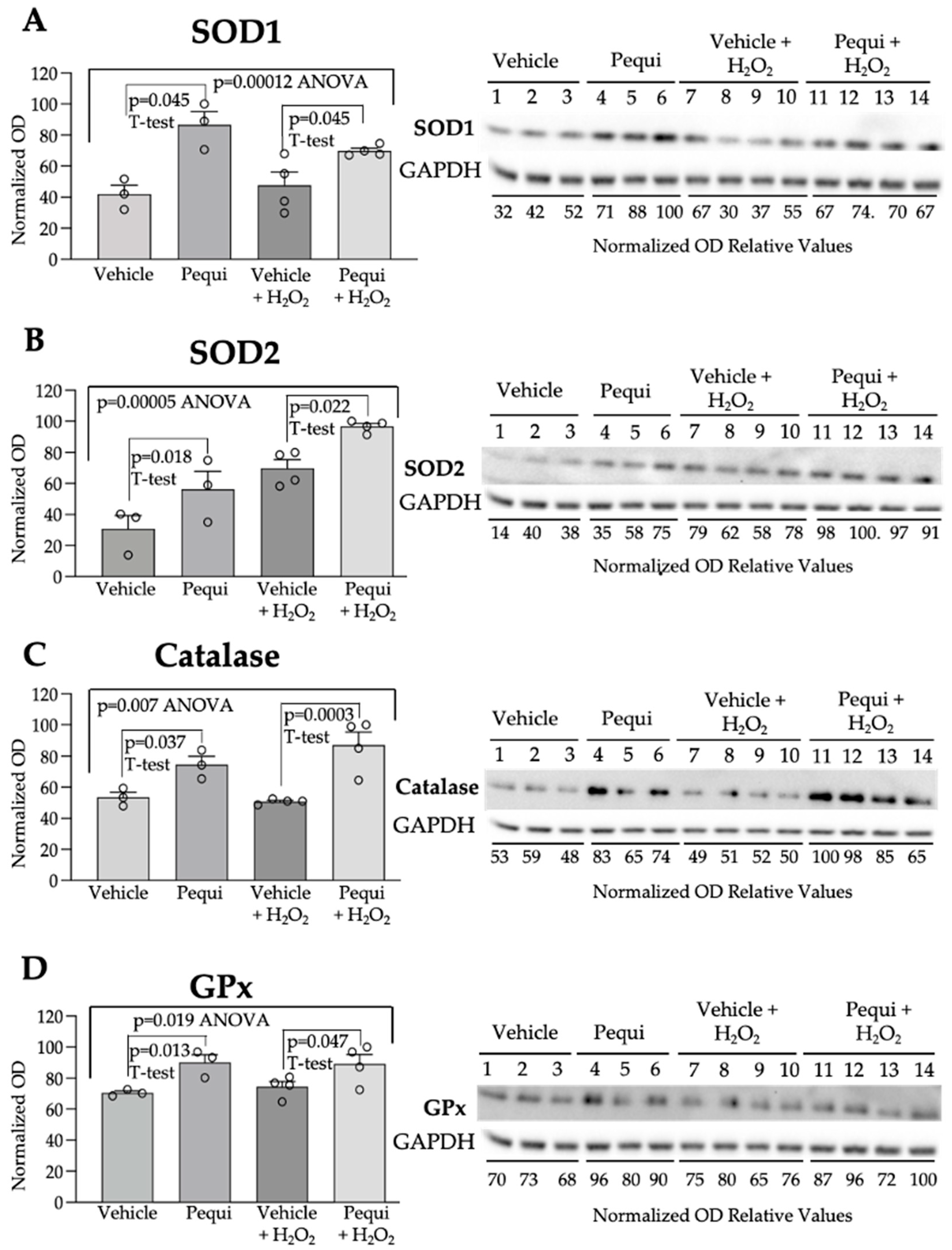
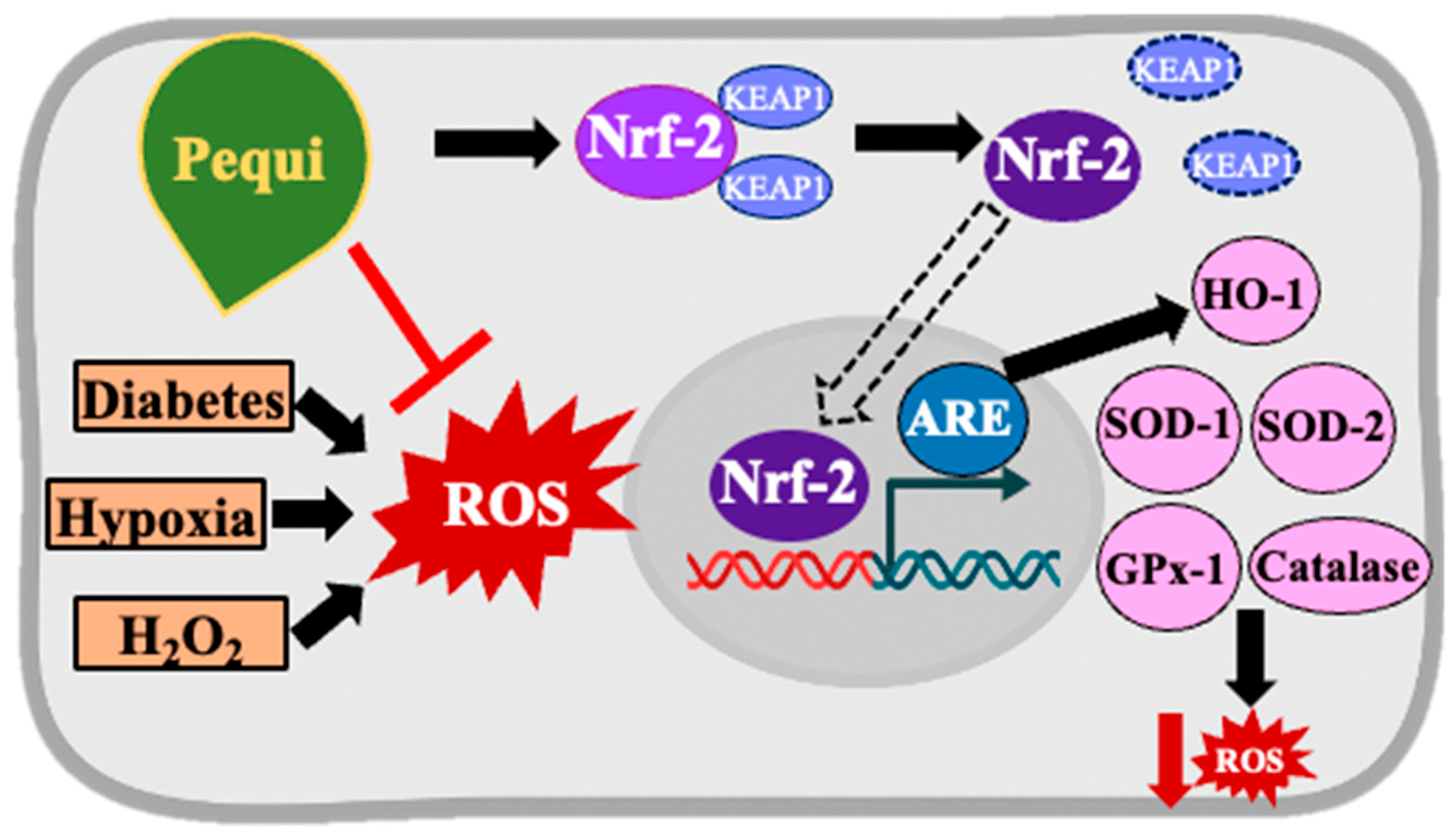
3.4. Pequi Extract-Induced Increased Expression of Antioxidant Enzymes Is Associated with the Nrf2 Pathway in D-HCAECs
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ROS | Reactive oxygen species |
| DM | Diabetes mellitus |
| T2DM | Type 2 diabetes mellitus |
| EC/ECs | Endothelial cell(s) |
| HCAEC | Human coronary artery endothelial cell |
| D-HCAEC | Diabetic human coronary artery endothelial cell |
| mito-ROS | Mitochondrial reactive oxygen species |
| Nrf2 | Nuclear factor (erythroid-derived 2)-like 2 |
| HO-1 | Heme-oxygenase-1 |
| SOD1 | Superoxide dismutase 1 |
| SOD2 | Superoxide dismutase 2 |
| GPx1/GPx | Glutathione peroxidase 1 |
| CAT | Catalase |
| DAPI | 4′,6-Diamidino-2-Phenylindole (nuclear stain) |
| DCF | 2′,7′-Dichlorofluorescein |
| H2DCF-DA | 2,7-Dichlorodihydrofluorescein diacetate |
| DMSO | Dimethyl sulfoxide |
| HBSS | Hank’s Balanced Salt Solution |
| MIC | Modular Incubator Chamber |
| WB | Western blot |
| ANOVA | Analysis of variance |
| ARE | Antioxidant response element |
| KEAP1 | Kelch-like ECH-associated protein 1 |
| HPLC-HRMS | High-Pressure Liquid Chromatography–High-Resolution Mass Spectrometry |
| LV | Left ventricle |
| ATP | Adenosine Triphosphate |
| HUVECs | Human umbilical vein endothelial cells |
| NIH | National Institutes of Health |
References
- Sabe, S.A.; Feng, J.; Sellke, F.W.; Abid, M.R. Mechanisms and Clinical Implications of Endothelium-Dependent Vasomotor Dysfunction in Coronary Microvasculature. Am. J. Physiol. Heart Circ. Physiol. 2022, 322, H819–H841. [Google Scholar] [CrossRef]
- Hayden, M.R. Endothelial Activation and Dysfunction in Metabolic Syndrome, Type 2 Diabetes and Coronavirus Disease 2019. J. Int. Med. Res. 2020, 48, 7. [Google Scholar] [CrossRef]
- Centers for Disease Control Type 2 Diabetes. 2024. Available online: https://www.cdc.gov/diabetes/about/about-type-2-diabetes.html (accessed on 19 August 2025).
- Daryabor, G.; Atashzar, M.R.; Kabelitz, D.; Meri, S.; Kalantar, K. The Effects of Type 2 Diabetes Mellitus on Organ Metabolism and the Immune System. Front. Immunol. 2020, 11, 1582. [Google Scholar] [CrossRef]
- Schindler, T.H.; Dilsizian, V. Coronary Microvascular Dysfunction. JACC Cardiovasc. Imaging 2020, 13, 140–155. [Google Scholar] [CrossRef]
- Severino, P.; D’Amato, A.; Netti, L.; Pucci, M.; Infusino, F.; Maestrini, V.; Mancone, M.; Fedele, F. Myocardial Ischemia and Diabetes Mellitus: Role of Oxidative Stress in the Connection between Cardiac Metabolism and Coronary Blood Flow. J. Diabetes Res. 2019, 21, 9489826. [Google Scholar] [CrossRef]
- Suganya, N.; Bhakkiyalakshmi, E.; Sarada, D.V.L.; Ramkumar, K.M. Reversibility of Endothelial Dysfunction in Diabetes: Role of Polyphenols. Br. J. Nutr. 2016, 116, 223–246. [Google Scholar] [CrossRef] [PubMed]
- Mirabelli, M.; Chiefari, E.; Arcidiacono, B.; Corigliano, D.M.; Brunetti, F.S.; Maggisano, V.; Russo, D.; Foti, D.P.; Brunetti, A. Mediterranean Diet Nutrients to Turn the Tide against Insulin Resistance and Related Diseases. Nutrients 2020, 12, 1066. [Google Scholar] [CrossRef] [PubMed]
- Den Hartogh, D.J.; Gabriel, A.; Tsiani, E. Antidiabetic Properties of Curcumin II: Evidence from in Vivo Studies. Nutrients 2020, 12, 118. [Google Scholar] [CrossRef]
- Roesler, R.; Catharino, R.R.; Malta, L.G.; Eberlin, M.N.; Pastore, G. Antioxidant Activity of Caryocar Brasiliense (Pequi) and Characterization of Components by Electrospray Ionization Mass Spectrometry. Food Chem. 2008, 110, 711–717. [Google Scholar] [CrossRef]
- Roesler, R.; Lorencini, M.; Pastore, G. Brazilian Cerrado Antioxidant Sources: Cytotoxicity and Phototoxicity in Vitro. Ciência E Tecnol. Aliment. 2010, 30, 814–821. [Google Scholar] [CrossRef]
- Souza, M.R.; de Carvalho, R.K.; de Carvalho, L.S.; de Sá, S.; Andersen, M.L.; de Araújo, E.G.; Mazaro-Costa, R. Effects of Subchronic Exposure to Caryocar Brasiliense Peel Ethanolic Extract on Male Reproductive Functions in Swiss Mice. Reprod. Toxicol. 2019, 87, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Grosso, G. Effects of Polyphenol-Rich Foods on Human Health. Nutrients 2018, 10, 1089. [Google Scholar] [CrossRef]
- Boodhwani, M.; Nakai, Y.; Mieno, S.; Voisine, P.; Bianchi, C.; Araujo, E.G.; Feng, J.; Michael, K.; Li, J.; Sellke, F.W. Hypercholesterolemia Impairs the Myocardial Angiogenic Response in a Swine Model of Chronic Ischemia: Role of Endostatin and Oxidative Stress. Ann. Thorac. Surg. 2006, 81, 634–641. [Google Scholar] [CrossRef]
- Shafique, E.; Choy, W.C.; Liu, Y.; Feng, J.; Cordeiro, B.; Lyra, A.; Arafah, M.; Yassin-Kassab, A.; Zanetti, A.V.D.; Clements, R.T.; et al. Oxidative Stress Improves Coronary Endothelial Function through Activation of the Pro-Survival Kinase AMPK. Aging 2013, 5, 515–530. [Google Scholar] [CrossRef]
- Shafique, E. Redox plays a critical role in the paradoxical effects of N. oxidase-derived R. on coronary endothelium; Torina, A.; Reichert, K.; Colantuono, B.; Nur, N.; Zeeshan, K.; Ravichandran, V.; Liu, Y.; Feng, J.; Zeeshan, K.; et al. Mitochondrial Redox Plays a Critical Role in the Paradoxical Effects of NADPH Oxidase-Derived ROS on Coronary Endothelium. Cardiovasc. Res. 2017, 113, 234–246. [Google Scholar] [CrossRef]
- Teixeira, R.B.; Albro, J.H.; Sabra, M.; Abedin, T.; Tucker, A.N.; Sidharth, R.; Sellke, F.W.; Wipf, P.; Abid, M.R. Mitochondria-Targeted ROS Scavenger JP4-039 Improves Cardiac Function in a Post-Myocardial Infarction Animal Model and Induces Angiogenesis in Vitro. PLoS ONE 2025, 20, e0320703. [Google Scholar] [CrossRef]
- Braga, K.M.S.; Araujo, E.G.; Sellke, F.W.; Abid, M.R. Pequi Fruit Extract Increases Antioxidant Enzymes and Reduces Oxidants in Human Coronary Artery Endothelial Cells. Antioxidants 2022, 11, 474. [Google Scholar] [CrossRef]
- Frey, R.S.; Ushio–Fukai, M.; Malik, A.B. NADPH Oxidase-Dependent Signaling in Endothelial Cells: Role in Physiology and Pathophysiology. Antioxid. Redox Signal 2009, 11, 791–810. [Google Scholar] [CrossRef]
- Frank, W.; Sellke, M.R.A. Antioxidant Therapy: Is It Your Gateway to Improved Cardiovascular Health? Pharm. Anal. Acta 2014, 6, 323. [Google Scholar] [CrossRef]
- Xu, C.M.; Karbasiafshar, C.; Brinck-Teixeira, R.; Broadwin, M.; Sellke, F.W.; Abid, M.R. Diabetic State of Human Coronary Artery Endothelial Cells Results in Altered Effects of Bone Mesenchymal Stem Cell-Derived Extracellular Vesicles. Physiol. Rep. 2023, 11, e15866. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Li, J.; Yuan, Z. Effect of Antioxidant Vitamin Supplementation on Cardiovascular Outcomes: A Meta-Analysis of Randomized Controlled Trials. PLoS ONE 2013, 8, e56803. [Google Scholar] [CrossRef]
- Cebova, M.; Pechanova, O. Protective Effects of Polyphenols against Ischemia/Reperfusion Injury. Molecules 2020, 25, 3469. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Shen, Y.; Kim, I.M.; Weintraub, N.L.; Tang, Y. The Impaired Bioenergetics of Diabetic Cardiac Microvascular Endothelial Cells. Front. Endocrinol. 2021, 12, 1. [Google Scholar] [CrossRef]
- Guo, Z.; Wan, X.; Luo, Y.; Liang, F.; Jiang, S.; Yuan, X.; Mo, Z. The Vicious Circle of UHRF1 Down-Regulation and KEAP1/NRF2/HO-1 Pathway Impairment Promotes Oxidative Stress-Induced Endothelial Cell Apoptosis in Diabetes. Diabet. Med. 2022, 40, e15026. [Google Scholar] [CrossRef] [PubMed]
- Raish, M.; Ahmad, A.; Bin Jardan, Y.A.; Shahid, M.; Alkharfy, K.M.; Ahad, A.; Ansari, M.A.; Abdelrahman, I.A.; Al-Jenoobi, F.I. Sinapic Acid Ameliorates Cardiac Dysfunction and Cardiomyopathy by Modulating NF-ΚB and Nrf2/HO-1 Signaling Pathways in Streptozocin-Induced Diabetic Rats. Biomed. Pharmacother. 2022, 145, 112412. [Google Scholar] [CrossRef]
- Xu, G.; Zhao, X.; Fu, J.; Wang, X. Resveratrol Increase Myocardial Nrf2 Expression in Type 2 Diabetic Rats and Alleviate Myocardial Ischemia/Reperfusion Injury (MIRI). Ann. Palliat. Med. 2019, 8, 565–575. [Google Scholar] [CrossRef]
- Yao, H.; Sun, J.; Wei, J.; Zhang, X.; Chen, B.; Lin, Y. Kaempferol Protects Blood Vessels from Damage Induced by Oxidative Stress and Inflammation in Association with the Nrf2/HO-1 Signaling Pathway. Front. Pharmacol. 2020, 11, 1118. [Google Scholar] [CrossRef]
- Alshehri, A.S.; El-Kott, A.F.; Eleawa, S.M.; El-Gerbed, M.S.A.; Khalifa, H.S.; El-Kenawy, A.E.; Albadrani, G.M.; Abdel-Daim, M.M. Kaempferol Protects against Streptozotocin-Induced Diabetic Cardiomyopathy in Rats by a Hypoglycemic Effect and Upregulating Sirt1. J. Physiol. Pharmacol. 2021, 72, 339–355. [Google Scholar] [CrossRef]
- De Bock, K.; Georgiadou, M.; Schoors, S.; Kuchnio, A.; Wong, B.W.; Cantelmo, A.R.; Quaegebeur, A.; Ghesquière, B.; Cauwenberghs, S.; Eelen, G.; et al. Role of PFKFB3-Driven Glycolysis in Vessel Sprouting. Cell 2013, 154, 651–663. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhao, X.; Zheng, H.; Cai, F. GW28-e0635 Quercetin Retards Progression of Diabetic Cardiomyopathy through Modulations of SIRT1 and AMP-Activated Protein Kinase. J. Am. Coll. Cardiol. 2017, 70 (Suppl. S16), C63–C64. [Google Scholar] [CrossRef]
- Castillo, R.L.; Herrera, E.A.; Gonzalez-Candia, A.; Reyes-Farias, M.; De La Jara, N.; Peña, J.P.; Carrasco-Pozo, C. Quercetin Prevents Diastolic Dysfunction Induced by a High-Cholesterol Diet: Role of Oxidative Stress and Bioenergetics in Hyperglycemic Rats. Oxid. Med. Cell Longev. 2018, 2018, 7239123. [Google Scholar] [CrossRef] [PubMed]
- Puri, V.; Nagpal, M.; Singh, I.; Singh, M.; Dhingra, G.A.; Huanbutta, K.; Dheer, D.; Sharma, A.; Sangnim, T. A Comprehensive Review on Nutraceuticals: Therapy Support and Formulation Challenges. Nutrients 2022, 14, 4637. [Google Scholar] [CrossRef] [PubMed]
| Identified Compound | Molecular Formula | RT (Min) | Standard RT (Min) | Detected Mass | Calculated Mass | Error (ppm) | Fragments (m/z) |
|---|---|---|---|---|---|---|---|
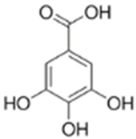 Gallic Acid | C7H6O5 | 14.25 | 14.26 | 169.01349 | 169.01370 | 2.013 | 169.01349 125.02338 |
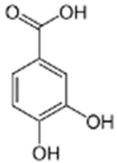 Protocatechuic Acid | C7H6O4 | 19.09 | 19.15 | 153.01843 | 153.01879 | 1.273 | 153.01843 109.0283 |
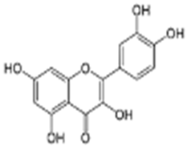 Quercetin | C15H10O7 | 27.78 | 27.78 | 301.03561 | 301.03561 | 4.585 | 301.03561 178.99792 151.00279 |
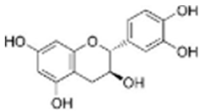 Catechin | C15H14O6 | 20.05 | 20.01 | 289.07199 | 289.07122 | 4.585 | 289.07199 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Braga, K.M.S.; Araujo, E.G.; Sellke, F.W.; Abid, M.R. Pequi (Caryocar brasiliense, Camb) Bark Extract Reduces ROS Production in Diabetic Human Coronary Endothelial Cells. Antioxidants 2025, 14, 1167. https://doi.org/10.3390/antiox14101167
Braga KMS, Araujo EG, Sellke FW, Abid MR. Pequi (Caryocar brasiliense, Camb) Bark Extract Reduces ROS Production in Diabetic Human Coronary Endothelial Cells. Antioxidants. 2025; 14(10):1167. https://doi.org/10.3390/antiox14101167
Chicago/Turabian StyleBraga, Karla M. S., Eugenio G. Araujo, Frank W. Sellke, and M. Ruhul Abid. 2025. "Pequi (Caryocar brasiliense, Camb) Bark Extract Reduces ROS Production in Diabetic Human Coronary Endothelial Cells" Antioxidants 14, no. 10: 1167. https://doi.org/10.3390/antiox14101167
APA StyleBraga, K. M. S., Araujo, E. G., Sellke, F. W., & Abid, M. R. (2025). Pequi (Caryocar brasiliense, Camb) Bark Extract Reduces ROS Production in Diabetic Human Coronary Endothelial Cells. Antioxidants, 14(10), 1167. https://doi.org/10.3390/antiox14101167










