Changes in the Activities of Antioxidant Enzymes in the Fat Body and Hemolymph of Apis mellifera L. Due to Pollen Monodiets
Abstract
1. Introduction
| Taxon | Total Protein Content [%] | Fatty Acid Composition | Phenolic and Flavonoid Compounds | Literature |
|---|---|---|---|---|
| Corylus sp. | No literature data available | |||
| Brassica napus | 20 | TS: 47.6 g/100 g TP: 36.7 g/100 g Fat: 6.56 g/100 g | TFC: 2.9–4.9 FRAP: 8.3–9.3 DPPH: 12.8–13.5 | [13,14,23] |
| Pinus sp. | 10.84 | TS: 13.6 mg/g TP: 13.2 mg/g Fat: 7.3 g/100g | TFC: 2–6 mg/g TPC: 1.9–3.5 mg/kg | [19] |
| Phacelia sp. | 27.44 | Fat: 5.35% | TPC: 27.5 mgGAE/g TFC: 3.58 mgQE/g FRAP: 8.16 mgAAE/g DPPH: 10.39 mgTE/g | [13,14] |
| Fagopyrum sp. | 11.4–14.3 | TS: 37.2 g/100 g TP: 21.6 g/100 g Fat: 5.15 g/100 g | TFC: 0.24 ppm, | [16,23] |
| Solidago sp. | >20 | No literature data available | [45] | |
2. Materials and Methods
2.1. Selection of Pollen Loads, Palynological Analyses, and Preparation of Pollen Monodiets
2.2. Analyses of Active Substance Residues in Pollen Loads
2.3. Obtaining One-Day-Old Bees and the Experiment
2.4. Laboratory Analyses
2.4.1. Obtaining Biological Material for Research
2.4.2. Biochemical Analyses
- Superoxide dismutase (SOD) activity according to the method described in the commercial kit SOD Assay Kit, Sigma Aldrich, Schnelldorf, Germany, no. 19160-1KT-F;
- Glutathione S-transferase (GST) activity according to the method described in the commercial kit Glutathione S-transferase Assay Kit, Sigma Aldrich, Schnelldorf, Germany, no. MAK 435-1KT;
- Glutathione peroxidase (GPx) activity according to the method described in the commercial kit Glutathione Peroxidase Assay Kit, Sigma Aldrich, Schnelldorf, Germany, no. MAK437-1KT;
- Catalase (CAT) activity according to the method described in the commercial kit Catalase Assay Kit, Cayman Chemical Company, East Ellsworth Road Ann Arbor, USA, Item: 707002;
- Total antioxidant capacity (TAC) according to the method described in the commercial kit Antioxidant Assay Kit, Cayman Chemical Company, East Ellsworth Road Ann Arbor, USA, Item: 709001.
2.5. Statistical Analyses
3. Results
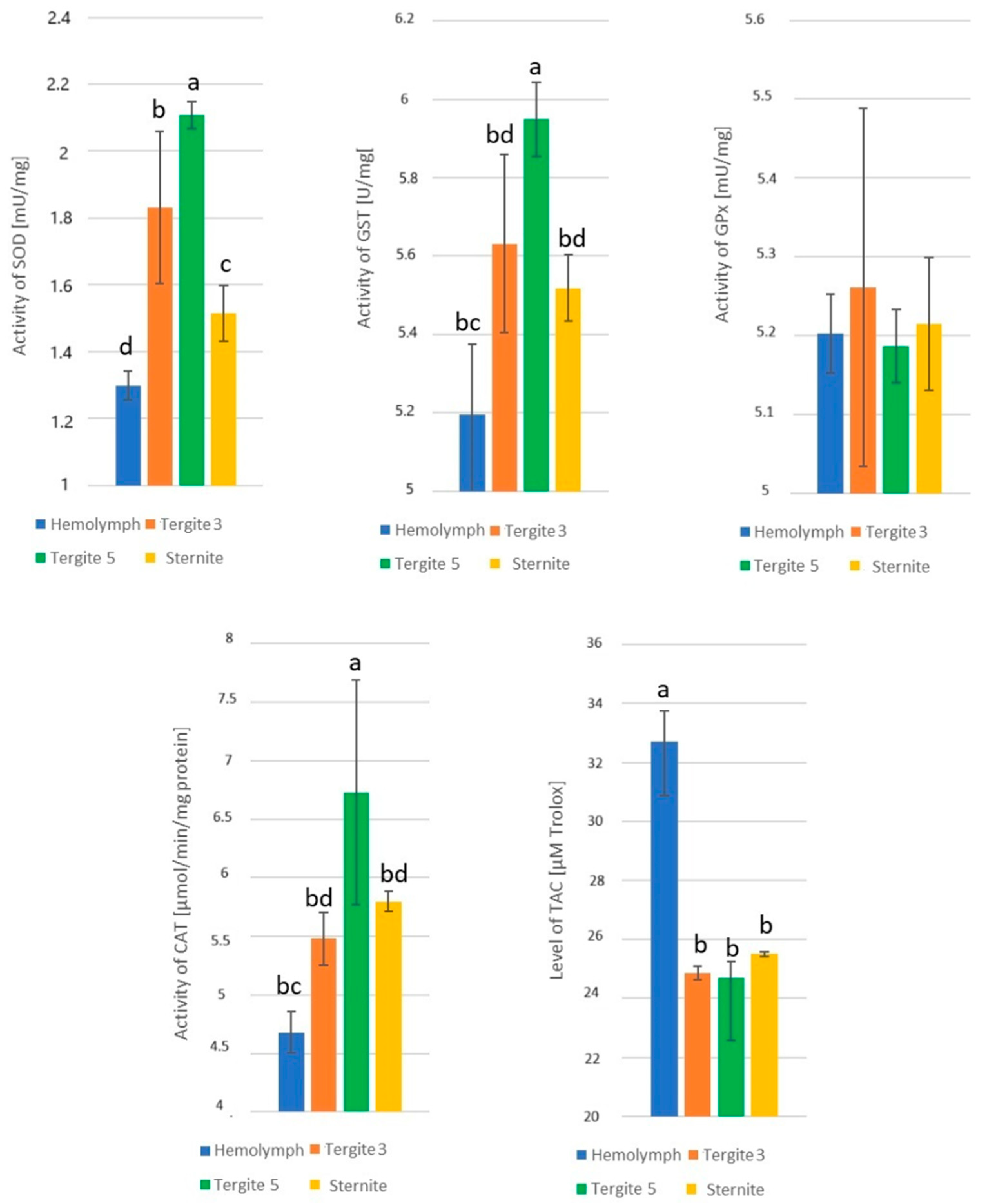
3.1. Activities of Superoxide Dismutase (SOD), Glutathione S-Transferase (GST), Glutathione Peroxidase (GPx), Catalase (CAT), and Total Antioxidant Capacity (TAC) Levels in One-Day-Old Workers
3.2. The Effect of the Tissue and Location of the Fat Body on the Enzyme Activities
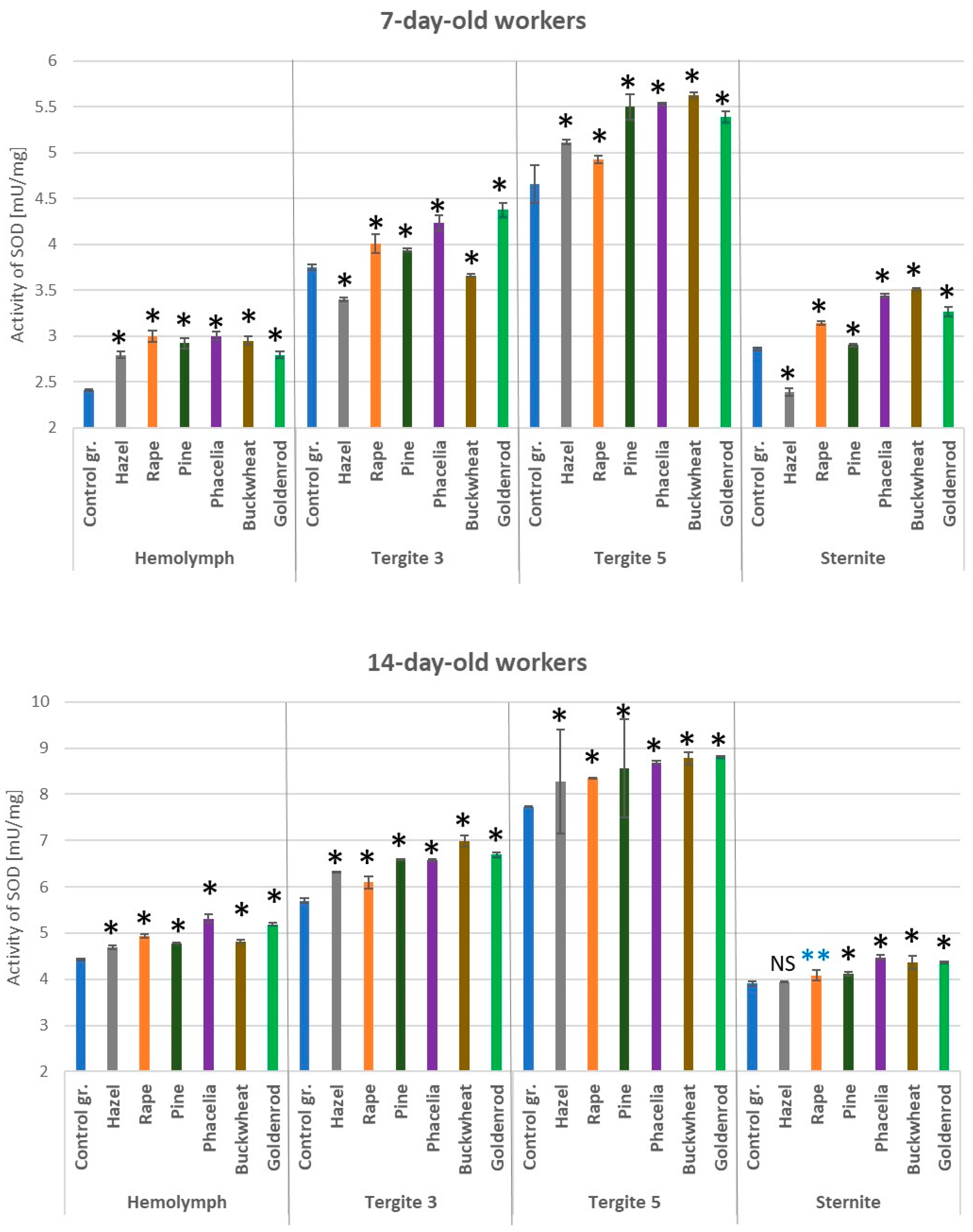
3.3. Activities of Superoxide Dismutase
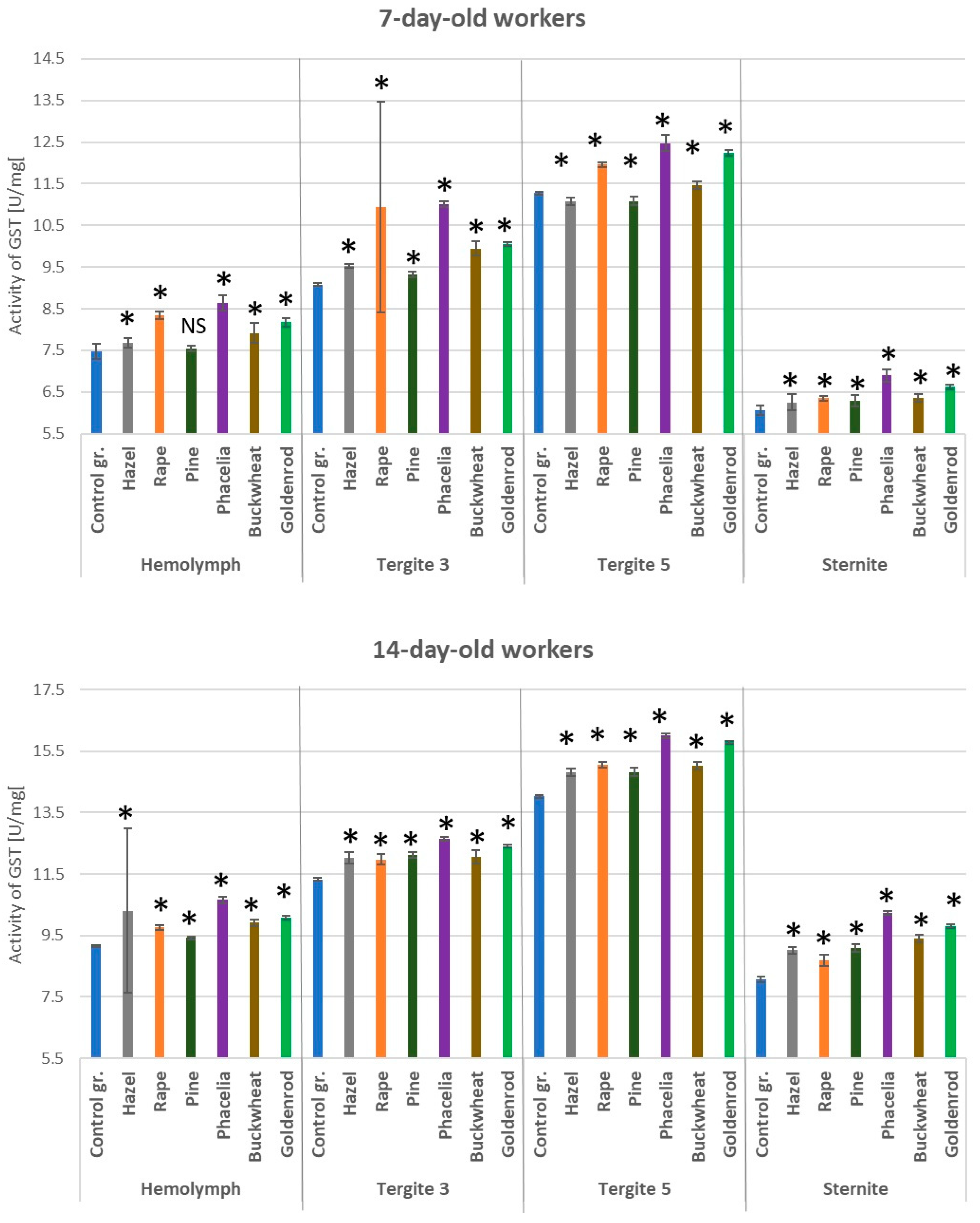
3.4. Activities of Glutathione S-Transferase
3.5. Activities of Glutathione Peroxidase
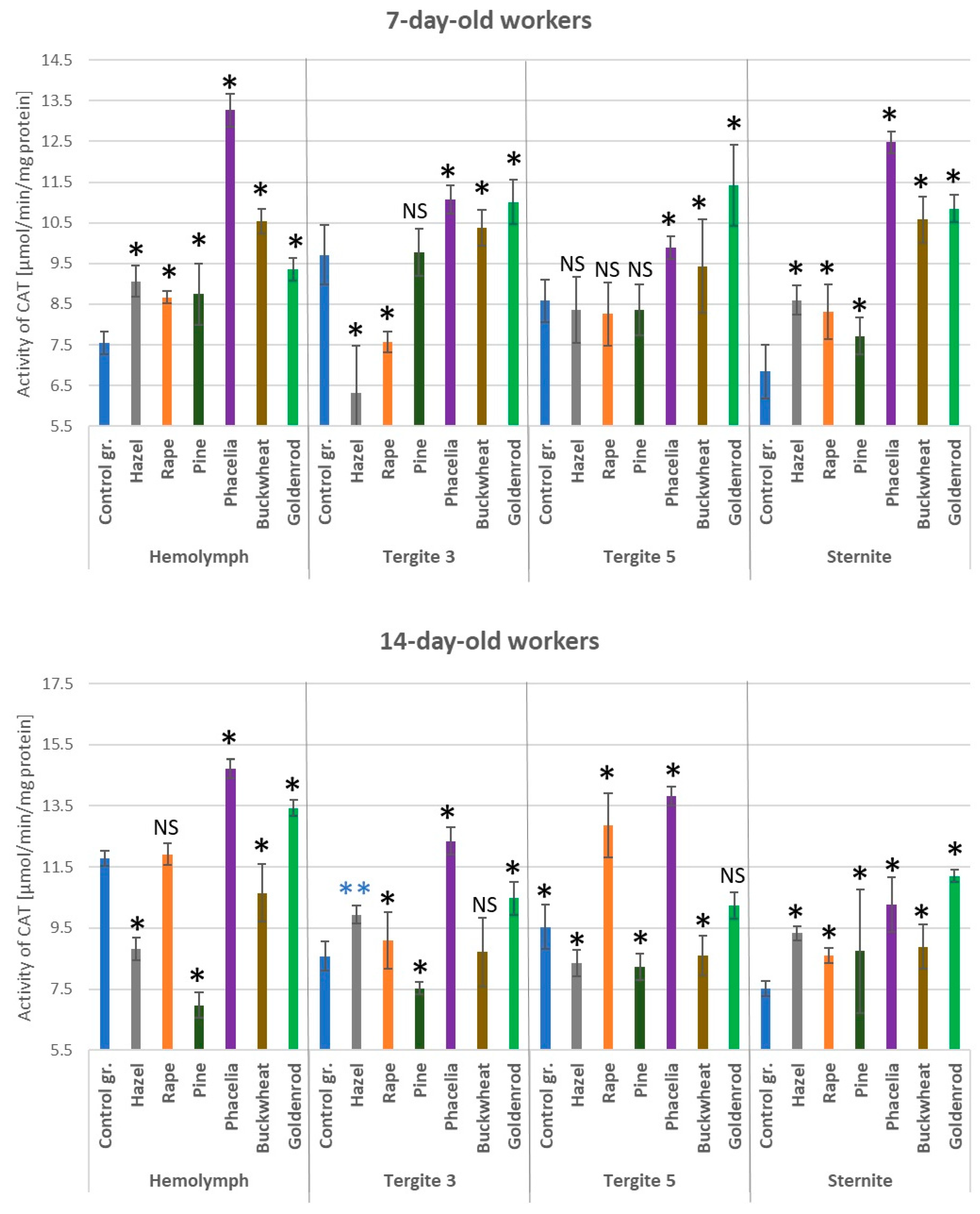
3.6. Activities of Catalase
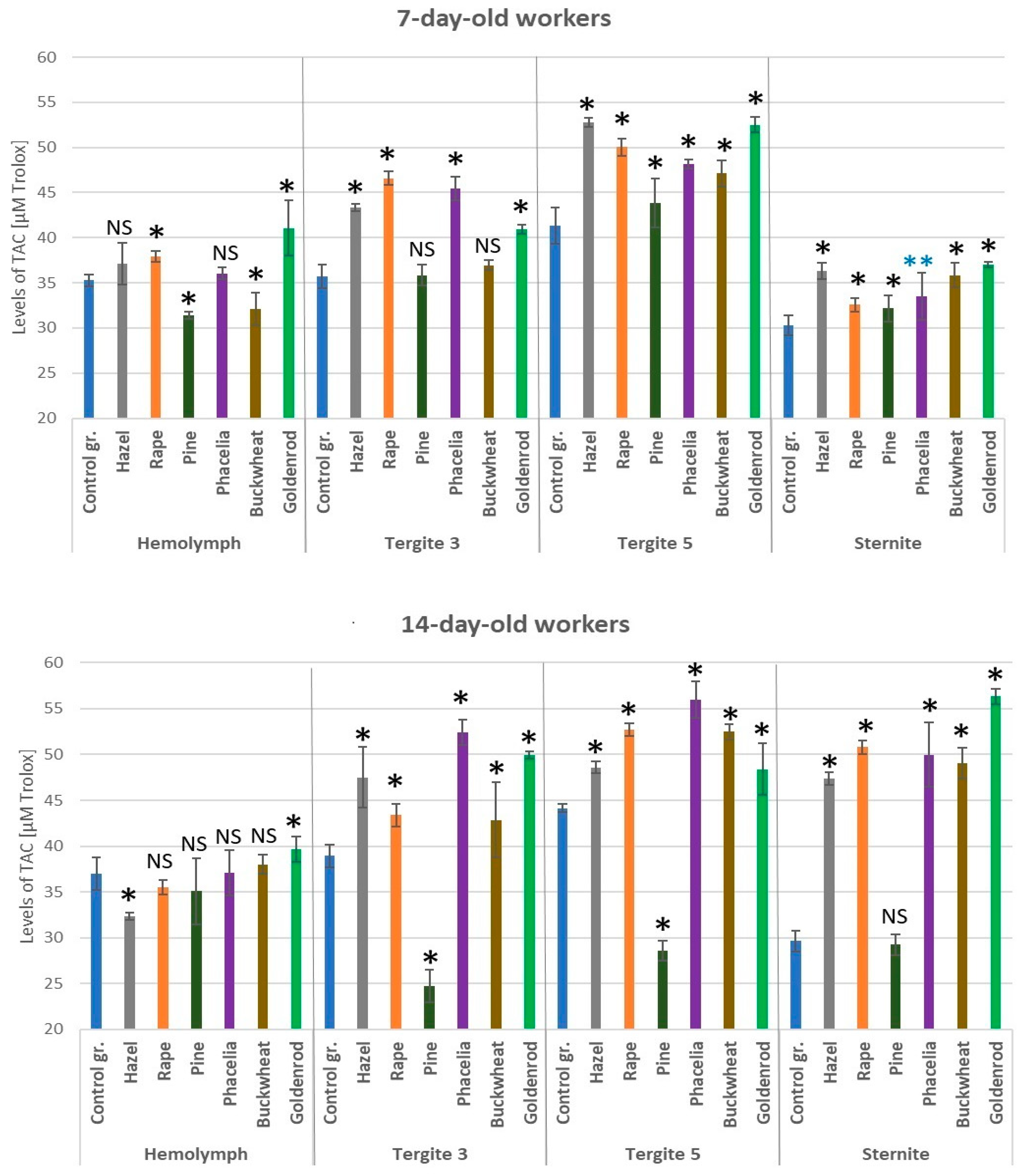
3.7. Levels of Total Antioxidant Capacity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Frias, B.E.D.; Barbosa, C.D.; Lourenço, A.P. Pollen Nutrition in Honey Bees (Apis mellifera): Impact on Adult Health. Apidologie 2016, 47, 15–25. [Google Scholar] [CrossRef]
- Di Pasquale, G.; Salignon, M.; Le Conte, Y.; Belzunces, L.P.; Decourtye, A.; Kretzschmar, A.; Suchail, S.; Brunet, J.L.; Alaux, C. Influence of Pollen Nutrition on Honey Bee Health: Do Pollen Quality and Diversity Matter? PLoS ONE 2013, 8, e72016. [Google Scholar] [CrossRef] [PubMed]
- Alaux, C.; Ducloz, F.; Crauser, D.; Le Conte, Y. Diet Effects on Honeybee Immunocompetence. Biol. Lett. 2010, 6, 562–565. [Google Scholar] [CrossRef]
- Bryś, M.S.; Staniec, B.; Strachecka, A. The Effect of Pollen Monodiets on Fat Body Morphology Parameters and Energy Substrate Levels in the Fat Body and Hemolymph of Apis mellifera L. Workers. Sci. Rep. 2024, 14, 15177. [Google Scholar] [CrossRef] [PubMed]
- DeGrandi-Hoffman, G.; Gage, S.L.; Corby-Harris, V.; Carroll, M.; Chambers, M.; Graham, H.; Watkins deJong, E.; Hidalgo, G.; Calle, S.; Azzouz-Olden, F.; et al. Connecting the Nutrient Composition of Seasonal Pollens with Changing Nutritional Needs of Honey Bee (Apis mellifera L.). Colonies. J. Insect Physiol. 2018, 109, 114–124. [Google Scholar] [CrossRef]
- Rodríguez-Flores, M.S.; Escuredo, O.; Seijo, M.C.; Rojo, S.; Vilas-Boas, M.; Falcão, S.I. Phenolic Profile of Castanea Bee Pollen from the Northwest of the Iberian Peninsula. Separations 2023, 10, 270. [Google Scholar] [CrossRef]
- Alimoglu, G.; Guzelmeric, E.; Yuksel, P.I.; Celik, C.; Deniz, I.; Yesilada, E. Monofloral and Polyfloral Bee Pollens: Comparative Evaluation of Their Phenolics and Bioactivity Profiles. LWT 2021, 142, 110973. [Google Scholar] [CrossRef]
- Fatrcová-Šramková, K.; Nôžková, J.; Kačániová, M.; Máriássyová, M.; Rovná, K.; Stričík, M. Antioxidant and Antimicrobial Properties of Monofloral Bee Pollen. J. Environ. Sci. Health B 2013, 48, 133–138. [Google Scholar] [CrossRef]
- Dinu, L.D.; Gatea, F.; Roaming Israel, F.; Lakicevic, M.; Dedović, N.; Vamanu, E. The Modulation Effect of a Fermented Bee Pollen Postbiotic on Cardiovascular Microbiota and Therapeutic Perspectives. Biomedicines 2023, 11, 2712. [Google Scholar] [CrossRef]
- El Ghouizi, A.; Bakour, M.; Laaroussi, H.; Ousaaid, D.; El Menyiy, N.; Hano, C.; Lyoussi, B. Bee Pollen as Functional Food: Insights into Its Composition and Therapeutic Properties. Antioxidants 2023, 12, 557. [Google Scholar] [CrossRef]
- Nguyen, H.C.; Liu, L.-C.; Wu, M.-C.; Lin, T.-P.; Yang, C.-Y.; Huang, M.-Y. Chemical constituents, antioxidant, and anticancer activities of bee pollen from various floral sources in Taiwan. Naturae Bot. Horti Agrobot. Cluj-Napoca 2022, 50, 12644. [Google Scholar] [CrossRef]
- Mǎrghitaş, L.A.; Stanciu, O.G.; Dezmirean, D.S.; Bobiş, O.; Popescu, O.; Bogdanov, S.; Campos, M.G. In Vitro Antioxidant Capacity of Honeybee-Collected Pollen of Selected Floral Origin Harvested from Romania. Food Chem. 2009, 115, 878–883. [Google Scholar] [CrossRef]
- Vergun, O. Nutritional Composition of Phacelia Tanacetifolia Benth. Bee Pollen and Inflorescences. Agrobiodiversity Improv. Nutr. Health Life Qual. 2023, 7, 95–104. [Google Scholar] [CrossRef]
- Végh, R.; Sipiczki, G.; Csóka, M. Investigating the Antioxidant and Color Properties of Bee Pollens of Various Plant Sources. Chem. Biodivers. 2023, 20, e202300126. [Google Scholar] [CrossRef] [PubMed]
- Owayss, A.A.; Shebl, M.A.; Iqbal, J.; Awad, A.M.; Raweh, H.S.; Alqarni, A.S. Phacelia Tanacetifolia Can Enhance Conservation of Honey Bees and Wild Bees in the Drastic Hot-Arid Subtropical Central Arabia. J. Apic. Res. 2020, 59, 569–582. [Google Scholar] [CrossRef]
- Nešović, M.; Gašić, U.; Tosti, T.; Horvacki, N.; Šikoparija, B.; Nedić, N.; Blagojević, S.; Ignjatović, L.; Tešić, Ž. Polyphenol Profile of Buckwheat Honey, Nectar and Pollen: Polyphenolics in Buckwheat. R. Soc. Open Sci. 2020, 7, 201576. [Google Scholar] [CrossRef]
- Kostić, A.; Milinčić, D.D.; Gašić, U.M.; Nedić, N.; Stanojević, S.P.; Tešić, Ž.L.; Pešić, M.B. Polyphenolic Profile and Antioxidant Properties of Bee-Collected Pollen from Sunflower (Helianthus annuus L.) Plant. LWT 2019, 112, 1091. [Google Scholar] [CrossRef]
- Czigle, S.; Filep, R.; Balažová, E.; Szentgyörgyi, H.; Balázs, V.L.; Kocsis, M.; Purger, D.; Papp, N.; Farkas, Á. Antioxidant Capacity Determination of Hungarian-, Slovak-, and Polish-Origin Goldenrod Honeys. Plants 2022, 11, 792. [Google Scholar] [CrossRef]
- Cheng, Y.; Wang, Z.; Quan, W.; Xue, C.; Qu, T.; Wang, T.; Chen, Q.; Wang, Z.; Zeng, M.; Qin, F.; et al. Pine Pollen: A Review of Its Chemical Composition, Health Effects, Processing, and Food Applications. Trends Food Sci. Technol. 2023, 138, 599–614. [Google Scholar] [CrossRef]
- Lee, K.-H.; Kim, A.-J.; Choi, E.-M. Antioxidant and antiinflammatory activity of pine pollen extract in vitro. Phytother. Res. 2009, 23, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Alves, T.; Trivellato, M.; Freitas, T.; Kato, A.; Gomes, C.; Ferraz, Y.; Serafim, J.; De Jong, D.; Prado, E.; Vicente, E.; et al. Pollen Contaminated with a Triple-Action Fungicide Induced Oxidative Stress and Reduced Bee Longevity Though with Less Impact on Lifespan in Bees from Well Fed Colonies. Environ. Toxicol. Pharmacol. 2024, 112, 104587. [Google Scholar] [CrossRef] [PubMed]
- Almasri, H.; Liberti, J.; Brunet, J.L.; Engel, P.; Belzunces, L.P. Mild Chronic Exposure to Pesticides Alters Physiological Markers of Honey Bee Health without Perturbing the Core Gut Microbiota. Sci. Rep. 2022, 12, 4281. [Google Scholar] [CrossRef]
- Yang, K.; Wu, D.; Ye, X.; Liu, D.; Chen, J.; Sun, P. Characterization of Chemical Composition of Bee Pollen in China. J. Agric. Food Chem. 2013, 61, 708–718. [Google Scholar] [CrossRef] [PubMed]
- Thakur, M.; Nanda, V. Composition and Functionality of Bee Pollen: A Review. Trends Food Sci. Technol. 2020, 98, 82–106. [Google Scholar] [CrossRef]
- Denisow, B.; Denisow-Pietrzyk, M. Biological and Therapeutic Properties of Bee Pollen: A Review. J. Sci. Food Agric. 2016, 96, 4303–4309. [Google Scholar] [CrossRef] [PubMed]
- Nicolson, S.W.; Da Silva Das Neves, S.; Human, H.; Pirk, C.W.W. Digestibility and Nutritional Value of Fresh and Stored Pollen for Honey Bees (Apis mellifera Scutellata). J. Insect Physiol. 2018, 107, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Carroll, M.J.; Brown, N.; Goodall, C.; Downs, A.M.; Sheenan, T.H.; Anderson, K.E. Honey Bees Preferentially Consume Freshlystored Pollen. PLoS ONE 2017, 12, e0249458. [Google Scholar] [CrossRef] [PubMed]
- Moliné, M.P.; Vázquez, M.M.; Moran Giardini, P.; Domínguez, E.; Fernández, N.J.; Damiani, N.; Quintana, S.; Gende, L.B. Immune and Antioxidant Gene Expression Stimulation of Apis mellifera (Hymenoptera: Apidae) Larvae by Saccharomyces Cerevisiae from the Brewering Industry. J. Appl. Entomol. 2024, 148, 382–390. [Google Scholar] [CrossRef]
- Skowronek, P.; Strachecka, A. Cannabidiol (CBD) Supports the Honeybee Worker Organism by Activating the Antioxidant System. Antioxidants 2023, 12, 279. [Google Scholar] [CrossRef]
- Skowronek, P.; Wójcik, Ł.; Strachecka, A. Impressive Impact of Hemp Extract on Antioxidant System in Honey Bee (Apis mellifera) Organism. Antioxidants 2022, 11, 707. [Google Scholar] [CrossRef] [PubMed]
- Strachecka, A.; Kuszewska, K.; Olszewski, K.; Skowronek, P.; Grzybek, M.; Grabowski, M.; Paleolog, J.; Woyciechowski, M. Activities of Antioxidant and Proteolytic Systems and Biomarkers in the Fat Body and Hemolymph of Young Apis mellifera Females. Animals 2022, 12, 1121. [Google Scholar] [CrossRef]
- Santos, D.E.; Souza, A.d.O.; Tibério, G.J.; Alberici, L.C.; Hartfelder, K. Differential Expression of Antioxidant System Genes in Honey Bee (Apis mellifera L.) Caste Development Mitigates ROS-Mediated Oxidative Damage in Queen Larvae. Genet. Mol. Biol. 2020, 43, e20200173. [Google Scholar] [CrossRef]
- Birch-Machin, M.A.; Bowman, A. Oxidative Stress and Ageing. Br. J. Dermatol. 2016, 175, 26–29. [Google Scholar] [CrossRef]
- Wang, H.; Lei, L.; Chen, W.; Chi, X.; Han, K.; Wang, Y.; Ma, L.; Liu, Z.; Xu, B. The Comparison of Antioxidant Performance, Immune Performance, IIS Activity and Gut Microbiota Composition between Queen and Worker Bees Revealed the Mechanism of Different Lifespan of Female Casts in the Honeybee. Insects 2022, 13, 772. [Google Scholar] [CrossRef] [PubMed]
- Seehuus, S.C.; Norberg, K.; Gimsa, U.; Krekling, T.; Amdam, G.V. Reproductive Protein Protects Functionally Sterile Honey Bee Workers from Oxidative Stress. Proc. Natl. Acad. Sci. USA 2006, 103, 962–967. [Google Scholar] [CrossRef] [PubMed]
- Weirich, G.; Collins, A.; Williams, V.; Weirich, G.F.; Collins, A.M.; Williams, V.P. Antioxidant Enzymes in the Honey Bee, Apis Mel-Lifera. Apidologie 2002, 33, 3–14. [Google Scholar] [CrossRef]
- Jovanovic, N.M.; Glavinic, U.; Ristanic, M.; Vejnovic, B.; Ilic, T.; Stevanovic, J.; Stanimirovic, Z. Effects of Plant-Based Supplement on Oxidative Stress of Honey Bees (Apis mellifera) Infected with Nosema Ceranae. Animals 2023, 13, 3543. [Google Scholar] [CrossRef] [PubMed]
- Dmochowska-Ślęzak, K.; Dmitryjuk, M.; Zaobidna, E.; Żółtowska, K. The Antioxidant Defense System of Varroa Destructor Facilitates the Infestation of Apis mellifera. J. Apic. Sci. 2016, 60, 163–165. [Google Scholar] [CrossRef][Green Version]
- Dziechciarz, P.; Strachecka, A.; Borsuk, G.; Olszewski, K. Effect of Rearing in Small-Cell Combs on Activities of Catalase and Superoxide Dismutase and Total Antioxidant Capacity in the Hemolymph of Apis mellifera Workers. Antioxidants 2023, 12, 709. [Google Scholar] [CrossRef]
- Paleolog, J.; Wilde, J.; Miszczak, A.; Gancarz, M.; Strachecka, A. Antioxidation Defenses of Apis mellifera Queens and Workers Respond to Imidacloprid in Different Age-Dependent Ways: Old Queens Are Resistant, Foragers Are Not. Animals 2021, 11, 1246. [Google Scholar] [CrossRef]
- Migdał, P.; Murawska, A.; Strachecka, A.; Bieńkowski, P.; Roman, A. Changes in the Honeybee Antioxidant System after 12 h of Exposure to Electromagnetic Field Frequency of 50 Hz and Variable Intensity. Insects 2020, 11, 713. [Google Scholar] [CrossRef]
- Tahir, F.; Goblirsch, M.; Adamczyk, J.; Karim, S.; Alburaki, M. Honey Bee Apis mellifera L. Responses to Oxidative Stress Induced by Pharmacological and Pesticidal Compounds. Front. Bee Sci. 2023, 1, 1275862. [Google Scholar] [CrossRef]
- Corona, M.; Robinson, G.E. Genes of the Antioxidant System of the Honey Bee: Annotation and Phylogeny. Insect. Mol. Biol. 2006, 15, 687–701. [Google Scholar] [CrossRef]
- Farjan, M.; Dmitryjuk, M.; LipiŃski, Z.; Biernat-łOpieŃska, E.; Zółtowska, K. Supplementation of the Honey Bee Diet with Vitamin C: The Effect on the Antioxidative System of Apis mellifera Carnica Brood at Different Stages. J. Apic. Res. 2012, 51, 263–270. [Google Scholar] [CrossRef]
- Jachuła, J.; Denisow, B.; Strzałkowska-Abramek, M. Does an Invader Have a Bright Side? Floral Reward in Two Solidago Species. J. Apic. Res. 2020, 59, 599–608. [Google Scholar] [CrossRef]
- Filipiak, M.; Kuszewska, K.; Asselman, M.; Denisow, B.; Stawiarz, E.; Woyciechowski, M.; Weiner, J. Ecological Stoichiometry of the Honeybee: Pollen Diversity and Adequate Species Composition Are Needed to Mitigate Limitations Imposed on the Growth and Development of Bees by Pollen Quality. PLoS ONE 2017, 12, e0183236. [Google Scholar] [CrossRef]
- Filipiak, Z.M.; Denisow, B.; Stawiarz, E.; Filipiak, M. Unravelling the Dependence of a Wild Bee on Floral Diversity and Composition Using a Feeding Experiment. Sci. Total Environ. 2022, 820, 153326. [Google Scholar] [CrossRef] [PubMed]
- Altaye, S.Z.; Pirk, C.W.W.; Crewe, R.M.; Nicolson, S.W. Convergence of Carbohydrate-Biased Intake Targets in Caged Worker Honeybees Fed Different Protein Sources. J. Exp. Biol. 2010, 213, 3311–3318. [Google Scholar] [CrossRef]
- Et, A. Fast and Easy Multiresidue Method. Employing Acetonitrile Extraction/Partitioning and “Dispersive Solid-Phase Extraction” for the Determination of Pesticide Residues in Produce. J. AOAC Int. 2003, 86, 412–431. [Google Scholar]
- Kaila, L.; Ketola, J.; Toivonen, M.; Loukola, O.; Hakala, K.; Raiskio, S.; Hurme, T.; Jalli, M. Pesticide Residues in Honeybee-Collected Pollen: Does the EU Regulation Protect Honeybees from Pesticides? Environ. Sci. Pollut. Res. 2022, 29, 18225–18244. [Google Scholar] [CrossRef] [PubMed]
- Łoś, A.; Strachecka, A. Fast and Cost-Effective Biochemical Spectrophotometric Analysis of Solution of Insect “Blood” and Body Surface Elution. Sensors 2018, 18, 1494. [Google Scholar] [CrossRef] [PubMed]
- Strachecka, A.; Olszewski, K.; Kuszewska, K.; Chobotow, J.; Wójcik, Ł.; Paleolog, J.; Woyciechowski, M. Segmentation of the Subcuticular Fat Body in Apis mellifera Females with Different Reproductive Potentials. Sci. Rep. 2021, 11, 1–12. [Google Scholar] [CrossRef]
- Yazlovytska, L.S.; Karavan, V.V.; Domaciuk, M.; Panchuk, I.I.; Borsuk, G.; Volkov, R.A. Increased Survival of Honey Bees Consuming Pollen and Beebread Is Associated with Elevated Biomarkers of Oxidative Stress. Front. Ecol. Evol. 2023, 11, 1098350. [Google Scholar] [CrossRef]
- Kramer, B.H.; Nehring, V.; Buttstedt, A.; Heinze, J.; Korb, J.; Libbrecht, R.; Meusemann, K.; Paxton, R.J.; Séguret, A.; Schaub, F.; et al. Oxidative Stress and Senescence in Social Insects: A Significant but Inconsistent Link? Philos. Trans. R. Soc. B Biol. Sci. 2021, 376, 20190732. [Google Scholar] [CrossRef] [PubMed]
- Spremo, J.; Purać, J.; Čelić, T.; Đorđievski, S.; Pihler, I.; Kojić, D.; Vukašinović, E. Assessment of Oxidative Status, Detoxification Capacity and Immune Responsiveness in Honey Bees with Ageing. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2024, 298, 111735. [Google Scholar] [CrossRef]
- Białecka, N.; Garbacz, K.; Berbeć, E.; Murawska, A.; Madras-Majewska, B.; Migdał, P. Changes in Selected Biochemical Markers of Honey Bees Exposed to Fermented Common Tansy Solution (Tanacetum vulgare L.). Animals 2024, 14, 2857. [Google Scholar] [CrossRef]
- Brejcha, M.; Prušáková, D.; Sábová, M.; Peska, V.; Černý, J.; Kodrík, D.; Konopová, B.; Čapková Frydrychová, R. Seasonal Changes in Ultrastructure and Gene Expression in the Fat Body of Worker Honey Bees. J. Insect Physiol. 2023, 146, 104504. [Google Scholar] [CrossRef]
- Hsu, C.Y.; Hsieh, Y.S. Oxidative Stress Decreases in the Trophocytes and Fat Cells of Worker Honeybees during Aging. Biogerontology 2014, 15, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Bryś, M.S.; Strachecka, A. The Key Role of Amino Acids in Pollen Quality and Honey Bee Physiology—A Review. Molecules 2024, 29, 2605. [Google Scholar] [CrossRef] [PubMed]
- Ara Begum, H.; Idrees, A.; Afzal, A.; Iqbal, J.; Qadir, Z.A.; Shahzad, M.F.; Li, Z.; Salah Shebl Ibrahim, S.; Alkahtani, J.; Li, J. Impact of Different Pollen Protein Diets on the Physiology of Apis mellifera L. (Hymenoptera: Apidae) Workers from Essential Plant Sources. J. King Saud. Univ. Sci. 2023, 35, 102511. [Google Scholar] [CrossRef]
- Jachuła, J.; Denisow, B.; Wrzesień, M. Habitat Heterogeneity Helps to Mitigate Pollinator Nectar Sugar Deficit and Discontinuity in an Agricultural Landscape. Sci. Total Environ. 2021, 782, 146909. [Google Scholar] [CrossRef]
- Scofield, S.L.; Amdam, G.V. Fat Body Lipogenic Capacity in Honey Bee Workers Is Affected by Age, Social Role and Dietary Protein. J. Exp. Biol. 2024, 227, jeb247777. [Google Scholar] [CrossRef]
- Vasilevskaya, N. Pollution of the Environment and Pollen: A Review. Stresses 2022, 2, 515–530. [Google Scholar] [CrossRef]
- Barbieri, D.; Gabriele, M.; Summa, M.; Colosimo, R.; Leonardi, D.; Domenici, V.; Pucci, L. Antioxidant, Nutraceutical Properties, and Fluorescence Spectral Profiles of Bee Pollen Samples from Different Botanical Origins. Antioxidants 2020, 9, 1001. [Google Scholar] [CrossRef]
- Sandmann, G. Antioxidant Protection from UV-and Light-Stress Related to Carotenoid Structures. Antioxidants 2019, 8, 219. [Google Scholar] [CrossRef]
- Krishnan, N.; Kodrík, D. Antioxidant Enzymes in Spodoptera Littoralis (Boisduval): Are They Enhanced to Protect Gut Tissues during Oxidative Stress? J. Insect Physiol. 2006, 52, 11–20. [Google Scholar] [CrossRef]
- Likhanov, A.; Oliinyk, M.; Pashkevych, N.; Churilov, A.; Kozyr, M. The Role of Flavonoids in Invasion Strategy of Solidago canadensis L. Plants 2021, 10, 1748. [Google Scholar] [CrossRef] [PubMed]
- Moroń, D.; Marjańska, E.; Skórka, P.; Lenda, M.; Woyciechowski, M. Invader–Pollinator Paradox: Invasive Goldenrods Benefit from Large Size Pollinators. Divers. Distrib. 2021, 27, 632–641. [Google Scholar] [CrossRef]
- Lenda, M.; Skórka, P.; Kuszewska, K.; Moroń, D.; Bełcik, M.; Baczek Kwinta, R.; Janowiak, F.; Duncan, D.H.; Vesk, P.A.; Possingham, H.P.; et al. Misinformation, Internet Honey Trading and Beekeepers Drive a Plant Invasion. Ecol. Lett. 2021, 24, 165–169. [Google Scholar] [CrossRef]
- Shakir, S.K.; Irfan, S.; Akhtar, B.; Rehman, S.; Daud, M.K.; Taimur, N.; Azizullah, A. Pesticide-Induced Oxidative Stress and Antioxidant Responses in Tomato (Solanum lycopersicum) Seedlings. Ecotoxicology 2018, 27, 919–935. [Google Scholar] [CrossRef] [PubMed]
- Jabłońska-Trypuć, A.; Wiater, J. Protective Effect of Plant Compounds in Pesticides Toxicity. J. Environ. Health Sci. Eng. 2022, 20, 1035–1045. [Google Scholar] [CrossRef] [PubMed]
- Murawska, A.; Migdał, P.; Roman, A. Effects of Plant Protection Products on Biochemical Markers in Honey Bees. Agriculture 2021, 11, 648. [Google Scholar] [CrossRef]
- Karise, R.; Raimets, R.; Bartkevics, V.; Pugajeva, I.; Pihlik, P.; Keres, I.; Williams, I.H.; Viinalass, H.; Mänd, M. Are Pesticide Residues in Honey Related to Oilseed Rape Treatments? Chemosphere 2017, 188, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Farjan, M.; Łopieńska-Biernat, E.; Lipiński, Z.; Dmitryjuk, M.; ZóŁtowska, K. Supplementing with Vitamin C the Diet of Honeybees (Apis mellifera Carnica) Parasitized with Varroa Destructor: Effects on Antioxidative Status. Parasitology 2014, 141, 770–776. [Google Scholar] [CrossRef] [PubMed]
- Strachecka, A.J.; Olszewski, K.; Paleolog, J. Curcumin Stimulates Biochemical Mechanisms of Apis mellifera Resistance and Extends the Apian Life-Span. J. Apic. Sci. 2015, 59, 129–141. [Google Scholar] [CrossRef]
- Hacke, A.C.M.; Lima, D.; de Costa, F.; Deshmukh, K.; Li, N.; Chow, A.M.; Marques, J.A.; Pereira, R.P.; Kerman, K. Probing the antioxidant activity of Δ9-tetrahydrocannabinol and cannabidiol in Cannabis sativa extracts. Analyst 2019, 144, 4952–4961. [Google Scholar] [CrossRef]
- Syama, P.S.; Sreeranjit Kumar, C.V. Evidence of Diet Supplementation with Vitamin C Protecting Honeybees from Imidacloprid Induced Peroxidative Damage: A Study with Apis Cerana Indica. Sociobiology 2022, 69, e7763. [Google Scholar] [CrossRef]
- Romanovskaja, D.; Baksiene, E.; Razukas, A. Impact of Climate Change on Phenology of Entomophilous Plants and Honey Bee (Apis mellifera L.). Preprints 2023, 2023050787. [Google Scholar] [CrossRef]



| No. | Pollen Load Samples | Active Substance | Mean ± SD [mg/kg] |
|---|---|---|---|
| 1. | Hazel | Anthraquinone | 0.053 ± 0.027 |
| 2. | Rape | Acetamiprid | 0.025 ± 0.013 |
| Azoxystrobin | 0.15 ± 0.07 | ||
| Thiminamethoxam | 0.009 ± 0.004 | ||
| 3. | Pine | <LOQ | |
| 4. | Phacelia | <LOQ | |
| 5. | Buckwheat | <LOQ | |
| 6. | Goldenrod | <LOQ |
| Groups | Age of Workers | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 7-Day-Old | 14-Day-Old | |||||||||
| SOD | GST | GPx | CAT | TAC | SOD | GST | GPx | CAT | TAC | |
| Control gr. | H = 36.67 p = 0.000 | F = 416.70 p = 0.000 | F = 222.34 p = 0.000 | F = 46.81 p = 0.000 | F = 110.42 p = 0.000 | H = 36.73 p = 0.000 | H = 36.73 p = 0.000 | H = 33.04 p = 0.000 | F = 153.71 p = 0.000 | F = 227.25 p = 0.000 |
| Hazel | H = 36.64 p = 0.000 | H = 36.65 p = 0.000 | H = 35.52 p = 0.000 | H = 22.09 p = 0.000 | H = 34.91 p = 0.000 | H = 36.72 p = 0.000 | H = 32.69 p = 0.000 | H = 34.12 p = 0.000 | F = 15.05 p = 0.000 | H = 33.61 p = 0.000 |
| Rape | H = 36.61 p = 0.000 | H = 35.35 p = 0.000 | H = 34.96 p = 0.000 | H = 20.29 p = 0.000 | H = 36.63 p = 0.000 | H = 36.64 p = 0.000 | H = 36.65 p = 0.000 | H = 36.66 p = 0.000 | H = 32.51 p = 0.000 | H = 36.21 p = 0.000 |
| Pine | H = 33.76 p = 0.000 | H = 36.65 p = 0.000 | H = 35.28 p = 0.000 | F = 19.88 p = 0.000 | H = 34.56 p = 0.000 | H = 36.67 p = 0.000 | H = 36.66 p = 0.000 | H = 35.73 p = 0.000 | H = 15.79 p = 0.000 | H = 33.89 p = 0.000 |
| Phacelia | H = 36.65 p = 0.000 | H = 36.63 p = 0.000 | H = 36.47 p = 0.000 | H = 35.49 p = 0.000 | H = 34.78 p = 0.000 | H = 36.62 p = 0.000 | H = 36.65 p = 0.000 | H = 36.62 p = 0.000 | H = 36.64 p = 0.000 | H = 30.30 p = 0.000 |
| Buckwheat | H = 36.63 p = 0.000 | H = 36.71 p = 0.000 | H = 36.61 p = 0.000 | H = 10.84 p = 0.013 | H = 33.46 p = 0.000 | H = 36.65 p = 0.000 | H = 36.64 p = 0.000 | H = 34.82 p = 0.000 | H = 22.82 p = 0.000 | H = 34.43 p = 0.000 |
| Goldenrod | H = 36.61 p = 0.000 | H = 36.68 p = 0.000 | H = 33.08 p = 0.000 | F = 22.06 p = 0.000 | H = 34.30 p = 0.000 | H = 36.65 p = 0.000 | H = 36.71 p = 0.000 | H = 36.63 p = 0.000 | H = 31.78 p = 0.000 | H = 33.06 p = 0.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bryś, M.S.; Olszewski, K.; Bartoń, M.; Strachecka, A. Changes in the Activities of Antioxidant Enzymes in the Fat Body and Hemolymph of Apis mellifera L. Due to Pollen Monodiets. Antioxidants 2025, 14, 69. https://doi.org/10.3390/antiox14010069
Bryś MS, Olszewski K, Bartoń M, Strachecka A. Changes in the Activities of Antioxidant Enzymes in the Fat Body and Hemolymph of Apis mellifera L. Due to Pollen Monodiets. Antioxidants. 2025; 14(1):69. https://doi.org/10.3390/antiox14010069
Chicago/Turabian StyleBryś, Maciej Sylwester, Krzysztof Olszewski, Maciej Bartoń, and Aneta Strachecka. 2025. "Changes in the Activities of Antioxidant Enzymes in the Fat Body and Hemolymph of Apis mellifera L. Due to Pollen Monodiets" Antioxidants 14, no. 1: 69. https://doi.org/10.3390/antiox14010069
APA StyleBryś, M. S., Olszewski, K., Bartoń, M., & Strachecka, A. (2025). Changes in the Activities of Antioxidant Enzymes in the Fat Body and Hemolymph of Apis mellifera L. Due to Pollen Monodiets. Antioxidants, 14(1), 69. https://doi.org/10.3390/antiox14010069







