Nanotechnological Approaches to Enhance the Potential of α-Lipoic Acid for Application in the Clinic
Abstract
1. Introduction
1.1. Chemical Structure of α-Lipoic Acid
1.2. Antioxidant Activity of α-Lipoic Acid
1.3. α-Lipoic Acid as a Therapeutic Agent
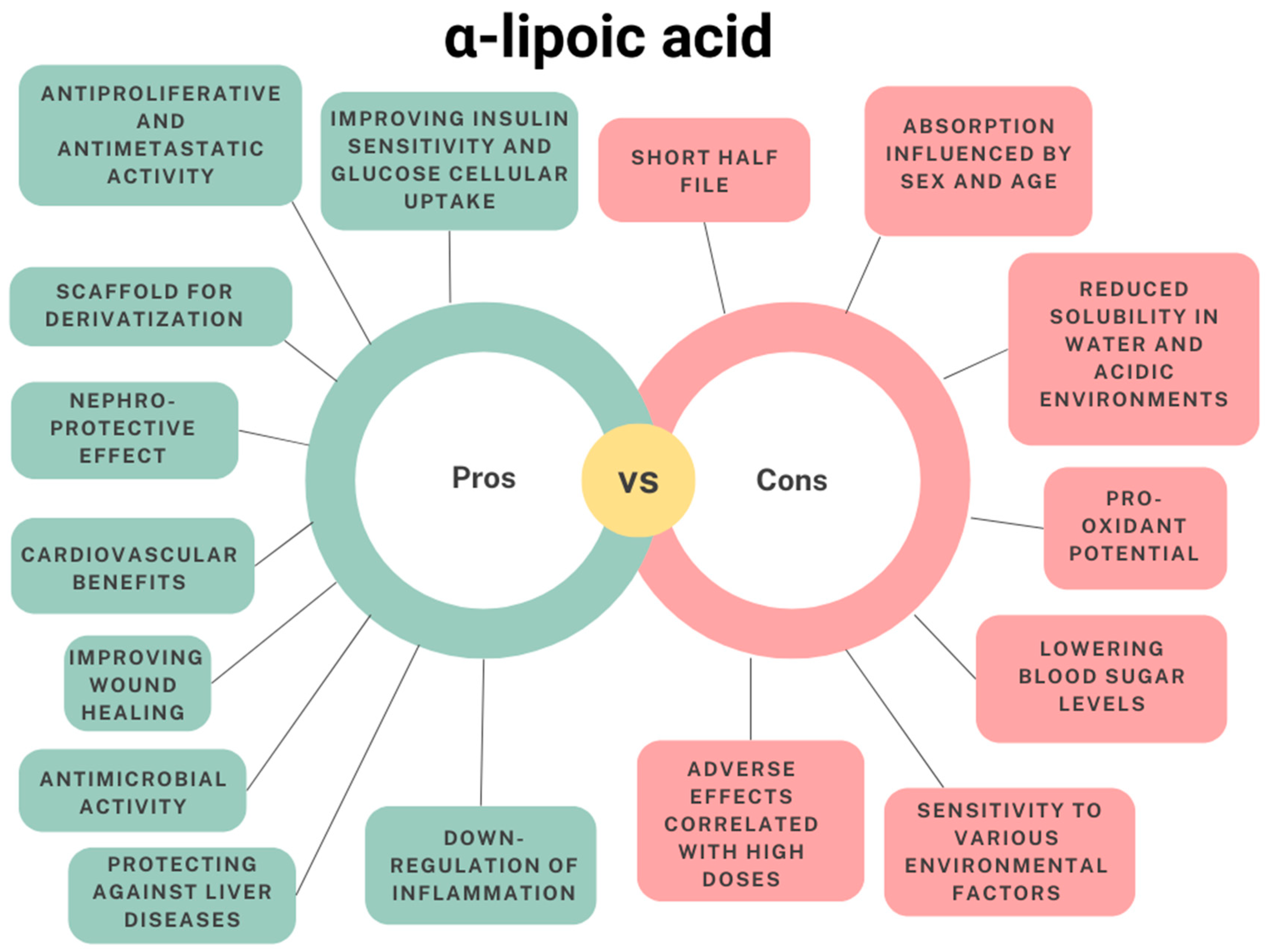
1.4. Limitations and Drawbacks in the Therapeutic Use of α-Lipoic Acid
1.5. Nanomedicine as a Strategy to Improve α-Lipoic Acid
2. Enhancement of α-Lipoic Acid Properties and Activity When Encapsulated in Nanoparticles
2.1. Silica Nanoparticles
2.2. Lipid-Based Nanoparticles (Lipid Carriers, Solid Lipid Nanoparticles, Nanoemulsions, Liposomes, Micelles)
2.3. Nanocapsules and Nanospheres
2.4. Polymeric Nanoparticles
2.5. Cyclodextrins
| Nanoplatform | Goal | Reference |
|---|---|---|
| Silica nanoparticles | Stabilise | [93,94] |
| Lipid-based nanoparticles | Prolong the release | [98,100,101,102,103,106,108,110] |
| Stabilise | [99,103,107,110] | |
| Improve solubility | [104] | |
| Improve bioavailability | [105] | |
| Improve antioxidant activity | [108] | |
| Nanocapsules and nanospheres | Counteract pro-oxidant activity | [43] |
| Preserve antioxidant activity | [111] | |
| Improve the stability | [114] | |
| Prolong the release | [111,113,114] | |
| Polymeric nanoparticle | Improve the solubility and bioavailability | [118,119,120] |
| Improve the release | [120] | |
| Cyclodextrins | Improve the stability and bioavailability | [76,126,127] |
3. Exploring the Role of α-Lipoic Acid in Therapeutic Nanoplatforms
3.1. Renewing Tissues: Nanoparticles and α-Lipoic Acid in Regenerative Medicine
3.2. Exploring the Use of α-Lipoic Acid-Containing Nanoparticles in Tumour Fighting
| Tumour Target | Molecule | Nanoplatform | Goal | Reference |
|---|---|---|---|---|
| Acidic pH and Overexpressed ASGPR * | Dimethylmaleic acid—PEG and lactobionic acid | Polymeric nanoparticle | Delivery of Doxorubicin | [138] |
| Overexpressed ASGPR and folate receptor | Pullulan and folic acid | Polymeric nanoparticle | Delivery of Paclitaxel | [148] |
| Overexpressed folate receptor | Folic acid | Silica hybrid Magnetic nanoparticles | Delivery of Doxorubicin | [143] |
| Acidic pH | Histidine | Polymeric nanoparticle | Delivery of Doxorubicin | [139] |
| Dimethylmaleic anhydride | Polymeric nanoparticle | Delivery of Doxorubicin | [140] | |
| Histidine—PEG | Liposomes | Delivery of VEGF *** siRNA and Etoposide | [141] | |
| PEG | Albumin-based nanocarrier | Delivery of Doxorubicin | [142] | |
| CD44 receptor overexpression | Hyaluronic acid | Polymeric nanoparticle | Delivery 17α-Methyltestosterone | [134] |
| Hyaluronic acid | Polymeric nanoparticle | Delivery of Doxorubicin | [135] | |
| Tumour esterase overexpression | Chlorambucil | Polymeric nanoparticle | Delivery of Doxorubicin and Chlorambucil | [136] |
| αvβ3 receptor overexpression | cRGD peptide ** | Micelle | Delivery of Doxorubicin | [137] |
3.3. Exploring Miscellaneous Applications of Nanoparticles and α-Lipoic Acid
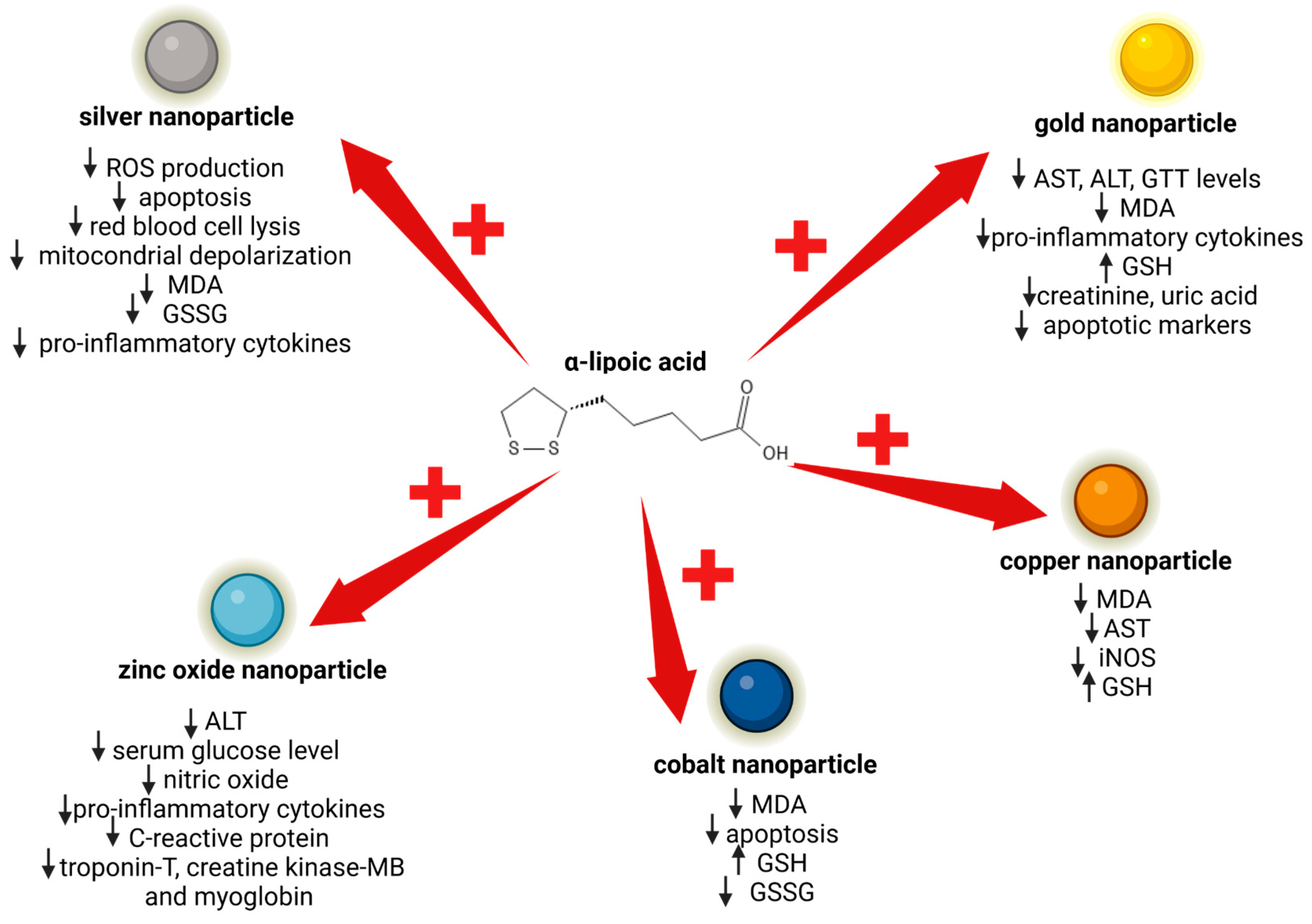
4. Protective Role of α-Lipoic Acid against Nanoparticle Cytotoxicity
4.1. α-Lipoic Acid and Silver Nanoparticle Cytotoxicity
4.2. α-Lipoic Acid and Gold Nanoparticle Renal and Hepatic Toxicity
4.3. α-Lipoic Acid and the Inhibition of Toxicity Induced by Other Types of Nanoparticles
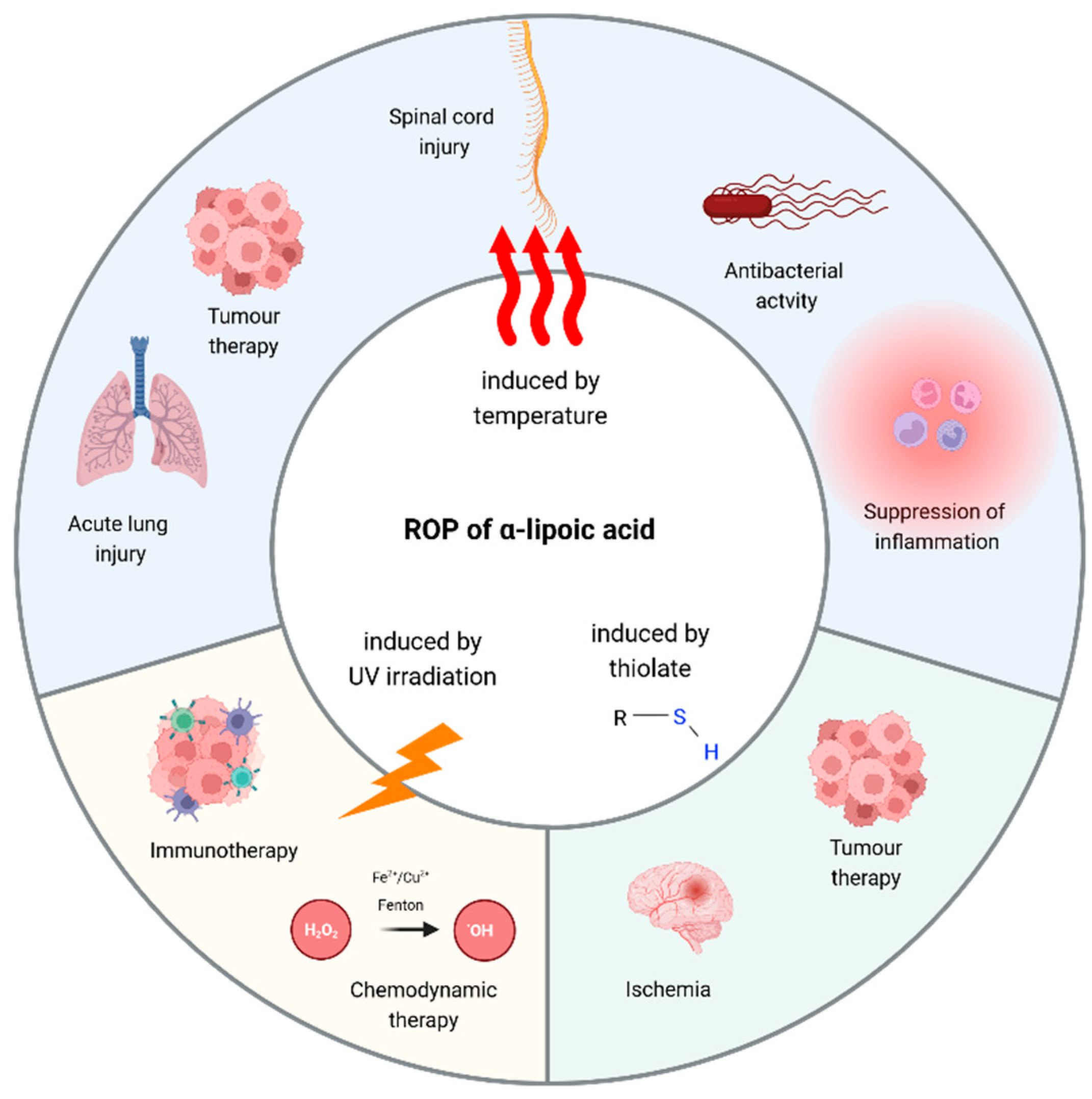
5. Poly(α-Lipoic Acid)-Based Polymeric Nanoparticles: Insights and Perspectives
5.1. Poly(α-Lipoic Acid) Nanoparticles Obtained by Thermal Polymerization
5.2. Poly(α-Lipoic Acid) Nanoparticles Obtained by Thiolate-Initiated Polymerization
5.3. Poly(α-Lipoic Acid) Nanoparticles Obtained by UV light
5.4. Harnessing Poly(α-Lipoic Acid) in Hydrogel Engineering
6. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, J.-Q.; Ling, X.; Wang, H.-J.; Chen, F.-E. α-Lipoic Acid Chemistry: The Past 70 Years. RSC Adv. 2023, 13, 36346–36363. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Li, W.; Huang, S.; Zhao, L.; Zhang, J.; Ji, C.; Ma, Q. R- Is Superior to S-Form of α-Lipoic Acid in Anti-Inflammatory and Antioxidant Effects in Laying Hens. Antioxidants 2022, 11, 1530. [Google Scholar] [CrossRef] [PubMed]
- Lechner, S.; Steimbach, R.R.; Wang, L.; Deline, M.L.; Chang, Y.-C.; Fromme, T.; Klingenspor, M.; Matthias, P.; Miller, A.K.; Médard, G.; et al. Chemoproteomic Target Deconvolution Reveals Histone Deacetylases as Targets of (R)-Lipoic Acid. Nat. Commun. 2023, 14, 3548. [Google Scholar] [CrossRef] [PubMed]
- Pacini, A.; Tomassoni, D.; Trallori, E.; Micheli, L.; Amenta, F.; Ghelardini, C.; Di Cesare Mannelli, L.; Traini, E. Comparative Assessment of the Activity of Racemic and Dextrorotatory Forms of Thioctic (Alpha-Lipoic) Acid in Low Back Pain: Preclinical Results and Clinical Evidences From an Open Randomized Trial. Front. Pharmacol. 2021, 12, 607572. [Google Scholar] [CrossRef] [PubMed]
- Uchida, R.; Okamoto, H.; Ikuta, N.; Terao, K.; Hirota, T. Investigation of Enantioselective Membrane Permeability of α-Lipoic Acid in Caco-2 and MDCKII Cell. Int. J. Mol. Sci. 2016, 17, 155. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.R.; Thiagaraj, H.V.; Seaver, B.; Parker, K.K. Differential Activity of Lipoic Acid Enantiomers in Cell Culture. J. Herb. Pharmacother. 2005, 5, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Shay, K.P.; Moreau, R.F.; Smith, E.J.; Smith, A.R.; Hagen, T.M. Alpha-Lipoic Acid as a Dietary Supplement: Molecular Mechanisms and Therapeutic Potential. Biochim. Biophys. Acta (BBA) Gen. Subj. 2009, 1790, 1149–1160. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Berkay Yılmaz, Y.; Antika, G.; Boyunegmez Tumer, T.; Fawzi Mahomoodally, M.; Lobine, D.; Akram, M.; Riaz, M.; Capanoglu, E.; Sharopov, F.; et al. Insights on the Use of α-Lipoic Acid for Therapeutic Purposes. Biomolecules 2019, 9, 356. [Google Scholar] [CrossRef]
- Amenta, F.; Buccioni, M.; Ben, D.D.; Lambertucci, C.; Navia, A.M.; Ngouadjeu Ngnintedem, M.A.; Ricciutelli, M.; Spinaci, A.; Volpini, R.; Marucci, G. Ex-Vivo Absorption Study of Lysine R-Lipoate Salt, a New Pharmaceutical Form of R-ALA. Eur. J. Pharm. Sci. 2018, 118, 200–207. [Google Scholar] [CrossRef]
- Lucarini, E.; Trallori, E.; Tomassoni, D.; Amenta, F.; Ghelardini, C.; Pacini, A.; Di Cesare Mannelli, L. Toxicological Profile of the Pain-Relieving Antioxidant Compound Thioctic Acid in Its Racemic and Enantiomeric Forms. Antioxidants 2020, 9, 749. [Google Scholar] [CrossRef]
- Tripathi, A.K.; Ray, A.K.; Mishra, S.K.; Bishen, S.M.; Mishra, H.; Khurana, A. Molecular and Therapeutic Insights of Alpha-Lipoic Acid as a Potential Molecule for Disease Prevention. Rev. Bras. Farmacogn. 2023, 33, 272–287. [Google Scholar] [CrossRef] [PubMed]
- Castañeda-Arriaga, R.; Alvarez-Idaboy, J.R. Lipoic Acid and Dihydrolipoic Acid. A Comprehensive Theoretical Study of Their Antioxidant Activity Supported by Available Experimental Kinetic Data. J. Chem. Inf. Model. 2014, 54, 1642–1652. [Google Scholar] [CrossRef] [PubMed]
- Bjørklund, G.; Aaseth, J.; Crisponi, G.; Rahman, M.; Chirumbolo, S. Insights on Alpha Lipoic and Dihydrolipoic Acids as Promising Scavengers of Oxidative Stress and Possible Chelators in Mercury Toxicology. J. Inorg. Biochem. 2019, 195, 111–119. [Google Scholar] [CrossRef]
- Gurer, H.; Ozgunes, H.; Oztezcan, S.; Ercal, N. Antioxidant Role of α-Lipoic Acid in Lead Toxicity. Free Radic. Biol. Med. 1999, 27, 75–81. [Google Scholar] [CrossRef]
- Ou, P.; Tritschler, H.J.; Wolff, S.P. Thioctic (Lipoic) Acid: A Therapeutic Metal-Chelating Antioxidant? Biochem. Pharmacol. 1995, 50, 123–126. [Google Scholar] [CrossRef] [PubMed]
- Koufaki, M.; Detsi, A.; Kiziridi, C. Multifunctional Lipoic Acid Conjugates. CMC 2009, 16, 4728–4742. [Google Scholar] [CrossRef] [PubMed]
- Gorąca, A.; Huk-Kolega, H.; Piechota, A.; Kleniewska, P.; Ciejka, E.; Skibska, B. Lipoic Acid—Biological Activity and Therapeutic Potential. Pharmacol. Rep. 2011, 63, 849–858. [Google Scholar] [CrossRef]
- Packer, L.; Cadenas, E. Lipoic Acid: Energy Metabolism and Redox Regulation of Transcription and Cell Signaling. J. Clin. Biochem. Nutr. 2010, 48, 26–32. [Google Scholar] [CrossRef]
- Smith, A.R.; Shenvi, S.V.; Widlansky, M.; Suh, J.H.; Hagen, T.M. Lipoic Acid as a Potential Therapy for Chronic Diseases Associated with Oxidative Stress. Curr. Med. Chem. 2004, 11, 1135–1146. [Google Scholar] [CrossRef]
- Tibullo, D.; Li Volti, G.; Giallongo, C.; Grasso, S.; Tomassoni, D.; Anfuso, C.D.; Lupo, G.; Amenta, F.; Avola, R.; Bramanti, V. Biochemical and Clinical Relevance of Alpha Lipoic Acid: Antioxidant and Anti-Inflammatory Activity, Molecular Pathways and Therapeutic Potential. Inflamm. Res. 2017, 66, 947–959. [Google Scholar] [CrossRef]
- Namazi, N.; Larijani, B.; Azadbakht, L. Alpha-Lipoic Acid Supplement in Obesity Treatment: A Systematic Review and Meta-Analysis of Clinical Trials. Clin. Nutr. 2018, 37, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Bobe, G.; Michels, A.J.; Zhang, W.-J.; Purnell, J.Q.; Woffendin, C.; Pereira, C.; Vita, J.A.; Thomas, N.O.; Traber, M.G.; Frei, B.; et al. A Randomized Controlled Trial of Long-Term (R)-α-Lipoic Acid Supplementation Promotes Weight Loss in Overweight or Obese Adults without Altering Baseline Elevated Plasma Triglyceride Concentrations. J. Nutr. 2020, 150, 2336–2345. [Google Scholar] [CrossRef] [PubMed]
- Golbidi, S.; Badran, M.; Laher, I. Diabetes and Alpha Lipoic Acid. Front. Pharmacol. 2011, 2, 69. [Google Scholar] [CrossRef]
- Ziegler, D. Pathogenetic Treatments for Diabetic Peripheral Neuropathy. Diabetes Res. Clin. Pract. 2023, 206, 110764. [Google Scholar] [CrossRef] [PubMed]
- Derosa, G.; D’Angelo, A.; Romano, D.; Maffioli, P. A Clinical Trial about a Food Supplement Containing α-Lipoic Acid on Oxidative Stress Markers in Type 2 Diabetic Patients. Int. J. Mol. Sci. 2016, 17, 1802. [Google Scholar] [CrossRef] [PubMed]
- Capece, U.; Moffa, S.; Improta, I.; Di Giuseppe, G.; Nista, E.C.; Cefalo, C.M.A.; Cinti, F.; Pontecorvi, A.; Gasbarrini, A.; Giaccari, A.; et al. Alpha-Lipoic Acid and Glucose Metabolism: A Comprehensive Update on Biochemical and Therapeutic Features. Nutrients 2022, 15, 18. [Google Scholar] [CrossRef] [PubMed]
- Kamt, S.F.; Liu, J.; Yan, L.-J. Renal-Protective Roles of Lipoic Acid in Kidney Disease. Nutrients 2023, 15, 1732. [Google Scholar] [CrossRef]
- Basu, P.P.; Shah, N.J.; Aloysius, M.M.; Brown, R.S., Jr. Effect of Vitamin E and Alpha Lipoic Acid in Nonalcoholic Fatty Liver Disease: A Randomized, Placebo-Controlled, Open-Label, Prospective Clinical Trial (VAIN Trial). OJGas 2014, 4, 199–207. [Google Scholar] [CrossRef]
- Kaya-Dagistanli, F.; Tanriverdi, G.; Altinok, A.; Ozyazgan, S.; Ozturk, M. The Effects of Alpha Lipoic Acid on Liver Cells Damages and Apoptosis Induced by Polyunsaturated Fatty Acids. Food Chem. Toxicol. 2013, 53, 84–93. [Google Scholar] [CrossRef]
- Longhitano, L.; Distefano, A.; Musso, N.; Bonacci, P.; Orlando, L.; Giallongo, S.; Tibullo, D.; Denaro, S.; Lazzarino, G.; Ferrigno, J.; et al. (+)-Lipoic Acid Reduces Mitochondrial Unfolded Protein Response and Attenuates Oxidative Stress and Aging in an in Vitro Model of Non-Alcoholic Fatty Liver Disease. J. Transl. Med. 2024, 22, 82. [Google Scholar] [CrossRef]
- Uskoković, A.; Dinić, S.; Jovanović, J.A.; Poznanović, G.; Vidaković, M.; Mihailović, M. Liver Diseases: Epigenetic Mechanisms, Oxidative Stress and Use of Alpha-Lipoic Acid. In Handbook of Nutrition, Diet, and Epigenetics; Patel, V., Preedy, V., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 1–21. ISBN 978-3-319-31143-2. [Google Scholar]
- Bustamante, J. α-Lipoic Acid in Liver Metabolism and Disease. Free Radic. Biol. Med. 1998, 24, 1023–1039. [Google Scholar] [CrossRef] [PubMed]
- El-Maadawy, W.; Hammam, O.; Seif el-Din, S.; El-Lakkany, N. α-Lipoic Acid Modulates Liver Fibrosis: A Cross Talk between TGF-Β1, Autophagy, and Apoptosis. Hum. Exp. Toxicol. 2020, 39, 440–450. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.-F.; Sun, A.-J. Cardioprotective Effect of Alpha-Lipoic Acid and Its Mechanisms. Cardiol. Plus 2020, 5, 109–117. [Google Scholar] [CrossRef]
- Wollin, S.D.; Jones, P.J.H. α-Lipoic Acid and Cardiovascular Disease. J. Nutr. 2003, 133, 3327–3330. [Google Scholar] [CrossRef] [PubMed]
- Skibska, B.; Goraca, A. The Protective Effect of Lipoic Acid on Selected Cardiovascular Diseases Caused by Age-Related Oxidative Stress. Oxidative Med. Cell. Longev. 2015, 2015, 313021. [Google Scholar] [CrossRef] [PubMed]
- Hajizadeh-Sharafabad, F.; Sharifi Zahabi, E. Role of Alpha-Lipoic Acid in Vascular Function: A Systematic Review of Human Intervention Studies. Crit. Rev. Food Sci. Nutr. 2022, 62, 2928–2941. [Google Scholar] [CrossRef] [PubMed]
- Rochette, L.; Ghibu, S. Mechanics Insights of Alpha-Lipoic Acid against Cardiovascular Diseases during COVID-19 Infection. Int. J. Mol. Sci. 2021, 22, 7979. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.; Singh, T.G.; Dahiya, R.S.; Abdel-Daim, M.M. α-Lipoic Acid, an Organosulfur Biomolecule a Novel Therapeutic Agent for Neurodegenerative Disorders: An Mechanistic Perspective. Neurochem. Res. 2022, 47, 1853–1864. [Google Scholar] [CrossRef] [PubMed]
- Kaur, D.; Behl, T.; Sehgal, A.; Singh, S.; Sharma, N.; Chigurupati, S.; Alhowail, A.; Abdeen, A.; Ibrahim, S.F.; Vargas-De-La-Cruz, C.; et al. Decrypting the Potential Role of α-Lipoic Acid in Alzheimer’s Disease. Life Sci. 2021, 284, 119899. [Google Scholar] [CrossRef]
- Xie, H.; Yang, X.; Cao, Y.; Long, X.; Shang, H.; Jia, Z. Role of Lipoic Acid in Multiple Sclerosis. CNS Neurosci. Ther. 2022, 28, 319–331. [Google Scholar] [CrossRef]
- Liu, W.; Shi, L.; Li, S. The Immunomodulatory Effect of Alpha-Lipoic Acid in Autoimmune Diseases. BioMed Res. Int. 2019, 2019, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Külkamp-Guerreiro, I.C.; Souza, M.N.; Bianchin, M.D.; Isoppo, M.; Freitas, J.S.; Alves, J.A.; Piovezan, A.P.; Pohlmann, A.R.; Guterres, S.S. Evaluation of Lipoic Acid Topical Application on Rats Skin Wound Healing. Acta Cir. Bras. 2013, 28, 708–715. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lateef, H.; Aslam, M.N.; Stevens, M.J.; Varani, J. Pretreatment of Diabetic Rats with Lipoic Acid Improves Healing of Subsequently-Induced Abrasion Wounds. Arch. Dermatol. Res. 2005, 297, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Xiao, Y.; Jiang, Y.; Luo, J.; Yuan, J.; Yan, J.; Tong, Q. Alpha-Lipoic Acid Promotes Intestinal Epithelial Injury Repair by Regulating MAPK Signaling Pathways. Mediat. Inflamm. 2022, 2022, 1894379. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.; Rodrigues, P.M.; Pintado, M.; Tavaria, F.K. A Systematic Review of Natural Products for Skin Applications: Targeting Inflammation, Wound Healing, and Photo-Aging. Phytomedicine 2023, 115, 154824. [Google Scholar] [CrossRef] [PubMed]
- De Bengy, A.-F.; Decorps, J.; Martin, L.S.; Pagnon, A.; Chevalier, F.P.; Sigaudo-Roussel, D.; Fromy, B. Alpha-Lipoic Acid Supplementation Restores Early Age-Related Sensory and Endothelial Dysfunction in the Skin. Biomedicines 2022, 10, 2887. [Google Scholar] [CrossRef] [PubMed]
- Beitner, H. Randomized, Placebo-Controlled, Double Blind Study on the Clinical Efficacy of a Cream Containing 5%alpha-Lipoic Acid Related to Photoageing of Facial Skin. Br. J. Dermatol. 2003, 149, 841–849. [Google Scholar] [CrossRef] [PubMed]
- Farhat, D.; Lincet, H. Lipoic Acid a Multi-Level Molecular Inhibitor of Tumorigenesis. Biochim. Biophys. Acta (BBA) Rev. Cancer 2020, 1873, 188317. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, K.M.; Sharma, G.; Takkar, S.; Kaushal, J.B.; Pothuraju, R.; Chakravarti, B.; Batra, S.K.; Siddiqui, J.A. α-Lipoic Acid Modulates Prostate Cancer Cell Growth and Bone Cell Differentiation. Sci. Rep. 2024, 14, 4404. [Google Scholar] [CrossRef]
- Chakravarti, B.; Rajput, S.; Raza, S.; Rajak, S.; Tewari, A.; Gupta, P.; Upadhyay, A.; Chattopadhyay, N.; Sinha, R.A. Lipoic Acid Blocks Autophagic Flux and Impairs Cellular Bioenergetics in Breast Cancer and Reduces Stemness. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2022, 1868, 166455. [Google Scholar] [CrossRef]
- Peng, P.; Zhang, X.; Qi, T.; Cheng, H.; Kong, Q.; Liu, L.; Cao, X.; Ding, Z. Alpha-lipoic Acid Inhibits Lung Cancer Growth via mTOR-mediated Autophagy Inhibition. FEBS Open Bio 2020, 10, 607–618. [Google Scholar] [CrossRef] [PubMed]
- Bossio, S.; Perri, A.; Gallo, R.; De Bartolo, A.; Rago, V.; La Russa, D.; Di Dio, M.; La Vignera, S.; Calogero, A.E.; Vitale, G.; et al. Alpha-Lipoic Acid Reduces Cell Growth, Inhibits Autophagy, and Counteracts Prostate Cancer Cell Migration and Invasion: Evidence from In Vitro Studies. Int. J. Mol. Sci. 2023, 24, 17111. [Google Scholar] [CrossRef] [PubMed]
- Hermann, R.; Mungo, J.; Cnota, P.J.; Ziegler, D. Enantiomer-Selective Pharmacokinetics, Oral Bioavailability, and Sex Effects of Various Alpha-Lipoic Acid Dosage Forms. CPAA 2014, 6, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Hermann, R.; Niebch, G.; Borbe, H.O.; Fieger-Büschges, H.; Ruus, P.; Nowak, H.; Riethmüller-Winzen, H.; Peukert, M.; Blume, H. Enantioselective Pharmacokinetics and Bioavailability of Different Racemic α-Lipoic Acid Formulations in Healthy Volunteers. Eur. J. Pharm. Sci. 1996, 4, 167–174. [Google Scholar] [CrossRef]
- Gleiter, C.H.; Schug, B.S.; Hermann, R.; Elze, M.; Blume, H.H.; Gundert-Remy, U. Influence of Food Intake on the Bioavailability of Thioctic Acid Enantiomers. Eur. J. Clin. Pharmacol. 1996, 50, 513–514. [Google Scholar] [CrossRef] [PubMed]
- Breithaupt-Grögler, K.; Niebch, G.; Schneider, E.; Erb, K.; Hermann, R.; Blume, H.H.; Schug, B.S.; Belz, G.G. Dose-Proportionality of Oral Thioctic Acid—Coincidence of Assessments via Pooled Plasma and Individual Data. Eur. J. Pharm. Sci. 1999, 8, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Teichert, J.; Hermann, R.; Ruus, P.; Preiss, R. Plasma Kinetics, Metabolism, and Urinary Excretion of Alpha-Lipoic Acid Following Oral Administration in Healthy Volunteers. J. Clin. Pharmacol. 2003, 43, 1257–1267. [Google Scholar] [CrossRef] [PubMed]
- Brufani, M.; Figliola, R. (R)-α-Lipoic Acid Oral Liquid Formulation: Pharmacokinetic Parameters and Therapeutic Efficacy. Acta Biomed. 2014, 85, 108–115. [Google Scholar]
- Keith, D.J.; Butler, J.A.; Bemer, B.; Dixon, B.; Johnson, S.; Garrard, M.; Sudakin, D.L.; Christensen, J.M.; Pereira, C.; Hagen, T.M. Age and Gender Dependent Bioavailability of R- and R,S-α-Lipoic Acid: A Pilot Study. Pharmacol. Res. 2012, 66, 199–206. [Google Scholar] [CrossRef]
- Lalić-Popović, M.N.; Vuković, M.M.; Jovičić-Bata, J.N.; Čanji-Panić, J.M.; Todorović, N.B. Comparison of Formulation Characteristics of Drugs and Dietary Supplements Containing Alpha-Lipoic Acid Relevant to Therapeutic Efficacy. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 3159–3170. [Google Scholar] [CrossRef]
- Pop, A.; Crișan, S.; Bârcă, M.; Ciobanu, A.-M.; Varlas, V.; Pop, C.; Pali, M.-A.; Cauni, D.; Ozon, E.; Udeanu, D.; et al. Evaluation of Dissolution Profiles of a Newly Developed Solid Oral Immediate-Release Formula Containing Alpha-Lipoic Acid. Processes 2021, 9, 176. [Google Scholar] [CrossRef]
- Di Martino, P. Human Bioavailability and Pharmacokinetic Profile of Different Formulations Delivering Alpha Lipoic Acid. J. Clin. Cell. Immunol. 2012, 1, 1–6. [Google Scholar] [CrossRef]
- Derosa, G.; D’Angelo, A.; Preti, P.; Maffioli, P. Safety and Efficacy of Alpha Lipoic Acid During 4 Years of Observation: A Retrospective, Clinical Trial in Healthy Subjects in Primary Prevention. DDDT 2020, 14, 5367–5374. [Google Scholar] [CrossRef] [PubMed]
- Parente, E.; Colannino, G.; Picconi, O.; Monastra, G. Safety of Oral Alpha-Lipoic Acid Treatment in Pregnant Women: A Retrospective Observational Study. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 4219–4227. [Google Scholar] [PubMed]
- Cremer, D.R.; Rabeler, R.; Roberts, A.; Lynch, B. Safety Evaluation of α-Lipoic Acid (ALA). Regul. Toxicol. Pharmacol. 2006, 46, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Fogacci, F.; Rizzo, M.; Krogager, C.; Kennedy, C.; Georges, C.M.G.; Knežević, T.; Liberopoulos, E.; Vallée, A.; Pérez-Martínez, P.; Wenstedt, E.F.E.; et al. Safety Evaluation of α-Lipoic Acid Supplementation: A Systematic Review and Meta-Analysis of Randomized Placebo-Controlled Clinical Studies. Antioxidants 2020, 9, 1011. [Google Scholar] [CrossRef] [PubMed]
- Moretti, R.; Angeletti, C.; Minora, S. Multiple Organ Failure and Shock Following Acute Alpha Lipoic Acid (ALA) Intoxication. Clin. Toxicol. 2019, 57, 749–751. [Google Scholar] [CrossRef] [PubMed]
- Halabi, Z.; El Helou, C.; Al Balushi, H.; Gittinger, M.; Steck, A.R.; Kaakour, A.; Abu-Alfa, A.; El Zahran, T. Alpha Lipoic Acid Toxicity: The First Reported Mortality in an Adult Patient After Multiorgan Failure. J. Emerg. Med. 2023, 64, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Gatti, M.; Ippoliti, I.; Poluzzi, E.; Antonazzo, I.C.; Moro, P.A.; Moretti, U.; Menniti-Ippolito, F.; Mazzanti, G.; De Ponti, F.; Raschi, E. Assessment of Adverse Reactions to α-Lipoic Acid Containing Dietary Supplements through Spontaneous Reporting Systems. Clin. Nutr. 2021, 40, 1176–1185. [Google Scholar] [CrossRef]
- Theodosis-Nobelos, P.; Papagiouvannis, G.; Tziona, P.; Rekka, E.A. Lipoic Acid. Kinetics and Pluripotent Biological Properties and Derivatives. Mol. Biol. Rep. 2021, 48, 6539–6550. [Google Scholar] [CrossRef]
- Vigil, M.; Berkson, B.M.; Garcia, A.P. Adverse Effects of High Doses of Intravenous Alpha Lipoic Acid on Liver Mitochondria. Glob. Adv. Health Med. 2014, 3, 25–27. [Google Scholar] [CrossRef] [PubMed]
- Usacheva, A.M.; Chernikov, A.V.; Karmanova, E.E.; Bruskov, V.I. Pharmacological Aspects of the Use of Lipoic Acid (Review). Pharm. Chem. J. 2022, 55, 1138–1146. [Google Scholar] [CrossRef]
- Çakatay, U. Pro-Oxidant Actions of α-Lipoic Acid and Dihydrolipoic Acid. Med. Hypotheses 2006, 66, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Bhatti, F.; Mankhey, R.W.; Asico, L.; Quinn, M.T.; Welch, W.J.; Maric, C. Mechanisms of Antioxidant and Pro-Oxidant Effects of α-Lipoic Acid in the Diabetic and Nondiabetic Kidney. Kidney Int. 2005, 67, 1371–1380. [Google Scholar] [CrossRef]
- Ikuta, N.; Sugiyama, H.; Shimosegawa, H.; Nakane, R.; Ishida, Y.; Uekaji, Y.; Nakata, D.; Pallauf, K.; Rimbach, G.; Terao, K.; et al. Analysis of the Enhanced Stability of R(+)-Alpha Lipoic Acid by the Complex Formation with Cyclodextrins. Int. J. Mol. Sci. 2013, 14, 3639–3655. [Google Scholar] [CrossRef] [PubMed]
- Nishiura, H. A Novel Nano-Capsule of α-Lipoic Acid as a Template of Core-Shell Structure Constructed by Self-Assembly. J. Nanomed. Nanotechnol. 2012, 4, 2. [Google Scholar] [CrossRef]
- Wada, N.; Wakami, H.; Konishi, T.; Matsugo, S. The Degradation and Regeneration of α-Lipoic Acid under the Irradiation of UV Light in the Existence of Homocysteine. J. Clin. Biochem. Nutr. 2009, 44, 218–222. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.R.; Edwards, J.O. Effect of Solvent on the Photolysis of .Alpha.-Lipoic Acid. J. Org. Chem. 1969, 34, 3131–3135. [Google Scholar] [CrossRef] [PubMed]
- Moghimi, S.M.; Hunter, A.C.; Murray, J.C. Nanomedicine: Current Status and Future Prospects. FASEB J. 2005, 19, 311–330. [Google Scholar] [CrossRef]
- Haleem, A.; Javaid, M.; Singh, R.P.; Rab, S.; Suman, R. Applications of Nanotechnology in Medical Field: A Brief Review. Glob. Health J. 2023, 7, 70–77. [Google Scholar] [CrossRef]
- Malik, S.; Muhammad, K.; Waheed, Y. Emerging Applications of Nanotechnology in Healthcare and Medicine. Molecules 2023, 28, 6624. [Google Scholar] [CrossRef] [PubMed]
- Ng, P.Q.; Ling, L.S.C.; Chellian, J.; Madheswaran, T.; Panneerselvam, J.; Kunnath, A.P.; Gupta, G.; Satija, S.; Mehta, M.; Hansbro, P.M.; et al. Applications of Nanocarriers as Drug Delivery Vehicles for Active Phytoconstituents. CPD 2020, 26, 4580–4590. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.; Roy, P.; Sharma, R.; Kasana, R.; Rathore, P.; Gupta, T.K. Recent Nanotheranostic Approaches in Cancer Research. Clin. Exp. Med. 2024, 24, 8. [Google Scholar] [CrossRef] [PubMed]
- Shafaei, N.; Khorshidi, S.; Karkhaneh, A. The Immune-Stealth Polymeric Coating on Drug Delivery Nanocarriers: In Vitro Engineering and in Vivo Fate. J. Biomater. Appl. 2023, 38, 159–178. [Google Scholar] [CrossRef] [PubMed]
- Aljabali, A.A.; Obeid, M.A.; Bashatwah, R.M.; Serrano-Aroca, Á.; Mishra, V.; Mishra, Y.; El-Tanani, M.; Hromić-Jahjefendić, A.; Kapoor, D.N.; Goyal, R.; et al. Nanomaterials and Their Impact on the Immune System. Int. J. Mol. Sci. 2023, 24, 2008. [Google Scholar] [CrossRef] [PubMed]
- Papini, E.; Tavano, R.; Mancin, F. Opsonins and Dysopsonins of Nanoparticles: Facts, Concepts, and Methodological Guidelines. Front. Immunol. 2020, 11, 567365. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, C.; Oliveira, C.; Benfeito, S.; Soares, P.; Garrido, J.; Borges, F. Nanotechnology and Antioxidant Therapy: An Emerging Approach for Neurodegenerative Diseases. CMC 2014, 21, 4311–4327. [Google Scholar] [CrossRef] [PubMed]
- Cartaya, A.; Maiocchi, S.; Bahnson, E.M. Nanotherapies for Treatment of Cardiovascular Disease: A Case for Antioxidant Targeted Delivery. Curr. Pathobiol. Rep. 2019, 7, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Liu, H.; Song, L. Novel Drug Delivery Systems Targeting Oxidative Stress in Chronic Obstructive Pulmonary Disease: A Review. J. Nanobiotechnol. 2020, 18, 145. [Google Scholar] [CrossRef]
- Zhu, X.; Yuan, W.; Li, Z.; Lin, Y.; Li, W.; Ji, L.; Wang, D.; Zhang, H.; Wang, Y. Progress of Research on Antioxidants and Carriers for Skin Wound Repair. Processes 2023, 11, 2069. [Google Scholar] [CrossRef]
- Omran, B.; Baek, K.-H. Nanoantioxidants: Pioneer Types, Advantages, Limitations, and Future Insights. Molecules 2021, 26, 7031. [Google Scholar] [CrossRef] [PubMed]
- Dolinina, E.S.; Akimsheva, E.Y.; Parfenyuk, E.V. Development of Novel Silica-Based Formulation of α-Lipoic Acid: Evaluation of Photo and Thermal Stability of the Encapsulated Drug. Pharmaceutics 2020, 12, 228. [Google Scholar] [CrossRef] [PubMed]
- Dolinina, E.S.; Parfenyuk, E.V. Development of Novel Oral Formulations of Disulfide Antioxidants Based on Porous Silica for Controlled Release of the Drugs. Materials 2021, 14, 963. [Google Scholar] [CrossRef] [PubMed]
- Tenchov, R.; Bird, R.; Curtze, A.E.; Zhou, Q. Lipid Nanoparticles─From Liposomes to mRNA Vaccine Delivery, a Landscape of Research Diversity and Advancement. ACS Nano 2021, 15, 16982–17015. [Google Scholar] [CrossRef] [PubMed]
- Naseri, N.; Valizadeh, H.; Zakeri-Milani, P. Solid Lipid Nanoparticles and Nanostructured Lipid Carriers: Structure, Preparation and Application. Adv. Pharm. Bull. 2015, 5, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Viegas, C.; Patrício, A.B.; Prata, J.M.; Nadhman, A.; Chintamaneni, P.K.; Fonte, P. Solid Lipid Nanoparticles vs. Nanostructured Lipid Carriers: A Comparative Review. Pharmaceutics 2023, 15, 1593. [Google Scholar] [CrossRef] [PubMed]
- Ruktanonchai, U.; Bejrapha, P.; Sakulkhu, U.; Opanasopit, P.; Bunyapraphatsara, N.; Junyaprasert, V.; Puttipipatkhachorn, S. Physicochemical Characteristics, Cytotoxicity, and Antioxidant Activity of Three Lipid Nanoparticulate Formulations of Alpha-Lipoic Acid. AAPS PharmSciTech 2009, 10, 227. [Google Scholar] [CrossRef] [PubMed]
- Dhaundiyal, A.; Jena, S.K.; Samal, S.K.; Sonvane, B.; Chand, M.; Sangamwar, A.T. Alpha-Lipoic Acid–Stearylamine Conjugate-Based Solid Lipid Nanoparticles for Tamoxifen Delivery: Formulation, Optimization, in-Vivo Pharmacokinetic and Hepatotoxicity Study. J. Pharm. Pharmacol. 2016, 68, 1535–1550. [Google Scholar] [CrossRef] [PubMed]
- Kothari, I.R.; Mazumdar, S.; Sharma, S.; Italiya, K.; Mittal, A.; Chitkara, D. Docetaxel and Alpha-Lipoic Acid Co-Loaded Nanoparticles for Cancer Therapy. Ther. Deliv. 2019, 10, 227–240. [Google Scholar] [CrossRef]
- Wang, J.; Xia, Q. Alpha-Lipoic Acid-Loaded Nanostructured Lipid Carrier: Sustained Release and Biocompatibility to HaCaT Cells in Vitro. Drug Deliv. 2014, 21, 328–341. [Google Scholar] [CrossRef]
- Shchelkonogov, V.A.; Alyaseva, S.O.; Lotosh, N.Y.; Baranova, O.A.; Chekanov, A.V.; Solov’eva, E.Y.; Kamyshinskii, R.A.; Vasilov, R.G.; Shastina, N.S.; Korepanova, E.A.; et al. Lipoic Acid Nanoforms Based on Phosphatidylcholine: Production and Characteristics. Eur. Biophys. J. 2020, 49, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Çoban, Ö.; Yıldırım, S.; Bakır, T. Alpha-Lipoic Acid and Cyanocobalamin Co-Loaded Nanoemulsions: Development, Characterization, and Evaluation of Stability. J. Pharm. Innov. 2022, 17, 510–520. [Google Scholar] [CrossRef]
- Badr-Eldin, S.M.; Fahmy, U.A.; Aldawsari, H.M.; Ahmed, O.A.A.; Alhakamy, N.A.; Okbazghi, S.Z.; El-Moselhy, M.A.; Alghaith, A.F.; Anter, A.; Matouk, A.I.; et al. Optimized Self-Nanoemulsifying Delivery System Based on Plant-Derived Oil Augments Alpha-Lipoic Acid Protective Effects Against Experimentally Induced Gastric Lesions. Dose-Response 2021, 19, 155932582110012. [Google Scholar] [CrossRef] [PubMed]
- Halder, S.; Mibe, Y.; Rikimura, S.; Kuromi, K.; Sato, H.; Onoue, S. Strategic Application of Liposomal System to R-α-Lipoic Acid for the Improvement of Nutraceutical Properties. Drug Dev. Ind. Pharm. 2022, 48, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Curcio, M.; Cirillo, G.; Amato, R.; Guidotti, L.; Amantea, D.; De Luca, M.; Nicoletta, F.P.; Iemma, F.; Garcia-Gil, M. Encapsulation of Alpha-Lipoic Acid in Functional Hybrid Liposomes: Promising Tool for the Reduction of Cisplatin-Induced Ototoxicity. Pharmaceuticals 2022, 15, 394. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Wang, Q.; Li, T.; Xia, N.; Xia, Q. Multilayer Emulsions as a Strategy for Linseed Oil and A-lipoic Acid Micro-encapsulation: Study on Preparation and In Vitro Characterization. J. Sci. Food Agric. 2018, 98, 3513–3523. [Google Scholar] [CrossRef]
- Zhao, G.D.; Sun, R.; Ni, S.L.; Xia, Q. Development and Characterisation of a Novel Chitosan-Coated Antioxidant Liposome Containing Both Coenzyme Q10 and Alpha-Lipoic Acid. J. Microencapsul. 2015, 32, 157–165. [Google Scholar] [CrossRef]
- Li, W.; Peng, J.; Yang, Q.; Chen, L.; Zhang, L.; Chen, X.; Qian, Z. α-Lipoic Acid Stabilized DTX/IR780 Micelles for Photoacoustic/Fluorescence Imaging Guided Photothermal Therapy/Chemotherapy of Breast Cancer. Biomater. Sci. 2018, 6, 1201–1216. [Google Scholar] [CrossRef]
- Jung, S.Y.; Yoo, J.; Yang, K.-J.; Jang, S.; Yi, G.; Kim, D.-K.; Koo, H. Intratympanic Administration of Alpha-Lipoic Acid-Loaded Pluronic F-127 Nanoparticles Ameliorates Acute Hearing Loss. Nanomed. Nanotechnol. Biol. Med. 2021, 32, 102329. [Google Scholar] [CrossRef]
- Xia, N.; Liu, T.; Wang, Q.; Xia, Q.; Bian, X. In Vitro Evaluation of α-Lipoic Acid-Loaded Lipid Nanocapsules for Topical Delivery. J. Microencapsul. 2017, 34, 571–581. [Google Scholar] [CrossRef]
- Külkamp, I.C.; Rabelo, B.D.; Berlitz, S.J.; Isoppo, M.; Bianchin, M.D.; Schaffazick, S.R.; Pohlmann, A.R.; Guterres, S.S. Nanoencapsulation Improves the <I>In Vitro</I> Antioxidant Activity of Lipoic Acid. J. Biomed. Nanotechnol. 2011, 7, 598–607. [Google Scholar] [CrossRef] [PubMed]
- Davies, S.; Contri, R.V.; Guterres, S.S.; Pohlmann, A.R.; Guerreiro, I.C.K. Simultaneous Nanoencapsulation of Lipoic Acid and Resveratrol with Improved Antioxidant Properties for the Skin. Colloids Surf. B Biointerfaces 2020, 192, 111023. [Google Scholar] [CrossRef] [PubMed]
- Rageh, M.M.; El-Gebaly, R.H. Antioxidant Activities of α-Lipoic Acid Free and Nano-Capsule Inhibit the Growth of Ehrlich Carcinoma. Mol. Biol. Rep. 2019, 46, 3141–3148. [Google Scholar] [CrossRef] [PubMed]
- EL-Gebaly, R.H.; Rageh, M.M.; Maamoun, I.K. Radio-Protective Potential of Lipoic Acid Free and Nano-Capsule against 99mTc-MIBI Induced Injury in Cardio Vascular Tissue. XST 2019, 27, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Kubota, Y.; Musashi, M.; Nagasawa, T.; Shimura, N.; Igarashi, R.; Yamaguchi, Y. Novel Nanocapsule of A-lipoic Acid Reveals Pigmentation Improvement: α-Lipoic Acid Stimulates the Proliferation and Differentiation of Keratinocyte in Murine Skin by Topical Application. Exp. Dermatol. 2019, 28, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Gogoi, P.; Dutta, A.; Ramteke, A.; Maji, T.K. Preparation, Characterization and Cytotoxic Applications of Curcumin-(±) α-lipoic Acid Coloaded Phosphorylated Chitosan Nanoparticles in MDA MB 231 Breast Cancer Cell Line. Polym. Adv. Technol. 2020, 31, 2827–2841. [Google Scholar] [CrossRef]
- Metwaly, H.H.; Fathy, S.A.; Abdel Moneim, M.M.; Emam, M.A.; Soliman, A.F.; El-Naggar, M.E.; Omara, E.A.; El-Bana, M.A. Chitosan and Solid Lipid Nanoparticles Enhance the Efficiency of Alpha-Lipoic Acid against Experimental Neurotoxicity. Toxicol. Mech. Methods 2022, 32, 268–279. [Google Scholar] [CrossRef] [PubMed]
- Campos, P.M.; Praça, F.G.; Mussi, S.V.; Figueiredo, S.A.; Fantini, M.C.D.A.; Fonseca, M.J.V.; Torchilin, V.P.; Bentley, M.V.L.B. Liquid Crystalline Nanodispersion Functionalized with Cell-Penetrating Peptides Improves Skin Penetration and Anti-Inflammatory Effect of Lipoic Acid after in Vivo Skin Exposure to UVB Radiation. Drug Deliv. Transl. Res. 2020, 10, 1810–1828. [Google Scholar] [CrossRef]
- Park, C.H.; Lee, K.-U.; Park, J.-Y.; Koh, E.-H.; Kim, H.-S.; Lee, J. Lipoic Acid Nanoparticles: Effect of Polymeric Stabilizer on Appetite Suppression. Pharmazie 2010, 65, 580–584. [Google Scholar]
- Del Valle, E.M.M. Cyclodextrins and Their Uses: A Review. Process Biochem. 2004, 39, 1033–1046. [Google Scholar] [CrossRef]
- Muankaew, C.; Loftsson, T. Cyclodextrin-Based Formulations: A Non-Invasive Platform for Targeted Drug Delivery. Basic Clin. Pharmacol. Toxicol. 2018, 122, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Uekama, K.; Hirayama, F.; Arima, H. Recent Aspect of Cyclodextrin-Based Drug Delivery System. J. Incl. Phenom. Macrocycl. Chem. 2006, 56, 3–8. [Google Scholar] [CrossRef]
- Ikuta, N.; Tanaka, A.; Otsubo, A.; Ogawa, N.; Yamamoto, H.; Mizukami, T.; Arai, S.; Okuno, M.; Terao, K.; Matsugo, S. Spectroscopic Studies of R(+)-α-Lipoic Acid—Cyclodextrin Complexes. Int. J. Mol. Sci. 2014, 15, 20469–20485. [Google Scholar] [CrossRef] [PubMed]
- Ikuta, N.; Terao, K.; Matsugo, S. Stabilized R -α-Lipoic Acid by Encapsulation Using Cyclodextrins. In Impact of Nanoscience in the Food Industry; Elsevier: Amsterdam, The Netherlands, 2018; pp. 351–366. ISBN 978-0-12-811441-4. [Google Scholar]
- Uchida, R.; Iwamoto, K.; Nagayama, S.; Miyajima, A.; Okamoto, H.; Ikuta, N.; Fukumi, H.; Terao, K.; Hirota, T. Effect of γ-Cyclodextrin Inclusion Complex on the Absorption of R-α-Lipoic Acid in Rats. Int. J. Mol. Sci. 2015, 16, 10105–10120. [Google Scholar] [CrossRef] [PubMed]
- Ikuta, N.; Okamoto, H.; Furune, T.; Uekaji, Y.; Terao, K.; Uchida, R.; Iwamoto, K.; Miyajima, A.; Hirota, T.; Sakamoto, N. Bioavailability of an R-α-Lipoic Acid/γ-Cyclodextrin Complex in Healthy Volunteers. Int. J. Mol. Sci. 2016, 17, 949. [Google Scholar] [CrossRef]
- Leu, J.-G.; Chen, S.-A.; Chen, H.-M.; Wu, W.-M.; Hung, C.-F.; Yao, Y.-D.; Tu, C.-S.; Liang, Y.-J. The Effects of Gold Nanoparticles in Wound Healing with Antioxidant Epigallocatechin Gallate and α-Lipoic Acid. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 767–775. [Google Scholar] [CrossRef]
- Chen, S.-A.; Chen, H.-M.; Yao, Y.-D.; Hung, C.-F.; Tu, C.-S.; Liang, Y.-J. Topical Treatment with Anti-Oxidants and Au Nanoparticles Promote Healing of Diabetic Wound through Receptor for Advance Glycation End-Products. Eur. J. Pharm. Sci. 2012, 47, 875–883. [Google Scholar] [CrossRef] [PubMed]
- Türkez, H.; Yıldırım, Ö.Ç.; Öner, S.; Kadı, A.; Mete, A.; Arslan, M.E.; Şahin, İ.O.; Yapça, Ö.E.; Mardinoğlu, A. Lipoic Acid Conjugated Boron Hybrids Enhance Wound Healing and Antimicrobial Processes. Pharmaceutics 2022, 15, 149. [Google Scholar] [CrossRef]
- Martínez-García, K.D.; Zertuche-Arias, T.; Bernáldez-Sarabia, J.; Iñiguez, E.; Kretzchmar, T.; Camacho-Villegas, T.A.; Lugo-Fabres, P.H.; Licea Navarro, A.F.; Bravo-Madrigal, J.; Castro-Ceseña, A.B. Radical Scavenging, Hemocompatibility, and Antibacterial Activity against MDR Acinetobacter Baumannii in Alginate-Based Aerogels Containing Lipoic Acid-Capped Silver Nanoparticles. ACS Omega 2024, 9, 2350–2361. [Google Scholar] [CrossRef]
- Attia, M.; Essa, E.A.; Zaki, R.M.; Elkordy, A.A. An Overview of the Antioxidant Effects of Ascorbic Acid and Alpha Lipoic Acid (in Liposomal Forms) as Adjuvant in Cancer Treatment. Antioxidants 2020, 9, 359. [Google Scholar] [CrossRef]
- Fahmy, U.A.; Aljaeid, B.M. Combined Strategy for Suppressing Breast Carcinoma MCF-7 Cell Lines by Loading Simvastatin on Alpha Lipoic Acid Nanoparticles. Expert Opin. Drug Deliv. 2016, 13, 1653–1660. [Google Scholar] [CrossRef]
- Ling, L.; Ismail, M.; Du, Y.; Yao, C.; Li, X. Lipoic Acid-Derived Cross-Linked Liposomes for Reduction-Responsive Delivery of Anticancer Drug. Int. J. Pharm. 2019, 560, 246–260. [Google Scholar] [CrossRef]
- Maiti, B.; Kumar, K.; Moitra, P.; Kondaiah, P.; Bhattacharya, S. Reduction Responsive Nanovesicles Derived from Novel α-Tocopheryl–Lipoic Acid Conjugates for Efficacious Drug Delivery to Sensitive and Drug Resistant Cancer Cells. Bioconjug. Chem. 2018, 29, 255–266. [Google Scholar] [CrossRef]
- Sauraj; Kumar, A.; Kumar, B.; Kulshreshtha, A.; Negi, Y.S. Redox-Sensitive Nanoparticles Based on Xylan-Lipoic Acid Conjugate for Tumor Targeted Drug Delivery of Niclosamide in Cancer Therapy. Carbohydr. Res. 2021, 499, 108222. [Google Scholar] [CrossRef] [PubMed]
- Veider, F.; Sanchez Armengol, E.; Bernkop-Schnürch, A. Charge-Reversible Nanoparticles: Advanced Delivery Systems for Therapy and Diagnosis. Small 2024, 20, 2304713. [Google Scholar] [CrossRef]
- Chen, W.; Yang, S.; Li, F.; Qu, C.; Liu, Y.; Wang, Y.; Wang, D.; Zhang, X. Programmed pH/Reduction-Responsive Nanoparticles for Efficient Delivery of Antitumor Agents in Vivo. Acta Biomater. 2018, 81, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Chen, W.; You, B.; Liu, Y.; Yang, S.; Yuan, Z.; Zhu, W.; Li, J.; Qu, C.; Zhou, Y.; et al. Enhanced Cellular Internalization and On-Demand Intracellular Release of Doxorubicin by Stepwise pH-/Reduction-Responsive Nanoparticles. ACS Appl. Mater. Interfaces 2016, 8, 32146–32158. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Wang, Y.; Ren, Z.; Chen, M.; Chen, W.; Zhang, X. Stepwise pH/Reduction-Responsive Polymeric Conjugates for Enhanced Drug Delivery to Tumor. Mater. Sci. Eng. C 2018, 82, 234–243. [Google Scholar] [CrossRef]
- Li, F.; Wang, Y.; Chen, W.; Wang, D.; Zhou, Y.; You, B.; Liu, Y.; Qu, C.; Yang, S.; Chen, M.; et al. Co-Delivery of VEGF siRNA and Etoposide for Enhanced Anti-Angiogenesis and Anti-Proliferation Effect via Multi-Functional Nanoparticles for Orthotopic Non-Small Cell Lung Cancer Treatment. Theranostics 2019, 9, 5886–5898. [Google Scholar] [CrossRef]
- Toosi Moghadam, F.; Mamashli, F.; Khoobi, M.; Ghasemi, A.; Pirhaghi, M.; Delavari, B.; Mahmoudi Aznaveh, H.; Nikkhah, M.; Saboury, A.A. A Dual Responsive Robust Human Serum Albumin-based Nanocarrier for Doxorubicin. Biotechnol. App. Biochem. 2022, 69, 2496–2506. [Google Scholar] [CrossRef]
- Birlik Demirel, G.; Aygul, E.; Dag, A.; Atasoy, S.; Cimen, Z.; Cetin, B. Folic Acid-Conjugated pH and Redox-Sensitive Ellipsoidal Hybrid Magnetic Nanoparticles for Dual-Triggered Drug Release. ACS Appl. Bio Mater. 2020, 3, 4949–4961. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, S.; Kashanian, S.; Bahrami, Y.; Cruz, L.J.; Motiei, M. Redox-Sensitive and Hyaluronic Acid-Functionalized Nanoparticles for Improving Breast Cancer Treatment by Cytoplasmic 17α-Methyltestosterone Delivery. Molecules 2020, 25, 1181. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Meng, F.; Deng, C.; Mao, X.; Zhong, Z. Targeted Inhibition of Human Hematological Cancers in Vivo by Doxorubicin Encapsulated in Smart Lipoic Acid-Crosslinked Hyaluronic Acid Nanoparticles. Drug Deliv. 2017, 24, 1482–1490. [Google Scholar] [CrossRef] [PubMed]
- Poursani, E.; Cirillo, G.; Curcio, M.; Vittorio, O.; De Luca, M.; Leggio, A.; Nicoletta, F.P.; Iemma, F. Dual-Responsive Chondroitin Sulfate Self-Assembling Nanoparticles for Combination Therapy in Metastatic Cancer Cells. Int. J. Pharm. X 2024, 7, 100235. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Qiu, M.; Zhang, J.; Sun, H.; Deng, C.; Zhong, Z. Integrated Multifunctional Micelles Co-Self-Assembled from Polypeptides Conjugated with Natural Ferulic Acid and Lipoic Acid for Doxorubicin Delivery. ChemPhysChem 2018, 19, 2070–2077. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Chaurasiya, B.; Wu, D.; Wang, H.; Du, Y.; Tu, J.; Webster, T.J.; Sun, C. Versatile Redox-Sensitive Pullulan Nanoparticles for Enhanced Liver Targeting and Efficient Cancer Therapy. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 1005–1017. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, M.; Bigdeli, B.; Jalili-baleh, L.; Baharifar, H.; Akrami, M.; Dehghani, S.; Goliaei, B.; Amani, A.; Lotfabadi, A.; Rashedi, H.; et al. Curcumin-Lipoic Acid Conjugate as a Promising Anticancer Agent on the Surface of Gold-iron Oxide Nanocomposites: A pH-Sensitive Targeted Drug Delivery System for Brain Cancer Theranostics. Eur. J. Pharm. Sci. 2018, 114, 175–188. [Google Scholar] [CrossRef] [PubMed]
- Mondal, A.; Das, S.; Ali, S.M.; Kolay, S.; Sengupta, A.; Molla, M.R. Bioderived Lipoic Acid-Based Dynamic Covalent Nanonetworks of Poly(Disulfide)s: Enhanced Encapsulation Stability and Cancer Cell-Selective Delivery of Drugs. Bioconjug. Chem. 2023, 34, 489–500. [Google Scholar] [CrossRef] [PubMed]
- Dzwonek, M.; Załubiniak, D.; Piątek, P.; Cichowicz, G.; Męczynska-Wielgosz, S.; Stępkowski, T.; Kruszewski, M.; Więckowska, A.; Bilewicz, R. Towards Potent but Less Toxic Nanopharmaceuticals—Lipoic Acid Bioconjugates of Ultrasmall Gold Nanoparticles with an Anticancer Drug and Addressing Unit. RSC Adv. 2018, 8, 14947–14957. [Google Scholar] [CrossRef]
- Cheng, M.; Zhang, Y.; Zhang, X.; Wang, W.; Yuan, Z. One-Pot Synthesis of Acid-Induced in Situ Aggregating Theranostic Gold Nanoparticles with Enhanced Retention in Tumor Cells. Biomater. Sci. 2019, 7, 2009–2022. [Google Scholar] [CrossRef]
- Emami, F.; Banstola, A.; Vatanara, A.; Lee, S.; Kim, J.O.; Jeong, J.-H.; Yook, S. Doxorubicin and Anti-PD-L1 Antibody Conjugated Gold Nanoparticles for Colorectal Cancer Photochemotherapy. Mol. Pharm. 2019, 16, 1184–1199. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Cheng, R.; Cheng, L.; Zhong, Z. Lipoyl Ester Terminated Star PLGA as a Simple and Smart Material for Controlled Drug Delivery Application. Biomacromolecules 2018, 19, 1368–1373. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Qiu, M.; Deng, C.; Cheng, R.; Zhong, Z. Targeted and Reduction-Sensitive Cross-Linked PLGA Nanotherapeutics for Safer and Enhanced Chemotherapy of Malignant Melanoma. ACS Biomater. Sci. Eng. 2020, 6, 2621–2629. [Google Scholar] [CrossRef]
- Tudose, M.; Culita, D.C.; Musuc, A.M.; Somacescu, S.; Ghica, C.; Chifiriuc, M.C.; Bleotu, C. Lipoic Acid Functionalized SiO2@Ag Nanoparticles. Synthesis, Characterization and Evaluation of Biological Activity. Mater. Sci. Eng. C 2017, 79, 499–506. [Google Scholar] [CrossRef]
- Abdelkader, N.F.; El-Batal, A.I.; Amin, Y.M.; Hawas, A.M.; Hassan, S.H.M.; Eid, N.I. Neuroprotective Effect of Gold Nanoparticles and Alpha-Lipoic Acid Mixture against Radiation-Induced Brain Damage in Rats. Int. J. Mol. Sci. 2022, 23, 9640. [Google Scholar] [CrossRef]
- Xi, Y.; Pan, W.; Liu, Y.; Liu, J.; Xu, G.; Su, Y.; Chen, D.; Ye, X. α-Lipoic Acid Loaded Hollow Gold Nanoparticles Designed for Osteoporosis Treatment: Preparation, Characterization and in Vitro Evaluation. Artif. Cells Nanomed. Biotechnol. 2023, 51, 131–138. [Google Scholar] [CrossRef]
- Sawie, H.G.; Khadrawy, Y.A.; El-Gizawy, M.M.; Mourad, H.H.; Omara, E.A.; Hosny, E.N. Effect of Alpha-Lipoic Acid and Caffeine-Loaded Chitosan Nanoparticles on Obesity and Its Complications in Liver and Kidney in Rats. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2023, 396, 3017–3031. [Google Scholar] [CrossRef]
- Hosny, E.N.; Sawie, H.G.; Abou-Seif, H.S.; Khadrawy, Y.A. Effect of Caffeine-Chitosan Nanoparticles and α-Lipoic Acid on the Cardiovascular Changes Induced in Rat Model of Obesity. Int. Immunopharmacol. 2024, 129, 111627. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, O.A.A. Development and Single Dose Clinical Pharmacokinetics Investigation of Novel Zein Assisted- Alpha Lipoic Acid Nanoencapsulation of Vardenafil. Sci. Rep. 2018, 8, 15802. [Google Scholar] [CrossRef]
- Cai, J.; Chen, J.; Zeng, Q.; Liu, J.; Zhang, Y.; Cheng, H.; Yao, S.; Chen, Q. Assessment of the Efficacy of α-Lipoic Acid in Treatment of Diabetes Mellitus Patients with Erectile Dysfunction: A Protocol for Systematic Review and Meta-Analysis. Medicine 2020, 99, e22161. [Google Scholar] [CrossRef]
- Bruna, T.; Maldonado-Bravo, F.; Jara, P.; Caro, N. Silver Nanoparticles and Their Antibacterial Applications. Int. J. Mol. Sci. 2021, 22, 7202. [Google Scholar] [CrossRef] [PubMed]
- Ferdous, Z.; Nemmar, A. Health Impact of Silver Nanoparticles: A Review of the Biodistribution and Toxicity Following Various Routes of Exposure. Int. J. Mol. Sci. 2020, 21, 2375. [Google Scholar] [CrossRef] [PubMed]
- Hajtuch, J.; Santos-Martinez, M.J.; Wojcik, M.; Tomczyk, E.; Jaskiewicz, M.; Kamysz, W.; Narajczyk, M.; Inkielewicz-Stepniak, I. Lipoic Acid-Coated Silver Nanoparticles: Biosafety Potential on the Vascular Microenvironment and Antibacterial Properties. Front. Pharmacol. 2022, 12, 733743. [Google Scholar] [CrossRef] [PubMed]
- Cotton, G.C.; Gee, C.; Jude, A.; Duncan, W.J.; Abdelmoneim, D.; Coates, D.E. Efficacy and Safety of Alpha Lipoic Acid-Capped Silver Nanoparticles for Oral Applications. RSC Adv. 2019, 9, 6973–6985. [Google Scholar] [CrossRef] [PubMed]
- Tohamy, H.G.; Lebda, M.A.; Sadek, K.M.; Elfeky, M.S.; El-Sayed, Y.S.; Samak, D.H.; Hamed, H.S.; Abouzed, T.K. Biochemical, Molecular and Cytological Impacts of Alpha-Lipoic Acid and Ginkgo Biloba in Ameliorating Testicular Dysfunctions Induced by Silver Nanoparticles in Rats. Environ. Sci. Pollut. Res. 2022, 29, 38198–38211. [Google Scholar] [CrossRef] [PubMed]
- Lebda, M.A.; Sadek, K.M.; Tohamy, H.G.; Abouzed, T.K.; Shukry, M.; Umezawa, M.; El-Sayed, Y.S. Potential Role of α-Lipoic Acid and Ginkgo Biloba against Silver Nanoparticles-Induced Neuronal Apoptosis and Blood-Brain Barrier Impairments in Rats. Life Sci. 2018, 212, 251–260. [Google Scholar] [CrossRef] [PubMed]
- An, X.; Liu, L.; Schaefer, M.; Yan, B.; Scholz, C.; Hillmer, S.; Wang, K.; Luo, Y.; Ji, H.; Gladkich, J.; et al. Alpha-Lipoic Acid Prevents Side Effects of Therapeutic Nanosilver without Compromising Cytotoxicity in Experimental Pancreatic Cancer. Cancers 2021, 13, 4770. [Google Scholar] [CrossRef] [PubMed]
- Alshammari, G.M.; Al-Ayed, M.S.; Abdelhalim, M.A.; Al-Harbi, L.N.; Yahya, M.A. Effects of Antioxidant Combinations on the Renal Toxicity Induced Rats by Gold Nanoparticles. Molecules 2023, 28, 1879. [Google Scholar] [CrossRef] [PubMed]
- Abdelhalim, M.A.K.; Qaid, H.A.Y.; Al-Mohy, Y.H.; Ghannam, M.M. The Protective Roles of Vitamin E and α-Lipoic Acid Against Nephrotoxicity, Lipid Peroxidation, and Inflammatory Damage Induced by Gold Nanoparticles. IJN 2020, 15, 729–734. [Google Scholar] [CrossRef]
- Abdelhalim, M.; Moussa, S.; Qaid, H.; Al-Ayed, M. Potential Effects of Different Natural Antioxidants on Inflammatory Damage and Oxidative-Mediated Hepatotoxicity Induced by Gold Nanoparticles. IJN 2018, 13, 7931–7938. [Google Scholar] [CrossRef]
- Al-Rasheed, N.M.; Al-Rasheed, N.M.; Abdel Baky, N.A.; Faddah, L.M.; Fatani, A.J.; Hasan, I.H.; Mohamad, R.A. Prophylactic Role of α-Lipoic Acid and Vitamin E against Zinc Oxide Nanoparticles Induced Metabolic and Immune Disorders in Rat’s Liver. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 1813–1828. [Google Scholar]
- Baky, N.A.; Faddah, L.; Al-Rasheed, N.; Al-Rasheed, N.; Fatani, A. Induction of Inflammation, DNA Damage and Apoptosis in Rat Heart after Oral Exposure to Zinc Oxide Nanoparticles and the Cardioprotective Role of α-lipoic Acid and Vitamin E. Drug Res. 2013, 63, 228–236. [Google Scholar] [CrossRef]
- Deore, M.S.; S, K.; Naqvi, S.; Kumar, A.; Flora, S.J.S. Alpha-Lipoic Acid Protects Co-Exposure to Lead and Zinc Oxide Nanoparticles Induced Neuro, Immuno and Male Reproductive Toxicity in Rats. Front. Pharmacol. 2021, 12, 626238. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, W.; Ni, D.; Zhou, Z.; Gu, J.; Zhang, W.; Sun, H.; Liu, F. Alpha Lipoic Acid Antagonizes Cytotoxicity of Cobalt Nanoparticles by Inhibiting Ferroptosis-like Cell Death. J. Nanobiotechnol. 2020, 18, 141. [Google Scholar] [CrossRef]
- Khalaf, A.; Zaki, A.; Galal, M.; Ogaly, H.; Ibrahim, M.; Hassan, A. The Potential Protective Effect of α-Lipoic Acid against Nanocopper Particle–Induced Hepatotoxicity in Male Rats. Hum. Exp. Toxicol. 2017, 36, 881–891. [Google Scholar] [CrossRef] [PubMed]
- Levkovskyi, I.O.; Mochizuki, S.; Zheng, A.; Zhang, X.; Zhang, F. Lipoic Acid-Based Poly(Disulfide)s: Synthesis and Biomedical Applications. Nano Trans. Med. 2023, 2, 100006. [Google Scholar] [CrossRef]
- Nuyken, O.; Pask, S. Ring-Opening Polymerization—An Introductory Review. Polymers 2013, 5, 361–403. [Google Scholar] [CrossRef]
- Bang, E.-K.; Lista, M.; Sforazzini, G.; Sakai, N.; Matile, S. Poly(Disulfide)s. Chem. Sci. 2012, 3, 1752. [Google Scholar] [CrossRef]
- Yu, Q.; Fang, Z.; Luan, S.; Wang, L.; Shi, H. Biological Applications of Lipoic Acid-Based Polymers: An Old Material with New Promise. J. Mater. Chem. B 2024, 12, 4574–4583. [Google Scholar] [CrossRef]
- Yang, H.; Shen, W.; Liu, W.; Chen, L.; Zhang, P.; Xiao, C.; Chen, X. PEGylated Poly(α-Lipoic Acid) Loaded with Doxorubicin as a pH and Reduction Dual Responsive Nanomedicine for Breast Cancer Therapy. Biomacromolecules 2018, 19, 4492–4503. [Google Scholar] [CrossRef]
- Li, Y.; Hou, H.; Zhang, P.; Zhang, Z. Co-Delivery of Doxorubicin and Paclitaxel by Reduction/pH Dual Responsive Nanocarriers for Osteosarcoma Therapy. Drug Deliv. 2020, 27, 1044–1053. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, Y.; Chen, Y.; Zhang, X.; Chen, X. A Convergent Fabrication of Programmed pH/Reduction-Responsive Nanoparticles for Efficient Dual Anticancer Drugs Delivery for Ovarian Cancer Treatment. J. Exp. Nanosci. 2023, 18, 2193400. [Google Scholar] [CrossRef]
- Liu, Z.; Shen, N.; Tang, Z.; Zhang, D.; Ma, L.; Yang, C.; Chen, X. An Eximious and Affordable GSH Stimulus-Responsive Poly(α-Lipoic Acid) Nanocarrier Bonding Combretastatin A4 for Tumor Therapy. Biomater. Sci. 2019, 7, 2803–2811. [Google Scholar] [CrossRef]
- Liu, Z.; Tang, Z.; Zhang, D.; Wu, J.; Si, X.; Shen, N.; Chen, X. A Novel GSH Responsive Poly(Alpha-Lipoic Acid) Nanocarrier Bonding with the Honokiol-DMXAA Conjugate for Combination Therapy. Sci. China Mater. 2020, 63, 307–315. [Google Scholar] [CrossRef]
- Lin, F.; Liu, Y.; Luo, W.; Liu, S.; Wang, Y.; Gu, R.; Liu, W.; Xiao, C. Minocycline-Loaded Poly(α-Lipoic Acid)–Methylprednisolone Prodrug Nanoparticles for the Combined Anti-Inflammatory Treatment of Spinal Cord Injury. IJN 2022, 17, 91–104. [Google Scholar] [CrossRef]
- Su, R.; Wang, H.; Xiao, C.; Tao, Y.; Li, M.; Chen, Z. Venetoclax Nanomedicine Alleviates Acute Lung Injury via Increasing Neutrophil Apoptosis. Biomater. Sci. 2021, 9, 4746–4754. [Google Scholar] [CrossRef]
- Banik, S.; Yamada, K.; Sato, H.; Onoue, S. Development of Poly(Lipoic Acid) Nanoparticles with Improved Oral Bioavailability and Hepatoprotective Effects of Quercetin. Mol. Pharm. 2022, 19, 1468–1476. [Google Scholar] [CrossRef]
- Han, L.; Zang, T.; Tan, L.; Liang, D.; Long, T.; Liu, X.; Shen, X.; Ren, H.; Li, Z.; Lu, Z.; et al. Self-Assembly of H2S-Responsive Nanoprodrugs Based on Natural Rhein and Geraniol for Targeted Therapy against Salmonella Typhimurium. J. Nanobiotechnol. 2023, 21, 483. [Google Scholar] [CrossRef]
- Han, L.; Liu, X.-W.; Zang, T.; Ren, H.; Liang, D.-S.; Bai, S.-C.; Li, C.; Liao, X.-P.; Liu, Y.-H.; Zhang, C.; et al. H2S Responsive PEGylated Poly (Lipoic Acid) with Ciprofloxacin for Targeted Therapy of Salmonella. J. Control. Release 2022, 351, 896–906. [Google Scholar] [CrossRef]
- Carmine, A.; Domoto, Y.; Sakai, N.; Matile, S. Comparison of Lipoic and Asparagusic Acid for Surface-Initiated Disulfide-Exchange Polymerization. Chem. Eur. J. 2013, 19, 11558–11563. [Google Scholar] [CrossRef]
- Wang, L.; Jing, P.; Tan, J.; Liao, C.; Chen, Y.; Yu, Y.; Zhang, S. “One-Stitch” Bioorthogonal Prodrug Activation Based on Cross-Linked Lipoic Acid Nanocapsules. Biomaterials 2021, 273, 120823. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Zhang, J.; Cheng, R.; Deng, C.; Meng, F.; Xie, F.; Zhong, Z. Reversibly Crosslinked Hyaluronic Acid Nanoparticles for Active Targeting and Intelligent Delivery of Doxorubicin to Drug Resistant CD44+ Human Breast Tumor Xenografts. J. Control. Release 2015, 205, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Mondal, U.K.; Barchi, J.J. Isolipoic Acid-Linked Gold Nanoparticles Bearing the Thomsen Friedenreich Tumor-Associated Carbohydrate Antigen: Stability and in Vitro Studies. Front. Chem. 2022, 10, 1002146. [Google Scholar] [CrossRef] [PubMed]
- Trzciński, J.W.; Morillas-Becerril, L.; Scarpa, S.; Tannorella, M.; Muraca, F.; Rastrelli, F.; Castellani, C.; Fedrigo, M.; Angelini, A.; Tavano, R.; et al. Poly(Lipoic Acid)-Based Nanoparticles as Self-Organized, Biocompatible, and Corona-Free Nanovectors. Biomacromolecules 2021, 22, 467–480. [Google Scholar] [CrossRef] [PubMed]
- Bellini, C.; Antonucci, S.; Morillas-Becerril, L.; Scarpa, S.; Tavano, R.; Mancin, F.; Di Lisa, F.; Papini, E. Nanoparticles Based on Cross-Linked Poly(Lipoic Acid) Protect Macrophages and Cardiomyocytes from Oxidative Stress and Ischemia Reperfusion Injury. Antioxidants 2022, 11, 907. [Google Scholar] [CrossRef] [PubMed]
- Castellani, C.; Radu, C.M.; Morillas-Becerril, L.; Barison, I.; Menato, F.; Do Nascimento, T.M.; Fedrigo, M.; Giarraputo, A.; Virzì, G.M.; Simioni, P.; et al. Poly(Lipoic Acid)-Based Nanoparticles as a New Therapeutic Tool for Delivering Active Molecules. Nanomed. Nanotechnol. Biol. Med. 2022, 45, 102593. [Google Scholar] [CrossRef] [PubMed]
- Gu, F.; Hu, C.; Tai, Z.; Yao, C.; Tian, J.; Zhang, L.; Xia, Q.; Gong, C.; Gao, Y.; Gao, S. Tumour Microenvironment-Responsive Lipoic Acid Nanoparticles for Targeted Delivery of Docetaxel to Lung Cancer. Sci. Rep. 2016, 6, 36281. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Chen, Y.; Zhang, J.; Liao, C.; Zhang, S. Cross-Linked (R)-(+)-Lipoic Acid Nanoparticles with Prodrug Loading for Synergistic Cancer Therapy. J. Mater. Chem. B 2021, 9, 1583–1591. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Dai, X.; Tan, J.; Wu, X.; Zhou, X.; Liao, C.; Zhang, S. Quaternary Ammonium Salts Anchored on Cross-Linked (R)-(+)-Lipoic Acid Nanoparticles for Drug-Resistant Tumor Therapy. ACS Appl. Mater. Interfaces 2021, 13, 56850–56857. [Google Scholar] [CrossRef]
- Hei, M.-W.; Zhan, Y.-R.; Chen, P.; Zhao, R.-M.; Tian, X.-L.; Yu, X.-Q.; Zhang, J. Lipoic Acid-Based Poly(Disulfide)s as Versatile Biomolecule Delivery Vectors and the Application in Tumor Immunotherapy. Mol. Pharm. 2023, 20, 3210–3222. [Google Scholar] [CrossRef]
- Jia, C.; Guo, Y.; Wu, F. Chemodynamic Therapy via Fenton and Fenton-Like Nanomaterials: Strategies and Recent Advances. Small 2022, 18, 2103868. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Zhou, X.; Zhang, S. Iron-Doped Cross-Linked Lipoic Acid Nano-Aggregates for Ferroptosis-Mediated Cancer Treatment. Acta Biomater. 2023, 159, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Cui, R.; Li, B.; Liao, C.; Zhang, S. Copper-Mediated Chemodynamic Therapy with Ultra-Low Copper Consumption by Doping Cupric Ion on Cross-Linked (R)-(+)-Lipoic Acid Nanoparticles. Regen. Biomater. 2023, 10, rbad021. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Zhang, X.; Zhou, S.; Li, J.; Pan, L.; Liao, C.; Wang, Z.; Chen, Y.; Shen, G.; Li, L.; et al. Pretargeted Radiotherapy and Synergistic Treatment of Metastatic, Castration-Resistant Prostate Cancer Using Cross-Linked, PSMA-Targeted Lipoic Acid Nanoparticles. J. Mater. Chem. B 2024, 12, 2324–2333. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Cui, R.; Liao, C.; Zhang, S. Cross-Linked Lipoic Acid Nanoparticles with Indole-3-Methanol Loading for the PTEN-Mediated TNBC Treatment. J. Mater. Sci. Technol. 2024, 181, 198–208. [Google Scholar] [CrossRef]
- Cely-Pinto, M.; Wang, B.; Scaiano, J.C. Understanding α-Lipoic Acid Photochemistry Helps to Control the Synthesis of Plasmonic Gold Nanostructures. Photochem. Photobiol. Sci. 2023, 22, 1299–1307. [Google Scholar] [CrossRef] [PubMed]
- Pourshahrestani, S.; Zeimaran, E.; Kadri, N.A.; Mutlu, N.; Boccaccini, A.R. Polymeric Hydrogel Systems as Emerging Biomaterial Platforms to Enable Hemostasis and Wound Healing. Adv. Healthc. Mater. 2020, 9, 2000905. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhou, Y.; Zhang, J.; Liang, H.; Chen, X.; Tan, H. Natural Polymer-Based Hydrogels: From Polymer to Biomedical Applications. Pharmaceutics 2023, 15, 2514. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Mei, L.; Wang, D.; Jia, P.; Zhou, Q.; Liu, W. A Self-Stabilized and Water-Responsive Deliverable Coenzyme-Based Polymer Binary Elastomer Adhesive Patch for Treating Oral Ulcer. Nat. Commun. 2023, 14, 7707. [Google Scholar] [CrossRef]
- Qi, Y.; Xu, C.; Zhang, Z.; Zhang, Q.; Xu, Z.; Zhao, X.; Zhao, Y.; Cui, C.; Liu, W. Wet Environment-Induced Adhesion and Softening of Coenzyme-Based Polymer Elastic Patch for Treating Periodontitis. Bioact. Mater. 2024, 35, 259–273. [Google Scholar] [CrossRef]
- Du, J.; Wang, F.; Li, J.; Yang, Y.; Guo, D.; Zhang, Y.; Yang, A.; He, X.; Cheng, Y. Green Polymer Hydrogels from a Natural Monomer with Inherent Antioxidative Capability for Efficient Wound Healing and Spinal Cord Injury Treatment. Biomater. Sci. 2023, 11, 3683–3694. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Deng, Z.; Li, T.; Bu, J.; Wang, D.; Wang, J.; Liu, M.; Li, J.; Yang, Y.; Zhong, S. Fabrication, GSH-Responsive Drug Release, and Anticancer Properties of Thioctic Acid-Based Intelligent Hydrogels. Colloids Surf. B Biointerfaces 2022, 217, 112703. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Fan, G.; Zhu, L.; Zhang, Y.; Wang, X.; Qin, J.; Lu, K.; Hu, J.; Ma, J. A Lipoic Acid Supramolecular Polymer-Based Hydrogel with Self-Regulating ROS, Reduced Blood Sugar, and Antibacterial Ability for Improved Diabetic Wound Healing. J. Sci. Adv. Mater. Devices 2024, 9, 100677. [Google Scholar] [CrossRef]
- Yu, H.; Wang, Y.; Yang, H.; Peng, K.; Zhang, X. Injectable Self-Healing Hydrogels Formed via Thiol/Disulfide Exchange of Thiol Functionalized F127 and Dithiolane Modified PEG. J. Mater. Chem. B 2017, 5, 4121–4127. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Zhang, B.; Gao, G.; Xiao, C.; Li, G. Single Component Pluronic F127-Lipoic Acid Hydrogels with Self-Healing and Multi-Responsive Properties. Eur. Polym. J. 2019, 115, 346–355. [Google Scholar] [CrossRef]
- Wang, T.; Fang, H.; Yalikun, S.; Li, J.; Pan, Y.; Zhang, K.; Yin, J.; Cui, H. Pluronic F127-Lipoic Acid Adhesive Nanohydrogel Combining with Ce 3+ /Tannic Acid/Ulinastatin Nanoparticles for Promoting Wound Healing. Biomacromolecules 2024, 25, 924–940. [Google Scholar] [CrossRef]
- Luo, Y.; Zhou, X.; Liu, C.; Lu, R.; Jia, M.; Li, P.; Zhang, S. Scavenging ROS and Inflammation Produced during Treatment to Enhance the Wound Repair Efficacy of Photothermal Injectable Hydrogel. Biomater. Adv. 2022, 141, 213096. [Google Scholar] [CrossRef]


| Method | Mechanism | Advantages | Limitations |
|---|---|---|---|
| Thermal polymerization | Free radical polymerization |
|
|
| Thiolate-initiated polymerization | Thiol–disulfide exchange |
|
|
| Photo-initiated polymerization | Free radical polymerization |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bellini, C.; Mancin, F.; Papini, E.; Tavano, R. Nanotechnological Approaches to Enhance the Potential of α-Lipoic Acid for Application in the Clinic. Antioxidants 2024, 13, 706. https://doi.org/10.3390/antiox13060706
Bellini C, Mancin F, Papini E, Tavano R. Nanotechnological Approaches to Enhance the Potential of α-Lipoic Acid for Application in the Clinic. Antioxidants. 2024; 13(6):706. https://doi.org/10.3390/antiox13060706
Chicago/Turabian StyleBellini, Chiara, Fabrizio Mancin, Emanuele Papini, and Regina Tavano. 2024. "Nanotechnological Approaches to Enhance the Potential of α-Lipoic Acid for Application in the Clinic" Antioxidants 13, no. 6: 706. https://doi.org/10.3390/antiox13060706
APA StyleBellini, C., Mancin, F., Papini, E., & Tavano, R. (2024). Nanotechnological Approaches to Enhance the Potential of α-Lipoic Acid for Application in the Clinic. Antioxidants, 13(6), 706. https://doi.org/10.3390/antiox13060706








