Antioxidant Activity of Bilirubin in Micellar and Liposomal Systems Is pH-Dependent
Abstract
1. Introduction
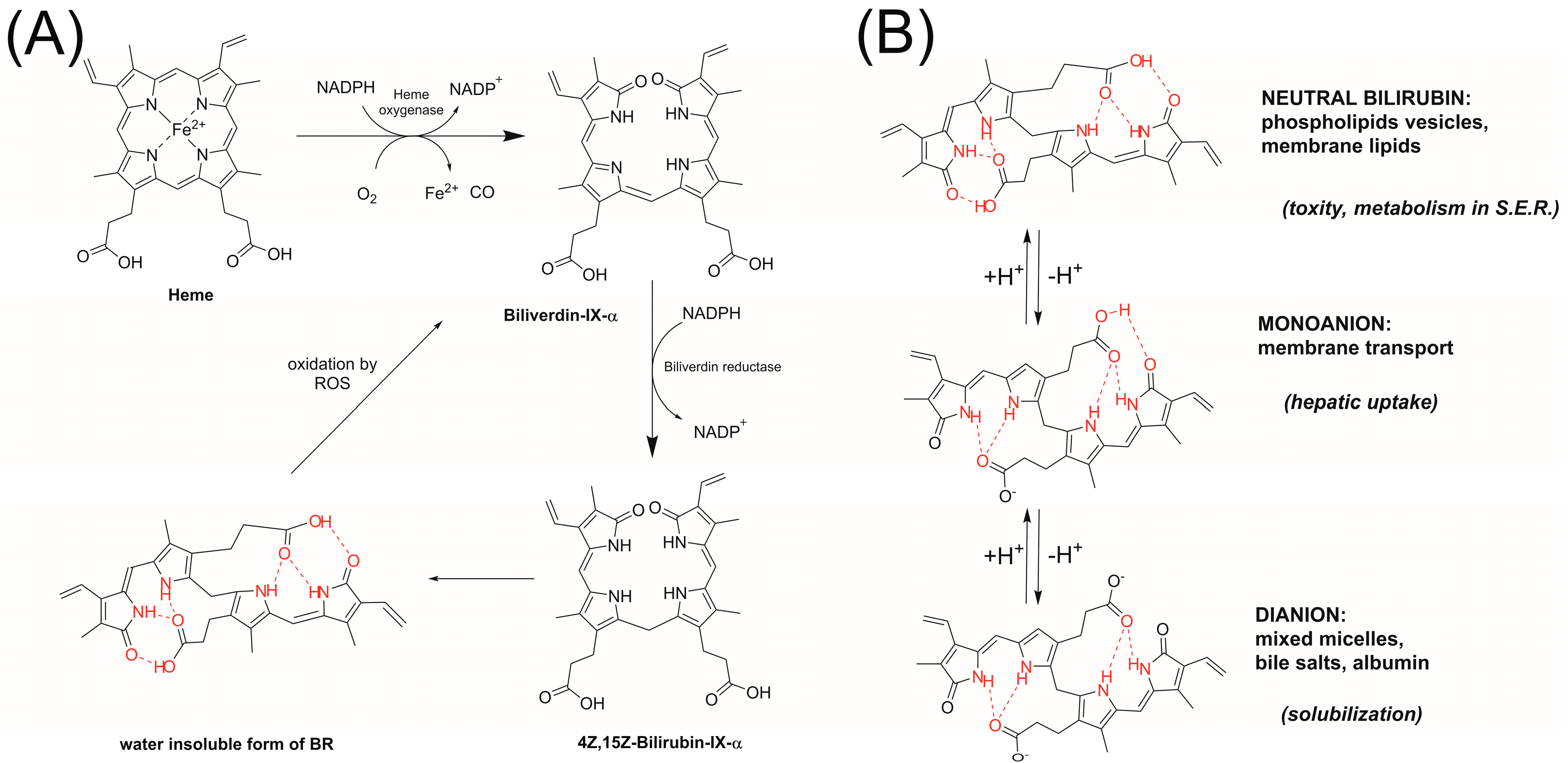
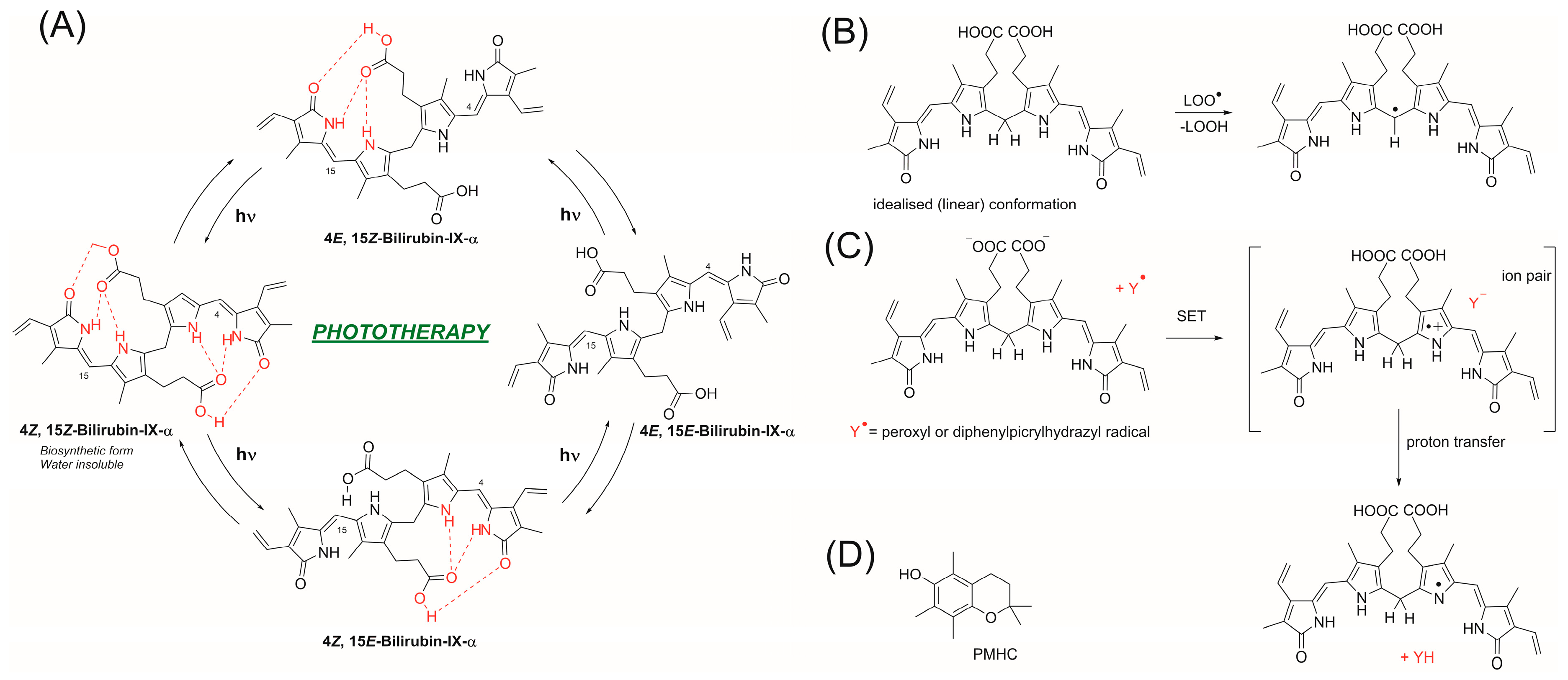
2. Materials and Methods
3. Results and Discussion
3.1. Results Obtained in Micellar Systems
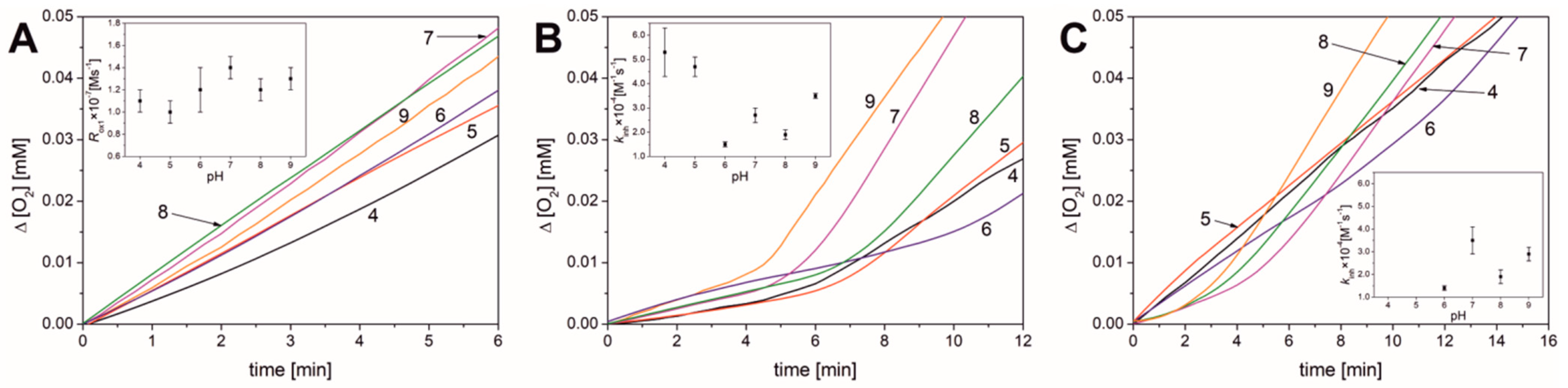
3.2. Results Obtained for Liposomal Systems
3.3. Influence of pH on the Mechanism of Bilirubin Action
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Person, R.V.; Peterson, B.R.; Lightner, D.A. Bilirubin conformational analysis and circular dichroism. J. Am. Chem. Soc. 1994, 116, 42–59. [Google Scholar] [CrossRef]
- Ostrow, J.D.; Mukerjee, P.; Tiribelli, C. Structure and binding of unconjugated bilirubin: Relevance for physiological and pathophysiological function. J. Lipid Res. 1994, 35, 1715–1737. [Google Scholar] [CrossRef] [PubMed]
- Ostrow, J.D.; Mukerjee, P. Revalidation and rationale for high pKa values of unconjugated bilirubin. BMC Biochem. 2007, 8, 7. [Google Scholar] [CrossRef] [PubMed]
- Lightner, D.A.; McDonagh, A.F. Molecular mechanisms of phototherapy for neonatal jaundice. Acc. Chem. Res. 1984, 17, 417–424. [Google Scholar] [CrossRef]
- Ziberna, L.; Martelanc, M.; Franko, M.; Passamonti, S. Bilirubin is an endogenous antioxidant in human vascular endothelial cells. Sci. Rep. 2016, 6, 29240. [Google Scholar] [CrossRef] [PubMed]
- Nocentini, A.; Bonardi, A.; Pratesi, S.; Gratteri, P.; Dani, C.; Supuran, C.T. Pharmaceutical strategies for preventing toxicity and promoting antioxidant and anti-inflammatory actions of bilirubin. J. Enzyme Inhib. Med. Chem. 2022, 37, 487–501. [Google Scholar] [CrossRef] [PubMed]
- Itoh, S.; Okada, H.; Kuboi, T.; Kusaka, T. Phototherapy for neonatal hyperbilirubinemia. Pediatr. Int. 2017, 59, 959–966. [Google Scholar] [CrossRef] [PubMed]
- Capková, N.; Pospíšilová, V.; Fedorová, V.; Raška, J.; Pospíšilová, K.; Dal Ben, M.; Dvořák, A.; Viktorová, J.; Bohačiaková, D.; Vítek, L. The effects of bilirubin and lumirubin on the differentiation of human pluripotent cell-derived neural stem cells. Antioxidants 2021, 10, 1532. [Google Scholar] [CrossRef] [PubMed]
- Hatfield, G.L.; Barclay, L.R.C. Bilirubin as an Antioxidant: Kinetic Studies of the Reaction of Bilirubin with Peroxyl Radicals in Solution, Micelles, and Lipid Bilayers. Org. Lett. 2004, 6, 1539–1542. [Google Scholar] [CrossRef] [PubMed]
- Chepelev, L.L.; Beshara, C.S.; MacLean, P.D.; Hatfield, G.L.; Rand, A.A.; Thompson, A.; Wright, J.S.; Barclay, L.R.C. Polypyrroles as Antioxidants: Kinetic Studies on Reactions of Bilirubin and Biliverdin Dimethyl Esters and Synthetic Model Compounds with Peroxyl Radicals in Solution. Chemical Calculations on Selected Typical Structures. J. Org. Chem. 2006, 71, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Vitek, L.; Bellarosa, C.; Tiribelli, C. Induction of Mild Hyperbilirubinemia: Hype or Real Therapeutic Opportunity? Clin. Pharmacol. Ther. 2019, 106, 568–575. [Google Scholar] [CrossRef] [PubMed]
- Adin, C.A. Bilirubin as a therapeutic molecule: Challenges and opportunities. Antioxidants 2021, 10, 1536. [Google Scholar] [CrossRef] [PubMed]
- Stocker, R.; Yamamoto, Y.; McDonagh, A.F.; Glazer, A.N.; Ames, B.N. Bilirubin is an antioxidant of possible physiological importance. Science 1987, 235, 1043–1046. [Google Scholar] [CrossRef] [PubMed]
- MacLean, P.D.; Drake, E.C.; Ross, L.; Barclay, C. Bilirubin as an antioxidant in micelles and lipid bilayers: Its contribution to the total antioxidant capacity of human blood plasma. Free Radical Biol. Med. 2007, 43, 600–609. [Google Scholar] [CrossRef] [PubMed]
- Sedlak, T.W.; Saleh, M.; Higginson, D.S.; Paul, B.D.; Juluri, K.R.; Snyder, S.H. Bilirubin and glutathione have complementary antioxidant and cytoprotective roles. Proc. Natl. Acad. Sci. USA 2009, 106, 5171–5176. [Google Scholar] [CrossRef] [PubMed]
- Sedlak, T.W.; Snyder, S.H. Bilirubin benefits: Cellular protection by a biliverdin reductase antioxidant cycle. Pediatrics 2004, 113, 1776–1782. [Google Scholar] [CrossRef] [PubMed]
- Grebowski, J.; Konopko, A.; Krokosz, A.; DiLabio, G.A.; Litwinienko, G. Antioxidant activity of highly hydroxylated fullerene C60 and its interactions with the analogue of α-tocopherol. Free Radic. Biol. Med. 2020, 160, 734–744. [Google Scholar] [CrossRef] [PubMed]
- Jodko-Piórecka, K.; Sikora, B.; Kluzek, M.; Przybylski, P.; Litwinienko, G. Antiradical Activity of Dopamine, L-DOPA, Adrenaline, and Noradrenaline in Water/Methanol and in Liposomal Systems. J. Org. Chem. 2022, 87, 1791–1804. [Google Scholar] [CrossRef] [PubMed]
- Jodko-Piorecka, K.; Litwinienko, G. Antioxidant activity of dopamine and L-DOPA in lipid micelles and their cooperation with an analogue of alpha-tocopherol. Free Radic. Biol. Med. 2015, 83, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Konopko, A.; Litwinienko, G. Unexpected Role of pH and Microenvironment on the Antioxidant and Synergistic Activity of Resveratrol in Model Micellar and Liposomal Systems. J. Org. Chem. 2021, 87, 1698–1709. [Google Scholar] [CrossRef] [PubMed]
- Konopko, A.; Kusio, J.; Litwinienko, G. Antioxidant Activity of Metal Nanoparticles Coated with Tocopherol-Like Residues—The Importance of Studies in Homo-and Heterogeneous Systems. Antioxidants 2020, 9, 5. [Google Scholar] [CrossRef] [PubMed]
- Amorati, R.; Baschieri, A.; Valgimigli, L. Measuring antioxidant activity in bioorganic samples by the differential oxygen uptake apparatus: Recent advances. J. Chem. 2017, 2017, 6369358. [Google Scholar] [CrossRef]
- Barclay, L.R.C.; Baskin, K.A.; Dakin, K.A.; Locke, S.J.; Vinqvist, M.R. The antioxidant activities of phenolic antioxidants in free radical peroxidation of phospholipid membranes. Can. J. Chem. 1990, 68, 2258–2269. [Google Scholar] [CrossRef]
- Barclay, L.R.C.; Baskin, K.A.; Locke, S.J.; Schaefer, T.D. Benzophenone-photosensitized autoxidation of linoleate in solution and sodium dodecyl sulfate micelles. Can. J. Chem. 1987, 65, 2529–2540. [Google Scholar] [CrossRef]
- Chiang, Y.; Whipple, E.B. The Protonation of Pyrroles. J. Am. Chem. Soc. 1963, 85, 2763–2767. [Google Scholar] [CrossRef]
- Perrin, D.D. Dissociation Constants of Organic Bases in Aqueous Solution; International Union of Pure and Applied Chemistry (IUPAC), Butterworth & CO.: London, UK, 1969. [Google Scholar]
- Lide, D.R. CRC Handbook of Chemistry and Physics, 89th ed.; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Boiadjiev, S.E.; Watters, K.; Wolf, S.; Lai, B.N.; Welch, W.H.; McDonagh, A.F.; Lightner, D.A. pKa and Aggregation of Bilirubin: Titrimetric and Ultracentrifugation Studies on Water-Soluble Pegylated Conjugates of Bilirubin and Fatty Acids. Biochemistry 2004, 43, 15617–15632. [Google Scholar] [CrossRef] [PubMed]
- Lightner, D.A.; Holmes, D.L.; McDonagh, A.F. On the acid dissociation constants of bilirubin and biliverdin: pKa values from 13C NMR spectroscopy. J. Biol. Chem. 1996, 271, 2397–2405. [Google Scholar] [CrossRef] [PubMed]
- Trull, F.; Boiadjiev, S.; Lightner, D.; McDonagh, A. Aqueous dissociation constants of bile pigments and sparingly soluble carboxylic acids by 13C NMR in aqueous dimethyl sulfoxide: Effects of hydrogen bonding. J. Lipid Res. 1997, 38, 1178–1188. [Google Scholar] [CrossRef] [PubMed]
- Mukerjee, P.; Ostrow, J.D. Review: Bilirubin pKa studies; new models and theories indicate high pKa values in water, dimethylformamide and DMSO. BMC Biochem. 2010, 11, 15. [Google Scholar] [CrossRef] [PubMed]
- Stocker, R.; Glazer, A.N.; Ames, B.N. Antioxidant activity of albumin-bound bilirubin. Proc. Natl. Acad. Sci. USA 1987, 84, 5918–5922. [Google Scholar] [CrossRef] [PubMed]
- Kurtin, W.E.; Heo, R.; Breimeir, D.J.; Tran, N.T.-V.; Elizondo, E.; Salas, R.E.; Morales, M.; Huang, L.; Frank, B. Effects of pH on the absorption, emission and light scattering spectroscopy of bilirubin and xanthobilirubic acid in sodium taurocholate solution. J. Chem. Soc. Perkin Trans. 2 1998, 1677–1682. [Google Scholar] [CrossRef]
- Snelgrove, D.W.; Lusztyk, J.; Banks, J.T.; Mulder, P.; Ingold, K.U. Kinetic solvent effects on hydrogen-atom abstractions: Reliable, quantitative predictions via a single empirical equation. J. Am. Chem. Soc. 2001, 123, 469–477. [Google Scholar] [CrossRef]
- Litwinienko, G.; Ingold, K.U. Solvent effects on the rates and mechanisms of reaction of phenols with free radicals. Acc. Chem. Res. 2007, 40, 222–230. [Google Scholar] [CrossRef] [PubMed]
- de Heer, M.I.; Mulder, P.; Korth, H.-G.; Ingold, K.U.; Lusztyk, J. Hydrogen atom abstraction kinetics from intramolecularly hydrogen bonded ubiquinol-0 and other (poly) methoxy phenols. J. Am. Chem. Soc. 2000, 122, 2355–2360. [Google Scholar] [CrossRef]
- Litwinienko, G.; Dilabio, G.A.; Mulder, P.; Korth, H.G.; Ingold, K.U. Intramolecular and intermolecular hydrogen bond formation by some ortho-substituted phenols: Some surprising results from an experimental and theoretical investigation. J. Phys. Chem. A 2009, 113, 6275–6288. [Google Scholar] [CrossRef] [PubMed]
- Bianco, A.; Dvořák, A.; Capková, N.; Gironde, C.; Tiribelli, C.; Furger, C.; Vitek, L.; Bellarosa, C. The Extent of Intracellular Accumulation of Bilirubin Determines Its Anti- or Pro-Oxidant Effect. Int. J. Mol. Sci. 2020, 21, 8101. [Google Scholar] [CrossRef] [PubMed]
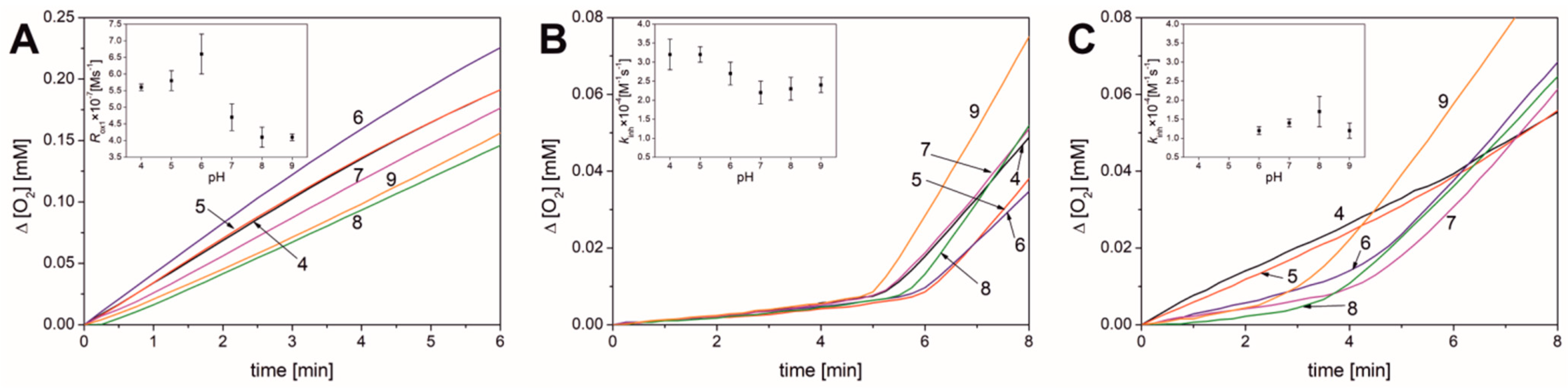
| Parameter | pH | |||||
|---|---|---|---|---|---|---|
| 4.0 | 5.0 | 6.0 | 7.0 | 8.0 | 9.0 | |
| none | ||||||
| Rox1 × 107 [M s−1] | 5.6 ± 0.1 | 5.8 ± 0.3 | 6.6 ± 0.6 | 4.7 ± 0.4 | 4.1 ± 0.3 | 4.1 ± 0.1 |
| νox1 b | 90 ± 2 | 110 ± 6 | 122 ± 11 | 86 ± 7 | 80 ± 6 | 69 ± 2 |
| PMHC | ||||||
| τ [min] | 5.4 ± 0.1 | 6.3 ± 0.3 | 6.1 ± 0.4 | 6.1 ± 0.1 | 6.5 ± 0.4 | 5.6 ± 0.3 |
| Ri × 109 [M s−1] | 6.2 ± 0.2 | 5.3 ± 0.2 | 5.5 ± 0.4 | 5.5 ± 0.1 | 5.1 ± 0.3 | 6.0 ± 0.3 |
| Rinh × 108 [M s−1] | 2.3 ± 0.2 | 2.5 ± 0.1 | 3.0 ± 0.7 | 2.7 ± 0.5 | 2.7 ± 0.4 | 3.3 ± 0.5 |
| Rox2 × 107 [M s−1] | 2.5 ± 0.1 | 2.3 ± 0.2 | 2.2 ± 0.1 | 3.0 ± 0.1 | 3.3 ± 0.2 | 4.4 ± 0.4 |
| νinh a | 3.7 ± 0.3 | 4.7 ± 0.4 | 5.4 ± 0.8 | 5.0 ± 0.9 | 5.2 ± 0.9 | 5.4 ± 0.7 |
| νox b | 39 ± 1 | 44 ± 3 | 41 ± 4 | 55 ± 2 | 65 ± 2 | 73 ± 4 |
| kinh × 10−4 [M−1 s−1] | 3.2 ± 0.4 | 3.2 ± 0.2 | 2.7 ± 0.3 | 2.2 ± 0.3 | 2.3 ± 0.3 | 2.4 ± 0.2 |
| Bilirubin | ||||||
| τ [min] | - | - | 4.9 ± 0.2 | 5.4 ± 0.1 | 5.0 ± 0.3 | 4.5 ± 0.6 |
| n | - | - | 1.8 ± 0.1 | 2.0 ± 0.1 | 1.9 ± 0.1 | 1.7 ± 0.2 |
| Rinh × 108 [M s−1] | 11 ± 1 | 11 ± 1 | 5.8 ± 0.4 | 4.1 ± 0.8 | 3.5 ± 0.7 | 5.3 ± 0.4 |
| Rox2 × 107 [M s−1] | 1.4 ± 0.1 | 1.9 ± 0.1 | 2.5 ± 0.1 | 2.7 ± 0.3 | 2.4 ± 0.2 | 3.7 ± 0.3 |
| νinh a | 22 ± 1 c | 35 ± 3 c | 10.7 ± 0.7 | 7.4 ± 1.4 | 6.7 ±1.4 | 8.8 ± 0.7 |
| νox2 b | 46 ± 1 | 49 ± 6 | 46 ± 3 | 62 ± 4 | ||
| kinh × 10−4 [M−1 s−1] | - | - | 1.2 ± 0.1 | 1.4 ± 0.1 | 1.7 ± 0.4 | 1.2 ± 0.2 |
| Parameter | pH | |||||
|---|---|---|---|---|---|---|
| 4.0 | 5.0 | 6.0 | 7.0 | 8.0 | 9.0 | |
| None | ||||||
| Rox1 × 108 [M s−1] | 11 ± 5 | 10 ± 1 | 12 ± 2 | 14 ± 1 | 12 ± 1 | 13 ± 1 |
| νox1 b | 17 ± 1 | 18 ± 1 | 30 ± 4 | 26 ± 1 | 27 ± 2 | 19 ± 1 |
| PMHC | ||||||
| τ [min] | 5.4 ± 0.1 | 6.1 ± 0.4 | 8.2 ± 0.6 | 6.2 ± 0.9 | 7.7 ± 0.2 | 4.8 ± 0.5 |
| Ri × 109 [M s−1] | 6.1 ± 0.1 | 5.5 ± 0.4 | 4.1 ± 0.3 | 5.5 ± 0.7 | 4.3 ± 0.1 | 6.9 ± 0.7 |
| Rinh × 108 [M s−1] | 1.8 ± 0.4 | 2.1 ± 0.5 | 2.5 ± 0.4 | 2.9 ± 0.2 | 2.7 ± 0.1 | 3.2 ± 0.5 |
| Rox2 × 108 [M s−1] | 5.9 ± 0.4 | 6.3 ± 0.3 | 6.2 ± 0.5 | 14.8 ± 0.7 | 12.0 ± 0.9 | 13.6 ± 0.9 |
| νinh a | 3.0 ± 0.7 | 3.8 ± 0.7 | 6.2 ± 0.4 | 5.4 ± 1.0 | 6.2 ± 0.1 | 4.6 ± 0.3 |
| νox2 b | 10 ± 1 | 12 ± 1 | 15 ± 2 | 28 ± 4 | 28 ± 1 | 20 ± 2 |
| kinh × 10−4 [M−1 s−1] | 5.3 ± 1.0 | 4.7 ± 0.4 | 1.5 ± 0.1 | 2.7 ± 0.3 | 1.9 ± 0.2 | 3.5 ± 0.1 |
| Bilirubin | ||||||
| τ [min] | - | - | 7.0 ± 0.7 | 5.1 ± 0.2 | 5.6 ± 0.4 | 4.0 ± 0.5 |
| n | - | - | 2.3 ± 0.2 | 1.7 ± 0.1 | 1.9 ± 0.1 | 1.3 ± 0.2 |
| Rinh × 108 [M s−1] | 5.5 ± 0.2 c | 5.8 ± 0.2 c | 4.9 ± 0.1 | 3.4 ± 0.6 | 4.4 ± 0.4 | 4.1 ± 0.5 |
| Rox2 × 108 [M s−1] | 5.6 ± 0.1 | 5.8 ± 0.4 | 7.5 ± 0.6 | 10.3 ± 1.4 | 10.9 ± 0.4 | 12.3 ± 0.6 |
| νinh a | 9 ± 1 c | 11 ± 1 c | 12.1 ± 0.2 | 6.3 ± 1.1 | 10.2 ± 0.9 | 6.0 ± 0.7 |
| νox2 b | - | - | 18 ± 2 | 19 ± 2 | 25 ± 1 | 18 ± 1 |
| kinh × 10−4 [M−1 s−1] | - | - | 1.4 ± 0.1 | 3.5 ± 0.6 | 1.9 ± 0.3 | 2.9 ± 0.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Przybylski, P.; Żebrowski, M.; Witkowski, W.; Cybularczyk-Cecotka, M.; Litwinienko, G. Antioxidant Activity of Bilirubin in Micellar and Liposomal Systems Is pH-Dependent. Antioxidants 2024, 13, 426. https://doi.org/10.3390/antiox13040426
Przybylski P, Żebrowski M, Witkowski W, Cybularczyk-Cecotka M, Litwinienko G. Antioxidant Activity of Bilirubin in Micellar and Liposomal Systems Is pH-Dependent. Antioxidants. 2024; 13(4):426. https://doi.org/10.3390/antiox13040426
Chicago/Turabian StylePrzybylski, Paweł, Michał Żebrowski, Wojciech Witkowski, Martyna Cybularczyk-Cecotka, and Grzegorz Litwinienko. 2024. "Antioxidant Activity of Bilirubin in Micellar and Liposomal Systems Is pH-Dependent" Antioxidants 13, no. 4: 426. https://doi.org/10.3390/antiox13040426
APA StylePrzybylski, P., Żebrowski, M., Witkowski, W., Cybularczyk-Cecotka, M., & Litwinienko, G. (2024). Antioxidant Activity of Bilirubin in Micellar and Liposomal Systems Is pH-Dependent. Antioxidants, 13(4), 426. https://doi.org/10.3390/antiox13040426






