Ferroptosis in Liver Disease: Natural Active Compounds and Therapeutic Implications
Abstract
1. Introduction
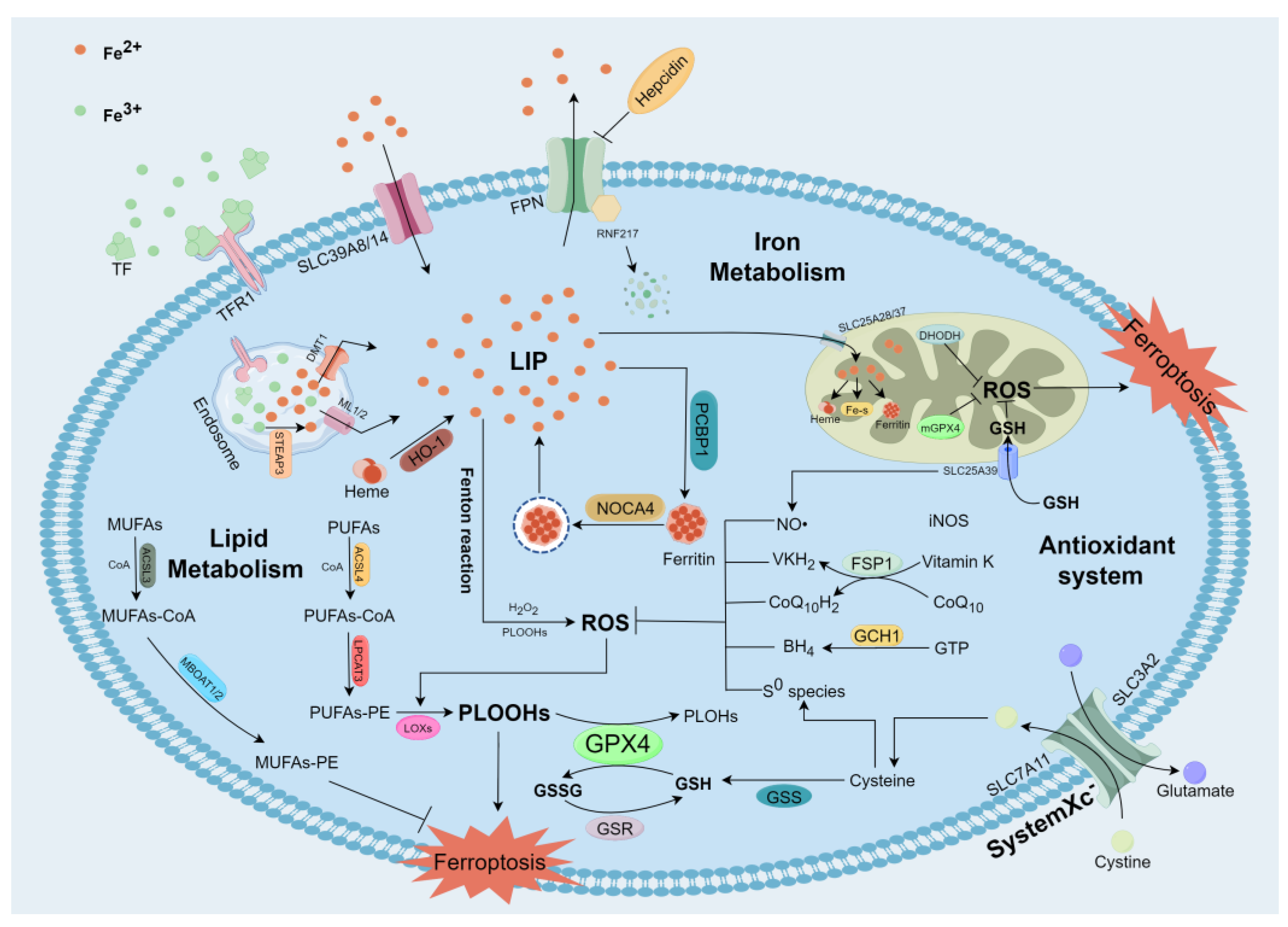
2. The Mechanisms of Ferroptosis
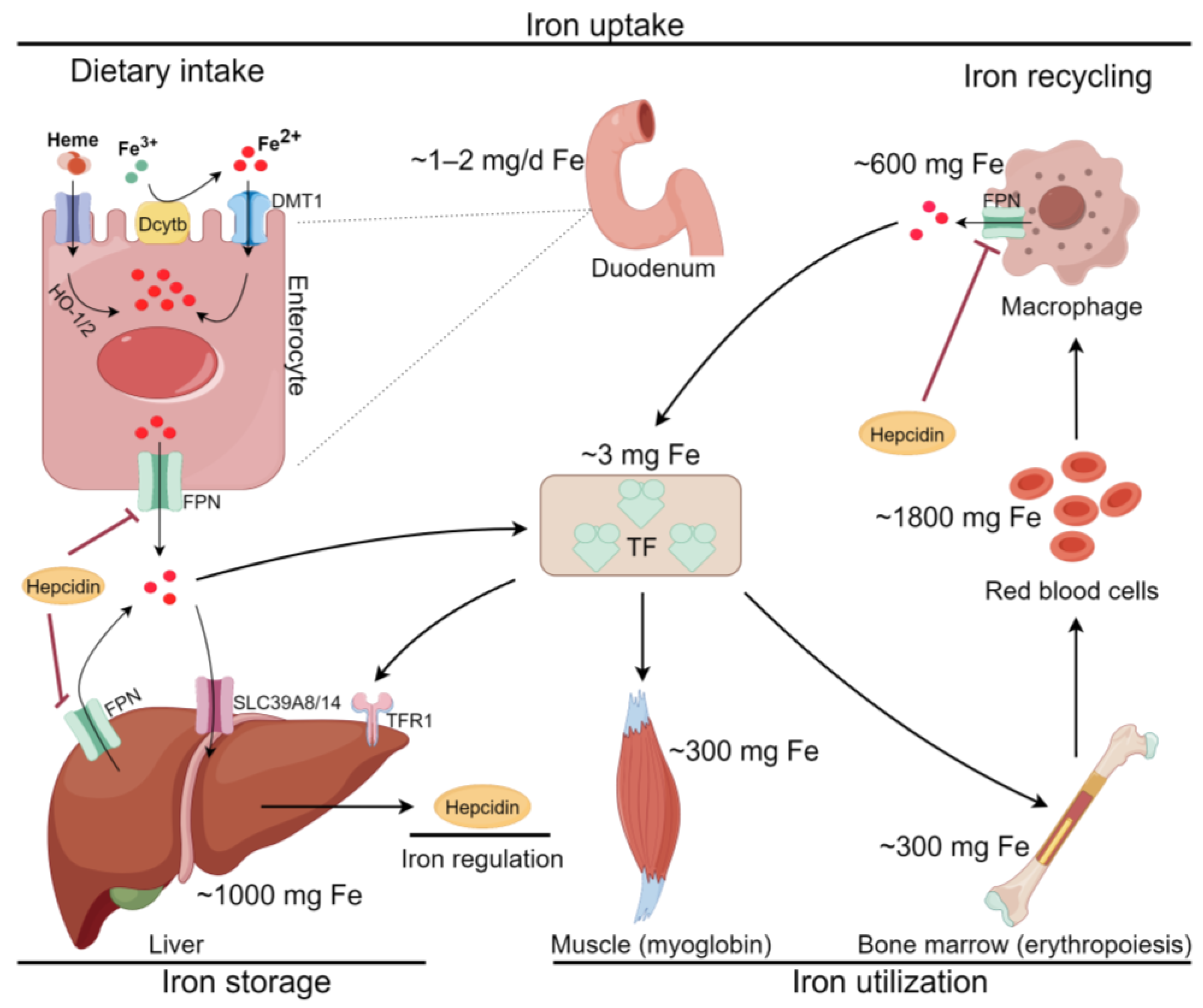
2.1. Iron Metabolism and Ferroptosis
2.2. Lipid Metabolism and Ferroptosis
2.3. Antioxidant System and Ferroptosis
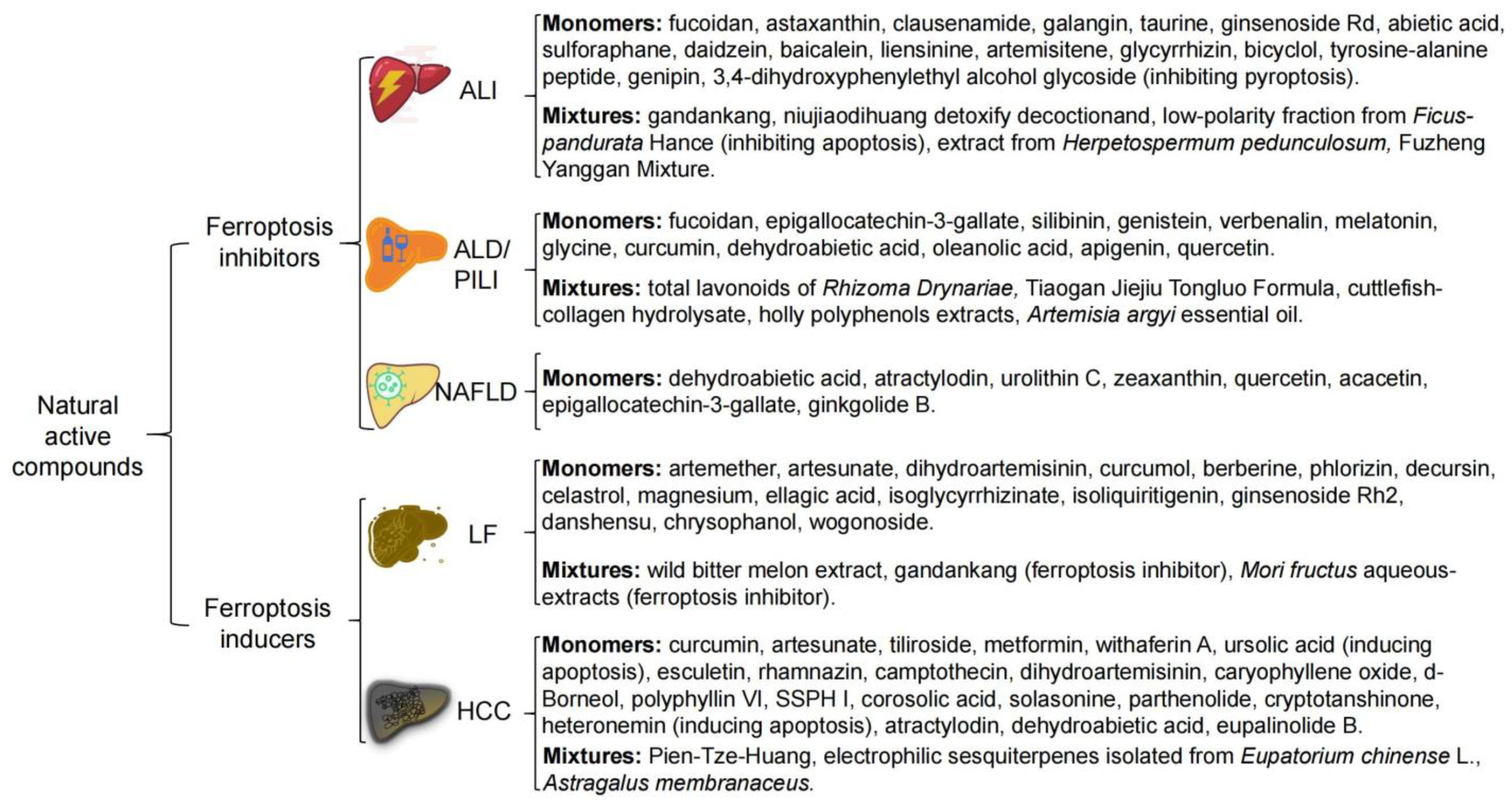
3. NAC Treat Liver Disease by Targeting Ferroptosis
3.1. Acute Liver Injury (ALI)
3.2. Alcohol and Environmental Pollutants-Induced Liver Disease
3.3. Non-Alcoholic Fatty Liver Disease (NAFLD)
3.4. Liver Fibrosis (LF)
3.5. Hepatocellular Carcinoma (HCC)
4. Discussion and Prospects
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; Amelio, I.; Andrews, D.W.; et al. Molecular Mechanisms of Cell Death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018, 25, 486–541. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Kang, R.; Berghe, T.V.; Vandenabeele, P.; Kroemer, G. The Molecular Machinery of Regulated Cell Death. Cell Res. 2019, 29, 347–364. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Chen, X.; Kang, R.; Kroemer, G. Ferroptosis: Molecular Mechanisms and Health Implications. Cell Res. 2021, 31, 107–125. [Google Scholar] [CrossRef] [PubMed]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An Iron-Dependent Form of Non-Apoptotic Cell Death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, Y.; Min, J.; Wang, F. Zooming in and out of Ferroptosis in Human Disease. Front. Med. 2023, 17, 173–206. [Google Scholar] [CrossRef]
- Devarbhavi, H.; Asrani, S.K.; Arab, J.P.; Nartey, Y.A.; Pose, E.; Kamath, P.S. Global Burden of Liver Disease: 2023 Update. Hepatology 2023, 79, 516–537. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, X.; Ge, C.; Min, J.; Wang, F. The Multifaceted Role of Ferroptosis in Liver Disease. Cell Death Differ. 2022, 29, 467–480. [Google Scholar] [CrossRef]
- Ali, N.; Ferrao, K.; Mehta, K.J. Liver Iron Loading in Alcohol-Associated Liver Disease. Am. J. Pathol. 2023, 193, 1427–1439. [Google Scholar] [CrossRef]
- Hirako, I.C.; Antunes, M.M.; Rezende, R.M.; Hojo-Souza, N.S.; Figueiredo, M.M.; Dias, T.; Nakaya, H.; Menezes, G.B.; Gazzinelli, R.T. Uptake of Plasmodium Chabaudi Hemozoin Drives Kupffer Cell Death and Fuels Superinfections. Sci. Rep. 2022, 12, 19805. [Google Scholar] [CrossRef]
- Kojima, H.; Hirao, H.; Kadono, K.; Ito, T.; Yao, S.; Torgerson, T.; Dery, K.J.; Kitajima, H.; Ogawa, T.; Kaldas, F.M.; et al. Cold Stress-Induced Ferroptosis in Liver Sinusoidal Endothelial Cells Determines Liver Transplant Injury and Outcomes. JCI Insight 2024, 9, e174354. [Google Scholar]
- Zhang, S.; Hu, R.; Geng, Y.; Chen, K.; Wang, L.; Imam, M.U. The Regulatory Effects and the Signaling Pathways of Natural Bioactive Compounds on Ferroptosis. Foods 2021, 10, 2952. [Google Scholar] [CrossRef] [PubMed]
- Ma, N.; Zhang, Z.; Liao, F.; Jiang, T.; Tu, Y. The Birth of Artemisinin. Pharmacol. Ther. 2020, 216, 107658. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Chen, Z.; Ding, Y.; Tang, Y.; Cheng, Y. Protective Effect of Traditional Chinese Medicine on Non-Alcoholic Fatty Liver Disease and Liver Cancer by Targeting Ferroptosis. Front. Nutr. 2022, 9, 1033129. [Google Scholar] [CrossRef]
- Zhou, Z.; Li, J.; Zhang, X. Natural Flavonoids and Ferroptosis: Potential Therapeutic Opportunities for Human Diseases. J. Agric. Food Chem. 2023, 71, 5902–5916. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A Review of Programmed Cell Death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Torti, S.V.; Torti, F.M. Iron and Cancer: More Ore to Be Mined. Nat. Rev. Cancer 2013, 13, 342–355. [Google Scholar] [CrossRef]
- Wang, F.; Lv, H.; Zhao, B.; Zhou, L.; Wang, S.; Luo, J.; Liu, J.; Shang, P. Iron and Leukemia: New Insights for Future Treatments. J. Exp. Clin. Cancer Res. CR 2019, 38, 406. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-Y.; Babitt, J.L. Liver Iron Sensing and Body Iron Homeostasis. Blood 2019, 133, 18–29. [Google Scholar] [CrossRef]
- Gao, Z.; Wang, D.; Zhang, H.; Yang, J.; Li, M.; Lu, H.; Shen, H.; Tang, Y. An Iron-Deficient Diet Prevents Alcohol- or Diethylnitrosamine-Induced Acute Hepatotoxicity in Mice by Inhibiting Ferroptosis. Curr. Res. Food Sci. 2022, 5, 2171–2177. [Google Scholar] [CrossRef]
- Arredondo, M.; Núñez, M.T. Iron and Copper Metabolism. Mol. Aspects Med. 2005, 26, 313–327. [Google Scholar] [CrossRef]
- Muckenthaler, M.U.; Rivella, S.; Hentze, M.W.; Galy, B. A Red Carpet for Iron Metabolism. Cell 2017, 168, 344–361. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Chu, H.; Zhu, Q.; Yang, L. Ferroptosis in Non-Alcoholic Liver Disease: Molecular Mechanisms and Therapeutic Implications. Front. Nutr. 2023, 10, 1090338. [Google Scholar] [CrossRef] [PubMed]
- Galaris, D.; Barbouti, A.; Pantopoulos, K. Iron Homeostasis and Oxidative Stress: An Intimate Relationship. Biochim. Biophys. Acta BBA -Mol. Cell Res. 2019, 1866, 118535. [Google Scholar] [CrossRef] [PubMed]
- Gulec, S.; Anderson, G.J.; Collins, J.F. Mechanistic and Regulatory Aspects of Intestinal Iron Absorption. Am. J. Physiol. Gastrointest. Liver Physiol. 2014, 307, G397–G409. [Google Scholar] [CrossRef] [PubMed]
- Fillebeen, C.; Gkouvatsos, K.; Fragoso, G.; Calvé, A.; Garcia-Santos, D.; Buffler, M.; Becker, C.; Schümann, K.; Ponka, P.; Santos, M.M.; et al. Mice Are Poor Heme Absorbers and Do Not Require Intestinal Hmox1 for Dietary Heme Iron Assimilation. Haematologica 2015, 100, e334–e337. [Google Scholar] [CrossRef] [PubMed]
- West, A.R.; Oates, P.S. Mechanisms of Heme Iron Absorption: Current Questions and Controversies. World J. Gastroenterol. 2008, 14, 4101–4110. [Google Scholar] [CrossRef]
- Donovan, A.; Lima, C.A.; Pinkus, J.L.; Pinkus, G.S.; Zon, L.I.; Robine, S.; Andrews, N.C. The Iron Exporter Ferroportin/Slc40a1 Is Essential for Iron Homeostasis. Cell Metab. 2005, 1, 191–200. [Google Scholar] [CrossRef]
- Nemeth, E.; Tuttle, M.S.; Powelson, J.; Vaughn, M.B.; Donovan, A.; Ward, D.M.; Ganz, T.; Kaplan, J. Hepcidin Regulates Cellular Iron Efflux by Binding to Ferroportin and Inducing Its Internalization. Science 2004, 306, 2090–2093. [Google Scholar] [CrossRef]
- Zheng, K.; Dong, Y.; Yang, R.; Liang, Y.; Wu, H.; He, Z. Regulation of Ferroptosis by Bioactive Phytochemicals: Implications for Medical Nutritional Therapy. Pharmacol. Res. 2021, 168, 105580. [Google Scholar] [CrossRef]
- Xiao, Y.; Xu, Z.; Cheng, Y.; Huang, R.; Xie, Y.; Tsai, H.-I.; Zha, H.; Xi, L.; Wang, K.; Cheng, X.; et al. Fe3+-Binding Transferrin Nanovesicles Encapsulating Sorafenib Induce Ferroptosis in Hepatocellular Carcinoma. Biomater. Res. 2023, 27, 63. [Google Scholar] [CrossRef]
- Ohgami, R.S.; Campagna, D.R.; Greer, E.L.; Antiochos, B.; McDonald, A.; Chen, J.; Sharp, J.J.; Fujiwara, Y.; Barker, J.E.; Fleming, M.D. Identification of a Ferrireductase Required for Efficient Transferrin-Dependent Iron Uptake in Erythroid Cells. Nat. Genet. 2005, 37, 1264–1269. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Ardehali, H.; Min, J.; Wang, F. The Molecular and Metabolic Landscape of Iron and Ferroptosis in Cardiovascular Disease. Nat. Rev. Cardiol. 2023, 20, 7–23. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, A.M.; Chirillo, R.; Aversa, I.; Sacco, A.; Costanzo, F.; Biamonte, F. Ferroptosis and Cancer: Mitochondria Meet the “Iron Maiden” Cell Death. Cells 2020, 9, 1505. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Li, L.; Zhang, Y.; Ai, R.; Li, D.; Dou, Y.; Hou, M.; Zhao, D.; Zhao, S.; Nan, Y. Heme Oxygenase 1 Alleviates Nonalcoholic Steatohepatitis by Suppressing Hepatic Ferroptosis. Lipids Health Dis. 2023, 22, 99. [Google Scholar] [CrossRef] [PubMed]
- Dang, R.; Wang, M.; Li, X.; Wang, H.; Liu, L.; Wu, Q.; Zhao, J.; Ji, P.; Zhong, L.; Licinio, J.; et al. Edaravone Ameliorates Depressive and Anxiety-like Behaviors via Sirt1/Nrf2/HO-1/Gpx4 Pathway. J. Neuroinflamm. 2022, 19, 41. [Google Scholar] [CrossRef]
- Rouault, T.A. The Role of Iron Regulatory Proteins in Mammalian Iron Homeostasis and Disease. Nat. Chem. Biol. 2006, 2, 406–414. [Google Scholar] [CrossRef]
- Bayeva, M.; Chang, H.-C.; Wu, R.; Ardehali, H. When Less Is More: Novel Mechanisms of Iron Conservation. Trends Endocrinol. Metab. TEM 2013, 24, 569–577. [Google Scholar] [CrossRef]
- Bayeva, M.; Khechaduri, A.; Puig, S.; Chang, H.-C.; Patial, S.; Blackshear, P.J.; Ardehali, H. mTOR Regulates Cellular Iron Homeostasis through Tristetraprolin. Cell Metab. 2012, 16, 645–657. [Google Scholar] [CrossRef]
- Liang, D.; Minikes, A.M.; Jiang, X. Ferroptosis at the Intersection of Lipid Metabolism and Cellular Signaling. Mol. Cell 2022, 82, 2215–2227. [Google Scholar] [CrossRef]
- Shah, R.; Shchepinov, M.S.; Pratt, D.A. Resolving the Role of Lipoxygenases in the Initiation and Execution of Ferroptosis. ACS Cent. Sci. 2018, 4, 387–396. [Google Scholar] [CrossRef]
- Nagarajan, S.R.; Butler, L.M.; Hoy, A.J. The Diversity and Breadth of Cancer Cell Fatty Acid Metabolism. Cancer Metab. 2021, 9, 2. [Google Scholar] [CrossRef]
- Krümmel, B.; von Hanstein, A.-S.; Plötz, T.; Lenzen, S.; Mehmeti, I. Differential Effects of Saturated and Unsaturated Free Fatty Acids on Ferroptosis in Rat β-Cells. J. Nutr. Biochem. 2022, 106, 109013. [Google Scholar] [CrossRef]
- Dierge, E.; Debock, E.; Guilbaud, C.; Corbet, C.; Mignolet, E.; Mignard, L.; Bastien, E.; Dessy, C.; Larondelle, Y.; Feron, O. Peroxidation of N-3 and n-6 Polyunsaturated Fatty Acids in the Acidic Tumor Environment Leads to Ferroptosis-Mediated Anticancer Effects. Cell Metab. 2021, 33, 1701–1715.e5. [Google Scholar] [CrossRef]
- Tesfay, L.; Paul, B.T.; Konstorum, A.; Deng, Z.; Cox, A.O.; Lee, J.; Furdui, C.M.; Hegde, P.; Torti, F.M.; Torti, S.V. Stearoyl-CoA Desaturase 1 Protects Ovarian Cancer Cells from Ferroptotic Cell Death. Cancer Res. 2019, 79, 5355–5366. [Google Scholar] [CrossRef]
- Yamane, D.; Hayashi, Y.; Matsumoto, M.; Nakanishi, H.; Imagawa, H.; Kohara, M.; Lemon, S.M.; Ichi, I. FADS2-Dependent Fatty Acid Desaturation Dictates Cellular Sensitivity to Ferroptosis and Permissiveness for Hepatitis C Virus Replication. Cell Chem. Biol. 2022, 29, 799–810.e4. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wu, K.; Lei, Y.; Huang, M.; Cheng, L.; Guan, H.; Lin, J.; Zhong, M.; Wang, X.; Zheng, Z. Inhibition of Fatty Acid β-Oxidation by Fatty Acid Binding Protein 4 Induces Ferroptosis in HK2 Cells Under High Glucose Conditions. Endocrinol. Metab. 2023, 38, 226–244. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Traer, E.; Zimmerman, G.A.; McIntyre, T.M.; Prescott, S.M. Cloning, Expression, and Chromosomal Localization of Human Long-Chain Fatty Acid-CoA Ligase 4 (FACL4). Genomics 1998, 49, 327–330. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Li, Y. The Interaction between Ferroptosis and Lipid Metabolism in Cancer. Signal Transduct. Target. Ther. 2020, 5, 108. [Google Scholar] [CrossRef] [PubMed]
- Ji, Q.; Fu, S.; Zuo, H.; Huang, Y.; Chu, L.; Zhu, Y.; Hu, J.; Wu, Y.; Chen, S.; Wang, Y.; et al. ACSL4 Is Essential for Radiation-Induced Intestinal Injury by Initiating Ferroptosis. Cell Death Discov. 2022, 8, 332. [Google Scholar] [CrossRef] [PubMed]
- Grube, J.; Woitok, M.M.; Mohs, A.; Erschfeld, S.; Lynen, C.; Trautwein, C.; Otto, T. ACSL4-Dependent Ferroptosis Does Not Represent a Tumor-Suppressive Mechanism but ACSL4 Rather Promotes Liver Cancer Progression. Cell Death Dis. 2022, 13, 704. [Google Scholar] [CrossRef] [PubMed]
- Reed, A.; Ichu, T.-A.; Milosevich, N.; Melillo, B.; Schafroth, M.A.; Otsuka, Y.; Scampavia, L.; Spicer, T.P.; Cravatt, B.F. LPCAT3 Inhibitors Remodel the Polyunsaturated Phospholipid Content of Human Cells and Protect from Ferroptosis. ACS Chem. Biol. 2022, 17, 1607–1618. [Google Scholar] [CrossRef]
- Liang, D.; Feng, Y.; Zandkarimi, F.; Wang, H.; Zhang, Z.; Kim, J.; Cai, Y.; Gu, W.; Stockwell, B.R.; Jiang, X. Ferroptosis Surveillance Independent of GPX4 and Differentially Regulated by Sex Hormones. Cell 2023, 186, 2748–2764.e22. [Google Scholar] [CrossRef]
- Fan, X.; Wang, X.; Hui, Y.; Zhao, T.; Mao, L.; Cui, B.; Zhong, W.; Sun, C. Genipin Protects against Acute Liver Injury by Abrogating Ferroptosis via Modification of GPX4 and ALOX15-Launched Lipid Peroxidation in Mice. Apoptosis Int. J. Program. Cell Death 2023, 28, 1469–1483. [Google Scholar] [CrossRef]
- Rochette, L.; Dogon, G.; Rigal, E.; Zeller, M.; Cottin, Y.; Vergely, C. Lipid Peroxidation and Iron Metabolism: Two Corner Stones in the Homeostasis Control of Ferroptosis. Int. J. Mol. Sci. 2022, 24, 449. [Google Scholar] [CrossRef]
- Brigelius-Flohé, R.; Maiorino, M. Glutathione Peroxidases. Biochim. Biophys. Acta 2013, 1830, 3289–3303. [Google Scholar] [CrossRef]
- Yang, W.S.; SriRamaratnam, R.; Welsch, M.E.; Shimada, K.; Skouta, R.; Viswanathan, V.S.; Cheah, J.H.; Clemons, P.A.; Shamji, A.F.; Clish, C.B.; et al. Regulation of Ferroptotic Cancer Cell Death by GPX4. Cell 2014, 156, 317–331. [Google Scholar] [CrossRef]
- Dixon, S.J.; Pratt, D.A. Ferroptosis: A Flexible Constellation of Related Biochemical Mechanisms. Mol. Cell 2023, 83, 1030–1042. [Google Scholar] [CrossRef]
- Yuan, S.; Wei, C.; Liu, G.; Zhang, L.; Li, J.; Li, L.; Cai, S.; Fang, L. Sorafenib Attenuates Liver Fibrosis by Triggering Hepatic Stellate Cell Ferroptosis via HIF-1α/SLC7A11 Pathway. Cell Prolif. 2022, 55, e13158. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wan, Y.; Jiang, Y.; Zhang, L.; Cheng, W. GPX4: The Hub of Lipid Oxidation, Ferroptosis, Disease and Treatment. Biochim. Biophys. Acta BBA -Rev. Cancer 2023, 1878, 188890. [Google Scholar] [CrossRef] [PubMed]
- Ingold, K.U.; Pratt, D.A. Advances in Radical-Trapping Antioxidant Chemistry in the 21st Century: A Kinetics and Mechanisms Perspective. Chem. Rev. 2014, 114, 9022–9046. [Google Scholar] [CrossRef] [PubMed]
- Bersuker, K.; Hendricks, J.M.; Li, Z.; Magtanong, L.; Ford, B.; Tang, P.H.; Roberts, M.A.; Tong, B.; Maimone, T.J.; Zoncu, R.; et al. The CoQ Oxidoreductase FSP1 Acts Parallel to GPX4 to Inhibit Ferroptosis. Nature 2019, 575, 688–692. [Google Scholar] [CrossRef]
- Doll, S.; Freitas, F.P.; Shah, R.; Aldrovandi, M.; da Silva, M.C.; Ingold, I.; Goya Grocin, A.; Xavier da Silva, T.N.; Panzilius, E.; Scheel, C.H.; et al. FSP1 Is a Glutathione-Independent Ferroptosis Suppressor. Nature 2019, 575, 693–698. [Google Scholar] [CrossRef]
- Mishima, E.; Ito, J.; Wu, Z.; Nakamura, T.; Wahida, A.; Doll, S.; Tonnus, W.; Nepachalovich, P.; Eggenhofer, E.; Aldrovandi, M.; et al. A Non-Canonical Vitamin K Cycle Is a Potent Ferroptosis Suppressor. Nature 2022, 608, 778–783. [Google Scholar] [CrossRef]
- Nakamura, T.; Hipp, C.; Santos Dias Mourão, A.; Borggräfe, J.; Aldrovandi, M.; Henkelmann, B.; Wanninger, J.; Mishima, E.; Lytton, E.; Emler, D.; et al. Phase Separation of FSP1 Promotes Ferroptosis. Nature 2023, 619, 371–377. [Google Scholar] [CrossRef]
- Werner, E.R.; Blau, N.; Thöny, B. Tetrahydrobiopterin: Biochemistry and Pathophysiology. Biochem. J. 2011, 438, 397–414. [Google Scholar] [CrossRef] [PubMed]
- Kraft, V.A.N.; Bezjian, C.T.; Pfeiffer, S.; Ringelstetter, L.; Müller, C.; Zandkarimi, F.; Merl-Pham, J.; Bao, X.; Anastasov, N.; Kössl, J.; et al. GTP Cyclohydrolase 1/Tetrahydrobiopterin Counteract Ferroptosis through Lipid Remodeling. ACS Cent. Sci. 2020, 6, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Mao, C.; Liu, X.; Zhang, Y.; Lei, G.; Yan, Y.; Lee, H.; Koppula, P.; Wu, S.; Zhuang, L.; Fang, B.; et al. DHODH-Mediated Ferroptosis Defence Is a Targetable Vulnerability in Cancer. Nature 2021, 593, 586–590. [Google Scholar] [CrossRef] [PubMed]
- Zi, L.; Ma, W.; Zhang, L.; Qiao, B.; Qiu, Z.; Xu, J.; Zhang, J.; Ye, Y.; Yang, Y.; Dong, K.; et al. Uridine Inhibits Hepatocellular Carcinoma Cell Development by Inducing Ferroptosis. J. Clin. Med. 2023, 12, 3552. [Google Scholar] [CrossRef] [PubMed]
- Villa, E.; Ali, E.S.; Sahu, U.; Ben-Sahra, I. Cancer Cells Tune the Signaling Pathways to Empower de Novo Synthesis of Nucleotides. Cancers 2019, 11, 688. [Google Scholar] [CrossRef]
- Mishima, E.; Nakamura, T.; Zheng, J.; Zhang, W.; Mourão, A.S.D.; Sennhenn, P.; Conrad, M. DHODH Inhibitors Sensitize to Ferroptosis by FSP1 Inhibition. Nature 2023, 619, E9–E18. [Google Scholar] [CrossRef] [PubMed]
- Ida, T.; Sawa, T.; Ihara, H.; Tsuchiya, Y.; Watanabe, Y.; Kumagai, Y.; Suematsu, M.; Motohashi, H.; Fujii, S.; Matsunaga, T.; et al. Reactive Cysteine Persulfides and S-Polythiolation Regulate Oxidative Stress and Redox Signaling. Proc. Natl. Acad. Sci. USA 2014, 111, 7606–7611. [Google Scholar] [CrossRef]
- Ezeriņa, D.; Takano, Y.; Hanaoka, K.; Urano, Y.; Dick, T.P. N-Acetyl Cysteine Functions as a Fast-Acting Antioxidant by Triggering Intracellular H2S and Sulfane Sulfur Production. Cell Chem. Biol. 2018, 25, 447–459.e4. [Google Scholar] [CrossRef]
- Barayeu, U.; Schilling, D.; Eid, M.; Xavier da Silva, T.N.; Schlicker, L.; Mitreska, N.; Zapp, C.; Gräter, F.; Miller, A.K.; Kappl, R.; et al. Hydropersulfides Inhibit Lipid Peroxidation and Ferroptosis by Scavenging Radicals. Nat. Chem. Biol. 2023, 19, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Barayeu, U.; Schilling, D.; Dick, T.P.; Pratt, D.A. Emergence of (Hydro)Persulfides as Suppressors of Lipid Peroxidation and Ferroptotic Cell Death. Curr. Opin. Chem. Biol. 2023, 76, 102353. [Google Scholar] [CrossRef] [PubMed]
- Erdélyi, K.; Ditrói, T.; Johansson, H.J.; Czikora, Á.; Balog, N.; Silwal-Pandit, L.; Ida, T.; Olasz, J.; Hajdú, D.; Mátrai, Z.; et al. Reprogrammed Transsulfuration Promotes Basal-like Breast Tumor Progression via Realigning Cellular Cysteine Persulfidation. Proc. Natl. Acad. Sci. USA 2021, 118, e2100050118. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.-H.; Lei, H.-X.; Yu, D.; Zhu, H.; Hao, M.-Z.; Cui, R.-H.; Meng, X.-S.; Sheng, X.-H.; Zhang, L. Endogenous Chemicals Guard Health through Inhibiting Ferroptotic Cell Death. BioFactors 2023, ahead of print. [Google Scholar] [CrossRef]
- Kapralov, A.A.; Yang, Q.; Dar, H.H.; Tyurina, Y.Y.; Anthonymuthu, T.S.; Kim, R.; St Croix, C.M.; Mikulska-Ruminska, K.; Liu, B.; Shrivastava, I.H.; et al. Redox Lipid Reprogramming Commands Susceptibility of Macrophages and Microglia to Ferroptotic Death. Nat. Chem. Biol. 2020, 16, 278–290. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhu, J.-Y.; Zang, X.; Zhai, Y.-Z. The Emerging Role of Ferroptosis in Liver Diseases. Front. Cell Dev. Biol. 2021, 9, 801365. [Google Scholar] [CrossRef] [PubMed]
- Capelletti, M.M.; Manceau, H.; Puy, H.; Peoc’h, K. Ferroptosis in Liver Diseases: An Overview. Int. J. Mol. Sci. 2020, 21, 4908. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wang, Y.; Jiang, R.; Xue, R.; Yin, X.; Wu, M.; Meng, Q. Ferroptosis in Liver Disease: New Insights into Disease Mechanisms. Cell Death Discov. 2021, 7, 276. [Google Scholar] [CrossRef]
- Stravitz, R.T.; Fontana, R.J.; Karvellas, C.; Durkalski, V.; McGuire, B.; Rule, J.A.; Tujios, S.; Lee, W.M. Acute Liver Failure Study Group. Future Directions in Acute Liver Failure. Hepatology 2023, 78, 1266–1289. [Google Scholar] [CrossRef]
- Grek, A.; Arasi, L. Acute Liver Failure. AACN Adv. Crit. Care 2016, 27, 420–429. [Google Scholar] [CrossRef]
- Liu, J.; Huang, C.; Liu, J.; Meng, C.; Gu, Q.; Du, X.; Yan, M.; Yu, Y.; Liu, F.; Xia, C. Nrf2 and Its Dependent Autophagy Activation Cooperatively Counteract Ferroptosis to Alleviate Acute Liver Injury. Pharmacol. Res. 2023, 187, 106563. [Google Scholar] [CrossRef]
- Yamada, N.; Karasawa, T.; Kimura, H.; Watanabe, S.; Komada, T.; Kamata, R.; Sampilvanjil, A.; Ito, J.; Nakagawa, K.; Kuwata, H.; et al. Ferroptosis Driven by Radical Oxidation of N-6 Polyunsaturated Fatty Acids Mediates Acetaminophen-Induced Acute Liver Failure. Cell Death Dis. 2020, 11, 144. [Google Scholar] [CrossRef]
- Lőrincz, T.; Jemnitz, K.; Kardon, T.; Mandl, J.; Szarka, A. Ferroptosis Is Involved in Acetaminophen Induced Cell Death. Pathol. Oncol. Res. POR 2015, 21, 1115–1121. [Google Scholar] [CrossRef]
- Dodson, M.; Castro-Portuguez, R.; Zhang, D.D. NRF2 Plays a Critical Role in Mitigating Lipid Peroxidation and Ferroptosis. Redox Biol. 2019, 23, 101107. [Google Scholar] [CrossRef]
- Wang, Y.-Q.; Wei, J.-G.; Tu, M.-J.; Gu, J.-G.; Zhang, W. Fucoidan Alleviates Acetaminophen-Induced Hepatotoxicity via Oxidative Stress Inhibition and Nrf2 Translocation. Int. J. Mol. Sci. 2018, 19, 4050. [Google Scholar] [CrossRef]
- An, Y.; Luo, Q.; Han, D.; Guan, L. Abietic Acid Inhibits Acetaminophen-Induced Liver Injury by Alleviating Inflammation and Ferroptosis through Regulating Nrf2/HO-1 Axis. Int. Immunopharmacol. 2023, 118, 110029. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Hua, S.; Deng, J.; Du, Z.; Zhang, D.; Liu, Z.; Khan, N.U.; Zhou, M.; Chen, Z. Astaxanthin Activated the Nrf2/HO-1 Pathway to Enhance Autophagy and Inhibit Ferroptosis, Ameliorating Acetaminophen-Induced Liver Injury. ACS Appl. Mater. Interfaces 2022, 14, 42887–42903. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Liu, C.-Y.; Wang, T.; Yu, H.-M.; Ouyang, S.-H.; Wu, Y.-P.; Gong, H.-B.; Ma, X.-H.; Jiao, G.-L.; Fu, L.-L.; et al. (+)-Clausenamide Protects against Drug-Induced Liver Injury by Inhibiting Hepatocyte Ferroptosis. Cell Death Dis. 2020, 11, 781. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Wu, R.; Wang, F.; Li, S.; Li, L.; Li, Y.; Qin, P.; Wei, M.; Yang, J.; Wu, J.; et al. Liberation of Daidzein by Gut Microbial β-Galactosidase Suppresses Acetaminophen-Induced Hepatotoxicity in Mice. Cell Host Microbe 2023, 31, 766–780.e7. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Yang, L.; Gao, H.; Zhuo, Y.; Tu, Z.; Wang, Y.; Xun, J.; Zhang, Q.; Zhang, L.; Wang, X. 3,4-Dihydroxyphenylethyl Alcohol Glycoside Reduces Acetaminophen-Induced Acute Liver Failure in Mice by Inhibiting Hepatocyte Ferroptosis and Pyroptosis. PeerJ 2022, 10, e13082. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Liu, C.-Y.; He, C.-C.; Zhao, J.; Sun, Y.-H.; Xu, H.-S.; Cai, X.-Q.; Li, Y.-F.; Hiroshi, K.; He, R.-R. [Protective effect of Fuzheng Yanggan Mixture on drug-induced liver injury]. Zhongguo Zhong Yao Za Zhi Zhongguo Zhongyao Zazhi China J. Chin. Mater. Medica 2018, 43, 4685–4691. [Google Scholar] [CrossRef]
- Li, J.; Lu, Q.; Peng, M.; Liao, J.; Zhang, B.; Yang, D.; Huang, P.; Yang, Y.; Zhao, Q.; Han, B.; et al. Water Extract from Herpetospermum Pedunculosum Attenuates Oxidative Stress and Ferroptosis Induced by Acetaminophen via Regulating Nrf2 and NF-κB Pathways. J. Ethnopharmacol. 2023, 305, 116069. [Google Scholar] [CrossRef]
- Li, X.; Ma, N.; Xu, J.; Zhang, Y.; Yang, P.; Su, X.; Xing, Y.; An, N.; Yang, F.; Zhang, G.; et al. Targeting Ferroptosis: Pathological Mechanism and Treatment of Ischemia-Reperfusion Injury. Oxid. Med. Cell. Longev. 2021, 2021, 1587922. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Li, Y.; Kang, M.; Chang, C.; Wei, H.; Zhang, C.; Chen, Y. Multiple Forms of Cell Death: A Focus on the PI3K/AKT Pathway. J. Cell. Physiol. 2023, 238, 2026–2038. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Xu, J.; Gao, N.; Tian, J.; Song, T. Dexmedetomidine Attenuates Myocardial Ischemia-Reperfusion Injury via Inhibiting Ferroptosis by the cAMP/PKA/CREB Pathway. Mol. Cell. Probes 2023, 68, 101899. [Google Scholar] [CrossRef]
- Chen, K.; Xue, R.; Geng, Y.; Zhang, S. Galangin Inhibited Ferroptosis through Activation of the PI3K/AKT Pathway in Vitro and in Vivo. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2022, 36, e22569. [Google Scholar] [CrossRef]
- Qiu, S.; Li, X.; Zhang, J.; Shi, P.; Cao, Y.; Zhuang, Y.; Tong, L. Neutrophil Membrane-Coated Taurine Nanoparticles Protect against Hepatic Ischemia-Reperfusion Injury. Eur. J. Pharmacol. 2023, 949, 175712. [Google Scholar] [CrossRef]
- Li, Y.; Yu, P.; Fu, W.; Wang, S.; Zhao, W.; Ma, Y.; Wu, Y.; Cui, H.; Yu, X.; Fu, L.; et al. Ginsenoside Rd Inhibited Ferroptosis to Alleviate CCl4-Induced Acute Liver Injury in Mice via cGAS/STING Pathway. Am. J. Chin. Med. 2023, 51, 91–105. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Yu, Z.; Zhou, L.; Wang, X.; Hui, Y.; Mao, L.; Fan, X.; Wang, B.; Zhao, X.; Sun, C. Regulating Nrf2-GPx4 Axis by Bicyclol Can Prevent Ferroptosis in Carbon Tetrachloride-Induced Acute Liver Injury in Mice. Cell Death Discov. 2022, 8, 380. [Google Scholar] [CrossRef]
- Wei, Y.-Y.; Wang, H.-R.; Fan, Y.-M.; Gu, J.-H.; Zhang, X.-Y.; Gong, X.-H.; Hao, Z.-H. Acute Liver Injury Induced by Carbon Tetrachloride Reversal by Gandankang Aqueous Extracts through Nuclear Factor Erythroid 2-Related Factor 2 Signaling Pathway. Ecotoxicol. Environ. Saf. 2023, 251, 114527. [Google Scholar] [CrossRef]
- Dai, C.; Li, H.; Wang, Y.; Tang, S.; Velkov, T.; Shen, J. Inhibition of Oxidative Stress and ALOX12 and NF-κB Pathways Contribute to the Protective Effect of Baicalein on Carbon Tetrachloride-Induced Acute Liver Injury. Antioxidants 2021, 10, 976. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Huang, Q.; Lv, M.; Ma, W.; Sun, J.; Zhong, X.; Hu, R.; Ma, M.; Han, Z.; Zhang, W.; et al. Role of Liensinine in Sensitivity of Activated Macrophages to Ferroptosis and in Acute Liver Injury. Cell Death Discov. 2023, 9, 189. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Xiao, C.; Feng, S.; Bai, J. Artemisitene Alters LPS-Induced Oxidative Stress, Inflammation and Ferroptosis in Liver Through Nrf2/HO-1 and NF-kB Pathway. Front. Pharmacol. 2023, 14, 1177542. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, Q.; Shi, C.; Jiao, F.; Gong, Z. Mechanism of Glycyrrhizin on Ferroptosis during Acute Liver Failure by Inhibiting Oxidative Stress. Mol. Med. Rep. 2019, 20, 4081–4090. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Si, W.; Zeng, J.; Huang, L.; Huang, Z.; Zhao, L.; Liu, J.; Zhu, M.; Kuang, W. Niujiaodihuang Detoxify Decoction Inhibits Ferroptosis by Enhancing Glutathione Synthesis in Acute Liver Failure Models. J. Ethnopharmacol. 2021, 279, 114305. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Pang, X.; Peng, W.; Zhan, X.; Chen, C.; Zhao, W.; Zeng, C.; Mei, Q.; Chen, Q.; Kuang, W.; et al. Liver Protection of a Low-Polarity Fraction from Ficus Pandurata Hance, Prepared by Supercritical CO2 Fluid Extraction, on CCl4-Induced Acute Liver Injury in Mice via Inhibiting Apoptosis and Ferroptosis Mediated by Strengthened Antioxidation. Molecules 2023, 28, 2078. [Google Scholar] [CrossRef] [PubMed]
- Samson, N.; Ablasser, A. The cGAS–STING Pathway and Cancer. Nat. Cancer 2022, 3, 1452–1463. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.; Zhong, X.; Meng, X.; Li, S.; Qian, X.; Lu, H.; Cai, J.; Zhang, Y.; Wang, M.; Ye, Z.; et al. Mitochondria-Localized cGAS Suppresses Ferroptosis to Promote Cancer Progression. Cell Res. 2023, 33, 299–311. [Google Scholar] [CrossRef]
- Siregar, A.S.; Nyiramana, M.M.; Kim, E.-J.; Cho, S.B.; Woo, M.S.; Lee, D.K.; Hong, S.-G.; Han, J.; Kang, S.S.; Kim, D.R.; et al. Oyster-Derived Tyr-Ala (YA) Peptide Prevents Lipopolysaccharide/D-Galactosamine-Induced Acute Liver Failure by Suppressing Inflammatory, Apoptotic, Ferroptotic, and Pyroptotic Signals. Mar. Drugs 2021, 19, 614. [Google Scholar] [CrossRef] [PubMed]
- Macías-Rodríguez, R.U.; Inzaugarat, M.E.; Ruiz-Margáin, A.; Nelson, L.J.; Trautwein, C.; Cubero, F.J. Reclassifying Hepatic Cell Death during Liver Damage: Ferroptosis—A Novel Form of Non-Apoptotic Cell Death? Int. J. Mol. Sci. 2020, 21, 1651. [Google Scholar] [CrossRef]
- Shi, J.-F.; Liu, Y.; Wang, Y.; Gao, R.; Wang, Y.; Liu, J. Targeting Ferroptosis, a Novel Programmed Cell Death, for the Potential of Alcohol-Related Liver Disease Therapy. Front. Pharmacol. 2023, 14, 1194343. [Google Scholar] [CrossRef] [PubMed]
- Ding, Q.; Pi, A.; Hao, L.; Xu, T.; Zhu, Q.; Shu, L.; Yu, X.; Wang, W.; Si, C.; Li, S. Genistein Protects against Acetaldehyde-Induced Oxidative Stress and Hepatocyte Injury in Chronic Alcohol-Fed Mice. J. Agric. Food Chem. 2023, 71, 1930–1943. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Wu, A.; Tan, L.; Tang, D.; Chen, W.; Lai, X.; Gu, K.; Chen, J.; Chen, D.; Tang, Q. Epigallocatechin-3-Gallate Alleviates Liver Oxidative Damage Caused by Iron Overload in Mice through Inhibiting Ferroptosis. Nutrients 2023, 15, 1993. [Google Scholar] [CrossRef]
- El Rashed, Z.; Lupidi, G.; Kanaan, H.; Grasselli, E.; Canesi, L.; Khalifeh, H.; Demori, I. Antioxidant and Antisteatotic Activities of a New Fucoidan Extracted from Ferula Hermonis Roots Harvested on Lebanese Mountains. Molecules 2021, 26, 1161. [Google Scholar] [CrossRef]
- Xue, M.; Tian, Y.; Sui, Y.; Zhao, H.; Gao, H.; Liang, H.; Qiu, X.; Sun, Z.; Zhang, Y.; Qin, Y. Protective Effect of Fucoidan against Iron Overload and Ferroptosis-Induced Liver Injury in Rats Exposed to Alcohol. Biomed. Pharmacother. 2022, 153, 113402. [Google Scholar] [CrossRef]
- Song, X.-Y.; Liu, P.-C.; Liu, W.-W.; Zhou, J.; Hayashi, T.; Mizuno, K.; Hattori, S.; Fujisaki, H.; Ikejima, T. Silibinin Inhibits Ethanol- or Acetaldehyde-Induced Ferroptosis in Liver Cell Lines. Toxicol. Vitro Int. J. Publ. Assoc. BIBRA 2022, 82, 105388. [Google Scholar] [CrossRef]
- Eiyama, A.; Okamoto, K. PINK1/Parkin-Mediated Mitophagy in Mammalian Cells. Curr. Opin. Cell Biol. 2015, 33, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Du, C.; Xu, C.; Wang, Q.; Wang, Z.; Zhu, Q.; Lv, X.; Zhang, L.; Li, J.; Huang, C.; et al. Verbenalin Attenuates Hepatic Damage and Mitochondrial Dysfunction in Alcohol-Associated Steatohepatitis by Regulating MDMX/PPARα-Mediated Ferroptosis. J. Ethnopharmacol. 2023, 307, 116227. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Zhang, J.; Han, J.; Lei, Y.; Cao, Z.; Pan, J.; Pan, Z.; Zhang, Z.; Qu, N.; Luo, H.; et al. Tiaogan Jiejiu Tongluo Formula Attenuated Alcohol-Induced Chronic Liver Injury by Regulating Lipid Metabolism in Rats. J. Ethnopharmacol. 2023, 317, 116838. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yang, M.; Kang, R.; Klionsky, D.J.; Tang, D. Autophagic Degradation of the Circadian Clock Regulator Promotes Ferroptosis. Autophagy 2019, 15, 2033–2035. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, R.; Wang, Z.; Chen, Z.; Wang, G.; Guan, S.; Lu, J. Melatonin Prevents against Ethanol-Induced Liver Injury by Mitigating Ferroptosis via Targeting Brain and Muscle ARNT-like 1 in Mice Liver and HepG2 Cells. J. Agric. Food Chem. 2022, 70, 12953–12967. [Google Scholar] [CrossRef]
- He, P.; Hua, H.; Tian, W.; Zhu, H.; Liu, Y.; Xu, X. Holly (Ilex Latifolia Thunb.) Polyphenols Extracts Alleviate Hepatic Damage by Regulating Ferroptosis Following Diquat Challenge in a Piglet Model. Front. Nutr. 2020, 7, 604328. [Google Scholar] [CrossRef] [PubMed]
- Hua, H.; Xu, X.; Tian, W.; Li, P.; Zhu, H.; Wang, W.; Liu, Y.; Xiao, K. Glycine Alleviated Diquat-Induced Hepatic Injury via Inhibiting Ferroptosis in Weaned Piglets. Anim. Biosci. 2022, 35, 938–947. [Google Scholar] [CrossRef]
- Cui, W.; Zhou, H.; Zhang, J.; Zhang, J.; Wu, D.; Rong, Y.; Liu, F.; Liu, J.; Liu, H.; Wei, B.; et al. Hepatoprotective Effect of Artemisia Argyi Essential Oil on Bisphenol A-Induced Hepatotoxicity via Inhibition of Ferroptosis in Mice. Environ. Toxicol. 2023, 38, 2416–2428. [Google Scholar] [CrossRef] [PubMed]
- Mahlooji, M.A.; Heshmati, A.; Kheiripour, N.; Ghasemi, H.; Asl, S.S.; Solgi, G.; Ranjbar, A.; Hosseini, A. Evaluation of Protective Effects of Curcumin and Nanocurcumin on Aluminium Phosphide-Induced Subacute Lung Injury in Rats: Modulation of Oxidative Stress through SIRT1/FOXO3 Signalling Pathway. Drug Res. 2022, 72, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Yin, K.; Zhang, Y.; Lu, H.; Hou, L.; Zhao, H.; Xing, M. Novel Pathways of Fluoride-Induced Hepatotoxicity: P53-Dependent Ferroptosis Induced by the SIRT1/FOXOs Pathway and Nrf2/HO-1 Pathway. Comp. Biochem. Physiol. Toxicol. Pharmacol. CBP 2023, 264, 109526. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, X.; Liang, C.; Pei, T.; Guo, M.; Wang, J.; Zhang, J. α-Lipoic Acid Alleviated Fluoride-Induced Hepatocyte Injury via Inhibiting Ferroptosis. J. Agric. Food Chem. 2022, 70, 15962–15971. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, W.; Ni, D.; Zhou, Z.; Gu, J.-H.; Zhang, W.; Sun, H.; Liu, F. Alpha Lipoic Acid Antagonizes Cytotoxicity of Cobalt Nanoparticles by Inhibiting Ferroptosis-like Cell Death. J. Nanobiotechnol. 2020, 18, 141. [Google Scholar] [CrossRef]
- Wu, L.; Dong, B.; Chen, Q.; Wang, Y.; Han, D.; Zhu, X.; Liu, H.; Zhang, Z.; Yang, Y.; Xie, S.; et al. Effects of Curcumin on Oxidative Stress and Ferroptosis in Acute Ammonia Stress-Induced Liver Injury in Gibel Carp (Carassius Gibelio). Int. J. Mol. Sci. 2023, 24, 6441. [Google Scholar] [CrossRef]
- Miao, Z.; Miao, Z.; Teng, X.; Xu, S. Melatonin Alleviates Lead-Induced Fatty Liver in the Common Carps (Cyprinus Carpio) via Gut-Liver Axis. Environ. Pollut. Barking Essex 1987 2023, 317, 120730. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, C.; Ma, X.; Zhao, J.; Li, S.; Liu, C.; Tang, Y.; Zhou, J.; Chen, J.; Li, X.; Li, W. Oleanolic Acid Inhibits Mercury Chloride Induced-Liver Ferroptosis by Regulating ROS/Iron Overload. Ecotoxicol. Environ. Saf. 2023, 258, 114973. [Google Scholar] [CrossRef] [PubMed]
- Du, B.; Deng, G.; Zaman, F.; Ma, H.; Li, X.; Chen, J.; Li, T.; Huang, Y. Antioxidant Cuttlefish Collagen Hydrolysate against Ethyl Carbamate-Induced Oxidative Damage. RSC Adv. 2021, 11, 2337–2345. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Li, Y.; Li, J.; Chen, W. Ethyl Carbamate Triggers Ferroptosis in Liver through Inhibiting GSH Synthesis and Suppressing Nrf2 Activation. Redox Biol. 2022, 53, 102349. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Yao, Y.; Chen, L.; Miao, Z.; Xu, S. Apigenin Ameliorates Di(2-Ethylhexyl) Phthalate-Induced Ferroptosis: The Activation of Glutathione Peroxidase 4 and Suppression of Iron Intake. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2022, 164, 113089. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Lin, L.; Wang, S.; Ding, W.; Zhang, C.; Shaukat, A.; Xu, B.; Yue, K.; Zhang, C.; Liu, F. Total Flavonoids of Rhizoma Drynariae Mitigates Aflatoxin B1-Induced Liver Toxicity in Chickens via Microbiota-Gut-Liver Axis Interaction Mechanisms. Antioxidants 2023, 12, 819. [Google Scholar] [CrossRef]
- Huang, T.; Zhang, K.; Wang, J.; He, K.; Zhou, X.; Nie, S. Quercetin Alleviates Acrylamide-Induced Liver Injury by Inhibiting Autophagy-Dependent Ferroptosis. J. Agric. Food Chem. 2023, 71, 7427–7439. [Google Scholar] [CrossRef] [PubMed]
- Han, S.K.; Baik, S.K.; Kim, M.Y. Non-Alcoholic Fatty Liver Disease: Definition and Subtypes. Clin. Mol. Hepatol. 2023, 29, S5–S16. [Google Scholar] [CrossRef]
- Ji, J.; Wu, L.; Wei, J.; Wu, J.; Guo, C. The Gut Microbiome and Ferroptosis in MAFLD. J. Clin. Transl. Hepatol. 2023, 11, 174–187. [Google Scholar] [CrossRef]
- Zhao, J.; Hu, Y.; Peng, J. Targeting Programmed Cell Death in Metabolic Dysfunction-Associated Fatty Liver Disease (MAFLD): A Promising New Therapy. Cell. Mol. Biol. Lett. 2021, 26, 17. [Google Scholar] [CrossRef]
- Zhao, S.; Guo, Y.; Yin, X. Autophagy, Ferroptosis, Apoptosis and Pyroptosis in Metabolic Dysfunction-Associated Steatotic Liver Disease. Front. Biosci. Landmark Ed. 2024, 29, 30. [Google Scholar] [CrossRef]
- Pierantonelli, I.; Svegliati-Baroni, G. Nonalcoholic Fatty Liver Disease: Basic Pathogenetic Mechanisms in the Progression From NAFLD to NASH. Transplantation 2019, 103, e1–e13. [Google Scholar] [CrossRef]
- Loguercio, C.; De Girolamo, V.; de Sio, I.; Tuccillo, C.; Ascione, A.; Baldi, F.; Budillon, G.; Cimino, L.; Di Carlo, A.; Di Marino, M.P.; et al. Non-Alcoholic Fatty Liver Disease in an Area of Southern Italy: Main Clinical, Histological, and Pathophysiological Aspects. Hepatology 2001, 35, 568–574. [Google Scholar] [CrossRef]
- Ma, C.; Han, L.; Zhu, Z.; Heng Pang, C.; Pan, G. Mineral Metabolism and Ferroptosis in Non-Alcoholic Fatty Liver Diseases. Biochem. Pharmacol. 2022, 205, 115242. [Google Scholar] [CrossRef]
- Qi, J.; Kim, J.-W.; Zhou, Z.; Lim, C.-W.; Kim, B. Ferroptosis Affects the Progression of Nonalcoholic Steatohepatitis via the Modulation of Lipid Peroxidation-Mediated Cell Death in Mice. Am. J. Pathol. 2020, 190, 68–81. [Google Scholar] [CrossRef]
- Qiu, M.; Xiao, F.; Wang, T.; Piao, S.; Zhao, W.; Shao, S.; Yan, M.; Zhao, D. Protective Effect of Hedansanqi Tiaozhi Tang against Non-Alcoholic Fatty Liver Disease in Vitro and in Vivo through Activating Nrf2/HO-1 Antioxidant Signaling Pathway. Phytomedicine 2020, 67, 153140. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Fan, Y.; Loor, J.J.; Liang, Y.; Lv, H.; Sun, X.; Jia, H.; Xu, C. Aloin Protects Mice from Diet-Induced Non-Alcoholic Steatohepatitis via Activation of Nrf2/HO-1 Signaling. Food Funct. 2021, 12, 696–705. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.-J.; Kim, W.; Bae, J.-M.; Gim, J.; Kim, S.-J. Dehydroabietic Acid Is a Novel Survivin Inhibitor for Gastric Cancer. Plants 2021, 10, 1047. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Kang, Y.-G.; Kim, Y.-J.; Lee, T.R.; Yoo, B.C.; Jo, M.; Kim, J.H.; Kim, J.-H.; Kim, D.; Cho, J.Y. Dehydroabietic Acid Suppresses Inflammatory Response Via Suppression of Src-, Syk-, and TAK1-Mediated Pathways. Int. J. Mol. Sci. 2019, 20, 1593. [Google Scholar] [CrossRef]
- da Silva, K.R.; Damasceno, J.L.; Inácio, M.d.O.; Abrão, F.; Ferreira, N.H.; Tavares, D.C.; Ambrosio, S.R.; Veneziani, R.C.S.; Martins, C.H.G. Antibacterial and Cytotoxic Activities of Pinus Tropicalis and Pinus Elliottii Resins and of the Diterpene Dehydroabietic Acid Against Bacteria That Cause Dental Caries. Front. Microbiol. 2019, 10, 987. [Google Scholar] [CrossRef]
- Gao, G.; Xie, Z.; Li, E.-W.; Yuan, Y.; Fu, Y.; Wang, P.; Zhang, X.; Qiao, Y.; Xu, J.; Hölscher, C.; et al. Dehydroabietic Acid Improves Nonalcoholic Fatty Liver Disease through Activating the Keap1/Nrf2-ARE Signaling Pathway to Reduce Ferroptosis. J. Nat. Med. 2021, 75, 540–552. [Google Scholar] [CrossRef]
- Xie, Z.; Gao, G.; Wang, H.; Li, E.; Yuan, Y.; Xu, J.; Zhang, Z.; Wang, P.; Fu, Y.; Zeng, H.; et al. Dehydroabietic Acid Alleviates High Fat Diet-Induced Insulin Resistance and Hepatic Steatosis through Dual Activation of PPAR-γ and PPAR-α. Biomed. Pharmacother. 2020, 127, 110155. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Jiang, Y.; Wu, D.; Cai, J.; Jiang, Z.; Zhou, Z.; Liu, L.; Ling, Q.; Wang, Q.; Zhao, G. Atractylodin Alleviates Nonalcoholic Fatty Liver Disease by Regulating Nrf2-Mediated Ferroptosis. Heliyon 2023, 9, e18321. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Chen, J.; Gao, Q.; Shan, X.; Wang, J.; Lv, Z. Study on the Attenuated Effect of Ginkgolide B on Ferroptosis in High Fat Diet Induced Nonalcoholic Fatty Liver Disease. Toxicology 2020, 445, 152599. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Tian, H.; Ji, Y.; Dong, L.; Liu, Y.; Wang, Y.; Gao, X.; Shi, H.; Li, H.; Yang, L. Urolithin C Reveals Anti-NAFLD Potential via AMPK-Ferroptosis Axis and Modulating Gut Microbiota. Naunyn. Schmiedebergs Arch. Pharmacol. 2023, 396, 2687–2699. [Google Scholar] [CrossRef] [PubMed]
- Castillo, V.; Figueroa, F.; González-Pizarro, K.; Jopia, P.; Ibacache-Quiroga, C. Probiotics and Prebiotics as a Strategy for Non-Alcoholic Fatty Liver Disease, a Narrative Review. Foods 2021, 10, 1719. [Google Scholar] [CrossRef]
- Jiang, L.; Hickman, J.H.; Wang, S.-J.; Gu, W. Dynamic Roles of P53-Mediated Metabolic Activities in ROS-Induced Stress Responses. Cell Cycle Georget. Tex 2015, 14, 2881–2885. [Google Scholar] [CrossRef]
- Ou, Y.; Wang, S.-J.; Li, D.; Chu, B.; Gu, W. Activation of SAT1 Engages Polyamine Metabolism with P53-Mediated Ferroptotic Responses. Proc. Natl. Acad. Sci. USA 2016, 113, E6806–E6812. [Google Scholar] [CrossRef]
- Liu, H.; Yan, J.; Guan, F.; Jin, Z.; Xie, J.; Wang, C.; Liu, M.; Liu, J. Zeaxanthin Prevents Ferroptosis by Promoting Mitochondrial Function and Inhibiting the P53 Pathway in Free Fatty Acid-Induced HepG2 Cells. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2023, 1868, 159287. [Google Scholar] [CrossRef]
- Moore, M.P.; Cunningham, R.P.; Meers, G.M.; Johnson, S.A.; Wheeler, A.A.; Ganga, R.R.; Spencer, N.M.; Pitt, J.B.; Diaz-Arias, A.; Swi, A.I.A.; et al. Compromised Hepatic Mitochondrial Fatty Acid Oxidation and Reduced Markers of Mitochondrial Turnover in Human NAFLD. Hepatology 2022, 76, 1452–1465. [Google Scholar] [CrossRef] [PubMed]
- Shum, M.; Ngo, J.; Shirihai, O.S.; Liesa, M. Mitochondrial Oxidative Function in NAFLD: Friend or Foe? Mol. Metab. 2021, 50, 101134. [Google Scholar] [CrossRef] [PubMed]
- Simões, I.C.M.; Fontes, A.; Pinton, P.; Zischka, H.; Wieckowski, M.R. Mitochondria in Non-Alcoholic Fatty Liver Disease. Int. J. Biochem. Cell Biol. 2018, 95, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.-B.; Chu, X.-L.; Jin, Y.-X.; Jiang, J.-J.; Zhao, X.; Yu, M. Epigallocatechin Gallate Alleviates High-Fat Diet-Induced Hepatic Lipotoxicity by Targeting Mitochondrial ROS-Mediated Ferroptosis. Front. Pharmacol. 2023, 14, 1148814. [Google Scholar] [CrossRef]
- Jiang, J.-J.; Zhang, G.-F.; Zheng, J.-Y.; Sun, J.-H.; Ding, S.-B. Targeting Mitochondrial ROS-Mediated Ferroptosis by Quercetin Alleviates High-Fat Diet-Induced Hepatic Lipotoxicity. Front. Pharmacol. 2022, 13, 876550. [Google Scholar] [CrossRef]
- Wu, C.; Du, M.; Yu, R.; Cheng, Y.; Wu, B.; Fu, J.; Tan, W.; Zhou, Q.; Balawi, E.; Liao, Z.B. A Novel Mechanism Linking Ferroptosis and Endoplasmic Reticulum Stress via the circPtpn14/miR-351-5p/5-LOX Signaling in Melatonin-Mediated Treatment of Traumatic Brain Injury. Free Radic. Biol. Med. 2022, 178, 271–294. [Google Scholar] [CrossRef]
- Liou, C.-J.; Wu, S.-J.; Shen, S.-C.; Chen, L.-C.; Chen, Y.-L.; Huang, W.-C. Acacetin Protects against Non-Alcoholic Fatty Liver Disease by Regulating Lipid Accumulation and Inflammation in Mice. Int. J. Mol. Sci. 2022, 23, 4687. [Google Scholar] [CrossRef]
- Jiang, Z.; Sun, H.; Miao, J.; Sheng, Q.; Xu, J.; Gao, Z.; Zhang, X.; Song, Y.; Chen, K. The Natural Flavone Acacetin Protects against High-Fat Diet-Induced Lipid Accumulation in the Liver via the Endoplasmic Reticulum Stress/Ferroptosis Pathway. Biochem. Biophys. Res. Commun. 2023, 640, 183–191. [Google Scholar] [CrossRef]
- Miao, J.; Yao, S.; Sun, H.; Jiang, Z.; Gao, Z.; Xu, J.; Chen, K. Protective Effect of Water-Soluble Acacetin Prodrug on APAP-Induced Acute Liver Injury Is Associated with Upregulation of PPARγ and Alleviation of ER Stress. Int. J. Mol. Sci. 2023, 24, 11320. [Google Scholar] [CrossRef]
- Bataller, R.; Brenner, D.A. Liver Fibrosis. J. Clin. Invest. 2005, 115, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Aydın, M.M.; Akçalı, K.C. Liver Fibrosis. Turk. J. Gastroenterol. Off. J. Turk. Soc. Gastroenterol. 2018, 29, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Pei, Q.; Yi, Q.; Tang, L. Liver Fibrosis Resolution: From Molecular Mechanisms to Therapeutic Opportunities. Int. J. Mol. Sci. 2023, 24, 9671. [Google Scholar] [CrossRef] [PubMed]
- Kitsugi, K.; Noritake, H.; Matsumoto, M.; Hanaoka, T.; Umemura, M.; Yamashita, M.; Takatori, S.; Ito, J.; Ohta, K.; Chida, T.; et al. Simvastatin Inhibits Hepatic Stellate Cells Activation by Regulating the Ferroptosis Signaling Pathway. Biochim. Biophys. Acta Mol. Basis Dis. 2023, 1869, 166750. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Guo, N.; Yang, T.; Yan, J.; Wang, W.; Li, X. The Potential Mechanisms by Which Artemisinin and Its Derivatives Induce Ferroptosis in the Treatment of Cancer. Oxid. Med. Cell. Longev. 2022, 2022, 1458143. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, Z.; Li, M.; Wang, F.; Jia, Y.; Zhang, F.; Shao, J.; Chen, A.; Zheng, S. P53-Dependent Induction of Ferroptosis Is Required for Artemether to Alleviate Carbon Tetrachloride-Induced Liver Fibrosis and Hepatic Stellate Cell Activation. IUBMB Life 2019, 71, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Jin, C.; Shen, M.; Wang, Z.; Tan, S.; Chen, A.; Wang, S.; Shao, J.; Zhang, F.; Zhang, Z.; et al. Iron Regulatory Protein 2 Is Required for Artemether -Mediated Anti-Hepatic Fibrosis through Ferroptosis Pathway. Free Radic. Biol. Med. 2020, 160, 845–859. [Google Scholar] [CrossRef]
- Kong, Z.; Liu, R.; Cheng, Y. Artesunate Alleviates Liver Fibrosis by Regulating Ferroptosis Signaling Pathway. Biomed. Pharmacother. 2019, 109, 2043–2053. [Google Scholar] [CrossRef]
- Shen, M.; Guo, M.; Li, Y.; Wang, Y.; Qiu, Y.; Shao, J.; Zhang, F.; Xu, X.; Yin, G.; Wang, S.; et al. m6A Methylation Is Required for Dihydroartemisinin to Alleviate Liver Fibrosis by Inducing Ferroptosis in Hepatic Stellate Cells. Free Radic. Biol. Med. 2022, 182, 246–259. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhao, T.; Wang, J.; Jiang, R.; Huang, J.; Li, W.; Wang, J. Curcumol Alleviates Liver Fibrosis through Inducing Autophagy and Ferroptosis in Hepatic Stellate Cells. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2022, 36, e22665. [Google Scholar] [CrossRef]
- Yi, J.; Wu, S.; Tan, S.; Qin, Y.; Wang, X.; Jiang, J.; Liu, H.; Wu, B. Berberine Alleviates Liver Fibrosis through Inducing Ferrous Redox to Activate ROS-Mediated Hepatic Stellate Cells Ferroptosis. Cell Death Discov. 2021, 7, 374. [Google Scholar] [CrossRef]
- Shi, Y.; Yan, T.; Lu, X.; Li, K.; Nie, Y.; Jiao, C.; Sun, H.; Li, T.; Li, X.; Han, D. Phloridzin Reveals New Treatment Strategies for Liver Fibrosis. Pharmaceuticals 2022, 15, 896. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.J.; Kim, D.H.; Kim, S.J.; Kim, J.; Jeong, S.-I.; Chung, C.H.; Yu, K.-Y.; Kim, S.-Y. Decursin Attenuates Hepatic Fibrogenesis through Interrupting TGF-Beta-Mediated NAD(P)H Oxidase Activation and Smad Signaling in Vivo and in Vitro. Life Sci. 2014, 108, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Que, R.; Cao, M.; Dai, Y.; Zhou, Y.; Chen, Y.; Lin, L. Decursin Ameliorates Carbon-Tetrachloride-Induced Liver Fibrosis by Facilitating Ferroptosis of Hepatic Stellate Cells. Biochem. Cell Biol. Biochim. Biol. Cell. 2022, 100, 378–386. [Google Scholar] [CrossRef] [PubMed]
- Kil, I.S.; Bae, S.H.; Rhee, S.G. Study of the Signaling Function of Sulfiredoxin and Peroxiredoxin III in Isolated Adrenal Gland: Unsuitability of Clonal and Primary Adrenocortical Cells. Methods Enzymol. 2013, 527, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Luo, P.; Liu, D.; Zhang, Q.; Yang, F.; Wong, Y.-K.; Xia, F.; Zhang, J.; Chen, J.; Tian, Y.; Yang, C.; et al. Celastrol Induces Ferroptosis in Activated HSCs to Ameliorate Hepatic Fibrosis via Targeting Peroxiredoxins and HO-1. Acta Pharm. Sin. B 2022, 12, 2300–2314. [Google Scholar] [CrossRef] [PubMed]
- Sui, M.; Jiang, X.; Chen, J.; Yang, H.; Zhu, Y. Magnesium Isoglycyrrhizinate Ameliorates Liver Fibrosis and Hepatic Stellate Cell Activation by Regulating Ferroptosis Signaling Pathway. Biomed. Pharmacother. 2018, 106, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, K.; Jia, R.; Xie, J.; Ma, L.; Hao, Z.; Zhang, W.; Mo, J.; Ren, F. Ferroportin-Dependent Ferroptosis Induced by Ellagic Acid Retards Liver Fibrosis by Impairing the SNARE Complexes Formation. Redox Biol. 2022, 56, 102435. [Google Scholar] [CrossRef] [PubMed]
- Deng, G.; Li, Y.; Ma, S.; Gao, Z.; Zeng, T.; Chen, L.; Ye, H.; Yang, M.; Shi, H.; Yao, X.; et al. Caveolin-1 Dictates Ferroptosis in the Execution of Acute Immune-Mediated Hepatic Damage by Attenuating Nitrogen Stress. Free Radic. Biol. Med. 2020, 148, 151–161. [Google Scholar] [CrossRef]
- Li, X.; Sun, R.; Liu, R. Natural Products in Licorice for the Therapy of Liver Diseases: Progress and Future Opportunities. Pharmacol. Res. 2019, 144, 210–226. [Google Scholar] [CrossRef]
- Huang, S.; Wang, Y.; Xie, S.; Lai, Y.; Mo, C.; Zeng, T.; Kuang, S.; Zhou, C.; Zeng, Z.; Chen, Y.; et al. Isoliquiritigenin Alleviates Liver Fibrosis through Caveolin-1-Mediated Hepatic Stellate Cells Ferroptosis in Zebrafish and Mice. Phytomed. Int. J. Phytother. Phytopharm. 2022, 101, 154117. [Google Scholar] [CrossRef]
- Chen, S.; He, Z.; Xie, W.; Chen, X.; Lin, Z.; Ma, J.; Liu, Z.; Yang, S.; Wang, Y. Ginsenoside Rh2 Attenuates CDAHFD-Induced Liver Fibrosis in Mice by Improving Intestinal Microbial Composition and Regulating LPS-Mediated Autophagy. Phytomed. Int. J. Phytother. Phytopharm. 2022, 101, 154121. [Google Scholar] [CrossRef]
- Lang, Z.; Yu, S.; Hu, Y.; Tao, Q.; Zhang, J.; Wang, H.; Zheng, L.; Yu, Z.; Zheng, J. Ginsenoside Rh2 Promotes Hepatic Stellate Cell Ferroptosis and Inactivation via Regulation of IRF1-Inhibited SLC7A11. Phytomed. Int. J. Phytother. Phytopharm. 2023, 118, 154950. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.-H.; Huang, J.-H.; Sun, M.-S.; Tzeng, I.-S.; Hsu, Y.-C.; Kuo, C.-Y. Wild Bitter Melon Extract Regulates LPS-Induced Hepatic Stellate Cell Activation, Inflammation, Endoplasmic Reticulum Stress, and Ferroptosis. Evid.-Based Complement. Altern. Med. ECAM 2021, 2021, 6671129. [Google Scholar] [CrossRef]
- Kuo, C.-Y.; Chiu, V.; Hsieh, P.-C.; Huang, C.-Y.; Huang, S.J.; Tzeng, I.-S.; Tsai, F.-M.; Chen, M.-L.; Liu, C.-T.; Chen, Y.-R. Chrysophanol Attenuates Hepatitis B Virus X Protein-Induced Hepatic Stellate Cell Fibrosis by Regulating Endoplasmic Reticulum Stress and Ferroptosis. J. Pharmacol. Sci. 2020, 144, 172–182. [Google Scholar] [CrossRef]
- Wang, C.; Su, Z.; Xu, J.-H.; Ko, C.-Y. Danshensu Attenuated Lipopolysaccharide-Induced LX-2 and T6 Cells Activation through Regulation of Ferroptosis. Food Sci. Nutr. 2023, 11, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Wei, C.; Yuan, S.; Zhang, Z.; Li, J.; Zhang, L.; Wang, G.; Fang, L. Wogonoside Attenuates Liver Fibrosis by Triggering Hepatic Stellate Cell Ferroptosis through SOCS1/P53/SLC7A11 Pathway. Phytother. Res. PTR 2022, 36, 4230–4243. [Google Scholar] [CrossRef]
- Wu, A.; Feng, B.; Yu, J.; Yan, L.; Che, L.; Zhuo, Y.; Luo, Y.; Yu, B.; Wu, D.; Chen, D. Fibroblast Growth Factor 21 Attenuates Iron Overload-Induced Liver Injury and Fibrosis by Inhibiting Ferroptosis. Redox Biol. 2021, 46, 102131. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Gao, C.; Wang, H.; Zhang, Y.; Gu, J.; Zhang, X.; Gong, X.; Hao, Z. Mori Fructus Aqueous Extracts Attenuates Liver Injury by Inhibiting Ferroptosis via the Nrf2 Pathway. J. Anim. Sci. Biotechnol. 2023, 14, 56. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, H.; Zhang, Y.; Gu, J.; Zhang, X.; Gong, X.; Hao, Z. Comprehensive Effect of Carbon Tetrachloride and Reversal of Gandankang Formula in Mice Liver: Involved in Oxidative Stress, Excessive Inflammation, and Intestinal Microflora. Antioxidants 2022, 11, 2234. [Google Scholar] [CrossRef]
- Tang, D.; Kroemer, G.; Kang, R. Ferroptosis in Hepatocellular Carcinoma: From Bench to Bedside. Hepatology 2023, ahead of print. [Google Scholar] [CrossRef]
- Bekric, D.; Ocker, M.; Mayr, C.; Stintzing, S.; Ritter, M.; Kiesslich, T.; Neureiter, D. Ferroptosis in Hepatocellular Carcinoma: Mechanisms, Drug Targets and Approaches to Clinical Translation. Cancers 2022, 14, 1826. [Google Scholar] [CrossRef]
- Casini, A.; Leone, S.; Vaccaro, R.; Vivacqua, G.; Ceci, L.; Pannarale, L.; Franchitto, A.; Onori, P.; Gaudio, E.; Mancinelli, R. The Emerging Role of Ferroptosis in Liver Cancers. Life 2022, 12, 2128. [Google Scholar] [CrossRef]
- Li, X.; Meng, F.; Wang, H.; Sun, L.; Chang, S.; Li, G.; Chen, F. Iron Accumulation and Lipid Peroxidation: Implication of Ferroptosis in Hepatocellular Carcinoma. Front. Endocrinol. 2024, 14, 1319969. [Google Scholar] [CrossRef] [PubMed]
- Nie, J.; Lin, B.; Zhou, M.; Wu, L.; Zheng, T. Role of Ferroptosis in Hepatocellular Carcinoma. J. Cancer Res. Clin. Oncol. 2018, 144, 2329–2337. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, X.-M.; Shi, X.-H.; Ye, K.; Fu, X.-L.; Wang, X.; Guo, S.-M.; Ma, J.-Q.; Xu, F.-F.; Sun, H.-M.; et al. Sorafenib Triggers Ferroptosis via Inhibition of HBXIP/SCD Axis in Hepatocellular Carcinoma. Acta Pharmacol. Sin. 2023, 44, 622–634. [Google Scholar] [CrossRef]
- Huang, W.; Chen, K.; Lu, Y.; Zhang, D.; Cheng, Y.; Li, L.; Huang, W.; He, G.; Liao, H.; Cai, L.; et al. ABCC5 Facilitates the Acquired Resistance of Sorafenib through the Inhibition of SLC7A11-Induced Ferroptosis in Hepatocellular Carcinoma. Neoplasia 2021, 23, 1227–1239. [Google Scholar] [CrossRef]
- Sun, X.; Ou, Z.; Chen, R.; Niu, X.; Chen, D.; Kang, R.; Tang, D. Activation of the P62-Keap1-NRF2 Pathway Protects against Ferroptosis in Hepatocellular Carcinoma Cells. Hepatology 2016, 63, 173–184. [Google Scholar] [CrossRef]
- Zou, C.-G.; Banerjee, R. Tumor Necrosis Factor-Alpha-Induced Targeted Proteolysis of Cystathionine Beta-Synthase Modulates Redox Homeostasis. J. Biol. Chem. 2003, 278, 16802–16808. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Li, L.; Li, M.; Zhang, X.; Zhang, Y.; Ran, J.; Li, L. miR-21-5p Inhibits Ferroptosis in Hepatocellular Carcinoma Cells by Regulating the AKT/mTOR Signaling Pathway through MELK. J. Immunol. Res. 2023, 2023, 8929525. [Google Scholar] [CrossRef]
- Wang, Z.; Li, M.; Liu, Y.; Qiao, Z.; Bai, T.; Yang, L.; Liu, B. Dihydroartemisinin Triggers Ferroptosis in Primary Liver Cancer Cells by Promoting and Unfolded Protein Response-induced Upregulation of CHAC1 Expression. Oncol. Rep. 2021, 46, 240. [Google Scholar] [CrossRef]
- Su, Y.; Zhao, D.; Jin, C.; Li, Z.; Sun, S.; Xia, S.; Zhang, Y.; Zhang, Z.; Zhang, F.; Xu, X.; et al. Dihydroartemisinin Induces Ferroptosis in HCC by Promoting the Formation of PEBP1/15-LO. Oxid. Med. Cell. Longev. 2021, 2021, 3456725. [Google Scholar] [CrossRef]
- Cui, Z.; Wang, H.; Li, S.; Qin, T.; Shi, H.; Ma, J.; Li, L.; Yu, G.; Jiang, T.; Li, C. Dihydroartemisinin Enhances the Inhibitory Effect of Sorafenib on HepG2 Cells by Inducing Ferroptosis and Inhibiting Energy Metabolism. J. Pharmacol. Sci. 2022, 148, 73–85. [Google Scholar] [CrossRef]
- Wong, K.H.; Yang, D.; Chen, S.; He, C.; Chen, M. Development of Nanoscale Drug Delivery Systems of Dihydroartemisinin for Cancer Therapy: A Review. Asian J. Pharm. Sci. 2022, 17, 475–490. [Google Scholar] [CrossRef]
- Su, Y.; Zhang, Z.; Lee, L.T.O.; Peng, L.; Lu, L.; He, X.; Zhang, X. Amphiphilic Dendrimer Doping Enhanced pH-Sensitivity of Liposomal Vesicle for Effective Co-Delivery toward Synergistic Ferroptosis-Apoptosis Therapy of Hepatocellular Carcinoma. Adv. Healthc. Mater. 2023, 12, e2202663. [Google Scholar] [CrossRef]
- Huang, D.; Xu, D.; Chen, W.; Wu, R.; Wen, Y.; Liu, A.; Lin, L.; Lin, X.; Wang, X. Fe-MnO2 Nanosheets Loading Dihydroartemisinin for Ferroptosis and Immunotherapy. Biomed. Pharmacother. 2023, 161, 114431. [Google Scholar] [CrossRef]
- Liu, X.; Wu, Z.; Guo, C.; Guo, H.; Su, Y.; Chen, Q.; Sun, C.; Liu, Q.; Chen, D.; Mu, H. Hypoxia Responsive Nano-Drug Delivery System Based on Angelica Polysaccharide for Liver Cancer Therapy. Drug Deliv. 2022, 29, 138–148. [Google Scholar] [CrossRef]
- Li, Z.-J.; Dai, H.-Q.; Huang, X.-W.; Feng, J.; Deng, J.-H.; Wang, Z.-X.; Yang, X.-M.; Liu, Y.-J.; Wu, Y.; Chen, P.-H.; et al. Artesunate Synergizes with Sorafenib to Induce Ferroptosis in Hepatocellular Carcinoma. Acta Pharmacol. Sin. 2021, 42, 301–310. [Google Scholar] [CrossRef]
- Yang, C.; Lu, T.; Liu, M.; Yuan, X.; Li, D.; Zhang, J.; Zhou, L.; Xu, M. Tiliroside Targets TBK1 to Induce Ferroptosis and Sensitize Hepatocellular Carcinoma to Sorafenib. Phytomed. Int. J. Phytother. Phytopharm. 2023, 111, 154668. [Google Scholar] [CrossRef]
- Hu, Z.; Zhao, Y.; Li, L.; Jiang, J.; Li, W.; Mang, Y.; Gao, Y.; Dong, Y.; Zhu, J.; Yang, C.; et al. Metformin Promotes Ferroptosis and Sensitivity to Sorafenib in Hepatocellular Carcinoma Cells via ATF4/STAT3. Mol. Biol. Rep. 2023, 50, 6399–6413. [Google Scholar] [CrossRef]
- Li, H.; Yu, Y.; Liu, Y.; Luo, Z.; Law, B.Y.K.; Zheng, Y.; Huang, X.; Li, W. Ursolic Acid Enhances the Antitumor Effects of Sorafenib Associated with Mcl-1-Related Apoptosis and SLC7A11-Dependent Ferroptosis in Human Cancer. Pharmacol. Res. 2022, 182, 106306. [Google Scholar] [CrossRef]
- Elkateb, A.S.; Nofal, S.; Ali, S.A.; Atya, H.B. Camptothecin Sensitizes Hepatocellular Carcinoma Cells to Sorafenib- Induced Ferroptosis Via Suppression of Nrf2. Inflammation 2023, 46, 1493–1511. [Google Scholar] [CrossRef]
- Zhang, Y.; Tan, Y.; Liu, S.; Yin, H.; Duan, J.; Fan, L.; Zhao, X.; Jiang, B. Implications of Withaferin A for the Metastatic Potential and Drug Resistance in Hepatocellular Carcinoma Cells via Nrf2-Mediated EMT and Ferroptosis. Toxicol. Mech. Methods 2023, 33, 47–55. [Google Scholar] [CrossRef]
- Xiu, Z.; Li, Y.; Fang, J.; Han, J.; Li, S.; Li, Y.; Yang, X.; Song, G.; Li, Y.; Jin, N.; et al. Inhibitory Effects of Esculetin on Liver Cancer Through Triggering NCOA4 Pathway-Mediation Ferritinophagy in Vivo and in Vitro. J. Hepatocell. Carcinoma 2023, 10, 611–629. [Google Scholar] [CrossRef]
- Xiu, Z.; Zhu, Y.; Han, J.; Li, Y.; Yang, X.; Yang, G.; Song, G.; Li, S.; Li, Y.; Cheng, C.; et al. Caryophyllene Oxide Induces Ferritinophagy by Regulating the NCOA4/FTH1/LC3 Pathway in Hepatocellular Carcinoma. Front. Pharmacol. 2022, 13, 930958. [Google Scholar] [CrossRef]
- Li, J.; Yuan, J.; Li, Y.; Wang, J.; Xie, Q.; Ma, R.; Wang, J.; Ren, M.; Lu, D.; Xu, Z. D-Borneol Enhances Cisplatin Sensitivity via Autophagy Dependent EMT Signaling and NCOA4-Mediated Ferritinophagy. Phytomedicine 2022, 106, 154411. [Google Scholar] [CrossRef]
- Zhu, Z.-H.; Xu, X.-T.; Shen, C.-J.; Yuan, J.-T.; Lou, S.-Y.; Ma, X.-L.; Chen, X.; Yang, B.; Zhao, H.-J. A Novel Sesquiterpene Lactone Fraction from Eupatorium chinense L. Suppresses Hepatocellular Carcinoma Growth by Triggering Ferritinophagy and Mitochondrial Damage. Phytomed. Int. J. Phytother. Phytopharm. 2023, 112, 154671. [Google Scholar] [CrossRef]
- Huang, D.; Dong, X.; Li, J.; Chen, Y.; Zhou, Y.; Chen, Q.; Sun, Y. Steroidal Saponin SSPH I Induces Ferroptosis in HepG2 Cells via Regulating Iron Metabolism. Med. Oncol. 2023, 40, 132. [Google Scholar] [CrossRef]
- Yan, X.; Liu, Y.; Li, C.; Mao, X.; Xu, T.; Hu, Z.; Zhang, C.; Lin, N.; Lin, Y.; Zhang, Y. Pien-Tze-Huang Prevents Hepatocellular Carcinoma by Inducing Ferroptosis via Inhibiting SLC7A11-GSH-GPX4 Axis. Cancer Cell Int. 2023, 23, 109. [Google Scholar] [CrossRef]
- Mei, F.; Liu, Y.; Zheng, S. Rhamnazin Inhibits Hepatocellular Carcinoma Cell Aggressiveness in Vitro via Glutathione Peroxidase 4-Dependent Ferroptosis. Tohoku J. Exp. Med. 2022, 258, 111–120. [Google Scholar] [CrossRef]
- Huang, Q.; Li, J.; Ma, M.; Lv, M.; Hu, R.; Sun, J.; Zhong, X.; Sun, X.; Feng, W.; Ma, W.; et al. High-throughput Screening Identification of a Small-molecule Compound That Induces Ferroptosis and Attenuates the Invasion and Migration of Hepatocellular Carcinoma Cells by Targeting the STAT3/GPX4 Axis. Int. J. Oncol. 2023, 62, 42. [Google Scholar] [CrossRef]
- Peng, Y.; Li, N.; Tang, F.; Qian, C.; Jia, T.; Liu, J.; Xu, Y. Corosolic Acid Sensitizes Ferroptosis by Upregulating HERPUD1 in Liver Cancer Cells. Cell Death Discov. 2022, 8, 376. [Google Scholar] [CrossRef]
- Jin, M.; Shi, C.; Li, T.; Wu, Y.; Hu, C.; Huang, G. Solasonine Promotes Ferroptosis of Hepatoma Carcinoma Cells via Glutathione Peroxidase 4-Induced Destruction of the Glutathione Redox System. Biomed. Pharmacother. 2020, 129, 110282. [Google Scholar] [CrossRef]
- LoBianco, F.V.; Krager, K.J.; Johnson, E.; Godwin, C.O.; Allen, A.R.; Crooks, P.A.; Compadre, C.M.; Borrelli, M.J.; Aykin-Burns, N. Parthenolide Induces Rapid Thiol Oxidation That Leads to Ferroptosis in Hepatocellular Carcinoma Cells. Front. Toxicol. 2022, 4, 936149. [Google Scholar] [CrossRef]
- Liu, J.-L.; Tong, L.; Luo, Y.; Gao, Y.-J. [Cryptotanshinone May Induce Ferroptosis of Human Liver Cancer HepG2 Cells]. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 2021, 43, 366–370. [Google Scholar] [CrossRef]
- Chang, W.-T.; Bow, Y.-D.; Fu, P.-J.; Li, C.-Y.; Wu, C.-Y.; Chang, Y.-H.; Teng, Y.-N.; Li, R.-N.; Lu, M.-C.; Liu, Y.-C.; et al. A Marine Terpenoid, Heteronemin, Induces Both the Apoptosis and Ferroptosis of Hepatocellular Carcinoma Cells and Involves the ROS and MAPK Pathways. Oxid. Med. Cell. Longev. 2021, 2021, 7689045. [Google Scholar] [CrossRef]
- Wang, Y.-F.; Feng, J.-Y.; Zhao, L.-N.; Zhao, M.; Wei, X.-F.; Geng, Y.; Yuan, H.-F.; Hou, C.-Y.; Zhang, H.-H.; Wang, G.-W.; et al. Aspirin Triggers Ferroptosis in Hepatocellular Carcinoma Cells through Restricting NF-κB P65-Activated SLC7A11 Transcription. Acta Pharmacol. Sin. 2023, 44, 1712–1724. [Google Scholar] [CrossRef]
- He, Y.; Fang, D.; Liang, T.; Pang, H.; Nong, Y.; Tang, L.; Yang, Z.; Lu, C.; Han, X.; Zhao, S.; et al. Atractylodin May Induce Ferroptosis of Human Hepatocellular Carcinoma Cells. Ann. Transl. Med. 2021, 9, 1535. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, H.; Mu, J.; Han, M.; Cao, Z.; Dong, F.; Wang, T.; Pan, L.; Luo, W.; Li, J.; et al. Eupalinolide B Inhibits Hepatic Carcinoma by Inducing Ferroptosis and ROS-ER-JNK Pathway. Acta Biochim. Biophys. Sin. 2022, 54, 974–986. [Google Scholar] [CrossRef]
- Xiahou, Z.; Han, J. Effects of Dehydroabietic Acid on Nontarget Lipidomics and Proteomics of HepG2. Front. Pharmacol. 2022, 13, 1015240. [Google Scholar] [CrossRef]
- Liu, Z.; Ma, H.; Lai, Z. The Role of Ferroptosis and Cuproptosis in Curcumin against Hepatocellular Carcinoma. Molecules 2023, 28, 1623. [Google Scholar] [CrossRef]
- Liu, Z.; Ma, H.; Lai, Z. Revealing the Potential Mechanism of Astragalus Membranaceus Improving Prognosis of Hepatocellular Carcinoma by Combining Transcriptomics and Network Pharmacology. BMC Complement. Med. Ther. 2021, 21, 263. [Google Scholar] [CrossRef]
- Wu, X.; Wang, J.; Li, B.; Gong, M.; Cao, C.; Song, L.; Qin, L.; Wang, Y.; Zhang, Y.; Li, Y. Chlorogenic Acid, Rutin, and Quercetin from Lysimachia Christinae Alleviate Triptolide-Induced Multi-Organ Injury in Vivo by Modulating Immunity and AKT/mTOR Signal Pathway to Inhibit Ferroptosis and Apoptosis. Toxicol. Appl. Pharmacol. 2023, 467, 116479. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Chen, S.; Han, S.; Luo, L.; Shen, F.; Huang, Z. Toosendanin Induced Hepatotoxicity via Triggering PERK-eIF2α-ATF4 Mediated Ferroptosis. Toxicol. Lett. 2023, 377, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Zhong, Y.; Liu, J.; Zheng, S.; Lin, L.; Lin, X.; Liang, B.; Huang, Y.; Xian, H.; Li, Z.; et al. An Adverse Outcome Pathway-Based Approach to Assess Aurantio-Obtusin-Induced Hepatotoxicity. Toxicology 2022, 478, 153293. [Google Scholar] [CrossRef]
- Li, P.; Zhang, L.; Guo, Z.; Kang, Q.; Chen, C.; Liu, X.; Ma, Q.; Zhang, J.; Hu, Y.; Wang, T. Epimedium Koreanum Nakai-Induced Liver Injury-A Mechanistic Study Using Untargeted Metabolomics. Front. Pharmacol. 2022, 13, 934057. [Google Scholar] [CrossRef]
| Pollutants | Active Compounds | Potential Therapeutic Targets | Ref. |
|---|---|---|---|
| Ammonia | Curcumin | ACSL4, PTGS2, SLC7A1 | [131] |
| Lead | Melatonin | Gut-liver axis, AMPK | [132] |
| Mercuric chloride | Oleanolic acid | GPX4, SOD1, NRF2, SLC7A11, TFR1 | [133] |
| Ethyl carbamate | Cuttlefish collagen hydrolysate | GSH, HO-1 | [134] |
| Di(2-ethylhexyl) phthalate | Apigenin | System Xc-, GPX4, TFR1, FTH1, FTL, ACSL4, LPCAT3, and PTGS2 | [136] |
| Aflatoxin B1 | Total flavonoids of Rhizoma Drynariae | GSH, GPX4, Microbiota-Gut-Liver Axis Interaction | [137] |
| Acrylamide | Quercetin | NCOA4, FTH1 | [138] |
| Active Compounds | Targets | Synergy Mechanism | Ref. |
|---|---|---|---|
| Artesunate | Lysosomal cathepsin B/L activity | Induces oxidative stress and lysosome-mediated ferritinophagy | [217] |
| Tiliroside | TANK-binding kinase 1 (TBK1) | Promotes KEAP1-mediated NRF2 degradation and inhibits the expression of the downstream target protein of NRF2 | [218] |
| Metformin | ATF4/STAT3 | Increases Fe2+, ROS, and lipid peroxidation | [219] |
| Ursolic acid | MCL-1 and SLC7A11 | Reduces the synthesis of GSH, increases ROS and lipid peroxidation accumulation | [220] |
| Camptothecin | NRF2 and SLC7A11. | Increases lipid peroxidation and iron concentration, decreases TAC, GPX4, and GSR activity | [221] |
| Withaferin A | KEAP1/NRF2 | Mitigates NRF2 signaling activation-mediated epithelial to mesenchymal transition (EMT) and SLC7A11 expression. | [222] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Z.; Zhu, Y.; Liu, W.; Balasubramanian, B.; Xu, X.; Yao, J.; Lei, X. Ferroptosis in Liver Disease: Natural Active Compounds and Therapeutic Implications. Antioxidants 2024, 13, 352. https://doi.org/10.3390/antiox13030352
Wu Z, Zhu Y, Liu W, Balasubramanian B, Xu X, Yao J, Lei X. Ferroptosis in Liver Disease: Natural Active Compounds and Therapeutic Implications. Antioxidants. 2024; 13(3):352. https://doi.org/10.3390/antiox13030352
Chicago/Turabian StyleWu, Zhili, Yanru Zhu, Wenchao Liu, Balamuralikrishnan Balasubramanian, Xiao Xu, Junhu Yao, and Xinjian Lei. 2024. "Ferroptosis in Liver Disease: Natural Active Compounds and Therapeutic Implications" Antioxidants 13, no. 3: 352. https://doi.org/10.3390/antiox13030352
APA StyleWu, Z., Zhu, Y., Liu, W., Balasubramanian, B., Xu, X., Yao, J., & Lei, X. (2024). Ferroptosis in Liver Disease: Natural Active Compounds and Therapeutic Implications. Antioxidants, 13(3), 352. https://doi.org/10.3390/antiox13030352









