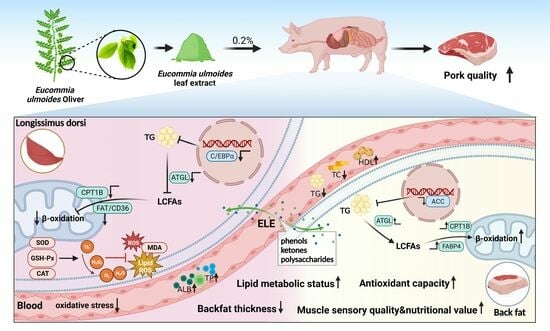
Effects of Dietary Eucommia ulmoides Leaf Extract Supplementation on Growth Performance, Meat Quality, Antioxidant Capacity, and Lipid Metabolism of Finishing Pigs
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Diets
2.2. Sample Collection
2.3. Growth Performance, Carcass Traits, and Meat Quality
2.4. Muscle Chemical Composition
2.5. Serum Biochemical Indexes
2.6. Serum and Longissimus Dorsi Muscle Antioxidant Indexes
2.7. Fatty Acid Composition
2.8. Amino Acid Composition and Taste Activity Value (TAV)
2.9. Relative mRNA Expression
2.10. Statistical Analysis
3. Results
3.1. Growth Performance and Carcass Characteristics
3.2. Meat Quality
3.3. Muscle Chemical Composition
3.4. Serum Biochemical Indexes
3.5. Antioxidant Enzyme Activities and MDA Content
3.6. Fatty Acid Composition
3.7. Free Amino Acid Composition of Muscle and Taste Activity Value
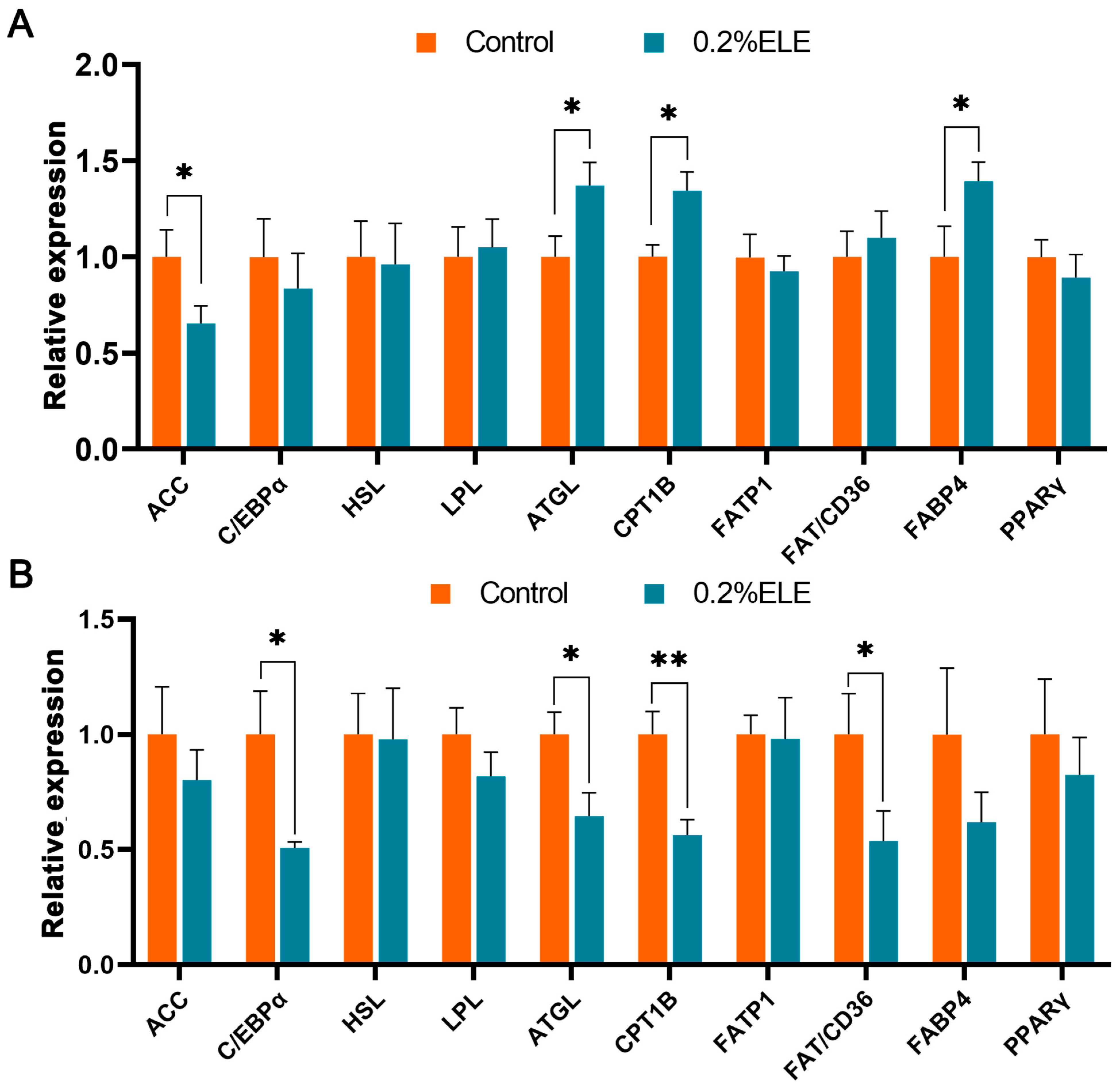
3.8. Lipid Metabolism-Related Gene mRNA Levels
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Calayag, A.M.B.; Paclibare, P.A.P.; Santos, P.D.M.; Bautista, C.A.C.; Rivera, W.L. Molecular characterization and antimicrobial resistance of Salmonella enterica from swine slaughtered in two different types of Philippine abattoir. Food Microbiol. 2017, 65, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Luo, J.Q.; Yu, B.; Zheng, P.; Huang, Z.Q.; Mao, X.B.; He, J.; Yu, J.; Chen, J.L.; Chen, D.W. Dietary resveratrol supplementation improves meat quality of finishing pigs through changing muscle fiber characteristics and antioxidative status. Meat Sci. 2015, 102, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Bradford, H.; McKernan, C.; Elliott, C.; Dean, M. Consumers’ perceptions and willingness to purchase pork labelled ‘raised without antibiotics’. Appetite 2022, 171, 105900. [Google Scholar] [CrossRef]
- Aaslyng, M.D.; Hviid, M. Meat quality in the Danish pig population anno 2018. Meat Sci. 2020, 163, 108034. [Google Scholar] [CrossRef]
- Aaslyng, M.D.; Oksama, M.; Olsen, E.V.; Bejerholm, C.; Baltzer, M.; Andersen, G.; Bredie, W.L.P.; Byrne, D.V.; Gabrielsen, G. The impact of sensory quality of pork on consumer preference. Meat Sci. 2007, 76, 61–73. [Google Scholar] [CrossRef]
- Wyness, L. The role of red meat in the diet: Nutrition and health benefits. Proc. Nutr. Soc. 2016, 75, 227–232. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, M.; Yan, E.; Wang, Y.; Ma, C.; Zhang, P.; Yin, J. Dietary Malic Acid Supplementation Induces Skeletal Muscle Fiber-Type Transition of Weaned Piglets and Further Improves Meat Quality of Finishing Pigs. Front. Nutr. 2022, 8, 825495. [Google Scholar] [CrossRef]
- Xu, M.; Chen, X.; Huang, Z.; Chen, D.; Li, M.; He, J.; Chen, H.; Zheng, P.; Yu, J.; Luo, Y.; et al. Effects of dietary grape seed proanthocyanidin extract supplementation on meat quality, muscle fiber characteristics and antioxidant capacity of finishing pigs. Food Chem. 2022, 367, 130781. [Google Scholar] [CrossRef]
- Wang, L.Y.; Huang, Y.Q.; Wang, Y.Z.; Shan, T.Z. Effects of Polyunsaturated Fatty Acids Supplementation on the Meat Quality of Pigs: A Meta-Analysis. Front. Nutr. 2021, 8, 746765. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Liu, Y.; Duan, G.; Han, M.; Gong, S.; Yang, Z.; Duan, Y.; Guo, Q.; Chen, Q.; Li, F. The Effect of Dietary Leucine Supplementation on Antioxidant Capacity and Meat Quality of Finishing Pigs under Heat Stress. Antioxidants 2022, 11, 1373. [Google Scholar] [CrossRef]
- Zhu, C.; Yang, J.; Nie, X.; Wu, Q.; Wang, L.; Jiang, Z. Influences of Dietary Vitamin E, Selenium-Enriched Yeast, and Soy Isoflavone Supplementation on Growth Performance, Antioxidant Capacity, Carcass Traits, Meat Quality and Gut Microbiota in Finishing Pigs. Antioxidants 2022, 11, 1510. [Google Scholar] [CrossRef]
- Wen, W.; Chen, X.; Huang, Z.; Chen, D.; Yu, B.; He, J.; Luo, Y.; Yan, H.; Chen, H.; Zheng, P.; et al. Dietary lycopene supplementation improves meat quality, antioxidant capacity and skeletal muscle fiber type transformation in finishing pigs. Anim. Nutr. 2022, 8, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Gaschler, M.M.; Stockwell, B.R. Lipid peroxidation in cell death. Biochem. Biophys. Res. Commun. 2017, 482, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.Z.; Yan, Z.M.; Song, B.; Zheng, C.B.; Duan, Y.H.; Kong, X.F.; Deng, J.P.; Li, F.N. Dietary supplementation with betaine or glycine improves the carcass trait, meat quality and lipid metabolism of finishing mini-pigs. Anim. Nutr. 2021, 7, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Fu, R.; Zhang, H.; Chen, D.; Tian, G.; Zheng, P.; He, J.; Yu, J.; Mao, X.; Huang, Z.; Pu, J.; et al. Long-Term Dietary Supplementation with Betaine Improves Growth Performance, Meat Quality and Intramuscular Fat Deposition in Growing-Finishing Pigs. Foods 2023, 12, 494. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Y.; Tang, L.; He, J.W.; Li, J.; Wang, Y.Z. Ethnobotany, Phytochemistry and Pharmacological Properties of Eucommia ulmoides: A Review. Am. J. Chin. Med. 2019, 47, 259–300. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Liu, J.; Guo, S.; Yang, F.; Bu, R.; Lu, J.; Xue, P. Metabolomics comparison of chemical components and metabolic regulations in different parts of Eucommia ulmoides Oliv. Arab. J. Chem. 2022, 15, 104304. [Google Scholar] [CrossRef]
- He, X.; Wang, J.; Li, M.; Hao, D.; Yang, Y.; Zhang, C.; He, R.; Tao, R. Eucommia ulmoides Oliv.: Ethnopharmacology, phytochemistry and pharmacology of an important traditional Chinese medicine. J. Ethnopharmacol. 2014, 151, 78–92. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.F.; He, D.; Wang, Y.; Zeng, W.; Zhang, C.; Lu, Y.; Su, N.; Kong, Y.H.; Xing, X.H. Chemical constituents, biological functions and pharmacological effects for comprehensive utilization of Eucommia ulmoides Oliver. Food Sci. Hum. Wellness 2019, 8, 177–188. [Google Scholar] [CrossRef]
- Zhao, J.S.; Deng, W.; Liu, H.W. Effects of chlorogenic acid-enriched extract from Eucommia ulmoides leaf on performance, meat quality, oxidative stability, and fatty acid profile of meat in heat-stressed broilers. Poult. Sci. 2019, 98, 3040–3049. [Google Scholar] [CrossRef]
- Liu, H.W.; Zhao, J.S.; Li, K.; Deng, W. Effects of chlorogenic acids-enriched extract from Eucommia ulmoides leaves on growth performance, stress response, antioxidant status and meat quality of lambs subjected or not to transport stress. Anim. Feed Sci. Technol. 2018, 238, 47–56. [Google Scholar] [CrossRef]
- Sun, W.T.; He, M.; Xu, X.Y.; Li, X.Q.; Pan, W.Q.; Leng, X.J. Comparison study of three compounds in Eucommia ulmoides on growth, flesh quality of grass carp (Ctenopharyngodon idella). Aquac. Nutr. 2019, 25, 906–916. [Google Scholar] [CrossRef]
- Yang, Y.H.; Li, F.N.; Guo, Q.P.; Wang, W.L.; Zhang, L.Y.; Yin, Y.J.; Gong, S.M.; Han, M.M.; Yin, Y.L. Effects of Different Supplemental Levels of Eucommia ulmoides Leaf Extract in the Diet on Carcass Traits and Lipid Metabolism in Growing-Finishing Pigs. Front. Vet. Sci. 2022, 8, 828165. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, F.; Duan, Y.; Guo, Q.; Zhang, L.; Yang, Y.; Yin, Y.; Han, M.; Gong, S.; Li, J.; et al. Effects of Dietary Chlorogenic Acid Supplementation Derived from Lonicera macranthoides Hand-Mazz on Growth Performance, Free Amino Acid Profile, and Muscle Protein Synthesis in a Finishing Pig Model. Oxidative Med. Cell. Longev. 2022, 2022, 6316611. [Google Scholar] [CrossRef] [PubMed]
- Council, N.R. Nutrient Requirements of Swine: Eleventh Revised Edition; The National Academies Press: Washington, DC, USA, 2012; p. 420. [Google Scholar]
- Zheng, C.; Zhang, S.; Duan, Y.; Li, F.; Song, B.; Guo, Q.; Zheng, J.; Zhang, L.; Lian, G.; Duan, G. Dietary beta-hydroxy-beta-methyl butyrate supplementation improves meat quality of Bama Xiang mini-pigs through manipulation of muscle fiber characteristics. J. Funct. Foods 2022, 88, 104885. [Google Scholar] [CrossRef]
- National Pork Producers Council (NPPC). Official Color and Marbling Standards; National Pork Producers Council: Des Moines, IA, USA, 1999. [Google Scholar]
- AOAC. Official Methods of Analysis, 18th ed.; AOAC: Rockville, MD, USA, 2007. [Google Scholar]
- Paszczyk, B.; Łuczyńska, J. The Comparison of Fatty Acid Composition and Lipid Quality Indices in Hard Cow, Sheep, and Goat Cheeses. Foods 2020, 9, 1667. [Google Scholar] [CrossRef]
- Liu, T.-T.; Xia, N.; Wang, Q.-Z.; Chen, D.-W. Identification of the Non-Volatile Taste-Active Components in Crab Sauce. Foods 2019, 8, 324. [Google Scholar] [CrossRef]
- Zhang, L.; Li, F.; Guo, Q.; Duan, Y.; Wang, W.; Yang, Y.; Yin, Y.; Gong, S.; Han, M.; Yin, Y. Different Proportions of Branched-Chain Amino Acids Modulate Lipid Metabolism in a Finishing Pig Model. J. Agric. Food Chem. 2021, 69, 7037–7048. [Google Scholar] [CrossRef]
- Hosoo, S.; Koyama, M.; Watanabe, A.; Ishida, R.; Hirata, T.; Yamaguchi, Y.; Yamasaki, H.; Wada, K.; Higashi, Y.; Nakamura, K. Preventive effect of Eucommia leaf extract on aortic media hypertrophy in Wistar-Kyoto rats fed a high-fat diet. Hypertens. Res. 2017, 40, 546–551. [Google Scholar] [CrossRef]
- Xu, Z.; Tang, M.; Li, Y.; Liu, F.; Li, X.; Dai, R. Antioxidant Properties of Du-zhong (Eucommia ulmoides Oliv.) Extracts and Their Effects on Color Stability and Lipid Oxidation of Raw Pork Patties. J. Agric. Food Chem. 2010, 58, 7289–7296. [Google Scholar] [CrossRef]
- Li, Q.; Feng, Y.; He, W.; Wang, L.; Wang, R.; Dong, L.; Wang, C. Post-screening characterisation and in vivo evaluation of an anti-inflammatory polysaccharide fraction from Eucommia ulmoides. Carbohydr. Polym. 2017, 169, 304–314. [Google Scholar] [CrossRef]
- Xiao, D.; Yuan, D.; Tan, B.; Wang, J.; Liu, Y.; Tan, B. The Role of Nrf2 Signaling Pathway in Eucommia ulmoides Flavones Regulating Oxidative Stress in the Intestine of Piglets. Oxidative Med. Cell. Longev. 2019, 2019, 9719618. [Google Scholar] [CrossRef]
- Lee, S.D.; Kim, H.Y.; Song, Y.M.; Jung, H.J.; Ji, S.Y.; Jang, H.D.; Ryu, J.W.; Park, J.C.; Moon, H.K.; Kim, I.C. The effect of Eucommia ulmoides leaf supplementation on the growth performance, blood and meat quality parameters in growing and finishing pigs. Anim. Sci. J. 2010, 80, 41–45. [Google Scholar] [CrossRef]
- Zhou, Y.; Ruan, Z.; Li, X.L.; Mi, S.M.; Jiang, M.; Liu, W.H.; Yang, H.S.; Wu, X.; Jiang, G.L.; Yin, Y.L. Eucommia ulmoides Oliver leaf polyphenol supplementation improves meat quality and regulates myofiber type in finishing pigs1. J. Anim. Sci. 2016, 94, 164–168. [Google Scholar] [CrossRef]
- Pringle, T.D.; Williams, S.E. Carcass traits, cut yields, and compositional end points in high-lean-yielding pork carcasses: Effects of 10th rib backfat and loin eye area. J. Anim. Sci. 2001, 79, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Suman, S.P.; Joseph, P. Myoglobin chemistry and meat color. Annu. Rev. Food Sci. Technol. 2012, 4, 79–99. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.-L.; Yen, G.-C. Antioxidant actions of du-zhong (Eucommia ulmoides Oliv.) toward oxidative damage in biomolecules. Life Sci. 2001, 66, 1387–1400. [Google Scholar] [CrossRef]
- Andrés-Bello, A.; Barreto-Palacios, V.; García-Segovia, P.; Mir-Bel, J.; Martínez-Monzó, J. Effect of pH on Color and Texture of Food Products. Food Eng. Rev. 2013, 5, 158–170. [Google Scholar] [CrossRef]
- Archile-Contreras, A.C.; Purslow, P.P. Oxidative stress may affect meat quality by interfering with collagen turnover by muscle fibroblasts. Food Res. Int. 2010, 44, 582–588. [Google Scholar] [CrossRef]
- Li, Y.J.; Li, L.Y.; Li, J.L.; Zhang, L.; Gao, F.; Zhou, G.H. Effects of dietary supplementation with ferulic Acid or vitamin e individually or in combination on meat quality and antioxidant capacity of finishing pigs. Asian-Australas. J. Anim. Sci. 2015, 28, 374–381. [Google Scholar] [CrossRef]
- Sies, H. Oxidative Stress: Concept and Some Practical Aspects. Antioxidants 2020, 9, 852. [Google Scholar] [CrossRef]
- Lim, H.; Oh, C.; Park, M.-S.; Park, H.-B.; Ahn, C.; Bae, W.K.; Yoo, K.H.; Hong, S. Hint from an Enzymatic Reaction: Superoxide Dismutase Models Efficiently Suppress Colorectal Cancer Cell Proliferation. J. Am. Chem. Soc. 2023, 145, 16058–16068. [Google Scholar] [CrossRef] [PubMed]
- Xianyong, M.; Dun, D.; Weidong, C. Chapter 9: Inhibitors and Activators of SOD, GSH-Px, and CAT. In Enzyme Inhibitors and Activators; Murat, S., Ed.; IntechOpen: Rijeka, Croatia, 2017. [Google Scholar]
- Cordiano, R.; Di Gioacchino, M.; Mangifesta, R.; Panzera, C.; Gangemi, S.; Minciullo, P.L. Malondialdehyde as a Potential Oxidative Stress Marker for Allergy-Oriented Diseases: An Update. Molecules 2023, 28, 5979. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhao, J.; Deng, W.; Li, K.; Liu, H. Effects of chlorogenic acid-enriched extract from Eucommia ulmoides Oliver leaf on growth performance and quality and oxidative status of meat in finishing pigs fed diets containing fresh or oxidized corn oil. J. Anim. Physiol. Anim. Nutr. 2019, 104, 1116–1125. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Cao, A.; Li, H.; Zhao, Y.; Feng, J. Effects of Eucommia ulmoides leaf extracts on growth performance, antioxidant capacity and intestinal function in weaned piglets. J. Anim. Physiol. Anim. Nutr. 2020, 104, 1169–1177. [Google Scholar] [CrossRef]
- Kim, J.-H.; Ju, M.-G.; Lee, H.-J.; Yeon, S.-J.; Lee, C.-H. Effect of dietary processed sulfur supplementation on water-soluble flavor precursors, free amino acids, and taste characteristics of pork during refrigerated storage. J. Sci. Food Agric. 2018, 98, 4937–4944. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Chen, X.; Chen, D.; Yu, B.; Yin, J.; Huang, Z. Effects of dietary apple polyphenol supplementation on carcass traits, meat quality, muscle amino acid and fatty acid composition in finishing pigs. Food Funct. 2019, 10, 7426–7434. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Wang, W.; Tang, X.; Huang, R.; Li, F.; Su, W.; Yin, Y.; Wen, C.; Liu, J. Comparison of the meat quality and fatty acid profile of muscles in finishing Xiangcun Black pigs fed varied dietary energy levels. Anim. Nutr. 2022, 11, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Rotzoll, N.; Dunkel, A.; Hofmann, T. Quantitative Studies, Taste Reconstitution, and Omission Experiments on the Key Taste Compounds in Morel Mushrooms (Morchella deliciosa Fr.). J. Agric. Food Chem. 2006, 54, 2705–2711. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, Y.; Jing, L.; Xiao, N.; Wu, X.; Shi, W. Changes in Protein Degradation and Non-Volatile Flavor Substances of Swimming Crab (Portunus trituberculatus) during Steaming. Foods 2022, 11, 3502. [Google Scholar] [CrossRef]
- Wu, G. Functional amino acids in growth, reproduction, and health. Adv. Nutr. 2010, 1, 31–37. [Google Scholar] [CrossRef]
- Moro, J.; Tomé, D.; Schmidely, P.; Demersay, T.-C.; Azzout-Marniche, D. Histidine: A Systematic Review on Metabolism and Physiological Effects in Human and Different Animal Species. Nutrients 2020, 12, 1414. [Google Scholar] [CrossRef] [PubMed]
- Solon-Biet, S.M.; Cogger, V.C.; Pulpitel, T.; Wahl, D.; Clark, X.; Bagley, E.; Gregoriou, G.C.; Senior, A.M.; Wang, Q.-P.; Brandon, A.E.; et al. Branched chain amino acids impact health and lifespan indirectly via amino acid balance and appetite control. Nat. Metab. 2019, 1, 532–545. [Google Scholar] [CrossRef]
- Bassil, M.S.; Hwalla, N.; Obeid, O.A. Meal pattern of male rats maintained on histidine-, leucine-, or tyrosine-supplemented diet. Obesity 2007, 15, 616–623. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, Y.; Yu, M.; Tian, Z.; Deng, J.; Ma, X.; Yin, Y. Magnolol Supplementation Alters Serum Parameters, Immune Homeostasis, Amino Acid Profiles, and Gene Expression of Amino Acid Transporters in Growing Pigs. Int. J. Mol. Sci. 2023, 24, 13952. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Q.; Dai, C.; Li, J.; Huang, P.; Li, Y.; Ding, X.; Huang, J.; Hussain, T.; Yang, H. Effects of dietary energy on growth performance, carcass characteristics, serum biochemical index, and meat quality of female Hu lambs. Anim. Nutr. 2020, 6, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-Y.; Lee, G.-H.; Lee, M.-R.; Kim, H.-K.; Kim, N.-y.; Kim, S.-H.; Lee, Y.-C.; Kim, H.-R.; Chae, H.-J. Eucommia ulmoides Oliver extract, aucubin, and geniposide enhance lysosomal activity to regulate ER stress and hepatic lipid accumulation. PLoS ONE 2013, 8, e81349. [Google Scholar] [CrossRef] [PubMed]
- Park, S.A.; Choi, M.-S.; Kim, M.-J.; Jung, U.J.; Kim, H.-J.; Park, K.-K.; Noh, H.J.; Park, H.-M.; Park, Y.B.; Lee, J.-S.; et al. Hypoglycemic and hypolipidemic action of Du-zhong (Eucommia ulmoides Oliver) leaves water extract in C57BL/KsJ-db/db mice. J. Ethnopharmacol. 2006, 107, 412–417. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Sun, Y.; Han, Y.; Chen, X.; Gong, P.; Zhai, P.; Yao, W.; Ba, Q.; Wang, H. Eucommia bark/leaf extract improves HFD-induced lipid metabolism disorders via targeting gut microbiota to activate the Fiaf-LPL gut-liver axis and SCFAs-GPR43 gut-fat axis. Phytomed. Int. J. Phytother. Phytopharm. 2023, 110, 154652. [Google Scholar] [CrossRef]
- Zhang, C.; Luo, J.Q.; Zheng, P.; Yu, B.; Huang, Z.Q.; Mao, X.B.; He, J.; Yu, J.; Chen, J.L.; Chen, D.W. Differential expression of lipid metabolism-related genes and myosin heavy chain isoform genes in pig muscle tissue leading to different meat quality. Animal 2015, 9, 1073–1080. [Google Scholar] [CrossRef]
- Inserra, L.; Luciano, G.; Bella, M.; Scerra, M.; Cilione, C.; Basile, P.; Lanza, M.; Priolo, A. Effect of including carob pulp in the diet of fattening pigs on the fatty acid composition and oxidative stability of pork. Meat Sci. 2014, 100, 256–261. [Google Scholar] [CrossRef] [PubMed]
- López, S.; Bermúdez, B.; Pacheco, Y.M.; Villar, J.; Abia, R.; Muriana, F.J.G. Distinctive postprandial modulation of β cell function and insulin sensitivity by dietary fats: Monounsaturated compared with saturated fatty acids. Am. J. Clin. Nutr. 2008, 88, 638–644. [Google Scholar] [CrossRef] [PubMed]
- Honkavaara, M. Influence of Porcine Stress and Breed on the Fatty-Acid Profiles of Subcutaneous and Intramuscular Total Lipids. Fleischwirtschaft 1989, 69, 1484–1487. [Google Scholar]
- Piedrafita, J.; Christian, L.L.; Lonergan, S.M. Fatty acid profiles in three stress genotypes of swine and relationships with performance, carcass and meat quality traits. Meat Sci. 2001, 57, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Sacks, F.M.; Lichtenstein, A.H.; Wu, J.H.Y.; Appel, L.J.; Creager, M.A.; Kris-Etherton, P.M.; Miller, M.; Rimm, E.B.; Rudel, L.L.; Robinson, J.G.; et al. Dietary Fats and Cardiovascular Disease: A Presidential Advisory From the American Heart Association. Circulation 2017, 136, e1–e23. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, J.; Gong, H.; Cui, L.; Zhang, W.; Ma, J.; Chen, C.; Ai, H.; Xiao, S.; Huang, L.; et al. Genetic correlation of fatty acid composition with growth, carcass, fat deposition and meat quality traits based on GWAS data in six pig populations. Meat Sci. 2019, 150, 47–55. [Google Scholar] [CrossRef]
- Wood, J.D.; Enser, M.; Fisher, A.V.; Nute, G.R.; Sheard, P.R.; Richardson, R.I.; Hughes, S.I.; Whittington, F.M. Fat deposition, fatty acid composition and meat quality: A review. Meat Sci. 2008, 78, 343–358. [Google Scholar] [CrossRef]
- Abdullah, M.M.H.; Jew, S.; Jones, P.J.H. Health benefits and evaluation of healthcare cost savings if oils rich in monounsaturated fatty acids were substituted for conventional dietary oils in the United States. Nutr. Rev. 2017, 75, 163–174. [Google Scholar] [CrossRef]
- Jenkins, D.J.A.; Chiavaroli, L.; Wong, J.M.W.; Kendall, C.; Lewis, G.F.; Vidgen, E.; Connelly, P.W.; Leiter, L.A.; Josse, R.G.; Lamarche, B. Adding monounsaturated fatty acids to a dietary portfolio of cholesterol-lowering foods in hypercholesterolemia. Can. Med. Assoc. J. 2010, 182, 1961–1967. [Google Scholar] [CrossRef]
- Parthasarathy, S.; Khoo, J.C.; Miller, E.; Barnett, J.; Witztum, J.L.; Steinberg, D. Low density lipoprotein rich in oleic acid is protected against oxidative modification: Implications for dietary prevention of atherosclerosis. Proc. Natl. Acad. Sci. USA 1990, 87, 3894–3898. [Google Scholar] [CrossRef]
- Becker, S.L.; Humphrey, D.C.; Karriker, L.A.; Brown, J.T.; Skoland, K.J.; Greiner, L.L. The effects of dietary essential fatty acid ratios and linoleic acid level in grow-finish pigs. J. Anim. Sci. 2023, 101, skad263. [Google Scholar] [CrossRef]
- Van Name, M.A.; Savoye, M.; Chick, J.M.; Galuppo, B.T.; Feldstein, A.E.; Pierpont, B.; Johnson, C.; Shabanova, V.; Ekong, U.; Valentino, P.L.; et al. A Low ω-6 to ω-3 PUFA Ratio (n-6:n-3 PUFA) Diet to Treat Fatty Liver Disease in Obese Youth. J. Nutr. 2020, 150, 2314–2321. [Google Scholar] [CrossRef]
- Simopoulos, A. An Increase in the Omega-6/Omega-3 Fatty Acid Ratio Increases the Risk for Obesity. Nutrients 2016, 8, 128. [Google Scholar] [CrossRef]
- Cameron, N.D.; Enser, M.; Nute, G.R.; Whittington, F.M.; Penman, J.C.; Fisken, A.C.; Perry, A.M.; Wood, J.D. Genotype with nutrition interaction on fatty acid composition of intramuscular fat and the relationship with flavour of pig meat. Meat Sci. 2000, 55, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zheng, W.; Li, X.; Liang, G.; Ye, N.; Liu, Y.; Li, A.; Liu, X.; Zhang, R.; Cheng, J.; et al. Investigation of Obesity-Alleviation Effect of Eurycoma longifolia on Mice Fed with a High-Fat Diet through Metabolomics Revealed Enhanced Decomposition and Inhibition of Accumulation of Lipids. J. Proteome Res. 2021, 20, 2714–2724. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yao, W.; Sun, Y.; Han, Y.; Chen, X.; Gong, P.; Zhai, P.; Pei, S.; Xie, J.; Ba, Q.; et al. Eucommia Bark/Leaf Extract Improves Lipid Metabolism Disorders by Affecting Intestinal Microbiota and Microbiome–Host Interaction in HFD Mice. J. Agric. Food Chem. 2023, 71, 3297–3314. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, F.; Guo, Q.; Duan, Y.; Wang, W.; Yang, Y.; Yin, Y.; Gong, S.; Han, M.; Yin, Y. Balanced branched-chain amino acids modulate meat quality by adjusting muscle fiber type conversion and intramuscular fat deposition in finishing pigs. J. Sci. Food Agric. 2022, 102, 3796–3807. [Google Scholar] [CrossRef] [PubMed]
- Jiao, A.; Diao, H.; Yu, B.; He, J.; Yu, J.; Zheng, P.; Luo, Y.; Luo, J.; Wang, Q.; Wang, H.; et al. Infusion of short chain fatty acids in the ileum improves the carcass traits, meat quality and lipid metabolism of growing pigs. Anim. Nutr. 2021, 7, 94–100. [Google Scholar] [CrossRef]
- Bak, A.M.; Moller, A.B.; Vendelbo, M.H.; Nielsen, T.S.; Viggers, R.; Rungby, J.; Pedersen, S.B.; Jorgensen, J.O.L.; Jessen, N.; Moller, N. Differential regulation of lipid and protein metabolism in obese vs. lean subjects before and after a 72-h fast. Am. J. Physiol. Endocrinol. Metab. 2016, 311, E224–E235. [Google Scholar] [CrossRef]
- Cho, Y.-L.; Park, J.-G.; Kang, H.J.; Kim, W.; Cho, M.J.; Jang, J.-H.; Kwon, M.-G.; Kim, S.; Lee, S.-H.; Lee, J.; et al. Ginkgetin, a biflavone from Ginkgo biloba leaves, prevents adipogenesis through STAT5-mediated PPAR gamma and C/EBP alpha regulation. Pharmacol. Res. 2019, 139, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Holloway, G.P.; Luiken, J.J.F.P.; Glatz, J.F.C.; Spriet, L.L.; Bonen, A. Contribution of FAT/CD36 to the regulation of skeletal muscle fatty acid oxidation: An overview. Acta Physiol. 2008, 194, 293–309. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Li, Z.; Rong, T.; Wang, G.; Liu, Z.; Chen, W.; Li, J.; Li, J.; Ma, X. Different dietary starch sources alter the carcass traits, meat quality, and the profile of muscle amino acid and fatty acid in finishing pigs. J. Anim. Sci. Biotechnol. 2020, 11, 78. [Google Scholar] [CrossRef] [PubMed]
| Ingredients (%) | 61~90 kg | 91~120 kg |
|---|---|---|
| Corn | 39.70 | 40.00 |
| Wheat | 33.70 | 39.10 |
| Soybean meal | 12.60 | 6.90 |
| Wheat bran | 6.00 | 6.00 |
| Biological feed | 4.00 | 4.00 |
| Premix 1 | 4.00 | 4.00 |
| Total | 100.00 | 100.00 |
| Nutrient levels,% 2 | ||
| Digestible energy, MJ/kg | 13.48 | 13.31 |
| Crude protein | 13.94 | 12.05 |
| Lysine | 0.90 | 0.82 |
| Methionine | 0.27 | 0.26 |
| Threonine | 0.61 | 0.56 |
| Tryptophan | 0.15 | 0.14 |
| Valine | 0.54 | 0.44 |
| Calcium | 0.66 | 0.57 |
| Available phosphorus | 0.34 | 0.26 |
| Fatty acid profile (g/100 g FAME) 3 | ||
| C14:0 | 0.05 | 0.05 |
| C16:0 | 15.35 | 15.63 |
| C16:1 | 0.22 | 0.23 |
| C18:0 | 1.65 | 1.53 |
| C18:1 | 24.68 | 24.43 |
| C18:2 | 54.35 | 54.49 |
| C18:3 | 3.70 | 3.65 |
| Genes 1 | Primer Sequences | Product Length/bp | Acc.Num. |
|---|---|---|---|
| ACC | F: 5′-AGCAAGGTCGAGACCGAAAG-3′ R: 5′-TAAGACCACCGGCGGATAGA-3′ | 208 | NM_001114269 |
| C/EBPα | F: 5′-GCAGAGATCCCTATAAACCAGC-3′ | 170 | XM_003127015 |
| R: 5′-TTCAAAGCCCCAAGTCCC-3′ | |||
| HSL | F: 5′-CACAAGGGCTGCTTCTACGG-3′ R: 5′-AAGCGGCCACTGGTGAAGAG-3′ | 195 | HM591297 |
| LPL | F: 5′-CTCGTGCTCAGATGCCCTAC-3′ R: 5′-GGCAGGGTGAAAGGGATGTT-3′ | 148 | NM_214286 |
| ATGL | F: 5′-TCACCAACACCAGCATCCA-3′ R: 5′-GCACATCTCTCGAAGCACCA-3′ | 62 | NM_001099930.1 |
| CPT1B | F: 5′-GACAAGTCCTTCACCCTCATCGC-3′ R: 5′-GGGTTTGGTTTGCCCAGACAG-3′ | 176 | NM_001007191 |
| FATP1 | F: 5′-ACCACTCCTACCGCATGCAG-3′ R: 5′-CCACGATGTTCCCTGCCGAGT-3′ | 208 | NM_001083931 |
| FAT/CD36 | F: 5′-CTGGTGCTGTCATTGGAGCAG-3′ R: 5′-CTGTCTGTAAACTTCCGTGCCTGTT-3′ | 160 | NM_001044622.1 |
| FABP4 | F: 5′-CAGGAAAGTCAAGAGCACCA-3′ R: 5′-TCGGGACAATACATCCAACA-3′ | 147 | NM_001002817 |
| PPARγ | F: 5′-TCGGGACAATACATCCAACA-3′ R: 5′-GACACAGGCTCCACTTTGATG-3′ | 381 | NM_214379 |
| GAPDH | F: 5′-CAAAGTGGACATTGTCGCCATCA-3′ R: 5′-AGCTTCCCATTCTCAGCCTTGACT-3′ | 140 | NM_001206359 |
| Items 1 | CON | ELE | SEM | p-Value |
|---|---|---|---|---|
| Initial body weight, kg | 75.19 | 74.38 | 0.77 | 0.61 |
| Final body weight, kg | 124.66 | 124.33 | 1.13 | 0.89 |
| ADFI, g | 2466.3 | 2480.1 | 20.4 | 0.75 |
| ADG, g | 824.4 | 832.5 | 10.1 | 0.69 |
| F/G | 3.00 | 2.98 | 0.03 | 0.84 |
| Carcass weight, kg | 95.50 | 94.88 | 0.61 | 0.63 |
| Carcass yield, % | 75.35 | 76.33 | 0.45 | 0.30 |
| Carcass straight length, cm | 101.83 | 99.06 | 0.89 | 0.12 |
| Carcass oblique length, cm | 85.28 | 84.24 | 0.67 | 0.45 |
| Loin-eye area, cm2 | 28.48 | 30.02 | 0.67 | 0.26 |
| Backfat thickness, cm | 2.38 | 2.16 | 0.52 | 0.07 |
| Liver, kg | 1.68 | 1.74 | 0.48 | 0.48 |
| Perirenal fat weight, kg | 1.58 | 1.79 | 1.27 | 0.42 |
| Items 1 | CON | ELE | SEM | p-Value |
|---|---|---|---|---|
| After slaughter 45 min | ||||
| Lightness (L*) | 52.08 | 48.02 | 0.90 | 0.02 |
| Redness (a*) | 12.66 | 12.78 | 0.17 | 0.73 |
| Yellowness (b*) | 5.40 | 4.70 | 0.19 | 0.07 |
| pH45min | 5.71 | 5.98 | 0.06 | 0.02 |
| After slaughter 24 h | ||||
| Lightness (L*) | 57.72 | 54.78 | 0.62 | 0.01 |
| Redness (a*) | 13.20 | 13.57 | 0.19 | 0.33 |
| Yellowness (b*) | 8.18 | 8.02 | 0.23 | 0.57 |
| pH24h | 5.30 | 5.43 | 0.03 | 0.03 |
| Pressurization loss, % | 29.14 | 24.19 | 1.06 | 0.01 |
| Cooking loss, % | 41.49 | 41.86 | 0.42 | 0.66 |
| Drip loss, % | 2.74 | 2.23 | 0.12 | 0.03 |
| Shear force, N | 69.80 | 68.33 | 1.38 | 0.61 |
| Marbling score | 0.90 | 1.00 | 0.06 | 0.58 |
| Meat color score | 2.20 | 3.40 | 0.24 | 0.01 |
| Items 1 | CON | ELE | SEM | p-Value |
|---|---|---|---|---|
| Dry matter, % | 25.59 | 25.86 | 0.17 | 0.44 |
| Intramuscular fat, % | 1.32 | 1.86 | 0.18 | 0.14 |
| Crude protein, % | 21.61 | 22.19 | 0.19 | 0.13 |
| Inosine monophosphate, mg/g | 2.79 | 3.30 | 0.10 | 0.01 |
| Items 1 | CON | ELE | SEM | p-Value |
|---|---|---|---|---|
| ALB, g/L | 44.31 | 49.65 | 1.22 | 0.02 |
| TP, g/L | 73.22 | 79.23 | 1.20 | <0.01 |
| BUN, mmol/L | 3.97 | 4.40 | 0.22 | 0.35 |
| GLU, mmol/L | 6.54 | 6.67 | 0.31 | 0.84 |
| TAG, mmol/L | 0.70 | 0.55 | 0.04 | 0.06 |
| TC, mmol/L | 3.02 | 2.40 | 0.11 | <0.01 |
| LDL-C, mmol/L | 1.60 | 1.39 | 0.08 | 0.10 |
| HDL-C, mmol/L | 0.94 | 1.22 | 0.06 | 0.02 |
| Items 1 | CON | ELE | SEM | p-Value |
|---|---|---|---|---|
| Serum | ||||
| GSH-PX, U/mL | 122.03 | 154.56 | 5.50 | <0.01 |
| SOD, U/mL | 103.41 | 149.23 | 7.76 | <0.01 |
| CAT, U/mL | 11.00 | 12.83 | 0.52 | 0.08 |
| MDA, nmol/mL | 8.96 | 7.41 | 0.44 | 0.08 |
| Longissimus dorsi muscle | ||||
| GSH-PX, U/mL | 144.38 | 165.87 | 5.09 | 0.03 |
| SOD, U/mL | 130.99 | 180.67 | 7.25 | <0.01 |
| CAT, U/mL | 14.84 | 14.88 | 0.37 | 0.96 |
| MDA, nmol/mL | 11.44 | 8.00 | 0.52 | <0.01 |
| Items 1 | CON | ELE | SEM | p-Value |
|---|---|---|---|---|
| C10:0 | 0.13 | 0.14 | 0.01 | 0.44 |
| C12:0 | 0.08 | 0.09 | 0.00 | 0.14 |
| C14:0 | 1.11 | 1.35 | 0.06 | 0.04 |
| C16:0 | 24.92 | 25.47 | 0.28 | 0.34 |
| C16:1 | 2.92 | 3.27 | 0.23 | 0.47 |
| C17:0 | 0.24 | 0.18 | 0.02 | 0.11 |
| C18:0 | 15.07 | 13.97 | 0.29 | 0.05 |
| C18:1 trans-9 | 0.13 | 0.14 | 0.00 | 0.25 |
| C18:1 cis-9 | 33.43 | 38.76 | 1.20 | 0.02 |
| C18:2n-6 | 14.46 | 11.30 | 0.94 | 0.09 |
| C20:0 | 0.16 | 0.17 | 0.01 | 0.54 |
| C18:3n-6 | 0.15 | 0.09 | 0.01 | 0.04 |
| C20:1 | 0.54 | 0.66 | 0.03 | 0.05 |
| C18:3n-3 | 0.46 | 0.46 | 0.02 | 1.00 |
| C20:2 | 0.35 | 0.35 | 0.02 | 0.93 |
| C20:3n-6 | 0.62 | 0.36 | 0.06 | 0.02 |
| C20:3n-3 | 0.08 | 0.09 | 0.00 | 0.83 |
| C20:4n-6 | 4.66 | 2.84 | 0.48 | 0.05 |
| C24:0 | 0.35 | 0.21 | 0.03 | 0.04 |
| C22:6n-3 | 0.12 | 0.09 | 0.01 | 0.11 |
| SFA 2 | 42.07 | 41.59 | 0.34 | 0.50 |
| UFA 3 | 57.93 | 58.41 | 0.34 | 0.50 |
| MUFA 4 | 37.02 | 42.82 | 1.38 | 0.03 |
| PUFA 5 | 20.91 | 15.56 | 1.46 | 0.06 |
| n-3 PUFA 6 | 0.66 | 0.64 | 0.03 | 0.45 |
| n-6 PUFA 7 | 19.89 | 14.60 | 1.43 | 0.06 |
| SFA/PUFA | 2.13 | 2.85 | 0.24 | 0.14 |
| n-6/n-3 PUFA | 29.62 | 23.39 | 1.60 | 0.05 |
| AI 8 | 0.51 | 0.53 | 0.01 | 0.30 |
| TI 9 | 1.35 | 1.33 | 0.02 | 0.75 |
| h/H 10 | 2.07 | 2.01 | 0.04 | 0.44 |
| Items 1 | Threshold Value (mg/100 g) | Content (mg/100 g) | TAV 2 | p-Value(content) | p-Value(TAV) | ||||
|---|---|---|---|---|---|---|---|---|---|
| CON | ELE | SEM | CON | ELE | SEM | ||||
| Umami AAs 3 | / | 7.62 | 8.89 | 0.42 | / | / | / | 0.13 | / |
| Glutamic, Glu | 30 | 6.75 | 7.60 | 0.35 | 0.23 | 0.25 | 0.01 | 0.24 | 0.24 |
| Aspartic, Asp | 100 | 0.87 | 1.30 | 0.10 | 0.01 | 0.01 | 0.00 | 0.02 | 0.02 |
| Sweet AAs 4 | / | 103.49 | 119.39 | 4.02 | / | / | / | 0.04 | / |
| Glycine, Gly | 130 | 42.13 | 45.63 | 2.35 | 0.32 | 0.35 | 0.02 | 0.48 | 0.48 |
| Alanine, Ala | 60 | 36.75 | 46.42 | 2.49 | 0.61 | 0.77 | 0.04 | 0.05 | 0.05 |
| Serine, Ser | 150 | 10.08 | 11.56 | 0.38 | 0.07 | 0.08 | 0.00 | 0.05 | 0.05 |
| Threonine, Thr | 260 | 14.53 | 15.78 | 0.45 | 0.06 | 0.06 | 0.00 | 0.18 | 0.18 |
| Proline, Pro | 300 | 6.86 | 7.31 | 0.17 | 0.02 | 0.02 | 0.00 | 0.19 | 0.19 |
| Lysine, Lys | / | 5.25 | 5.82 | 0.27 | 0.10 | 0.12 | 0.01 | 0.31 | / |
| Bitter AAs 5 | / | 56.10 | 57.08 | 1.30 | / | / | / | 0.70 | / |
| Tryptophan, Trp | 90 | 2.67 | 2.95 | 0.11 | / | / | / | 0.23 | / |
| Arginine, Arg | 50 | 11.54 | 7.19 | 0.91 | 0.23 | 0.14 | 0.02 | 0.01 | 0.01 |
| Histidine, His | 20 | 3.32 | 4.98 | 0.34 | 0.17 | 0.25 | 0.02 | 0.01 | 0.01 |
| Valine, Val | 30 | 9.73 | 11.33 | 0.52 | 0.24 | 0.28 | 0.01 | 0.13 | 0.13 |
| Methionine, Met | / | 7.76 | 6.61 | 0.33 | 0.26 | 0.22 | 0.01 | 0.09 | 0.09 |
| Isoleucine, Ile | 40 | 5.74 | 6.85 | 0.19 | 0.06 | 0.08 | 0.00 | <0.01 | <0.01 |
| Leucine, Leu | 90 | 10.9 | 12.20 | 0.44 | 0.06 | 0.06 | 0.00 | 0.13 | 0.14 |
| Tyrosine, Tyr | / | 6.41 | 7.51 | 0.21 | / | / | / | 0.01 | / |
| Phenylalanine, Phe | 190 | 7.13 | 7.93 | 0.25 | 0.08 | 0.09 | 0.00 | 0.11 | 0.11 |
| Cysteine, Cys | / | 2.57 | 1.66 | 0.28 | / | / | / | 0.08 | / |
| Total, | / | 190.98 | 210.6 | 5.14 | / | / | / | 0.05 | / |
| EAAs 6 | / | 67.03 | 74.45 | 1.56 | / | / | / | 0.07 | / |
| NEAAs | / | 123.95 | 135.55 | 3.87 | / | / | / | 0.07 | / |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, M.; Yin, Y.; Gong, S.; Shi, H.; Li, Q.; Lian, X.; Duan, Y.; Li, F.; Guo, Q. Effects of Dietary Eucommia ulmoides Leaf Extract Supplementation on Growth Performance, Meat Quality, Antioxidant Capacity, and Lipid Metabolism of Finishing Pigs. Antioxidants 2024, 13, 320. https://doi.org/10.3390/antiox13030320
Han M, Yin Y, Gong S, Shi H, Li Q, Lian X, Duan Y, Li F, Guo Q. Effects of Dietary Eucommia ulmoides Leaf Extract Supplementation on Growth Performance, Meat Quality, Antioxidant Capacity, and Lipid Metabolism of Finishing Pigs. Antioxidants. 2024; 13(3):320. https://doi.org/10.3390/antiox13030320
Chicago/Turabian StyleHan, Mengmeng, Yunju Yin, Saiming Gong, Hanjing Shi, Qilong Li, Xiao Lian, Yehui Duan, Fengna Li, and Qiuping Guo. 2024. "Effects of Dietary Eucommia ulmoides Leaf Extract Supplementation on Growth Performance, Meat Quality, Antioxidant Capacity, and Lipid Metabolism of Finishing Pigs" Antioxidants 13, no. 3: 320. https://doi.org/10.3390/antiox13030320
APA StyleHan, M., Yin, Y., Gong, S., Shi, H., Li, Q., Lian, X., Duan, Y., Li, F., & Guo, Q. (2024). Effects of Dietary Eucommia ulmoides Leaf Extract Supplementation on Growth Performance, Meat Quality, Antioxidant Capacity, and Lipid Metabolism of Finishing Pigs. Antioxidants, 13(3), 320. https://doi.org/10.3390/antiox13030320