Assessment of Six Blackberry Cultivars Using a Combination of Metabolomics, Biological Activity, and Network Pharmacology Approaches
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Plant Materials
2.3. Sample Preparation
2.4. Untargeted Analysis Using LC-MS/MS
2.5. Targeted Analysis Using LC-MS/MS
2.6. Analysis Based on Network Pharmacology
2.7. Determination of Total Phenolic, Flavonoid, and Anthocyanin Contents and Antioxidant Activities
2.8. Cell Culture and Cell Viability
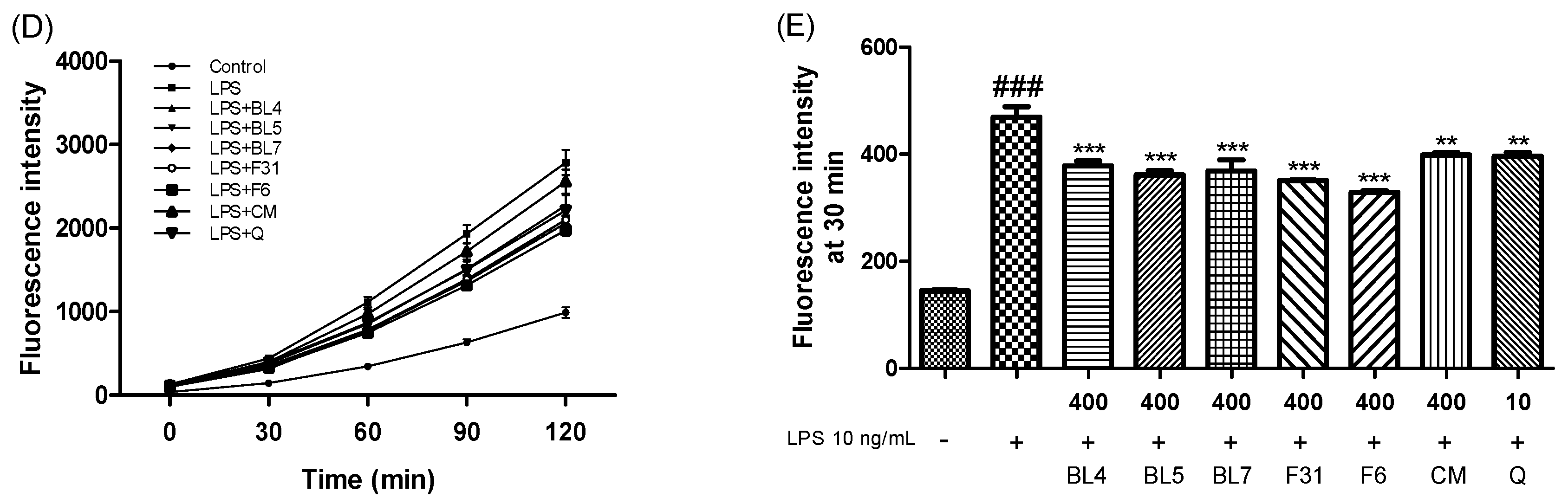
2.9. Measurement of Nitric Oxide (NO), Tumor Necrosis Factor-α (TNF-α), Protective Effect, and Intercellular Reactive Oxygen Species (ROS) Levels
2.10. Data Processing and Statistical Analysis
3. Results and Discussion
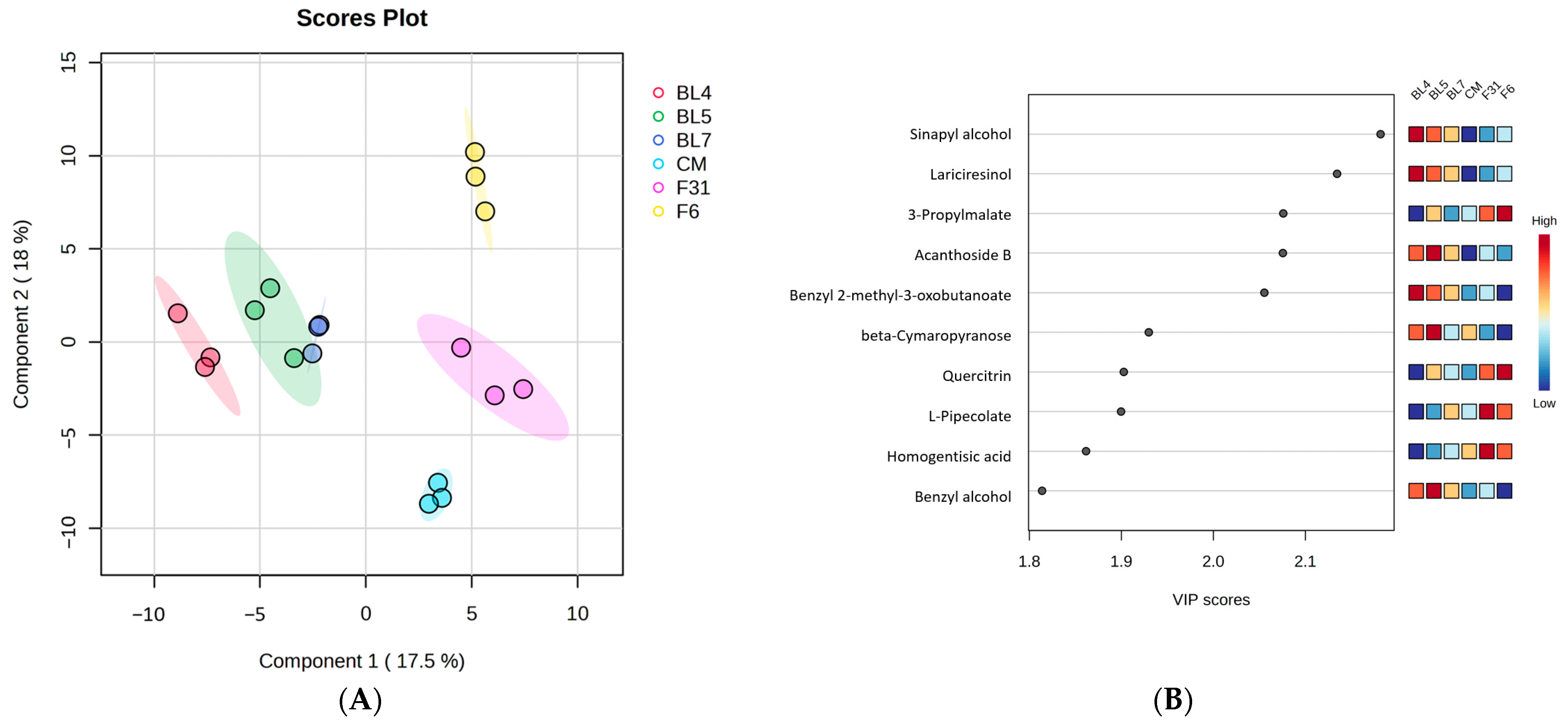
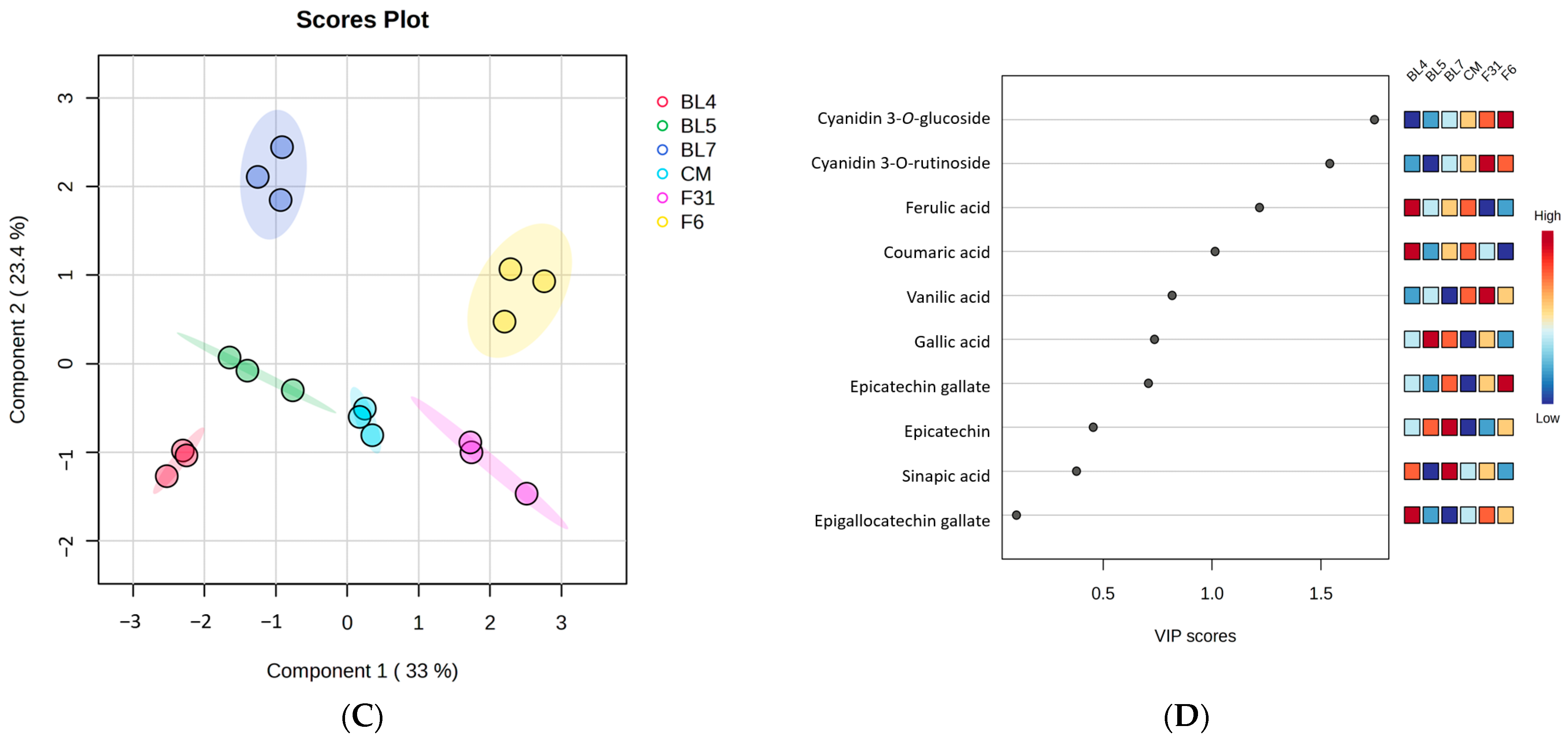
3.1. Metabolite Profiling of Six Blackberry Cultivars
| BL4 | BL5 | BL7 | F31 (Caddo) | F6 (Kiowa) | CM (Navaho) | |
|---|---|---|---|---|---|---|
| Flavonoid content (µg/g dry weight) | ||||||
| Cyanidin-3-glucoside | 1201.7 ± 47.4 e | 1688.1 ± 171.5 d | 2245.6 ± 80.5 c | 2672.5 ± 197.7 b | 3530.8 ± 197.8 a | 2595.2 ± 71.5 b |
| Cyanidin-3-rutinoside | 5.8 ± 0.3 c | 4.3 ± 0.2 c | 9.3 ± 1.1 c | 620.5 ± 43.2 a | 444.7 ± 104.5 b | 397.1 ± 31.3 b |
| Epicatechin | 551.6 ± 119.3 b | 1006.2 ± 35.4 a | 1041.2 ± 75.6 a | 419.3 ± 117.2 b | 713.8 ± 409.3 ab | 28.8 ± 3.0 c |
| Epicatechin gallate | 0.114 ± 0.010 c | 0.106 ± 0.025 c | 0.162 ± 0.030 b | 0.115 ± 0.019 c | 0.253 ± 0.042 a | 0.021 ± 0.001 d |
| Epigallocatechin gallate | 0.342 ± 0.037 a | 0.103 ± 0.025 c | 0.072 ± 0.011 c | 0.335 ± 0.019 a | 0.203 ± 0.070 b | 0.193 ± 0.017 b |
| Phenolic acid content (µg/g dry weight) | ||||||
| Gallic acid | 0.012 ± 0.003 b | 0.029 ± 0.015 a | 0.019 ± 0.008 ab | 0.013 ± 0.004 b | 0.007 ± 0.003 b | 0.006 ± 0.001 b |
| Vanillic acid | 0.014 ± 0.005 b | 0.017 ± 0.008 b | 0.010 ± 0.004 b | 0.047 ± 0.026 a | 0.021 ± 0.006 b | 0.044 ± 0.005 a |
| Coumaric acid | 0.057 ± 0.005 a | 0.021 ± 0.004 c | 0.027 ± 0.005 c | 0.024 ± 0.004 c | 0.020 ± 0.006 c | 0.042 ± 0.005 b |
| Ferulic acid | 0.060 ± 0.002 a | 0.041 ± 0.012 b | 0.042 ± 0.005 b | 0.026 ± 0.004 c | 0.028 ± 0.009 c | 0.057 ± 0.002 a |
| Sinapic acid | 0.029 ± 0.004 b | 0.018 ± 0.004 c | 0.057 ± 0.006 a | 0.027 ± 0.006 bc | 0.019 ± 0.006 c | 0.022 ± 0.003 bc |
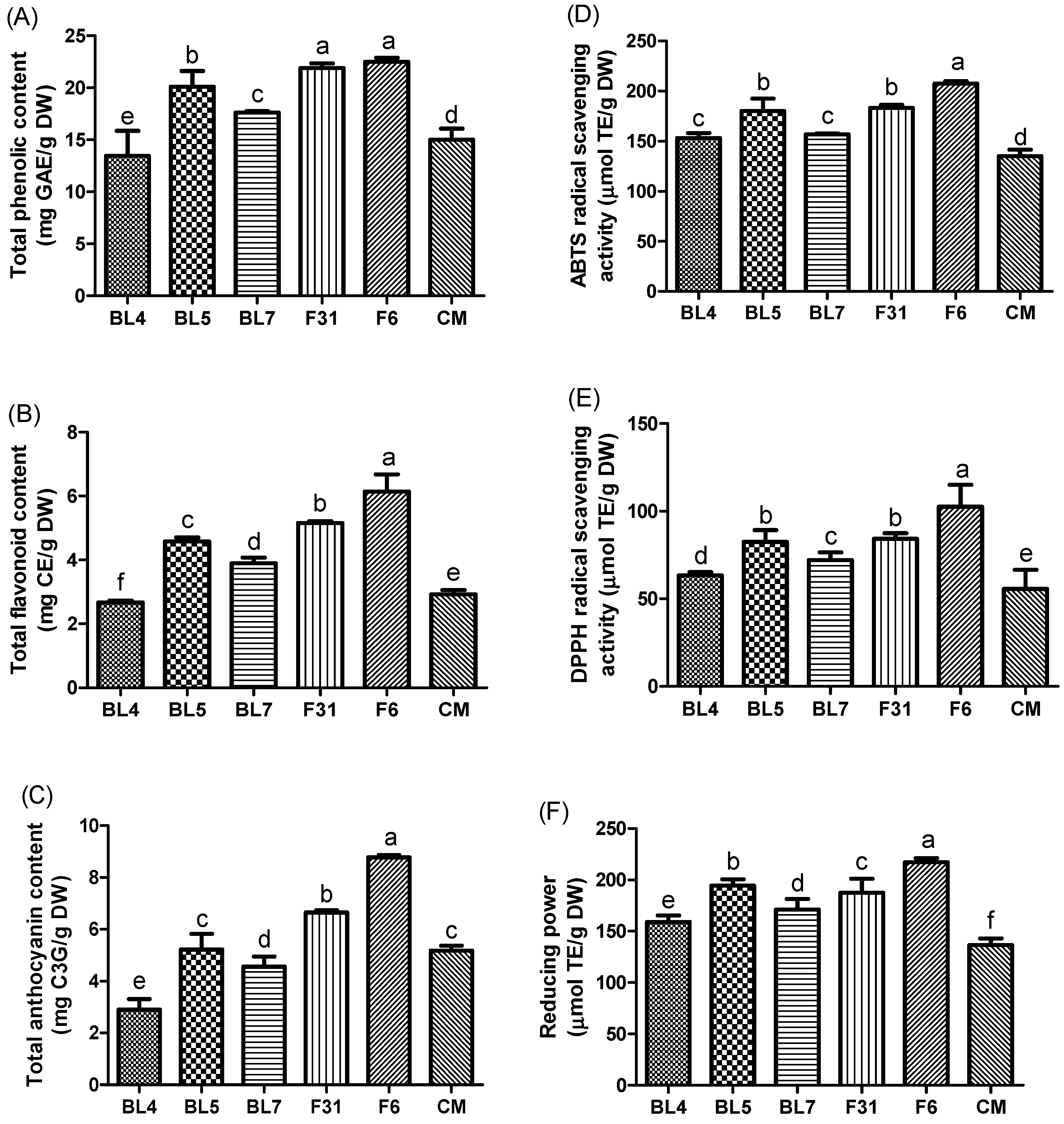
3.2. Antioxidant Contents and Activities of Six Blackberry Cultivars
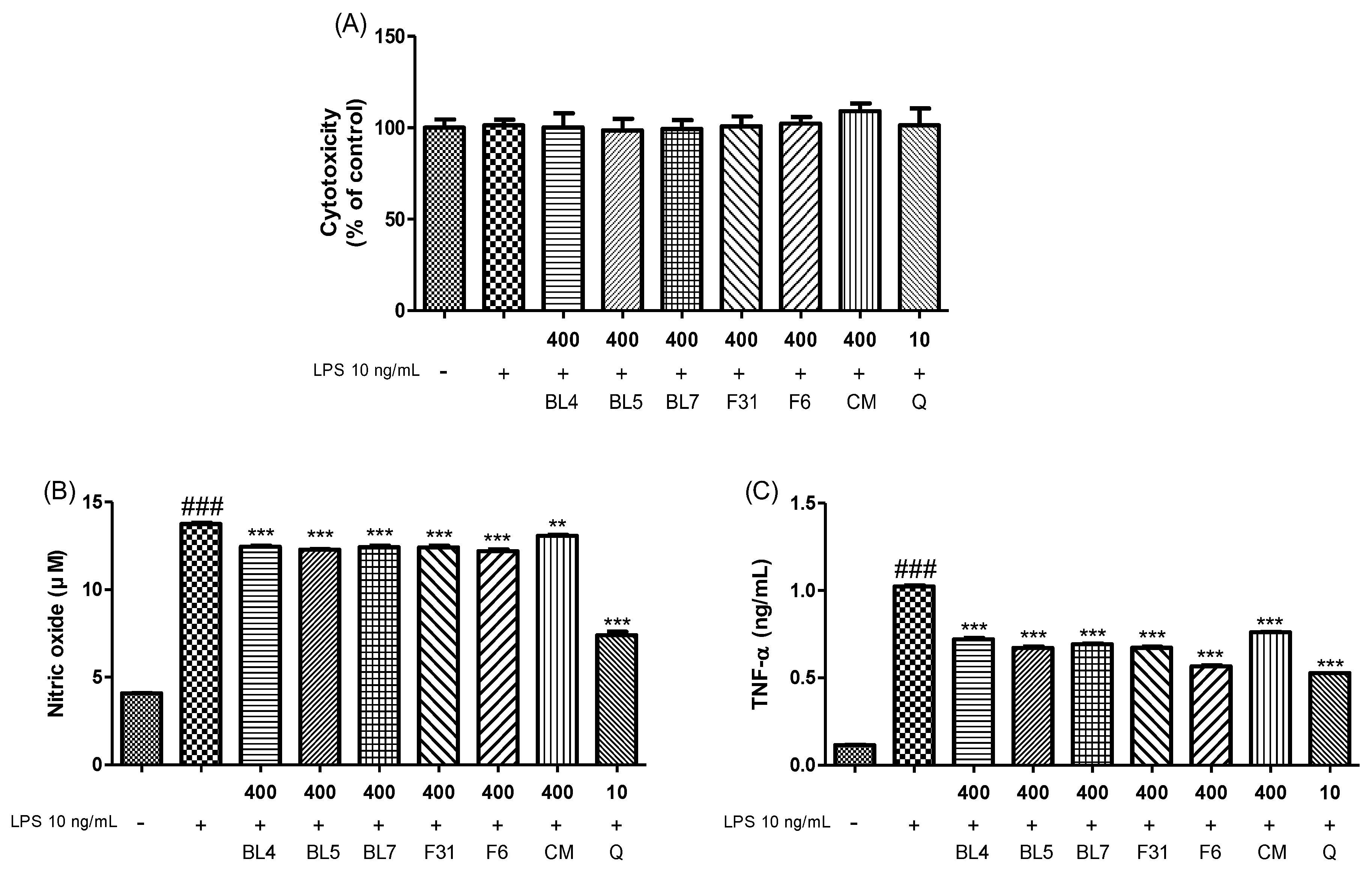
3.3. Anti-Inflammatory Activity of Six Blackberry Cultivars
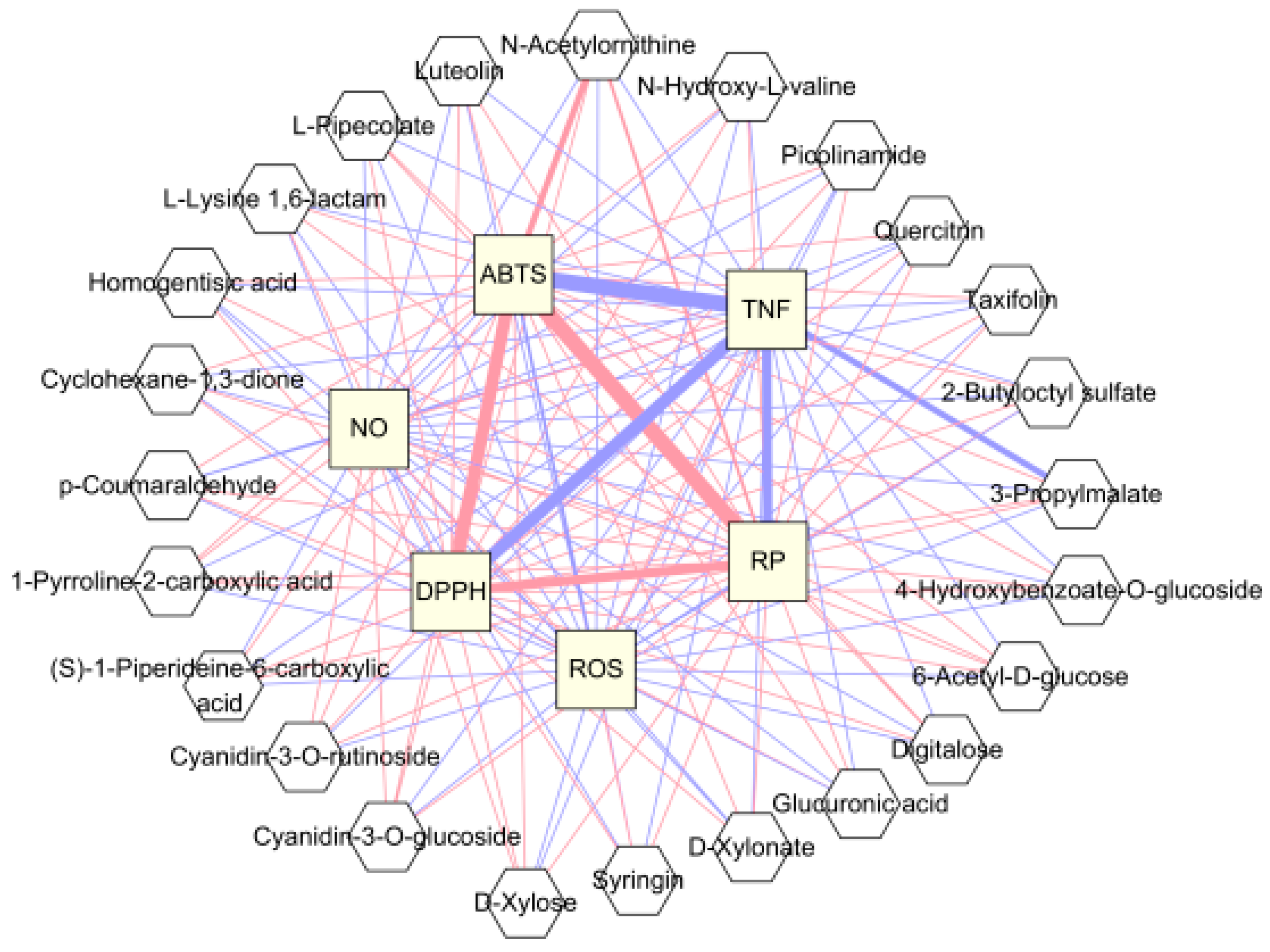
3.4. Correlation between Blackberry Metabolites and Biological Activities and Significant Pathway of Metabolites
3.5. Identification of Target Proteins of Selected Blackberry Metabolites Using Network Pharmacology
3.6. GO and KEGG Enrichment Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Takachi, R.; Inoue, M.; Ishihara, J.; Kurahashi, N.; Iwasaki, M.; Sasazuki, S.; Iso, H.; Tsubono, Y.; Tsugane, S.; JPHC Study Group. Fruit and Vegetable Intake and Risk of Total Cancer and Cardiovascular Disease: Japan Public Health Center-Based Prospective Study. Am. J. Epidemiol. 2008, 167, 59–70. [Google Scholar] [CrossRef]
- Nijveldt, R.J.; van Nood, E.; van Hoorn, D.E.; Boelens, P.G.; van Norren, K.; van Leeuwen, P.A. Flavonoids: A Review of Probable Mechanisms of Action and Potential Applications. Am. J. Clin. Nutr. 2001, 74, 418–425. [Google Scholar] [CrossRef]
- Halvorsen, B.L.; Carlsen, M.H.; Phillips, K.M.; Bøhn, S.K.; Holte, K.; Jacobs, D.R., Jr.; Blomhoff, R. Content of Redox-Active Compounds (Ie, Antioxidants) in Foods Consumed in the United States. Am. J. Clin. Nutr. 2006, 84, 95–135. [Google Scholar] [CrossRef]
- Kaume, L.; Howard, L.R.; Devareddy, L. The Blackberry Fruit: A Review on Its Composition and Chemistry, Metabolism and Bioavailability, and Health Benefits. J. Agric. Food Chem. 2012, 60, 5716–5727. [Google Scholar] [CrossRef]
- Strik, B.C.; Finn, C.E.; Clark, J.R.; Pilar Bañados, M. Worldwide Production of Blackberries. Acta. Hortic. 2008, 777, 209–218. [Google Scholar] [CrossRef]
- Clark, J.R.; Finn, C.E. Blackberry Cult Ivation in the World. Rev. Bras. Frutic. 2014, 36, 46–57. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, C.; Huang, Z.; Lyu, L.; Li, W.; Wu, W. Integrative Analysis of the Metabolome and Transcriptome Provides Insights into the Mechanisms of Flavonoid Biosynthesis in Blackberry. Food Res. Int. 2022, 153, 110948. [Google Scholar] [CrossRef] [PubMed]
- Sariburun, E.; Şahin, S.; Demir, C.; Türkben, C.; Uylaşer, V. Phenolic Content and Antioxidant Activity of Raspberry and Blackberry Cultivars. J. Food Sci. 2010, 75, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Solverson, P.; Albaugh, G.P.; Harrison, D.J.; Luthria, D.L.; Baer, D.J.; Novotny, J.A. High-Dose Administration of Purified Cyanidin-3-Glucose or a Blackberry Extract Causes Improved Mitochondrial Function but Reduced Content in 3T3-L1 Adipocytes. Food Front. 2022, 3, 276–284. [Google Scholar] [CrossRef]
- Heinonen, I.M.; Meyer, A.S.; Frankel, E.N. Antioxidant Activity of Berry Phenolics on Human Low-Density Lipoprotein and Liposome Oxidation. J. Agric. Food Chem. 1998, 46, 4107–4112. [Google Scholar] [CrossRef]
- Wang, S.Y.; Jiao, H. Scavenging Capacity of Berry Crops on Superoxide Radicals, Hydrogen Peroxide, Hydroxyl Radicals, and Singlet Oxygen. J. Agric. Food Chem. 2000, 48, 5677–5684. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yu, Q.; Cheng, H.; Ge, Y.; Liu, H.; Ye, X.; Chen, Y. Metabolomic Approach for the Authentication of Berry Fruit Juice by Liquid Chromatography Quadrupole Time-of-Flight Mass Spectrometry Coupled to Chemometrics. J. Agric. Food Chem. 2018, 66, 8199–8208. [Google Scholar] [CrossRef] [PubMed]
- Schrimpe-Rutledge, A.C.; Codreanu, S.G.; Sherrod, S.D.; McLean, J.A. Untargeted Metabolomics Strategies—Challenges and Emerging Directions. J. Am. Soc. Mass Spectrom. 2016, 27, 1897–1905. [Google Scholar] [CrossRef] [PubMed]
- Cajka, T.; Fiehn, O. Toward Merging Untargeted and Targeted Methods in Mass Spectrometry-Based Metabolomics and Lipidomics. Anal. Chem. 2016, 88, 524–545. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Lee, M.Y.; Shon, J.C.; Kwon, Y.S.; Liu, K.-H.; Lee, C.H.; Ku, K.-M. Untargeted and Targeted Metabolomics Analyses of Blackberries–Understanding Postharvest Red Drupelet Disorder. Food Chem. 2019, 300, 125169. [Google Scholar] [CrossRef]
- Wu, Y.; Huang, X.; Yang, H.; Zhang, S.; Lyu, L.; Li, W.; Wu, W. Analysis of Flavonoid-Related Metabolites in Different Tissues and Fruit Developmental Stages of Blackberry Based on Metabolome Analysis. Food Res. Int. 2023, 163, 112313. [Google Scholar] [CrossRef]
- Fan-Chiang, H.-J.; Wrolstad, R.E. Sugar and Nonvolatile Acid Composition of Blackberries. J. AOAC Int. 2010, 93, 956–965. [Google Scholar] [CrossRef]
- Makarkina, M.; Gruner, L.; Vetrova, O.; Matnasarova, D. The Accumulation of Sugars and Organic Acids in Blackberry Fruit in the Conditions of Central Russia. BIO Web Conf. 2021, 36, 02006. [Google Scholar] [CrossRef]
- Siriwoharn, T.; Wrolstad, R.E.; Finn, C.E.; Pereira, C.B. Influence of Cultivar, Maturity, and Sampling on Blackberry (Rubus L. Hybrids) Anthocyanins, Polyphenolics, and Antioxidant Properties. J. Agric. Food Chem. 2004, 52, 8021–8030. [Google Scholar] [CrossRef]
- Mattioli, R.; Francioso, A.; Mosca, L.; Silva, P. Anthocyanins: A Comprehensive Review of Their Chemical Properties and Health Effects on Cardiovascular and Neurodegenerative Diseases. Molecules 2020, 25, 3809. [Google Scholar] [CrossRef]
- Poornima, P.; Kumar, J.D.; Zhao, Q.; Blunder, M.; Efferth, T. Network Pharmacology of Cancer: From Understanding of Complex Interactomes to the Design of Multi-Target Specific Therapeutics from Nature. Pharmacol. Res. 2016, 111, 290–302. [Google Scholar] [CrossRef]
- Tao, W.; Xu, X.; Wang, X.; Li, B.; Wang, Y.; Li, Y.; Yang, L. Network Pharmacology-Based Prediction of the Active Ingredients and Potential Targets of Chinese Herbal Radix Curcumae Formula for Application to Cardiovascular Disease. J. Ethnopharmacol. 2013, 145, 1–10. [Google Scholar] [CrossRef]
- Lai, X.; Wang, X.; Hu, Y.; Su, S.; Li, W.; Li, S. Editorial: Network Pharmacology and Traditional Medicine. Front. Pharmacol. 2020, 11, 1194. [Google Scholar] [CrossRef]
- Kürbitz, C.; Heise, D.; Redmer, T.; Goumas, F.; Arlt, A.; Lemke, J.; Rimbach, G.; Kalthoff, H.; Trauzold, A. Epicatechin Gallate and Catechin Gallate Are Superior to Epigallocatechin Gallate in Growth Suppression and Anti-Inflammatory Activities in Pancreatic Tumor Cells. Cancer Sci. 2011, 102, 728–734. [Google Scholar] [CrossRef]
- Huang, C.-C.; Fang, J.-Y.; Wu, W.-B.; Chiang, H.-S.; Wei, Y.-J.; Hung, C.-F. Protective Effects of (−)-Epicatechin-3-Gallate on UVA-Induced Damage in HaCaT Keratinocytes. Arch. Dermatol. Res. 2005, 296, 473–481. [Google Scholar] [CrossRef]
- Tang, J.; Diao, P.; Shu, X.; Li, L.; Xiong, L. Quercetin and Quercitrin Attenuates the Inflammatory Response and Oxidative Stress in LPS-Induced RAW264.7 Cells: In Vitro Assessment and a Theoretical Model. BioMed Res. Int. 2019, 2019, e7039802. [Google Scholar] [CrossRef]
- Wang, Z.; Gmitter, F.G., Jr.; Grosser, J.W.; Wang, Y. Natural Sweeteners and Sweetness-Enhancing Compounds Identified in Citrus Using an Efficient Metabolomics-Based Screening Strategy. J. Agric. Food Chem. 2022, 70, 10593–10603. [Google Scholar] [CrossRef] [PubMed]
- Suh, D.H.; Jung, E.S.; Lee, G.M.; Lee, C.H. Distinguishing Six Edible Berries Based on Metabolic Pathway and Bioactivity Correlations by Non-Targeted Metabolite Profiling. Front. Plant Sci. 2018, 9, 1462. [Google Scholar] [CrossRef] [PubMed]
- Sung, J.; Lee, J. Antioxidant and Antiproliferative Activities of Grape Seeds from Different Cultivars. Food Sci. Biotechnol. 2010, 19, 321–326. [Google Scholar] [CrossRef]
- Lee, H.; Lee, J. Anti-Diabetic Effect of Hydroxybenzoic Acid Derivatives in Free Fatty Acid-Induced HepG2 Cells via miR-1271/IRS1/PI3K/AKT/FOXO1 Pathway. J. Food Biochem. 2021, 45, e13993. [Google Scholar] [CrossRef] [PubMed]
- Cebulak, T.; Oszmiański, J.; Kapusta, I.; Lachowicz, S. Effect of Abiotic Stress Factors on Polyphenolic Content in the Skin and Flesh of Pear by UPLC-PDA-Q/TOF-MS. Eur. Food Res. Technol. 2019, 245, 2715–2725. [Google Scholar] [CrossRef]
- Chen, X.; Cai, W.; Xia, J.; Yu, H.; Wang, Q.; Pang, F.; Zhao, M. Metabolomic and Transcriptomic Analyses Reveal That Blue Light Promotes Chlorogenic Acid Synthesis in Strawberry. J. Agric. Food Chem. 2020, 68, 12485–12492. [Google Scholar] [CrossRef] [PubMed]
- Fayek, N.M.; Farag, M.A.; Saber, F.R. Metabolome Classification via GC/MS and UHPLC/MS of Olive Fruit Varieties Grown in Egypt Reveal Pickling Process Impact on Their Composition. Food Chem. 2021, 339, 127861. [Google Scholar] [CrossRef] [PubMed]
- Veberic, R.; Stampar, F.; Schmitzer, V.; Cunja, V.; Zupan, A.; Koron, D.; Mikulic-Petkovsek, M. Changes in the contents of anthocyanins and other compounds in blackberry fruits due to freezing and long-term frozen storage. J. Agric. Food Chem. 2014, 62, 6926–6935. [Google Scholar] [CrossRef]
- Robinson, J.A.; Bierwirth, J.E.; Greenspan, P.; Pegg, R.B. Blackberry Polyphenols: Review of Composition, Quantity, and Health Impacts from in Vitro and in Vivo Studies. J. Food Bioact. 2020, 9, 9217. [Google Scholar] [CrossRef]
- Schulz, M.; Seraglio, S.K.T.; Della Betta, F.; Nehring, P.; Valese, A.C.; Daguer, H.; Gonzaga, L.V.; Costa, A.C.O.; Fett, R. Blackberry (Rubus ulmifolius Schott): Chemical Composition, Phenolic Compounds and Antioxidant Capacity in Two Edible Stages. Food Res. Int. 2019, 122, 627–634. [Google Scholar] [CrossRef]
- da Rosa, C.G.; Borges, C.D.; Zambiazi, R.C.; Rutz, J.K.; da Luz, S.R.; Krumreich, F.D.; Benvenutti, E.V.; Nunes, M.R. Encapsulation of the Phenolic Compounds of the Blackberry (Rubus fruticosus). LWT—Food Sci. Technol. 2014, 58, 527–533. [Google Scholar] [CrossRef]
- Sellappan, S.; Akoh, C.C.; Krewer, G. Phenolic Compounds and Antioxidant Capacity of Georgia-Grown Blueberries and Blackberries. J. Agric. Food Chem. 2002, 50, 2432–2438. [Google Scholar] [CrossRef]
- Hassimotto, N.M.A.; da Mota, R.V.; Cordenunsi, B.R.; Lajolo, F.M. Physico-Chemical Characterization and Bioactive Compounds of Blackberry Fruits (Rubus Sp.) Grown in Brazil. Food Sci. Technol. 2008, 28, 702–708. [Google Scholar] [CrossRef]
- Pantelidis, G.E.; Vasilakakis, M.; Manganaris, G.A.; Diamantidis, G. Antioxidant Capacity, Phenol, Anthocyanin and Ascorbic Acid Contents in Raspberries, Blackberries, Red Currants, Gooseberries and Cornelian Cherries. Food Chem. 2007, 102, 777–783. [Google Scholar] [CrossRef]
- Wang, S.Y.; Lin, H.S. Antioxidant Activity in Fruits and Leaves of Blackberry, Raspberry, and Strawberry Varies with Cultivar and Developmental Stage. J. Agric. Food Chem. 2000, 48, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Cuevas-Rodríguez, E.O.; Yousef, G.G.; García-Saucedo, P.A.; López-Medina, J.; Paredes-López, O.; Lila, M.A. Characterization of Anthocyanins and Proanthocyanidins in Wild and Domesticated Mexican Blackberries (Rubus Spp.). J. Agric. Food Chem. 2010, 58, 7458–7464. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-S. Antioxidant Activities of Selected Berries and Their Free, Esterified, and Insoluble-Bound Phenolic Acid Contents. Prev. Nutr. Food Sci. 2018, 23, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Krzepiłko, A.; Prażak, R.; Święciło, A. Chemical Composition, Antioxidant and Antimicrobial Activity of Raspberry, Blackberry and Raspberry-Blackberry Hybrid Leaf Buds. Molecules 2021, 26, 327. [Google Scholar] [CrossRef]
- Forrester, S.J.; Kikuchi, D.S.; Hernandes, M.S.; Xu, Q.; Griendling, K.K. Reactive Oxygen Species in Metabolic and Inflammatory Signaling. Circ. Res. 2018, 122, 877–902. [Google Scholar] [CrossRef]
- Roy, A.; Das, S.; Chatterjee, I.; Roy, S.; Chakraborty, R. Anti-Inflammatory Effects of Different Dietary Antioxidants. In Plant Antioxidants and Health; Reference Series in Phytochemistry; Ekiert, H.M., Ramawat, K.G., Arora, J., Eds.; Springer: Cham, Switzerland, 2022; pp. 573–597. [Google Scholar] [CrossRef]
- Wu, L.; Ashraf, M.H.N.; Facci, M.; Wang, R.; Paterson, P.G.; Ferrie, A.; Juurlink, B.H.J. Dietary Approach to Attenuate Oxidative Stress, Hypertension, and Inflammation in the Cardiovascular System. Proc. Natl. Acad. Sci. USA 2004, 101, 7094–7099. [Google Scholar] [CrossRef]
- Ma, Z.; Du, B.; Li, J.; Yang, Y.; Zhu, F. An Insight into Anti-Inflammatory Activities and Inflammation Related Diseases of Anthocyanins: A Review of Both In Vivo and In Vitro Investigations. Int. J. Mol. Sci. 2021, 22, 11076. [Google Scholar] [CrossRef]
- Wongwichai, T.; Teeyakasem, P.; Pruksakorn, D.; Kongtawelert, P.; Pothacharoen, P. Anthocyanins and Metabolites from Purple Rice Inhibit IL-1β-Induced Matrix Metalloproteinases Expression in Human Articular Chondrocytes through the NF-κB and ERK/MAPK Pathway. Biomed. Pharmacother. 2019, 112, 108610. [Google Scholar] [CrossRef]
- Bernatova, I.; Liskova, S. Mechanisms Modified by (−)-Epicatechin and Taxifolin Relevant for the Treatment of Hypertension and Viral Infection: Knowledge from Preclinical Studies. Antioxidants 2021, 10, 467. [Google Scholar] [CrossRef] [PubMed]
- Aziz, N.; Kim, M.-Y.; Cho, J.Y. Anti-Inflammatory Effects of Luteolin: A Review of in Vitro, in Vivo, and in Silico Studies. J. Ethnopharmacol. 2018, 225, 342–358. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Liu, K.; Wang, J.; Shao, H. Syringin Exerts Anti-Inflammatory and Antioxidant Effects by Regulating SIRT1 Signaling in Rat and Cell Models of Acute Myocardial Infarction. Immun. Inflamm. Dis. 2023, 11, e775. [Google Scholar] [CrossRef] [PubMed]
- Ginting, C.; Lister, I.N.; Girsang, E.; Mutia, M.; Lubis, Y.; Amalia, A.; Rizal, R.; Widowati, W. Anti-Inflammatory Activity of Quercitrin on Hypoxia-Induced EA.Hy926. J. Phys. Conf. Ser. 2019, 1374, 012033. [Google Scholar] [CrossRef]
- Li, X.; Jiang, Q.; Wang, T.; Liu, J.; Chen, D. Comparison of the Antioxidant Effects of Quercitrin and Isoquercitrin: Understanding the Role of the 6″-OH Group. Molecules 2016, 21, 1246. [Google Scholar] [CrossRef]
- Kotanidou, A.; Xagorari, A.; Bagli, E.; Kitsanta, P.; Fotsis, T.; Papapetropoulos, A.; Roussos, C. Luteolin Reduces Lipopolysaccharide-Induced Lethal Toxicity and Expression of Proinflammatory Molecules in Mice. Am. J. Respir. Crit. Care Med. 2002, 165, 818–823. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Kitts, D. Demonstrating the Relationship between the Phytochemical Profile of Different Teas with Relative Antioxidant and Anti-Inflammatory Capacities. Funct. Foods Health Dis. 2017, 7, 375–395. [Google Scholar] [CrossRef][Green Version]
- Cheynier, V.; Comte, G.; Davies, K.M.; Lattanzio, V.; Martens, S. Plant Phenolics: Recent Advances on Their Biosynthesis, Genetics, and Ecophysiology. Plant Physiol. Biochem. 2013, 72, 1–20. [Google Scholar] [CrossRef]
- Seong, E.; Heo, H.; Sang Jeong, H.; Lee, H.; Lee, J. Enhancement of Bioactive Compounds and Biological Activities of Centella Asiatica through Ultrasound Treatment. Ultrason. Sonochem. 2023, 94, 106353. [Google Scholar] [CrossRef]
- Yu, J.; Lee, H.; Heo, H.; Jeong, H.S.; Sung, J.; Lee, J. Sucrose-Induced Abiotic Stress Improves the Phytochemical Profiles and Bioactivities of Mung Bean Sprouts. Food Chem. 2023, 400, 134069. [Google Scholar] [CrossRef]
- Sim, U.; Sung, J.; Lee, H.; Heo, H.; Jeong, H.S.; Lee, J. Effect of Calcium Chloride and Sucrose on the Composition of Bioactive Compounds and Antioxidant Activities in Buckwheat Sprouts. Food Chem. 2020, 312, 126075. [Google Scholar] [CrossRef]
- He, X.; Li, Y.; Deng, B.; Lin, A.; Zhang, G.; Ma, M.; Wang, Y.; Yang, Y.; Kang, X. The PI3K/AKT Signalling Pathway in Inflammation, Cell Death and Glial Scar Formation after Traumatic Spinal Cord Injury: Mechanisms and Therapeutic Opportunities. Cell Prolif. 2022, 55, e13275. [Google Scholar] [CrossRef]
- Wu, D.; Wang, Z.; Zhang, J.; Robinson, A.G.; Lyu, B.; Chen, Z.; Wang, C.; Wei, B.; Xia, X.; Zhang, Q.; et al. Apoptotic Caspase Inhibits Innate Immune Signaling by Cleaving NF-κBs in Both Mammals and Flies. Cell Death Dis. 2022, 13, 731. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Kim, H.J.; Heo, H.; Kim, M.; Jeon, Y.; Lee, H.; Lee, J. Comparison of the Antihypertensive Activity of Phenolic Acids. Molecules 2022, 27, 6185. [Google Scholar] [CrossRef] [PubMed]
- Bougaki, M.; Searles, R.J.; Kida, K.; Yu, J.; Buys, E.S.; Ichinose, F. Nos3 Protects against Systemic Inflammation and Myocardial Dysfunction in Murine Polymicrobial Sepsis. Shock 2010, 34, 281–290. [Google Scholar] [CrossRef]
- Schonthaler, H.B.; Guinea-Viniegra, J.; Wagner, E.F. Targeting Inflammation by Modulating the Jun/AP-1 Pathway. Ann. Rheum. Dis. 2011, 70, i109–i112. [Google Scholar] [CrossRef] [PubMed]



| Gene ID | Protein Names | Interacting Compounds | Combined Score |
|---|---|---|---|
| ABCA1 | Phospholipid-transporting ATPase ABCA1 | Mevalonic acid | 0.832 |
| ABCB1 | ATP-dependent translocase ABCB1 | Gallic acid | 0.824 |
| ADIPOQ | Adiponectin | trans-Cinnamic acid | 0.800 |
| AHR | Aryl hydrocarbon receptor | Cyanidin-3-glucoside | 0.700 |
| AKT1 | RAC-alpha serine/threonine-protein kinase | Epigallocatechin gallate | 0.964 |
| Luteolin | 0.856 | ||
| ATM | Serine-protein kinase ATM | Gallic acid | 0.824 |
| CASP3 | Caspase-3 subunit p12 | Gallic acid | 0.745 |
| Luteolin | 0.947 | ||
| CXCL2 | C-X-C motif chemokine 2 | Isoferulic acid | 0.657 |
| CYCS | Cytochrome c | Ferulic acid | 0.819 |
| CYP3A4 | Cytochrome P450 3A4 | Quercitrin | 0.800 |
| EGFR | Epidermal growth factor receptor | Luteolin | 0.869 |
| GATA3 | Trans-acting T-cell-specific transcription factor GATA-3 | Gallic acid | 0.800 |
| HMOX1 | Heme oxygenase 1 | Epicatechin | 0.838 |
| Genipin | 0.840 | ||
| IL6 | Interleukin-6 | Epicatechin | 0.800 |
| JUN | Transcription factor Jun | Gallic acid | 0.823 |
| Luteolin | 0.946 | ||
| MAPK1 | Mitogen-activated protein kinase 1 | Caffeic acid | 0.800 |
| MAPK8 | Mitogen-activated protein kinase 8 | Caffeic acid | 0.818 |
| Epigallocatechin gallate | 0.961 | ||
| Luteolin | 0.951 | ||
| MIF | Macrophage migration inhibitory factor | Caffeic acid | 0.958 |
| MMP1 | Matrix metalloproteinase 1 | Genipin | 0.800 |
| MMP2 | Matrix metalloproteinase 2 | Gallic acid | 0.951 |
| MMP9 | Matrix metalloproteinase 9 | Luteolin | 0.949 |
| NOS1 | Nitric oxide synthase 1, neuronal | Epicatechin | 0.813 |
| NOS2 | Nitric oxide synthase 2, inducible | Epicatechin | 0.794 |
| NOS3 | Nitric oxide synthase 3, endothelial | Cyanidin-3-glucoside | 0.816 |
| Epicatechin | 0.818 | ||
| Epigallocatechin gallate | 0.958 | ||
| NQO1 | NAD(P)H dehydrogenase [quinone] 1 | Genipin | 0.849 |
| Taxifolin | 0.700 | ||
| SERPINE1 | Plasminogen activator inhibitor 1 | Gallic acid | 0.800 |
| STAT3 | Signal transducer and activator of transcription 3 | Genipin | 0.800 |
| TP53 | Cellular tumor antigen p53 | Epigallocatechin gallate | 0.958 |
| TTR | Transthyretin | Epigallocatechin gallate | 0.961 |
| VEGFA | Vascular endothelial growth factor A | Epigallocatechin gallate | 0.959 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.; Wang, Z.; Deng, Z.; Wang, Y. Assessment of Six Blackberry Cultivars Using a Combination of Metabolomics, Biological Activity, and Network Pharmacology Approaches. Antioxidants 2024, 13, 319. https://doi.org/10.3390/antiox13030319
Lee H, Wang Z, Deng Z, Wang Y. Assessment of Six Blackberry Cultivars Using a Combination of Metabolomics, Biological Activity, and Network Pharmacology Approaches. Antioxidants. 2024; 13(3):319. https://doi.org/10.3390/antiox13030319
Chicago/Turabian StyleLee, Hana, Zhixin Wang, Zhanao Deng, and Yu Wang. 2024. "Assessment of Six Blackberry Cultivars Using a Combination of Metabolomics, Biological Activity, and Network Pharmacology Approaches" Antioxidants 13, no. 3: 319. https://doi.org/10.3390/antiox13030319
APA StyleLee, H., Wang, Z., Deng, Z., & Wang, Y. (2024). Assessment of Six Blackberry Cultivars Using a Combination of Metabolomics, Biological Activity, and Network Pharmacology Approaches. Antioxidants, 13(3), 319. https://doi.org/10.3390/antiox13030319







