Supercritical Fluid Extraction of Bioactive Components from Apple Peels and Their Modulation of Complex I Activity in Isolated Mitochondria
Abstract
1. Introduction
2. Materials and Methods
2.1. Apple Collection and Apple Peel Preparation
2.2. Supercritical Fluid Extraction
2.3. Total Antioxidant Capacity (TAC) Assay
2.4. Quantification of Antioxidant Compounds
2.4.1. Total Phenol (TP) Determination
2.4.2. Total Flavonoid (TF) Determination
2.4.3. Total Anthocyanin (TA) Determination
2.4.4. Ascorbic Acid (AA) Determination
2.5. HPLC/DAD Analysis
2.6. Evaluating Biological Activity
2.6.1. Cell Cultures
2.6.2. Mitochondrial Isolation
2.6.3. Complex I Activity Measurements
2.6.4. Statistical Analysis
3. Results and Discussion
3.1. Supercritical Fluid Extraction
3.2. Quantification of Antioxidant Compounds
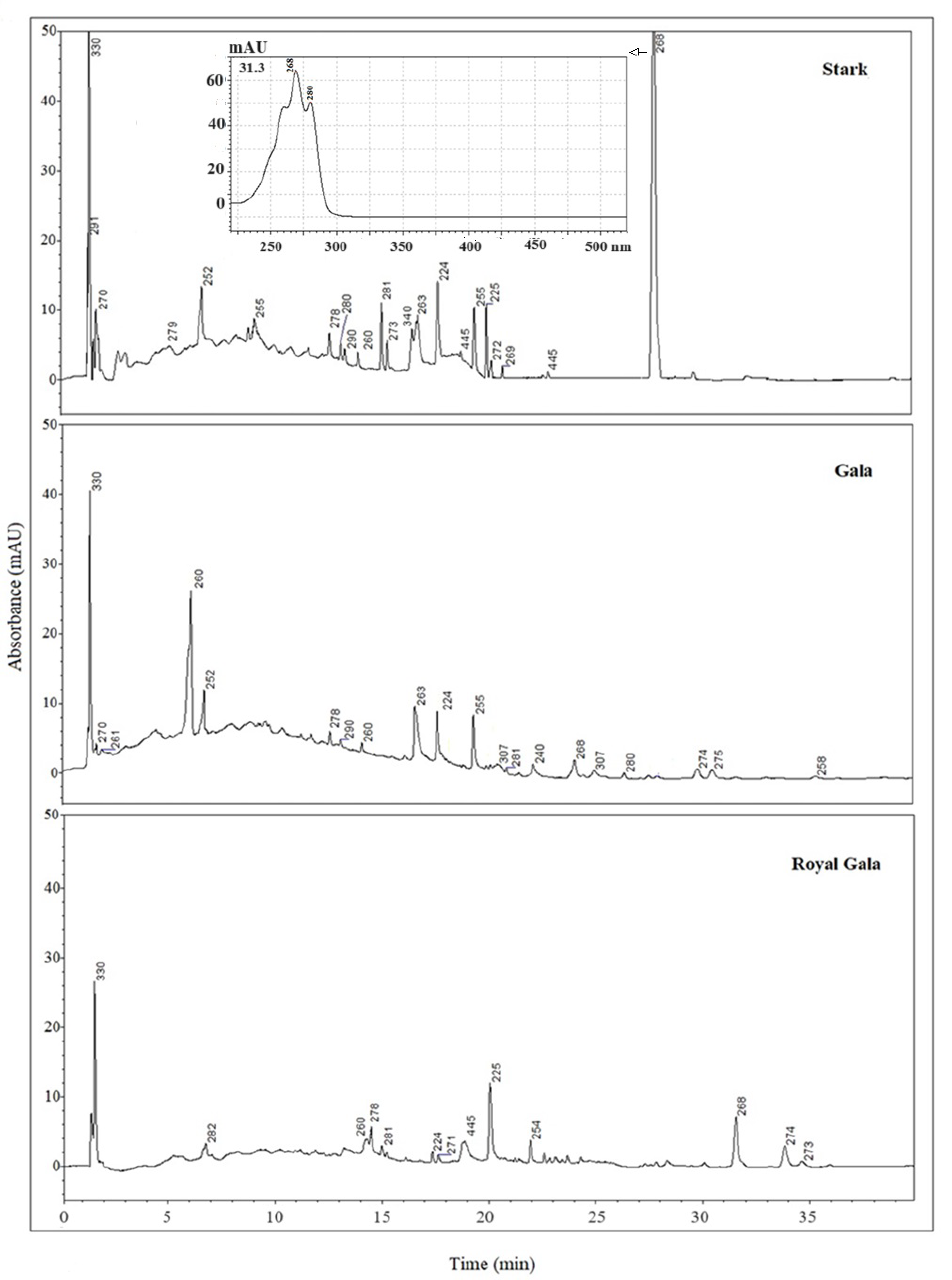
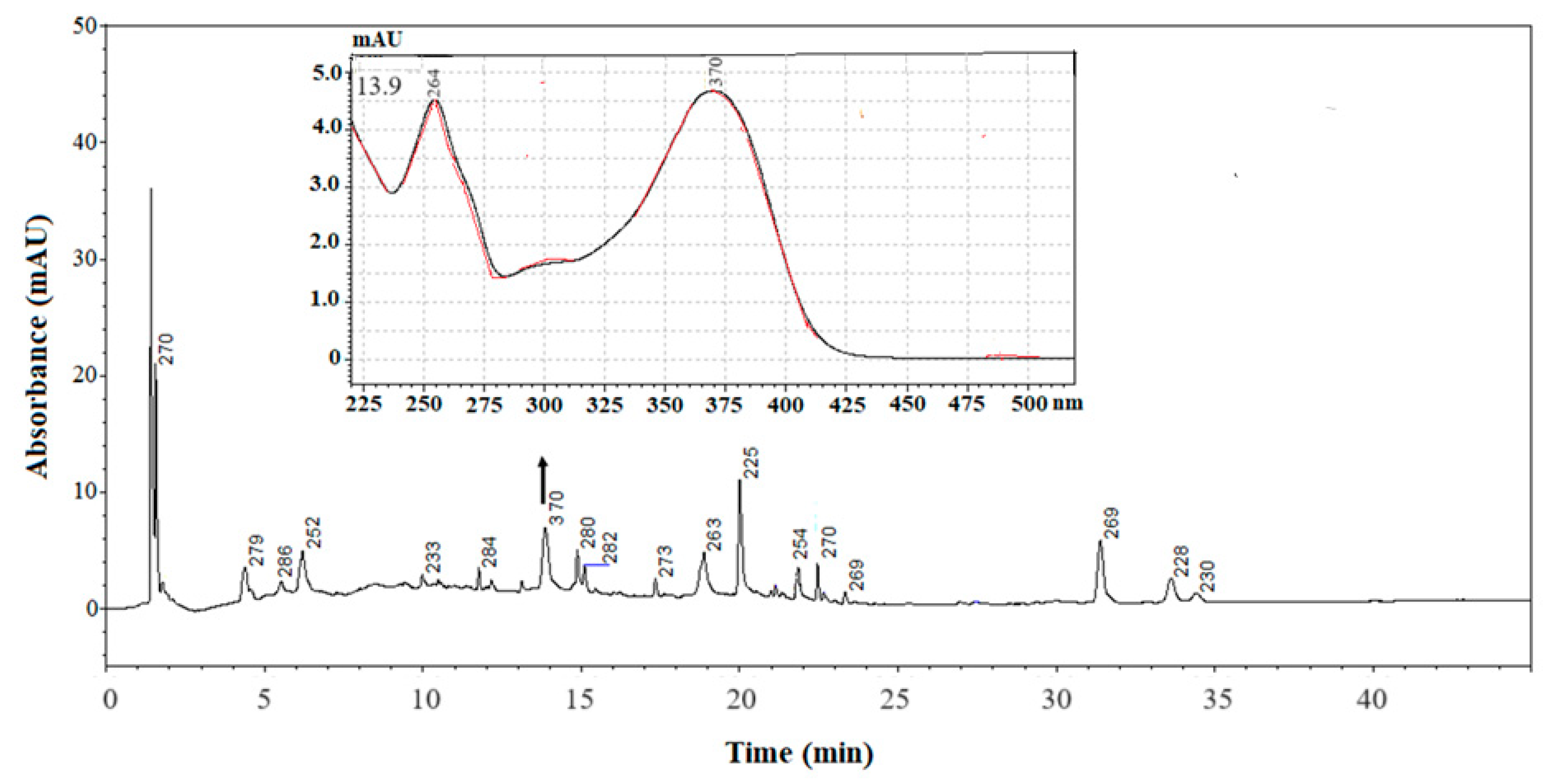
3.3. HPLC/DAD Analysis
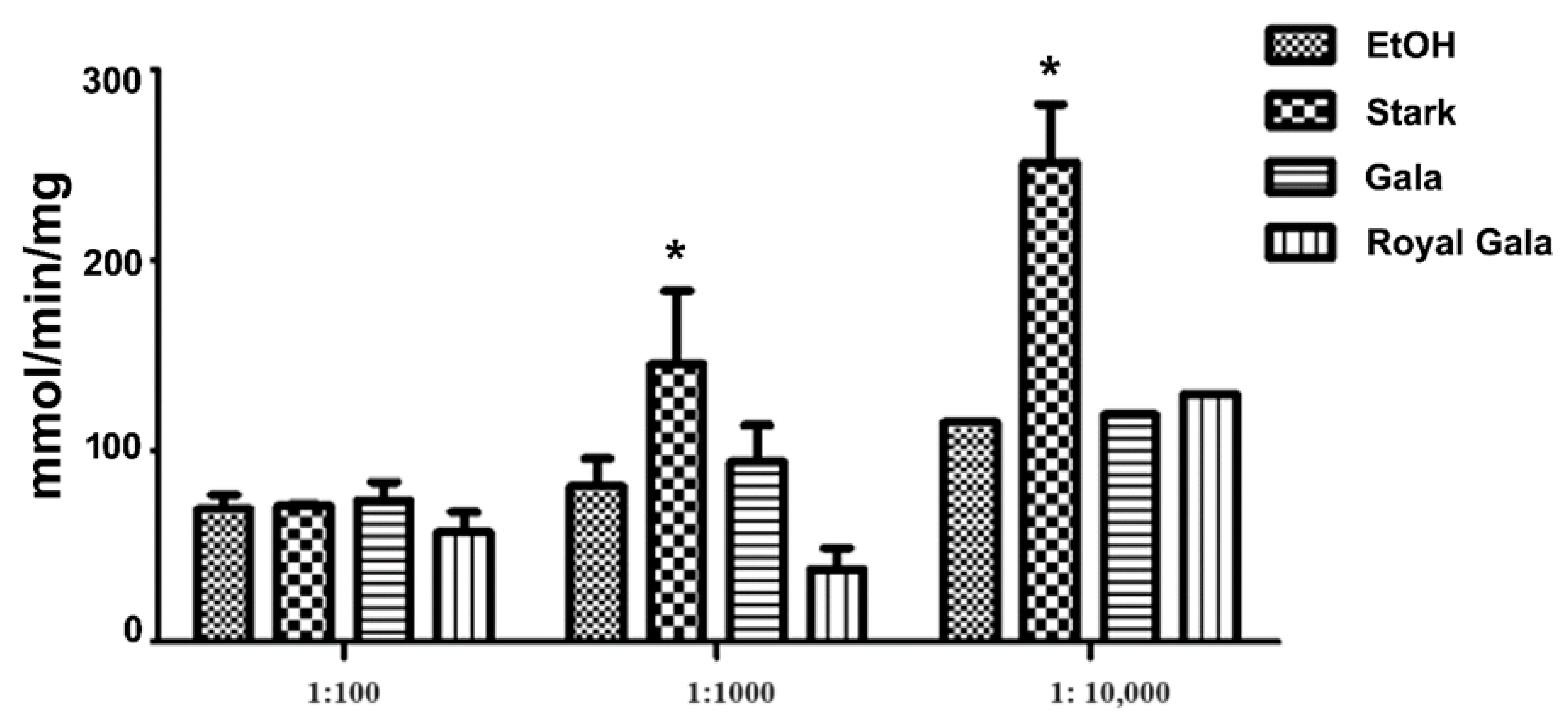
3.4. Evaluating Biological Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Carpena, M.; da Pereira, R.; Garcia-Perez, P.; Otero, P.; Soria-Lopez, A.; Chamorro, F.; Alcaide-Sancho, J.; Fraga-Corral, M.; Prieto, M.A.; Simal-Gandara, J. An Overview of Food Bioactive Compounds and Their Properties. In Membrane Separation of Food Bioactive Ingredients. Food Bioactive Ingredients; Jafari, S.M., Castro-Muñoz, R., Eds.; Springer: Cham, Switzerland, 2021. [Google Scholar]
- Cai, H.L.; You, S.Y.; Xu, Z.Y.; Li, Z.M.; Guo, J.J.; Ren, Z.Y.; Fu, C.L. Novel extraction methods and potential applications of polyphenols in fruit waste: A review. J. Food Meas. Charact. 2021, 15, 3250–3261. [Google Scholar] [CrossRef]
- Putra, N.R.; Rizkiyah, D.N.; Abdul Aziz, A.H.; Che Yunus, M.A.; Veza, I.; Harny, I.; Tirta, A. Waste to Wealth of Apple Pomace Valorization by Past and Current Extraction Processes: A Review. Sustainability 2023, 15, 830. [Google Scholar] [CrossRef]
- Vetralla, M.; Ferrentino, G.; Zambon, A.; Spilimbergo, S. A Study about the Effects of Supercritical Carbon Dioxide Drying on Apple Pieces. Int. J. Food Eng. 2018, 4, 186–190. [Google Scholar] [CrossRef]
- Aresta, A.; Cotugno, P.; De Vietro, N.; Massari, F.; Zambonin, C. Determination of Polyphenols and Vitamins in Wine-Making by-Products by Supercritical Fluid Extraction (SFE). Anal. Lett. 2020, 53, 2585–2595. [Google Scholar] [CrossRef]
- Garofulic, I.E.; Zoric, Z.; Pedisic, S.; Brncic, M.; Dragovic-Uzelac, V. UPLC-MS2 profiling of blackthorn flower polyphenols Isolated by ultrasound-assisted extraction. J. Food Sci. 2018, 83, 2782–2789. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.L.; Chen, Y.T. Polyphenol supplementation benefits human health via gut microbiota: A systematic review via meta-analysis. J. Fuoncional. Food 2020, 66, 103829. [Google Scholar] [CrossRef]
- Fernandes, P.A.R.; Wessel, D.F.; Coimbra, M.A.; Cardoso, S.M. Apple (Malus domestica) By-products: Chemistry, Functionality and Industrial Applications. In Mediterranean Fruits Bio-Wastes; Ramadan, M.F., Farag, M.A., Eds.; Springer: Cham, Switzerland, 2022. [Google Scholar]
- De la Peña-Armada, R.; Mateos-Aparicio, I. Sustainable Approaches Using Green Technologies for Apple By-Product Valorisation as A New Perspective into the History of the Apple. Molecules 2022, 27, 6937. [Google Scholar] [CrossRef] [PubMed]
- Martău, G.A.; Teleky, B.-E.; Ranga, F.; Pop, I.D.; Vodnar, D.C. Apple Pomace as a Sustainable Substrate in Sourdough Fermentation. Front. Microbiol. 2021, 12, 742020. [Google Scholar] [CrossRef]
- Chen, Z.; Yu, L.; Liu, W.; Zhang, J.; Wang, N.; Chen, X. Research progress of fruit color development in apple (Malus domestica Borkh.). Plant Physiol. Biochem. 2021, 162, 267–279. [Google Scholar] [CrossRef] [PubMed]
- Vasylieva, N.; James, H. Production and trade patterns in the world apple market. Innov. Mark. 2021, 17, 16–25. [Google Scholar] [CrossRef]
- Fotirić Akšić, M.; Dabić Zagorac, D.; Gašić, U.; Tosti, T.; Natić, M.; Meland, M. Analysis of apple fruit (Malus × domestica Borkh.) quality attributes obtained from organic and integrated production systems. Sustainability 2022, 14, 5300. [Google Scholar] [CrossRef]
- Rabetafika, H.N.; Bchir, B.; Blecker, C.; Richel, A. Fractionation of apple by-products as source of new ingredients: Current situation and perspectives. Trends Food Sci. Technol. 2014, 40, 99–114. [Google Scholar] [CrossRef]
- Rupasinghe, V.H.P.; Wang, L.; Huber, M.G.; Pitts, L.N. Effect of baking on dietary fibre and phenolics of muffins incorporated with apple skin powder. Food Chem. 2008, 107, 1217–1437. [Google Scholar] [CrossRef]
- Shehzadi, K.; Rubab, Q.; Asad, L.; Ishfaq, M.; Shafique, B.; Nawaz Ranjha, M.M.A.; Mahmood, S.; Mueen-Ud-Din, G.; Javaid, T.; Sabtain, B.; et al. Critical Review on Presence of Polyphenols in Commercial Varieties of Apple Peel, their Extraction and Health Benefits. J. Bio. Sci. Res. 2020, 6, 1–8. [Google Scholar]
- Hidalgo, A.; Brandolini, A.; Čanadanovic-Brunet, J.; Ćetkovic, G.; Tumbas-Šaponjac, V. Microencapsulates and extracts from red beetroot pomace modify antioxidant capacity, heat damage and colour of pseudocereals-enriched einkorn water biscuits. Food Chem. 2018, 268, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Wilson, A.M.; Castro-Arredondo, I.S.; Espinosa-Plascencia, A.; Robles-Burgueño, M.R.; Balandrán-Quintana, R.R.; Bermúdez-Almada, M.C. Chemical composition and antioxidant-prooxidant potential of a polyphenolic extract and a proanthocyanidin-rich fraction of apple skin. Heliyon 2016, 2, e00073. [Google Scholar] [CrossRef] [PubMed]
- Henríquez, C.; Speisky, H.; Chiffelle, I.; Valenzuela, T.; Araya, M.; Simpson, R.; Almonacid, S. Development of an ingredient containing apple peel, as a source of polyphenols and dietary fiber. J. Food Sci. 2010, 75, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Massini, L.; Rico, D.; Martin-Diana, A.B.; Barry-Ryan, C. Valorisation of apple peels. Eur. J. Food Res. Rev. 2013, 3, 1–15. [Google Scholar]
- Kaur, M.; Kaur, M.; Kaur, H. Apple peel as a source of dietary fiber and antioxidants: Effect on batter rheology and nutritional composition, textural and sensory quality attributes of muffins. J. Food Meas. Charact. 2022, 16, 2411–2421. [Google Scholar] [CrossRef]
- Kschonsek, J.; Wolfram, T.; Stöckl, A.; Böhm, V. Polyphenolic compounds analysis of old and new apple cultivars and contribution of polyphenolic profile to the in vitro antioxidant capacity. Antioxidants 2018, 7, 20. [Google Scholar] [CrossRef]
- Vicki Brower, V. An Apple a Day May Be Safer Than Vitamins. J. Natl. Cancer Inst. 2008, 100, 770–772. [Google Scholar] [CrossRef]
- Lemmens, E.; Alos, E.; Rymenants, M.; De Storme, N.; Keulemans, W. Dynamics of ascorbic acid content in apple (Malus × domestica) during fruit development and storage. Plant Physiol. Biochem. 2020, 151, 47–51. [Google Scholar] [CrossRef]
- Awad, M.A.; De Jager, A.; Van Westing, L.M. Flavonoid and chlorogenic acid levels in apple fruit: Characterisation of variation. Sci. Hortic. 2000, 83, 249–263. [Google Scholar] [CrossRef]
- Wiczkowski, W.; Piskuła, M.K. Food flavonoids. Pol. J. Food Nutr. Sci. 2004, 13, 101–114. [Google Scholar]
- Aizpurua-Olaizola, O.; Ormazabal, M.; Vallejo, A.; Olivares, M.; Navarro, P.; Etxebarria, N.; Usobiaga, A. Optimization of supercritical fluid consecutive extractions of fatty acids and polyphenols from Vitis vinifera grape wastes. J. Food Sci. 2015, 80, E101–E107. [Google Scholar] [CrossRef] [PubMed]
- Punzi, G.; Porcelli, V.; Ruggiu, M.; Hossain, M.F.; Menga, A.; Scarcia, P.; Castegna, A.; Gorgoglione, R.; Pierri, C.L.; Laera, L.; et al. SLC25A10 biallelic mutations in intractable epileptic encephalopathy with complex I deficiency. Hum. Mol. Genet. 2018, 27, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Wallace, D.C. Mitochondrial DNA in aging and disease. Sci. Am. 1997, 277, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Di Mauro, S.; Schon, E.A. Mitochondrial DNA mutations in human disease. Am. J. Med. Genet. 2001, 106, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.W.; Han, J.Z.; Li, H.R.; Zhang, X.; Liu, L.L.; Chen, F.; Zeng, B. Human Embryonic Kidney Cells: A Vehicle for Biopharmaceutical Manufacturing, Structural Biology, and Electrophysiology. Cell Tissues Organs 2018, 205, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Al-Duais, M.; Müller, L.; Böhm, V.; Jetschke, G. Antioxidant capacity and total phenolics of cyphostemma digitatum before and after processing: Use of different assays. Eur. Food. Res. Technol. 2009, 228, 813–821. [Google Scholar] [CrossRef]
- Medina, B. Simple and rapid method for the analysis of phenolic compounds in beverages and grains. J. Agric. Food Chem. 2011, 59, 1565–1571. [Google Scholar] [CrossRef] [PubMed]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Lee, J.; Durst, R.W.; Wrolstad, R.E. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: Collaborative study. J AOAC Int. 2005, 88, 1269–1278. [Google Scholar] [CrossRef] [PubMed]
- Palacino, J.J.; Sagi, D.; Goldberg, M.S.; Krauss, S.; Motz, C.; Wacker, M.; Klose, J.; Shen, J. Mitochondrial Dysfunction and Oxidative Damage in parkin-deficient Mice. J. Biol. Chem. 2004, 279, 18614–18622. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Spinazzi, M.; Casarin, A.; Pertegato, V.; Salviati, L.; Angelini, C. Assessment of mitochondrial respiratory chain enzymatic activities on tissues and cultured cells. Nat. Protoc. 2012, 31, 1235–1246. [Google Scholar] [CrossRef]
- Gegenschatz, S.A.; Chiappini, F.A.; Teglia, C.M.; Munoz de la Pena, A.; Goicoechea, H.C. Binding the gap between experiments, statistics, and method comparison: A tutorial for computing limits of detection and quantification in univariate calibration for complex samples. Anal. Chim. Acta 2021, 1209, 339342. [Google Scholar] [CrossRef]
- Da Porto, C.; Natolino, A. Supercritical fluid extraction of polyphenols from grape seed (Vitis vinifera): Study on process variables and kinetics. J. Supercrit. Fluids 2017, 130, 239–245. [Google Scholar] [CrossRef]
- Pandey, J.; Bastola, T.; Tripathi, J.; Tripathi, M.; Kumar, R.; Rokaya, R.K.; Dhakal Bhawana, R.; Ravin, D.C.; Bhandari, R.; Poudel, A. Estimation of Total Quercetin and Rutin Content in Malus domestica of Nepalese Origin by HPLC Method and Determination of Their Antioxidative Activity. J. Food Qual. 2020, 2020, 8853426. [Google Scholar] [CrossRef]
- Tempio, A.; Niso, M.; Laera, L.; Trisolini, L.; Favia, M.; Ciranna, L.; Marzulli, D.; Petrosillo, G.; Pierri, C.L.; Lacivita, E.; et al. Mitochondrial Membranes of Human SH-SY5Y Neuroblastoma Cells Express Serotonin 5-HT7 Receptor. Int. J. Mol. Sci. 2020, 21, 9629. [Google Scholar] [CrossRef]
- Tragni, V.; Primiano, G.; Tummolo, A.; Cafferati Beltrame, L.; La Piana, G.; Sgobba, M.N.; Cavalluzzi, M.M.; Paterno, G.; Gorgoglione, R.; Volpicella, M.; et al. Personalized Medicine in Mitochondrial Health and Disease: Molecular Basis of Therapeutic Approaches Based on Nutritional Supplements and Their Analogs. Molecules 2022, 27, 3494. [Google Scholar] [CrossRef] [PubMed]
- Gęgotek, A.; Skrzydlewska, E. Antioxidative and Anti-Inflammatory Activity of Ascorbic Acid. Antioxidants 2022, 11, 1993. [Google Scholar] [CrossRef] [PubMed]
- Chun, J.; Lee, J.; Ye, L.; Exler, J.; Eitenmiller, R.R. Tocopherol and tocotrienol contents of raw and processed fruits and vegetables in the United States diet. J. Food Compos. Anal. 2006, 19, 196–204. [Google Scholar] [CrossRef]
- Dismore, M.L.; Haytowitz, D.B.; Gebhardt, S.E.; Peterson, J.W.; Booth, S.L. Vitamin K content of nuts and fruits in the US diet. Res. Prof. Brief 2003, 103, 1650–1652. [Google Scholar] [CrossRef]
- Carrasco-Pozo, C.; Mizgier, M.L.; Speisky, H.; Gotteland, M. Differential protective effects of quercetin, resveratrol, rutin and epigallocatechin gallate against mitochondrial dysfunction induced by indomethacin in Caco-2 cells. Chem.-Biol. Interact. 2012, 195, 199–205. [Google Scholar] [CrossRef]
- Skemiene, K.; Liobikas, J.; Borutaite, V. Anthocyanins as substrates for mitochondrial complex I—Protective effect against heart ischemic injury. FEBS J. 2015, 282, 963–971. [Google Scholar] [CrossRef]
| Cultivar | TAC mmole Trolox/100 g (n = 3) | TPs mg GA/100 g (n = 3) | TFs mg GA/100 g (n = 3) | TAs mg CG/100 g (n = 3) | AA mg/100 g (n = 3) |
|---|---|---|---|---|---|
| Stark | 5.74 ± 0.07 | 379 ± 68 | 120 ± 2 | 7.0 ± 0.3 | 43.75 ± 6.05 |
| Gala | 2.20 ± 0.04 | 152 ± 25 | 90 ± 1 | 5.0 ± 0.3 | 9.02 ± 1.65 |
| Royal Gala | 5.95 ± 0.89 | 136 ± 14 | 115 ± 2 | 4.8 ± 0.1 | 25.59 ± 1.96 |
| RT (min) | Compound | λmax (nm) | Presence in SC-CO2 Extract |
|---|---|---|---|
| 2.0 ± 0.1 | Gallic acid | 270 | Stark and Gala |
| 5.0 ± 0.1 | (+)-Catechin | 279 | Stark |
| 5.6 ± 0.1 | Vanillic acid | 260 | Gala |
| 9.1 ± 0.1 | Rutin | 255 | Stark |
| 12.4 ± 0.1 | trans-Resveratrol | 307 | |
| 13.8 ± 0.1 | Quercetin | 370 | |
| 14.3 ± 0.2 | Cinnamic acid | 278 | Royal Gala |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aresta, A.; De Vietro, N.; Cotugno, P.; Pierri, C.L.; Trisolini, L.; Zambonin, C. Supercritical Fluid Extraction of Bioactive Components from Apple Peels and Their Modulation of Complex I Activity in Isolated Mitochondria. Antioxidants 2024, 13, 307. https://doi.org/10.3390/antiox13030307
Aresta A, De Vietro N, Cotugno P, Pierri CL, Trisolini L, Zambonin C. Supercritical Fluid Extraction of Bioactive Components from Apple Peels and Their Modulation of Complex I Activity in Isolated Mitochondria. Antioxidants. 2024; 13(3):307. https://doi.org/10.3390/antiox13030307
Chicago/Turabian StyleAresta, Antonella, Nicoletta De Vietro, Pietro Cotugno, Ciro Leonardo Pierri, Lucia Trisolini, and Carlo Zambonin. 2024. "Supercritical Fluid Extraction of Bioactive Components from Apple Peels and Their Modulation of Complex I Activity in Isolated Mitochondria" Antioxidants 13, no. 3: 307. https://doi.org/10.3390/antiox13030307
APA StyleAresta, A., De Vietro, N., Cotugno, P., Pierri, C. L., Trisolini, L., & Zambonin, C. (2024). Supercritical Fluid Extraction of Bioactive Components from Apple Peels and Their Modulation of Complex I Activity in Isolated Mitochondria. Antioxidants, 13(3), 307. https://doi.org/10.3390/antiox13030307










