The Utilization of an Aloe Vera Rind By-Product: Deep Eutectic Solvents as Eco-Friendly and Recyclable Extraction Media of Polyphenolic Compounds
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Preparation of Deep Eutectic Solvents
2.3. Plant Material
2.4. Ultrasound-Assisted Extraction of Polyphenols from Aloe Vera Rinds
2.5. Determination of Total Phenolic Content
2.6. Determination of Radical Scavenging Activity by DPPH
2.7. Determination of Ferric Reducing Antioxidant Power (FRAP)
2.8. Experimental Design and Statistical Analysis
2.9. Quantification of Polyphenolic Compounds through HPLC
2.10. Recovery of Polyphenols and DESs through Solid-Phase Extraction
2.10.1. Optimization of Polyphenol Recovery—Selection of SPE Cartridges
2.10.2. Recovery of Polyphenols and DESs from the Rind Extract
3. Results and Discussion
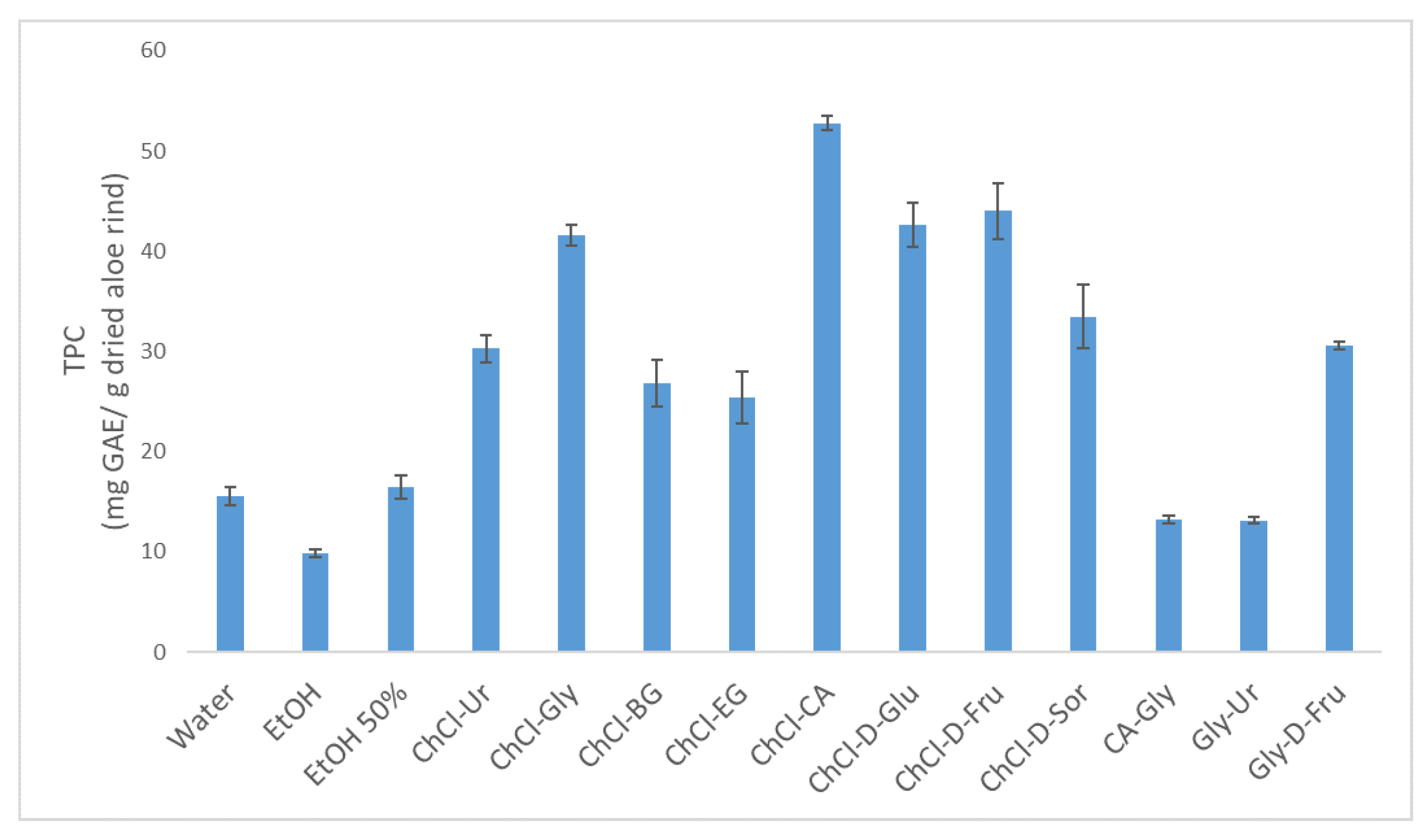
3.1. Selection of a Suitable Green Solvent for Phenolic Extraction
3.2. Optimization of the Antioxidant Extraction Procedure from Aloe Rinds
3.2.1. Statistical Analysis and Model Fitting
3.2.2. Effect of Process Variables
3.2.3. Optimal Conditions and Model Validation
3.3. Phenolic Profile of Aloe Rind Extracts—HPLC Analysis
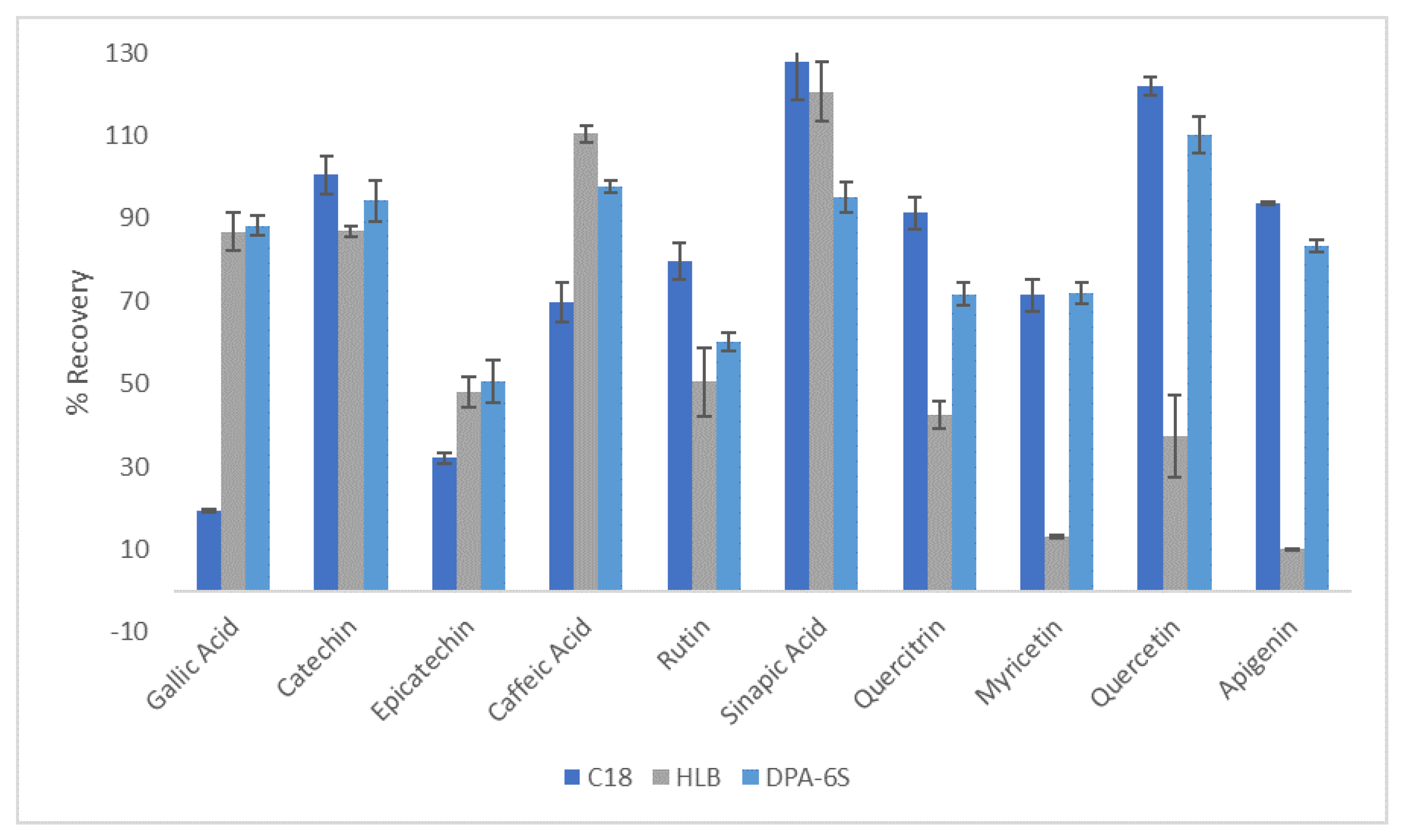
3.4. Recovery of Polyphenols and DESs from Aloe Rind Extracts
3.5. Green Evaluation Using Green Metrics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kumar, S.; Yadav, J.P. Ethnobotanical and Pharmacological Properties of Aloe Vera: A Review. J. Med. Plant Res. 2014, 8, 1387–1398. [Google Scholar] [CrossRef]
- Kumar, R.; Singh, A.K.; Gupta, A.; Bishayee, A.; Pandey, A.K. Therapeutic Potential of Aloe Vera—A Miracle Gift of Nature. Phytomedicine 2019, 60, 152996. [Google Scholar] [CrossRef] [PubMed]
- Sahu, P.K.; Giri, D.D.; Singh, R.; Pandey, P.; Gupta, S. Therapeutic and Medicinal Uses of Aloe Vera: A Review. Pharmacol. Pharm. 2013, 4, 599–610. [Google Scholar] [CrossRef]
- Radha, M.H.; Laxmipriya, N.P. Evaluation of Biological Properties and Clinical Effectiveness of Aloe Vera: A Systematic Review. J. Tradit. Complement. Med. 2015, 5, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Javed, S.; Atta-Ur, R. Aloe Vera Gel in Food, Health Products, and Cosmetics Industry, 1st ed.; Elsevier B.V.: Amsterdam, The Netherlands, 2014; Volume 41, ISBN 9780444632944. [Google Scholar]
- Bozzi, A.; Perrin, C.; Austin, S.; Vera, F.A. Quality and Authenticity of Commercial Aloe Vera Gel Powders. Food Chem. 2007, 103, 22–30. [Google Scholar] [CrossRef]
- Ramachandra, C.T.; Srinivasa Rao, P. Processing of Aloe Vera Leaf Gel: A Review. Am. J. Agric. Biol. Sci. 2008, 3, 502–510. [Google Scholar] [CrossRef]
- Guo, X.; Mei, N. Aloe Vera: A Review of Toxicity and Adverse Clinical Effects. J. Environ. Sci. Health 2016, 34, 77–96. [Google Scholar] [CrossRef] [PubMed]
- Esposito, B.; Sessa, M.R.; Sica, D.; Malandrino, O. Towards Circular Economy in the Agri-Food Sector. A Systematic Literature Review. Sustainability 2020, 12, 7401. [Google Scholar] [CrossRef]
- Gullón, P.; Gullón, B.; Romaní, A.; Rocchetti, G.; Manuel, J. Trends in Food Science & Technology Smart Advanced Solvents for Bioactive Compounds Recovery from Agri-Food by-Products: A Review. Trends Food Sci. Technol. 2020, 101, 182–197. [Google Scholar] [CrossRef]
- Ozturk, B.; Parkinson, C.; Gonzalez-Miquel, M. Extraction of Polyphenolic Antioxidants from Orange Peel Waste Using Deep Eutectic Solvents. Sep. Purif. Technol. 2018, 206, 1–13. [Google Scholar] [CrossRef]
- Christou, A.; Stavrou, I.J.; Kapnissi-Christodoulou, C.P. Continuous and Pulsed Ultrasound-Assisted Extraction of Carob’s Antioxidants: Processing Parameters Optimization and Identification of Polyphenolic Composition. Ultrason. Sonochem. 2021, 76, 105630. [Google Scholar] [CrossRef]
- Pal, C.B.T.; Jadeja, G.C. Microwave-Assisted Deep Eutectic Solvent Extraction of Phenolic Antioxidants from Onion (Allium Cepa L.) Peel: A Box–Behnken Design Approach for Optimization. J. Food Sci. Technol. 2019, 56, 4211–4223. [Google Scholar] [CrossRef] [PubMed]
- López, A.; De Tangil, M.S.; Vega-orellana, O.; Ramírez, A.S.; Rico, M. Phenolic Constituents, Antioxidant and Preliminary Antimycoplasmic Activities of Leaf Skin and Flowers of Aloe Vera (L.) Burm. f. (Syn. A. Barbadensis Mill.) from the Canary Islands (Spain). Molecules 2013, 18, 4942–4954. [Google Scholar] [CrossRef]
- Solaberrieta, I.; Jimenez, A.; Garrigos, M.C. Valorization of Aloe Vera Skin By-Products to Obtain Bioactive Compounds by Microwave-Assisted Extraction: Antioxidant Activity and Chemical Composition. Antioxidants 2022, 11, 1058. [Google Scholar] [CrossRef] [PubMed]
- Stavrou, I.J.; Christou, A.; Kapnissi-Christodoulou, C.P. Polyphenols in Carobs: A Review on Their Composition, Antioxidant Capacity and Cytotoxic Effects, and Health Impact. Food Chem. 2018, 269, 355–374. [Google Scholar] [CrossRef] [PubMed]
- Khurana, S.; Venkataraman, K.; Hollingsworth, A.; Piche, M.; Tai, T.C. Polyphenols: Benefits to the Cardiovascular System in Health and in Aging. Nutrients 2013, 5, 3779–3827. [Google Scholar] [CrossRef]
- Scalbert, A.; Manach, C.; Morand, C.; Rémésy, C.; Jiménez, L. Dietary Polyphenols and the Prevention of Diseases. Crit. Rev. Food Sci. Nutr. 2005, 45, 287–306. [Google Scholar] [CrossRef] [PubMed]
- Ningappa, M.B.; Dinesha, R.; Srinivas, L. Antioxidant and Free Radical Scavenging Activities of Polyphenol-Enriched Curry Leaf (Murraya Koenigii L.) Extracts. Food Chem. 2008, 106, 720–728. [Google Scholar] [CrossRef]
- Quispe, C.; Villalobos, M.; Bórquez, J.; Simirgiotis, M. Chemical Composition and Antioxidant Activity of Aloe Vera from the Pica Oasis (Tarapacá, Chile) by UHPLC-Q/Orbitrap/MS/MS. J. Chem. 2018, 2018, 1–12. [Google Scholar] [CrossRef]
- Añibarro-Ortega, M.; Pinela, J.; Barros, L.; Ciric, A.; Silva, S.P.; Coelho, E.; Mocan, A.; Calhelha, R.C.; Sokovic, M.; Coimbra, M.A.; et al. Compositional Features and Bioactive Properties of Aloe Vera Leaf (Fillet, Mucilage and Rind) and Flower. Antioxidants 2019, 8, 444. [Google Scholar] [CrossRef]
- Miladi, S.; Mohamed, D. In Vitro Antioxidant Activities of Aloe Vera Leaf Skin Extracts. J. La Société Chim. Tunis. 2008, 10, 101–109. [Google Scholar]
- Kammoun, M.; Miladi, S.; Ali, Y.B.; Damak, M.; Gargouri, Y.; Bezzine, S. In Vitro Study of the PLA2 Inhibition and Antioxidant Activities of Aloe Vera Leaf Skin Extracts. Lipids Health Dis. 2011, 10, 30. [Google Scholar] [CrossRef]
- Añibarro-Ortega, M.; Pinela, J.; Ćirić, A.; Lopes, E.; Molina, A.K.; Calhelha, R.C.; Soković, M.; Ferreira, O.; Ferreira, I.C.F.R.; Barros, L. Extraction of Aloesin from Aloe Vera Rind Using Alternative Green Solvents: Process Optimization and Biological Activity Assessment. Biology 2021, 10, 951. [Google Scholar] [CrossRef] [PubMed]
- Ameer, K.; Shahbaz, H.M.; Kwon, J.H. Green Extraction Methods for Polyphenols from Plant Matrices and Their Byproducts: A Review. Compr. Rev. Food Sci. Food Saf. 2017, 16, 295–315. [Google Scholar] [CrossRef] [PubMed]
- Talmaciu, A.I.; Volf, I.; Popa, V.I. A Comparative Analysis of the “Green” Techniques Applied for Polyphenols Extraction from Bioresources. Chem. Biodiv. 2015, 12, 1635–1651. [Google Scholar] [CrossRef]
- Ramić, M.; Vidović, S.; Zeković, Z.; Vladić, J.; Cvejin, A.; Pavlić, B. Modeling and Optimization of Ultrasound-Assisted Extraction of Polyphenolic Compounds from Aronia Melanocarpa by-Products from Filter-Tea Factory. Ultrason. Sonochem. 2015, 23, 360–368. [Google Scholar] [CrossRef]
- Ajila, C.M.; Brar, S.K.; Verma, M.; Tyagi, R.D.; Godbout, S.; Valéro, J.R. Extraction and Analysis of Polyphenols: Recent Trends. Crit. Rev. Biotechnol. 2011, 31, 227–249. [Google Scholar] [CrossRef] [PubMed]
- Torres-Valenzuela, L.S.; Ballesteros-Gómez, A.; Rubio, S. Green Solvents for the Extraction of High Added-Value Compounds from Agri-Food Waste. Food Eng. Rev. 2020, 12, 83–100. [Google Scholar] [CrossRef]
- Choi, Y.H.; Verpoorte, R. Green Solvents for the Extraction of Bioactive Compounds from Natural Products Using Ionic Liquids and Deep Eutectic Solvents. Curr. Opin. Food Sci. 2019, 26, 87–93. [Google Scholar] [CrossRef]
- Liu, Y.; Friesen, J.B.; McAlpine, J.B.; Lankin, D.C.; Chen, S.N.; Pauli, G.F. Natural Deep Eutectic Solvents: Properties, Applications, and Perspectives. J. Nat. Prod. 2018, 81, 679–690. [Google Scholar] [CrossRef]
- El Achkar, T.; Greige-Gerges, H.; Fourmentin, S. Basics and Properties of Deep Eutectic Solvents: A Review. Environ. Chem. Lett. 2021, 19, 3397–3408. [Google Scholar] [CrossRef]
- Pena-Pereira, F.; Namieśnik, J. Ionic Liquids and Deep Eutectic Mixtures: Sustainable Solvents for Extraction Processes. ChemSusChem 2014, 7, 1784–1800. [Google Scholar] [CrossRef] [PubMed]
- Hansen, B.B.; Spittle, S.; Chen, B.; Poe, D.; Zhang, Y.; Klein, J.M.; Horton, A.; Adhikari, L.; Zelovich, T.; Doherty, B.W.; et al. Deep Eutectic Solvents: A Review of Fundamentals and Applications. Chem. Rev. 2021, 121, 1232–1285. [Google Scholar] [CrossRef] [PubMed]
- Shishov, A.; Bulatov, A.; Locatelli, M.; Carradori, S.; Andruch, V. Application of Deep Eutectic Solvents in Analytical Chemistry. A Review. Microchem. J. 2017, 135, 33–38. [Google Scholar] [CrossRef]
- Bajkacz, S.; Adamek, J.; Sobska, A. Application of Deep Eutectic Solvents and Ionic Liquids in the Extraction of Catechins from Tea. Molecules 2020, 25, 3216. [Google Scholar] [CrossRef]
- Tan, T.; Zhang, M.; Wan, Y.; Qiu, H. Utilization of Deep Eutectic Solvents as Novel Mobile Phase Additives for Improving the Separation of Bioactive Quaternary Alkaloids. Talanta 2016, 149, 85–90. [Google Scholar] [CrossRef]
- Bener, M.; Sen, F.B.; Onem, A.N.; Bekdeser, B.; Celik, S.E.; Lalikoglu, M.; Asci, Y.S.; Capanoglu, E.; Apak, R. Microwave-Assisted Extraction of Antioxidant Compounds from by-Products of Turkish Hazelnut (Corylus Avellana L.) Using Natural Deep Eutectic Solvents: Modeling, Optimization and Phenolic Characterization. Food Chem. 2022, 385, 132633. [Google Scholar] [CrossRef]
- Cai, C.; Li, F.; Liu, L.; Tan, Z. Deep Eutectic Solvents Used as the Green Media for the Efficient Extraction of Caffeine from Chinese Dark Tea. Sep. Purif. Technol. 2019, 227, 115723. [Google Scholar] [CrossRef]
- Mouratoglou, E.; Malliou, V.; Makris, D.P. Novel Glycerol-Based Natural Eutectic Mixtures and Their Efficiency in the Ultrasound-Assisted Extraction of Antioxidant Polyphenols from Agri-Food Waste Biomass. Waste Biomass Valorization 2016, 7, 1377–1387. [Google Scholar] [CrossRef]
- Xu, B.J.; Chang, S.K.C. A Comparative Study on Phenolic Profiles and Antioxidant Activities of Legumes as Affected by Extraction Solvents. J. Food Sci. 2007, 72, S159–S166. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of ’Antioxidant Power’: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Kumar, N.; Bhandari, P.; Singh, B.; Gupta, A.P.; Kaul, V.K. Reversed Phase-HPLC for Rapid Determination of Polyphenols in Flowers of Rose Species. J. Sep. Sci. 2008, 31, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Pinto, D.; Vieira, E.F.; Peixoto, A.F.; Freire, C.; Freitas, V.; Costa, P.; Delerue-Matos, C.; Rodrigues, F. Optimizing the Extraction of Phenolic Antioxidants from Chestnut Shells by Subcritical Water Extraction Using Response Surface Methodology. Food Chem. 2021, 334, 127521. [Google Scholar] [CrossRef]
- Xia, B.; Yan, D.; Bai, Y.; Xie, J.; Cao, Y.; Liao, D.; Lin, L. Determination of Phenolic Acids in Prunella Vulgaris L.: A Safe and Green Extraction Method Using Alcohol-Based Deep Eutectic Solvents. Anal. Methods 2015, 7, 9354–9364. [Google Scholar] [CrossRef]
- Vilková, M.; Justyna, P.; Andruch, V. The Role of Water in Deep Eutectic Solvent-Base Extraction. J. Mol. Liq. 2020, 304, 112747. [Google Scholar] [CrossRef]
- Garcia-salas, P.; Morales-soto, A.; Segura-carretero, A.; Fernández-gutiérrez, A. Phenolic-Compound-Extraction Systems for Fruit and Vegetable Samples. Molecules 2010, 15, 8813–8826. [Google Scholar] [CrossRef]
- Dai, J.; Mumper, R.J. Plant Phenolics: Extraction, Analysis and Their Antioxidant and Anticancer Properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef]
- Vural, N.; Cavuldak, Ö.A.; Anlı, R.E. Multi Response Optimisation of Polyphenol Extraction Conditions from Grape Seeds by Using Ultrasound Assisted Extraction (UAE). Sep. Sci. Technol. 2018, 53, 1540–1551. [Google Scholar] [CrossRef]
- Hsieh, Y.; Li, Y.; Pan, Z.; Chen, Z.; Lu, J.; Yuan, J. Ultrasonication-Assisted Synthesis of Alcohol-Based Deep Eutectic Solvents for Extraction of Active Compounds from Ginger. Ultrason. Sonochem. 2020, 63, 104915. [Google Scholar] [CrossRef] [PubMed]
- Alrugaibah, M.; Yagiz, Y.; Gu, L. Use Natural Deep Eutectic Solvents as Efficient Green Reagents to Extract Procyanidins and Anthocyanins from Cranberry Pomace and Predictive Modeling by RSM and Artificial Neural Networking. Sep. Purif. Technol. 2021, 255, 117720. [Google Scholar] [CrossRef]
- Bertolo, M.R.V.; Martins, V.C.A.; Plepis, A.M.G.; Junior, S.B. Utilization of Pomegranate Peel Waste: Natural Deep Eutectic Solvents as a Green Strategy to Recover Valuable Phenolic Compounds. J. Clean. Prod. 2021, 327, 129471. [Google Scholar] [CrossRef]
- Hossen, M.; Lokman, M.; Mitra, K.; Hossain, B. Phytochemicals and In-Vitro Antioxidant Activity Analysis of Aloe Vera by-Products (Skin) in Different Solvent Extract. J. Agric. Food Res. 2022, 10, 100460. [Google Scholar] [CrossRef]
- Hu, Q.; Hu, Y.; Xu, J. Free Radical-Scavenging Activity of Aloe Vera (Aloe Barbadensis Miller) Extracts by Supercritical Carbon Dioxide Extraction. Food Chem. 2005, 91, 85–90. [Google Scholar] [CrossRef]
- Bushra, S.; Farooq, A.; Muhammad, A. Effect of Extraction Solvent/Technique on the Antioxidant Activity of Selected Medicinal Plant Extracts. Molecules 2009, 14, 2167–2180. [Google Scholar] [CrossRef]
- Papagiannopoulos, M.; Wollseifen, H.R.; Mellenthin, A.; Haber, B.; Galensa, R. Identification and Quantification of Polyphenols in Carob Fruits (Ceratonia Siliqua L.) and Derived Products by HPLC-UV-ESI/MSn. J. Agric. Food Chem. 2004, 52, 3784–3791. [Google Scholar] [CrossRef] [PubMed]
- Lucini, L.; Pellizzoni, M.; Pellegrino, R.; Pietro, G.; Colla, G. Phytochemical Constituents and in Vitro Radical Scavenging Activity of Different Aloe Species. Food Bioprod. Process. Chem. 2015, 170, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Min, K.; Sang, M.; Woo, M.; Zhao, J.; Jin, Y.; Lee, D.; Won, S.; Hoon, J.; Lee, J. Tailoring and Recycling of Deep Eutectic Solvents as Sustainable and Efficient Extraction Media. J. Chromatogr. A 2015, 1424, 10–17. [Google Scholar] [CrossRef]
- Nam, M.W.; Zhao, J.; Lee, M.S.; Jeong, J.H.; Lee, J. Enhanced Extraction of Bioactive Natural Products Using Tailor-Made Deep Eutectic Solvents: Application to Flavonoid Extraction from Flos Sophorae. Green Chem. 2015, 17, 1718–1727. [Google Scholar] [CrossRef]
- Dai, Y.; Van Spronsen, J.; Witkamp, G.J.; Verpoorte, R.; Choi, Y.H. Ionic Liquids and Deep Eutectic Solvents in Natural Products Research: Mixtures of Solids as Extraction Solvents. J. Nat. Prod. 2013, 76, 2162–2173. [Google Scholar] [CrossRef]
- Zhou, P.; Wang, X.; Liu, P.; Huang, J.; Wang, C.; Pan, M. Enhanced Phenolic Compounds Extraction from Morus Alba L. Leaves by Deep Eutectic Solvents Combined with Ultrasonic-Assisted Extraction. Ind. Crop. Prod. 2018, 120, 147–154. [Google Scholar] [CrossRef]
- Huang, Y.; Feng, F.; Jiang, J.; Qiao, Y.; Wu, T.; Voglmeir, J.; Chen, Z. Green and Efficient Extraction of Rutin from Tartary Buckwheat Hull by Using Natural Deep Eutectic Solvents. Food Chem. 2017, 221, 1400–1405. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Yang, M.; Cao, F.; Wang, J.; Su, E. Tailor-Made Hydrophobic Deep Eutectic Solvents for Cleaner Extraction of Polyprenyl Acetates from Ginkgo Biloba Leaves. J. Clean. Prod. 2017, 152, 399–405. [Google Scholar] [CrossRef]
- Cui, Q.; Liu, J.; Wang, L.; Kang, Y.; Meng, Y.; Jiao, J.; Fu, Y. Sustainable Deep Eutectic Solvents Preparation and Their Ef Fi Ciency in Extraction and Enrichment of Main Bioactive Fl Avonoids from Sea Buckthorn Leaves. J. Clean. Prod. 2018, 184, 826–835. [Google Scholar] [CrossRef]
- Plaza, A.; Tapia, X.; Yañez, C.; Vilches, F.; Candia, O.; Cabezas, R. Obtaining Hydroxytyrosol from Olive Mill Waste Using Deep Eutectic Solvents and Then Supercritical—CO2. Waste Biomass Valorization 2020, 11, 6273–6284. [Google Scholar] [CrossRef]
- Della Posta, S.; Gallo, V.; Gentili, A.; Fanali, C. Strategies for the Recovery of Bioactive Molecules from Deep Eutectic Solvents Extracts. Trends Anal. Chem. 2022, 157, 116798. [Google Scholar] [CrossRef]
- Fu, N.; Lv, R.; Guo, Z.; Guo, Y.; You, X.; Tang, B.; Han, D.; Yan, H.; Ho, K. Environmentally Friendly and Non-Polluting Solvent Pretreatment of Palm Samples for Polyphenol Analysis Using Choline Chloride Deep Eutectic Solvents. J. Chromatogr. A 2017, 1492, 1–11. [Google Scholar] [CrossRef]
- Pena-Pereira, F.; Wojnowski, W.; Tobiszewski, M. AGREE—Analytical GREEnness Metric Approach and Software. Anal. Chem. 2020, 92, 10076–10082. [Google Scholar] [CrossRef]
- Wojnowski, W.; Tobiszewski, M.; Pena-Pereira, F.; Psillakis, E. AGREEprep—Analytical Greenness Metric for Sample Preparation. TrAC Trends Anal. Chem. 2022, 149, 116553. [Google Scholar] [CrossRef]
- Mammana, S.B.; Gagliardi, L.G.; Silva, M.F. Sustainable Sample Preparation Method Based on Hydrophobic Natural Deep Eutectic Solvents. Chemometric Tools and Green Metrics for Ibuprofen in Groundwater. Sep. Purif. Technol. 2022, 303, 122240. [Google Scholar] [CrossRef]

| HΒA | HBD | Abbreviation | Molar Ratio |
|---|---|---|---|
| Choline Chloride | Ethylene Glycol | ChCl-EG | 1:2 |
| Glycerol | ChCl-Gly | 1:1 | |
| Urea | ChCl-Ur | 1:2 | |
| 1,3-Butylene Glycol | ChCl-BG | 1:2 | |
| Citric Acid | ChCl-CA | 1:1 | |
| D-Glucose | ChCl-D-Glu | 2:1 | |
| D-Fructose | ChCl-D-Fru | 2:1 | |
| D-Sorbitol | ChCl-D-Sor | 1:1 | |
| Citric Acid | Glycerol | CA-Gly | 1:2 |
| Glycerol | Urea | Gly-Ur | 1:1 |
| D-Fructose | Gly-D-Fru | 3:1 |
| Run | Independent Factors | Responses | ||||
|---|---|---|---|---|---|---|
| Time (min) | Solvent/Solid (mL/g) | % DES | TPC | DPPH | FRAP | |
| 1 | 15 | 100 | 60 | 42.1 | 7.4 | 799 |
| 2 | 10 | 150 | 60 | 45.8 | 11.9 | 1570 |
| 3 | 20 | 150 | 60 | 52.1 | 11.0 | 1423 |
| 4 | 15 | 200 | 60 | 64.6 | 14.3 | 1132 |
| 5 | 20 | 100 | 70 | 40.5 | 8.3 | 1338 |
| 6 | 10 | 100 | 70 | 49.7 | 8.4 | 1655 |
| 7 | 15 | 150 | 70 | 53.8 | 11.8 | 1889 |
| 8 | 15 | 150 | 70 | 53.9 | 11.8 | 1889 |
| 9 | 15 | 150 | 70 | 53.8 | 11.7 | 1889 |
| 10 | 15 | 150 | 70 | 53.8 | 11.8 | 1889 |
| 11 | 20 | 200 | 70 | 65.4 | 16.8 | 2234 |
| 12 | 10 | 200 | 70 | 69.5 | 17.1 | 2339 |
| 13 | 15 | 100 | 80 | 36.6 | 8.3 | 1846 |
| 14 | 10 | 150 | 80 | 45.9 | 12.6 | 2234 |
| 15 | 20 | 150 | 80 | 52.0 | 12.6 | 1907 |
| 16 | 15 | 200 | 80 | 69.7 | 16.0 | 2535 |
| Source | Sum of Squares (SS) | Degree of Freedom (DF) | Mean Square (MS) | F-Value | p-Value |
|---|---|---|---|---|---|
| TPC model | 20.59 | <0.0001 *** | |||
| A | 0.1 | 1 | 0.1 | 0.01 | 0.9437 |
| B | 1256.5 | 1 | 1256.5 | 61.77 | <0.0001 *** |
| C | 0.1 | 1 | 0.1 | 0.01 | 0.9780 |
| Residuals | 244.1 | 12 | 20.34 | ||
| R2 = 0.8373, R2adj = 0.797, R2pred = 0.757 | |||||
| DPPH model | 103.69 | <0.0001 *** | |||
| A | 0.2 | 1 | 0.2 | 1.25 | 0.3070 |
| B | 125.9 | 1 | 125.9 | 886.43 | <0.0001 *** |
| C | 3.1 | 1 | 3.1 | 21.76 | 0.0034 ** |
| AB | 0.0 | 1 | 0.0 | 0.03 | 0.8588 |
| AC | 0.2 | 1 | 0.2 | 1.73 | 0.2370 |
| BC | 0.1 | 1 | 0.1 | 0.86 | 0.3908 |
| A2 | 2.1 | 1 | 2.1 | 14.33 | 0.0091 ** |
| B2 | 0.1 | 1 | 0.1 | 0.98 | 0.3602 |
| C2 | 0.8 | 1 | 0.8 | 5.85 | 0.0497 * |
| Residuals | 0.9 | 6 | 0.1 | ||
| R2 = 0.994, R2adj = 0.984, R2pred = 0.899 | |||||
| FRAP model | 18.26 | <0.0001 *** | |||
| A | 1.0 × 105 | 1 | 1.0 × 105 | 2.14 | 0.1688 |
| B | 8.5 × 105 | 1 | 8.5 × 105 | 18.07 | 0.0011 ** |
| C | 1.6 × 105 | 1 | 1.6 × 105 | 34.56 | <0.0001 *** |
| Residuals | 5.6 × 105 | 12 | 46,798.8 | ||
| R2 = 0.8203, R2adj = 0.775, R2pred = 0.733 | |||||
| Dependent Variables | Predicted Value | Experimental Value | Percentage Error (%) |
|---|---|---|---|
| TPC (mg GAE/g) | 63.56 | 64.96 ± 3.04 | 2.20 |
| DPPH (mM TE/g) | 15.51 | 15.69 ± 0.35 | 1.19 |
| FRAP (μM TE/g) | 2203 | 2217 ± 39 | 0.62 |
| Compound | Concentration (mg/g Dried Aloe Rind) | % Content |
|---|---|---|
| Gallic acid | 1.37 ± 0.14 | 14.2 |
| Catechin | 1.44 ± 0.09 | 14.9 |
| Epicatechin | 0.91 ± 0.04 | 9.4 |
| Caffeic acid | 0.21 ± 0.02 | 2.2 |
| Rutin | 1.20 ± 0.04 | 12.4 |
| Sinapic acid | ND | 0 |
| Quercitrin | ND | 0 |
| Myricetin | 2.45 ± 0.04 | 25.4 |
| Quercetin | 0.98 ± 0.03 | 10.1 |
| Apigenin | 1.11 ± 0.04 | 11.4 |
| Total | 9.67 | 100 |
| Extraction Method | Extraction Time | Extraction Solvent | Mass of Aloe Rind | Solvent Volume | Detection System | AGREE | AGREEprep | Ref. |
|---|---|---|---|---|---|---|---|---|
| Soxhlet | 12 h (4 × 3 h) | Hex, Ace, EtOH, MeOH | 10 g | 700 mL (4 × 175 mL) | HPLC-DAD | 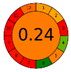 |  | [14] |
| MAE | 36.6 min | 80% EtOH | 1.5 g | 50 mL | HPLC-ESI-MS/MS |  | 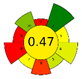 | [15] |
| Stirring | 3 days | Acidic MeOH | 100 g | 500 mL | UV | 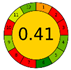 | 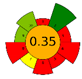 | [53] |
| UAE | 16.5 min | 74% DES (ChCl-CA) | 0.052 g | 10 mL | HPLC-PDA | 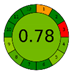 | 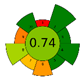 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ioannou, G.D.; Ioannou, K.A.; Christou, A.; Stavrou, I.J.; Kapnissi-Christodoulou, C.P. The Utilization of an Aloe Vera Rind By-Product: Deep Eutectic Solvents as Eco-Friendly and Recyclable Extraction Media of Polyphenolic Compounds. Antioxidants 2024, 13, 162. https://doi.org/10.3390/antiox13020162
Ioannou GD, Ioannou KA, Christou A, Stavrou IJ, Kapnissi-Christodoulou CP. The Utilization of an Aloe Vera Rind By-Product: Deep Eutectic Solvents as Eco-Friendly and Recyclable Extraction Media of Polyphenolic Compounds. Antioxidants. 2024; 13(2):162. https://doi.org/10.3390/antiox13020162
Chicago/Turabian StyleIoannou, Georgia D., Katerina A. Ioannou, Atalanti Christou, Ioannis J. Stavrou, and Constantina P. Kapnissi-Christodoulou. 2024. "The Utilization of an Aloe Vera Rind By-Product: Deep Eutectic Solvents as Eco-Friendly and Recyclable Extraction Media of Polyphenolic Compounds" Antioxidants 13, no. 2: 162. https://doi.org/10.3390/antiox13020162
APA StyleIoannou, G. D., Ioannou, K. A., Christou, A., Stavrou, I. J., & Kapnissi-Christodoulou, C. P. (2024). The Utilization of an Aloe Vera Rind By-Product: Deep Eutectic Solvents as Eco-Friendly and Recyclable Extraction Media of Polyphenolic Compounds. Antioxidants, 13(2), 162. https://doi.org/10.3390/antiox13020162







