Metabolism of Phenolic Compounds and Antioxidant Activity in Different Tissue Parts of Post-Harvest Chive (Allium schoenoprasum L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Treatments
2.2. Preparation of Extracts
2.3. The Folin–Ciocalteu Assay and Detection of AsA
2.4. UHPLC-MS/MS Method
2.5. Determination of Enzyme Activities Involved in Phenolic Compounds Biosynthesis and Degradation
2.6. Determination of Relative Gene Expression
2.7. Determination of Antioxidant Activities
2.8. Statistical Analysis
3. Results and Discussion
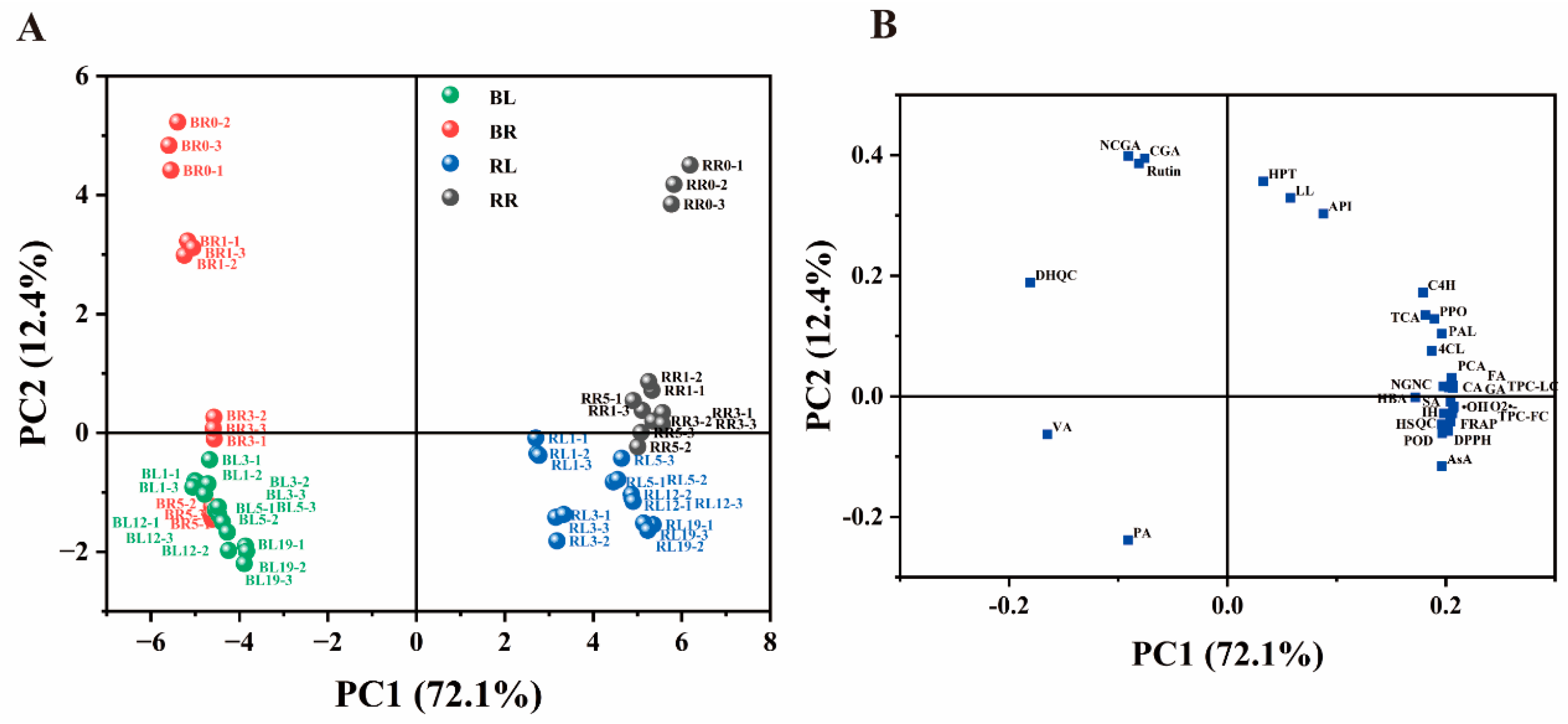
3.1. Principal Component Analysis
3.2. Dynamics of Antioxidant Compounds
3.2.1. Dynamics of TPC-FC and AsA
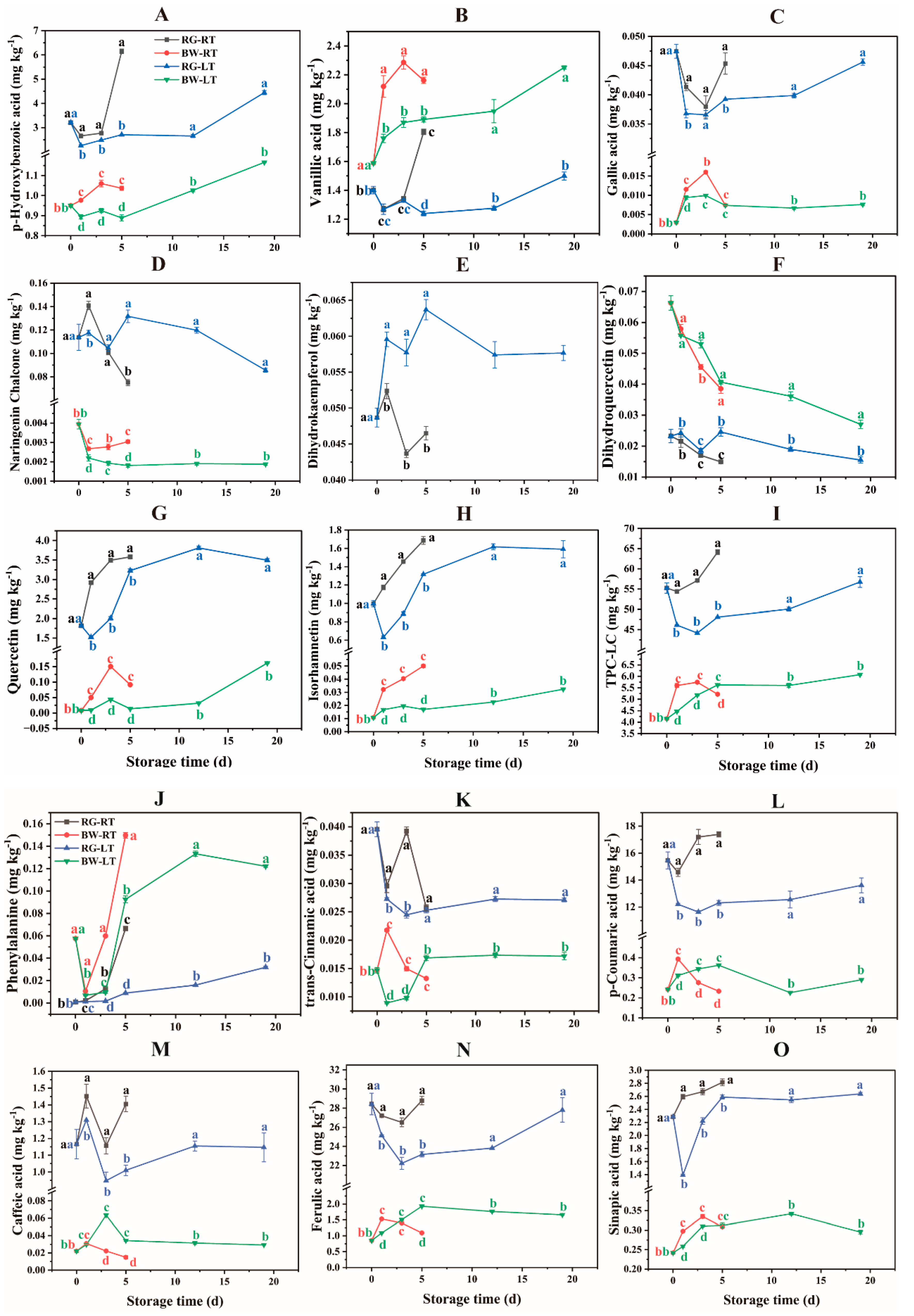
3.2.2. Dynamics of Phenolic Compounds Determined Using LC–MS
3.3. Dynamics of Enzyme Activities Involved in the Metabolism of Phenolic Compounds in Post-Harvest Chive
3.4. Dynamics of Gene Expression
3.5. Dynamics of Antioxidant Activities in Post-Harvest Chive
3.6. Dynamics of Other Nutrients
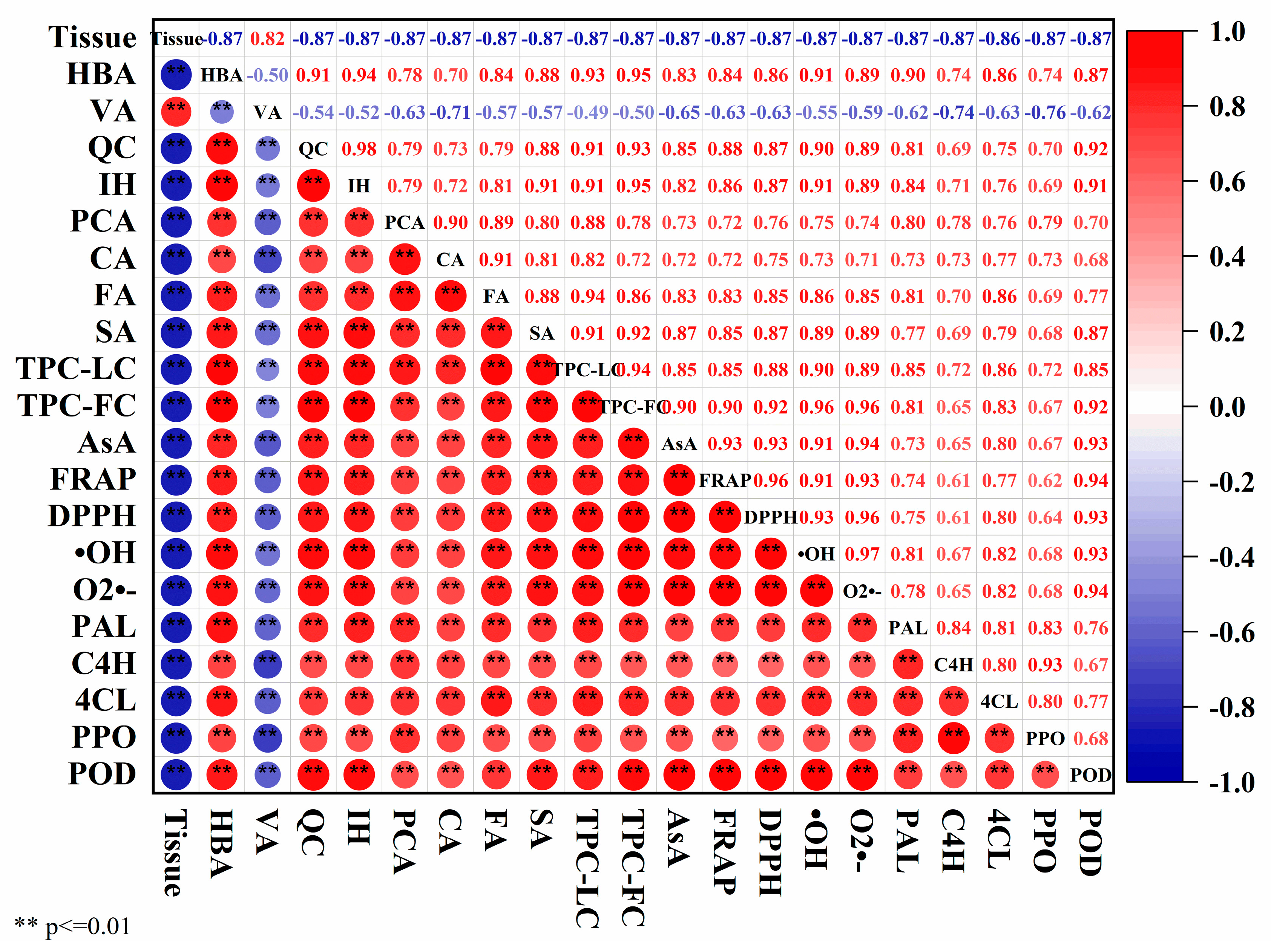
3.7. Correlation Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abdelrahman, M.; Ariyanti, N.A.; Sawada, Y.; Tsuji, F.; Hirata, S.; Hang, T.T.M.; Okamoto, M.; Yamada, Y.; Tsugawa, H.; Hirai, M.Y.; et al. Metabolome-based discrimination analysis of shallot landraces and bulb onion cultivars associated with differences in the amino acid and flavonoid profiles. Molecules 2020, 25, 5300. [Google Scholar] [CrossRef]
- Dai, X.; Jia, C.; Lu, J.; Yu, Z. The dynamics of bioactive compounds and their contributions to the antioxidant activity of postharvest chive (Allium schoenoprasum L.). Food Res. Int. 2023, 174, 113600. [Google Scholar] [CrossRef]
- Zhang, F.; Xie, Y.; Shi, J.; Jiang, L. Effects of 1-methylcyclopropene treatment on phenolic metabolism in postharvest Gynura bicolor DC. Sci. Hortic. 2022, 293, 110668. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, D. Comprehensive metabolomics and antioxidant activity of Allium species viz. Allium semenovii, A. sativum and A. cepa: An important spice. Food Res. Int. 2023, 166, 112584. [Google Scholar] [CrossRef]
- Mollica, A.; Zengin, G.; Locatelli, M.; Picot-Allain, C.M.N.; Mahomoodally, M.F. Multidirectional investigations on different parts of Allium scorodoprasum L. subsp. rotundum (L.) Stearn: Phenolic components, in vitro biological, and in silico propensities. Food Res. Int. 2018, 108, 641–649. [Google Scholar] [CrossRef]
- Biernacka, B.; Dziki, D.; Kozlowska, J.; Kowalska, I.; Soluch, A. Dehydrated at different conditions and powdered leek as a concentrate of biologically active substances: Antioxidant activity and phenolic compound profile. Materials 2021, 14, 6127. [Google Scholar] [CrossRef]
- Naheed, Z.; Cheng, Z.; Wu, C.; Wen, Y.; Ding, H. Total polyphenols, total flavonoids, allicin and antioxidant capacities in garlic scape cultivars during controlled atmosphere storage. Postharvest Biol. Technol. 2017, 131, 39–45. [Google Scholar] [CrossRef]
- Ferreira, F.S.; de Oliveira, V.S.; Chavez, D.W.H.; Chaves, D.S.; Riger, C.J.; Sawaya, A.; Guizellini, G.M.; Sampaio, G.R.; Torres, E.; Saldanha, T. Bioactive compounds of parsley (Petroselinum crispum), chives (Allium schoenoprasum L.) and their mixture (Brazilian cheiro-verde) as promising antioxidant and anti-cholesterol oxidation agents in a food system. Food Res. Int. 2022, 151, 110864. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Chauhan, G.; Krishan, P.; Shri, R. Allium schoenoprasum L.: A review of phytochemistry, pharmacology and future directions. Nat. Prod. Res. 2018, 32, 2202–2216. [Google Scholar] [CrossRef] [PubMed]
- Beretta, H.V.; Bannoud, F.; Insani, M.; Berli, F.; Hirschegger, P.; Galmarini, C.R.; Cavagnaro, P.F. Relationships between bioactive compound content and the antiplatelet and antioxidant activities of six allium vegetable species. Food Technol. Biotechnol. 2017, 55, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Kadyrbayeva, G.; Zagorska, J.; Grzegorczyk, A.; Gawel-Beben, K.; Strzepek-Gomolka, M.; Ludwiczuk, A.; Czech, K.; Kumar, M.; Koch, W.; Malm, A.; et al. The phenolic compounds profile and cosmeceutical significance of two Kazakh species of onions: Allium galanthum and A. turkestanicum. Molecules 2021, 26, 5491. [Google Scholar] [CrossRef] [PubMed]
- Lachowicz, S.; Kolniak-Ostek, J.; Oszmiański, J.; Wiśniewski, R. Comparison of phenolic content and antioxidant capacity of bear garlic (Allium ursinum L.) in different maturity stages. J. Food Process. Preserv. 2017, 41, e12921. [Google Scholar] [CrossRef]
- Gouda, M.; Nassarawa, S.S.; Gupta, S.D.; Sanusi, N.I.; Nasiru, M.M. Evaluation of carbon dioxide elevation on phenolic compounds and antioxidant activity of red onion (Allium cepa L.) during postharvest storage. Plant Physiol. Biochem. 2023, 200, 107752. [Google Scholar] [CrossRef] [PubMed]
- Bernaert, N.; De Clercq, H.; Van Bockstaele, E.; De Loose, M.; Van Droogenbroeck, B. Antioxidant changes during postharvest processing and storage of leek (Allium ampeloprasum var. porrum). Postharvest Biol. Technol. 2013, 86, 8–16. [Google Scholar] [CrossRef]
- Dai, X.; Yu, H.; Zhu, L.; Yu, Z. S-alk(en)ylcysteine sulfoxides biosynthesis and free amino acids profile in different parts of postharvest chive (Allium schoenoprasum L.). Sci. Hortic. 2022, 303, 111191. [Google Scholar] [CrossRef]
- Dai, X.; Yu, Z. Transcriptome analysis reveals the genes involved in S-alk(en)ylcysteine sulfoxide biosynthesis and its biosynthetic location in postharvest chive (Allium schoenoprasum L.). Food Res. Int. 2022, 158, 111548. [Google Scholar] [CrossRef]
- Beato, V.M.; Orgaz, F.; Mansilla, F.; Montano, A. Changes in phenolic compounds in garlic (Allium sativum L.) owing to the cultivar and location of growth. Plant Foods Hum. Nutr. 2011, 66, 218–223. [Google Scholar] [CrossRef]
- Kampfenkel, K.; Vanmontagu, M.; Inze, D. Extraction and determination of ascorbate and dehydroascorbate from plant tissue. Anal. Biochem. 1995, 225, 165–167. [Google Scholar] [CrossRef]
- Olivares, D.; Contreras, C.; Munoz, V.; Rivera, S.; Gonzalez-Aguero, M.; Retamales, J.; Defilippi, B.G. Relationship among color development, anthocyanin and pigment-related gene expression in ‘Crimson Seedless’ grapes treated with abscisic acid and sucrose. Plant Physiol. Biochem. 2017, 115, 286–297. [Google Scholar] [CrossRef] [PubMed]
- Emir, C.; Emir, A. Phytochemical analyses with LC-MS/MS and in vitro enzyme inhibitory activities of an endemic species “Allium stylosum O. Schwarz” (Amaryllidaceae). S. Afr. J. Bot. 2021, 136, 70–75. [Google Scholar] [CrossRef]
- Emir, A.; Emir, C.; Yildirim, H. Characterization of phenolic profile by LC-ESI-MS/MS and enzyme inhibitory activities of two wild edible garlic: Allium nigrum L. and Allium subhirsutum L. J. Food Biochem. 2020, 44, e13165. [Google Scholar] [CrossRef]
- Cecchi, L.; Ieri, F.; Vignolini, P.; Mulinacci, N.; Romani, A. Characterization of volatile and flavonoid composition of different cuts of dried onion (Allium cepa L.) by HS-SPME-GC-MS, HS-SPME-GCxGC-TOF and HPLC-DAD. Molecules 2020, 25, 408. [Google Scholar] [CrossRef]
- Castillejo, N.; Martínez-Zamora, L.; Gómez, P.A.; Pennisi, G.; Crepaldi, A.; Fernández, J.A.; Orsini, F.; Artés-Hernández, F. Postharvest yellow LED lighting affects phenolics and glucosinolates biosynthesis in broccoli sprouts. J. Food Compos. Anal. 2021, 103, 104101. [Google Scholar] [CrossRef]
- Zhou, D.; Sun, R.; Zhu, W.; Shi, Y.; Ni, S.; Wu, C.; Li, T. Impact of dielectric barrier discharge cold plasma on the quality and phenolic metabolism in blueberries based on metabonomic analysis. Postharvest Biol. Technol. 2023, 197, 112208. [Google Scholar] [CrossRef]
- Zhou, D.; Liu, Q.; Peng, J.; Tu, S.; Pan, L.; Tu, K. Metabolic analysis of phenolic profiles reveals the enhancements of anthocyanins and procyanidins in postharvest peach as affected by hot air and ultraviolet C. Postharvest Biol. Technol. 2020, 167, 111227. [Google Scholar] [CrossRef]
- Metsämuuronen, S.; Sirén, H. Bioactive phenolic compounds, metabolism and properties: A review on valuable chemical compounds in Scots pine and Norway spruce. Phytochem. Rev. 2019, 18, 623–664. [Google Scholar] [CrossRef]
- Carmona, L.; Alquezar, B.; Marques, V.V.; Pena, L. Anthocyanin biosynthesis and accumulation in blood oranges during postharvest storage at different low temperatures. Food Chem. 2017, 237, 7–14. [Google Scholar] [CrossRef]
- Hong, H.T.; Phan, A.D.T.; O’Hare, T.J. Temperature and maturity stages affect anthocyanin development and phenolic and sugar content of purple-pericarp supersweet sweetcorn during storage. J. Agric. Food Chem. 2021, 69, 922–931. [Google Scholar] [CrossRef] [PubMed]
- Vithana, M.D.K.; Singh, Z.; Johnson, S.K. Cold storage temperatures and durations affect the concentrations of lupeol, mangiferin, phenolic acids and other health-promoting compounds in the pulp and peel of ripe mango fruit. Postharvest Biol. Technol. 2018, 139, 91–98. [Google Scholar] [CrossRef]
- Zheng, X.; Jiang, H.; Silvy, E.M.; Zhao, S.; Chai, X.; Wang, B.; Li, Z.; Bi, Y.; Prusky, D. Candida oleophila proliferated and accelerated accumulation of suberin poly phenolic and lignin at wound sites of potato tubers. Foods 2021, 10, 1286. [Google Scholar] [CrossRef] [PubMed]
- Tsaniklidis, G.; Kafkaletou, M.; Delis, C.; Tsantili, E. The effect of postharvest storage temperature on sweet cherry (Prunus avium L.) phenolic metabolism and colour development. Sci. Hortic. 2017, 225, 751–756. [Google Scholar] [CrossRef]
- Dai, X.; Lu, Y.; Yang, Y.; Yu, Z. 1-Methylcyclopropene preserves the quality of chive (Allium schoenoprasum L.) by enhancing its antioxidant capacities and organosulfur profile during storage. Foods 2021, 10, 1792. [Google Scholar] [CrossRef]
- Tao, X.; Wu, Q.; Li, J.; Huang, S.; Cai, L.; Mao, L.; Luo, Z.; Li, L.; Ying, T. Exogenous methyl jasmonate regulates phenolic compounds biosynthesis during postharvest tomato ripening. Postharvest Biol. Technol. 2022, 184, 111760. [Google Scholar] [CrossRef]
- Liu, Q.; Luo, L.; Zheng, L. Lignins: Biosynthesis and biological functions in plants. Int. J. Mol. Sci. 2018, 19, 335. [Google Scholar] [CrossRef]
- Rocchetti, G.; Zhang, L.; Bocchi, S.; Giuberti, G.; Ak, G.; Elbasan, F.; Yildiztugay, E.; Ceylan, R.; Picot-Allain, M.C.N.; Mahomoodally, M.F.; et al. The functional potential of nine Allium species related to their untargeted phytochemical characterization, antioxidant capacity and enzyme inhibitory ability. Food Chem. 2022, 368, 130782. [Google Scholar] [CrossRef]
- Kantakhoo, J.; Ose, K.; Imahori, Y. Effects of hot water treatment to alleviate chilling injury and enhance phenolic metabolism in eggplant fruit during low temperature storage. Sci. Hortic. 2022, 304, 111325. [Google Scholar] [CrossRef]
- Younes, N.A.; Anik, T.R.; Rahman, M.M.; Wardany, A.A.; Dawood, M.F.A.; Tran, L.P.; Abdel Latef, A.A.H.; Mostofa, M.G. Effects of microbial biostimulants (Trichoderma album and Bacillus megaterium) on growth, quality attributes, and yield of onion under field conditions. Heliyon 2023, 9, e14203. [Google Scholar] [CrossRef] [PubMed]
- Younes, N.A.; Rahman, M.M.; Wardany, A.A.; Dawood, M.F.A.; Mostofa, M.G.; Keya, S.S.; Abdel Latef, A.A.H.; Tran, L.P. Antioxidants and bioactive compounds in licorice root extract potentially contribute to improving growth, bulb quality and yield of onion (Allium cepa). Molecules 2021, 26, 2633. [Google Scholar] [CrossRef] [PubMed]
- Emir, A.; Emir, C. Chemical profiles and biological properties of methanol extracts of Allium pallens L. from different localities in Turkey. Arch. Biol. Sci. 2020, 72, 193–201. [Google Scholar] [CrossRef]




 means a slight change. The thickness of the arrows indicates the extent of the change.
means a slight change. The thickness of the arrows indicates the extent of the change.  means inhibition by a low temperature.
means inhibition by a low temperature.
 means a slight change. The thickness of the arrows indicates the extent of the change.
means a slight change. The thickness of the arrows indicates the extent of the change.  means inhibition by a low temperature.
means inhibition by a low temperature.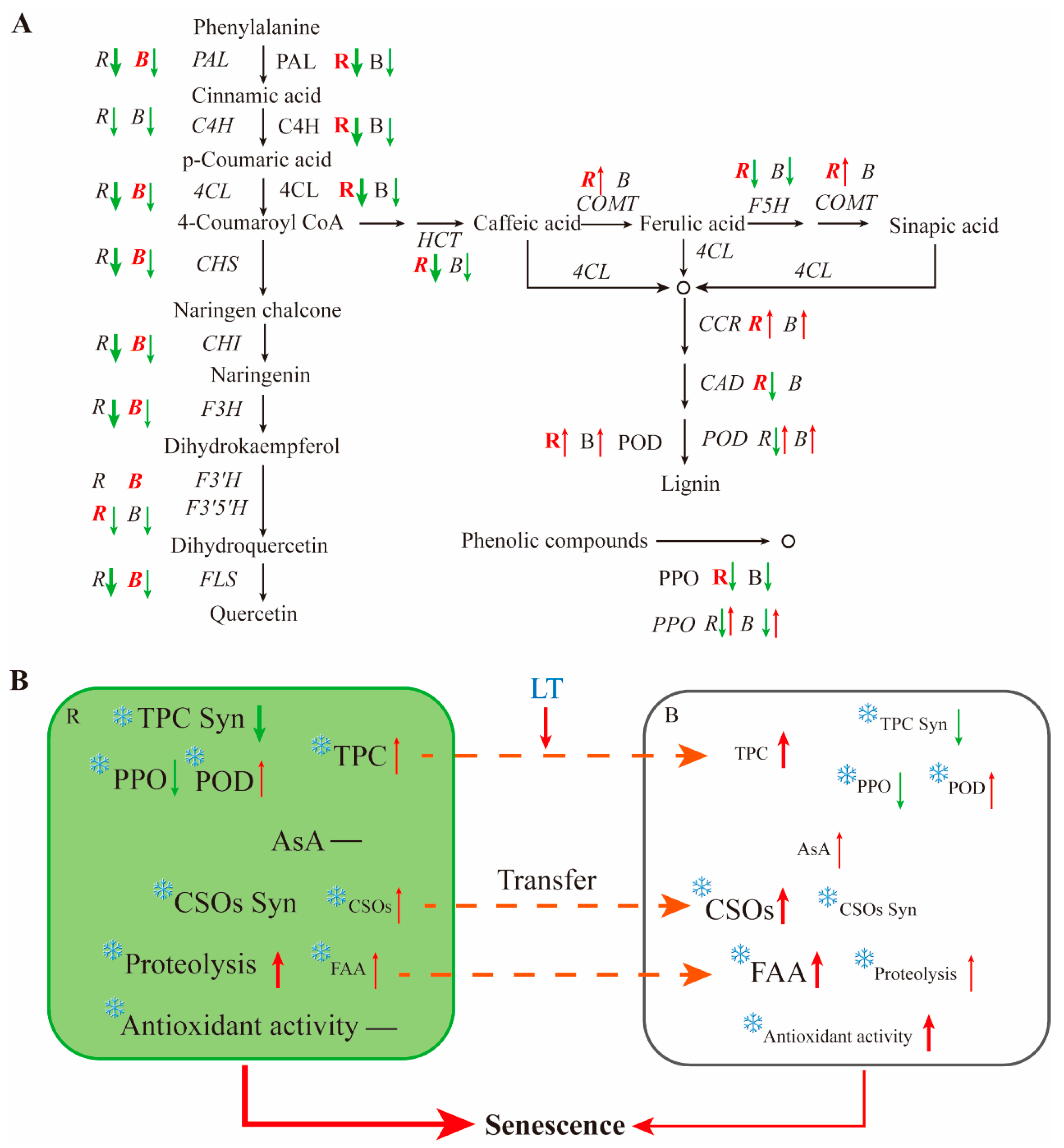
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, X.; Jia, C.; Lu, J.; Yu, Z. Metabolism of Phenolic Compounds and Antioxidant Activity in Different Tissue Parts of Post-Harvest Chive (Allium schoenoprasum L.). Antioxidants 2024, 13, 279. https://doi.org/10.3390/antiox13030279
Dai X, Jia C, Lu J, Yu Z. Metabolism of Phenolic Compounds and Antioxidant Activity in Different Tissue Parts of Post-Harvest Chive (Allium schoenoprasum L.). Antioxidants. 2024; 13(3):279. https://doi.org/10.3390/antiox13030279
Chicago/Turabian StyleDai, Xiaomei, Chonglei Jia, Jiaqi Lu, and Zhifang Yu. 2024. "Metabolism of Phenolic Compounds and Antioxidant Activity in Different Tissue Parts of Post-Harvest Chive (Allium schoenoprasum L.)" Antioxidants 13, no. 3: 279. https://doi.org/10.3390/antiox13030279
APA StyleDai, X., Jia, C., Lu, J., & Yu, Z. (2024). Metabolism of Phenolic Compounds and Antioxidant Activity in Different Tissue Parts of Post-Harvest Chive (Allium schoenoprasum L.). Antioxidants, 13(3), 279. https://doi.org/10.3390/antiox13030279






