Antioxidative Status and Meat Quality Traits as Affected by Dietary Supplementation of Finishing Pigs with Natural Phenolics
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Animal Handling, Experimental Design, Diets and Sampling
2.3. Ethical Statement
2.4. Feed Analyses
2.5. Antioxidant Activity and Phenolic Compound Content of the Supplement
2.6. Meat Quality Determination
2.6.1. Basic Chemical Composition
2.6.2. Water Holding Capacity
2.6.3. Drip Loss
2.6.4. Cooking Loss
2.6.5. Cutting Force (Texture)
2.6.6. pH Measurements
2.6.7. Marbling Score
2.6.8. Color Analysis
2.6.9. Determination of Surface Myoglobins
2.6.10. Determination of Total Myoglobin (Mb)
2.6.11. Thiobarbituric Acid Reagent Substances (TBARS)
2.6.12. Antioxidative Status (DPPH Antioxidant Activity, Enzyme Activity and Total Glutathione)
2.6.13. Sensory Analysis
2.7. Antioxidative Status of Blood and Liver Tissues
2.7.1. Determination of Enzyme Activities and Total Glutathione
2.7.2. Lipid Peroxidation
2.8. Statistical Analysis
3. Results
3.1. The Antioxidant Activity and Phenolic Content of the Supplement
3.2. Fresh Meat Quality Attributes
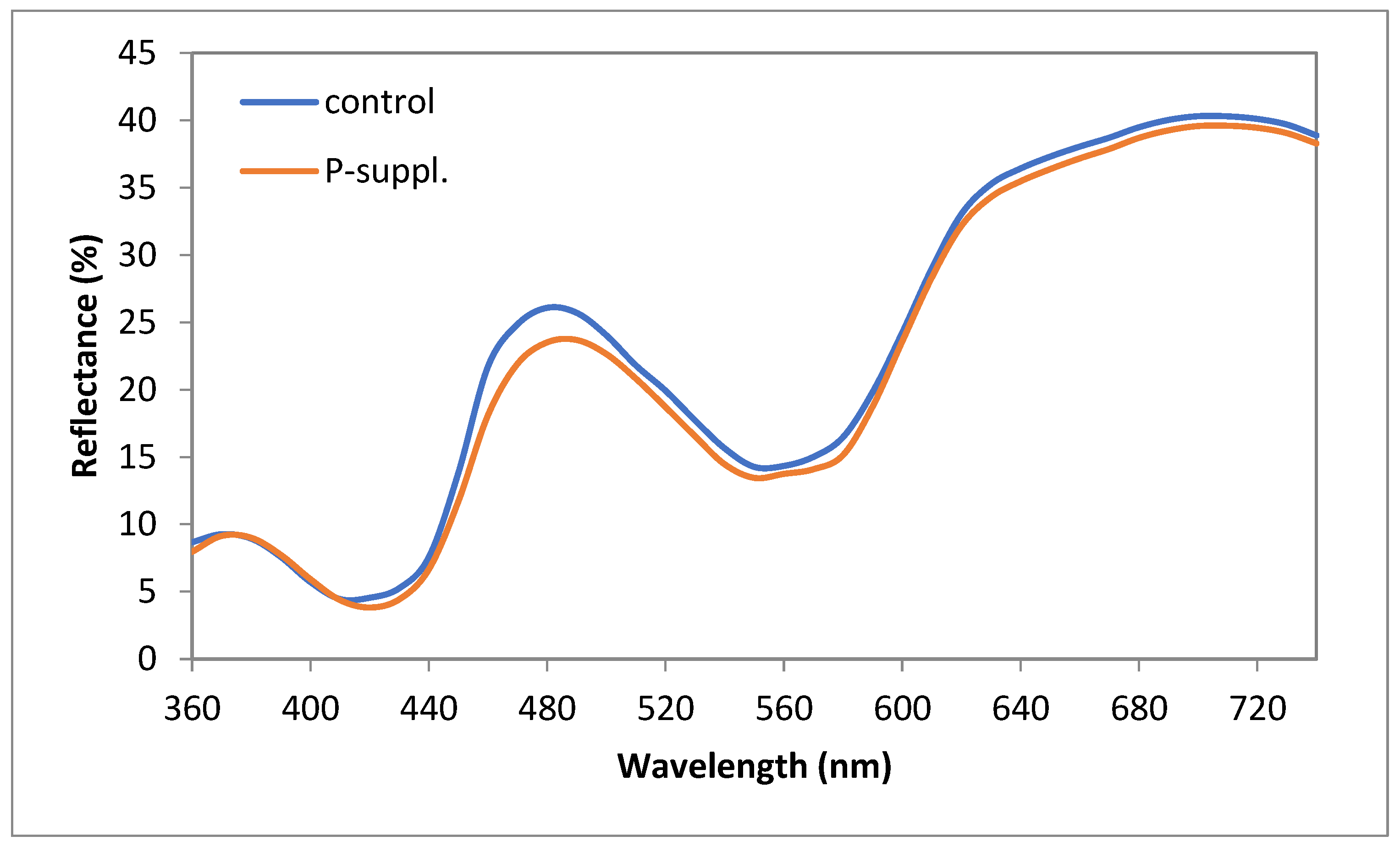
3.3. Color Profile of Fresh Meat
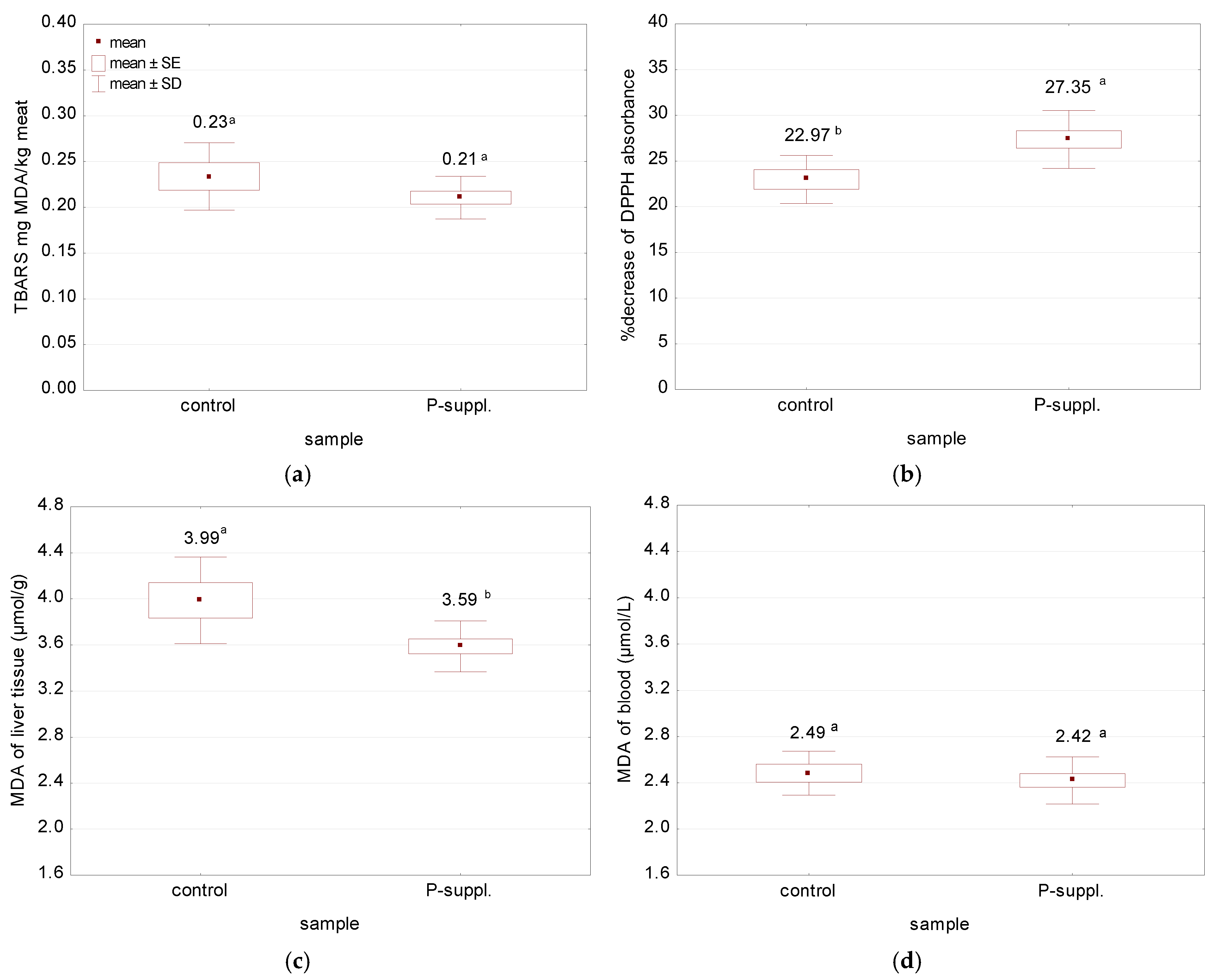
3.4. Oxidative Stability Parameters of Fresh Meat
3.5. Sensory Analysis of Fresh Meat
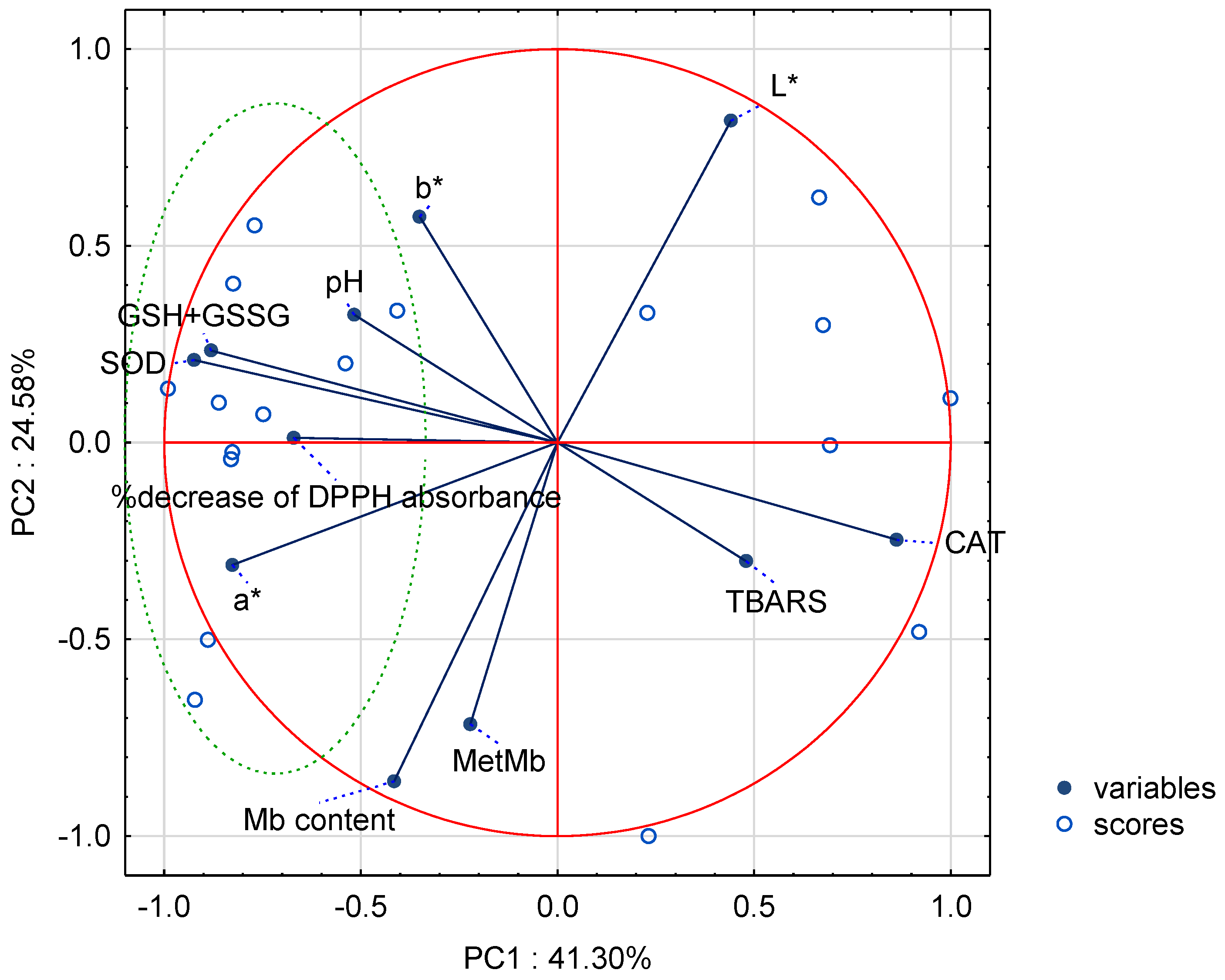
3.6. Multivariate Analysis of Fresh Meat Quality Parameters
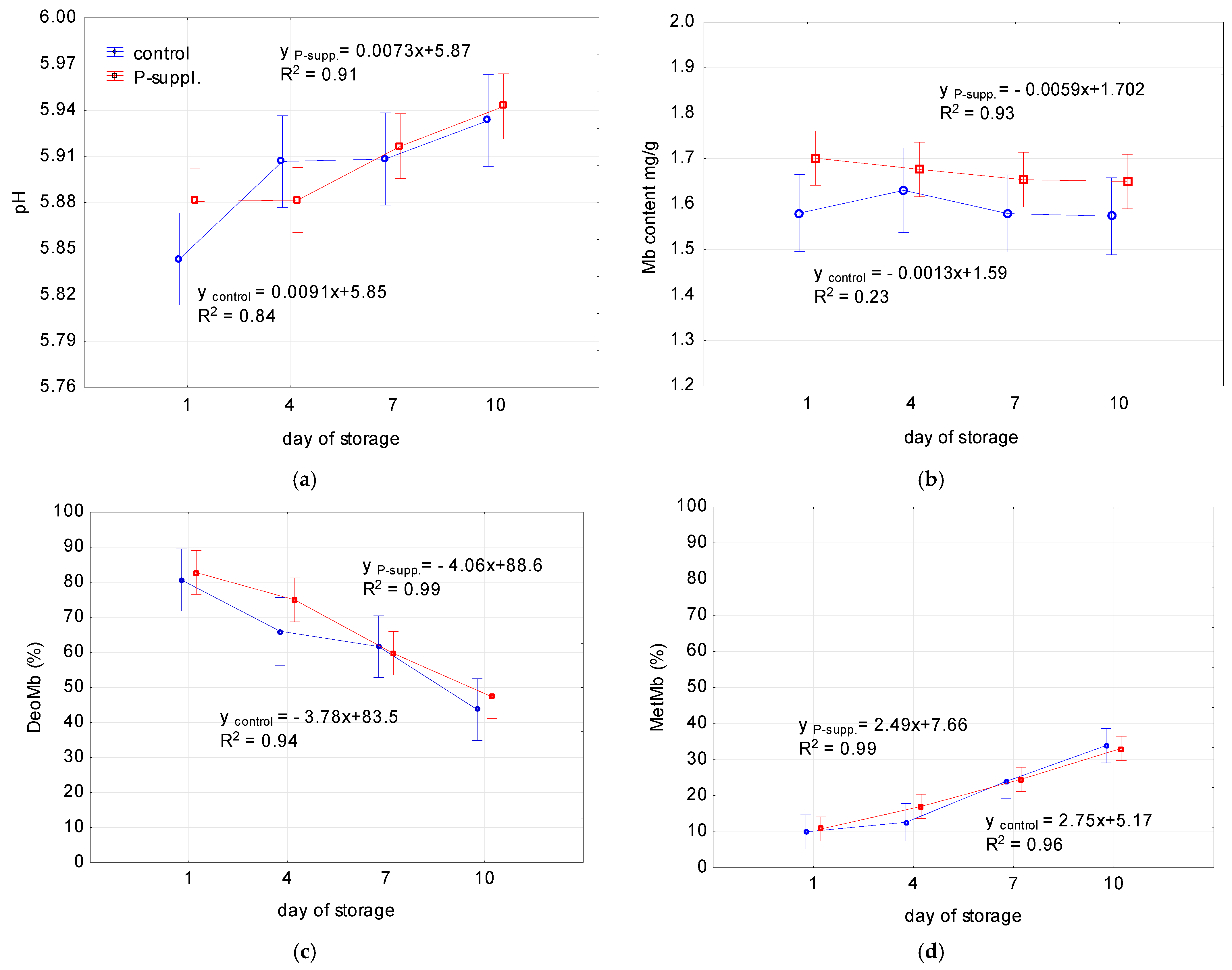
3.7. Effect of Storage Time on Meat Quality
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- OECD-FAO. OECD-FAO Agricultural Outlook 2023–2032; OECD: Paris, France; FAO: Rome, Italy, 2023; ISBN 9789264619333. [Google Scholar]
- Muzolf-Panek, M.; Kaczmarek, A.; Tomaszewska-Gras, J.; Cegielska-Radziejewska, R.; Majcher, M. Oxidative and microbiological stability of raw ground pork during chilled storage as affected by Plant extracts. Int. J. Food Prop. 2019, 22, 111–129. [Google Scholar] [CrossRef]
- Faustman, C.; Sun, Q.; Mancini, R.; Suman, S.P. Myoglobin and lipid oxidation interactions: Mechanistic bases and control. Meat Sci. 2010, 86, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Muzolf-Panek, M.; Waśkiewicz, A.; Kowalski, R.; Konieczny, P. The effect of blueberries on the oxidative stability of pork meatloaf during chilled storage. J. Food Process. Preserv. 2016, 40, 899–909. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, J.; Guo, X. Effects of antimicrobial and antioxidant activities of spice extracts on raw chicken meat quality. Food Sci. Hum. Wellness 2016, 5, 39–48. [Google Scholar] [CrossRef]
- Kaczmarek, A.M.; Muzolf-Panek, M. Predictive modelling of TBARS changes in the intramuscular lipid fraction of raw ground pork enriched with plant extracts. J. Food Sci. Technol. 2022, 59, 1756–1768. [Google Scholar] [CrossRef]
- Muzolf-Panek, M.; Kaczmarek, A. Chemometric analysis of fatty acid composition of raw chicken, beef, and pork meat with plant extract addition during refrigerated storage. Molecules 2021, 26, 4952. [Google Scholar] [CrossRef]
- Estévez, M.; Geraert, P.A.; Liu, R.; Delgado, J.; Mercier, Y.; Zhang, W. Sulphur amino acids, muscle redox status and meat quality: More than building blocks—Invited review. Meat Sci. 2020, 163, 108087. [Google Scholar] [CrossRef]
- Carvalho, R.H.; Ida, E.I.; Madruga, M.S.; Martínez, S.L.; Shimokomaki, M.; Estévez, M. Underlying connections between the redox system imbalance, protein oxidation and impaired quality traits in pale, soft and exudative (PSE) poultry meat. Food Chem. 2017, 215, 129–137. [Google Scholar] [CrossRef]
- Estévez, M. Protein carbonyls in meat systems: A review. Meat Sci. 2011, 89, 259–279. [Google Scholar] [CrossRef]
- Zhang, T.; Fan, L.; Yu, Y. The influence of myoglobin on the color of beef during the storage. Acad. J. Agric. Life Sci. 2020, 1, 9–19. [Google Scholar] [CrossRef]
- Zhang, W.; Xiao, S.; Ahn, D.U. Protein oxidation: Basic principles and implications for meat quality. Crit. Rev. Food Sci. Nutr. 2013, 53, 1191–1201. [Google Scholar] [CrossRef] [PubMed]
- Lund, M.N.; Heinonen, M.; Baron, C.P.; Estévez, M. Protein oxidation in muscle foods: A review. Mol. Nutr. Food Res. 2011, 55, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Ertbjerg, P. Effects of protein oxidation on the texture and water-holding of meat: A review. Crit. Rev. Food Sci. Nutr. 2019, 59, 3564–3578. [Google Scholar] [CrossRef] [PubMed]
- Hellwig, M. The Chemistry of Protein Oxidation in Food. Angew. Chemie Int. Ed. 2019, 58, 16742–16763. [Google Scholar] [CrossRef]
- Ge, Y.; Lin, S.; Li, B.; Yang, Y.; Tang, X.; Shi, Y.; Sun, J.; Le, G. Oxidized pork induces oxidative stress and inflammation by altering gut microbiota in mice. Mol. Nutr. Food Res. 2020, 64, e1901012. [Google Scholar] [CrossRef]
- Wang, Z.; He, Z.; Gan, X.; Li, H. Interrelationship among ferrous myoglobin, lipid and protein oxidations in rabbit meat during refrigerated and superchilled storage. Meat Sci. 2018, 146, 131–139. [Google Scholar] [CrossRef]
- Gagaoua, M.; Suman, S.P.; Purslow, P.P.; Lebret, B. The color of fresh pork: Consumers expectations, underlying farm-to-fork factors, myoglobin chemistry and contribution of proteomics to decipher the biochemical mechanisms. Meat Sci. 2023, 206, 109340. [Google Scholar] [CrossRef]
- King, D.A.; Hunt, M.C.; Barbut, S.; Claus, J.R.; Cornforth, D.P.; Joseph, P.; Kim, Y.H.; Lindahl, G.; Mancini, R.A.; Nair, M.N.; et al. AMSA guidlines for meat color measurement. Meat Muscle Biol. 2023, 6, 1–81. [Google Scholar] [CrossRef]
- Mancini, R.A.; Hunt, M.C. Current research in meat color. Meat Sci. 2005, 71, 100–121. [Google Scholar] [CrossRef]
- Domínguez, R.; Pateiro, M.; Gagaoua, M.; Barba, F.J.; Zhang, W.; Lorenzo, J.M. A comprehensive review on lipid oxidation in meat and meat products. Antioxidants 2019, 8, 429. [Google Scholar] [CrossRef]
- Ribeiro, J.S.; Santos, M.J.M.C.; Silva, L.K.R.; Pereira, L.C.L.; Santos, I.A.; da Silva Lannes, S.C.; da Silva, M.V. Natural antioxidants used in meat products: A brief review. Meat Sci. 2019, 148, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.H.; Kim, Y.J.; Park, Y.J.; Shin, D.M.; Choi, Y.S.; Lee, N.K.; Paik, H.D. Application of mixed natural preservatives to improve the quality of vacuum skin packaged beef during refrigerated storage. Meat Sci. 2020, 169, 108219. [Google Scholar] [CrossRef] [PubMed]
- Muzolf-Panek, M.; Stuper-Szablewska, K. Comprehensive study on the antioxidant capacity and phenolic profiles of black seed and other spices and herbs: Effect of solvent and time of extraction. J. Food Meas. Charact. 2021, 15, 4561–4574. [Google Scholar] [CrossRef]
- Oswell, N.J.; Thippareddi, H.; Pegg, R.B. Practical use of natural antioxidants in meat products in the U.S.: A review. Meat Sci. 2018, 145, 469–479. [Google Scholar] [CrossRef]
- Haugaard, P.; Hansen, F.; Jensen, M.; Grunert, K.G. Consumer attitudes toward new technique for preserving organic meat using herbs and berries. Meat Sci. 2014, 96, 126–135. [Google Scholar] [CrossRef]
- Martínez, L.; Ros, G.; Nieto, G. Effect of natural extracts obtained from food industry by-products on nutritional quality and shelf life of chicken nuggets enriched with organic Zn and Se provided in broiler diet. Poult. Sci. 2020, 99, 1491–1501. [Google Scholar] [CrossRef]
- Zahid, M.A.; Choi, J.Y.; Seo, J.K.; Parvin, R.; Ko, J.; Yang, H.S. Effects of clove extract on oxidative stability and sensory attributes in cooked beef patties at refrigerated storage. Meat Sci. 2020, 161, 107972. [Google Scholar] [CrossRef]
- Yang, X.Y.; Xu, B.C.; Lei, H.M.; Xin, L.U.O.; Zhu, L.X.; Zhang, Y.M.; Mao, Y.W.; Liang, R.R. Effects of grape seed extract on meat color and premature browning of meat patties in high-oxygen packaging. J. Integr. Agric. 2022, 21, 2445–2455. [Google Scholar] [CrossRef]
- Scerra, M.; Foti, F.; Caparra, P.; Cilione, C.; Rao, R.; Priolo, A.; Natalello, A.; Luciano, G.; Chies, L. Effect of feeding pigs with bergamot by-product on fatty acid composition and oxidative stability of meat and salami. Meat Sci. 2022, 183, 108662. [Google Scholar] [CrossRef]
- Xu, M.; Chen, X.; Huang, Z.; Chen, D.; Li, M.; He, J.; Chen, H.; Zheng, P.; Yu, J.; Luo, Y.; et al. Effects of dietary grape seed proanthocyanidin extract supplementation on meat quality, muscle fiber characteristics and antioxidant capacity of finishing pigs. Food Chem. 2022, 367, 130781. [Google Scholar] [CrossRef]
- Kafantaris, I.; Stagos, D.; Kotsampasi, B.; Hatzis, A.; Kypriotakis, A.; Gerasopoulos, K.; Makri, S.; Goutzourelas, N.; Mitsagga, C.; Giavasis, I.; et al. Grape pomace improves performance, antioxidant status, fecal microbiota and meat quality of piglets. Animal 2018, 12, 246–255. [Google Scholar] [CrossRef] [PubMed]
- Biondi, L.; Luciano, G.; Cutello, D.; Natalello, A.; Mattioli, S.; Priolo, A.; Lanza, M.; Morbidini, L.; Gallo, A.; Valenti, B. Meat quality from pigs fed tomato processing waste. Meat Sci. 2020, 159, 107940. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Jiang, J.; Yu, J.; Mao, X.; Yu, B.; Chen, D. Effect of dietary supplementation with mulberry (Morus alba L.) leaves on the growth performance, meat quality and antioxidative capacity of finishing pigs. J. Integr. Agric. 2019, 18, 143–151. [Google Scholar] [CrossRef]
- Cheng, C.; Liu, Z.; Zhou, Y.; Wei, H.; Zhang, X.; Xia, M.; Deng, Z.; Zou, Y.; Jiang, S.; Peng, J. Effect of oregano essential oil supplementation to a reduced-protein, amino acid-supplemented diet on meat quality, fatty acid composition, and oxidative stability of Longissimus thoracis muscle in growing-finishing pigs. Meat Sci. 2017, 133, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Bešlo, D.; Golubić, N.; Rastija, V.; Agić, D.; Karnaš, M.; Šubarić, D.; Lučić, B. Antioxidant Activity, Metabolism, and Bioavailability of Polyphenols in the Diet of Animals. Antioxidants 2023, 12, 1141. [Google Scholar] [CrossRef]
- O’Grady, M.N.; Carpenter, R.; Lynch, P.B.; O’Brien, N.M.; Kerry, J.P. Addition of grape seed extract and bearberry to porcine diets: Influence on quality attributes of raw and cooked pork. Meat Sci. 2008, 78, 438–446. [Google Scholar] [CrossRef]
- Menci, R.; Biondi, L.; Natalello, A.; Lanza, M.; Priolo, A.; Valenti, B.; Bertino, A.; Scerra, M.; Luciano, G. Feeding hazelnut skin to lambs delays lipid oxidation in meat. Meat Sci. 2023, 202, 109218. [Google Scholar] [CrossRef]
- Ponnampalam, E.N.; Kiani, A.; Santhiravel, S.; Holman, B.W.B.; Lauridsen, C.; Dunshea, F.R. The importance of dietary antioxidants on oxidative stress, meat and milk production, and their preservative aspects in farm animals: Antioxidant action, animal health, and product quality—Invited review. Animals 2022, 12, 3279. [Google Scholar] [CrossRef]
- Ghosh, N.; Chatterjee, S.; Sil, P.C. Chapter1.1—Evolution of antioxidants over times (including current global market and trend). In Antioxidants Effects in Health; Nabavi, S.M., Silva, A.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 3–32. ISBN 978-0-12-819096-8. [Google Scholar]
- Dal Bosco, A.; Mourvaki, E.; Cardinali, R.; Servili, M.; Sebastiani, B.; Ruggeri, S.; Mattioli, S.; Taticchi, A.; Esposto, S.; Castellini, C. Effect of dietary supplementation with olive pomaces on the performance and meat quality of growing rabbits. Meat Sci. 2012, 92, 783–788. [Google Scholar] [CrossRef]
- Deschepper, K.; Carles, G.M. Use of a Composition for Improving Slaughtering and/or Meat Quality Parameters. Patent No. WO2020254391A1, 24 December 2020. [Google Scholar]
- Flachowsky, G.; Gesellschaft für Ernährungsphysiologie Ausschuss für Bedarfsnormen. Recommendations for the Supply of Energy and Nutrients to Pigs; Staudacher, W., Ed.; Energy and Nutrient Requirements of Livestock; DLG-Verlag: Frankfurt am Main, Germany, 2008; ISBN 9783769007077. [Google Scholar]
- Animal Feed. Official Methods of Analysis of Association of Official Analytical Chemists; AOAC International: Gaithersburg, MD, USA, 2007. [Google Scholar]
- PN ISO 6449:1999; Animal Feeding Stuffs—Determination of Moisture and Other Volatile Matter Content. Polish Committee for Standarization: Warsaw, Poland, 1999.
- Szkudzińska, K.; Smutniak, I.; Rubaj, J.; Korol, W.; Bielecka, G. Method validation for determination of amino acids in feed by UPLC. Accredit. Qual. Assur. 2017, 22, 247–252. [Google Scholar] [CrossRef]
- Sánchez-Moreno, C.; Larrauri, J.A.; Saura-Calixto, F. A procedure to measure the antiradical efficiency of polyphenols. J. Sci. Food Agric. 1998, 270, 270–276. [Google Scholar] [CrossRef]
- Miller, N.J.; Rice-Evans, C.; Davies, M.; Gopinathan, V.; Milner, A. A novel method for measuring antioxidant capacity and its application to monitoring the antioxidant status in premature neonates. Clin. Sci. 1993, 84, 407. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “‘antioxidant power’”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Meda, A.; Lamien, C.E.; Romito, M.; Millogo, J.; Nacoulma, O.G. Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey, as well as their radical scavenging activity. Food Chem. 2005, 91, 571–577. [Google Scholar] [CrossRef]
- PN ISO 1442:2000; Meat and Meat Products—Determination of Moisture Content (Reference Method). Polish Committee for Standarization: Warsaw, Poland, 2000.
- PN ISO 1444:2000; Meat and Meat Products—Determinationof Free Fat Content. Polish committee for Standarization: Warsaw, Poland, 2000.
- PN 75/A-04018; Agricultural and Food Products: Determination of Nitrogen by the Kjeldalh Method and Conversion to Protein. Polish Committee for Standarization: Warsaw, Poland, 1975.
- Grau, R.; Hamm, R. Determination of water binding in meats by the pressing method. Fleischwirtschaft 1956, 8, 733–736. [Google Scholar]
- Pohja, N.; Niinivaara, F. Die Bestimmung der Wasserbindung des Fleisches mittels des Konstantdruck Methode. Fleischwirtschaft 1957, 9, 193–195. [Google Scholar]
- Baryłko-Pikielna, N.; Kossakowska, T.; Baldwin, Z. Wybór optymalnej metody przygotowania mięsa wołowego i wieprzowego do oceny sensorycznej [Choosing the right method to prepare beef and pork for sensory analysis]. Rocz. Inst. Przem. Mięsnago 1964, 1, 111–132. [Google Scholar]
- Krzywicki, K. Assessment of relative content of myoglobin, oxymyoglobin and metmyoglobin at the surface of beef. Meat Sci. 1979, 3, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Trout, G.R. Variation in myoglobin denaturation and color of cooked beef, pork, and turkey meat as influenced by pH, sodium chloride, sodium tripolyphosphate, and cooking temperature. J. Food Sci. 1989, 54, 536–540. [Google Scholar] [CrossRef]
- Mielnik, M.B.; Olsen, E.; Vogt, G.; Adeline, D.; Skrede, G. Grape seed extract as antioxidant in cooked, cold stored turkey meat. LWT Food Sci. Technol. 2006, 39, 191–198. [Google Scholar] [CrossRef]
- Muzolf-Panek, M.; Kaczmarek, A.; Tomaszewska-Gras, J.; Cegielska-Radziejewska, R.; Szablewski, T.; Majcher, M.; Stuper-Szablewska, K. A chemometric approach to oxidative stability and physicochemical quality of raw ground chicken meat affected by black seed and other spice extracts. Antioxidants 2020, 9, 903. [Google Scholar] [CrossRef] [PubMed]
- Hodges, D.M.; DeLong, J.M.; Forney, C.F.; Prange, R.K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Fasseas, M.K.; Mountzouris, K.C.; Tarantilis, P.A.; Polissiou, M.; Zervas, G. Antioxidant activity in meat treated with oregano and sage essential oils. Food Chem. 2008, 106, 1188–1194. [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Aebi, H. Oxygen Radicals in Biological Systems—Catalase In Vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar]
- Jollow, D.J.; Mitchell, J.R.; Zampaglione, N.; Gillette, J.R. Bromobenzene-induced liver necrosis. Protective role of glutathione and evidence for 3,4-bromobenzene oxide as the hepatotoxic metabolite. Pharmacology 1974, 11, 151–169. [Google Scholar] [CrossRef]
- PN ISO 4121:2013; Sensory Analysis—Methodology—Evaluation of Food Products by Methods Using Scales. Polish Committee for Standarization: Warsaw, Poland, 2013.
- ISO 6658:2017; Sensory Analysis—Methodology—Method of Investigating Sensitivity of Taste. International Organization for Standardization: Geneva, Switzerland, 2017.
- ISO 8586:2023; Sensory Analysis-Selection and Training of Sensory Assessors. International Organization for Standardization: Geneva, Switzerland, 2023.
- American Meat Science Association (AMSA). Research Guidelines for Cookery, Sensory Evaluation, and Instrumental Tenderness Measurements of Meat, 2nd ed.; American Meat Science Association: Champaign, IL, USA, 2015; ISBN 8005172672. [Google Scholar]
- Esterbauer, H.; Cheeseman, K.H. Determination of aldehydic lipid peroxidation products malonaldehyde and 4-hydroxynonenal. Methods Enzymol. 1990, 186, 407–421. [Google Scholar]
- Kim, H.; Kim, J.; Yan, F.; Cheng, H.; Brad Kim, Y.H. Effects of heat stress and probiotic supplementation on protein functionality and oxidative stability of ground chicken leg meat during display storage. J. Sci. Food Agric. 2017, 97, 5343–5351. [Google Scholar] [CrossRef] [PubMed]
- Cannata, S.; Engle, T.E.; Moeller, S.J.; Zerby, H.N.; Radunz, A.E.; Green, M.D.; Bass, P.D.; Belk, K.E. Effect of visual marbling on sensory properties and quality traits of pork loin. Meat Sci. 2010, 85, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Kalt, W.; Blumberg, J.B.; McDonald, J.E.; Vinqvist-Tymchuk, M.R.; Fillmore, S.A.E.; Graf, B.A.; O’leary, J.M.; Milbury, P.E. Identification of anthocyanins in the liver, eye, and brain of blueberry-fed pigs. J. Agric. Food Chem. 2008, 56, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Karamucki, T.; Jakubowska, M.; Rybarczyk, A.; Gardzielewska, J. The influence of myoglobin on the colour of minced pork loin. Meat Sci. 2013, 94, 234–238. [Google Scholar] [CrossRef]
- Fernández-López, J.; Pérez-Alvarez, J.A.; Sayas-Barberá, E.; Aranda-Catalá, V. Characterization of different states of myoglobin in pork using color parameters and reflectance ratios. J. Muscle Foods 2000, 11, 157–167. [Google Scholar] [CrossRef]
- Zareian, M.; Tybussek, T.; Silcock, P.; Bremer, P.; Beauchamp, J.; Böhner, N. Interrelationship among myoglobin forms, lipid oxidation and protein carbonyls in minced pork packaged under modified atmosphere. Food Packag. Shelf Life 2019, 20, 100311. [Google Scholar] [CrossRef]
- Muzolf-Panek, M.; Gliszczyńska-Świgło, A.; De Haan, L.; Aarts, J.M.M.J.G.; Szymusiak, H.; Vervoort, J.M.; Tyrakowska, B.; Rietjens, I.M.C.M. Role of catechin quinones in the induction of EpRE-mediated gene expression. Chem. Res. Toxicol. 2008, 21, 2352–2360. [Google Scholar] [CrossRef]


| Item | Grower | Finisher | |
|---|---|---|---|
| Control and P-Suppl. 1 | Control | P-Suppl. | |
| Composition (kg/ton) | |||
| Cereals and cereal grain co-products—barley, corn, wheat, wheat bran | 800.3 | 826.4 | 825.4 |
| Soybean meal and rapeseed meal | 160.0 | 140.0 | |
| Limestone | 14.7 | 12.0 | |
| Oil | 13.0 | 13.0 | |
| NaCl | 4.0 | 4.0 | |
| HCL-lysine | 3.5 | 2.4 | |
| Premix 0.2% 2 | 2.0 | 2.0 | |
| 1-calcium phosphate | 1.8 | 0.0 | |
| DL-methionine | 0.3 | 0.0 | |
| L-threonine | 0.2 | 0.0 | |
| Phytase—5000 FTU | 0.1 | 0.1 | |
| Endo-1,4-beta-xylanase—15,000 EPU | 0.1 | 0.1 | |
| P-suppl. 1 | 0.0 | 0.0 | 1.0 |
| Nutritional value (g/kg) | |||
| Crude protein | 162.1 | 148.7 | 149.1 |
| Crude fat | 36.4 | 34.1 | 33.8 |
| Crude fibre | 50.8 | 54.1 | 54.4 |
| Crude ash | 53.41 | 48.7 | 49.1 |
| Dry matter | 889.0 | 990.3 | 991.0 |
| Ca | 8.0 | 6.4 | 6.4 |
| P | 5.4 | 5.2 | 5.1 |
| Lysine | 10.2 | 8.6 | 8.6 |
| Methionine | 3.0 | 2.5 | 2.5 |
| Quality Attributes | Control | P-Suppl. | SE | p * |
|---|---|---|---|---|
| Fat (%) | 1.66 ± 0.70 | 1.66 ± 0.63 | 0.149 | 0.996 |
| Protein (%) | 24.85 ± 1.17 | 24.92 ± 0.89 | 0.226 | 0.893 |
| Water (%) | 72.53 ± 0.93 | 72.47 ± 1.11 | 0.253 | 0.905 |
| pH 45 min | 6.31 ± 0.32 | 6.50 ± 0.24 | 0.065 | 0.195 |
| pH 24 h | 5.50 ± 0.06 | 5.50 ± 0.11 | 0.022 | 0.944 |
| pH 1 | 5.84 ± 0.05 | 5.88 ± 0.06 | 0.058 | 0.204 |
| Water holding capacity (%) | 2.34 ± 0.93 | 2.34 ± 1.20 | 0.257 | 0.998 |
| Drip loss (%) | 6.00 ± 1.32 | 5.41 ± 1.71 | 0.371 | 0.471 |
| Cooking loss (%) | 32.45 ± 3.20 | 31.22 ± 5.90 | 1.199 | 0.644 |
| Cutting force (N/m2) | 183.42 ± 44.34 | 205.65 ± 68.20 | 14.35 | 0.482 |
| Marbling score | 1.50 ± 0.25 | 1.47 ± 0.32 | 0.069 | 0.829 |
| Color Parameter | Control | P-Suppl. | SE | p * |
|---|---|---|---|---|
| L* | 51.69 ± 3.03 | 50.53 ± 2.44 | 0.61 | 0.393 |
| a* | 4.52 ± 0.54 | 6.15 ± 1.16 | 0.30 | 0.005 |
| b* | 12.40 ± 1.02 | 13.07 ± 0.86 | 0.22 | 0.160 |
| h (°) | 69.91 ± 3.02 | 64.97 ± 4.15 | 1.04 | 0.020 |
| C | 13.21 ± 0.93 | 14.48 ± 1.03 | 0.27 | 0.021 |
| RI (a*/b*) | 0.37 ± 0.06 | 0.47 ± 0.09 | 0.02 | 0.021 |
| Abs 525 nm | 0.73 ± 0.07 | 0.76 ± 0.05 | 0.01 | 0.452 |
| Mb content (mg/g) | 1.58 ± 0.08 | 1.70 ± 0.07 | 0.03 | 0.012 |
| Homogenate | Oxidative Status | Control | P-Suppl. | SE | p * |
|---|---|---|---|---|---|
| blood | CAT (U/mL) | 2.46 ± 0.17 | 3.06 ± 0.17 | 0.078 | 0.000 |
| SOD (U/mL) | 14.49 ± 1.18 | 17.83 ± 1.72 | 0.525 | 0.001 | |
| GSH + GSSG (μmol/L) | 0.58 ± 0.04 | 0.68 ± 0.05 | 0.015 | 0.001 | |
| FRAP (μmol/L) | 9.70 ± 0.99 | 12.11 ± 0.83 | 0.342 | 0.000 | |
| liver | CAT (U/g) | 101.93 ± 3.82 | 111.42 ± 9.82 | 2.209 | 0.038 |
| SOD (U/g) | 122.76 ± 3.17 | 135.81 ± 3.65 | 1.693 | 0.000 | |
| GSH + GSSG (μmol/g) | 0.19 ± 0.012 | 0.23 ± 0.023 | 0.007 | 0.001 | |
| meat | CAT (U/g) | 22.34 ± 1.23 | 16.71 ± 0.92 | 0.685 | 0.000 |
| SOD (U/g) | 36.81 ± 0.80 | 43.44 ± 1.67 | 0.828 | 0.000 | |
| GSH + GSSG (nmol/g) | 239.29 ± 11.45 | 325.74 ± 13.78 | 10.329 | 0.000 |
| Sample | Days | L* | a* | b* | h (°) | C | RI (a*/b*) | Abs 525 nm |
|---|---|---|---|---|---|---|---|---|
| control | 1 | 51.69 ± 3.02 a,d | 4.52 ± 0.54 a,b | 12.40 ± 1.02 a,d,e | 69.91 ± 3.02 b,d | 13.21 ± 0.93 a,c,d | 0.37 ± 0.06 a,b | 0.73 ± 0.07 c,d |
| 4 | 52.36 ± 3.19 a–d | 5.24 ± 0.62 c–e | 12.57 ± 0.94 a–g | 67.30 ± 3.21 a,c | 13.64 ± 0.82 a,c,d | 0.42 ± 0.07 c,d | 0.71 ± 0.06 a–d | |
| 7 | 53.24 ± 2.30 b,c,e,f | 5.00 ± 0.69 c–e | 13.43 ± 0.89 c,h,i | 69.53 ± 3.52 b,d | 14.36 ± 0.71 b,e,f | 0.38 ± 0.07 a,b | 0.71 ± 0.04 a,b | |
| 10 | 53.62 ± 3.01 e,f | 5.81 ± 0.58 f–h | 13.04 ± 0.98 b–i | 65.90 ± 3.11 a,c | 14.29 ± 0.84 b,e,f | 0.45 ± 0.07 c,d | 0.70 ± 0.06 a,b | |
| P-suppl | 1 | 50.53 ± 2.44 a–c | 6.15 ± 1.16 e,h | 13.07 ± 0.86 e,g,i | 64.97 ± 4.15 a,b | 14.48 ± 1.03 d,f | 0.47 ± 0.09 b,d | 0.76 ± 0.05 b,d |
| 4 | 50.94 ± 2.39 a–c | 5.14 ± 0.95 a,c,f | 12.03 ± 0.75 a–c | 66.96 ± 4.20 c,d | 13.11 ± 0.76 a,b | 0.43 ± 0.09 a,c | 0.75 ± 0.05 a–d | |
| 7 | 51.64 ± 2.50 a–c | 5.76 ± 1.01 b,d,g | 12.23 ± 0.94 a–c | 64.87 ± 4.03 a,b | 13.55 ± 1.01 c,e | 0.47 ± 0.09 b,d | 0.74 ± 0.05 a,c | |
| 10 | 51.93 ± 2.50 d–f | 6.13 ± 0.8 e,h | 12.68 ± 0.86 d,f,h | 64.25 ± 3.36 b,d | 14.10 ± 0.86 d,f | 0.49 ± 0.07 b,d | 0.74 ± 0.05 a,c |
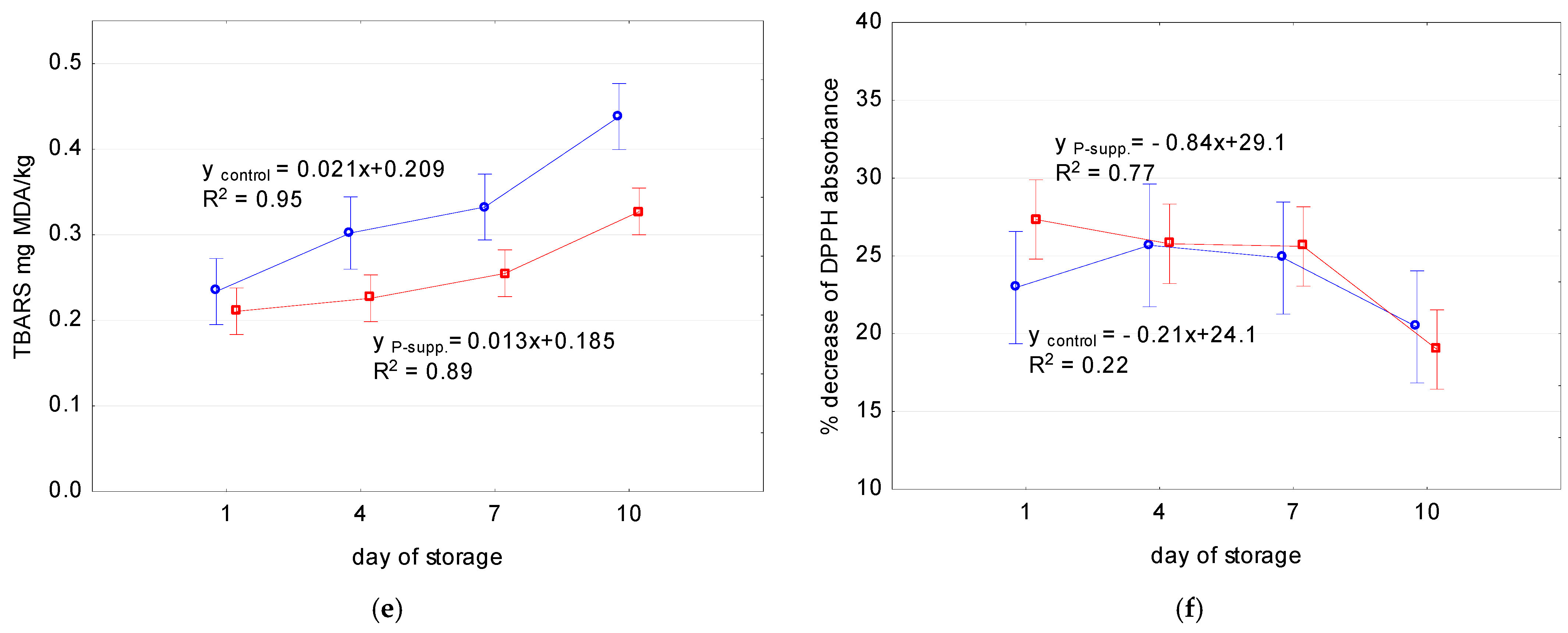
| Sample | Quality Attributes | Slope | Intercept | R2 | p (ANOVA) |
|---|---|---|---|---|---|
| control | pH | 0.0091 * | 5.85 * | 0.84 | 0.00153 |
| Mb content (mg/g) | −0.0013 | 1.59 * | 0.23 | 0.846 | |
| DeoMb | −3.78 * | 83.5 * | 0.94 | 0.000001 | |
| MetMb | 2.75 * | 5.17 * | 0.96 | 0.000001 | |
| TBARS (mg MDA/kg meat) | 0.021 * | 0.209 * | 0.95 | 0.00001 | |
| % decrease of DPPH absorbance | −0.21 | 24.13 * | 0.22 | 0.481 | |
| P-suppl. | pH | 0.0073 * | 5.87 * | 0.91 | 0.000013 |
| Mb content (mg/g) | −0.0059 | 1.702 * | 0.93 | 0.175 | |
| DeoMb | −4.06 * | 88.6 * | 0.99 | 0.000001 | |
| MetMb | 2.49 * | 7.66 * | 0.99 | 0.000001 | |
| TBARS (mg MDA/kg meat) | 0.013 * | 0.185 * | 0.89 | 0.000 | |
| % decrease of DPPH absorbance | −0.84 * | 29.1 * | 0.77 | 0.00013 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muzolf-Panek, M.; Zaworska-Zakrzewska, A.; Czech, A.; Lisiak, D.; Kasprowicz-Potocka, M. Antioxidative Status and Meat Quality Traits as Affected by Dietary Supplementation of Finishing Pigs with Natural Phenolics. Antioxidants 2024, 13, 1362. https://doi.org/10.3390/antiox13111362
Muzolf-Panek M, Zaworska-Zakrzewska A, Czech A, Lisiak D, Kasprowicz-Potocka M. Antioxidative Status and Meat Quality Traits as Affected by Dietary Supplementation of Finishing Pigs with Natural Phenolics. Antioxidants. 2024; 13(11):1362. https://doi.org/10.3390/antiox13111362
Chicago/Turabian StyleMuzolf-Panek, Małgorzata, Anita Zaworska-Zakrzewska, Anna Czech, Dariusz Lisiak, and Małgorzata Kasprowicz-Potocka. 2024. "Antioxidative Status and Meat Quality Traits as Affected by Dietary Supplementation of Finishing Pigs with Natural Phenolics" Antioxidants 13, no. 11: 1362. https://doi.org/10.3390/antiox13111362
APA StyleMuzolf-Panek, M., Zaworska-Zakrzewska, A., Czech, A., Lisiak, D., & Kasprowicz-Potocka, M. (2024). Antioxidative Status and Meat Quality Traits as Affected by Dietary Supplementation of Finishing Pigs with Natural Phenolics. Antioxidants, 13(11), 1362. https://doi.org/10.3390/antiox13111362










