Abstract
Heavy metals are often found in soil and can contaminate drinking water, posing a serious threat to human health. Molecular pathways and curation therapies for mitigating heavy metal toxicity have been studied for a long time. Recent studies on oxidative stress and aging have shown that the molecular foundation of cellular damage caused by heavy metals, namely, apoptosis, endoplasmic reticulum stress, and mitochondrial stress, share the same pathways as those involved in cellular senescence and aging. In recent aging studies, many types of heavy metal exposures have been used in both cellular and animal aging models. Chelation therapy is a traditional treatment for heavy metal toxicity. However, recently, various antioxidants have been found to be effective in treating heavy metal-induced damage, shifting the research focus to investigating the interplay between antioxidants and heavy metals. In this review, we introduce the molecular basis of heavy metal-induced cellular damage and its relationship with aging, summarize its clinical implications, and discuss antioxidants and other agents with protective effects against heavy metal damage.
1. Introduction
Heavy metals are those with a density >4.5 g/cm3 and include lead, chromium, cadmium, mercury, and arsenic [1]. Heavy metals are often found in soil and can contaminate drinking water, posing a serious threat to human health [2,3]. Heavy metals exist in natural sources such as volcanic eruption, natural deposits, or sea salt spray (lead), tectonic and hydrothermal events (chromium), dust storm or wildfire (cadmium), degassing or weathering of rock (mercury), and weathering of rock or microbial colonization (arsenic). People are exposed to heavy metals from mining, tanning, and textile dyeing (chromium), battery manufacturing (cadmium), pesticides and fertilizers (mercury), and smelting (arsenic) [4]. Heavy metal exposure damages many organs [5,6], and its effect in the prenatal stage is critical, especially during neural development [7]. Heavy metal toxicity depends on the absorbed dose, route of exposure, and duration of exposure—acute or chronic. Heavy metal toxicity can lead to various disorders and can result in excessive damage due to oxidative stress induced by free radical formation [8]. In this review, the molecular mechanisms underlying heavy metal-induced toxicity are discussed from the perspective of mitochondrial dysfunction related to oxidative stress and endoplasmic reticulum (ER) stress, including their interaction, as well as protective agents against heavy metal exposure.
2. Molecular Pathways in Heavy Metal-Induced Cytotoxicity and Relation with Aging
Heavy metals induce cytotoxicity by molecular mechanisms, including oxidative stress associated with mitochondrial dysfunction, apoptosis, necrosis, and ER stress, which are interconnected. Recent advances in systems biology and in vitro label-free proteomic approaches have revealed that metal exposure induces significant changes in protein expression during mitochondrial dysfunction, oxidative stress, ubiquitin proteome dysfunction, and mRNA splicing [9].
2.1. Oxidative Stress, Mitochondrial Stress, and Mitochondrial Dysfunction
Mitochondrial dysfunction is one of the main phenomena in heavy metal-induced cytotoxicity. Heavy metals can damage mitochondria both inside the organelle and on the organelle surface, and these mechanisms interact with each other.
2.1.1. Heavy Metals Affect Cellular Redox Homeostasis (Figure 1 and Figure 2)
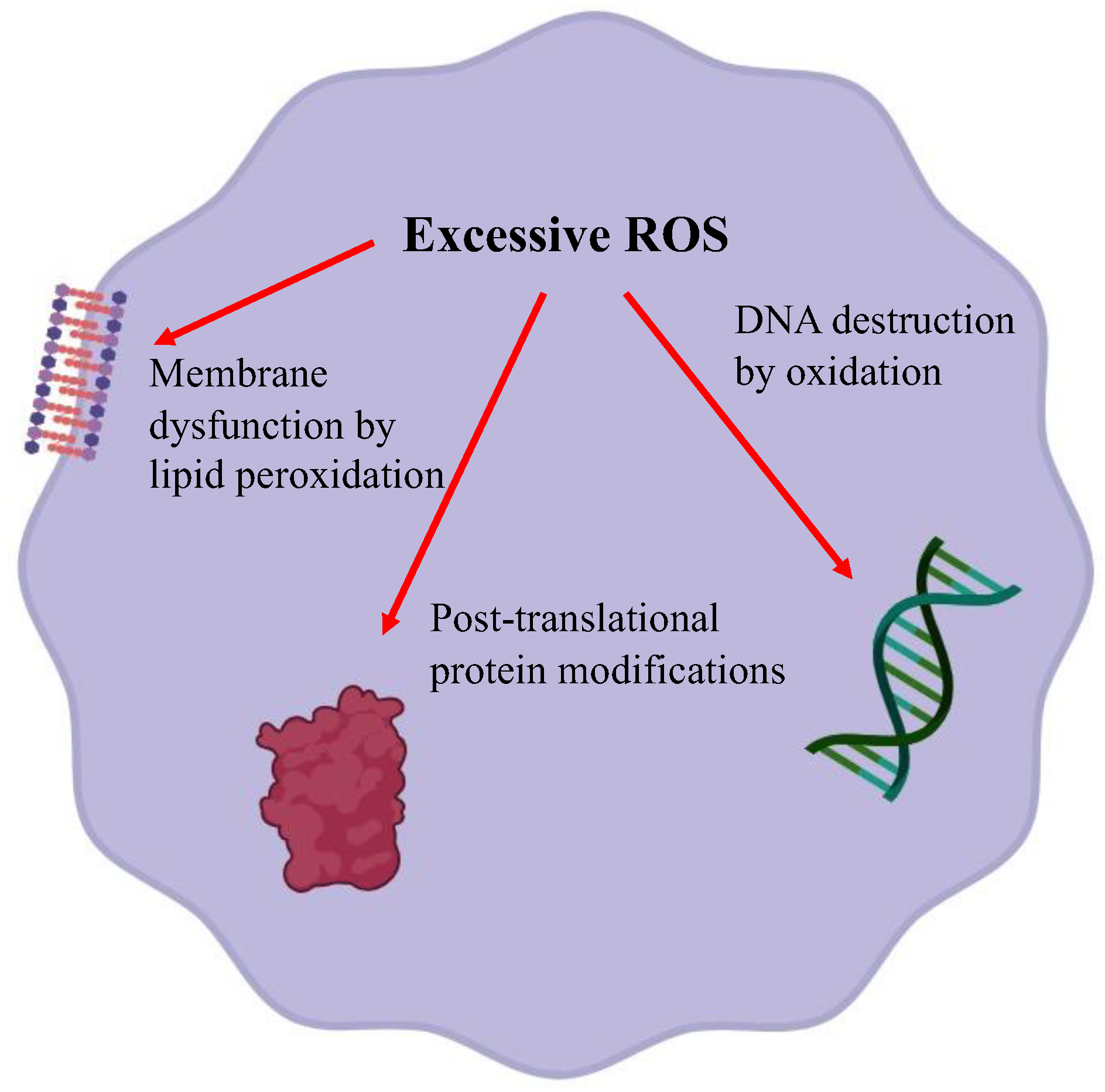
Normal cellular metabolism forms reactive oxygen species (ROS) and is controlled by antioxidant enzymes. An imbalance between ROS production and defense causes oxidative stress. Excessive ROS damages cells through three basic pathways: lipid peroxidation of membranes, oxidative modification of proteins, and DNA damage [10]. Lipids are abundant in cellular membranes, and lipid peroxidation by ROS causes membrane dysfunction, such as a decrease in membrane fluidity and an increase in membrane leakiness, leading to mutilation of membrane proteins, enzymes, and receptors. Proteins are also a target of ROS. ROS cause various post-translational protein modifications such as oxidation of sulfur-containing side chains, chlorination of side chain amines, oxidation of histidines and tryptophans, formation of dityrosine leading to oligomerization, fragmentation, destabilization, aggregation, and/or increased degradation of proteins [11]. DNA is susceptible to ROS attack, particularly guanine, which is easily oxidized to 8-hydroxyguanine and 8-hydroxy-2-deoxyguanosine. Such abnormalities lead to inappropriate protein formation and cell damage.
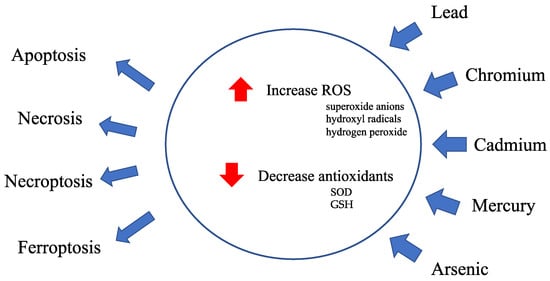
Figure 1.
Overview of heavy metal toxicity. Heavy metal exposure causes a disruption of cellular redox homeostasis.
Figure 1.
Overview of heavy metal toxicity. Heavy metal exposure causes a disruption of cellular redox homeostasis.
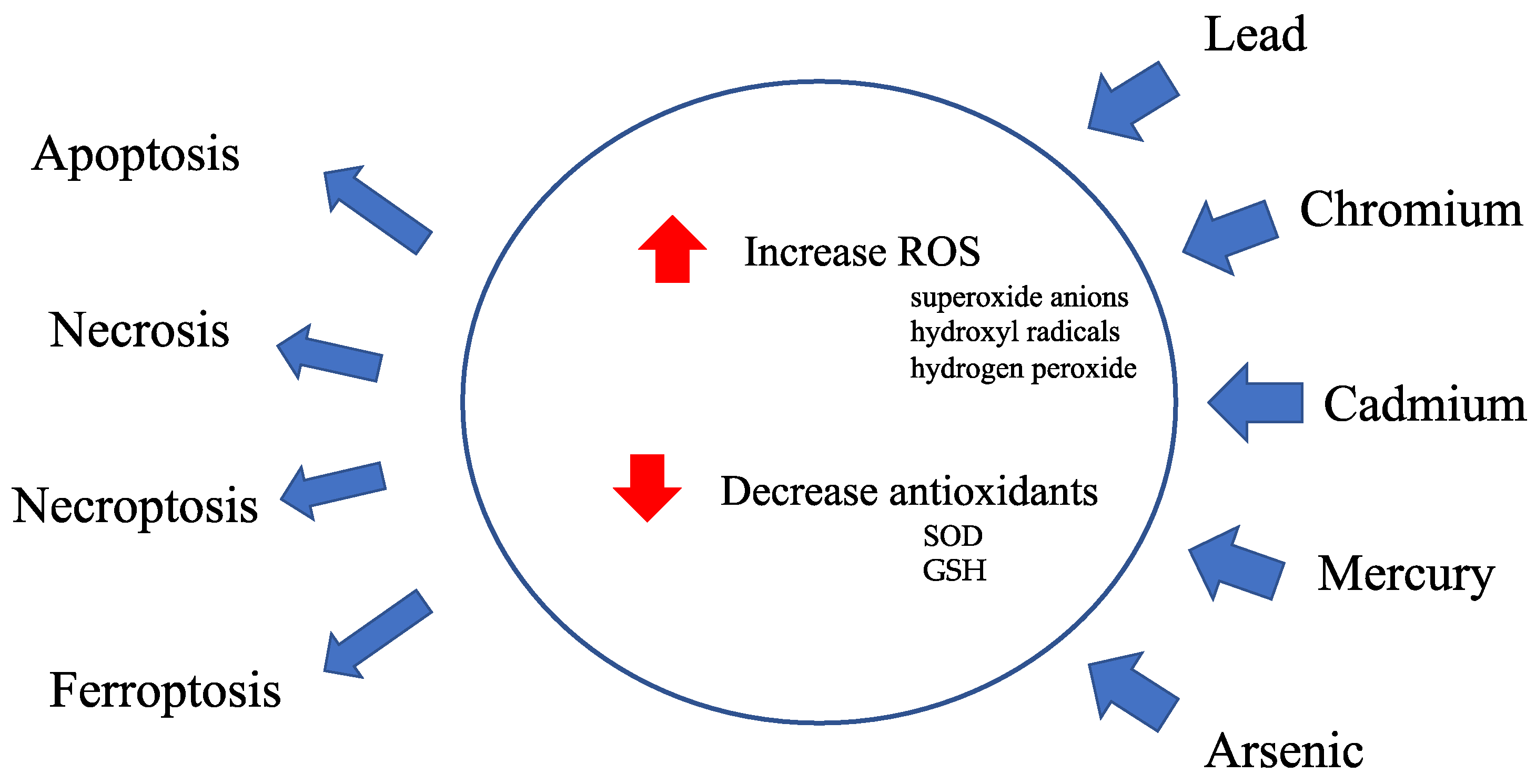
Figure 2.
An effect of excessive reactive oxygen species on membrane lipid peroxidation, oxidative modification of proteins and DNA damage.
Figure 2.
An effect of excessive reactive oxygen species on membrane lipid peroxidation, oxidative modification of proteins and DNA damage.
Antioxidants, including superoxide dismutase (SOD) and glutathione peroxidase, scavenge excessive ROS, such as superoxide anions, hydroxyl radicals, and hydrogen peroxide, produced in the mitochondria and protect against cellular damage from highly reactive ROS, preventing cellular damage caused by ROS. Heavy metals impair mitochondrial function by increasing ROS production and decreasing antioxidant activity.
ROS production is associated with heavy metal-induced mitochondrial damage. Increases in the expression of SOD1 and p62/Sequestosome 1 (SQSTM1) and an increase of cleaved caspase 3 are induced in the spleen following lead exposure, which corresponds to an increase in oxidative stress, apoptosis induction, and dysregulated autophagy [12]. In addition, lead increases ROS levels in the liver [13,14] and induces mitophagy via the phosphatase and tensin homolog (PTEN)-induced kinase 1 (PINK1)/Parkin pathway [15]. Lead damages the intracellular antioxidant system in hepatocytes [16] and blood [17], and an increase in ROS promotes DNA damage and inflammation [18,19,20] in hepatocytes [21,22], lungs [23], and plasma [24]. The increase in MDA levels upon Cd exposure suggests the promotion of lipid peroxidation by ROS in rat kidneys [25]. Mercury increases ROS production in erythrocytes [26] and neutrophils [27].
Heavy metals disturb the redox balance by decreasing antioxidant activity. Lead has the propensity to inhibit glutathione reductase, which converts oxidized glutathione (GSSG) to reduced glutathione (GSH) [28]. Cadmium damages mitochondrial function by reducing the activity of antioxidant enzymes. GSSG reductase activity is significantly decreased in the livers of cadmium-injected rats [29]. Cadmium also decreases SOD activity and GSH concentration in rat kidneys [25]. Mercury exposure dysregulates the oxidant detoxification system, thioredoxin1, and thioredoxin reductase1 redox system in neutrophils [27]. In summary, heavy metals disrupt the balance of antioxidants, increase harmful ROS, and impair mitochondrial function, disturbing cellular redox homeostasis.
Heavy metals affect the balance of the redox system through another pathway, ferroptosis. Ferroptosis was proposed as an iron-dependent form of non-apoptotic cell death in 2012 [30]. Ferroptosis is dependent on an intracellular level of free catalytically active iron [31]. Iron overload leads to the Fenton reaction, leading to overproduction of ROS as well as causing mitochondrial dysfunction by increased mitochondrial ROS production. ROS produced by iron overload results in cell death. The glutathione system plays a critical role in the regulation of ferroptosis. A key component is the Xc system, composed of SLC7A11 and SLC3A2, which facilitates the uptake of cystine while exporting cellular glutamate. Cystine is then converted to cysteine, an essential precursor for GSH synthesis, through the actions of GCL and GSS. The Xc system is a primary target of erastin, a ferroptosis inducer. Erastin not only interacts with the Xc system, but also binds to voltage-dependent anion channels 2 and 3 (VDAC2/3), leading to an increase in mitochondrial ROS generation [32]. GSH serves as a cofactor for the selenoprotein glutathione peroxidase 4 (GPX4), which plays a critical role in reducing lipid hydroperoxides to their corresponding alcohols [33]. These processes effectively prevent the harmful accumulation of lipoperoxidation products.
Arsenic increases cytoplasmic and mitochondrial iron accumulation through VDAC3 expression and down-regulation of SLC7A11 and GPX4 mRNA and protein expression in mouse brain and PC-12 cells [34]. It also induces ferroptosis in testicular cells, decreases GPX4, SLC7A11, Iron-Responsive Element-Binding Protein 2 (IREB2), and increases VDAC3 protein expression [35]. Cadmium induces ferroptosis in PC12 cells by increasing cellular iron content, repressing GPX4 expression [36]. This also happens in mouse seminiferous tubules and Leydig cells accompanying a significant decrease in GPX4 and an upregulation of SLC7A11 protein expression [37]. Mercury intoxication causes damage to rat astrocytes and rat liver cells through ferroptosis, with an increase in cytoplasmic ROS, lipid peroxidation, and decreased GPX4 activity [38]. Mercury also causes iron homeostasis. Hg exposure causes an increase free iron levels through heme degradation [39] or alteration of hypoxia-inducible factor-1α signaling [40], leading to ferroptosis. Lead exposure increases free iron levels in PC12 cells and causes ferroptosis [41]. Lead also affects SLC7A11 expression in neural stem cells [42] and inhibits GPX4 mRNA expression in chicken nerve tissue [43], all of which indicate ferroptosis.
2.1.2. Heavy Metal Toxicity Affects the Mitochondrial Surface
The mitochondrial electron transport chain (mtETC) is located in the inner mitochondrial membrane and maintains mitochondrial respiratory functions. This is inhibited by the blockade of enzyme activity upon heavy metal exposure [44], which promotes the opening of mitochondrial permeability transition pores (mPTP). Mitochondrial dysfunction induces the apoptotic pathway, and mPTP regulates apoptosis and necrosis. This means that the maintenance of the mitochondrial membrane potential (mMP) and ROS by mPTP plays an important role in a molecular pathway of heavy metal-induced cytotoxicity. Mutations in mitochondrial DNA (mtDNA) induce various phenotypes related to aging and cancer, which can also be induced by heavy metal exposure [45,46]. Histologically, mitochondrial dysfunction is characterized by mitochondrial edema. Lead promotes mPTP opening and harms the mMP, leading to a decrease in ATP production in rat proximal tubular cells [47]. Chronic lead exposure leads to mitochondrial edema in mice hepatocytes [48]. Mercury induces the release of cytochrome c from mitochondria, followed by mPTP opening [49]. Cadmium and mercury induce neurotoxicity via mETC dysfunction, intracellular ROS formation, mMP decrease, and mPTP opening mediated by mPTP assembly in the rat neuronal cell line PC12 [50]. Mercury and cadmium inhibit the mitochondrial respiratory chain and rapidly dissipate mMP, promoting both necrosis and apoptosis, which cause cell death in rat hepatoma cells [51]. Cadmium leads to mPTP opening and causes mitochondrial dysfunction in the rat liver [52]. Chromium induces mitochondrial dysfunction and mitophagy mediated by ATF4 via ER stress [53]. In conclusion, heavy metal exposure adversely affects various components of the mitochondrial surface, disrupting the mtETC, promoting mPTP opening, and influencing the mMP, all of which contribute to mitochondrial dysfunction.
2.2. Heavy Metal Exposure Induces ER Stress and Mitophagy (Figure 3)
Heavy metal exposure triggers ER stress by accumulating unfolded proteins in the endoplasmic reticulum (ER) damaged by heavy metal binding; this is called the unfolded protein response (UPR). In the ER stress pathway, the misfolded proteins induced by toxic agents are detected by binding immunoglobulin protein (BiP)/glucose-regulating protein 78 (GRP78) and multiple signaling pathways [54]. Pathways, including the protein kinase R (PKR)-like endoplasmic reticulum kinase (PERK)-eukaryotic initiation factor 2α (eIF2α)-activating transcription factor 4 (ATF4) pathway, inositol-requiring enzyme 1α (IRE1α)-X-box binding protein 1 (XBP1) pathway [55] and activating transcription factor 6 (ATF6) pathway, are subsequently activated, leading to autophagy or mitophagy [56]. Mitophagy controls mitochondrial function by processing damaged mitochondria for digestion and maintenance of mitochondrial health. Lead specifically binds to GPR78, the major downstream target of the UPR induced by ER stress [57,58]. Cadmium also induces ER stress and ferroptosis [59]. Cadmium activates the PERK-eIF2α-ATF4 pathway and triggers autophagy activation [60,61]. Mercury induces GRP78 overexpression in rat glioma cells, and the effect of mercury on ER stress is dose-dependent [62]. Chromium induces GPR78 overexpression, inducing ER stress. Chromium activates the PERK-eIF2α-ATF6 pathway, fostering crosstalk between ER stress and mitochondrial dysfunction [53].
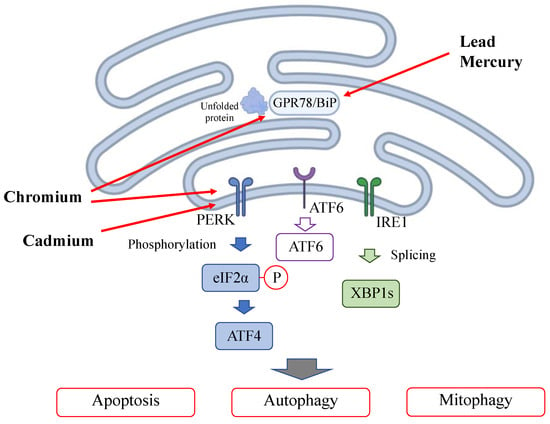
Figure 3.
Effect of heavy metals on the endoplasmic reticulum.
Figure 3.
Effect of heavy metals on the endoplasmic reticulum.
2.3. Heavy Metal Exposure Induces Apoptosis, Necrosis, and Necroptosis
Heavy metal exposure induces cell toxicity and death via apoptosis, necrosis, and necroptosis, all of which are part of a molecular cascade of heavy metal cytotoxicity. During necroptosis, two members of the receptor-interacting protein kinase (RIPK) family, RIPK1 and RIPK3, are activated to phosphorylate mixed lineage kinase domain-like protein (MLKL), which compromises the cell membrane to execute cell death. Apoptosis and necrosis can occur simultaneously because the signaling pathways are interconnected [63,64]. ER stress activates apoptosis of cells [65,66], and ER stress pathways are also activated by necroptosis [67]. The difference between these pathways, apoptosis or necroptosis, depends on the cell type and is an important therapeutic target [68]. Lead acetate induces the overexpression of interleukin 6 (IL-6) in the brain and excessive activation of nerve cells, leading to necrosis [69,70]. High doses of lead acetate elevate the levels of caspase 8, caspase 9, and Bax in the brain, kidney, and liver [4], whereas a low dose of lead induces the apoptotic pathway in the brain [71]. Lead also activates necroptosis induced cell death in an olfactory cell line, elevating PIPK3 and MLKL [72]. Mercury induces the release of cytochrome c from mitochondria into the cytosol, which activates caspase 3 and induces apoptosis in the human leukemia cell line HL-60 [49]. Cadmium intake increases the expression of RIPK1, RIPK3, and MLKL to activate the RIPK3-dependent necroptotic pathway [73]. Cadmium exposure increases the levels of proapoptotic proteins (Bax, B-cell lymphoma 2 (Bcl-2), caspase 3, and caspase 9) and pronecropotic proteins (RIP, RIP3, and MLKL) [74,75]. Cadmium induces apoptosis at low concentrations in a dose-dependent manner and necrosis at high concentrations [76]. The effects of cadmium on apoptosis and necrosis differ during the cell cycle. Cadmium induces apoptosis in the G0 and S phases and necrosis in the S and M phases but hardly induces apoptosis in the G1 phase [77]. Chromium induces apoptosis via two major pathways: p53-dependent and independent pathways. Chromium leads to an increase in p53 protein levels in human lung epithelium and fibroblasts, leading to apoptosis [78,79]. Chromium induces apoptosis independently of p53 activation by causing mitochondrial instability, leading to capase-3 activation and subsequent damage to the mitochondria [80].
2.4. Heavy Metal Exposure Affects MicroRNAs (Figure 4)
Heavy metals induce alterations in microRNAs (miRNAs) that regulate the expression of various genes and signaling pathways [81]. miRNAs are non-coding, short, single-stranded RNAs (21–23 nucleotide-long); they regulate gene expression through RNA silencing and mitochondrial function and homeostasis, as well as modulate cell metabolism by targeting known oncogenes and tumor suppressor genes of metabolism-related signaling pathways [82]. Several human cancers are caused by the dysregulation of miRNA function. Recent studies have confirmed that miRNA dysregulation plays a role in carcinogenesis in many tissues [83]. The mRNA expression codons differ between the nucleus and mitochondria [84], and research on the cross-interaction of expression with miRNA found a new axis, including the regulation of mitochondrial function by miRNA [85,86]. Cadmium exposure causes miRNA overexpression and has negative effects on the kidneys and other organs [87]. Cadmium alters miRNA expression in the renal cortex. Cadmium induces the overexpression of 44 and suppresses the expression of 54 miRNAs [88]. Cadmium overload positively and negatively affected several miRNAs (185 miRNAs) in renal epithelial cells [89]. Of the miRNAs suppressed by cadmium induction, miR-125a and miR-125b function as anti-apoptotic elements by increasing the expression of Bcl2 and decreasing that of Bax, Bak, caspase-9, and caspase-3 [90]. Cadmium also alters miRNA expression in other organs. Cadmium decreases the expression of 12 miRNAs in liver cells (HepG2 cells) and impairs liver functions, such as lipid and fatty acid transportation, cholesterol metabolism, and fatty acid oxidation [91]. In the spleen, cadmium decreases miR-33-5p and increases AMPK, leading to AMPK-mediated autophagy [92]. Cadmium upregulated miR-101 and miR-144 in human bronchial epithelial cells and targeted cystic fibrosis transmembrane conductance regulator (CFTR), leading to respiratory damage [93]. miR-193, mi-R221, and miR-222 are upregulated in ovarian tissues exposed to cadmium [94], leading to apoptosis [95].
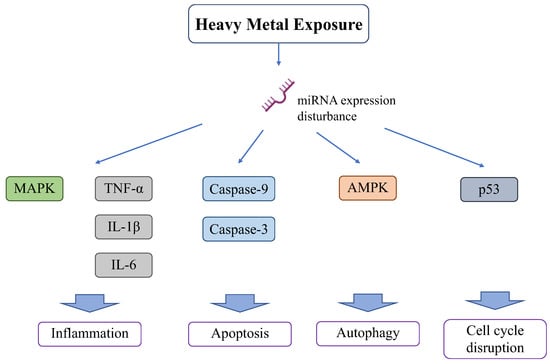
Figure 4.
Effect of heavy metals on miRNA.
Figure 4.
Effect of heavy metals on miRNA.
Other heavy metals also affect miRNAs and damage various organs. Lead promotes inflammation and apoptosis in mammary glands via the circRNA-05280/miR-146a/IRAK1 pathway [96]. Lead also increases miR-155 and activates inflammation in the MAPK pathway or production of inflammatory cytokines, such as TNF-α, IL-1β, and IL-6 [97].
2.5. Heavy Metal Exposure Affects Tumor and Senescence Pathways Involving p16/p21/p53
Heavy metal exposure impairs the p16/p21/p53 transcription pathway, which plays a critical role in cellular stress responses [98]. Heavy metal exposure alters the activation or inactivation of p53 both directly and indirectly through ROS [99]. Cadmium disrupts native p53 conformation, inhibits DNA binding, and downregulates the transcriptional activation of p21 in human breast cancer MCF7 cells [100]. Cadmium regulates p21/p53/p16 protein expression and mitochondrial dysfunction, leading to cellular senescence in bone marrow-derived mesenchymal stromal cells [101]. Long-term exposure to cadmium downregulates p16 via FNA hypermethylation in lymphocytes [102]. Lead exposure alters the promoter methylation rates of p16 in children [103]. Chromium exposure induces p16 methylation, decreases its expression, and damages lung cells [104]. Chromium increases CpG1 methylation levels of p16 and epigenetic modifications that damage human bronchial epithelial cells [105].
2.6. Relation with Aging
Aging is the process by which cells and organs cease to work properly or lose their function. Aging can be caused by similar mitochondrial dysfunction as occurs in heavy metal toxicity. ROS overproduction causes irregular signaling in mitochondria [106], acceleration of membrane instability by lipid peroxidation [107], and dysfunction of the DNA repair system [108], leading to malfunction of biological molecules and accumulation of irregular proteins and lipids. All this leads to cellular senescence [109].
Aging is also associated with ER stress. Accumulation of UPR plays a vital role in the cellular dysfunction which occurs in aging [110] These UPRs alter solubility, function inappropriately, and accumulate as plaques in various organs [111]
3. Pathophysiology of Heavy Metal-Induced Clinical Disorders
Considering the various pathways involved in heavy metal cytotoxicity, the pathophysiology of clinical disorders caused by heavy metal exposure involves several organs, exhibiting acute and chronic toxicity. In addition, complex interactions occur between each heavy metal, and their detrimental effects vary in different organisms. The new Bayesian network approach can be extended to incorporate information about metal–organism interactions [112]. A mixture of heavy metals results in an increased toxic effect compared with individual components at the same concentration [113].
3.1. Manifestation of Acute Heavy Metal Toxicity
The acute toxicity of heavy metal exposure induces various diseases and symptoms, including abdominal pain, anorexia, dyspepsia, diarrhea, fatigue, anxiety, numbness, memory and concentration difficulties, leukopenia, and thrombocytopenia.
Acute exposure to cadmium damages the respiratory system. Cadmium-containing nanoparticles accumulate in the lungs and penetrate the membranes of organelles, including mitochondria, damaging not only the lungs but also other organs [114]. One case report suggested that a patient with acute cadmium inhalation developed severe pneumonitis and died within 25 days [115].
Acute toxicity of arsenic causes gastrointestinal damage. Symptoms begin with a garlic-like taste, followed by dysphagia and severe vomiting. Acute paralytic syndrome occurs after the first symptom and ends with death within a few hours [116].
High-dose lead exposure causes acute hemolytic anemia. Lead inhibits the enzymes aminolevulinic acid synthetase (ALAS), aminolevulinic acid dehydratase (ALAD), and ferrochelatase, which are essential for heme synthesis, and their absence can cause anemia [117].
The acute toxicity of nickel originates from its combination with thiols, which results in the formation of Ni-thiol complexes [118]. These complexes generate free radicals that damage the body [119]. The clinical symptoms can be divided into two stages: immediate and delayed. Immediate effects include vomiting, irritation, headaches, and insomnia. Delayed effects include vertigo, palpitations, coughing, cyanosis, chest tightness, and lassitude [120].
Acute cadmium exposure can cause liver damage. Acute hepatotoxicity involves two pathways: the first, caused by the direct effects of cadmium, leads to initial injury, and the second causes subsequent injury due to inflammation. Primary injury appears to be caused by the binding of cadmium to sulfhydryl groups on critical mitochondrial molecules, causing oxidative stress, mitochondrial permeability transition, and mitochondrial dysfunction. Secondary injury is caused by the activation of Kupffer cells by triggering a cascade of events involving several types of liver cells and a large number of inflammatory and cytotoxic mediators [121].
3.2. Manifestation of Chronic Heavy Metal Toxicity (Figure 5)
Chronic toxicity due to heavy metal exposure affects a broad range of organs and manifests as neurological deterioration, cardiovascular diseases, reproductive problems, nephropathy, respiratory dysfunction, bronchitis, pulmonary edema, asthma, emphysema, hepatitis, anemia, hyperpigmentation, and cancer. In the following sections, we delve into each manifestation of chronic heavy metal toxicity.
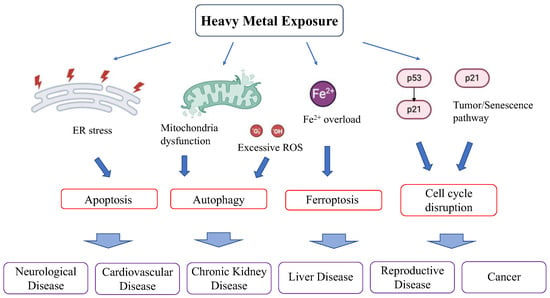
Figure 5.
Effect of heavy metals on clinical disease.
Figure 5.
Effect of heavy metals on clinical disease.
3.2.1. Neurological Symptoms
Heavy metals induce neurodegeneration through dyshomeostasis, ROS formation, and mitochondrial dysfunction [122]. Heavy metal exposure deteriorates mitochondrial quality. Calcium transporters in the mitochondrial membrane stabilize the mMP, and the disruption of calcium homeostasis triggers apoptosis in cells, which causes neurogenerative damage. Recent system biology analyses revealed that changes in protein expression associated with heavy metal exposure are related to neurodegenerative diseases, such as Parkinson’s disease and Alzheimer’s disease [9,123]. For example, PINK1/Parkin has a regulatory role in cleaning damaged mitochondria and underscores the importance of maintaining mitochondrial function in neural tissues, especially concerning Parkinson’s disease.
Among heavy metals, lead easily crosses the blood–brain barrier and acts as an alternative to calcium ions, which leads to interference with the normal action of calcium ions in the brain [124], affecting the uptake, release, and binding of GABA in the rat brain [125], calcium release through ryanodine receptors, and calcium signaling, thereby causing neurotoxicity in the rat brain [126].
Cadmium-induced oxidative stress has severe implications for neurological damage. ROS levels are increased in neuronal cell types, and mTOR and MAPK pathways are activated and are one of the causes of cytotoxicity in these cells [127]. Cadmium may be a risk factor for Alzheimer’s disease [128].
Mercury also damages neural cells. Methyl mercury is lipid-soluble and, therefore, easily crosses the blood-brain barrier. Mercury induces neurogenerative damage through oxidative stress and mitochondrial dysfunction, which can cause neurodegenerative diseases, such as Alzheimer’s disease [129,130] and amyotrophic lateral sclerosis [131]. Mercury is also considered a cause of Minamata disease, a neurodegenerative disease that occurs in Japan [132].
Heavy metals cause neuronal damage as individual metals, but mixtures of heavy metals also are harmful. Simultaneous exposure to lead, mercury, and cadmium causes greater brain damage in mice with reduced motor coordination and impaired learning and memory abilities than exposure to individual metals [133].
3.2.2. Cardiovascular Symptoms
Heavy metal exposure induces hypertension through oxidative stress, impaired nitric oxide signaling, modified vascular response to neurotransmitters, disturbed vascular muscle Ca2+ signaling, renal damage, and interference with the renin-angiotensin system, whose mechanism involves multiple axes owing to the complexity of the vascular system [134]. Blood pressure regulation is related to calcium signaling and renal function. Mitochondrial dysfunction in the myocardial tissue triggers cardiovascular dysfunction.
Lead increases the levels of endothelin, norepinephrine, angiotensin-converting enzyme, and thromboxane [135], leading to increased blood pressure and organ hypoperfusion. An animal study suggested that lead intoxication results in hypertension, dyslipidemia, atherosclerosis, and cardiac complications [136]. In humans, chronic lead exposure is associated with hypertension [137]. A meta-analysis suggested that a two-fold increase in blood lead levels was associated with a rise of 1–1.25 mmHg in systolic blood pressure and 0.6 mmHg in diastolic blood pressure [138]. A human autopsy study revealed that lead exposure was associated with aortic atherosclerosis [139]. Elevated blood or bone lead levels are correlated with increased cardiovascular mortality [140].
Cadmium induces endothelial dysfunction and accelerates the formation of atherosclerotic plaques, which cause cardiovascular damage [141]. Cadmium exposure can also cause hypertension [142]. Cadmium exposure increases the risk of coronary heart disease, stroke, and peripheral arterial diseases [143].
3.2.3. Chronic Kidney Disease
Heavy metal exposure leads to chronic kidney disease [144]. Lead accumulates in the kidney, and lead exposure inhibits glomerular development, resulting in renal dysfunction [145]. Chronic exposure to lead causes histopathological changes in the kidneys, such as progressive tubulointerstitial nephritis, characterized by the infiltration of leukocytes, interstitial fibrosis, and tubular atrophy [146]. Epidemiological studies have suggested that high serum levels of lead are associated with a higher risk of renal injury [147]. Furthermore, a high level of serum creatinine has been correlated with serum levels of lead [148].
Cadmium has a long half-life and tends to accumulate in the renal cortex for a long time [149]. Chronic exposure to cadmium has been associated with end-stage renal disease [150]. Cadmium also accumulates in the proximal tubules, causing Fanconi syndrome. Workers who work in cadmium-exposed areas tend to have kidney stones and tubulointerstitial nephritis [151].
Mercury induces neurological and nephrological damage. Mercury induces H2O2 formation and oxidative stress in rat kidney mitochondria [152] and causes glomerular and tubular dysfunction [153]. Arsenic also causes nephrological toxicity. Arsenic exposure induces ROS production in the kidneys and causes cellular damage and death [154]. An epidemiological study suggested that arsenic exposure is associated with albuminuria and proteinuria [155]. Arsenic in urine is associated with the prevalence of chronic kidney disease [156].
3.2.4. Hepatitis
Heavy metals cause long-term damage to the liver. Cadmium accumulates in the liver and causes various metabolic disturbances [157], such as increases in hepatic, mitochondrial, and microsomal lipid peroxidation, as well as the depletion of glutathione [158].
Although its exact mechanism of action remains unknown, lead induces hepatotoxicity [48]. Lead accumulates in the liver and elevates aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels, leading to inflammation and hepatocyte death [20]. In India, lead-exposed individuals tend to have higher serum ASL, ALT, and bilirubin levels than healthy individuals [159], reflecting hepatotoxicity [160]. High-dose chromium exposure causes liver damage [161].
Arsenic destroys DNA, lipids, and proteins, damaging membranes, cells, and tissues in the liver [162]. Arsenic-intoxicated rat showed increased levels in thiobarbituric acid reactive substances, lipid hydroperoxides, protein carbonyl content and conjugated dienes, reduced DNA, decreased levels of the activities of membrane-bound ATPases, and increased levels of AST and ALT in the liver. Histological damage such as the extensive inflammation, dilated sinusoids, degeneration of hepatocytes with necrosis, vacuolization, and inflammatory cell infiltration also occurred. One study suggested that people who drink water containing more than 0.05 mg/L of arsenic show significantly more hepatomegaly than those who drink water containing less than 0.05 mg/L of arsenic [163].
3.2.5. Reproductive Problems
Heavy metals can damage the reproductive system. They affect the male [164] and female reproductive systems [165] through various mechanisms.
Lead can bind to histidine in protamine and change the molecule’s conformation [166], resulting in sperm destabilization [167] and fertility issues. Lead can decrease testosterone levels [168], and the ROS produced by lead can cause severe damage to sperm quality and quantity [169,170]. Lead decreases the levels of several hormones, such as estradiol and LH, that are vital for reproduction [171]. Lead also elevates metallo-matrix proteins, which causes abnormalities in the placenta [172]; lead-exposed females have a high percentage of miscarriages [173].
Arsenic can damage the reproductive system. Arsenic decreases sperm number and mobility and affect sperm DNA [174,175]. Women exposed to arsenic experience disturbances in sex hormones and infertility [175].
Cadmium blocks calcium channels that play an important role in sperm fertilization [176]. Cadmium affects the transcription of a sperm protein (CatSper) [177] and damages sperm function [178]. It also decreases sperm mobility, causing infertility [179].
Mercury produces ROS, which causes infertility. Mercury decreases the number of sperms and alters their shape [179]. Mercury induces an imbalance in sex hormones [180], and low-dose mercury exposure causes stillbirth, spontaneous abortion, and infertility [181].
3.2.6. Cancer
Heavy metals promote the production of ROS and chronic oxidative stress through various pathways including ferroptosis, which lay the groundwork conducive to carcinogenesis [182,183]. Three main mechanisms have been proposed to drive carcinogenesis: (1) disruption of cellular redox control, resulting in DNA damage, (2) suppression of essential DNA repair systems, resulting in genomic instability and accumulation of critical mutations; and (3) activation of oncogenic pathways and inactivation of tumor suppressors, disrupting the balance between cell proliferation and death [184].
Arsenic is a carcinogenic agent that induces oxidative DNA damage. It causes ROS production and breaks single-stranded and double-stranded DNA, forming 8-hydroxy-2′-deoxyguanosine (8-OHdG), which causes nucleotide conversion and tumor formation [185]. Bowen’s carcinoma is caused by arsenic exposure [186]; some lung tumors have also been reported to be associated with arsenic toxicity [187].
Cadmium has been implicated as a carcinogen and manifests its effects through several pathways. Cadmium produces ROS and increases the expression of c-Fos and c-Jun, which promote cancer [188]. Cadmium inhibits vital DNA mismatch repair systems and increases genomic instability, resulting in oncogenesis [189]. Cadmium can cause prostate cancer [190] and its inhalation can cause renal cancer [191].
Transcriptome analysis revealed that arsenic and cadmium exposure altered 167 genes which correlate with tumorigenesis, cell cycle, apoptosis, and oxidative stress in human lymphoblastoid cells [192]. Another study revealed that low-dose cadmium altered several gene expressions associated with maintaining cellular redox homeostasis such as increasing glutathione synthesis and antioxidant capacity, facilitating the survival or death response, and repairing damage or stimulating degradation [193].
4. Drugs for Protection against Heavy Metal-Induced Injury and Their Molecular Mechanisms
Chelating therapy is traditionally used to treat heavy metal toxicity. Moreover, while antioxidants are not generally used clinically, recent advances in molecular pathways have revealed that new compounds targeting oxidative stress effectively protect cells from heavy metal-induced cell damage (Table 1).
There are two types of protective drugs in terms of timing of use. Post-treatment drugs are used after exposure to heavy metals, whereas pretreatment intake is considered before exposure. Chelating agents and stoichiometric antioxidants are used for post-treatment strategies, and some antioxidants are used for pretreatment.
4.1. Posttreatment Drugs
4.1.1. Chelating Agents
Chelation therapy is the primary treatment used to reduce the toxic effects of heavy metals. Chelating agents bind toxic metal ions and form complex structures that are easily excreted from the body, removing them from intracellular and extracellular spaces [194]. Chelators mobilize metals in tissues that mediate their excretion through the kidneys in the urine or the liver in bile. Concerns regarding enterohepatic recirculation and renal reabsorption of heavy metals are overcome by the use of lipophilic chelators, which can excrete larger amounts of heavy metals than aqueous chelators via bile; this is because aqueous chelators facilitate transport within the blood and excretion via the kidney, whereas lipophilic chelators exhibit greater penetration of cellular membranes, including those within the central nervous system, to chelate intracellular elements [195]. The major chelating agents reported to be effective against heavy metal toxicity are British anti-Lewisite (BAL), dimercaptopropane-l-sulphonate (DMPS), meso-2,3-dimercaptosuccinic acid (DMSA), sodium 2,3, monoisoamyl DMSA (MiADMSA), monomethyl DMSA (MmDMSA), monocyclohexyl DMSA (MchDMSA), calcium disodium ethylenediamine tetraacetic acid (CaNa2EDTA), calcium trisodium diethylenetriaminepentaacetate, D-penicillamine, tetraethylenetetraamine (TETA) or trientine, nitrilotriacetic acid (NTA), deferoxamine (DFO), and deferiprone (L1). BAL was the first reported antidote with two sulfhydryl and hydroxyl groups to arsenical nerve gas [175]; the heavy metal binds to a thiol group, resulting in the formation of a stable complex and excretion from the kidney [196]. DMPS is a water-soluble analog of dimercaprol with fewer side effects than dimercaprol [197] and is mainly used to alleviate arsenic and mercury poisoning [198]. DMSA is an analog of dimercaprol with a sulfhydryl group and is a water-soluble, non-toxic, orally administered metal chelator that has been used as an antidote for heavy metal toxicity since the 1950s [199]. Other DMSA analogs have been developed to increase the excretion efficacy [200]. CaNa2EDTA is a water-soluble chelator in which the calcium atom is replaced by lead ions to form a water-soluble complex that is excreted from the kidney and is mainly used for treating lead toxicity [194]. Penicillamine is often used to chelate copper [201]. TETA and NTA are major chelators of copper [202]. DFO is an organic substance that binds tightly to trivalent ions and is used to treat aluminum toxicity [203]. Deferiprone is a major chelator of iron [196].
4.1.2. Stoichiometric Antioxidants
Oxidative stress is one of the primary contributing mechanisms in metal toxicity, offering a strong rationale for the exploration of antioxidant therapy as a treatment for heavy metal toxicity. Free radical scavenging (chain-terminating) antioxidants, such as vitamins E and C, are chemicals with large resonance-stabilized electron clouds that work as electron donors [204] and vitamin C works through the activation of biological antioxidant defenses, such as GSH-linked enzymes [205,206]. Astaxanthin also prevents oxidative stress induced by cadmium [207], copper [208], and cobalt [209] and alleviates cell damage caused by these metals. Selenium [210], and lycopene [211] have been reported as antioxidants against the toxicity of heavy metals, such as cadmium, lead, and chromium.
Other agents have also been reported to be effective in protecting against exposure to heavy metals. The use of probiotic yogurt may be an effective and affordable approach for combating toxic metal exposure through the protection of indigenous gut microbiota in humans, resulting in a faster decrease in copper (34.45%) and nickel (38.34%) levels in the blood [212]. Gossypin, a flavonoid glycoside originally isolated from Hibiscus vitifolius, has antioxidant and anti-inflammatory activities and has shown considerable promise for improving recovery in animal models [213].
4.2. Pretreatment Drugs
4.2.1. Antioxidants
Pretreatment with several antioxidants is effective in preventing heavy metal overload through direct detoxification of ROS and introduction of some antioxidant enzymes. Melatonin acts as an antioxidant and prevents cadmium and lead overload [214,215]. Coenzyme Q10 works as a NF-E2-related factor 2 (Nrf2) activator and antioxidant [213] Quercetin is a free radical scavenger that can alleviate oxidative stress and protect against lead-induced ER stress by modulating the phosphatidylinositol-3 kinase (PI3K)/protein kinase B (PKB, AKT) and inositol requiring 1 (IRE1)/c-Jun amino-terminal kinase (JNK) pathways in rat liver [216].
Curcumin, a bioactive substance found in turmeric, is widely used as a dietary supplement and has promising metal toxicity-ameliorating effects related to its intrinsic antioxidant activity [217]. Sinapic acid ameliorates cadmium-induced nephrotoxicity involving oxidative stress, apoptosis, and inflammation via NF-κB downregulation [218].
Mitochondrial protection is another target for protection against heavy metal toxicity. Melatonin reduces mitochondrial fission and prevents cadmium-induced cell damage [219]. Metformin also functions as a mitochondrial protection agent by reducing mitochondrial fission and fragmentation and suppressing lead-induced [220] and cadmium-induced toxicity [221].
4.2.2. Senolytic Drugs
Senolytic drugs that induce apoptosis in senescent cells effectively protect against heavy metal toxicity [222]. Senolytic drugs are classified as BCL family inhibitors, PI3K/AKT inhibitors, or FOXO regulators [223,224]. Heavy metals induce phosphorylation of Bcl-2 through activation of the JNK pathway, which is not associated with G2/M cell cycle arrest; hyperphosphorylated Bcl-2 protein can inhibit zinc-induced cell death compared to the hypo-phosphorylated mutant form, indicating that the regulation of Bcl-2 by phosphorylation is an important part of the cell response to heavy metal-induced stress [225]. ROS and heavy metal exposure activate AKT via the PI3K-dependent pathway [226].
Multiple molecules investigated in anti-aging research have been identified as senolytic drugs. Resveratrol prevents cadmium, lead, and manganese toxicity via the JNK or AKT pathways [227,228,229]. Fisetin is a natural flavonoid found in fruits and vegetables [230] and elicits senolytic activity through the AMPK/SIRT1, autophagy, mitochondrial apoptosis, and Rho GTPase signaling pathways, alleviating lead- and mercury-induced toxicity [231]. Mitoquinone (MitoQ) is an antioxidant molecule that targets mitochondrial ATP production and restores mMP, inhibiting lead-induced toxicity [232].

Table 1.
List of Compounds alleviating heavy metal toxicity.
Table 1.
List of Compounds alleviating heavy metal toxicity.
| Compound | Dose | Model | Mechanism | Other Information | References |
|---|---|---|---|---|---|
| Vitamin C | 500 mg/L drinking water 20–40 mg/kg | Cadmium-treated rat | Oxidative stress | Increases SOD and glutathione peroxidase, and regulates StAR gene expression | Gupta et al. [233], El-Neweshy et al. [234], Ayinde et al. [235] |
| Vitamin E | 100–300 mg/kg, 100 IU/kg | Cadmium-treated rat or rabbit | Oxidative stress Nrf-2 pathway | Increases SOD and glutathione peroxidase and regulates StAR gene expression. Decreases Bax and caspase-9 genes and increase Mfn1, Mfn2, and Bcl-2 Activates the Nrf-2 pathway. | Gupta et al. [233], Amanpour et al. [236], Fang et al. [237], El-Boshy et al. [238], Chen et al. [239], Beytut et al. [240], Ayinde et al. [235] |
| 80 μg/mL | Lead-treated PC12 cells | Oxidative stress | Decreases ROS levels. | Yang et al. [241] | |
| L-carnitine | 100 mg/kg | Lead-treated rats | Oxidative stress | Reduces MDA and elevates TAC levels. | Abdel-Emam et al. [242] |
| 10–100 mg/kg | Cadmium-treated rats or mice | Oxidative stress | Increases SOD, GSH, and CAT levels. | Iftikhar et al. [211], Abu-El-Zahab et al. [210] | |
| 1 mM | Nickel-treated Neuro-2a cells | Mitochondrial function | Decreases ROS and MDA levels, maintains the mitochondrial membrane potential, and increases mitochondrial DNA copy numbers and transcript levels. | He et al. [243] | |
| Folic acid | 0.4 mg/kg | Lead-treated rat | Oxidative stress | Downregulates Bc1-2 and upregulates Bax levels. | Quan et al. [244] |
| Astaxanthin | 10 mg/kg | Cadmium-treated mice | Oxidative stress | Increases CatSper1 and decreases DNA fragmentation. | Saberi et al. [207] |
| 100 mg/kg | Copper-treated rat | Oxidative stress | Increases G6PD, GR, and GST levels. | Bayramoglu et al. [208,245] | |
| 0.001–10 μM | Copper-treated RWPE-1 cells | Oxidative stress | Decreases MDA and increases MMP, SOD, GSH, and CAT levels. | Meng et al. [246] | |
| 1–20 μM | Cobalt-treated MG-63 cells | Oxidative stress | Regulates Bcl-2 and JNK. | Li et al. [209] | |
| Selenium | 0.87 mg/kg | Cadmium-treated mice | Oxidative stress | Increases SOD and CAT levels. | Abu-El-Zahab et al. [210] |
| Lycopene | 4 mg/kg | Cadmium-treated rats | Oxidative stress | Increases SOD, GSH, and CAT levels. | Iftikhar et al. [211] |
| Fisetin | 25 mg/kg or 50 mg/kg | Lead-treated mice | AMPK/SIRT or autophagy pathway | Inhibits apoptosis, decreases Aβ, and increases Aβ removal through neprilysin in the brains of mice. | Yang et al. [247]. |
| 30 mg/kg | Mercury-treated mice | Mitochondrial apoptosis Rho GTPase signaling | Prevents cytochrome c release, reduces ERK 1/2 and caspase 3 levels, and increases RhoA/Rac1/Cdc42 levels in the hippocampus. | Jacob et al. [248] | |
| Quercetin | 25–50 mg/kg | Lead-treated rat | IRE1/JNK and PI3K/Akt pathway | Decreases ROS and increases PI3K and PKB/Akt activity. | Liu et al. [249] |
| Curcumin | 15–400 mg/kg | Cadmium-treated mice or rats | Oxidative stress | Increases GSH, CAT, SOD and decrease MDA level. | Eybl et al. [250], Deevika et al. [251], Tarasub et al. [252], Akinyemi et al. [253], Kim et al. [254], Aktas et al. [255], Oguzturk et al. [256], Zoheb et al. [257], Algasham et al. [258], Sharm a et al. [259], Kukongviriyapan et al. [260] |
| 30–200 mg/kg | Lead-treated mice or rats | Oxidative stress Erk1/2 and JNK pathway | Increases GSH, CAT, and SOD and decreases MDA level. | Abubakar et al. [261,262], Changlek et al. [263], Zahid et al. [264], Alhusaini et al. [265], Soliman et al. [266], Dairam et al. [267] | |
| 5 μM | Copper-treated SH-SY5Y cells, | Oxidative stress | Increases SOD and decrease MDA level. Upregulates pro-caspase 3, pro-caspase 9, and PARP1 expression. | Xiang et al. [268], | |
| 30 mg/kg | Copper-treated rat | Oxidative stress | Increases SOD and GSH levels. Deceases NF-κB and activates the BCl-2/Bax pathway. | Abbaoui et al. [269,270], Yan et al. [271] | |
| Coenzyme Q10 | 20 mg/kg | Cadmium-treated rats | Oxidative stress | Increases SOD, GSH, and CAT levels. | Iftikhar et al. [211], Paunovic et al. [272] |
| 10 mg/kg | Lead-treated rats | Oxidative stress Nrf2/HO-1 pathway | Reduces Bax and caspase-3 and increases Bcl-2 levels. | Al-Megrin et al. [273], Yousef et al. [274], Mazandaran et al. [275] | |
| 10 mg/kg | Chromium-treated mice | Nrf2/HO-1/NQO1 pathway | Reduces LPO, GSH, TT, CAT, and PCC and increase SOD and GST levels. | Tripathi et al. [276] | |
| xanthophylls | 2.5–20 μM | Cobalt-treated Muller cells | Apoptosis and autophagy pathway | Increases Bcl-2 and decreases Bax, cleaved caspase-3, and LC3II levels. | Fung et al. [277] |
| Resveratrol | 20 mg/kg | Cadmium- and lead-treated albino mice | Akt cascade pathway | Reduces p-Akt and increases GSH. | Mitra et al. [227,278] |
| 400 mg/kg | Cadmium-treated birds | Nuclear xenobiotic receptor response and PINK1/Parkin-mediated Mitophagy | Restores VDAC1, Cyt C, and Sirt3 upregulation and Sirt1, PGC-1α, Nrf1, and TFAM transcription restrictions. | Zhang et al. [279] | |
| 1–100 μM | Cadmium-treated PC12 cells, TCMK-1 cells, MC3T3-E1 cells, ovine oocyte | PP2A/PP5-mediated Erk1/2 and JNK pathway and mTORC1-mediated S6K1/4E-BP1 pathway. ROS decrease and F actin assembly. | Inhibits phosphorylation of S6K1/4E-BP1, Akt, Erk1/2 and/or JNK/c-Jun and cleavage of caspase-3. Increases SIRT1, SOD1, GPX1. | Liu et al. [280,281], Fu et al. [282], Mei et al. [283], Piras et al. [284] | |
| 50 mg/kg | Lead-treated mice or rats | Autophagy pathway Neuroprotective pathway | Inhibits LC3 and Beclin-1 expression and promotes the Aβ degradation and Tau phosphorylation. Increases BDNF and SIRT1. | Bai et al. [228]. Wang et al. [285], Feng et al. [286] | |
| 5 and 10 μM | Copper-treated fibroblast | Autophagy pathway | Reduces carbonylated and polyubiquitinated proteins. | Matos et al. [287] | |
| 10–40 μM | Nickel-treated BEAS-2B cells | P38 MAPK, NLRP3, and NF-κB pathway. | Suppresses p38 MAPK, NF-κB signaling, and NLRP3. | Cao et al. [288] | |
| 10–30 μM | Manganese-treated PC12 cells or rat primary astrocytes | SIRT1 FOXO3a pathway ERK-MMP-9 pathway | Activates SIRT and FOXO3 decreases Bax. Suppresses ERK activity and decreases MMP-9. | Zhao et al. [229], Sun et al. [289], Latronico et al. [290] | |
| 30 mg/kg | Manganese-treated mice | Mitochondrial fragmentation | Activates the deacetylase activity of SIRT1, reduces PGC1α, and regulates DRP1. | Lei et al. [291], Lang et al. [292] | |
| 50 μM | Cobalt-treated cochlear hair cell | Sirtuin1 and NF-κB deacetylation | Activates sirtuin1 and deacetylates NF-κB. | Wang et al. [293] | |
| Lycium barbarum | 200, 400, and 600 mg/kg | Lead-treated mice | Oxidative stress and apoptosis | Decreases Nrf2 levels. | Xie et al. [294] |
| 10, 33.3, and 100 mg/kg or 300 mg/kg | Cadmium-induced mice | Oxidative stress | Increases SOD and GSH-Px levels. | Zhang et al. [295], Varoni et al. [296,297]. | |
| MitoQ | 500 μM | Lead-treated rats | Mitochondrial ATP production and mitochondrial membrane potential | Decreases caspase 3 and 9 activities, synaptosomal lipid peroxidation, and protein oxidation. | Maiti et al. [249] |
| Melatonin | 1 μM | Cadmium-treated HepG2 cells | Autophagy pathway, mitochondrial fission | Inhibits SIRT2-SOD activity and suppresses autophagy. Suppresses the SIRT1-PGC-1α pathway. | Pi et al. [219], Dong et al. [298] |
| 5–15 mg/kg | Cadmium-treated rats, hamsters, or mice | Oxidative stress, lipid peroxidation | Increases SOD, GSH, decrease MDA levels. | Kim et al. [28], Karbownik et al. [214], Eybl et al. [250], El-Sokkary et al. [25,299], Ji et al. [300], | |
| 10 μM | Lead-treated SH-SY5Y cells | Oxidative stress | Increases GSH levels and inhibits caspase3 activation | Suresh et al. [215] | |
| 10 mg/kg | Lead-treated rat | Oxidative stress, lipid peroxidation | Increases SOD and GSH levels. | El-Sokkary et al. [301] | |
| Rapamycin | 0.2 μg/mL, 100 nM–5 μM | Cadmium-treated rat pheochromocytoma (PC12) cells, human neuroblastoma SH-SY5Y cells, human placental trophoblasts, renal tubular cells. | mTORC1 and mTORC2 pathway, mitochondrial ROS-dependent neuronal apoptosis. | Downregulates Akt, S6K1, 4E-BP1. Reduces PP2A and suppresses the activation of JNK and Erk1/2 pathways. | Xu et al. [302,303], Yuan et al. [304], Zhu et al. [305], Kato et al. [306], Chen et al. [307], Fujiki et al. [308], Lee et al. [309] |
| 5 μM | Lead-treated rat proximal tubular (rPT) cells. | Autophagy pathway | Decreases LC3-II protein levels | Chu et al. [49] | |
| 1.5 mg/kg | Zinc-treated rats | mTOR pathway | Decreases mTOR/p70S6K and increases Nrf2/HO-1. | Lai et al. [310] | |
| 10–100 nM | Copper-treated chicken hepatocytes or RAW264.7 cells | Mitophagy through the PINK1/Parkin pathway, Akt/AMPK/mTOR pathway | Decreases p53, Bak1, Bax, Cyt C, and caspase3/cleaved-caspase3 mRNA and protein levels; increase Bcl2 mRNA. | Yang et al. [311,312], Luo et al. [313] | |
| 0.25 mg/kg | Iron-treated rats | Autophagy pathway | Decreases the ratio phospho-mTOR/total mTOR and restores LC3 II levels. | Uberti et al. [314] | |
| 500 nM | Cobalt-treated HT22 cells or A transformed cell line (RGC-5 cells) with some ganglion cell characteristics | Autophagy pathway | Promotes Beclin-1 expression, increases the conversion of LC3-I into LC3-II, and decreases Bax expression. | Zimmerman et al. [315], Olmo-Aguado et al. [316] | |
| 5 mg/kg | Manganese-treated mice | Autophagy pathway | Activates autophagy and decreased a-Syn oligomers. | Liu et al. [317] | |
| Metformin | 2 mM or 250 mg/kg | Lead-treated SH-SY cells or lead -induced rats | Mitochondrial fragmentation, methylglyoxal scavenger. | Increases AMPK/Nrf2 activation. Decreases methylglyoxal and D-lactate. | Yang et al. [220], Huang et al. [318] |
| 5 mM | Nickel-treated BESA-2B cells | AMPK pathway | Suppresses hexokinase 2 and activates lipocalin 2. | Kang et al. [319,320] | |
| 1 mM | Cadmium-treated spiral ganglion neuron | Autophagy pathway, ROS-dependent PP5/AMPK-JNK signaling pathway | Suppresses LC3-II and p62. Suppresses JNK, and inactive PP5 and AMP. | Li et al. [221], Chen et al. [321] | |
| 100 mg/kg | Cadmium-treated mice | Mitochondrial fission | Decreases Drp1and RB. | Zhang et al. [322] | |
| N-acetyl cysteine | 5 mM | Copper-treated GC-1 spg cells | AMPK-mTOR pathway | Reverses mTOR suppression induced by copper. | Guo et al. [323] |
| Ganoderma lucidum | 0.1, 0.5, or 1.0 g/kg | Cadmium-treated mice | Metallothionein | Increases metallothionein protein levels and inhibits oxidative stress. | Jin et al. [324] |
| 0.25 g/kg | Cadmium-treated rats | Oxidative stress and proinflammatory cytokines | Increases SOD, CAT, and GSH levels. Reduces TNF-α, and IL-1β. | Bin-Jumah et al. [325] |
Aβ; Amyloid β, AMPK; Adenosine monophosphate (AMP)-activated protein kinase, a-Syn; α-Synuclein, Bax; B-cell/CLL lymphoma 2 (Bcl-2)-associated X protein, Bcl; B-cell/CLL lymphoma, BDNF; Brain-derived neurotrophic factor, CAT; Catalase, Cyt C; Cytochrome c, DRP; Dynamin-related proteins, ERK; Extracellular signal-regulated kinase, FOXO; Forkhead box O, G6PD; Glucose-6-phosphate dehydrogenase, GPX; Glutathione peroxidase, GR; Gutathione reductase, GSH; Reduced glutathione, GST; Glutathione S-transferase, HO; Heme oxygenase, IL; Interleukin, JNK; Jun amino terminal kinase, LPO; Lipid peroxide, MAPK; Mitogen-activated protein kinase, MDA; Malondialdehyde, Mfn; Mitofusin, MMP; Matrix metalloproteinase, NF-κB; Nuclear factor kappa-light-chain-enhancer of activated B cells, NLRP; Nucleotide-binding oligomerization domain-like receptor pyrin-domain-containing protein, NQO1; Nicotinamide adenine dinucleotide phosphate (NAD(P)H) quinone oxidoreductase, Nrf; Nuclear factor, erythroid 2 (NF-E2)-related factor, PCC; protein carbonyl content, PGC; Peroxisome proliferator-activated receptor gamma (PPARγ) coactivator, PINK; Phosphatase and tensin homolog (PTEN) induced putative kinase, PP; Protein phosphatase, SIRT; Silent information regulator, SOD; Superoxide dismutase, StAR; Steroidogenic acute regulatory protein, TAC; Total Antioxidant Capacity, TFAM; Mitochondrial transcription factor A, TNF; Tumor necrosis factor, TOR; Target of Rapamycin, TT; Total thiols, VDAC; Voltage-dependent anion-selective channel.
5. Conclusions and Perspectives
Exposure to heavy metals induces various diseases through multiple molecular pathways, including apoptosis, endoplasmic reticulum stress, and mitochondrial stress, resulting in cell damage. These pathways are also implicated in cellular senescence and aging. Many types of heavy metal exposure have been used in aging models in both cell lines and animals. In addition to traditional chelation therapy for heavy metal toxicity, various antioxidants have been found to be effective in treating damage by heavy metal exposure. Advances in research on heavy metals can provide insights into the development of anti-aging and anti-cancer agents.
Author Contributions
Conceptualization, T.K. and T.Y.; methodology, H.K. and T.K.; software, H.K.; validation, H.K. and T.K.; formal analysis, H.K. and T.K.; investigation, H.K.; resources, H.K. and T.K.; data curation, H.K. and T.K.; writing—original draft preparation, H.K. and T.K.; writing—review and editing, T.K. and T.Y.; visualization, H.K.; supervision, T.Y.; project administration, T.Y.; funding acquisition, H.K. and T.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data presented in this study are openly available in the references.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jannetto, P.J.; Cowl, C.T. Elementary overview of heavy metals. Clin. Chem. 2023, 69, 336–349. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization; Fewtrell, L.; Kaufman, R.; Prüss-Üstün, A. Lead: Assessing the Environmental Burden of Diseases at National and Local Levels; World Health Organization: Geneva, Switzerland, 2003.
- World Health Organization; Poulin, J.; Gibb, H.; Prüss-Üstün, A. Mercury: Assessing the Environmental Burden of Disease at National and Local Levels; World Health Organization: Geneva, Switzerland, 2008.
- Rahman, Z.; Singh, V.P. The relative impact of toxic heavy metals (THMs) (arsenic (As), cadmium (Cd), chromium (Cr)(VI), mercury (Hg), and lead (Pb)) on the total environment: An overview. Environ. Monit. Assess. 2019, 191, 419. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Kumar, A.; Gupta, V.K.; Sharma, B. Biochemical and molecular bases of lead-induced toxicity in mammalian systems and possible mitigations. Chem. Res. Toxicol. 2018, 31, 1009–1021. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer (IARC). Summaries & Evaluations: Inorganic and Organic Lead. IARC Summary & Evaluation. 2006, 87. Available online: https://www.inchem.org/documents/iarc/vol87/volume87.pdf (accessed on 25 December 2023).
- Guo, X.; Jiang, S.; Xu, J.; Tian, Y.; Ouyang, F.; Yu, X.; Liu, J.; Yan, C.; Zhang, J.; Shanghai Birth Cohort. Effects of single and combined exposure to lead and stress during pregnancy on offspring neurodevelopment. Dev. Cogn. Neurosci. 2022, 56, 101124. [Google Scholar] [CrossRef] [PubMed]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Karri, V.; Ramos, D.; Martinez, J.B.; Odena, A.; Oliveira, E.; Coort, S.L.; Evelo, C.T.; Mariman, E.C.M.; Schuhmacher, M.; Kumar, V. Differential protein expression of hippocampal cells associated with heavy metals (Pb, As, and MeHg) neurotoxicity: Deepening into the molecular mechanism of neurodegenerative diseases. J. Proteom. 2018, 187, 106–125. [Google Scholar] [CrossRef]
- Kudryavtseva, A.V.; Krasnov, G.S.; Dmitriev, A.A.; Alekseev, B.Y.; Kardymon, O.L.; Sadritdinova, A.F.; Fedorova, M.S.; Pokrovsky, A.V.; Melnikova, N.V.; Kaprin, A.D.; et al. Mitochondrial dysfunction and oxidative stress in aging and cancer. Oncotarget 2016, 7, 44879–44905. [Google Scholar] [CrossRef]
- Dahl, J.U.; Gray, M.J.; Jakob, U. Protein quality control under oxidative stress conditions. J. Mol. Biol. 2015, 427, 1549–1563. [Google Scholar] [CrossRef]
- Corsetti, G.; Romano, C.; Stacchiotti, A.; Pasini, E.; Dioguardi, F.S. Endoplasmic reticulum stress and apoptosis triggered by sub-chronic lead exposure in mice spleen: A histopathological study. Biol. Trace Elem. Res. 2017, 178, 86–97. [Google Scholar] [CrossRef]
- Mudipalli, A. Lead hepatotoxicity & potential health effects. Indian J. Med. Res. 2007, 126, 518–527. [Google Scholar]
- Jia, Q.; Ha, X.; Yang, Z.; Hui, L.; Yang, X. Oxidative stress: A possible mechanism for lead-induced apoptosis and nephrotoxicity. Toxicol. Mech. Methods 2012, 22, 705–710. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Qi, Y.; Feng, Z.; Ma, L.; Gao, K.; Zhang, Y. Lead (Pb) induced ATM-dependent mitophagy via PINK1/Parkin pathway. Toxicol. Lett. 2018, 291, 92–100. [Google Scholar] [CrossRef]
- Almasmoum, H.; Refaat, B.; Ghaith, M.M.; Almaimani, R.A.; Idris, S.; Ahmad, J.; Abdelghany, A.H.; BaSalamah, M.A.; El-Boshy, M. Protective effect of vitamin D3 against lead induced hepatotoxicity, oxidative stress, immunosuppressive and calcium homeostasis disorders in rat. Environ. Toxicol. Pharmacol. 2019, 72, 103246. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, M.; Ghaznavi, R.; Keyhanmanesh, R.; Sadeghipour, H.R.; Naderi, R.; Mohammadi, H. Voluntary exercise prevents lead-induced elevation of oxidative stress and inflammation markers in male rat blood. Sci. World J. 2013, 2013, 320704. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Zhang, W.; Guo, J.; Zhu, Q.; Chen, H.; Xia, Y.; Zhu, G. Mitochondrion: A sensitive target for pb exposure. J. Toxicol. Sci. 2021, 46, 345–358. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.M.; Ma, J.Q.; Sun, Y.Z. Puerarin protects the rat liver against oxidative stress-mediated DNA damage and apoptosis induced by lead. Exp. Toxicol. Pathol. 2012, 64, 575–582. [Google Scholar] [CrossRef]
- Yuan, X.; Tang, C. The accumulation effect of lead on DNA damage in mice blood cells of three generations and the protection of selenium. J. Environ. Sci. Health A Tox. Hazard. Subst. Environ. Eng. 2001, 36, 501–508. [Google Scholar] [CrossRef]
- Renu, K.; Chakraborty, R.; Myakala, H.; Koti, R.; Famurewa, A.C.; Madhyastha, H.; Vellingiri, B.; George, A.; Valsala Gopalakrishnan, A. Molecular mechanism of heavy metals (Lead, chromium, arsenic, Mercury, Nickel and cadmium)—Induced hepatotoxicity—A review. Chemosphere 2021, 271, 129735. [Google Scholar] [CrossRef]
- Abd Allah, E.S.H.; Badary, D.M. Folic acid protects against lead acetate-induced hepatotoxicity by decreasing NF-κB, IL-1β production and lipid peroxidation mediataed cell injury. Pathophysiology 2017, 24, 39–44. [Google Scholar] [CrossRef]
- González Rendón, E.S.; Cano, G.G.; Alcaraz-Zubeldia, M.; Garibay-Huarte, T.; Fortoul, T.I. Lead inhalation and hepatic damage: Morphological and functional evaluation in mice. Toxicol. Ind. Health 2018, 34, 128–138. [Google Scholar] [CrossRef]
- Gonick, H.C.; Ding, Y.; Bondy, S.C.; Ni, Z.; Vaziri, N.D. Lead-induced hypertension: Interplay of nitric oxide and reactive oxygen species. Hypertension 1997, 30, 1487–1492. [Google Scholar] [CrossRef] [PubMed]
- El-Sokkary, G.H.; Nafady, A.A.; Shabash, E.H. Melatonin ameliorates cadmium-induced oxidative damage and morphological changes in the kidney of rat. Open Neuroendocrinol. J. 2009, 2, 1–9. [Google Scholar] [CrossRef]
- Perrone, P.; Spinelli, S.; Mantegna, G.; Notariale, R.; Straface, E.; Caruso, D.; Falliti, G.; Marino, A.; Manna, C.; Remigante, A.; et al. Mercury Chloride Affects Band 3 Protein-Mediated Anionic Transport in Red Blood Cells: Role of Oxidative Stress and Protective Effect of Olive Oil Polyphenols. Cells 2023, 12, 424. [Google Scholar] [CrossRef] [PubMed]
- Alshehri, S.; Ahmad, S.F.; Albekairi, N.A.; Alqarni, S.S.; Al-Harbi, N.O.; Al-Ayadhi, L.Y.; Attia, S.M.; Alfardan, A.S.; Bakheet, S.A.; Nadeem, A. Thioredoxin 1 and Thioredoxin Reductase 1 Redox System Is Dysregulated in Neutrophils of Subjects with Autism: In Vitro Effects of Environmental Toxicant, Methylmercury. Toxics 2023, 11, 739. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Cobbina, S.J.; Mao, G.; Xu, H.; Zhang, Z.; Yang, L. A review of toxicity and mechanisms of individual and mixtures of heavy metals in the environment. Environ. Sci. Pollut. Res. Int. 2016, 23, 8244–8259. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.Y.; Lee, M.J.; Lee, S.M.; Lee, W.C.; Kim, J.S. Effect of melatonin on cadmium-induced hepatotoxicity in male Sprague-Dawley rats. Tohoku J. Exp. Med. 1998, 186, 205–213. [Google Scholar] [CrossRef]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef]
- Aschner, M.; Skalny, A.V.; Martins, A.C.; Sinitskii, A.I.; Farina, M.; Lu, R.; Barbosa, F., Jr.; Gluhcheva, Y.G.; Santamaria, A.; Tinkov, A.A. Ferroptosis as a mechanism of non-ferrous metal toxicity. Arch Toxicol. 2022, 96, 2391–2417. [Google Scholar] [CrossRef]
- Lemasters, J.J. Evolution of Voltage-Dependent Anion Channel Function: From Molecular Sieve to Governator to Actuator of Ferroptosis. Front. Oncol. 2017, 7, 303. [Google Scholar] [CrossRef]
- Sui, X.; Zhang, R.; Liu, S.; Duan, T.; Zhai, L.; Zhang, M.; Han, X.; Xiang, Y.; Huang, X.; Lin, H.; et al. RSL3 Drives Ferroptosis Through GPX4 Inactivation and ROS Production in Colorectal Cancer. Front. Pharmacol. 2018, 9, 1371. [Google Scholar] [CrossRef]
- Xiao, J.; Zhang, S.; Tu, B.; Jiang, X.; Cheng, S.; Tang, Q.; Zhang, J.; Qin, X.; Wang, B.; Zou, Z.; et al. Arsenite induces ferroptosis in the neuronal cells via activation of ferritinophagy. Food Chem. Toxicol. 2021, 151, 112114. [Google Scholar] [CrossRef] [PubMed]
- Meng, P.; Zhang, S.; Jiang, X.; Cheng, S.; Zhang, J.; Cao, X.; Qin, X.; Zou, Z.; Chen, C. Arsenite induces testicular oxidative stress in vivo and in vitro leading to ferroptosis. Ecotoxicol. Environ. Saf. 2020, 194, 110360. [Google Scholar] [CrossRef] [PubMed]
- Hao, R.; Ge, J.; Song, X.; Li, F.; Sun-Waterhouse, D.; Li, D. Cadmium induces ferroptosis and apoptosis by modulating miR-34a-5p/Sirt1axis in PC12 cells. Environ. Toxicol. 2022, 37, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Zhou, J.; Wang, X.; Zhang, Y.; Wang, M.; Su, P. Cadmium attenuates testosterone synthesis by promoting ferroptosis and blocking autophagosome-lysosome fusion. Free Radic. Biol. Med. 2021, 176, 176–188. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Yang, B.; Zhang, Y.; Wang, S.; Li, F.; Xing, G.; Farina, M.; Zhang, Y.; Appiah-Kubi, K.; Tinkov, A.A.; et al. Ferroptosis contributes to methylmercury-induced cytotoxicity in rat primary astrocytes and Buffalo rat liver cells. Neurotoxicology 2022, 90, 228–236. [Google Scholar] [CrossRef]
- Ahmad, S.; Mahmood, R. Mercury chloride toxicity in human erythrocytes: Enhanced generation of ROS and RNS, hemoglobin oxidation, impaired antioxidant power, and inhibition of plasma membrane redox system. Environ. Sci. Pollut. Res. Int. 2019, 26, 5645–5657. [Google Scholar] [CrossRef]
- Chang, J.; Yang, B.; Zhou, Y.; Yin, C.; Liu, T.; Qian, H.; Xing, G.; Wang, S.; Li, F.; Zhang, Y.; et al. Acute Methylmercury Exposure and the Hypoxia-Inducible Factor-1α Signaling Pathway under Normoxic Conditions in the Rat Brain and Astrocytes in Vitro. Environ. Health Perspect. 2019, 127, 127006. [Google Scholar] [CrossRef]
- Zhou, F.; Chen, Y.; Fan, G.; Feng, C.; Du, G.; Zhu, G.; Li, Y.; Jiao, H.; Guan, L.; Wang, Z. Lead-induced iron overload and attenuated effects of ferroportin 1 overexpression in PC12 cells. Toxicol. In Vitro 2014, 28, 1339–1348. [Google Scholar] [CrossRef]
- Wagner, P.J.; Park, H.R.; Wang, Z.; Kirchner, R.; Wei, Y.; Su, L.; Stanfield, K.; Guilarte, T.R.; Wright, R.O.; Christiani, D.C.; et al. In Vitro Effects of Lead on Gene Expression in Neural Stem Cells and Associations between Up-regulated Genes and Cognitive Scores in Children. Environ. Health Perspect. 2017, 125, 721–729. [Google Scholar] [CrossRef]
- Zhu, Y.; Jiao, X.; An, Y.; Li, S.; Teng, X. Selenium against lead-induced apoptosis in chicken nervous tissues via mitochondrial pathway. Oncotarget. 2017, 8, 108130–108145. [Google Scholar] [CrossRef]
- Wang, Y.; Fang, J.; Leonard, S.S.; Rao, K.M.K. Cadmium inhibits the electron transfer chain and induces reactive oxygen species. Free Radic. Biol. Med. 2004, 36, 1434–1443. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.R.; Lin, P.D.; Rifas-Shiman, S.L.; Rahman, M.L.; Gold, D.R.; Baccarelli, A.A.; Claus Henn, B.; Amarasiriwardena, C.; Wright, R.O.; Coull, B.; et al. Prospective associations of early pregnancy metal mixtures with mitochondria DNA copy number and telomere length in maternal and cord blood. Environ. Health Perspect. 2021, 129, 117007. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.; Wang, C.; Hong, S.; Guan, X.; Meng, H.; Feng, Y.; Xiao, Y.; Zhou, Y.; Liu, C.; Zhong, G.; et al. Multiple metals exposure and blood mitochondrial DNA copy number: A cross-sectional study from the Dongfeng-Tongji cohort. Environ. Res. 2023, 216, 114509. [Google Scholar] [CrossRef] [PubMed]
- Chu, B.X.; Fan, R.F.; Lin, S.Q.; Yang, D.B.; Wang, Z.Y.; Wang, L. Interplay between autophagy and apoptosis in lead(II)-induced cytotoxicity of primary rat proximal tubular cells. J. Inorg. Biochem. 2018, 182, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Hegazy, A.M.S.; Fouad, U.A. Evaluation of lead hepatotoxicity; histological, histochemical and ultrastructural study. Forensic Med. Anat. Res. 2014, 02, 70–79. [Google Scholar] [CrossRef]
- Araragi, S.; Kondoh, M.; Kawase, M.; Saito, S.; Higashimoto, M.; Sato, M. Mercuric chloride induces apoptosis via a mitochondrial-dependent pathway in human leukemia cells. Toxicology 2003, 184, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Belyaeva, E.A.; Sokolova, T.V.; Emelyanova, L.V.; Zakharova, I.O. Mitochondrial electron transport chain in heavy metal-induced neurotoxicity: Effects of cadmium, mercury, and copper. Sci. World J. 2012, 2012, 136063. [Google Scholar] [CrossRef]
- Belyaeva, E.A.; Dymkowska, D.; Wieckowski, M.R.; Wojtczak, L. Mitochondria as an important target in heavy metal toxicity in rat hepatoma AS-30D cells. Toxicol. Appl. Pharmacol. 2008, 231, 34–42. [Google Scholar] [CrossRef]
- Belyaeva, E.A.; Korotkov, S.M. Mechanism of primary CD2+-induced rat liver mitochondria dysfunction: Discrete modes of CD2+ action on calcium and thiol-dependent domains. Toxicol. Appl. Pharmacol. 2003, 192, 56–68. [Google Scholar] [CrossRef]
- Dlamini, M.B.; Gao, Z.; Hasenbilige; Jiang, L.; Geng, C.; Li, Q.; Shi, X.; Liu, Y.; Cao, J. The crosstalk between mitochondrial dysfunction and endoplasmic reticulum stress promoted ATF4-mediated mitophagy induced by hexavalent chromium. Environ. Toxicol. 2021, 36, 1162–1172. [Google Scholar] [CrossRef]
- Ron, D.; Walter, P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat. Rev. Mol. Cell Biol. 2007, 8, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.H.; Li, H.; Yasumura, D.; Cohen, H.R.; Zhang, C.; Panning, B.; Shokat, K.M.; Lavail, M.M.; Walter, P. IRE1 signaling affects cell fate during the unfolded protein response. Science 2007, 318, 944–949. [Google Scholar] [CrossRef] [PubMed]
- Gao, K.; Zhang, C.; Tian, Y.; Naeem, S.; Zhang, Y.; Qi, Y. The role of endoplasmic reticulum stress in lead (Pb)-induced mitophagy of HEK293 cells. Toxicol. Ind. Health 2020, 36, 1002–1009. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Falahatpisheh, M.H.; Zheng, Y.; Ramos, K.S.; Tiffany-Castiglioni, E. Induction of 78 kD glucose-regulated protein (GRP78) expression and redox-regulated transcription factor activity by lead and mercury in C6 rat glioma cells. Neurotox. Res. 2001, 3, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Harris, E.D.; Zheng, Y.; Tiffany-Castiglioni, E. Lead targets GRP78, a molecular chaperone, in C6 rat glioma cells. Toxicol. Appl. Pharmacol. 2000, 163, 260–266. [Google Scholar] [CrossRef]
- Zhu, H.L.; Shi, X.T.; Xu, X.F.; Xiong, Y.W.; Yi, S.J.; Zhou, G.X.; Liu, W.B.; Huang, M.M.; Gao, L.; Zhang, C.; et al. Environmental cadmium exposure induces fetal growth restriction via triggering PERK-regulated mitophagy in placental trophoblasts. Environ. Int. 2021, 147, 106319. [Google Scholar] [CrossRef]
- Zhao, C.; Yu, D.; He, Z.; Bao, L.; Feng, L.; Chen, L.; Liu, Z.; Hu, X.; Zhang, N.; Wang, T.; et al. Endoplasmic reticulum stress-mediated autophagy activation is involved in cadmium-induced ferroptosis of renal tubular epithelial cells. Free Radic. Biol. Med. 2021, 175, 236–248. [Google Scholar] [CrossRef]
- He, Z.; Shen, P.; Feng, L.; Hao, H.; He, Y.; Fan, G.; Liu, Z.; Zhu, K.; Wang, Y.; Zhang, N.; et al. Cadmium induces liver dysfunction and ferroptosis through the endoplasmic stress-ferritinophagy axis. Ecotoxicol. Environ. Saf. 2022, 245, 114123. [Google Scholar] [CrossRef]
- Zhong, Y.; Wang, B.; Hu, S.; Wang, T.; Zhang, Y.; Wang, J.; Liu, Y.; Zhang, H. The role of endoplasmic reticulum stress in renal damage caused by acute mercury chloride poisoning. J. Toxicol. Sci. 2020, 45, 589–598. [Google Scholar] [CrossRef]
- Loos, B.; Engelbrecht, A.M. Cell death: A dynamic response concept. Autophagy 2009, 5, 590–603. [Google Scholar] [CrossRef]
- Chen, Q.; Kang, J.; Fu, C. The independence of and associations among apoptosis, autophagy, and necrosis. Signal Transduct. Target. Ther. 2018, 3, 18. [Google Scholar] [CrossRef] [PubMed]
- Tabas, I.; Ron, D. Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat. Cell Biol. 2011, 13, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Sano, R.; Reed, J.C. ER stress-induced cell death mechanisms. Biochim. Biophys. Acta 2013, 1833, 3460–3470. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Qi, W.; Geng, Y.; Wang, L.; Zhao, J.; Zhu, K.; Wu, G.; Zhang, Z.; Pan, H.; Qian, L.; et al. Necroptosis activates UPR sensors without disrupting their binding with GRP78. Proc. Natl. Acad. Sci. USA 2021, 118, e2110476118. [Google Scholar] [CrossRef] [PubMed]
- Kumar Pandey, V.K.; Mathur, A.; Fareed Khan, M.; Kakkar, P. Endoplasmic reticulum stress induces degradation of glucose transporter proteins during hyperglycemic hepatotoxicity: Role of PERK-eIF2α-ATF4 axis. Eur. J. Pharmacol. 2022, 926, 175012. [Google Scholar] [CrossRef]
- Chibowska, K.; Baranowska-Bosiacka, I.; Falkowska, A.; Gutowska, I.; Goschorska, M.; Chlubek, D. Effect of lead (Pb) on inflammatory processes in the brain. Int. J. Mol. Sci. 2016, 17, 2140. [Google Scholar] [CrossRef]
- Kasten-Jolly, J.; Heo, Y.; Lawrence, D.A. Central nervous system cytokine gene expression: Modulation by lead. J. Biochem. Mol. Toxicol. 2011, 25, 41–54. [Google Scholar] [CrossRef]
- Ahmed, M.B.; Ahmed, M.I.; Meki, A.R.; Abdraboh, N. Neurotoxic effect of lead on rats: Relationship to apoptosis. Int. J. Health Sci. 2013, 7, 192–199. [Google Scholar] [CrossRef]
- Han, B.; Kamogashira, T.; Kikuta, S.; Yamasoba, T. Endoplasmic reticulum stress associated with lead (Pb)-induced olfactory epithelium toxicity in an olfactory dark basal cell line. FEBS Open Bio. 2023; Online ahead of print. [Google Scholar] [CrossRef]
- Chen, X.; Bi, M.; Yang, J.; Cai, J.; Zhang, H.; Zhu, Y.; Zheng, Y.; Liu, Q.; Shi, G.; Zhang, Z. Cadmium exposure triggers oxidative stress, necroptosis, Th1/Th2 imbalance and promotes inflammation through the TNF-α/NF-κB pathway in swine small intestine. J. Hazard. Mater. 2022, 421, 126704. [Google Scholar] [CrossRef]
- Zhang, J.; Zheng, S.; Wang, S.; Liu, Q.; Xu, S. Cadmium-induced oxidative stress promotes apoptosis and necrosis through the regulation of the miR-216a-PI3K/AKT axis in common carp lymphocytes and antagonized by selenium. Chemosphere 2020, 258, 127341. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, S.; Liu, H.; Xu, S. MAPK/iNOS pathway is involved in swine kidney necrosis caused by cadmium exposure. Environ. Pollut. 2021, 274, 116497. [Google Scholar] [CrossRef] [PubMed]
- López, E.; Figueroa, S.; Oset-Gasque, M.J.; González, M.P. Apoptosis and necrosis: Two distinct events induced by cadmium in cortical neurons in culture. Br. J. Pharmacol. 2003, 138, 901–911. [Google Scholar] [CrossRef] [PubMed]
- Luo, T.; Yu, Q.; Dong, W.; Gong, Z.; Tan, Y.; Liu, W.; Zou, H.; Gu, J.; Yuan, Y.; Bian, J.; et al. Effect of cell cycle synchronization on cadmium-induced apoptosis and necrosis in NRK-52E cells. Cell Cycle 2020, 19, 3386–3397. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Leonard, S.S.; Ye, J.; Ding, M.; Shi, X. The role of hydroxyl radical as a messenger in Cr(VI)-induced p53 activation. Am. J. Physiol. Cell Physiol. 2000, 279, C868–C875. [Google Scholar] [CrossRef]
- Carlisle, D.L.; Pritchard, D.E.; Singh, J.; Patierno, S.R. Chromium(VI) induces p53-dependent apoptosis in diploid human lung and mouse dermal fibroblasts. Mol. Carcinog. 2000, 28, 111–118. [Google Scholar] [CrossRef]
- Pritchard, D.E.; Singh, J.; Carlisle, D.L.; Patierno, S.R. Cyclosporin A inhibits chromium(VI)-induced apoptosis and mitochondrial cytochrome c release and restores clonogenic survival in CHO cells. Carcinogenesis 2000, 21, 2027–2033. [Google Scholar] [CrossRef]
- Wallace, D.R.; Taalab, Y.M.; Heinze, S.; Tariba Lovaković, B.; Pizent, A.; Renieri, E.; Tsatsakis, A.; Farooqi, A.A.; Javorac, D.; Andjelkovic, M.; et al. Toxic-metal-induced alteration in miRNA expression profile as a proposed mechanism for disease development. Cells 2020, 9, 901. [Google Scholar] [CrossRef]
- Rosolen, D.; Nunes-Souza, E.; Marchi, R.; Tofolo, M.V.; Antunes, V.C.; Berti, F.C.B.; Fonseca, A.S.; Cavalli, L.R. MiRNAs action and impact on mitochondria function, metabolic reprogramming and chemoresistance of cancer cells: A systematic review. Biomedicines 2023, 11, 693. [Google Scholar] [CrossRef]
- Aalami, A.H.; Hoseinzadeh, M.; Hosseini Manesh, P.; Jiryai Sharahi, A.; Kargar Aliabadi, E. Carcinogenic effects of heavy metals by inducing dysregulation of microRNAs: A review. Mol. Biol. Rep. 2022, 49, 12227–12238. [Google Scholar] [CrossRef]
- Barrell, B.G.; Bankier, A.T.; Drouin, J. A different genetic code in human mitochondria. Nature 1979, 282, 189–194. [Google Scholar] [CrossRef]
- Das, S.; Ferlito, M.; Kent, O.A.; Fox-Talbot, K.; Wang, R.; Liu, D.; Raghavachari, N.; Yang, Y.; Wheelan, S.J.; Murphy, E.; et al. Nuclear miRNA regulates the mitochondrial genome in the heart. Circ. Res. 2012, 110, 1596–1603. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.Q.; Wang, S.Q.; Chen, Y.; Fu, L.Y.; Xu, Y.N.; Li, L.; Tao, L.; Shen, X.C. MicroRNAs regulating mitochondrial function in cardiac diseases. Front. Pharmacol. 2021, 12, 663322. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.; Dai, J.; Qu, T.; He, Z. Emerging roles of microRNAs and long noncoding RNAs in cadmium toxicity. Biol. Trace Elem. Res. 2020, 195, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Fay, M.J.; Alt, L.A.C.; Ryba, D.; Salamah, R.; Peach, R.; Papaeliou, A.; Zawadzka, S.; Weiss, A.; Patel, N.; Rahman, A.; et al. Cadmium nephrotoxicity is associated with altered microRNA expression in the rat renal cortex. Toxics 2018, 6, 16. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Gu, D.; Zhou, M.; Shi, H.; Yan, S.; Cai, Y. Regulatory role of miR-125a/b in the suppression by selenium of cadmium-induced apoptosis via the mitochondrial pathway in LLC-PK1 cells. Chem. Biol. Interact. 2016, 243, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Shen, H.; Li, X.; Li, Z.; Liu, Z.; Xu, J.; Ma, S.; Zhao, X.; Bai, X.; Li, M.; et al. MiR-125a-5p decreases after long non-coding RNA HOTAIR knockdown to promote cancer cell apoptosis by releasing caspase 2. Cell Death Dis. 2016, 7, e2137. [Google Scholar] [CrossRef] [PubMed]
- Fabbri, M.; Urani, C.; Sacco, M.G.; Procaccianti, C.; Gribaldo, L. Whole genome analysis and microRNAs regulation in HepG2 cells exposed to cadmium. ALTEX 2012, 29, 173–182. [Google Scholar] [CrossRef]
- Chen, M.; Li, X.; Fan, R.; Yang, J.; Jin, X.; Hamid, S.; Xu, S. Cadmium induces BNIP3-dependent autophagy in chicken spleen by modulating miR-33-AMPK axis. Chemosphere 2018, 194, 396–402. [Google Scholar] [CrossRef]
- Hassan, F.; Nuovo, G.J.; Crawford, M.; Boyaka, P.N.; Kirkby, S.; Nana-Sinkam, S.P.; Cormet-Boyaka, E. MiR-101 and miR-144 regulate the expression of the CFTR chloride channel in the lung. PLoS ONE 2012, 7, e50837. [Google Scholar] [CrossRef]
- Weng, S.; Wang, W.; Li, Y.; Li, H.; Lu, X.; Xiao, S.; Wu, T.; Xie, M.; Zhang, W. Continuous cadmium exposure from weaning to maturity induces downregulation of ovarian follicle development-related SCF/c-kit gene expression and the corresponding changes of DNA methylation/microRNA pattern. Toxicol. Lett. 2014, 225, 367–377. [Google Scholar] [CrossRef]
- Liu, K.X.; Chen, G.P.; Lin, P.L.; Huang, J.C.; Lin, X.; Qi, J.C.; Lin, Q.C. Detection and analysis of apoptosis- and autophagy-related miRNAs of mouse vascular endothelial cells in chronic intermittent hypoxia model. Life Sci. 2018, 193, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Lu, Q.; Cao, X.; Wang, K.; Wang, Y.; Wu, Y.; Yang, Z. Lead exposure promotes the inflammation via the circRNA-05280/miR-146a/IRAK1 axis in mammary gland. Ecotoxicol. Environ. Saf. 2022, 247, 114204. [Google Scholar] [CrossRef] [PubMed]
- Jing, H.; Zhang, Q.; Li, S.; Gao, X.J. Pb exposure triggers MAPK-dependent inflammation by activating oxidative stress and miRNA-155 expression in carp head kidney. Fish Shellfish Immunol. 2020, 106, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Mijit, M.; Caracciolo, V.; Melillo, A.; Amicarelli, F.; Giordano, A. Role of p53 in the regulation of cellular senescence. Biomolecules 2020, 10, 420. [Google Scholar] [CrossRef] [PubMed]
- Phatak, V.M.; Muller, P.A.J. Metal toxicity and the p53 protein: An intimate relationship. Toxicol. Res. 2015, 4, 576–591. [Google Scholar] [CrossRef]
- Méplan, C.; Mann, K.; Hainaut, P. Cadmium induces conformational modifications of wild-type p53 and suppresses p53 response to DNA damage in cultured cells. J. Biol. Chem. 1999, 274, 31663–31670. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Gu, R.; Ouyang, H.; Wang, L.; Shi, S.; Ji, Y.; Bao, B.; Liao, G.; Xu, B. Cadmium exposure induces osteoporosis through cellular senescence, associated with activation of NF-κB pathway and mitochondrial dysfunction. Environ. Pollut. 2021, 290, 118043. [Google Scholar] [CrossRef] [PubMed]
- Yuan, D.; Ye, S.; Pan, Y.; Bao, Y.; Chen, H.; Shao, C. Long-term cadmium exposure leads to the enhancement of lymphocyte proliferation via down-regulating p16 by DNA hypermethylation. Mutat. Res. 2013, 757, 125–131. [Google Scholar] [CrossRef]
- Yohannes, Y.B.; Nakayama, S.M.; Yabe, J.; Nakata, H.; Toyomaki, H.; Kataba, A.; Muzandu, K.; Ikenaka, Y.; Choongo, K.; Ishizuka, M. Blood lead levels and aberrant DNA methylation of the ALAD and p16 gene promoters in children exposed to environmental-lead. Environ. Res. 2020, 188, 109759. [Google Scholar] [CrossRef]
- Kondo, K.; Takahashi, Y.; Hirose, Y.; Nagao, T.; Tsuyuguchi, M.; Hashimoto, M.; Ochiai, A.; Monden, Y.; Tangoku, A. The reduced expression and aberrant methylation of p16(INK4a) in chromate workers with lung cancer. Lung Cancer 2006, 53, 295–302. [Google Scholar] [CrossRef]
- Hu, G.; Li, P.; Li, Y.; Wang, T.; Gao, X.; Zhang, W.; Jia, G. Methylation levels of P16 and TP53 that are involved in DNA strand breakage of 16HBE cells treated by hexavalent chromium. Toxicol. Lett. 2016, 249, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Genova, M.L.; Lenaz, G. The Interplay Between Respiratory Supercomplexes and ROS in Aging. Antioxid Redox Signal. 2015, 23, 208–238. [Google Scholar] [CrossRef] [PubMed]
- Bjelakovic, G.; Nikolova, D.; Gluud, C. Antioxidant supplements and mortality. Curr. Opin. Clin. Nutr. Metab. Care 2014, 17, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Fimognari, C. Role of Oxidative RNA Damage in Chronic-Degenerative Diseases. Oxid. Med. Cell Longev. 2015, 2015, 358713. [Google Scholar] [CrossRef]
- Barja, G. The mitochondrial free radical theory of aging. Prog. Mol. Biol. Transl. Sci. 2014, 127, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.S.; Yu, W.S.; Lim, L.W. Exploring ER stress response in cellular aging and neuroinflammation in Alzheimer’s disease. Ageing Res. Rev. 2021, 70, 1014–1017. [Google Scholar] [CrossRef]
- Youssef, S.A.; Capucchio, M.T.; Rofina, J.E.; Chambers, J.K.; Uchida, K.; Nakayama, H.; Head, E. Pathology of the Aging Brain in Domestic and Laboratory Animals, and Animal Models of Human Neurodegenerative Diseases. Vet. Pathol. 2016, 53, 327–348. [Google Scholar] [CrossRef] [PubMed]
- Perrelli, M.; Wu, R.; Liu, D.J.; Lucchini, R.G.; Del Bosque-Plata, L.; Vergare, M.J.; Akhter, M.P.; Ott, J.; Gragnoli, C. Heavy metals as risk factors for human diseases—A Bayesian network approach. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 9275–9310. [Google Scholar] [CrossRef]
- Gianì, F.; Masto, R.; Trovato, M.A.; Malandrino, P.; Russo, M.; Pellegriti, G.; Vigneri, P.; Vigneri, R. Heavy metals in the environment and thyroid cancer. Cancers 2021, 13, 4052. [Google Scholar] [CrossRef]
- Dumkova, J.; Vrlikova, L.; Vecera, Z.; Putnova, B.; Docekal, B.; Mikuska, P.; Fictum, P.; Hampl, A.; Buchtova, M. Inhaled Cadmium Oxide Nanoparticles: Their in Vivo Fate and Effect on Target Organs. Int. J. Mol. Sci. 2016, 17, 874. [Google Scholar] [CrossRef]
- Seidal, K.; Jörgensen, N.; Elinder, C.G.; Sjögren, B.; Vahter, M. Fatal cadmium-induced pneumonitis. Scand. J. Work Environ. Health 1993, 19, 429–431. [Google Scholar] [CrossRef] [PubMed]
- Civantos, D.P.; López Rodríguez, A.L.; Aguado-Borruey, J.M.; Narvaez, J.A.J. Fulminant malignant arrythmia and multiorgan failure in acute arsenic poisoning. Chest 1995, 108, 1774–1775. [Google Scholar] [CrossRef] [PubMed]
- Vij, A.G. Hemopoietic, Hemostatic and Mutagenic Effects of Lead and Possible Prevention by Zinc and Vitamin C. Al Ameen J. Med. Sci. 2009, 2, 27–36. [Google Scholar]
- Das, K.K.; Buchner, V. Effect of nickel exposure on peripheral tissues: Role of oxidative stress in toxicity and possible protection by ascorbic acid. Rev. Environ. Health 2007, 22, 157–173. [Google Scholar] [CrossRef] [PubMed]
- Das, K.K.; Das Gupta, A.D.; Dhundasi, S.A.; Patil, A.M.; Das, S.N.; Ambekar, J.G. Effect of L-Ascorbic Acid on Nickel-Induced Alterations in Serum Lipid Profiles and Liver Histopathology in Rats. J. Basic Clin. Physiol. Pharmacol. 2006, 17, 29–44. [Google Scholar] [CrossRef]
- Sunderman, F., Jr.; Coulston, F.; Eichhorn, G. Nickel; National Academy of Sciences: Washington, DC, USA, 1975. [Google Scholar]
- Rikans, L.E.; Yamano, T. Mechanisms of cadmium-mediated acute hepatotoxicity. J. Biochem. Mol. Toxicol. 2000, 14, 110–117. [Google Scholar] [CrossRef]
- Karri, V.; Schuhmacher, M.; Kumar, V. A systems toxicology approach to compare the heavy metal mixtures (Pb, As, MeHg) impact in neurodegenerative diseases. Food Chem. Toxicol. 2020, 139, 111257. [Google Scholar] [CrossRef]
- Jomova, K.; Vondrakova, D.; Lawson, M.; Valko, M. Metals, oxidative stress and neurodegenerative disorders. Mol. Cell. Biochem. 2010, 345, 91–104. [Google Scholar] [CrossRef]
- Dec, G. The Cellular Effect of Lead Poisoning and Its Clinical Picture. Geirget. Undergrad. J. Heal. Sci. 2014, 5, 1–19. [Google Scholar]
- Drew, C.A.; Spence, I.; Johnston, G.A.R. Effects of lead salts on the uptake, release, and binding of γ-aminobutyric acid: The importance of buffer composition. J. Neurochem. 1989, 52, 433–440. [Google Scholar] [CrossRef]
- Jia, Q.; Du, G.; Li, Y.; Wang, Z.; Xie, J.; Gu, J.; Yin, G.; Zhang, S.; Gao, Y.; Zhou, F.; et al. Pb2+ modulates ryanodine receptors from the endoplasmic reticulum in rat brain. Toxicol. Appl. Pharmacol. 2018, 338, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Liu, L.; Huang, S. Cadmium activates the mitogen-activated protein kinase (MAPK) pathway via induction of reactive oxygen species and inhibition of protein phosphatases 2A and 5. Free Radic. Biol. Med. 2008, 45, 1035–1044. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.F.; Yao, T.M.; Zhu, Z.L.; Wang, C.; Ji, L.N. Impacts of Cd(II) on the conformation and self-aggregation of Alzheimer’s tau fragment corresponding to the third repeat of microtubule-binding domain. Biochim. Biophys. Acta 2007, 1774, 1414–1421. [Google Scholar] [CrossRef] [PubMed]
- Monnet-Tschudi, F.; Zurich, M.G.; Boschat, C.; Corbaz, A.; Honegger, P. Involvement of environmental mercury and lead in the etiology of neurodegenerative diseases. Rev. Environ. Health 2006, 21, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Mutter, J.; Curth, A.; Naumann, J.; Deth, R.; Walach, H. Does inorganic mercury play a role in Alzheimer’s disease? A systematic review and an integrated molecular mechanism. J. Alzheimers Dis. 2010, 22, 357–374. [Google Scholar] [CrossRef] [PubMed]
- Johnson, F.O.; Atchison, W.D. The role of environmental mercury, lead and pesticide exposure in development of amyotrophic lateral sclerosis. Neurotoxicology 2009, 30, 761–765. [Google Scholar] [CrossRef] [PubMed]
- Harada, M. Minamata disease: Methylmercury poisoning in Japan caused by environmental pollution. Crit. Rev. Toxicol. 1995, 25, 1–24. [Google Scholar] [CrossRef]
- Pyatha, S.; Kim, H.; Lee, D.; Kim, K. Co-exposure to lead, mercury, and cadmium induces neurobehavioral impairments in mice by interfering with dopaminergic and serotonergic neurotransmission in the striatum. Front Public Health. 2023, 11, 1265864. [Google Scholar] [CrossRef]
- da Cunha Martins, A.; Carneiro, M.F.H.; Grotto, D.; Adeyemi, J.A.; Barbosa, F. Arsenic, cadmium, and mercury-induced hypertension: Mechanisms and epidemiological findings. J. Toxicol. Environ. Health B Crit. Rev. 2018, 21, 61–82. [Google Scholar] [CrossRef]
- Carmignani, M.; Boscolo, P.; Poma, A.; Volpe, A.R. Kininergic system and arterial hypertension following chronic exposure to inorganic lead. Immunopharmacology 1999, 44, 105–110. [Google Scholar] [CrossRef]
- Alissa, E.M.; Ferns, G.A. Heavy metal poisoning and cardiovascular disease. J. Toxicol. 2011, 2011, 870125. [Google Scholar] [CrossRef] [PubMed]
- Navas-Acien, A.; Guallar, E.; Silbergeld, E.K.; Rothenberg, S.J. Lead exposure and cardiovascular disease—A systematic review. Environ. Health Perspect. 2007, 115, 472–482. [Google Scholar] [CrossRef] [PubMed]
- Nawrot, T.S.; Thijs, L.; Den Hond, E.M.; Roels, H.A.; Staessen, J.A. An epidemiological re-appraisal of the association between blood pressure and blood lead: A meta-analysis. J. Hum. Hypertens. 2002, 16, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Lustberg, M.; Silbergeld, E. Blood lead levels and mortality. Arch. Intern. Med. 2002, 162, 2443–2449. [Google Scholar] [CrossRef]
- Navas-Acien, A.; Selvin, E.; Sharrett, A.R.; Calderon-Aranda, E.; Silbergeld, E.; Guallar, E. Lead, cadmium, smoking, and increased risk of peripheral arterial disease. Circulation 2004, 109, 3196–3201. [Google Scholar] [CrossRef] [PubMed]
- Messner, B.; Knoflach, M.; Seubert, A.; Ritsch, A.; Pfaller, K.; Henderson, B.; Shen, Y.H.; Zeller, I.; Willeit, J.; Laufer, G.; et al. Cadmium is a novel and independent risk factor for early atherosclerosis mechanisms and in vivo relevance. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 1392–1398. [Google Scholar] [CrossRef]
- Vallée, A.; Gabet, A.; Grave, C.; Blacher, J.; Olié, V. Associations between urinary cadmium levels, blood pressure, and hypertension: The ESTEBAN survey. Environ. Sci. Pollut. Res. Int. 2020, 27, 10748–10756. [Google Scholar] [CrossRef]
- Tellez-Plaza, M.; Jones, M.R.; Dominguez-Lucas, A.; Guallar, E.; Navas-Acien, A. Cadmium exposure and clinical cardiovascular disease: A systematic review topical collection on nutrition. Curr. Atheroscler. Rep. 2013, 15, 1–15. [Google Scholar] [CrossRef]
- Soderland, P.; Lovekar, S.; Weiner, D.E.; Brooks, D.R.; Kaufman, J.S. Chronic kidney disease associated with environmental toxins and exposures. Adv. Chronic Kidney Dis. 2010, 17, 254–264. [Google Scholar] [CrossRef]
- Swenberg, J.A.; Short, B.; Borghoff, S.; Strasser, J.; Charbonneau, M. The comparative pathobiology of α2u-globulin nephropathy. Toxicol. Appl. Pharmacol. 1989, 97, 35–46. [Google Scholar] [CrossRef]
- Goyer, R.A. Toxic and essential metal interactions. Annu. Rev. Nutr. 1997, 17, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Cárdenas, A.; Roels, H.; Bernard, A.M.; Barbon, R.; Buchet, J.P.; Lauwerys, R.R.; Roselló, J.; Ramis, I.; Mutti, A.; Franchini, I. Markers of early renal changes induced by industrial pollutants. II. Application to workers exposed to lead. Br. J. Ind. Med. 1993, 50, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Orr, S.E.; Bridges, C.C. Chronic kidney disease and exposure to nephrotoxic metals. Int. J. Mol. Sci. 2017, 18, 1039. [Google Scholar] [CrossRef] [PubMed]
- Prozialeck, W.C.; Edwards, J.R. Mechanisms of cadmium-induced proximal tubule injury: New insights with implications for biomonitoring and therapeutic interventions. J. Pharmacol. Exp. Ther. 2012, 343, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Hellström, L.; Elinder, C.G.; Dahlberg, B.; Lundberg, M.; Järup, L.; Persson, B.; Axelson, O. Cadmium exposure and end-stage renal disease. Am. J. Kidney Dis. 2001, 38, 1001–1008. [Google Scholar] [CrossRef]
- Järup, L.; Elinder, C.G. Incidence of renal stones among cadmium exposed battery workers. Br. J. Ind. Med. 1993, 50, 598–602. [Google Scholar] [CrossRef]
- Lund, B.O.; Miller, D.M.; Woods, J.S. Studies on Hg(II)-induced H2O2 formation and oxidative stress in vivo and in vitro in rat kidney mitochondria. Biochem. Pharmacol. 1993, 45, 2017–2024. [Google Scholar] [CrossRef]
- Bridges, C.C.; Joshee, L.; Zalups, R.K. Multidrug resistance proteins and the renal elimination of inorganic mercury mediated by 2,3-dimercaptopropane-1-sulfonic acid and meso-2,3-dimercaptosuccinic acid. J. Pharmacol. Exp. Ther. 2008, 324, 383–390. [Google Scholar] [CrossRef]
- Delnomdedieu, M.; Basti, M.M.; Styblo, M.; Otvos, J.D.; Thomas, D.J. Complexation of arsenic species in rabbit erythrocytes. Chem. Res. Toxicol. 1994, 7, 621–627. [Google Scholar] [CrossRef]
- Jha, V.; Garcia-Garcia, G.; Iseki, K.; Li, Z.; Naicker, S.; Plattner, B.; Saran, R.; Wang, A.Y.; Yang, C.W. Chronic kidney disease: Global dimension and perspectives. Lancet 2013, 382, 260–272. [Google Scholar] [CrossRef]
- Tsao, D.A.; Tseng, W.C.; Chang, H.R. RKIP expression of liver and kidney after arsenic exposure. Environ. Toxicol. 2017, 32, 1079–1082. [Google Scholar] [CrossRef] [PubMed]
- Renugadevi, J.; Prabu, S.M. Cadmium-induced hepatotoxicity in rats and the protective effect of naringenin. Exp. Toxicol. Pathol. 2010, 62, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Karmakar, R.; Bhattacharya, R.; Chatterjee, M. Biochemical, haematological and histopathological study in relation to time-related cadmium-induced hepatotoxicity in mice. BioMetals 2000, 13, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Haleagrahara, N.; Jackie, T.; Chakravarthi, S.; Rao, M.; Kulur, A. Protective effect of Etlingera elatior (torch ginger) extract on lead acetate—Induced hepatotoxicity in rats. J. Toxicol. Sci. 2010, 35, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Dongre, N.; Suryakar, A.; Patil, A.; Rathi, D.B. Occupational Lead Exposure in Automobile Workers in North Karnataka (India): Effect on Liver and Kidney Functions. Al Ameen J. Med. Sci. 2010, 3, 284–292. Available online: https://www.academia.edu/ (accessed on 25 December 2023).
- Xiao, F.; Feng, X.; Zeng, M.; Guan, L.; Hu, Q.; Zhong, C. Hexavalent chromium induces energy metabolism disturbance and p53-dependent cell cycle arrest via reactive oxygen species in L-02 hepatocytes. Mol. Cell. Biochem. 2012, 371, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Muthumani, M.; Prabu, S.M. Silibinin potentially protects arsenic-induced oxidative hepatic dysfunction in rats. Toxicol. Mech. Methods 2012, 22, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Mazumder, D.N. Effect of chronic intake of arsenic-contaminated water on liver. Toxicol. Appl. Pharmacol. 2005, 206, 169–175. [Google Scholar] [CrossRef]
- Wirth, J.J.; Mijal, R.S. Adverse effects of low level heavy metal exposure on male reproductive function. Syst. Biol. Reprod. Med. 2010, 56, 147–167. [Google Scholar] [CrossRef]
- Rzymski, P.; Tomczyk, K.; Rzymski, P.; Poniedziałek, B.; Opala, T.; Wilczak, M. Impact of heavy metals on the female reproductive system. Ann. Agric. Environ. Med. 2015, 22, 259–264. [Google Scholar] [CrossRef]
- Patel, N.; Chauhan, D.; Shahane, S.; Rai, D.; Ali Khan, M.Z.; Mishra, U.; Chaudhary, V.K. Contamination and Health Impact of Heavy Metals. In Water Pollution and Remediation: Heavy Metals; Springer: Berlin/Heidelberg, Germany, 2021; pp. 259–280. [Google Scholar] [CrossRef]
- Hutchison, J.M.; Rau, D.C.; DeRouchey, J.E. Role of disulfide bonds on DNA packaging forces in bull sperm chromatin. Biophys. J. 2017, 113, 1925–1933. [Google Scholar] [CrossRef] [PubMed]
- Pasha, H.F.; Rezk, N.A.; Selim, S.A.; Abd El Motteleb, D.M. Therapeutic effect of spermatogonial stem cell on testicular damage caused by lead in rats. Gene 2016, 592, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Xing, X.; Zhang, Y.; Zhang, C.; Wu, Y.; Chen, Y.; Meng, R.; Jia, H.; Cheng, Y.; Zhang, Y.; et al. Lead exposure activates the Nrf2/Keap1 pathway, aggravates oxidative stress, and induces reproductive damage in female mice. Ecotoxicol. Environ. Saf. 2021, 207, 111231. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Sharma, A. Cadmium toxicity: Effects on human reproduction and fertility. Rev. Environ. Health 2019, 34, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Flora, S.J.S.; Agrawal, S. Arsenic, cadmium, and lead. In Reproductive and Developmental Toxicology, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2017; Chapter 31; pp. 537–566. [Google Scholar] [CrossRef]
- Hsu, P.C.; Chang, H.Y.; Guo, Y.L.; Liu, Y.C.; Shih, T.S. Effect of smoking on blood lead levels in workers and role of reactive oxygen species in lead-induced sperm chromatin DNA damage. Fertil. Steril. 2009, 91, 1096–1103. [Google Scholar] [CrossRef] [PubMed]
- Wani, A.L.; Ara, A.; Usmani, J.A. Lead toxicity: A review. Interdiscip. Toxicol. 2015, 8, 55–64. [Google Scholar] [CrossRef]
- Chai, Z.; Zhao, C.; Jin, Y.; Wang, Y.; Zou, P.; Ling, X.; Yang, H.; Zhou, N.; Chen, Q.; Sun, L.; et al. Generating adverse outcome pathway (AOP) of inorganic arsenic-induced adult male reproductive impairment via integration of phenotypic analysis in comparative toxicogenomics database (CTD) and AOP wiki. Toxicol. Appl. Pharmacol. 2021, 411, 115370. [Google Scholar] [CrossRef]
- Zubair, M.; Ahmad, M.; Qureshi, Z.I. Review on arsenic-induced toxicity in male reproductive system and its amelioration. Andrologia 2017, 49, e12791. [Google Scholar] [CrossRef]
- Cobbina, S.J.; Chen, Y.; Zhou, Z.; Wu, X.; Zhao, T.; Zhang, Z.; Feng, W.; Wang, W.; Li, Q.; Wu, X.; et al. Toxicity assessment due to sub-chronic exposure to individual and mixtures of four toxic heavy metals. J. Hazard. Mater. 2015, 294, 109–120. [Google Scholar] [CrossRef]
- Hagar, H.; Al Malki, W. Betaine supplementation protects against renal injury induced by cadmium intoxication in rats: Role of oxidative stress and caspase-3. Environ. Toxicol. Pharmacol. 2014, 37, 803–811. [Google Scholar] [CrossRef]
- Ema, M.; Okuda, H.; Gamo, M.; Honda, K. A review of reproductive and developmental toxicity of silver nanoparticles in laboratory animals. Reprod. Toxicol. 2017, 67, 149–164. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; An, J.; Xie, D.; Gong, S.; Lian, X.; Liu, Z.; Shen, Y.; Li, Y. Suppression and recovery of reproductive behavior induced by early life exposure to mercury in zebrafish. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2021, 239, 108876. [Google Scholar] [CrossRef] [PubMed]
- Ommati, M.M.; Heidari, R. Amino acids ameliorate heavy metals-induced oxidative stress in male/female reproductive tissue. In Toxicology Oxidative Stress and Dietary Antioxidants; Elsevier: Amsterdam, The Netherlands, 2020; Chapter 37; pp. 371–386. [Google Scholar] [CrossRef]
- Pollack, A.Z.; Schisterman, E.F.; Goldman, L.R.; Mumford, S.L.; Albert, P.S.; Jones, R.L.; Wactawski-Wende, J. Cadmium, lead, and mercury in relation to reproductive hormones and anovulation in premenopausal women. Environ. Health Perspect. 2011, 119, 1156–1161. [Google Scholar] [CrossRef] [PubMed]
- Engwa, G.A.; Ferdinand, P.U.; Nwalo, F.N.; Unachukwu, M.N. Mechanism and health effects of heavy metal toxicity in humans. In Poisoning in the Modern World: New Tricks for an Old Dog? BoD–Books on Demand: Norderstedt, Germany, 2019; Volume 10, pp. 70–90. [Google Scholar] [CrossRef]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy metal toxicity and the environment. EXS 2012, 101, 133–164. [Google Scholar] [CrossRef]
- Parida, L.; Patel, T.N. Systemic impact of heavy metals and their role in cancer development: A review. Environ. Monit. Assess. 2023, 195, 766. [Google Scholar] [CrossRef]
- Qin, X.J.; Hudson, L.G.; Liu, W.; Ding, W.; Cooper, K.L.; Liu, K.J. Dual actions involved in arsenite-induced oxidative DNA damage. Chem. Res. Toxicol. 2008, 21, 1806–1813. [Google Scholar] [CrossRef]
- Kitchin, K.T. Recent advances in arsenic carcinogenesis: Modes of action, animal model systems, and methylated arsenic metabolites. Toxicol. Appl. Pharmacol. 2001, 172, 249–261. [Google Scholar] [CrossRef]
- Martinez, V.D.; Thu, K.L.; Vucic, E.A.; Hubaux, R.; Adonis, M.; Gil, L.; MacAulay, C.; Lam, S.; Lam, W.L. Whole-genome sequencing analysis identifies a distinctive mutational spectrum in an arsenic-related lung tumor. J. Thorac. Oncol. 2013, 8, 1451–1455. [Google Scholar] [CrossRef]
- Joseph, P.; Muchnok, T.K.; Klishis, M.L.; Roberts, J.R.; Antonini, J.M.; Whong, W.Z.; Ong, T. Cadmium-Induced Cell Transformation and tumorigenesis Are Associated with Transcriptional Activation of c-fos, c-Jun, and c-myc Proto-Oncogenes: Role of cellular calcium and reactive oxygen species. Toxicol. Sci. 2001, 61, 295–303. [Google Scholar] [CrossRef]
- Valko, M.; Morris, H.; Cronin, M.T. Metals, toxicity and oxidative stress. Curr. Med. Chem. 2005, 12, 1161–1208. [Google Scholar] [CrossRef]
- Goering, P.L.; Waalkes, M.P.; Klaassen, C.D. Toxicology of Cadmium; Springer: Berlin/Heidelberg, Germany, 1995; pp. 189–214. [Google Scholar] [CrossRef]
- Jin, T.; Nordberg, M.; Frech, W.; Dumont, X.; Bernard, A.; Ye, T.T.; Kong, Q.; Wang, Z.; Li, P.; Lundström, N.G.; et al. Cadmium biomonitoring and renal dysfunction among a population environmentally exposed to cadmium from smelting in China (ChinaCad). Biometals 2002, 15, 397–410. [Google Scholar] [CrossRef] [PubMed]
- Benton, M.A.; Rager, J.E.; Smeester, L.; Fry, R.C. Comparative genomic analyses identify common molecular pathways modulated upon exposure to low doses of arsenic and cadmium. BMC Genom. 2011, 12, 173. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, C.J.; Stapleton, S.R. Early sensing and gene expression profiling under a low dose of cadmium exposure. Biochimie 2009, 91, 329–343. [Google Scholar] [CrossRef] [PubMed]
- Flora, S.J.S.; Pachauri, V. Chelation in metal intoxication. Int. J. Environ. Res. Public Health 2010, 7, 2745–2788. [Google Scholar] [CrossRef] [PubMed]
- Sears, M.E. Chelation: Harnessing and enhancing heavy metal detoxification—A review. Sci. World J. 2013, 2013, 219840. [Google Scholar] [CrossRef]
- Kim, J.J.; Kim, Y.S.; Kumar, V. Heavy metal toxicity: An update of chelating therapeutic strategies. J. Trace Elem. Med. Biol. 2019, 54, 226–231. [Google Scholar] [CrossRef]
- Blaurock-Busch, E.; Busch, Y.M. Comparison of Chelating Agents DMPS, DMSA and EDTA for the Diagnosis and Treatment of Chronic Metal Exposure. Br. J. Med. Med. Res. 2014, 4, 1821. [Google Scholar] [CrossRef]
- Guha Mazumder, D.N.; De, B.K.; Santra, A.; Ghosh, N.; Das, S.; Lahiri, S.; Das, T. Randomized placebo-controlled trial of 2,3-dimercapto-1-propanesulfonate (DMPS) in therapy of chronic arsenicosis due to drinking arsenic-contaminated water. J. Toxicol. Clin. Toxicol. 2001, 39, 665–674. [Google Scholar] [CrossRef]
- Miller, A.L. Dimercaptosuccinic acid (DMSA), a non-toxic, water-soluble treatment for heavy metal toxicity. Altern. Med. Rev. 1998, 3, 199–207. Available online: http://www.ncbi.nlm.nih.gov/pubmed/9630737 (accessed on 25 December 2023).
- Aaseth, J.; Crisponi, G.; Anderson, O. Chelation Therapy in the Treatment of Metal Intoxication; Academic Press: Cambridge, MA, USA, 2016. [Google Scholar]
- Cao, Y.; Skaug, M.A.; Andersen, O.; Aaseth, J. Chelation therapy in intoxications with mercury, lead and copper. J. Trace Elem. Med. Biol. 2015, 31, 188–192. [Google Scholar] [CrossRef]
- Horn, N.; Møller, L.B.; Nurchi, V.M.; Aaseth, J. Chelating principles in Menkes and Wilson diseases: Choosing the right compounds in the right combinations at the right time. J. Inorg. Biochem. 2019, 190, 98–112. [Google Scholar] [CrossRef] [PubMed]
- Britton, R.S.; Leicester, K.L.; Bacon, B.R. Iron toxicity and chelation therapy. Int. J. Hematol. 2002, 76, 219–228. [Google Scholar] [CrossRef]
- Padayatty, S.J.; Katz, A.; Wang, Y.; Eck, P.; Kwon, O.; Lee, J.H.; Chen, S.; Corpe, C.; Dutta, A.; Dutta, S.K.; et al. Vitamin C as an antioxidant: Evaluation of its role in disease prevention. J. Am. Coll. Nutr. 2003, 22, 18–35. [Google Scholar] [CrossRef] [PubMed]
- Kaźmierczak-Barańska, J.; Boguszewska, K.; Adamus-Grabicka, A.; Karwowski, B.T. Two Faces of Vitamin C-Antioxidative and Pro-Oxidative Agent. Nutrients 2020, 12, 1501. [Google Scholar] [CrossRef] [PubMed]
- Traber, M.G.; Stevens, J.F. Vitamins C and E: Beneficial effects from a mechanistic perspective. Free Radic. Biol. Med. 2011, 51, 1000–1013. [Google Scholar] [CrossRef] [PubMed]
- Saberi, E.; Mohammadrezaei, F.M.; Jazayeri, O.; Fathi, N.; Moghadam, A.H. Astaxanthin induces the expression of CatSper1 gene and protects sperms in toxicity induced by cadmium in mice. Drug Res. 2021, 71, 512–519. [Google Scholar] [CrossRef]
- Bayramoglu Akkoyun, M.; Temel, Y.; Bengü, A.Ş.; Akkoyun, H.T. Ameliorative effects of astaxanthin against copper(II) ion-induced alteration of pentose phosphate pathway and antioxidant system enzymes in rats. Environ. Sci. Pollut. Res. Int. 2021, 28, 62919–62926. [Google Scholar] [CrossRef]
- Li, D.; Tong, W.; Liu, D.; Zou, Y.; Zhang, C.; Xu, W. Astaxanthin mitigates cobalt cytotoxicity in the MG-63 cells by modulating the oxidative stress. BMC Pharmacol. Toxicol. 2017, 18, 58. [Google Scholar] [CrossRef]
- Abu-El-Zahab, H.S.H.; Hamza, R.Z.; Montaser, M.M.; El-Mahdi, M.M.; Al-Harthi, W.A. Antioxidant, antiapoptotic, antigenotoxic, and hepatic ameliorative effects of L-carnitine and selenium on cadmium-induced hepatotoxicity and alterations in liver cell structure in male mice. Ecotoxicol. Environ. Saf. 2019, 173, 419–428. [Google Scholar] [CrossRef]
- Iftikhar, A.; Akhtar, M.F.; Saleem, A.; Riaz, A.; Zehravi, M.; Rahman, M.H.; Md Ashraf, G. Comparative potential of zinc sulfate, L-carnitine, lycopene, and coenzyme Q10 on cadmium-induced male infertility. Int. J. Endocrinol. 2022, 2022, 6266613. [Google Scholar] [CrossRef]
- Feng, P.; Yang, J.; Zhao, S.; Ling, Z.; Han, R.; Wu, Y.; Salama, E.S.; Kakade, A.; Khan, A.; Jin, W.; et al. Human supplementation with Pediococcus acidilactici GR-1 decreases heavy metals levels through modifying the gut microbiota and metabolome. Npj Biofilms Microbiomes 2022, 8, 63. [Google Scholar] [CrossRef] [PubMed]
- Gautam, P.; Flora, S.J.S. Oral supplementation of gossypin during lead exposure protects alteration in heme synthesis pathway and brain oxidative stress in rats. Nutrition 2010, 26, 563–570. [Google Scholar] [CrossRef]
- Karbownik, M.; Gitto, E.; Lewinski, A.; Reiter, R.J. Induction of lipid peroxidation in hamster organs by the carcinogen cadmium: Amelioration by melatonin. Cell Biol. Toxicol. 2001, 17, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Suresh, C.; Dennis, A.O.; Heinz, J.; Vemuri, M.C.; Chetty, C.S. Melatonin protection against lead-induced changes in human neuroblastoma cell cultures. Int. J. Toxicol. 2006, 25, 459–464. [Google Scholar] [CrossRef]
- Liu, C.M.; Zheng, G.H.; Ming, Q.L.; Sun, J.M.; Cheng, C. Protective effect of quercetin on lead-induced oxidative stress and endoplasmic reticulum stress in rat liver via the IRE1/JNK and PI3K/Akt pathway. Free Radic. Res. 2013, 47, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Smirnova, E.; Moniruzzaman, M.; Chin, S.; Sureshbabu, A.; Karthikeyan, A.; Do, K.; Min, T. A review of the role of curcumin in metal induced toxicity. Antioxidants 2023, 12, 243. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.A.; Raish, M.; Ahmad, A.; Alkharfy, K.M.; Ahmad, S.F.; Attia, S.M.; Alsaad, A.M.S.; Bakheet, S.A. Sinapic acid ameliorate cadmium-induced nephrotoxicity: In vivo possible involvement of oxidative stress, apoptosis, and inflammation via NF-κB downregulation. Environ. Toxicol. Pharmacol. 2017, 51, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Pi, H.; Xu, S.; Reiter, R.J.; Guo, P.; Zhang, L.; Li, Y.; Li, M.; Cao, Z.; Tian, L.; Xie, J.; et al. SIRT3-SOD2-mROS-dependent autophagy in cadmium-induced hepatotoxicity and salvage by melatonin. Autophagy 2015, 11, 1037–1051. [Google Scholar] [CrossRef]
- Yang, L.; Li, X.; Jiang, A.; Li, X.; Chang, W.; Chen, J.; Ye, F. Metformin alleviates lead-induced mitochondrial fragmentation via AMPK/Nrf2 activation in SH-SY5Y cells. Redox Biol. 2020, 36, 101626. [Google Scholar] [CrossRef]
- Li, Q.; Wang, L.; Ji, D.; Yu, W.; Zhang, Y.; Xiang, Y.; Zhou, C.; Wang, L.; Deng, P.; Pi, H.; et al. Metformin attenuates cadmium-induced degeneration of spiral ganglion neuron via restoring autophagic flux in primary culture. J. Inorg. Biochem. 2022, 221, 111901. [Google Scholar] [CrossRef]
- Vielee, S.T.; Wise, J.P. Among Gerontogens, heavy metals are a class of their own: A review of the evidence for cellular senescence. Brain Sci. 2023, 13, 500. [Google Scholar] [CrossRef] [PubMed]
- Sikora, E.; Bielak-Zmijewska, A.; Dudkowska, M.; Krzystyniak, A.; Mosieniak, G.; Wesierska, M.; Wlodarczyk, J. Cellular senescence in brain aging. Front. Aging Neurosci. 2021, 13, 646924. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Meng, P.; Ling, X.; Zhou, L. Advancements in therapeutic drugs targeting of senescence. Ther. Adv. Chronic Dis. 2020, 11, 2040622320964125. [Google Scholar] [CrossRef] [PubMed]
- Ondroušková, E.; Slovácková, J.; Pelková, V.; Procházková, J.; Soucek, K.; Benes, P.; Smarda, J. Heavy metals induce phosphorylation of the Bcl-2 protein by Jun N-terminal kinase. Biol. Chem. 2009, 390, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Eckers, A.; Klotz, L.O. Heavy metal ion-induced insulin-mimetic signaling. Redox Rep. 2009, 14, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.; Bhattacharyya, S.; Ray, S.; Saha, R.; Ghosh, P.; Rauth, S.; Mandal, S.; Banerjee, S.; Murmu, N. Resveratrol alleviates cadmium-induced damage and overexpression of epidermal growth factor receptor and its downstream signaling proteins in the reproductive system of male Swiss albino mice. J. Environ. Pathol. Toxicol. Oncol. 2016, 35, 73–90. [Google Scholar] [CrossRef]
- Bai, L.; Liu, R.; Wang, R.; Xin, Y.; Wu, Z.; Ba, Y.; Zhang, H.; Cheng, X.; Zhou, G.; Huang, H. Attenuation of Pb-induced Aβ generation and autophagic dysfunction via activation of SIRT1: Neuroprotective properties of resveratrol. Ecotoxicol. Environ. Saf. 2021, 222, 112511. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Liu, Y.; Zhu, G.; Liang, Y.; Liu, B.; Wu, Y.; Han, M.; Sun, W.; Han, Y.; Chen, G.; et al. SIRT1 downregulation mediated Manganese-induced neuronal apoptosis through activation of FOXO3a-Bim/PUMA axis. Sci. Total Environ. 2019, 646, 1047–1055. [Google Scholar] [CrossRef]
- Khan, N.; Syed, D.N.; Ahmad, N.; Mukhtar, H. Fisetin: A dietary antioxidant for health promotion. Antioxid. Redox Signal. 2013, 19, 151–162. [Google Scholar] [CrossRef]
- Zhu, Y.; Doornebal, E.J.; Pirtskhalava, T.; Giorgadze, N.; Wentworth, M.; Fuhrmann-Stroissnigg, H.; Niedernhofer, L.J.; Robbins, P.D.; Tchkonia, T.; Kirkland, J.L. New agents that target senescent cells: The flavone, fisetin, and the BCL-XL inhibitors, A1331852 and A1155463. Aging 2017, 9, 955–963. [Google Scholar] [CrossRef]
- Wani, W.Y.; Gudup, S.; Sunkaria, A.; Bal, A.; Singh, P.P.; Kandimalla, R.J.; Sharma, D.R.; Gill, K.D. Protective efficacy of mitochondrial targeted antioxidant MitoQ against dichlorvos induced oxidative stress and cell death in rat brain. Neuropharmacology 2011, 61, 1193–1201. [Google Scholar] [CrossRef] [PubMed]
- Sen Gupta, R.; Sen Gupta, E.; Dhakal, B.K.; Thakur, A.R.; Ahnn, J. Vitamin C and vitamin E protect the rat testes from cadmium-induced reactive oxygen species. Mol. Cells 2004, 17, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Shaban El-Neweshy, M.; Said El-Sayed, Y.S. Influence of vitamin C supplementation on lead-induced histopathological alterations in male rats. Exp. Toxicol. Pathol. 2011, 63, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Ayinde, O.C.; Ogunnowo, S.; Ogedegbe, R.A. Influence of vitamin C and vitamin E on testicular zinc content and testicular toxicity in lead exposed albino rats. BMC Pharmacol. Toxicol. 2012, 13, 17. [Google Scholar] [CrossRef]
- Amanpour, P.; Khodarahmi, P.; Salehipour, M. Protective effects of vitamin E on cadmium-induced apoptosis in rat testes. Naunyn Schmiedebergs Arch. Pharmacol. 2020, 393, 349–358. [Google Scholar] [CrossRef]
- Fang, J.; Xie, S.; Chen, Z.; Wang, F.; Chen, K.; Zuo, Z.; Cui, H.; Guo, H.; Ouyang, P.; Chen, Z.; et al. Protective effect of vitamin E on cadmium-induced renal oxidative damage and apoptosis in rats. Biol. Trace Elem. Res. 2021, 199, 4675–4687. [Google Scholar] [CrossRef]
- El-Boshy, M.E.; Risha, E.F.; Abdelhamid, F.M.; Mubarak, M.S.; Ben Hadda, T.B. Protective effects of selenium against cadmium induced hematological disturbances, immunosuppressive, oxidative stress and hepatorenal damage in rats. J. Trace Elem. Med. Biol. 2015, 29, 104–110. [Google Scholar] [CrossRef]
- Chen, Z.; Zuo, Z.; Chen, K.; Yang, Z.; Wang, F.; Fang, J.; Cui, H.; Guo, H.; Ouyang, P.; Chen, Z.; et al. Activated Nrf-2 pathway by vitamin E to attenuate testicular injuries of rats with sub-chronic cadmium exposure. Biol. Trace Elem. Res. 2022, 200, 1722–1735. [Google Scholar] [CrossRef]
- Beytut, E.; Yuce, A.; Kamiloglu, N.N.; Aksakal, M. Role of dietary vitamin E in cadmium-induced oxidative damage in rabbit’s blood, liver and kidneys. Int. J. Vitam. Nutr. Res. 2003, 73, 351–355. [Google Scholar] [CrossRef]
- Yang, L.; Shen, K.; Ji, D. Natural compounds attenuate heavy metal-induced PC12 cell damage. J. Int. Med. Res. 2020, 48, 300060520930847. [Google Scholar] [CrossRef]
- Abdel-Emam, R.A.; Ahmed, E.A. Ameliorative effect of L-carnitine on chronic lead-induced reproductive toxicity in male rats. Vet. Med. Sci. 2021, 7, 1426–1435. [Google Scholar] [CrossRef] [PubMed]
- Di He, M.D.; Xu, S.C.; Lu, Y.H.; Li, L.; Zhong, M.; Zhang, Y.W.; Wang, Y.; Li, M.; Yang, J.; Zhang, G.B.; et al. L-carnitine protects against nickel-induced neurotoxicity by maintaining mitochondrial function in Neuro-2a cells. Toxicol. Appl. Pharmacol. 2011, 253, 38–44. [Google Scholar] [CrossRef]
- Quan, F.S.; Yu, X.F.; Gao, Y.; Ren, W.Z. Protective effects of folic acid against central nervous system neurotoxicity induced by lead exposure in rat pups. Genet. Mol. Res. 2015, 14, 12466–12471. [Google Scholar] [CrossRef]
- Bayramoğlu Akkoyun, M.; Bengü, A.Ş.; Temel, Y.; Akkoyun, H.T.; Ekin, S.; Ciftci, M. The effect of astaxanthin and cadmium on rat erythrocyte G6PD, 6PGD, GR, and TrxR enzymes activities in vivo and on rat erythrocyte 6PGD enzyme activity in vitro. J. Biochem. Mol. Toxicol. 2018, 32, e22170. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.Z.; Ni, X.F.; Yu, H.N.; Wang, S.S.; Shen, S.R. Effects of astaxanthin on oxidative stress induced by Cu2+ in prostate cells. J. Zhejiang Univ. Sci. B 2017, 18, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Tian, Z.K.; Yang, H.X.; Feng, Z.J.; Sun, J.M.; Jiang, H.; Cheng, C.; Ming, Q.L.; Liu, C.M. Fisetin improves lead-induced neuroinflammation, apoptosis and synaptic dysfunction in mice associated with the AMPK/SIRT1 and autophagy pathway. Food Chem. Toxicol. 2019, 134, 110824. [Google Scholar] [CrossRef] [PubMed]
- Jacob, S.; Thangarajan, S. Fisetin impedes developmental methylmercury neurotoxicity via downregulating apoptotic signalling pathway and upregulating Rho GTPase signalling pathway in hippocampus of F1 generation rats. Int. J. Dev. Neurosci. 2018, 69, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Maiti, A.K.; Saha, N.C.; More, S.S.; Panigrahi, A.K.; Paul, G. Neuroprotective efficacy of mitochondrial antioxidant MitoQ in suppressing peroxynitrite-mediated mitochondrial dysfunction inflicted by lead toxicity in the rat brain. Neurotox. Res. 2017, 31, 358–372. [Google Scholar] [CrossRef] [PubMed]
- Eybl, V.; Kotyzová, D.; Bludovská, M. The effect of curcumin on cadmium-induced oxidative damage and trace elements level in the liver of rats and mice. Toxicol. Lett. 2004, 151, 79–85. [Google Scholar] [CrossRef]
- Deevika, B.; Asha, S.; Taju, G.; Nalini, T. Cadmium acetate induced nephrotoxicity and protective role of curcumin in rats. Asian J. Pharm. Clin. Res. 2012, 5 (Suppl. 3), 186–188. [Google Scholar]
- Tarasub, N.; Narula, K.; Devakul, N.A.W. Effects of curcumin on cadmium-induced hepatotoxicity in rats. Thai J. Toxicol. 2008, 23, 100. [Google Scholar]
- Akinyemi, A.J.; Onyebueke, N.; Faboya, O.A.; Onikanni, S.A.; Fadaka, A.; Olayide, I. Curcumin inhibits adenosine deaminase and arginase activities in cadmium-induced renal toxicity in rat kidney. J. Food Drug Anal. 2017, 25, 438–446. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S.; Lim, H.J.; Lim, J.S.; Son, J.Y.; Lee, J.; Lee, B.M.; Chang, S.C.; Kim, H.S. Curcumin ameliorates cadmium-induced nephrotoxicity in Sprague-Dawley rats. Food Chem. Toxicol. 2018, 114, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Aktas, C.; Kanter, M.; Erboga, M.; Ozturk, S. Anti-apoptotic effects of curcumin on cadmium-induced apoptosis in rat testes. Toxicol. Ind. Health 2012, 28, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Oguzturk, H.; Ciftci, O.; Aydin, M.; Timurkaan, N.; Beytur, A.; Yilmaz, F. Ameliorative effects of curcumin against acute cadmium toxicity on male reproductive system in rats. Andrologia 2012, 44, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Zoheb, S.M.; Prakash, A.; Rahal, A.; Mandil, R.; Gangwar, N.K.; Garg, S.K. Curcumin attenuates oxidative stress-induced altered histoarchitecture of testes in experimentally exposed rats to metal mixture (lead, arsenic, cadmium, mercury, iron, and copper) for 28 days. Toxicol. Environ. Chem. 2014, 96, 660–679. [Google Scholar] [CrossRef]
- Alghasham, A.; Salem, T.A.; Meki, A.R.M. Effect of cadmium-polluted water on plasma levels of tumor necrosis factor-α, interleukin-6 and oxidative status biomarkers in rats: Protective effect of curcumin. Food Chem. Toxicol. 2013, 59, 160–164. [Google Scholar] [CrossRef]
- Sharma, S.; Kumari, A. Amelioration of curcumin against cadmium induced oxidative stress in lung of albino mice. Int. J. Adv. Res. 2016, 4, 1020–1026. [Google Scholar] [CrossRef]
- Kukongviriyapan, U.; Apaijit, K.; Kukongviriyapan, V. Oxidative stress and cardiovascular dysfunction associated with cadmium exposure: Beneficial effects of curcumin and tetrahydrocurcumin. Tohoku J. Exp. Med. 2016, 239, 25–38. [Google Scholar] [CrossRef]
- Abubakar, K.; Mailafiya, M.M.; Chiroma, S.M.; Danmaigoro, A.; Zyoud, T.Y.T.; Abdul Rahim, E.; Abu Bakar Zakaria, M.Z. Ameliorative effect of curcumin on lead-induced hematological and hepatorenal toxicity in a rat model. J. Biochem. Mol. Toxicol. 2020, 34, e22483. [Google Scholar] [CrossRef]
- Abubakar, K.; Muhammad Mailafiya, M.; Danmaigoro, A.; Musa Chiroma, S.; Abdul Rahim, E.B.; Abu Bakar Zakaria, M.Z. Curcumin attenuates lead-induced cerebellar toxicity in rats via chelating activity and inhibition of oxidative stress. Biomolecules 2019, 9, 453. [Google Scholar] [CrossRef] [PubMed]
- Changlek, S.; Rana, M.N.; Phyu, M.P.; Karim, N.; Majima, H.J.; Tangpong, J. Curcumin suppresses lead-induced inflammation and memory loss in mouse model and in silico molecular docking. Foods 2022, 11, 856. [Google Scholar] [CrossRef]
- Zahid, M.; Rawat, P.S.; Singh, S.; Gupta, A.K.; Ahmad, R.; Mahdi, A.A.; Ahmad, M.K.; Mehrotra, S. Assessment of role and efficacy of curcumin and quercetin in preventing lead-induced oxidative stress in rats. Indian J. Clin. Biochem. 2022, 37, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Alhusaini, A.; Fadda, L.; Hasan, I.H.; Zakaria, E.; Alenazi, A.M.; Mahmoud, A.M. Curcumin ameliorates lead-induced hepatotoxicity by suppressing oxidative stress and inflammation, and modulating akt/GSK-3β signaling pathway. Biomolecules 2019, 9, 703. [Google Scholar] [CrossRef] [PubMed]
- Soliman, M.M.; Baiomy, A.A.; Yassin, M.H. Molecular and histopathological study on the ameliorative effects of curcumin against lead acetate-induced hepatotoxicity and Nephrototoxicity in Wistar rats. Biol. Trace Elem. Res. 2015, 167, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Dairam, A.; Limson, J.L.; Watkins, G.M.; Antunes, E.; Daya, S. Curcuminoids, curcumin, and demethoxycurcumin reduce lead-induced memory deficits in male Wistar rats. J. Agric. Food Chem. 2007, 55, 1039–1044. [Google Scholar] [CrossRef]
- Xiang, B.; Li, D.; Chen, Y.; Li, M.; Zhang, Y.; Sun, T.; Tang, S. Curcumin ameliorates copper-induced neurotoxicity through inhibiting oxidative stress and mitochondrial apoptosis in SH-SY5Y cells. Neurochem. Res. 2021, 46, 367–378. [Google Scholar] [CrossRef]
- Abbaoui, A.; Gamrani, H. Obvious anxiogenic-like effects of subchronic copper intoxication in rats, outcomes on spatial learning and memory and neuromodulatory potential of curcumin. J. Chem. Neuroanat. 2019, 96, 86–93. [Google Scholar] [CrossRef]
- Abbaoui, A.; Gamrani, H. Neuronal, astroglial and locomotor injuries in subchronic copper intoxicated rats are repaired by curcumin: A possible link with Parkinson’s disease. Acta Histochem. 2018, 120, 542–550. [Google Scholar] [CrossRef]
- Yan, F.S.; Sun, J.L.; Xie, W.H.; Shen, L.; Ji, H.F. Neuroprotective effects and mechanisms of curcumin–Cu(II) and –Zn(II) complexes systems and their pharmacological implications. Nutrients 2017, 10, 28. [Google Scholar] [CrossRef]
- Paunović, M.G.; Matić, M.M.; Ognjanović, B.I.; Saičić, Z.S. Antioxidative and haematoprotective activity of coenzyme Q10 and vitamin E against cadmium-induced oxidative stress in Wistar rats. Toxicol. Ind. Health 2017, 33, 746–756. [Google Scholar] [CrossRef] [PubMed]
- Al-Megrin, W.A.; Soliman, D.; Kassab, R.B.; Metwally, D.M.; Dina, D.M.; El-Khadragy, M.F. Coenzyme Q10 activates the antioxidant machinery and inhibits the inflammatory and apoptotic cascades against lead acetate-induced renal injury in rats. Front. Physiol. 2020, 11, 64. [Google Scholar] [CrossRef] [PubMed]
- Yousef, A.O.S.; Fahad, A.A.; Abdel Moneim, A.E.; Metwally, D.M.; El-Khadragy, M.F.; Kassab, R.B. The neuroprotective role of coenzyme Q10 against lead acetate-induced neurotoxicity is mediated by antioxidant, anti-inflammatory and anti-apoptotic activities. Int. J. Environ. Res. Public Health 2019, 16, 2859. [Google Scholar] [CrossRef]
- Mazandaran, A.A.; Khodarahmi, P. The protective role of coenzyme Q10 in metallothionein-3 expression in liver and kidney upon rats’ exposure to lead acetate. Mol. Biol. Rep. 2021, 48, 3107–3115. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, S.; Parmar, D.; Fathima, S.; Raval, S.; Singh, G. Coenzyme Q10, biochanin A and phloretin attenuate Cr(VI)-induced oxidative stress and DNA damage by stimulating Nrf2/HO-1 pathway in the experimental model. Biol. Trace Elem. Res. 2023, 201, 2427–2441. [Google Scholar] [CrossRef] [PubMed]
- Fung, F.K.C.; Law, B.Y.K.; Lo, A.C.Y. Lutein attenuates both apoptosis and autophagy upon cobalt (II) chloride-induced hypoxia in rat Műller cells. PLoS ONE 2016, 11, e0167828. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.; Patra, T.; Saha, D.; Ghosh, P.; Mustafi, S.M.; Varghese, A.C.; Murmu, N. Sub-chronic cadmium and lead compound exposure induces reproductive toxicity and development of testicular germ cell neoplasia in situ in murine model: Attenuative effects of resveratrol. J. Biochem. Mol. Toxicol. 2022, 36, e23058. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, C.; Ge, J.; Lv, M.W.; Talukder, M.; Guo, K.; Li, Y.H.; Li, J.L. Ameliorative effects of resveratrol against cadmium-induced nephrotoxicity via modulating nuclear xenobiotic receptor response and PINK1/Parkin-mediated mitophagy. Food Funct. 2020, 11, 1856–1868. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, R.; Sun, C.; Zhang, H.; Xu, C.; Liu, W.; Gao, W.; Huang, S.; Chen, L. Resveratrol prevents cadmium activation of ERK1/2 and JNK pathways from neuronal cell death via protein phosphatases 2A and 5. J. Neurochem. 2015, 135, 466–478. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, R.; Yang, L.; Ji, T.; Zhu, C.; Liu, B.; Zhang, H.; Xu, C.; Zhang, N.; Huang, S.; et al. Neuroprotection of resveratrol against cadmium-poisoning acts through dual inhibition of mTORC1/2 signaling. Neuropharmacology 2022, 219, 109236. [Google Scholar] [CrossRef]
- Fu, B.; Zhao, J.; Peng, W.; Wu, H.; Zhang, Y. Resveratrol rescues cadmium-induced mitochondrial injury by enhancing transcriptional regulation of PGC-1α and SOD2 via the Sirt3/FoxO3a pathway in TCMK-1 cells. Biochem. Biophys. Res. Commun. 2017, 486, 198–204. [Google Scholar] [CrossRef]
- Mei, W.; Song, D.; Wu, Z.; Yang, L.; Wang, P.; Zhang, R.; Zhu, X. Resveratrol protects MC3T3-E1 cells against cadmium-induced suppression of osteogenic differentiation by modulating the ERK1/2 and JNK pathways. Ecotoxicol. Environ. Saf. 2021, 214, 112080. [Google Scholar] [CrossRef] [PubMed]
- Piras, A.R.; Ariu, F.; Maltana, A.; Leoni, G.G.; Martino, N.A.; Mastrorocco, A.; Dell’Aquila, M.E.; Bogliolo, L. Protective effect of resveratrol against cadmium-induced toxicity on ovine oocyte in vitro maturation and fertilization. J. Anim. Sci. Biotechnol. 2022, 13, 83. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Wu, Z.; Liu, M.; Wu, Y.; Li, Q.; Ba, Y.; Zhang, H.; Cheng, X.; Zhou, G.; Huang, H. Resveratrol reverses hippocampal synaptic markers injury and SIRT1 inhibition against developmental Pb exposure. Brain Res. 2021, 1767, 147567. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Gu, J.; Zhou, F.; Li, J.; Zhu, G.; Guan, L.; Liu, H.; Du, G.; Feng, J.; Liu, D.; et al. The effect of lead exposure on expression of SIRT1 in the rat hippocampus. Environ. Toxicol. Pharmacol. 2016, 44, 84–92. [Google Scholar] [CrossRef]
- Matos, L.; Gouveia, A.M.; Almeida, H. Resveratrol attenuates copper-induced senescence by improving cellular proteostasis. Oxid. Med. Cell. Longev. 2017, 2017, 3793817. [Google Scholar] [CrossRef]
- Cao, X.; Tian, S.; Fu, M.; Li, Y.; Sun, Y.; Liu, J.; Liu, Y. Resveratrol protects human bronchial epithelial cells against nickel-induced toxicity via suppressing p38 MAPK, NF-κB signaling, and NLRP3 inflammasome activation. Environ. Toxicol. 2020, 35, 609–618. [Google Scholar] [CrossRef]
- Sun, Q.; Kang, R.R.; Chen, K.G.; Liu, K.; Ma, Z.; Liu, C.; Deng, Y.; Liu, W.; Xu, B. Sirtuin 3 is required for the protective effect of resveratrol on Manganese-induced disruption of mitochondrial biogenesis in primary cultured neurons. J. Neurochem. 2021, 156, 121–135. [Google Scholar] [CrossRef]
- Latronico, T.; Branà, M.T.; Merra, E.; Fasano, A.; Di Bari, G.; Casalino, E.; Liuzzi, G.M. Impact of manganese neurotoxicity on MMP-9 production and superoxide dismutase activity in rat primary astrocytes. Effect of resveratrol and therapeutical implications for the treatment of CNS diseases. Toxicol. Sci. 2013, 135, 218–228. [Google Scholar] [CrossRef]
- Lei, M.Y.; Cong, L.; Liu, Z.Q.; Liu, Z.F.; Ma, Z.; Liu, K.; Li, J.; Deng, Y.; Liu, W.; Xu, B. Resveratrol reduces DRP1-mediated mitochondrial dysfunction via the SIRT1-PGC1α signaling pathway in manganese-induced nerve damage in mice. Environ. Toxicol. 2022, 37, 282–298. [Google Scholar] [CrossRef]
- Lang, J.; Gao, L.; Wu, J.; Meng, J.; Gao, X.; Ma, H.; Yan, D. Resveratrol attenuated manganese-induced learning and memory impairments in mice through PGC-1Alpha-Mediated autophagy and microglial M1/M2 polarization. Neurochem. Res. 2022, 47, 3414–3427. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Du, B.; Yin, W.; Wang, X.; Zhu, W. Resveratrol attenuates CoCl2-induced cochlear hair cell damage through upregulation of sirtuin1 and NF-κB deacetylation. PLoS ONE 2013, 8, e80854. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Huang, Y.Y.; Chen, H.G.; Zhou, X. Study on the efficacy and mechanism of Lycium barbarum polysaccharide against lead-induced renal injury in mice. Nutrients 2021, 13, 2945. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, Q.; Zheng, G.; Chen, Y.; Huang, M.; Zhang, L.; Lin, X. Protective effect of Lycium barbarum polysaccharides against cadmium-induced testicular toxicity in male mice. Food Funct. 2017, 8, 2322–2330. [Google Scholar] [CrossRef] [PubMed]
- Varoni, M.V.; Gadau, S.D.; Pasciu, V.; Baralla, E.; Serra, E.; Palomba, D.; Demontis, M.P. Investigation of the effects of Lycium barbarum polysaccharides against cadmium induced damage in testis. Exp. Mol. Pathol. 2017, 103, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Varoni, M.V.; Pasciu, V.; Gadau, S.D.; Baralla, E.; Serra, E.; Palomba, D.; Demontis, M.P. Possible antioxidant effect of Lycium barbarum polysaccharides on hepatic cadmium-induced oxidative stress in rats. Environ. Sci. Pollut. Res. Int. 2017, 24, 2946–2955. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Yan, L.; Tan, Y.; Chen, S.; Zhang, K.; Gong, Z.; Liu, W.; Zou, H.; Song, R.; Zhu, J.; et al. Melatonin improves mitochondrial function by preventing mitochondrial fission in cadmium-induced rat proximal tubular cell injury via SIRT1–PGC-1α pathway activation. Ecotoxicol. Environ. Saf. 2022, 242, 113879. [Google Scholar] [CrossRef]
- El-Sokkary, G.H.; Nafady, A.A.; Shabash, E.H. Melatonin administration ameliorates cadmium-induced oxidative stress and morphological changes in the liver of rat. Ecotoxicol. Environ. Saf. 2010, 73, 456–463. [Google Scholar] [CrossRef]
- Ji, Y.L.; Wang, H.; Meng, C.; Zhao, X.; Zhang, C.; Zhang, Y.; Zhao, M.; Chen, Y.; Meng, X.; Xu, D. Melatonin alleviates cadmium-induced cellular stress and germ cell apoptosis in testes. J. Pineal Res. 2012, 52, 71–79. [Google Scholar] [CrossRef]
- El-Sokkary, G.H.; Kamel, E.S.; Reiter, R.J. Prophylactic effect of melatonin in reducing lead-induced neurotoxicity in the rat. Cell. Mol. Biol. Lett. 2003, 8, 461–470. [Google Scholar]
- Xu, C.; Liu, C.; Liu, L.; Zhang, R.; Zhang, H.; Chen, S.; Luo, Y.; Chen, L.; Huang, S. Rapamycin prevents cadmium-induced neuronal cell death via targeting both mTORC1 and mTORC2 pathways. Neuropharmacology 2015, 97, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Wang, X.; Zhu, Y.; Dong, X.; Liu, C.; Zhang, H.; Liu, L.; Huang, S.; Chen, L. Rapamycin ameliorates cadmium-induced activation of MAPK pathway and neuronal apoptosis by preventing mitochondrial ROS inactivation of PP2A. Neuropharmacology 2016, 105, 270–284. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Wang, Y.; Hu, F.F.; Jiang, C.Y.; Zhang, Y.J.; Yang, J.L.; Zhao, S.W.; Gu, J.H.; Liu, X.Z.; Bian, J.C.; et al. Cadmium activates reactive oxygen species-dependent AKT/mTOR and mitochondrial apoptotic pathways in neuronal cells. Biomed. Environ. Sci. 2016, 29, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.L.; Xu, X.F.; Shi, X.T.; Feng, Y.J.; Xiong, Y.W.; Nan, Y.; Zhang, C.; Gao, L.; Chen, Y.H.; Xu, D.X.; et al. Activation of autophagy inhibits cadmium-triggered apoptosis in human placental trophoblasts and mouse placenta. Environ. Pollut. 2019, 254, 112991. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Katoh, R.; Kitamura, M. Dual regulation of cadmium-induced apoptosis by mTORC1 through selective induction of IRE1 branches in unfolded protein response. PLoS ONE 2013, 8, e64344. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Liu, L.; Luo, Y.; Huang, S. MAPK and mTOR pathways are involved in cadmium-induced neuronal apoptosis. J. Neurochem. 2008, 105, 251–261. [Google Scholar] [CrossRef]
- Fujiki, K.; Inamura, H.; Matsuoka, M. PI3K signaling mediates diverse regulation of ATF4 expression for the survival of HK-2 cells exposed to cadmium. Arch. Toxicol. 2014, 88, 403–414. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.K.; Probst, S.; Santoyo-Sánchez, M.P.; Al-Hamdani, W.; Diebels, I.; von Sivers, J.K.; Kerek, E.; Prenner, E.J.; Thévenod, F. Initial autophagic protection switches to disruption of autophagic flux by lysosomal instability during cadmium stress accrual in renal NRK-52E cells. Arch. Toxicol. 2017, 91, 3225–3245. [Google Scholar] [CrossRef]
- Lai, C.; Chen, Z.; Ding, Y.; Chen, Q.; Su, S.; Liu, H.; Ni, R.; Tang, Z. Rapamycin attenuated zinc-induced tau phosphorylation and oxidative stress in rats: Involvement of dual mTOR/p70S6K and Nrf2/HO-1 pathways. Front. Immunol. 2022, 13, 782434. [Google Scholar] [CrossRef]
- Yang, F.; Liao, J.; Yu, W.; Qiao, N.; Guo, J.; Han, Q.; Li, Y.; Hu, L.; Pan, J.; Tang, Z. Exposure to copper induces mitochondria-mediated apoptosis by inhibiting mitophagy and the PINK1/parkin pathway in chicken (Gallus gallus) livers. J. Hazard. Mater. 2021, 408, 124888. [Google Scholar] [CrossRef]
- Yang, F.; Liao, J.; Pei, R.; Yu, W.; Han, Q.; Li, Y.; Guo, J.; Hu, L.; Pan, J.; Tang, Z. Autophagy attenuates copper-induced mitochondrial dysfunction by regulating oxidative stress in chicken hepatocytes. Chemosphere 2018, 204, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Song, Y.; Kang, J.; Wu, Y.; Wu, F.; Li, Y.; Dong, Q.; Wang, J.; Song, C.; Guo, H. mtROS-mediated Akt/AMPK/mTOR pathway was involved in Copper-induced autophagy and it attenuates Copper-induced apoptosis in RAW264.7 mouse monocytes. Redox Biol. 2021, 41, 101912. [Google Scholar] [CrossRef] [PubMed]
- Uberti, V.H.; de Freitas, B.S.; Molz, P.; Bromberg, E.; Schröder, N. Iron overload impairs autophagy: Effects of rapamycin in ameliorating iron-related memory deficits. Mol. Neurobiol. 2020, 57, 1044–1054. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, M.A.; Biggers, C.D.; Li, P.A. Rapamycin treatment increases hippocampal cell viability in an mTOR-independent manner during exposure to hypoxia mimetic, cobalt chloride. BMC Neurosci. 2018, 19, 82. [Google Scholar] [CrossRef]
- Del Olmo-Aguado, S.; Núñez-Álvarez, C.; Ji, D.; Manso, A.G.; Osborne, N.N. RTP801 immunoreactivity in retinal ganglion cells and its down-regulation in cultured cells protect them from light and cobalt chloride. Brain Res. Bull. 2013, 98, 132–144. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Yan, D.Y.; Tan, X.; Ma, Z.; Wang, C.; Deng, Y.; Liu, W.; Yang, T.Y.; Xu, Z.F.; Xu, B. Effect of the cross-talk between autophagy and endoplasmic reticulum stress on Mn-induced alpha-synuclein oligomerization. Environ. Toxicol. 2018, 33, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.S.; Li, Y.C.; Tsai, P.Y.; Lin, C.E.; Chen, C.M.; Chen, S.M.; Lee, J.A. Accumulation of methylglyoxal and d-lactate in Pb-induced nephrotoxicity in rats. Biomed. Chromatogr. 2017, 31, e3869. [Google Scholar] [CrossRef]
- Kang, Y.T.; Hsu, W.C.; Wu, C.H.; Hsin, I.L.; Wu, P.R.; Yeh, K.T.; Ko, J.L. Metformin alleviates nickel-induced autophagy and apoptosis via inhibition of hexokinase-2, activating lipocalin-2, in human bronchial epithelial cells. Oncotarget 2017, 8, 105536–105552. [Google Scholar] [CrossRef]
- Kang, Y.T.; Hsu, W.C.; Ou, C.C.; Tai, H.C.; Hsu, H.T.; Yeh, K.T.; Ko, J.L. Metformin mitigates nickel-elicited angiopoietin-like protein 4 expression via HIF-1α for lung tumorigenesis. Int. J. Mol. Sci. 2020, 21, 619. [Google Scholar] [CrossRef]
- Chen, X.; Wu, W.; Gong, B.; Hou, L.; Dong, X.; Xu, C.; Zhao, R.; Yu, Q.; Zhou, Z.; Huang, S.; et al. Metformin attenuates cadmium-induced neuronal apoptosis in vitro via blocking ROS-dependent PP5/AMPK-JNK signaling pathway. Neuropharmacology 2020, 175, 108065. [Google Scholar] [CrossRef]
- Zhang, S.; Che, L.; He, C.; Huang, J.; Guo, N.; Shi, J.; Lin, Y.; Lin, Z. Drp1 and RB interaction to mediate mitochondria-dependent necroptosis induced by cadmium in hepatocytes. Cell Death Dis. 2019, 10, 523. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Ouyang, Y.; Yin, H.; Cui, H.; Deng, H.; Liu, H.; Jian, Z.; Fang, J.; Zuo, Z.; Wang, X.; et al. Induction of autophagy via the ROS-dependent AMPK-mTOR pathway protects copper-induced spermatogenesis disorder. Redox Biol. 2022, 49, 102227. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Jin, F.; Jin, J.X.; Xu, J.; Tao, T.T.; Liu, J.; Huang, H.J. Protective effects of Ganoderma lucidum spore on cadmium hepatotoxicity in mice. Food Chem. Toxicol. 2013, 52, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Bin-Jumah, M.N.; Nadeem, M.S.; Gilani, S.J.; Imam, S.S.; Alshehri, S.; Kazmi, I. Novelkaraya gum micro-particles loaded Ganoderma lucidum polysaccharide regulate sex hormones, oxidative stress and inflammatory cytokine levels in cadmium induced testicular toxicity in experimental animals. Int. J. Biol. Macromol. 2022, 194, 338–346. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
