Dysregulation of Ceramide Metabolism Is Linked to Iron Deposition and Activation of Related Pathways in the Aorta of Atherosclerotic Miniature Pigs
Abstract
:1. Introduction
2. Materials and Methods
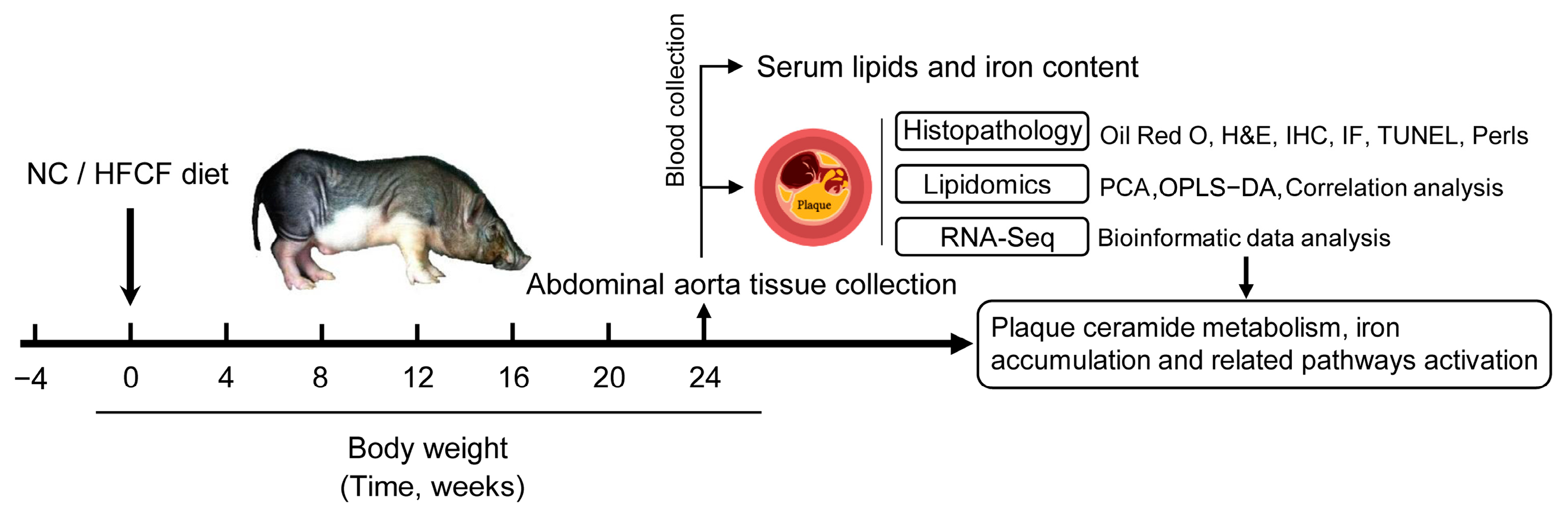
2.1. Animals
2.2. Biochemical Parameter Measurements
2.3. Histological Analysis
2.4. Lipid Sample Preparation and Lipidomic Profiling
2.5. Unsupervised Multivariate Data Analyses
2.6. cDNA Library Construction and RNA-Seq Analysis
2.7. Bioinformatic Analysis
2.8. Cell Culture
2.9. Quantitative Real-Time PCR Analysis
2.10. Western Blot Analysis
2.11. Immunohistochemistry and Immunofluorescence Staining
2.12. Statistical Analysis
3. Results
3.1. Phenotype of Atherosclerotic Pigs Induced by High-Fat, High-Cholesterol, and High-Fructose Diet
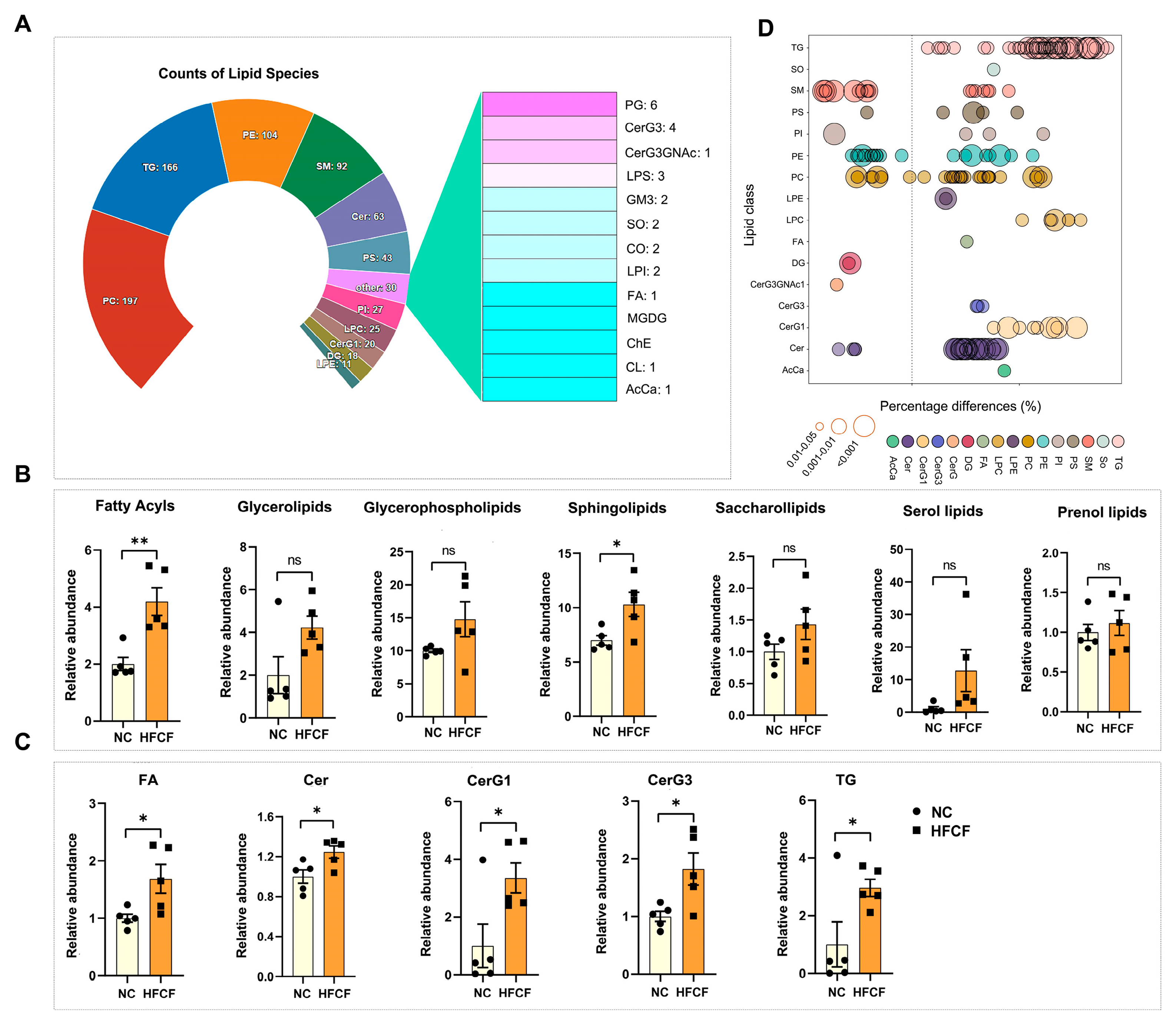
3.2. Alteration in Aortic Lipidomics in Atherosclerotic Pigs Induced by High-Fat, High-Cholesterol, and High-Fructose Diet
3.3. Significantly Increased Ceramide Metabolism, Inflammation, and Apoptosis-Related Genes Were Observed in the Aorta of Atherosclerotic Pigs Induced by High-Fat, High-Cholesterol, and High-Fructose Diet
3.4. Significantly Increased Iron Metabolism-Related Genes Were Observed in the Aorta of Atherosclerotic Pigs Induced by High-Fat, High-Cholesterol, and High-Fructose Diet
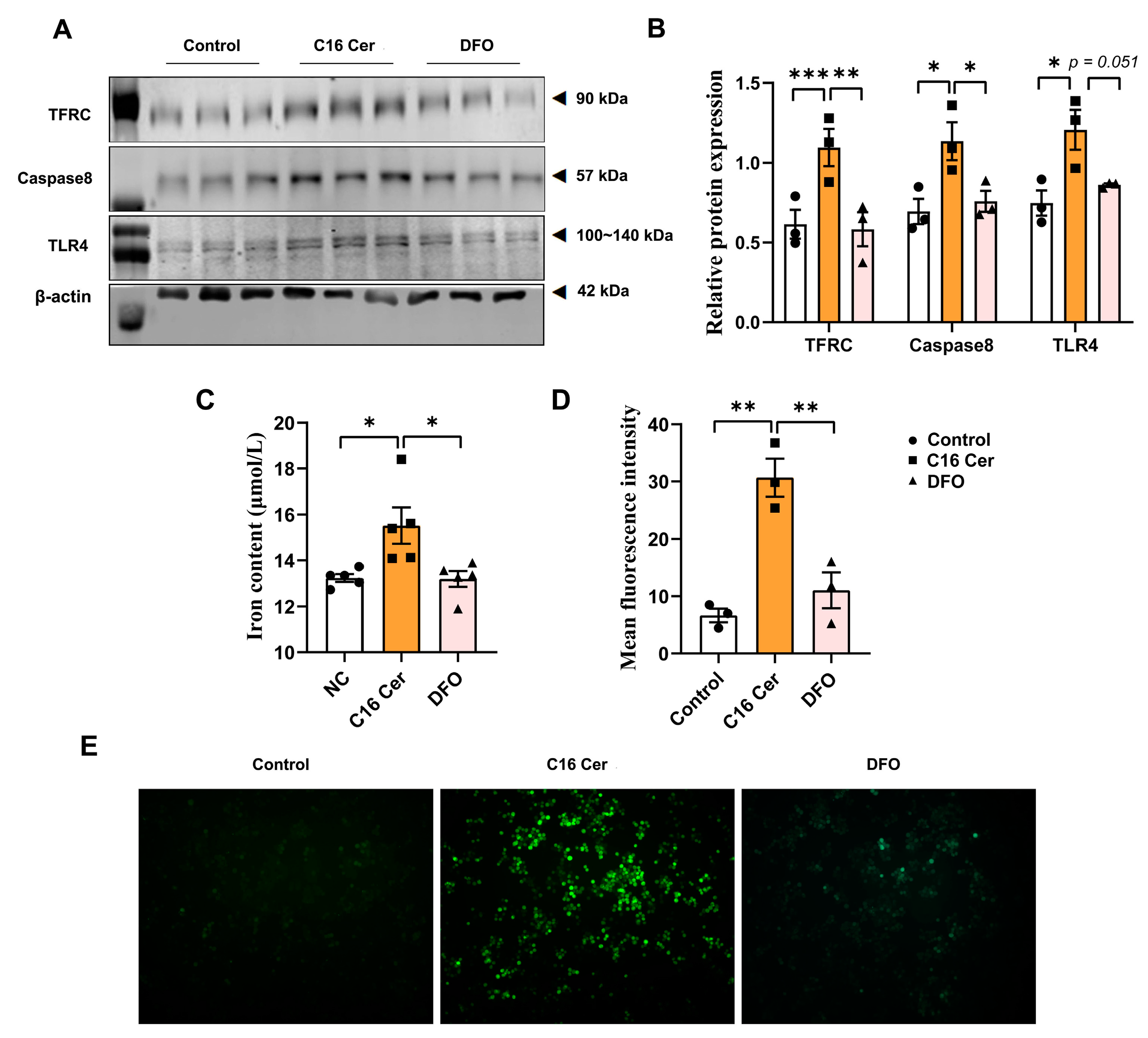
3.5. C16 Cer Increases ROS Production, Apoptosis, and Inflammatory Pathway Activation in Macrophages by Inducing Iron Overload
4. Discussion
4.1. Lipidomics Profiling of Atherosclerotic Plaques from Pigs and Other Mammals
4.2. Changes in PC and LPC in Pig Atherosclerotic Plaques
4.3. Changes in Gene Expression Related to Sphingolipid Metabolism in Pig Atherosclerotic Plaques
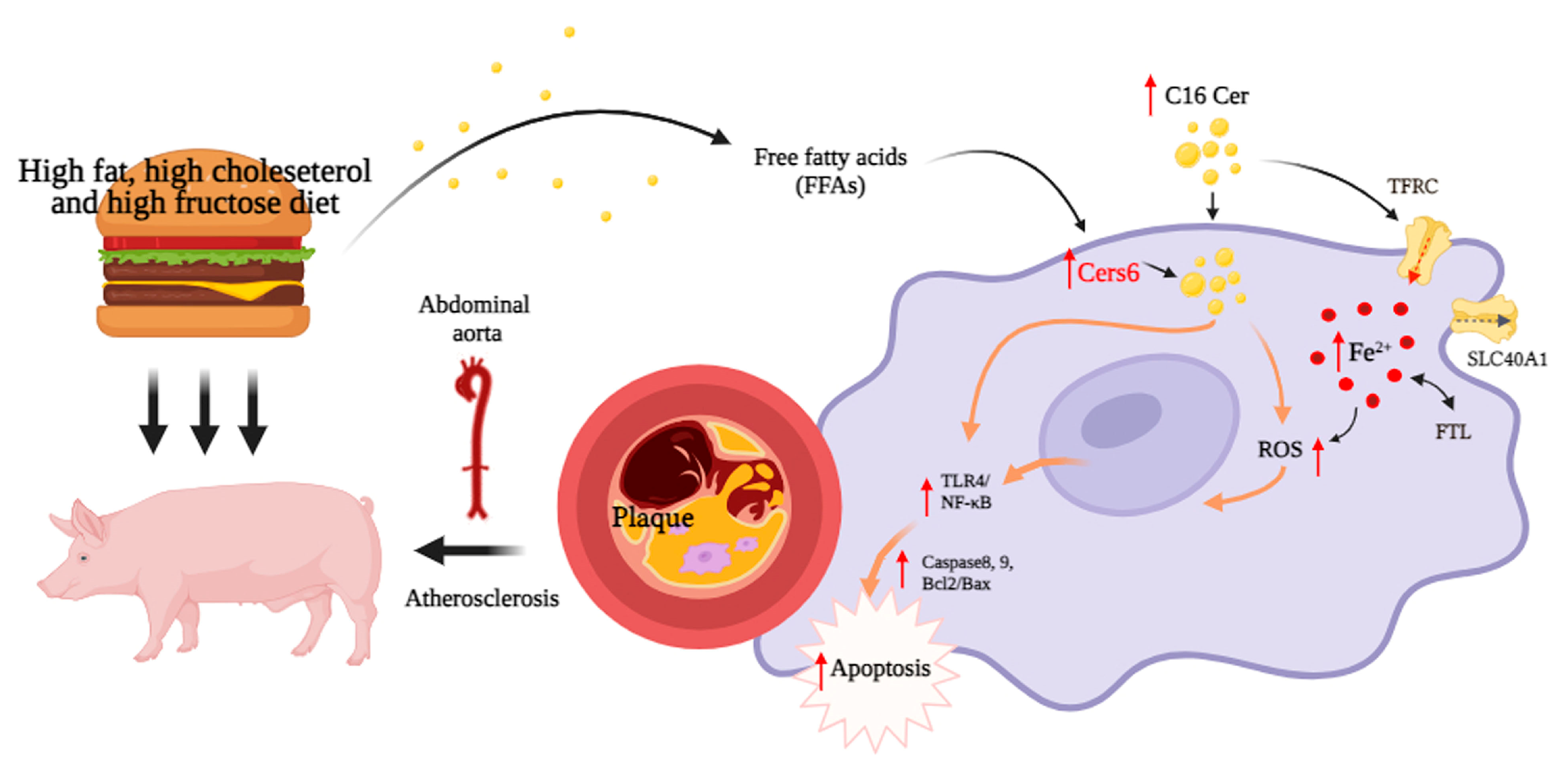
4.4. Dysregulation of Ceramide Metabolism Is Linked to Iron Deposition and Activation of Related Pathways in Macrophages
4.5. Therapeutic Potential of Ceramide
4.6. Limitations of this Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roeters van Lennep, J.E.; Tokgözoğlu, L.S.; Badimon, L.; Dumanski, S.M.; Gulati, M.; Hess, C.N.; Holven, K.B.; Kavousi, M.; Kayıkçıoğlu, M.; Lutgens, E.; et al. Women, lipids, and atherosclerotic cardiovascular disease: A call to action from the European Atherosclerosis Society. Eur. Heart J. 2023, 44, 4157–4173. [Google Scholar] [CrossRef] [PubMed]
- Roy, P.; Orecchioni, M.; Ley, K. How the immune system shapes atherosclerosis: Roles of innate and adaptive immunity. Nat. Rev. Immunol. 2022, 22, 251–265. [Google Scholar] [CrossRef] [PubMed]
- Ginsberg, H.N.; Packard, C.J.; Chapman, M.J.; Borén, J.; Aguilar-Salinas, C.A.; Averna, M.; Ference, B.A.; Gaudet, D.; Hegele, R.A.; Kersten, S.; et al. Triglyceride-rich lipoproteins and their remnants: Metabolic insights, role in atherosclerotic cardiovascular disease, and emerging therapeutic strategies-a consensus statement from the European Atherosclerosis Society. Eur. Heart J. 2021, 42, 4791–4806. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Friera, L.; Fuster, V.; López-Melgar, B.; Oliva, B.; García-Ruiz, J.M.; Mendiguren, J.; Bueno, H.; Pocock, S.; Ibáñez, B.; Fernández-Ortiz, A.; et al. Normal LDL-Cholesterol Levels Are Associated With Subclinical Atherosclerosis in the Absence of Risk Factors. J. Am. Coll. Cardiol. 2017, 70, 2979–2991. [Google Scholar] [CrossRef] [PubMed]
- Canfrán-Duque, A.; Rotllan, N.; Zhang, X.; Andrés-Blasco, I.; Thompson, B.M.; Sun, J.; Price, N.L.; Fernández-Fuertes, M.; Fowler, J.W.; Gómez-Coronado, D.; et al. Macrophage-Derived 25-Hydroxycholesterol Promotes Vascular Inflammation, Atherogenesis, and Lesion Remodeling. Circulation 2023, 147, 388–408. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.M.; Suoniemi, M.; Kardys, I.; Vihervaara, T.; de Boer, S.P.; Akkerhuis, K.M.; Sysi-Aho, M.; Ekroos, K.; Garcia-Garcia, H.M.; Oemrawsingh, R.M.; et al. Plasma concentrations of molecular lipid species in relation to coronary plaque characteristics and cardiovascular outcome: Results of the ATHEROREMO-IVUS study. Atherosclerosis 2015, 243, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Paapstel, K.; Kals, J.; Eha, J.; Tootsi, K.; Ottas, A.; Piir, A.; Jakobson, M.; Lieberg, J.; Zilmer, M. Inverse relations of serum phosphatidylcholines and lysophosphatidylcholines with vascular damage and heart rate in patients with atherosclerosis. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 44–52. [Google Scholar] [CrossRef]
- Jové, M.; Ayala, V.; Ramírez-Núñez, O.; Serrano, J.C.; Cassanyé, A.; Arola, L.; Caimari, A.; Del Bas, J.M.; Crescenti, A.; Pamplona, R.; et al. Lipidomic and metabolomic analyses reveal potential plasma biomarkers of early atheromatous plaque formation in hamsters. Cardiovasc. Res. 2013, 97, 642–652. [Google Scholar] [CrossRef]
- Tomas, L.; Edsfeldt, A.; Mollet, I.G.; Perisic Matic, L.; Prehn, C.; Adamski, J.; Paulsson-Berne, G.; Hedin, U.; Nilsson, J.; Bengtsson, E.; et al. Altered metabolism distinguishes high-risk from stable carotid atherosclerotic plaques. Eur. Heart J. 2018, 39, 2301–2310. [Google Scholar] [CrossRef]
- Björkegren, J.L.M.; Lusis, A.J. Atherosclerosis: Recent developments. Cell 2022, 185, 1630–1645. [Google Scholar] [CrossRef]
- Bai, T.; Li, M.; Liu, Y.; Qiao, Z.; Wang, Z. Inhibition of ferroptosis alleviates atherosclerosis through attenuating lipid peroxidation and endothelial dysfunction in mouse aortic endothelial cell. Free Rad. Biol. Med. 2020, 160, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Xu, Z.; You, W.; Zhou, Y.; Wang, L.; Huang, Y.; Shan, T. Cold exposure alters lipid metabolism of skeletal muscle through HIF-1α-induced mitophagy. BMC Biol. 2023, 21, 27. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Jiang, X.; Pan, Y.; Chen, L.; Zhang, L.; Zhu, K.; Cai, Y.; Ling, Y.; Chen, F.; Xu, X.; et al. Transcriptomic analysis of hepatic responses to testosterone deficiency in miniature pigs fed a high-cholesterol diet. BMC Genom. 2015, 16, 59. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Yu, C.; Fu, D.; Pan, Y.; Huang, J.; Rong, Y.; Deng, L.; Chen, J.; Chen, M. Differential metabolic and hepatic transcriptome responses of two miniature pig breeds to high dietary cholesterol. Life Sci. 2020, 250, 117514. [Google Scholar] [CrossRef]
- Oliveira, T.G.; Chan, R.B.; Bravo, F.V.; Miranda, A.; Silva, R.R.; Zhou, B.; Marques, F.; Pinto, V.; Cerqueira, J.J.; Di Paolo, G.; et al. The impact of chronic stress on the rat brain lipidome. Mol. Psychiatry 2016, 21, 80–88. [Google Scholar] [CrossRef]
- Bories, G.; Colin, S.; Vanhoutte, J.; Derudas, B.; Copin, C.; Fanchon, M.; Daoudi, M.; Belloy, L.; Haulon, S.; Zawadzki, C.; et al. Liver X receptor activation stimulates iron export in human alternative macrophages. Circ. Res. 2013, 113, 1196–1205. [Google Scholar] [CrossRef]
- Edsfeldt, A.; Dunér, P.; Ståhlman, M.; Mollet, I.G.; Asciutto, G.; Grufman, H.; Nitulescu, M.; Persson, A.F.; Fisher, R.M.; Melander, O.; et al. Sphingolipids Contribute to Human Atherosclerotic Plaque Inflammation. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 1132–1140. [Google Scholar] [CrossRef]
- Stegemann, C.; Drozdov, I.; Shalhoub, J.; Humphries, J.; Ladroue, C.; Didangelos, A.; Baumert, M.; Allen, M.; Davies, A.H.; Monaco, C.; et al. Comparative lipidomics profiling of human atherosclerotic plaques. Circ. Cardiovasc. Genet. 2011, 4, 232–242. [Google Scholar] [CrossRef]
- Jung, S.; Song, S.W.; Lee, S.; Kim, S.H.; Ann, S.J.; Cheon, E.J.; Yi, G.; Choi, E.Y.; Lee, S.H.; Joo, H.C.; et al. Metabolic phenotyping of human atherosclerotic plaques: Metabolic alterations and their biological relevance in plaque-containing aorta. Atherosclerosis 2018, 269, 21–28. [Google Scholar] [CrossRef]
- Chen, Y.; Wen, S.; Jiang, M.; Zhu, Y.; Ding, L.; Shi, H.; Dong, P.; Yang, J.; Yang, Y. Atherosclerotic dyslipidemia revealed by plasma lipidomics on ApoE−/− mice fed a high-fat diet. Atherosclerosis 2017, 262, 78–86. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, L.; Zhang, X.; Song, J.; Guo, Q.; Zhang, X.; Bai, D. Prediction model for different progressions of Atherosclerosis in ApoE−/− mice based on lipidomics. J. Pharm. Biomed Anal. 2022, 214, 114734. [Google Scholar] [CrossRef] [PubMed]
- Law, S.H.; Chan, H.C.; Ke, G.M.; Kamatam, S.; Marathe, G.K.; Ponnusamy, V.K.; Ke, L.Y. Untargeted Lipidomic Profiling Reveals Lysophosphatidylcholine and Ceramide as Atherosclerotic Risk Factors in apolipoprotein E Knockout Mice. Int. J. Mol. Sci. 2023, 24, 6956. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xiong, L.; Fan, L.; Diao, H.; Tang, M.; Luo, E.; Guo, W.; Yang, X.; Xing, S. Vascular lipidomics analysis reveales increased levels of phosphocholine and lysophosphocholine in atherosclerotic mice. Nutr. Metab. 2023, 20, 1. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Nagata, E.; Sasaki, K.; Harada-Shiba, M.; Kojo, S.; Kikuzaki, H. Increase in secretory sphingomyelinase activity and specific ceramides in the aorta of apolipoprotein E knockout mice during aging. Biol. Pharm. Bull 2013, 36, 1192–1196. [Google Scholar] [CrossRef] [PubMed]
- Chiesa, G.; Busnelli, M.; Parolini, C.; Manzini, S.; Ganzetti, G.S.; Dellera, F.; Suoniemi, M.; Hilvo, M.; Hurme, R.; Ekroos, K.; et al. Lipidomics of plasma, liver and aorta of Pcsk9-KO mice. Atherosclerosis 2023, 252, e103–e104. [Google Scholar] [CrossRef]
- Cao, J.; Goossens, P.; Martin-Lorenzo, M.; Dewez, F.; Claes, B.S.R.; Biessen, E.A.L.; Heeren, R.M.A.; Balluff, B. Atheroma-Specific Lipids in ldlr-/- and apoe-/- Mice Using 2D and 3D Matrix-Assisted Laser Desorption/Ionization Mass Spectrometry Imaging. J. Am. Soc. Mass. Spectrom. 2020, 31, 1825–1832. [Google Scholar] [CrossRef]
- Martin-Lorenzo, M.; Cao, J.; Van Kuijk, K.; Gijbels, M.J.; Claes, B.S.R.; Heeren, R.M.A.; Sluimer, J.; Alvarez-Llamas, G.; Balluff, B. In-situ lipid alterations of aortic atherosclerosis in LDLR-deficient mice using mass spectrometry imaging. Atherosclerosis 2022, 355, 76. [Google Scholar] [CrossRef]
- Wilensky, R.L.; Shi, Y.; Mohler, E.R., 3rd; Hamamdzic, D.; Burgert, M.E..; Li, J.; Postle, A.; Fenning, R.S.; Bollinger, J.G.; Hoffman, B.E.; et al. Inhibition of lipoprotein-associated phospholipase A2 reduces complex coronary atherosclerotic plaque development. Nat. Med. 2008, 14, 1059–1066. [Google Scholar] [CrossRef]
- De Keyzer, D.; Karabina, S.A.; Wei, W.; Geeraert, B.; Stengel, D.; Marsillach, J.; Camps, J.; Holvoet, P.; Ninio, E. Increased PAFAH and oxidized lipids are associated with inflammation and atherosclerosis in hypercholesterolemic pigs. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 2041–2046. [Google Scholar] [CrossRef]
- Wang, P.; Zeng, G.; Yan, Y.; Zhang, S.Y.; Dong, Y.; Zhang, Y.; Zhang, X.; Liu, H.; Zhang, Z.; Jiang, C.; et al. Disruption of adipocyte HIF-1α improves atherosclerosis through the inhibition of ceramide generation. Acta Pharm. Sin. B 2022, 12, 1899–1912. [Google Scholar] [CrossRef]
- Giussani, P.; Maceyka, M.; Le Stunff, H.; Mikami, A.; Lépine, S.; Wang, E.; Kelly, S.; Merrill, A.H., Jr.; Milstien, S.; Spiegel, S. Sphingosine-1-phosphate phosphohydrolase regulates endoplasmic reticulum-to-golgi trafficking of ceramide. Mol. Cell Biol. 2006, 26, 5055–5069. [Google Scholar] [CrossRef] [PubMed]
- Perisic, L.; Hedin, E.; Razuvaev, A.; Lengquist, M.; Osterholm, C.; Folkersen, L.; Gillgren, P.; Paulsson-Berne, G.; Ponten, F.; Odeberg, J.; et al. Profiling of atherosclerotic lesions by gene and tissue microarrays reveals PCSK6 as a novel protease in unstable carotid atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 2432–2443. [Google Scholar] [CrossRef] [PubMed]
- Weinstock, N.I.; Shin, D.; Dhimal, N.; Hong, X.; Irons, E.E.; Silvestri, N.J.; Reed, C.B.; Nguyen, D.; Sampson, O.; Cheng, Y.C.; et al. Macrophages Expressing GALC Improve Peripheral Krabbe Disease by a Mechanism Independent of Cross-Correction. Neuron 2020, 107, 65–81.e9. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Fan, Y.; Liu, J.; Li, Y.; Huan, C.; Bui, H.H.; Kuo, M.S.; Park, T.S.; Cao, G.; Jiang, X.C. Impact of sphingomyelin synthase 1 deficiency on sphingolipid metabolism and atherosclerosis in mice. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 1577–1584. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Li, H.; Chen, W.; Sum, Y.; Yu, J.; Chen, M.; Shu, G.; Qiao, E.; Guo, X.; Xu, M.; et al. Integrated application of transcriptome and metabolomics reveals potential therapeutic targets for the polarization of atherosclerotic macrophages. Biochim. Biophys. Acta Mol. Basis Dis. 2022, 1868, 166550. [Google Scholar] [CrossRef]
- Lu, S.; Natarajan, S.K.; Mott, J.L.; Kharbanda, K.K.; Harrison-Findik, D.D. Ceramide Induces Human Hepcidin Gene Transcription through JAK/STAT3 Pathway. PLoS ONE 2016, 11, e0147474. [Google Scholar] [CrossRef]
- Matsunaga, T.; Kotamraju, S.; Kalivendi, S.V.; Dhanasekaran, A.; Joseph, J.; Kalyanaraman, B. Ceramide-induced intracellular oxidant formation, iron signaling, and apoptosis in endothelial cells: Protective role of endogenous nitric oxide. J. Biol. Chem. 2004, 279, 28614–28624. [Google Scholar] [CrossRef]
- Akhiyat, N.; Vasile, V.; Ahmad, A.; Sara, J.D.; Nardi, V.; Lerman, L.O.; Jaffe, A.; Lerman, A. Plasma Ceramide Levels Are Elevated in Patients With Early Coronary Atherosclerosis and Endothelial Dysfunction. J. Am. Heart Assoc. 2022, 11, e022852. [Google Scholar] [CrossRef]
- Camell, C.D.; Nguyen, K.Y.; Jurczak, M.J.; Christian, B.E.; Shulman, G.I.; Shadel, G.S.; Dixit, V.D. Macrophage-specific de Novo Synthesis of Ceramide Is Dispensable for Inflammasome-driven Inflammation and Insulin Resistance in Obesity. J. Biol. Chem. 2015, 290, 29402–29413. [Google Scholar] [CrossRef]
- Park, T.S.; Rosebury, W.; Kindt, E.K.; Kowala, M.C.; Panek, R.L. Serine palmitoyltransferase inhibitor myriocin induces the regression of atherosclerotic plaques in hyperlipidemic ApoE-deficient mice. Pharmacol. Res. 2008, 58, 45–51. [Google Scholar] [CrossRef]
- Glaros, E.N.; Kim, W.S.; Quinn, C.M.; Jessup, W.; Rye, K.A.; Garner, B. Myriocin slows the progression of established atherosclerotic lesions in apolipoprotein E gene knockout mice. J. Lipid Res. 2008, 49, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Zietzer, A.; Düsing, P.; Reese, L.; Nickenig, G.; Jansen, F. Ceramide Metabolism in Cardiovascular Disease: A Network With High Therapeutic Potential. Arterioscler. Thromb. Vasc. Biol. 2022, 42, 1220–1228. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, Z.; Deng, L.; Fan, Y.; Ren, Y.; Ling, Y.; Tu, J.; Cai, Y.; Xu, X.; Chen, M. Dysregulation of Ceramide Metabolism Is Linked to Iron Deposition and Activation of Related Pathways in the Aorta of Atherosclerotic Miniature Pigs. Antioxidants 2024, 13, 4. https://doi.org/10.3390/antiox13010004
Cai Z, Deng L, Fan Y, Ren Y, Ling Y, Tu J, Cai Y, Xu X, Chen M. Dysregulation of Ceramide Metabolism Is Linked to Iron Deposition and Activation of Related Pathways in the Aorta of Atherosclerotic Miniature Pigs. Antioxidants. 2024; 13(1):4. https://doi.org/10.3390/antiox13010004
Chicago/Turabian StyleCai, Zhaowei, Liqun Deng, Yingying Fan, Yujie Ren, Yun Ling, Jue Tu, Yueqin Cai, Xiaoping Xu, and Minli Chen. 2024. "Dysregulation of Ceramide Metabolism Is Linked to Iron Deposition and Activation of Related Pathways in the Aorta of Atherosclerotic Miniature Pigs" Antioxidants 13, no. 1: 4. https://doi.org/10.3390/antiox13010004
APA StyleCai, Z., Deng, L., Fan, Y., Ren, Y., Ling, Y., Tu, J., Cai, Y., Xu, X., & Chen, M. (2024). Dysregulation of Ceramide Metabolism Is Linked to Iron Deposition and Activation of Related Pathways in the Aorta of Atherosclerotic Miniature Pigs. Antioxidants, 13(1), 4. https://doi.org/10.3390/antiox13010004




