Influence of Reactive Oxygen Species on Wound Healing and Tissue Regeneration in Periodontal and Peri-Implant Tissues in Diabetic Patients
Abstract
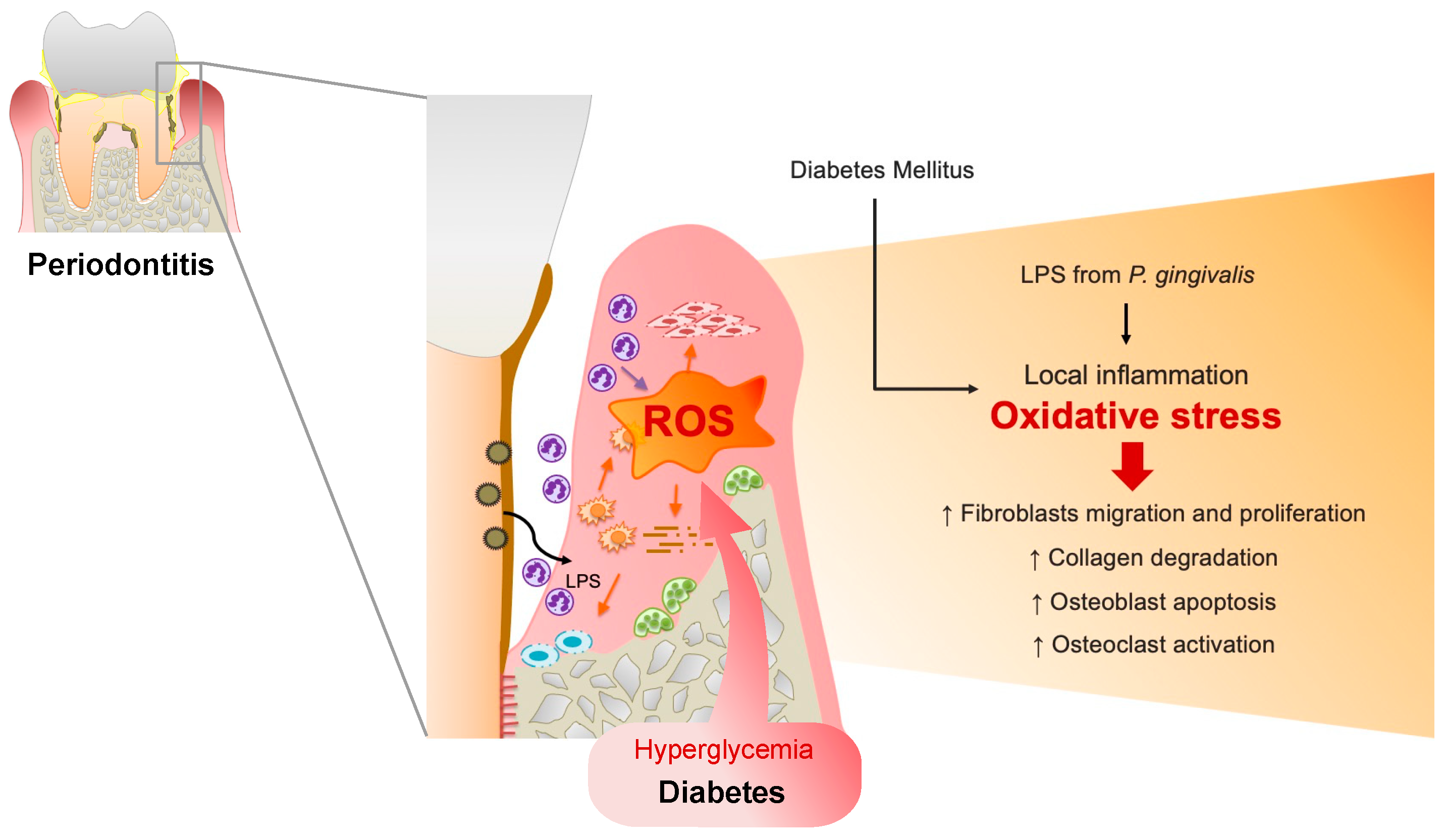
1. Introduction: Oxidative Stress in Periodontitis
1.1. Basic and Clinical Findings
1.2. Diabetes Mellitus (DM)-Induced ROS Production in Periodontal Tissues
2. Basic Research on the Influence of Oxidative Stress on Periodontitis
2.1. ROS Upregulation Impairs Wound Healing in the Periodontal Tissues
2.2. ROS Upregulation Inhibits the Osseointegration of Dental Implants
2.3. Antioxidants Recover Wound Healing In Vitro and In Vivo Studies
3. Clinical Study
3.1. Increased ROS in Patients with Periodontitis
3.2. Patients with Both Periodontitis and DM
3.3. Beneficial Effects of Antioxidants in Periodontal Therapy
3.4. Dental Implants and Antioxidant
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lushchak, V.I. Free radicals, reactive oxygen species, oxidative stress and its classification. Chem. Biol. Interact. 2014, 224, 164–175. [Google Scholar] [CrossRef] [PubMed]
- Chapple, I.L.; Matthews, J.B. The role of reactive oxygen and antioxidant species in periodontal tissue destruction. Periodontology 2000 2007, 43, 160–232. [Google Scholar] [CrossRef] [PubMed]
- Staudte, H.; Guntsch, A.; Volpel, A.; Sigusch, B.W. Vitamin C attenuates the cytotoxic effects of Porphyromonas gingivalis on human gingival fibroblasts. Arch. Oral. Biol. 2010, 55, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Golz, L.; Memmert, S.; Rath-Deschner, B.; Jager, A.; Appel, T.; Baumgarten, G.; Gotz, W.; Frede, S. LPS from P. gingivalis and hypoxia increases oxidative stress in periodontal ligament fibroblasts and contributes to periodontitis. Mediat. Inflamm. 2014, 2014, 986264. [Google Scholar] [CrossRef] [PubMed]
- Jepsen, S.; Caton, J.G.; Albandar, J.M.; Bissada, N.F.; Bouchard, P.; Cortellini, P.; Demirel, K.; de Sanctis, M.; Ercoli, C.; Fan, J.; et al. Periodontal manifestations of systemic diseases and developmental and acquired conditions: Consensus report of workgroup 3 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Clin. Periodontol. 2018, 89 (Suppl. S1), S237–S248. [Google Scholar] [CrossRef]
- Graziani, F.; Gennai, S.; Solini, A.; Petrini, M. A systematic review and meta-analysis of epidemiologic observational evidence on the effect of periodontitis on diabetes An update of the EFP-AAP review. J. Clin. Periodontol. 2018, 45, 167–187. [Google Scholar] [CrossRef]
- Pendyala, G.; Thomas, B.; Joshi, S.R. Evaluation of Total Antioxidant Capacity of Saliva in Type 2 Diabetic Patients with and without Periodontal Disease: A Case-Control Study. N. Am. J. Med. Sci. 2013, 5, 51–57. [Google Scholar] [CrossRef]
- Mizutani, K.; Park, K.; Mima, A.; Katagiri, S.; King, G.L. Obesity-associated Gingival Vascular Inflammation and Insulin Resistance. J. Dent. Res. 2014, 93, 596–601. [Google Scholar] [CrossRef]
- Schmidt, A.M.; Weidman, E.; Lalla, E.; Yan, S.D.; Hori, O.; Cao, R.; Brett, J.G.; Lamster, I.B. Advanced glycation endproducts (AGEs) induce oxidant stress in the gingiva: A potential mechanism underlying accelerated periodontal disease associated with diabetes. J. Periodontal Res. 1996, 31, 508–515. [Google Scholar] [CrossRef]
- Chopra, A.; Jayasinghe, T.N.; Eberhard, J. Are Inflamed Periodontal Tissues Endogenous Source of Advanced Glycation End-Products (AGEs) in Individuals with and without Diabetes Mellitus? A Systematic Review. Biomolecules 2022, 12, 642. [Google Scholar] [CrossRef]
- Chen, M.; Cai, W.; Zhao, S.; Shi, L.; Chen, Y.; Li, X.; Sun, X.; Mao, Y.; He, B.; Hou, Y.; et al. Oxidative stress-related biomarkers in saliva and gingival crevicular fluid associated with chronic periodontitis: A systematic review and meta-analysis. J. Clin. Periodontol. 2019, 46, 608–622. [Google Scholar] [CrossRef]
- Allen, E.M.; Matthews, J.B.; O’ Halloran, D.J.; Griffiths, H.R.; Chapple, I.L. Oxidative and inflammatory status in Type 2 diabetes patients with periodontitis. J. Clin. Periodontol. 2011, 38, 894–901. [Google Scholar] [CrossRef]
- Ray, P.D.; Huang, B.W.; Tsuji, Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal 2012, 24, 981–990. [Google Scholar] [CrossRef]
- Almerich-Silla, J.M.; Montiel-Company, J.M.; Pastor, S.; Serrano, F.; Puig-Silla, M.; Dasi, F. Oxidative Stress Parameters in Saliva and Its Association with Periodontal Disease and Types of Bacteria. Dis. Markers 2015, 2015, 653537. [Google Scholar] [CrossRef] [PubMed]
- Sczepanik, F.S.C.; Grossi, M.L.; Casati, M.; Goldberg, M.; Glogauer, M.; Fine, N.; Tenenbaum, H.C. Periodontitis is an inflammatory disease of oxidative stress: We should treat it that way. Periodontology 2000 2020, 84, 45–68. [Google Scholar] [CrossRef] [PubMed]
- Landzberg, M.; Doering, H.; Aboodi, G.M.; Tenenbaum, H.C.; Glogauer, M. Quantifying oral inflammatory load: Oral neutrophil counts in periodontal health and disease. J. Periodontal Res. 2015, 50, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Mo, L.; Niu, Y.; Li, X.; Zhou, X.; Xu, X. The Role of Reactive Oxygen Species and Autophagy in Periodontitis and Their Potential Linkage. Front. Physiol. 2017, 8, 439. [Google Scholar] [CrossRef]
- Kanzaki, H.; Wada, S.; Narimiya, T.; Yamaguchi, Y.; Katsumata, Y.; Itohiya, K.; Fukaya, S.; Miyamoto, Y.; Nakamura, Y. Pathways that Regulate ROS Scavenging Enzymes, and Their Role in Defense Against Tissue Destruction in Periodontitis. Front. Physiol. 2017, 8, 351. [Google Scholar] [CrossRef]
- Miyasaki, K.T. The neutrophil: Mechanisms of controlling periodontal bacteria. J. Periodontol. 1991, 62, 761–774. [Google Scholar] [CrossRef]
- Wang, Y.; Andrukhov, O.; Rausch-Fan, X. Oxidative Stress and Antioxidant System in Periodontitis. Front. Physiol. 2017, 8, 910. [Google Scholar] [CrossRef]
- Banasova, L.; Kamodyova, N.; Jansakova, K.; Tothova, L.; Stanko, P.; Turna, J.; Celec, P. Salivary DNA and markers of oxidative stress in patients with chronic periodontitis. Clin. Oral. Investig. 2015, 19, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Gornitsky, M.; Velly, A.M.; Yu, H.; Benarroch, M.; Schipper, H.M. Salivary DNA, lipid, and protein oxidation in nonsmokers with periodontal disease. Free Radic. Biol. Med. 2009, 46, 914–921. [Google Scholar] [CrossRef] [PubMed]
- Konopka, T.; Krol, K.; Kopec, W.; Gerber, H. Total antioxidant status and 8-hydroxy-2′-deoxyguanosine levels in gingival and peripheral blood of periodontitis patients. Arch. Immunol. Et Ther. Exp. 2007, 55, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Nassar, H.; Kantarci, A.; van Dyke, T.E. Diabetic periodontitis: A model for activated innate immunity and impaired resolution of inflammation. Periodontology 2000 2007, 43, 233–244. [Google Scholar] [CrossRef]
- Ohgi, S.; Johnson, P.W. Glucose modulates growth of gingival fibroblasts and periodontal ligament cells: Correlation with expression of basic fibroblast growth factor. J. Periodontal Res. 1996, 31, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Willershausen-Zonnchen, B.; Lemmen, C.; Hamm, G. Influence of high glucose concentrations on glycosaminoglycan and collagen synthesis in cultured human gingival fibroblasts. J. Clin. Periodontol. 1991, 18, 190–195. [Google Scholar] [CrossRef]
- Buranasin, P.; Mizutani, K.; Iwasaki, K.; Pawaputanon Na Mahasarakham, C.; Kido, D.; Takeda, K.; Izumi, Y. High glucose-induced oxidative stress impairs proliferation and migration of human gingival fibroblasts. PLoS ONE 2018, 13, e0201855. [Google Scholar] [CrossRef]
- Kido, D.; Mizutani, K.; Takeda, K.; Mikami, R.; Matsuura, T.; Iwasaki, K.; Izumi, Y. Impact of diabetes on gingival wound healing via oxidative stress. PLoS ONE 2017, 12, e0189601. [Google Scholar] [CrossRef]
- Desta, T.; Li, J.; Chino, T.; Graves, D.T. Altered fibroblast proliferation and apoptosis in diabetic gingival wounds. J. Dent. Res. 2010, 89, 609–614. [Google Scholar] [CrossRef]
- Zhu, C.; Shen, S.; Zhang, S.; Huang, M.; Zhang, L.; Chen, X. Autophagy in Bone Remodeling: A Regulator of Oxidative Stress. Front. Endocrinol. 2022, 13, 898634. [Google Scholar] [CrossRef]
- Agidigbi, T.S.; Kim, C. Reactive Oxygen Species in Osteoclast Differentiation and Possible Pharmaceutical Targets of ROS-Mediated Osteoclast Diseases. Int. J. Mol. Sci. 2019, 20, 3576. [Google Scholar] [CrossRef]
- Liu, R.; Bal, H.S.; Desta, T.; Krothapalli, N.; Alyassi, M.; Luan, Q.; Graves, D.T. Diabetes enhances periodontal bone loss through enhanced resorption and diminished bone formation. J. Dent. Res. 2006, 85, 510–514. [Google Scholar] [CrossRef] [PubMed]
- Terheyden, H.; Lang, N.P.; Bierbaum, S.; Stadlinger, B. Osseointegration--communication of cells. Clin. Oral. Implants Res. 2012, 23, 1127–1135. [Google Scholar] [CrossRef] [PubMed]
- Saito, N.; Mikami, R.; Mizutani, K.; Takeda, K.; Kominato, H.; Kido, D.; Ikeda, Y.; Buranasin, P.; Nakagawa, K.; Takemura, S.; et al. Impaired dental implant osseointegration in rat with streptozotocin-induced diabetes. J. Periodontal Res. 2022, 57, 412–424. [Google Scholar] [CrossRef] [PubMed]
- Mouraret, S.; Hunter, D.J.; Bardet, C.; Brunski, J.B.; Bouchard, P.; Helms, J.A. A pre-clinical murine model of oral implant osseointegration. Bone 2014, 58, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Lee, R.S.; Hamlet, S.; Doan, N.; Ivanovski, S.; Xiao, Y. Evaluation of the first maxillary molar post-extraction socket as a model for dental implant osseointegration research. Clin. Oral. Implants Res. 2016, 27, 1469–1478. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.H.; Hao, J.; Fretwurst, T.; Liu, M.; Kostenuik, P.; Giannobile, W.V.; Jin, Q. Sclerostin-Neutralizing Antibody Enhances Bone Regeneration Around Oral Implants. Tissue Eng. Part. A 2018, 24, 1672–1679. [Google Scholar] [CrossRef]
- Hou, M.; Lee, R.S.B.; Du, Z.; Hamlet, S.M.; Vaquette, C.; Ivanovski, S. The influence of high-dose systemic zoledronate administration on osseointegration of implants with different surface topography. J. Periodontal Res. 2019, 54, 633–643. [Google Scholar] [CrossRef]
- Feng, Y.F.; Wang, L.; Zhang, Y.; Li, X.; Ma, Z.S.; Zou, J.W.; Lei, W.; Zhang, Z.Y. Effect of reactive oxygen species overproduction on osteogenesis of porous titanium implant in the present of diabetes mellitus. Biomaterials 2013, 34, 2234–2243. [Google Scholar] [CrossRef]
- Sies, H. Oxidative stress: Oxidants and antioxidants. Exp. Physiol. 1997, 82, 291–295. [Google Scholar] [CrossRef]
- Ellis, S.D.; Tucci, M.A.; Serio, F.G.; Johnson, R.B. Factors for progression of periodontal diseases. J. Oral. Pathol. Med. 1998, 27, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Xi, X.; Li, Z.X.; Zhao, Y.; Liu, H.; Chen, S.; Liu, D.X. N-acetylcysteine promotes cyclic mechanical stress-induced osteogenic differentiation of periodontal ligament stem cells by down-regulating Nrf2 expression. J. Dent. Sci. 2022, 17, 750–762. [Google Scholar] [CrossRef] [PubMed]
- Pei, Y.; Liu, H.; Yang, Y.; Yang, Y.; Jiao, Y.; Tay, F.R.; Chen, J. Biological Activities and Potential Oral Applications of N-Acetylcysteine: Progress and Prospects. Oxid. Med. Cell Longev. 2018, 2018, 2835787. [Google Scholar] [CrossRef] [PubMed]
- Ueno, T.; Yamada, M.; Igarashi, Y.; Ogawa, T. N-acetyl cysteine protects osteoblastic function from oxidative stress. J. Biomed. Mater. Res. A 2011, 99, 523–531. [Google Scholar] [CrossRef]
- Orihuela-Campos, R.C.; Tamaki, N.; Mukai, R.; Fukui, M.; Miki, K.; Terao, J.; Ito, H.O. Biological impacts of resveratrol, quercetin, and N-acetylcysteine on oxidative stress in human gingival fibroblasts. J. Clin. Biochem. Nutr. 2015, 56, 220–227. [Google Scholar] [CrossRef]
- Kim, D.Y.; Jun, J.H.; Lee, H.L.; Woo, K.M.; Ryoo, H.M.; Kim, G.S.; Baek, J.H.; Han, S.B. N-acetylcysteine prevents LPS-induced pro-inflammatory cytokines and MMP2 production in gingival fibroblasts. Arch. Pharm. Res. 2007, 30, 1283–1292. [Google Scholar] [CrossRef]
- Kominato, H.; Takeda, K.; Mizutani, K.; Mikami, R.; Kido, D.; Buranasin, P.; Saito, N.; Takemura, S.; Nakagawa, K.; Nagasawa, T.; et al. Metformin accelerates wound healing by Akt phosphorylation of gingival fibroblasts in insulin-resistant prediabetes mice. J. Periodontol. 2022, 93, 256–268. [Google Scholar] [CrossRef]
- Mikami, R.; Mizutani, K.; Shioyama, H.; Matsuura, T.; Aoyama, N.; Suda, T.; Kusunoki, Y.; Takeda, K.; Izumi, Y.; Aida, J.; et al. Influence of aging on periodontal regenerative therapy using enamel matrix derivative: A 3-year prospective cohort study. J. Clin. Periodontol. 2022, 49, 123–133. [Google Scholar] [CrossRef]
- Miron, R.J.; Sculean, A.; Cochran, D.L.; Froum, S.; Zucchelli, G.; Nemcovsky, C.; Donos, N.; Lyngstadaas, S.P.; Deschner, J.; Dard, M.; et al. Twenty years of enamel matrix derivative: The past, the present and the future. J. Clin. Periodontol. 2016, 43, 668–683. [Google Scholar] [CrossRef]
- Hammarström, L. Enamel matrix, cementum development and regeneration. J. Clin. Periodontol. 1997, 24, 658–668. [Google Scholar] [CrossRef]
- Nokhbehsaim, M.; Deschner, B.; Winter, J.; Bourauel, C.; Jager, A.; Jepsen, S.; Deschner, J. Anti-inflammatory effects of EMD in the presence of biomechanical loading and interleukin-1beta in vitro. Clin. Oral. Investig. 2012, 16, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Villa, O.; Wohlfahrt, J.C.; Koldsland, O.C.; Brookes, S.J.; Lyngstadaas, S.P.; Aass, A.M.; Reseland, J.E. EMD in periodontal regenerative surgery modulates cytokine profiles: A randomised controlled clinical trial. Sci. Rep. 2016, 6, 23060. [Google Scholar] [CrossRef] [PubMed]
- Gestrelius, S.; Andersson, C.; Lidstrom, D.; Hammarstrom, L.; Somerman, M. In vitro studies on periodontal ligament cells and enamel matrix derivative. J. Clin. Periodontol. 1997, 24, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Keila, S.; Nemcovsky, C.E.; Moses, O.; Artzi, Z.; Weinreb, M. In vitro effects of enamel matrix proteins on rat bone marrow cells and gingival fibroblasts. J. Dent. Res. 2004, 83, 134–138. [Google Scholar] [CrossRef]
- Takeda, K.; Mizutani, K.; Matsuura, T.; Kido, D.; Mikami, R.; Noda, M.; Buranasin, P.; Sasaki, Y.; Izumi, Y. Periodontal regenerative effect of enamel matrix derivative in diabetes. PLoS ONE 2018, 13, e0207201. [Google Scholar] [CrossRef]
- Takeda, K.; Mizutani, K.; Matsuura, T.; Kido, D.; Mikami, R.; Buranasin, P.; Saito, N.; Kominato, H.; Takemura, S.; Nakagawa, K.; et al. Antioxidant effect of enamel matrix derivative for early phase of periodontal tissue regeneration in diabetes. J. Periodontol. 2022, 93, 1206–1217. [Google Scholar] [CrossRef]
- Fijany, A.; Sayadi, L.R.; Khoshab, N.; Banyard, D.A.; Shaterian, A.; Alexander, M.; Lakey, J.R.T.; Paydar, K.Z.; Evans, G.R.D.; Widgerow, A.D. Mesenchymal stem cell dysfunction in diabetes. Mol. Biol. Rep. 2019, 46, 1459–1475. [Google Scholar] [CrossRef]
- Luo, M.L.; Jiao, Y.; Gong, W.P.; Li, Y.; Niu, L.N.; Tay, F.R.; Chen, J.H. Macrophages enhance mesenchymal stem cell osteogenesis via down-regulation of reactive oxygen species. J. Dent. 2020, 94, 103297. [Google Scholar] [CrossRef]
- Yamawaki, I.; Taguchi, Y.; Komasa, S.; Tanaka, A.; Umeda, M. Effects of glucose concentration on osteogenic differentiation of type II diabetes mellitus rat bone marrow-derived mesenchymal stromal cells on a nano-scale modified titanium. J. Periodontal Res. 2017, 52, 761–771. [Google Scholar] [CrossRef]
- Mizutani, K.; Shioyama, H.; Matsuura, T.; Mikami, R.; Takeda, K.; Izumi, Y.; Aoki, A.; Iwata, T. Periodontal regenerative therapy in patients with type 2 diabetes using minimally invasive surgical technique with enamel matrix derivative under 3-year observation: A prospective cohort study. J. Periodontol. 2021, 92, 1262–1273. [Google Scholar] [CrossRef]
- Gharbi, A.; Hamila, A.; Bouguezzi, A.; Dandana, A.; Ferchichi, S.; Chandad, F.; Guezguez, L.; Miled, A. Biochemical parameters and oxidative stress markers in Tunisian patients with periodontal disease. BMC Oral. Health 2019, 19, 225. [Google Scholar] [CrossRef] [PubMed]
- Paredes-Sanchez, E.; Montiel-Company, J.M.; Iranzo-Cortes, J.E.; Almerich-Torres, T.; Bellot-Arcis, C.; Almerich-Silla, J.M. Meta-Analysis of the Use of 8-OHdG in Saliva as a Marker of Periodontal Disease. Dis. Markers 2018, 2018, 7916578. [Google Scholar] [CrossRef] [PubMed]
- Baltacioglu, E.; Sukuroglu, E. Protein carbonyl levels in serum, saliva and gingival crevicular fluid in patients with chronic and aggressive periodontitis. Saudi Dent. J. 2019, 31, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Panjamurthy, K.; Manoharan, S.; Ramachandran, C.R. Lipid peroxidation and antioxidant status in patients with periodontitis. Cell Mol. Biol. Lett. 2005, 10, 255–264. [Google Scholar]
- Toczewska, J.; Baczyńska, D.; Zalewska, A.; Maciejczyk, M.; Konopka, T. The mRNA expression of genes encoding selected antioxidant enzymes and thioredoxin, and the concentrations of their protein products in gingival crevicular fluid and saliva during periodontitis. Dent. Med. Probl. 2023, 60, 255–265. [Google Scholar] [CrossRef]
- Baumgartner-Parzer, S.M.; Wagner, L.; Pettermann, M.; Grillari, J.; Gessl, A.; Waldhausl, W. High-glucose--triggered apoptosis in cultured endothelial cells. Diabetes 1995, 44, 1323–1327. [Google Scholar] [CrossRef] [PubMed]
- Giugliano, D.; Ceriello, A.; Paolisso, G. Oxidative stress and diabetic vascular complications. Diabetes Care 1996, 19, 257–267. [Google Scholar] [CrossRef]
- Paolisso, G.; Giugliano, D. Oxidative stress and insulin action: Is there a relationship? Diabetologia 1996, 39, 357–363. [Google Scholar] [CrossRef]
- Sculley, D.V.; Langley-Evans, S.C. Periodontal disease is associated with lower antioxidant capacity in whole saliva and evidence of increased protein oxidation. Clin. Sci. 2003, 105, 167–172. [Google Scholar] [CrossRef]
- Simon, H.U.; Haj-Yehia, A.; Levi-Schaffer, F. Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis 2000, 5, 415–418. [Google Scholar] [CrossRef]
- Evans, J.L.; Goldfine, I.D.; Maddux, B.A.; Grodsky, G.M. Are oxidative stress-activated signaling pathways mediators of insulin resistance and beta-cell dysfunction? Diabetes 2003, 52, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Sanz, M.; Ceriello, A.; Buysschaert, M.; Chapple, I.; Demmer, R.T.; Graziani, F.; Herrera, D.; Jepsen, S.; Lione, L.; Madianos, P.; et al. Scientific evidence on the links between periodontal diseases and diabetes: Consensus report and guidelines of the joint workshop on periodontal diseases and diabetes by the International Diabetes Federation and the European Federation of Periodontology. J. Clin. Periodontol. 2018, 45, 138–149. [Google Scholar] [CrossRef] [PubMed]
- Altıngöz, S.M.; Kurgan, Ş.; Önder, C.; Serdar, M.A.; Ünlütürk, U.; Uyanık, M.; Başkal, N.; Tatakis, D.N.; Günhan, M. Salivary and serum oxidative stress biomarkers and advanced glycation end products in periodontitis patients with or without diabetes: A cross-sectional study. J. Periodontol. 2021, 92, 1274–1285. [Google Scholar] [CrossRef] [PubMed]
- Bullon, P.; Newman, H.N.; Battino, M. Obesity, diabetes mellitus, atherosclerosis and chronic periodontitis: A shared pathology via oxidative stress and mitochondrial dysfunction? Periodontology 2000 2014, 64, 139–153. [Google Scholar] [CrossRef] [PubMed]
- Smiley, C.J.; Tracy, S.L.; Abt, E.; Michalowicz, B.S.; John, M.T.; Gunsolley, J.; Cobb, C.M.; Rossmann, J.; Harrel, S.K.; Forrest, J.L.; et al. Systematic review and meta-analysis on the nonsurgical treatment of chronic periodontitis by means of scaling and root planing with or without adjuncts. J. Am. Dent. Assoc. 2015, 146, 508–524.e505. [Google Scholar] [CrossRef] [PubMed]
- Mailoa, J.; Lin, G.H.; Khoshkam, V.; MacEachern, M.; Chan, H.L.; Wang, H.L. Long-Term Effect of Four Surgical Periodontal Therapies and One Non-Surgical Therapy: A Systematic Review and Meta-Analysis. J. Periodontol. 2015, 86, 1150–1158. [Google Scholar] [CrossRef]
- Abou Sulaiman, A.E.; Shehadeh, R.M. Assessment of total antioxidant capacity and the use of vitamin C in the treatment of non-smokers with chronic periodontitis. J. Periodontol. 2010, 81, 1547–1554. [Google Scholar] [CrossRef]
- Kunsongkeit, P.; Okuma, N.; Rassameemasmaung, S.; Chaivanit, P. Effect of Vitamin C as an Adjunct in Nonsurgical Periodontal Therapy in Uncontrolled Type 2 Diabetes Mellitus Patients. Eur. J. Dent. 2019, 13, 444–449. [Google Scholar] [CrossRef][Green Version]
- Mathur, A.; Mathur, L.; Manohar, B.; Mathur, H.; Shankarapillai, R.; Shetty, N.; Bhatia, A. Antioxidant therapy as monotherapy or as an adjunct to treatment of periodontal diseases. J. Indian. Soc. Periodontol. 2013, 17, 21–24. [Google Scholar] [CrossRef]
- Taalab, M.R.; Mahmoud, S.A.; Moslemany, R.M.E.; Abdelaziz, D.M. Intrapocket application of tea tree oil gel in the treatment of stage 2 periodontitis. BMC Oral. Health 2021, 21, 239. [Google Scholar] [CrossRef]
- Raut, C.P.; Sethi, K.S. Comparative evaluation of co-enzyme Q10 and Melaleuca alternifolia as antioxidant gels in treatment of chronic periodontitis: A clinical study. Contemp. Clin. Dent. 2016, 7, 377–381. [Google Scholar] [CrossRef]
- Kaipa, V.R.K.; Asif, S.M.; Assiri, K.I.; Saquib, S.A.; Arem, S.A.; Sree, S.; Yassin, S.M.; Ibrahim, M.; Shariff, M.; Shamsudeen, S.M.; et al. Antioxidant effect of spirulina in chronic periodontitis. Medicine 2022, 101, e31521. [Google Scholar] [CrossRef] [PubMed]
- Merle, C.L.; Lenzen, C.; Schmalz, G.; Ziebolz, D. Systematic Review on Protocols of Coenzyme Q10 Supplementation in Non-Surgical Periodontitis Therapy. Nutrients 2023, 15, 1585. [Google Scholar] [CrossRef] [PubMed]
- Chatzopoulos, G.S.; Karakostas, P.; Kavakloglou, S.; Assimopoulou, A.; Barmpalexis, P.; Tsalikis, L. Clinical Effectiveness of Herbal Oral Care Products in Periodontitis Patients: A Systematic Review. Int. J. Environ. Res. Public Health 2022, 19, 10061. [Google Scholar] [CrossRef] [PubMed]
- Borgnakke, W.S.; Ylostalo, P.V.; Taylor, G.W.; Genco, R.J. Effect of periodontal disease on diabetes: Systematic review of epidemiologic observational evidence. J. Clin. Periodontol. 2013, 40 (Suppl. S14), S135–S152. [Google Scholar] [CrossRef]
- Mizutani, K.; Buranasin, P.; Mikami, R.; Takeda, K.; Kido, D.; Watanabe, K.; Takemura, S.; Nakagawa, K.; Kominato, H.; Saito, N.; et al. Effects of Antioxidant in Adjunct with Periodontal Therapy in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis. Antioxidants 2021, 10, 1304. [Google Scholar] [CrossRef]
- Mizutani, K.; Mikami, R.; Tsukui, A.; Nagai, S.; Pavlic, V.; Komada, W.; Iwata, T.; Aoki, A. Novel flapless esthetic procedure for the elimination of extended gingival metal tattoos adjacent to prosthetic teeth: Er:YAG laser micro-keyhole surgery. J. Prosthodont. Res. 2022, 66, 346–352. [Google Scholar] [CrossRef]
- Mijiritsky, E.; Ferroni, L.; Gardin, C.; Peleg, O.; Gultekin, A.; Saglanmak, A.; Delogu, L.G.; Mitrecic, D.; Piattelli, A.; Tatullo, M.; et al. Presence of ROS in Inflammatory Environment of Peri-Implantitis Tissue: In Vitro and In Vivo Human Evidence. J. Clin. Med. 2019, 9, 38. [Google Scholar] [CrossRef]
- González-Serrano, J.; López-Pintor, R.M.; Serrano, J.; Torres, J.; Hernández, G.; Sanz, M. Short-term efficacy of a gel containing propolis extract, nanovitamin C and nanovitamin E on peri-implant mucositis: A double-blind, randomized, clinical trial. J. Periodontal Res. 2021, 56, 897–906. [Google Scholar] [CrossRef]
- Li, X.; Tang, L.; Lin, Y.F.; Xie, G.F. Role of vitamin C in wound healing after dental implant surgery in patients treated with bone grafts and patients with chronic periodontitis. Clin. Implant. Dent. Relat. Res. 2018, 20, 793–798. [Google Scholar] [CrossRef]
- Dreyer, H.; Grischke, J.; Tiede, C.; Eberhard, J.; Schweitzer, A.; Toikkanen, S.E.; Glöckner, S.; Krause, G.; Stiesch, M. Epidemiology and risk factors of peri-implantitis: A systematic review. J. Periodontal Res. 2018, 53, 657–681. [Google Scholar] [CrossRef] [PubMed]
- Elangovan, S.; Brogden, K.A.; Dawson, D.V.; Blanchette, D.; Pagan-Rivera, K.; Stanford, C.M.; Johnson, G.K.; Recker, E.; Bowers, R.; Haynes, W.G.; et al. Body fat indices and biomarkers of inflammation: A cross-sectional study with implications for obesity and peri-implant oral health. Int. J. Oral. Maxillofac. Implant. 2014, 29, 1429–1434. [Google Scholar] [CrossRef] [PubMed][Green Version]
| Authors (Year) | Study Type | Treatment |
Treatment Duration | Sample Type | Results |
|---|---|---|---|---|---|
| Abou Sulaiman and Shehadeh (2010) [77] | RCT | SRP with vitamin C (2000 mg/d) | 1 month | 30 patients with ChP 30 healthy controls |
|
| Mathur et al. (2013) [79] | CH |
| 3 doses in 2 weeks | 30 patients with ChP 30 gingivitis patients 10 healthy controls |
|
| Raut and Sethi (2016) [81] | CH |
| 7 days | 15 patients with ChP (moderate to severe) |
|
| Kunsongkeit (2019) [78] | A double-blind, placebo-controlled, clinical trial |
| 2 months | 31 patients with ChP |
|
| Taalab [80] (2021) | RCT |
| 6 months | 30 patients with ChP |
|
| Kaipa (2022) [82] | CH |
| 90 days | 60 patients with ChP |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buranasin, P.; Kominato, H.; Mizutani, K.; Mikami, R.; Saito, N.; Takeda, K.; Iwata, T. Influence of Reactive Oxygen Species on Wound Healing and Tissue Regeneration in Periodontal and Peri-Implant Tissues in Diabetic Patients. Antioxidants 2023, 12, 1787. https://doi.org/10.3390/antiox12091787
Buranasin P, Kominato H, Mizutani K, Mikami R, Saito N, Takeda K, Iwata T. Influence of Reactive Oxygen Species on Wound Healing and Tissue Regeneration in Periodontal and Peri-Implant Tissues in Diabetic Patients. Antioxidants. 2023; 12(9):1787. https://doi.org/10.3390/antiox12091787
Chicago/Turabian StyleBuranasin, Prima, Hiromi Kominato, Koji Mizutani, Risako Mikami, Natsumi Saito, Kohei Takeda, and Takanori Iwata. 2023. "Influence of Reactive Oxygen Species on Wound Healing and Tissue Regeneration in Periodontal and Peri-Implant Tissues in Diabetic Patients" Antioxidants 12, no. 9: 1787. https://doi.org/10.3390/antiox12091787
APA StyleBuranasin, P., Kominato, H., Mizutani, K., Mikami, R., Saito, N., Takeda, K., & Iwata, T. (2023). Influence of Reactive Oxygen Species on Wound Healing and Tissue Regeneration in Periodontal and Peri-Implant Tissues in Diabetic Patients. Antioxidants, 12(9), 1787. https://doi.org/10.3390/antiox12091787






