Laser Biostimulation Induces Wound Healing-Promoter β2-Defensin Expression in Human Keratinocytes via Oxidative Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. In Vitro Laser Biomodulation
2.3. Role of Oxidative Stress in Laser Biostimulation
2.4. Analysis of hBD-1 and hBD-2 Production
2.5. Statistical Analysis
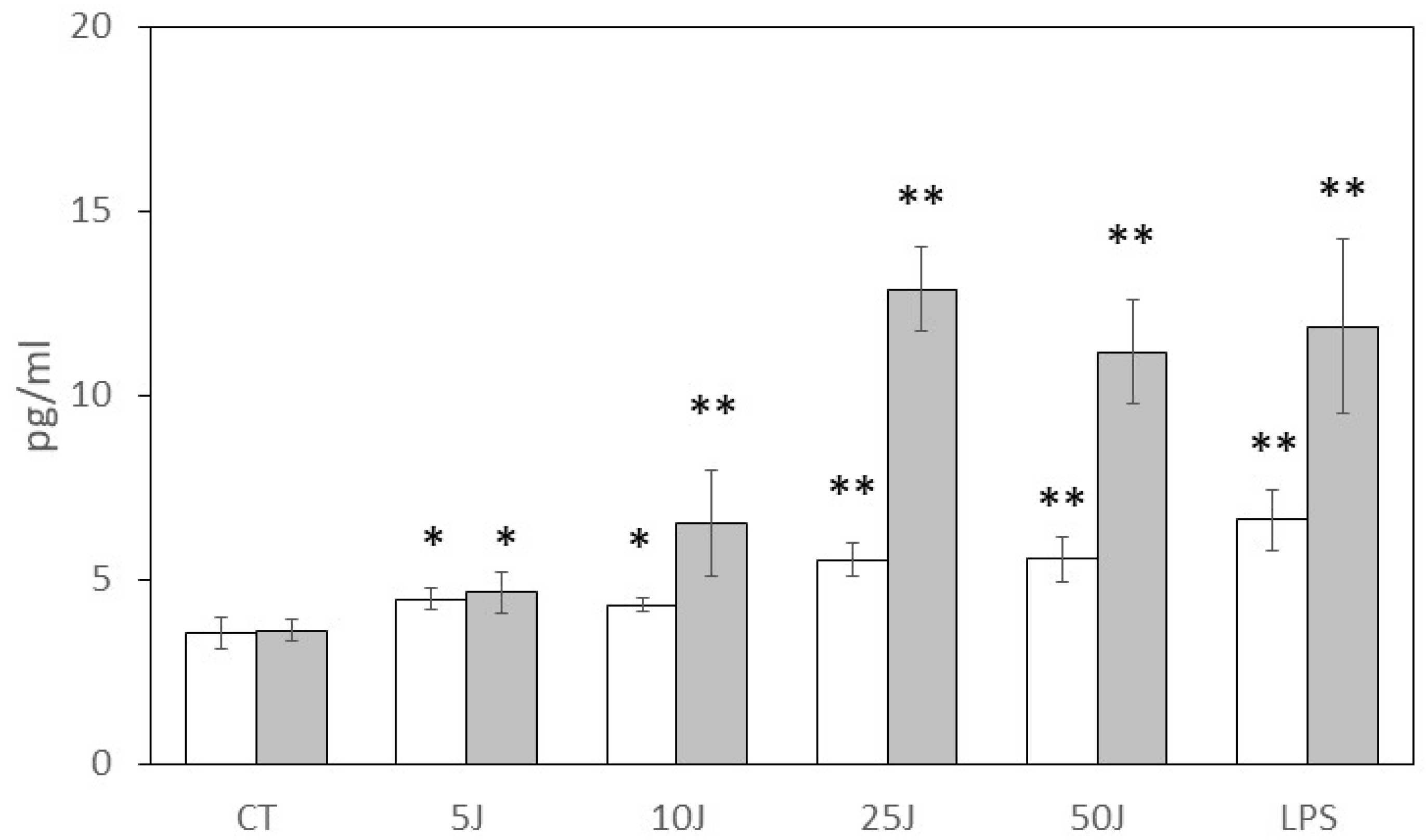
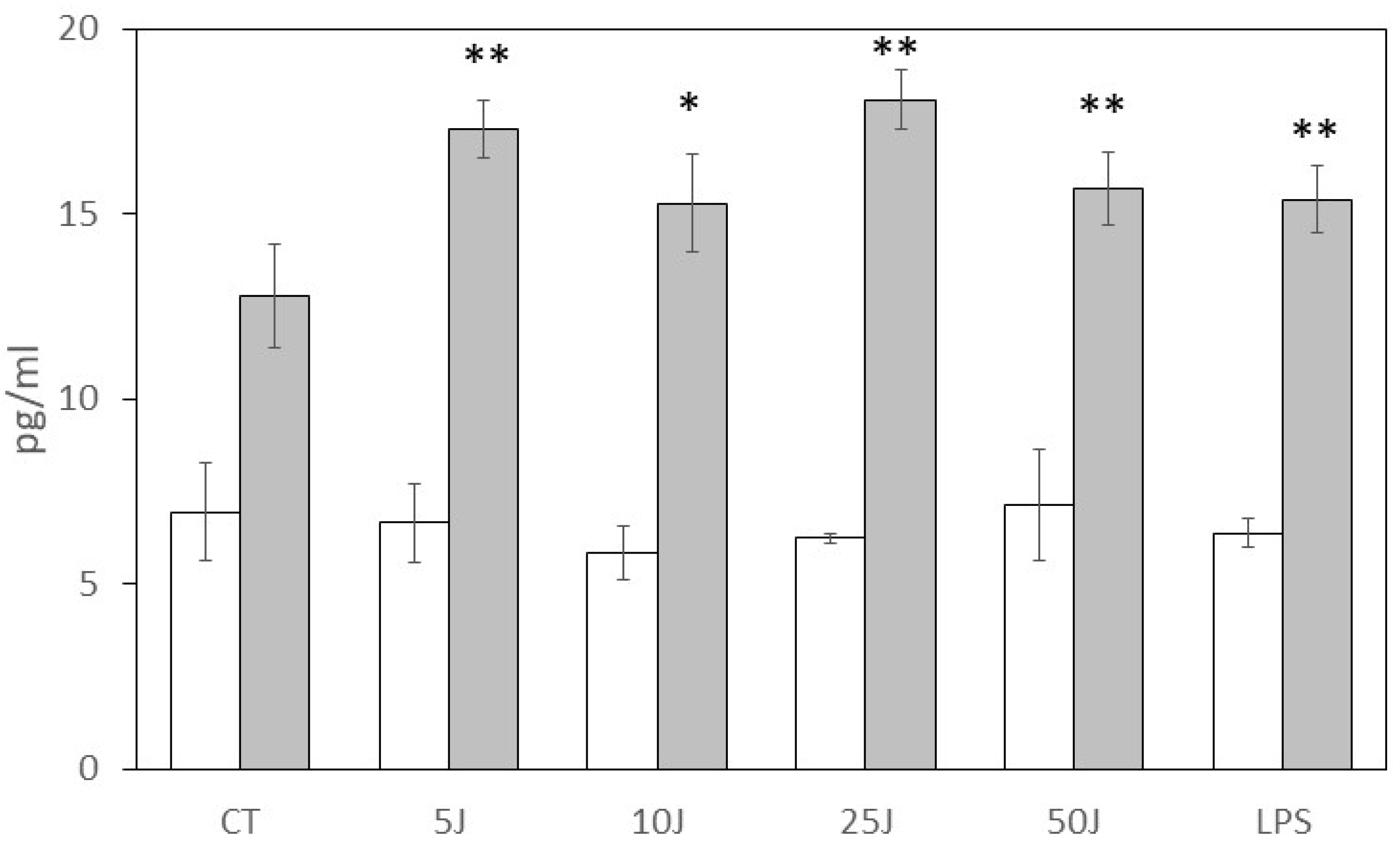
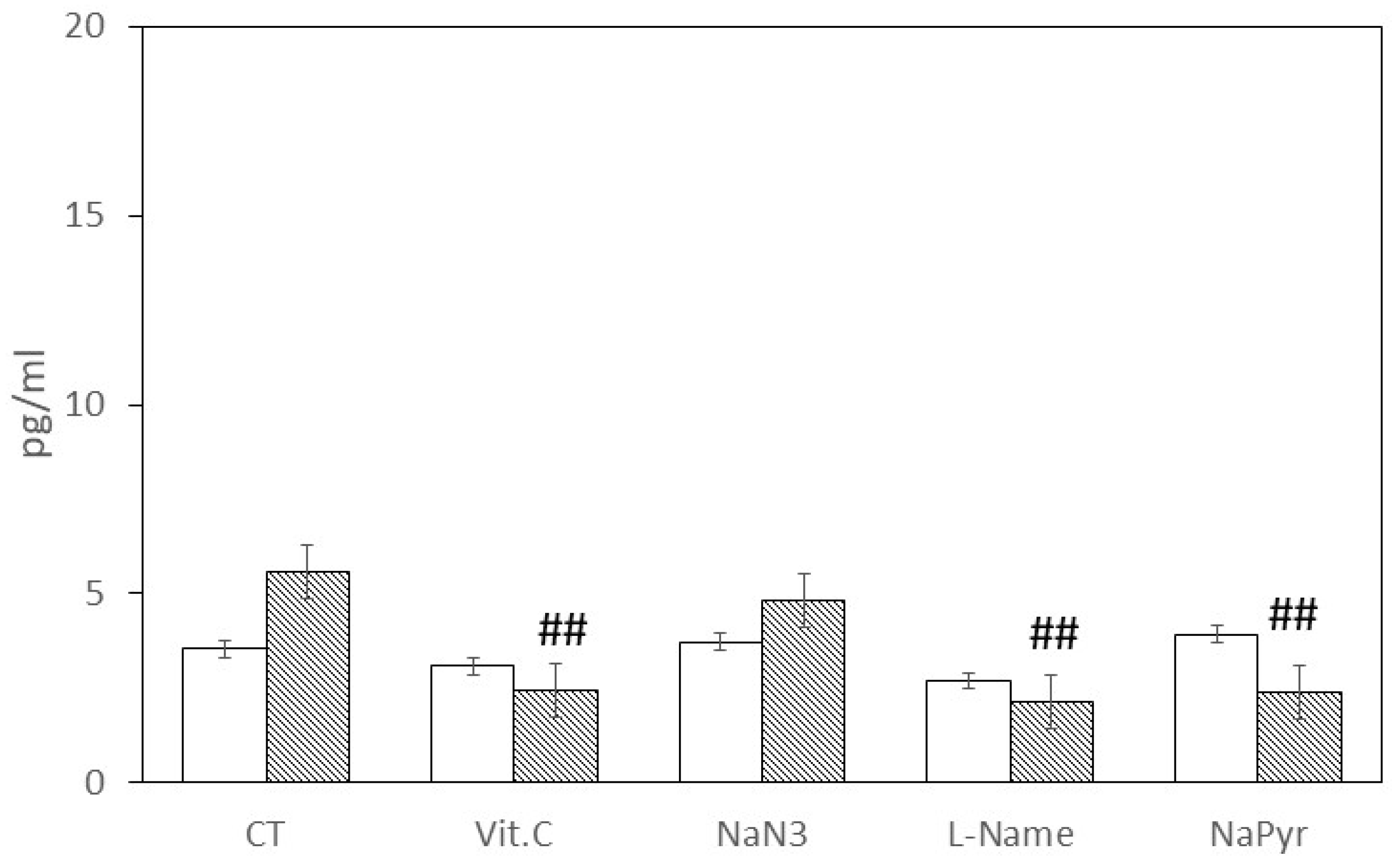
3. Results
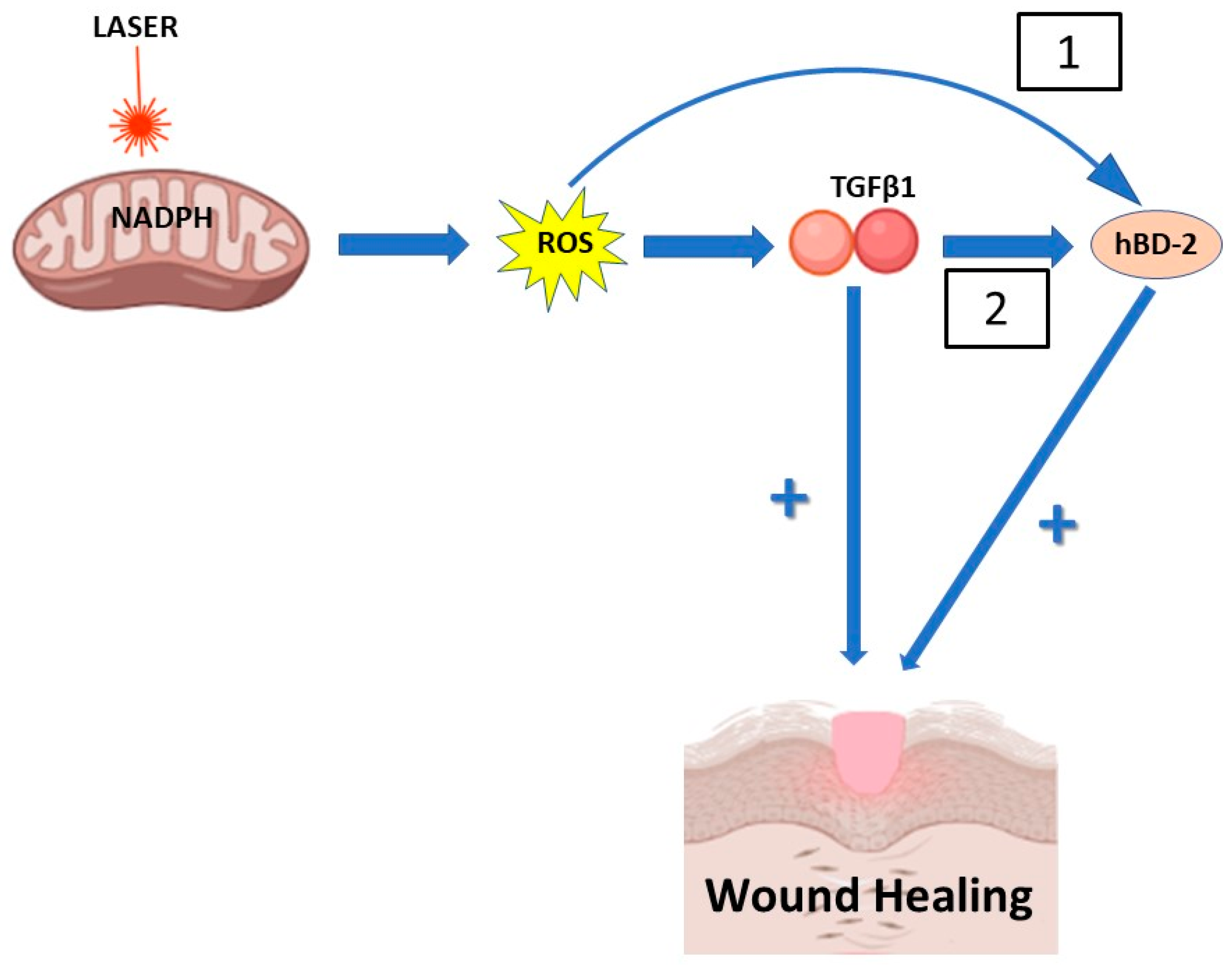
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- de Paula, V.S.; Ana Paula Valente, A.P. A dynamic overview of antimicrobial peptides and their complexes. Molecules 2018, 23, 2040. [Google Scholar] [CrossRef] [PubMed]
- Duits, L.A.; Ravensbergen, B.; Rademaker, M.; Hiemstra, P.S.; Nibbering, P.H. Expression of b-defensin 1 and 2 mRNA by human monocytes, macrophages and dendritic cells. Immunology 2002, 106, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Cunliffe, R.N. α-Defensins in the gastrointestinal tract. Mol. Immunol. 2003, 40, 463–467. [Google Scholar] [CrossRef]
- Taylor, K.; Barran, P.E.; Dorin, J.R. Structure–Activity Relationships in β-Defensin Peptides. Biopolymers 2008, 90, 1–7. [Google Scholar] [CrossRef]
- Yang, D.; Chertov, O.; Bykovskaia, S.N.; Chen, Q.; Buffo, M.J.; Shogan, J.; Anderson, M.; Schroder, J.M.; Wang, J.M.; Howard, O.M.; et al. Beta-defensins: Linking innate and adaptive immunity through dendritic and T cell CCR6. Science 1999, 286, 525–528. [Google Scholar] [CrossRef]
- Bensch, K.W.; Raida, M.; Mägert, H.-J.; Schulz-Knappe, P.; Forssmann, W.-G. hBD-I: A novel beta-defensin from human plasma. FEBS Lett. 1995, 368, 331–335. [Google Scholar] [CrossRef]
- Seo, S.J.; Ahn, S.W.; Hong, C.K.; Ro, B.I. Expression of β-defensins in human keratinocyte cell lines. J. Dermatol. Sci. 2001, 27, 183–191. [Google Scholar] [CrossRef]
- Harder, J.; Meyer-Hoffert, U.; Manuel Teran, L.; Schwichtenberg, L.; Bartels, J.; Maune, S.; Schröder, J.-M. Mucoid Pseudomonas aeruginosa, TNFα, and IL-1β, but Not IL-6, Induce Human β -Defensin-2 in Respiratory Epithelia. Am. J. Respir. Cell Mol. Biol. 2000, 22, 714–721. [Google Scholar] [CrossRef] [PubMed]
- Harder, J.; Bartels, J.; Christophers, E.; Schroder, J.M. Isolation and characterization of human beta-defensin-3, a novel human inducible peptide antibiotic. J. Biol. Chem. 2001, 276, 5707–5713. [Google Scholar] [CrossRef]
- Batoni, G.; Maisetta, G.; Esin, S.; Campa, M. Human β-defensin 3: A promising antimicrobial peptide. Med. Chem. 2006, 6, 1063–1073. [Google Scholar] [CrossRef]
- Dhople, V.; Krukemeyer, A.; Ramamoorthy, A. The human β-defensin 3, an antibacterial peptide with multiple biological functions. Biochim. Biophys. Acta 2006, 1758, 1499–1512. [Google Scholar] [CrossRef] [PubMed]
- Froy, O. Regulation of mammalian defensin expression by Toll-like receptor-dependent and independent signalling pathways. Cell. Microbiol. 2005, 7, 1387–1397. [Google Scholar] [CrossRef] [PubMed]
- Avci, P.; Gupta, A.; Sadasivam, M.; Vecchio, D.; Pam, Z.; Pam, N.; Hamblin, M.R. Low-level laser (light) terapy (LLLT) in skin: Stimulating, healing, restoring. Semin. Cutan. Med. Surg. 2013, 32, 41–52. [Google Scholar] [PubMed]
- Amaroli, A.; Pasquale, C.; Zekiy, A.; Utyuzh, A.; Benedicenti, S.; Signore, A.; Ravera, S. Photobiomodulation and oxidative stress: 980 nm diode laser light regulates mitochondrial activity and reactive oxygen species production. Oxidative Med. Cell. Longev. 2021, 6626286. [Google Scholar] [CrossRef]
- Hartmann, D.D.; Martins, R.P.; Silva, T.C.D.; Stefanello, S.T.; Courtes, A.A.; Gonçalves, D.F.; Furtado, A.B.V.; Duarte, B.S.L.; Signori, L.U.; Soares, F.A.A.; et al. Oxidative stress is involved in LLLT mechanism of action on skin healing in rats. Braz. J. Med. Biol. Res. 2021, 54, 10293. [Google Scholar] [CrossRef]
- Saenko, Y.V.; Glushchenko, E.S.; Zolotovskii, I.O.; Sholokhov, E.; Kurkov, A. Mitochondrial dependent oxidative stress in cell culture induced by laser radiation at 1265 nm. Lasers Med. Sci. 2016, 31, 405–413. [Google Scholar] [CrossRef]
- Migliario, M.; Tonello, S.; Rocchetti, V.; Rizzi, M.; Renò, F. Near infrared laser irradiation induces NETosis via oxidative stress and autophagy. Lasers Med. Sci. 2018, 33, 1919–1924. [Google Scholar] [CrossRef]
- Rupel, K.; Zupin, L.; Colliva, A.; Kamada, A.; Poropat, A.; Ottaviani, G.; Gobbo, M.; Fanfoni, L.; Gratton, R.; Santoro, M.; et al. Photobiomodulation at multiple wavelengths differentially modulates oxidative stress in vitro and in vivo. Oxidative Med. Cell. Longev. 2018, 6510159. [Google Scholar] [CrossRef]
- Martins, L.P.O.; Santos, F.F.D.; Costa, T.E.D.; Lacerda, A.C.R.; Santos, J.M.D.; Costa, K.B.; Santos, A.P.; Gaiad, T.P.; Pinfildi, C.E.; Rocha-Vieira, E.; et al. Photobiomodulation therapy (Light-Emitting Diode 630 nm) favored the oxidative stress and the preservation of articular cartilage in an induced knee osteoarthritis Model. Photobiomodul. Photomed. Laser Surg. 2021, 39, 272–279. [Google Scholar] [CrossRef]
- Hamblin, M.R. Mechanisms and applications of the anti-inflammatory effects of photobiomodulation. AIMS Biophys. 2017, 4, 337–361. [Google Scholar] [CrossRef]
- Hamblin, M.R. Mechanisms and Mitochondrial Redox Signaling in Photobiomodulation. Photochem. Photobiol. 2018, 94, 199–212. [Google Scholar] [CrossRef] [PubMed]
- Rizzi, M.; Migliario, M.; Tonello, S.; Rocchetti, V.; Renò, F. Photobiomodulation induces in vitro re-epithelialization via nitric oxide production. Lasers Med. Sci. 2018, 33, 1003–1008. [Google Scholar] [CrossRef]
- de Abreu, P.T.R.; de Arruda, J.A.A.; Mesquita, R.A.; Abreu, L.G.; Diniz, I.M.A.; Silva, T.A. Photobiomodulation effects on keratinocytes cultured in vitro: A critical review. Lasers Med. Sci. 2019, 34, 1725–1734. [Google Scholar] [CrossRef]
- Wilson, V.G. Growth and differentiation of HaCaT keratinocytes. Met. Mol. Biol. 2014, 1195, 33–41. [Google Scholar]
- Poli, R.; Parker, S.; Anagnostaki, E.; Mylona, V.; Lynch, E.; Grootveld, M. Laser analgesia associated with restorative dental care: A Systematic Review of the rationale, Techniques, and Energy dose considerations. Dent. J. 2020, 8, 128. [Google Scholar] [CrossRef] [PubMed]
- Passarella, S.; Karu, T. Absorption of monochromatic and narrow band radiation in the visible and near IR by both mitochondrial and non-mitochondrial photoacceptors results in photobiomodulation. J. Photochem. Photobiol. B 2014, 140, 344–358. [Google Scholar] [PubMed]
- Rizzi, M.; Migliario, M.; Rocchetti, V.; Tonello, S.; Renò, F. Near-infrared laser increases MDPC-23 odontoblast-like cells proliferation by activating redox sensitive pathways. J. Phochem. Photobiol. B 2016, 164, 283–288. [Google Scholar] [CrossRef]
- Migliario, M.; Sabbatini, M.; Mortellaro, C.; Renò, F. Near infrared low-level laser therapy and cell proliferation: The emerging role of redox sensitive signal transduction pathways. J. Biophotonics 2018, 11, e201800025. [Google Scholar] [CrossRef]
- George, S.; Hamblin, M.R.; Abrahamse, H. Effect of red ligh and near infrared laser on the generation of reactive oxygen species in primary dermal fibroblasts. J. Photochem. Photobiol. B 2018, 188, 60–68. [Google Scholar] [CrossRef]
- Arany, P.R.; Cho, A.; Hunt, T.D.; Sidhu, G.; Shin, K.; Hahm, E.; Huang, G.X.; Weaver, J.; Chen, A.C.; Padwa, B.L.; et al. Photoactivation of endogenous latent transforming growth factor–β1 directs dental stem cell differentiation for regeneration. Sci. Transl. Med. 2014, 6, 238ra69. [Google Scholar] [CrossRef]
- Gandhirajan, R.K.; Rödder, K.; Bodnar, Y.; Pasqual-Melo, G.; Emmert, S.; Griguer, C.E.; Weltmann, K.-D.; Bekeschus, S. Cytochrome C oxidase inhibition and cold plasma-derived oxidants synergize in melanoma cell death induction. Sci. Rep. 2018, 8, 12734. [Google Scholar] [CrossRef]
- Niyonsaba, F.; Ushio, H.; Nakano, N.; Ng, W.; Sayama, K.; Hashimoto, K.; Nagaoka, I.; Okomura, K.; Ogawa, H. Antimicrobial peptides human beta-defensins stimulate epidermal keratinocyte migration, proliferation and production of proinflammatory cytokines and chemokines. J. Investig. Dermatol. 2007, 127, 594–604. [Google Scholar] [CrossRef] [PubMed]
- Baroni, A.; Donnarumma, G.; Paoletti, I.; Longanesi-Cattani, I.; Bifulco, K.; Tufano, M.A.; Carriero, M.V. Antimicrobial human beta-defensin-2 stimulates migration, proliferation and tube formation of human umbilical vein endothelial cells. Peptides 2009, 30, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Butmarc, J.; Yufit, T.; Carson, P.; Falanga, V. Human beta-defensin-2 expression is increased in chronic wounds. Wound Repair Regen. 2004, 12, 439–443. [Google Scholar] [CrossRef] [PubMed]
- Roupé, K.M.; Nybo, M.; Sjöbring, U.; Alberius, P.; Schnidtchen, A.; Sørensen, O.E. Injury is a major inducer of epidermal innate immune responses during wound healing. J. Investig. Dermatol. 2010, 130, 1167–1177. [Google Scholar] [CrossRef]
- Galkowska, H.; Olszewski, W.L.; Wojewodzka, U. Expression of natural antimicrobial peptide beta-defensin-2 and Langerhans cell accumulation in epidermis from human non-healing leg ulcers. Folia Histochem. Cytobiol. 2005, 43, 133–136. [Google Scholar]
- Khan, I.; Rahman, S.U.; Tang, E.; Engel, K.; Hall, B.; Kulkarni, A.B.; Arany, P.R. Accelerated burn wound healing with photobiomodulation therapy involves activation of endogenous latent TGF-β1. Sci. Rep. 2021, 11, 13371. [Google Scholar] [CrossRef]
- Lichtman, M.K.; Otero-Vinas, M.; Falanga, V. Transforming growth factor beta (TGF-β) isoforms in wound healing and fibrosis. Wound Rep. Reg. 2016, 24, 215–222. [Google Scholar] [CrossRef]
- Liarte, S.; Bernabé-García, Á.; Nicolás, F.J. Role of TGF-β in skin chronic wounds: A keratinocyte perspective. Cells 2020, 9, 306. [Google Scholar] [CrossRef]
- Tang, E.; Khan, I.; Andreana, S.; Arany, P.R. Laser-activated transforming growth factor-β1 induces human β-defensin 2: Implications for laser therapies for periodontitis and peri-implantitis. J. Periodontal Res. 2017, 52, 360–367. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Migliario, M.; Yerra, P.; Gino, S.; Sabbatini, M.; Renò, F. Laser Biostimulation Induces Wound Healing-Promoter β2-Defensin Expression in Human Keratinocytes via Oxidative Stress. Antioxidants 2023, 12, 1550. https://doi.org/10.3390/antiox12081550
Migliario M, Yerra P, Gino S, Sabbatini M, Renò F. Laser Biostimulation Induces Wound Healing-Promoter β2-Defensin Expression in Human Keratinocytes via Oxidative Stress. Antioxidants. 2023; 12(8):1550. https://doi.org/10.3390/antiox12081550
Chicago/Turabian StyleMigliario, Mario, Preetham Yerra, Sarah Gino, Maurizio Sabbatini, and Filippo Renò. 2023. "Laser Biostimulation Induces Wound Healing-Promoter β2-Defensin Expression in Human Keratinocytes via Oxidative Stress" Antioxidants 12, no. 8: 1550. https://doi.org/10.3390/antiox12081550
APA StyleMigliario, M., Yerra, P., Gino, S., Sabbatini, M., & Renò, F. (2023). Laser Biostimulation Induces Wound Healing-Promoter β2-Defensin Expression in Human Keratinocytes via Oxidative Stress. Antioxidants, 12(8), 1550. https://doi.org/10.3390/antiox12081550









