Fish Skin Grafts Affect Adenosine and Methionine Metabolism during Burn Wound Healing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Sample Preparation and UPLC-MS/MS Analysis
2.3. Data Processing and Statistical Analysis
2.4. Feature Annotation/Identification of Metabolites
3. Results
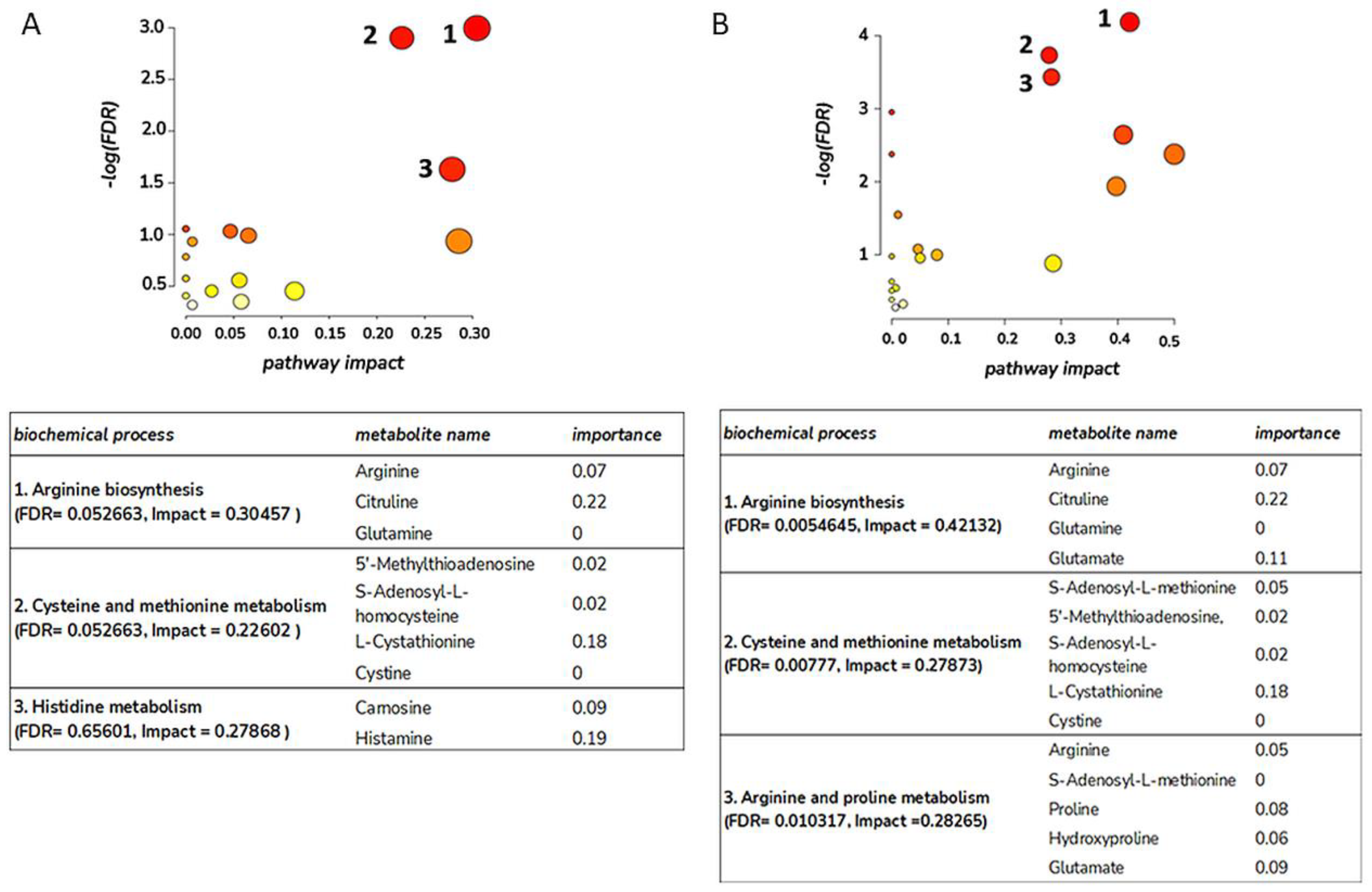
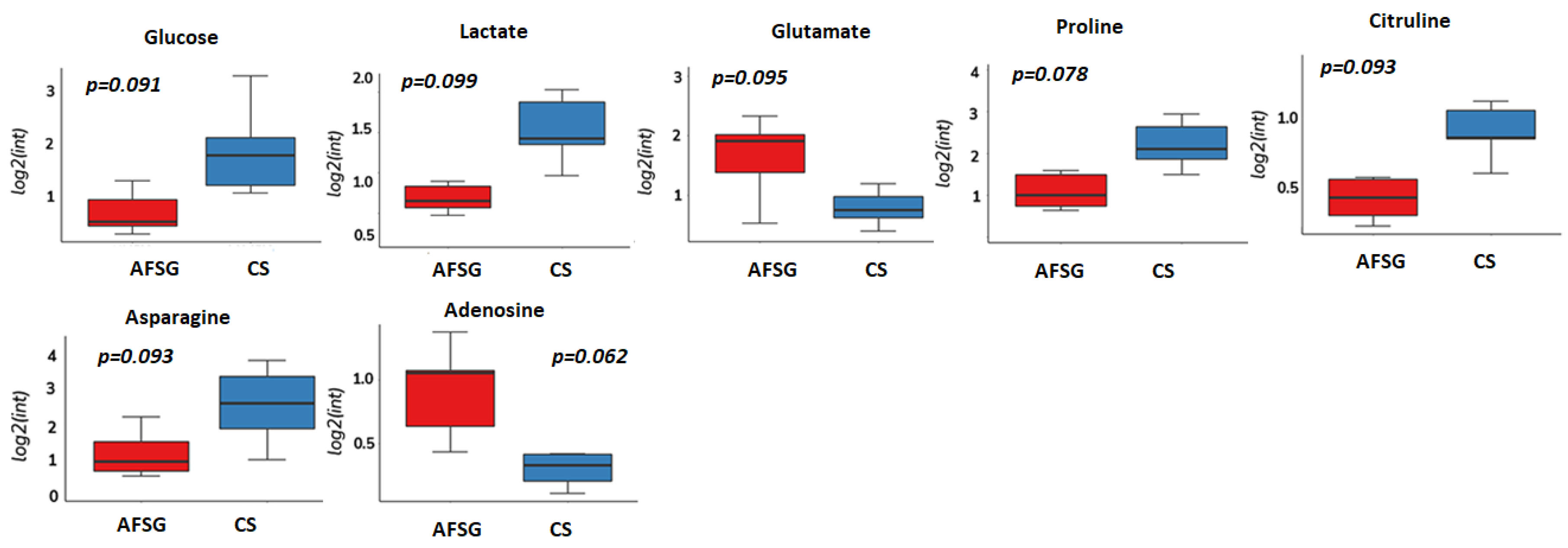
3.1. Amino Acid Metabolism Is Altered during Healing Irrespective of Burn Wound Depth
| Metabolite | Kegg ID | Mode | m/z | Retention Time | p-Value | Temporal Pattern |
|---|---|---|---|---|---|---|
| Allantoin | C02350 | neg | 157.0367 | 3.4 | 2.0 × 10−3 | U1 |
| Arginine | C00062 | pos | 175.1193 | 5.0 | 3.0 × 10−8 | U2 |
| Histamine | C00388 | pos | 95.0610 | 4.7 | 1.0 × 10−4 | U2 |
| 3,4-Dihydroxymandelic acid | C05580 | pos | 84.9606 | 5.8 | 8.0 × 10−3 | P2 |
| 5′-Methylthioadenosine | C00170 | pos | 298.1026 | 4.8 | 2.0 × 10−7 | P2 |
| Valine/Betain | C00183 | pos | 118.0866 | 3.8 | 8.0 × 10−11 | P2 |
| Carnosine | C00386 | pos | 151.1390 | 5.0 | 4.0 × 10−4 | P3 |
| Citrulline | C00327 | pos | 150.0772 | 4.6 | 7.0 × 10−3 | P2 |
| Cystathionine | C02291 | pos | 223.0759 | 5.4 | 1.0 × 10−5 | P1 |
| Cystine | C00491 | pos | 151.9845 | 5.4 | 8.0 × 10−5 | P2 |
| Cytidine | C00475 | neg | 242.0792 | 4.7 | 6.0 × 10−4 | P3 |
| Glutamine | C00064 | pos | 148.0799 | 4.8 | 6.0 × 10−3 | P2 |
| Glycerophosphocholine | C12181 | pos | 258.1105 | 4.7 | 8.0 × 10−3 | P1 |
| Guanosine | C00387 | pos | 328.0643 | 3.9 | 3.0 × 10−3 | P2 |
| Hypotaurine | C00519 | neg | 108.0121 | 4.5 | 9.0 × 10−10 | P2 |
| Glycogen/Maltotetraose | C02052 | neg | 665.2152 | 5.6 | 5.0 × 10−4 | P2 |
| Maltotriose | C01835 | neg | 549.1679 | 5.3 | 1.0 × 10−3 | P2 |
| Oxidized glutathione | C00051 | pos | 556.1392 | 5.4 | 3.0 × 10−3 | P2 |
| PI(18:2/20:3) | #N/A | pos | 887.5654 | 3.1 | 6.0 × 10−3 | P3 |
| pyrophosphate | C00013 | neg | 176.9356 | 4.4 | 7.0 × 10−3 | P3 |
| S-Adenosylhomocysteine | C00021 | pos | 238.0463 | 4.8 | 7.0 × 10−7 | P3 |
| LPC(18:2) | #N/A | pos | 564.2877 | 5.1 | 9.0 × 10−9 | D1 |
| LPC(20:4) | #N/A | pos | 588.6345 | 5.0 | 7.0 × 10−12 | D1 |
| Methyladenosine | C02494 | pos | 282.1220 | 3.4 | 4.0 × 10−4 | D1 |
| Metabolite | Kegg ID | Mode | m/z | Retention Time | p-Value | Temporal Pattern |
|---|---|---|---|---|---|---|
| Histamine | C00388 | pos | 95.06082 | 5.1 | 8.80 × 10−3 | U1 |
| Serine | C00065 | neg | 104.03505 | 4.6 | 6.62 × 10−5 | U1 |
| Hypotaurine | C00519 | pos/neg | 110.02764/108.01206 | 4.4 | 1.60 × 10−3 | U1 |
| Histidine | C00135 | pos/neg | 156.07728/154.06188 | 5.0 | 2.42 × 10−6 | U1 |
| Allantoin | C02350 | neg | 157.03668 | 3.4 | 1.90 × 10−3 | U1 |
| Citrulline | C00327 | neg | 174.08813 | 4.6 | 3.48 × 10−4 | U1 |
| Arginine | C00062 | pos | 175.11927 | 5.0 | 8.57 × 10−10 | U1 |
| Cystathionine | C02291 | neg | 221.06041 | 5.4 | 2.19 × 10−8 | P1 |
| Cytidine | C00475 | neg | 240.92911 | 4.0 | 9.00 × 10−3 | P1 |
| Hydroxyproline | C01157 | neg | 130.05016 | 4.4 | 1.17 × 10−5 | P3 |
| S-adenosyl methionine | C00019 | pos | 708.63496 | NA | 5.15 × 10−6 | D1 |
| Glutamine | C00064 | pos | 148.0799 | 4.8 | 3.25 × 10−5 | D2 |
| Pantothenic acid | C00864 | pos | 220.11887 | 2.5 | 5.20 × 10−3 | D2 |
| 5′-Deoxy-5′-methylthioadenosine | C00170 | pos | 298.10262 | 5.3 | 4.93 × 10−12 | D2 |
| Oxidized glutathione | C00051 | pos | 777.8926 | NA | 4.83 × 10−4 | D2 |
| Leukotriene C4 | C02166 | pos | 828.9304 | NA | 8.51 × 10−6 | D2 |
| Valine/Betaine | C00183 | pos | 118.08662 | 3.8 | 1.92 × 10−11 | D3 |
| Glutamate | C00025 | pos/neg | 148.06088/146.04521 | 4.4 | 3.20 × 10−7 | D3 |
| Cystine | C00491 | pos | 151.98453 | NA | 3.12 × 10−3 | D3 |
| 3,4-Dihydroxymandelic acid | C05580 | pos | 125.98727 | 5.8 | 5.15 × 10−6 | D3 |
| Guanine | C00242 | pos | 152.05761 | 5.4 | 2.44 × 10−4 | D3 |
| S-Adenosylhomocysteine | C00021 | pos | 238.04632 | 4.8 | 2.29 × 10−4 | D3 |
| N-acetyl aspartate | C01042 | neg | 359.99008 | 4.2 | 4.96 × 10−4 | D3 |
| CMP | C00055 | pos/neg | 324.06018/322.04423 | 5.3 | 5.00 × 10−3 | D3 |
| NADH | C00004 | pos | 362.0896 | 5.2 | 3.00 × 10−3 | D3 |
| GMP | C00144 | pos | 364.06663 | 5.3 | 6.00 × 10−4 | D3 |
| Proline | C00148 | neg | 385.93804 | NA | 3.00 × 10−3 | D3 |
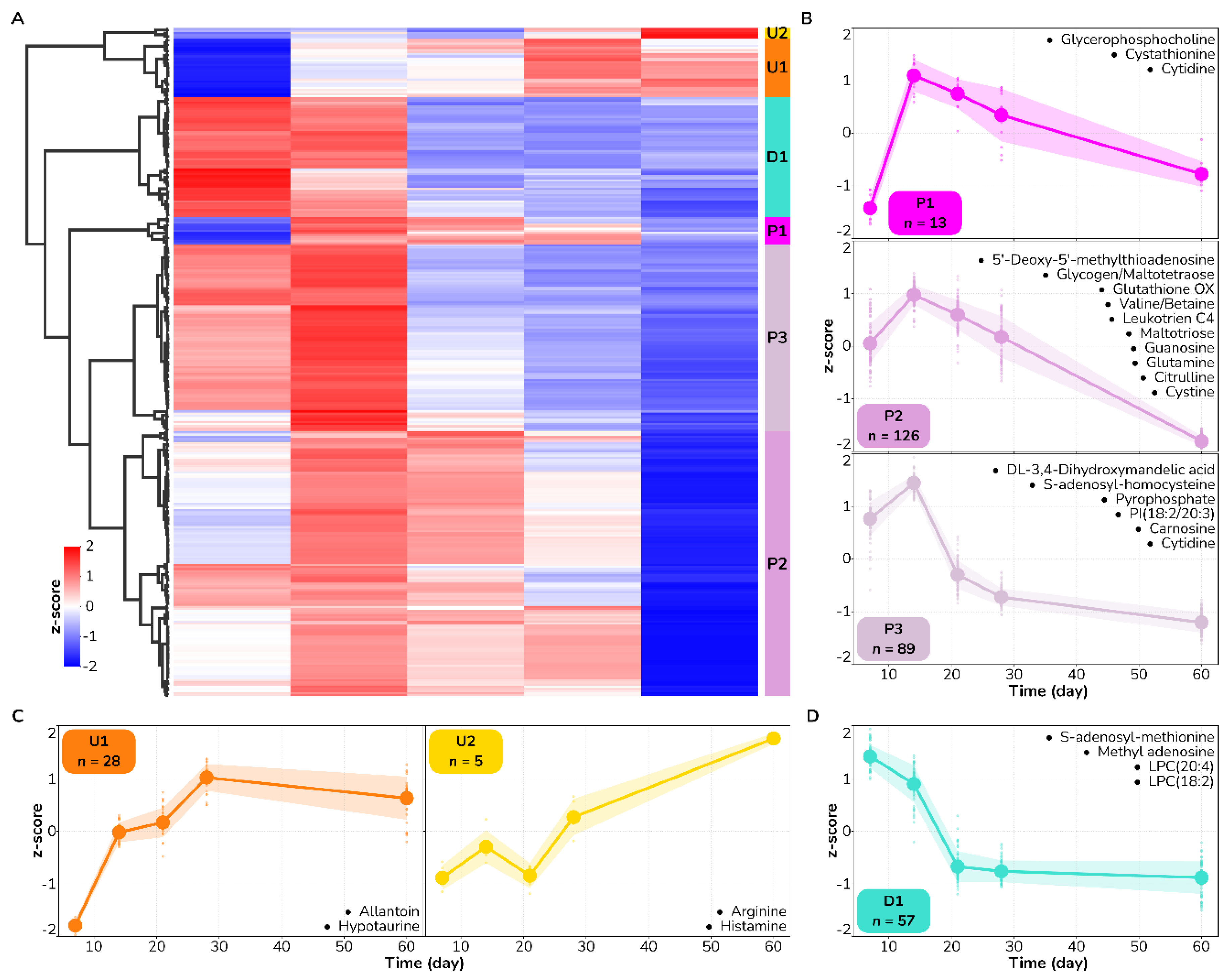
3.2. Metabolite Trajectories of Wound Healing Are Different across DPT and FT Burn Wounds
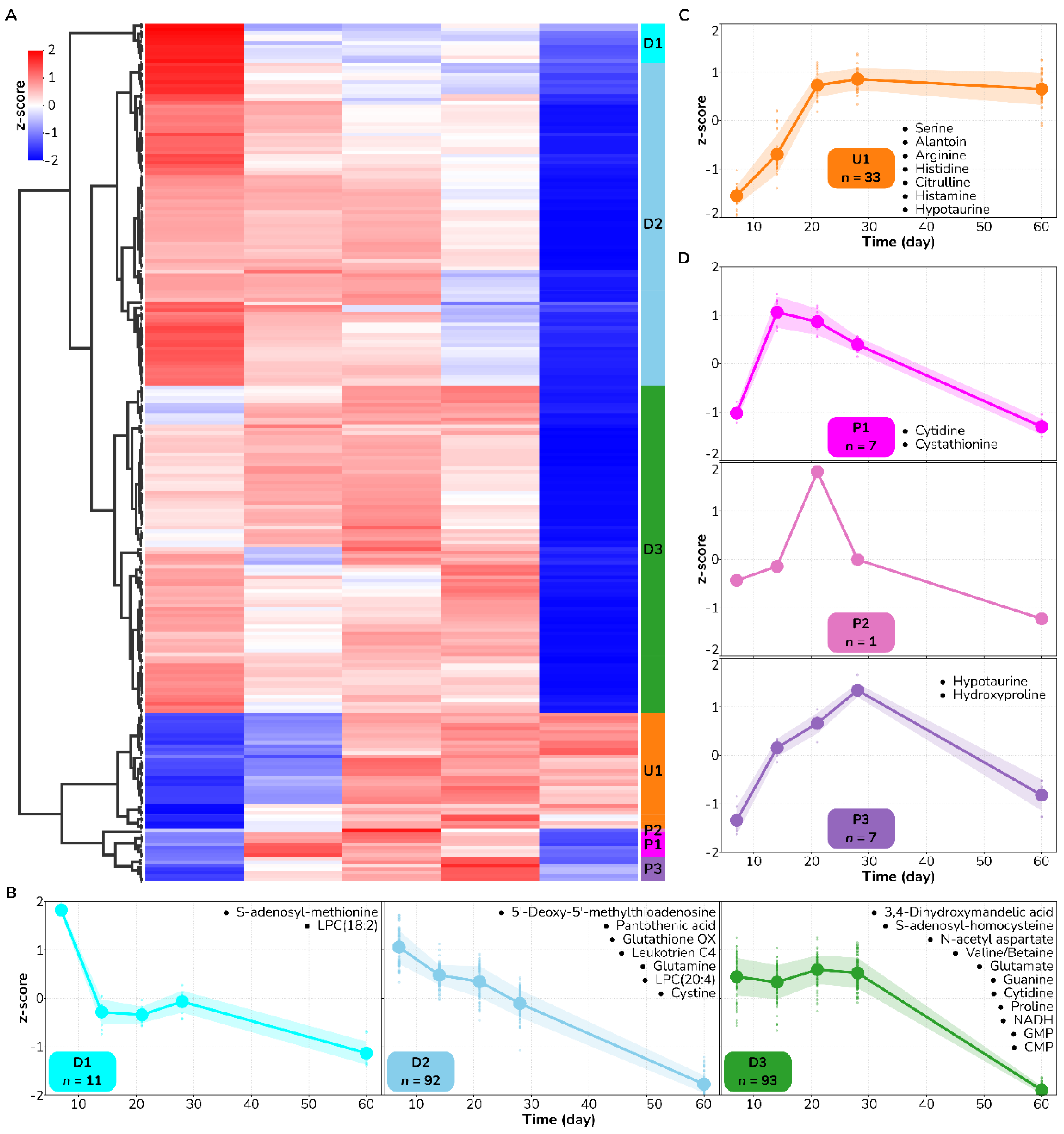
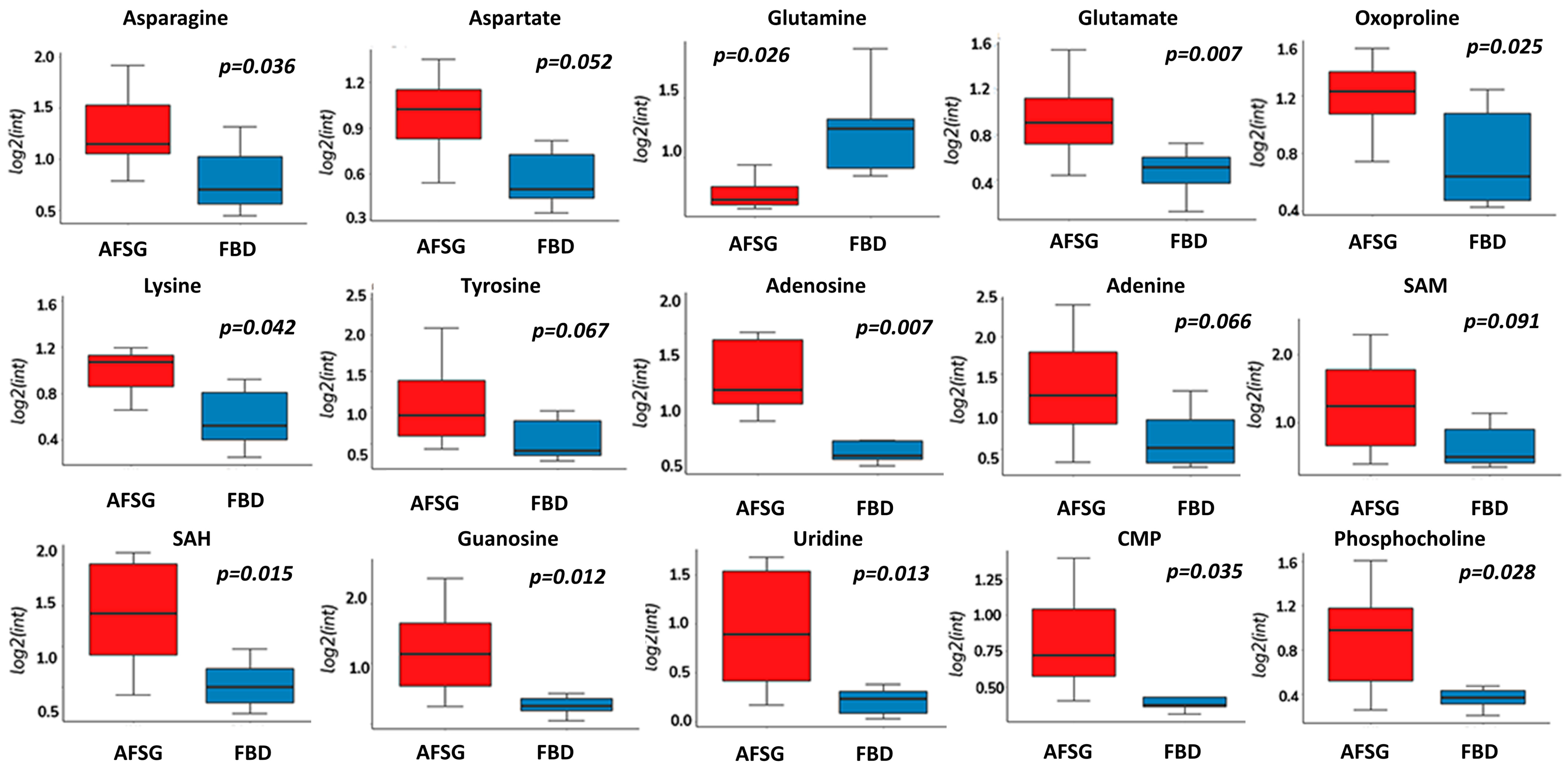
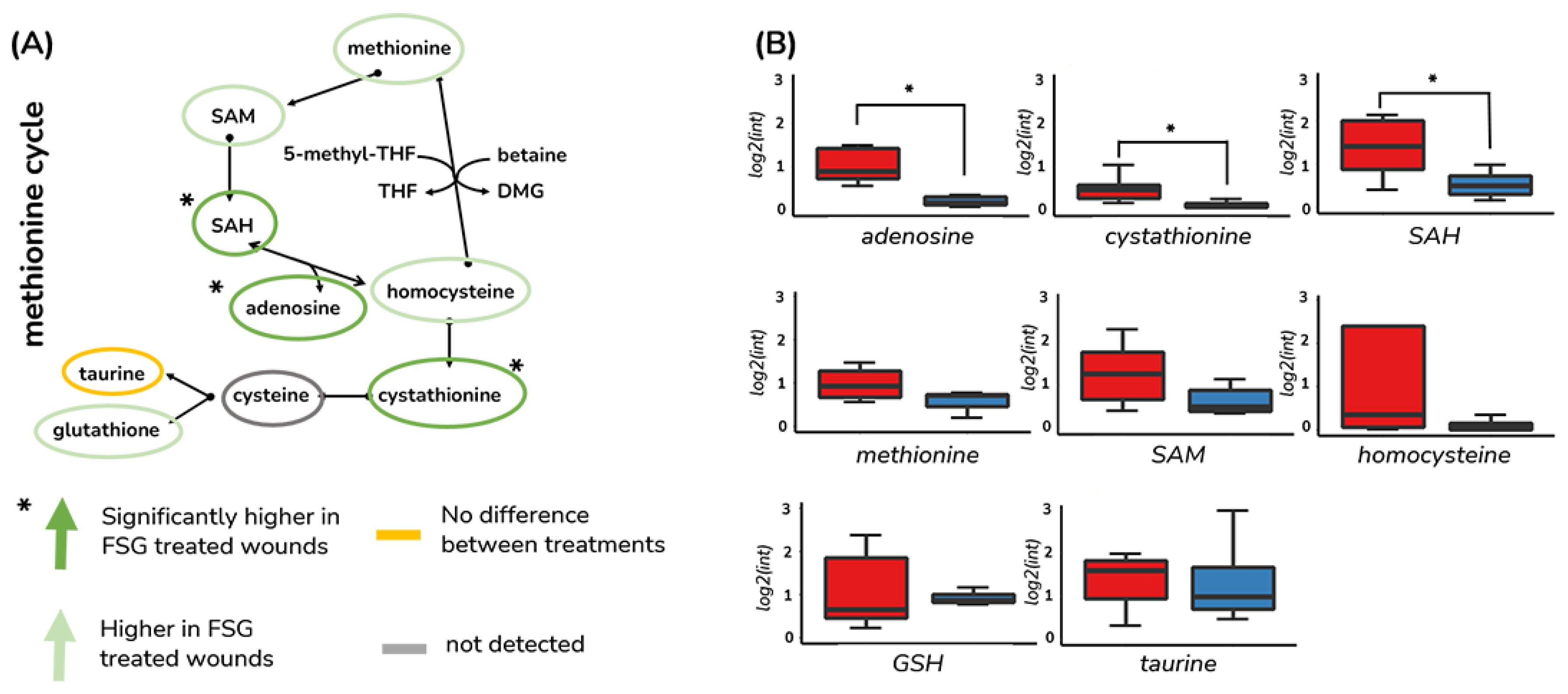
3.3. Methionine Salvage Is Affected by Burn Wound Treatment on Day 7
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Burns Report. Available online: https://www.who.int/news-room/fact-sheets/detail/burns (accessed on 20 January 2022).
- American Burn Association. Burn Incidence Fact Sheet. Available online: https://ameriburn.org/who-we-are/media/burn-incidence-fact-sheet/ (accessed on 20 January 2022).
- Yamamoto, T.; Iwase, H.; King, T.W.; Hara, H.; Cooper, D.K.C. Skin xenotransplantation: Historical review and clinical potential. Burns 2018, 44, 1738–1749. [Google Scholar] [CrossRef] [PubMed]
- Chua, A.W.C.; Khoo, Y.C.; Tan, B.K.; Tan, K.C.; Foo, C.L.; Chong, S.J. Skin tissue engineering advances in severe burns: Review and therapeutic applications. Burn. Trauma 2016, 4, s41038-016-0027-y. [Google Scholar] [CrossRef] [PubMed]
- Zeng, R.; Lin, C.; Lin, Z.; Chen, H.; Lu, W.; Lin, C.; Li, H. Approaches to cutaneous wound healing: Basics and future directions. Cell Tissue Res. 2018, 374, 217–232. [Google Scholar] [CrossRef] [PubMed]
- Stone Ii, R.; Natesan, S.; Kowalczewski, C.J.; Mangum, L.H.; Clay, N.E.; Clohessy, R.M.; Carlsson, A.H.; Tassin, D.H.; Chan, R.K.; Rizzo, J.A.; et al. Advancements in Regenerative Strategies Through the Continuum of Burn Care. Front. Pharmacol. 2018, 9, 672. [Google Scholar] [CrossRef] [PubMed]
- Assessment and Classification of Burn Injury. Available online: https://www.uptodate.com/contents/assessment-and-classification-of-burn-injury (accessed on 20 January 2022).
- Nisanci, M.; Eski, M.; Sahin, I.; Ilgan, S.; Isik, S. Saving the zone of stasis in burns with activated protein C: An experimental study in rats. Burns 2010, 36, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Kagan, R.J.; Peck, M.D.; Ahrenholz, D.H.; Hickerson, W.L.; Holmes, J.t.; Korentager, R.; Kraatz, J.; Pollock, K.; Kotoski, G. Surgical management of the burn wound and use of skin substitutes: An expert panel white paper. J. Burn. Care Res. 2013, 34, e60–e79. [Google Scholar] [CrossRef] [PubMed]
- Porter, C.; Hurren, N.M.; Herndon, D.N.; Borsheim, E. Whole body and skeletal muscle protein turnover in recovery from burns. Int. J. Burn. Trauma. 2013, 3, 9–17. [Google Scholar]
- Sommer, K.; Sander, A.L.; Albig, M.; Weber, R.; Henrich, D.; Frank, J.; Marzi, I.; Jakob, H. Delayed wound repair in sepsis is associated with reduced local pro-inflammatory cytokine expression. PLoS ONE 2013, 8, e73992. [Google Scholar] [CrossRef]
- van der Poll, T.; van de Veerdonk, F.L.; Scicluna, B.P.; Netea, M.G. The immunopathology of sepsis and potential therapeutic targets. Nat. Rev. Immunol. 2017, 17, 407–420. [Google Scholar] [CrossRef]
- Nitzschke, S.L.; Aden, J.K.; Serio-Melvin, M.L.; Shingleton, S.K.; Chung, K.K.; Waters, J.A.; King, B.T.; Burns, C.J.; Lundy, J.B.; Salinas, J.; et al. Wound healing trajectories in burn patients and their impact on mortality. J. Burn. Care Res. 2014, 35, 474–479. [Google Scholar] [CrossRef]
- Torres, M.J.M.; Peterson, J.M.; Wolf, S.E. Detection of Infection and Sepsis in Burns. Surg. Infect. 2021, 22, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Castilla, M.; Roca, O.; Masclans, J.R.; Barret, J.P. Recent Advances in Biomarkers in Severe Burns. Shock 2016, 45, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Lindley, L.E.; Stojadinovic, O.; Pastar, I.; Tomic-Canic, M. Biology and Biomarkers for Wound Healing. Plast. Reconstr. Surg. 2016, 138, 18S–28S. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Maheshwari, A.; Chandra, A. Biomarkers for wound healing and their evaluation. J. Wound Care 2016, 25, 46–55. [Google Scholar] [CrossRef]
- Kim, S.Y.; Nair, M.G. Macrophages in wound healing: Activation and plasticity. Immunol. Cell Biol. 2019, 97, 258–267. [Google Scholar] [CrossRef]
- Jeschke, M.G.; Chinkes, D.L.; Finnerty, C.C.; Kulp, G.; Suman, O.E.; Norbury, W.B.; Branski, L.K.; Gauglitz, G.G.; Mlcak, R.P.; Herndon, D.N. Pathophysiologic response to severe burn injury. Ann. Surg. 2008, 248, 387–401. [Google Scholar] [CrossRef]
- Hawksworth, J.S.; Stojadinovic, A.; Gage, F.A.; Tadaki, D.K.; Perdue, P.W.; Forsberg, J.; Davis, T.A.; Dunne, J.R.; Denobile, J.W.; Brown, T.S.; et al. Inflammatory biomarkers in combat wound healing. Ann. Surg. 2009, 250, 1002–1007. [Google Scholar] [CrossRef]
- Forsberg, J.A.; Potter, B.K.; Polfer, E.M.; Safford, S.D.; Elster, E.A. Do inflammatory markers portend heterotopic ossification and wound failure in combat wounds? Clin. Orthop. Relat. Res. 2014, 472, 2845–2854. [Google Scholar] [CrossRef]
- Carlton, M.; Voisey, J.; Parker, T.J.; Punyadeera, C.; Cuttle, L. A review of potential biomarkers for assessing physical and psychological trauma in paediatric burns. Burn. Trauma. 2021, 9, tkaa049. [Google Scholar] [CrossRef]
- Janak, J.C.; Stewart, I.J.; Sosnov, J.A.; Howard, J.T.; Siew, E.D.; Chan, M.M.; Wickersham, N.; Ikizler, T.A.; Chung, K.K. Urinary Biomarkers are Associated with Severity and Mechanism of Injury. Shock 2017, 47, 593–598. [Google Scholar] [CrossRef]
- Norbury, W.B.; Herndon, D.N.; Branski, L.K.; Chinkes, D.L.; Jeschke, M.G. Urinary cortisol and catecholamine excretion after burn injury in children. J. Clin. Endocrinol. Metab. 2008, 93, 1270–1275. [Google Scholar] [CrossRef] [PubMed]
- Zang, T.; Cuttle, L.; Broszczak, D.A.; Broadbent, J.A.; Tanzer, C.; Parker, T.J. Characterization of the Blister Fluid Proteome for Pediatric Burn Classification. J. Proteome Res. 2019, 18, 69–85. [Google Scholar] [CrossRef] [PubMed]
- Brown, N.J.; Kimble, R.M.; Rodger, S.; Ware, R.S.; McWhinney, B.C.; Ungerer, J.P.; Cuttle, L. Biological markers of stress in pediatric acute burn injury. Burns 2014, 40, 887–895. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Thompson, C.B. Metabolic regulation of cell growth and proliferation. Nat. Rev. Mol. Cell Biol. 2019, 20, 436–450. [Google Scholar] [CrossRef] [PubMed]
- Comino-Sanz, I.M.; López-Franco, M.D.; Castro, B.; Pancorbo-Hidalgo, P.L. The Role of Antioxidants on Wound Healing: A Review of the Current Evidence. J. Clin. Med. 2021, 10, 3558. [Google Scholar] [CrossRef] [PubMed]
- Kotronoulas, A.; de Lomana, A.L.G.; Karvelsson, S.T.; Heijink, M.; Stone Ii, R.; Giera, M.; Rolfsson, O. Lipid mediator profiles of burn wound healing: Acellular cod fish skin grafts promote the formation of EPA and DHA derived lipid mediators following seven days of treatment. Prostaglandins Leukot. Essent. Fat. Acids 2021, 175, 102358. [Google Scholar] [CrossRef] [PubMed]
- Cajka, T.; Fiehn, O. Toward Merging Untargeted and Targeted Methods in Mass Spectrometry-Based Metabolomics and Lipidomics. Anal. Chem. 2016, 88, 524–545. [Google Scholar] [CrossRef]
- Liu, X.R.; Zheng, X.F.; Ji, S.Z.; Lv, Y.H.; Zheng, D.Y.; Xia, Z.F.; Zhang, W.D. Metabolomic analysis of thermally injured and/or septic rats. Burns 2010, 36, 992–998. [Google Scholar] [CrossRef]
- Qi, P.; Abdullahi, A.; Stanojcic, M.; Patsouris, D.; Jeschke, M.G. Lipidomic analysis enables prediction of clinical outcomes in burn patients. Sci. Rep. 2016, 6, 38707. [Google Scholar] [CrossRef]
- Stone, R., 2nd; Saathoff, E.C.; Larson, D.A.; Wall, J.T.; Wienandt, N.A.; Magnusson, S.; Kjartansson, H.; Natesan, S.; Christy, R.J. Accelerated Wound Closure of Deep Partial Thickness Burns with Acellular Fish Skin Graft. Int. J. Mol. Sci. 2021, 22, 1590. [Google Scholar] [CrossRef]
- Smith, C.A.; Want, E.J.; O’Maille, G.; Abagyan, R.; Siuzdak, G. XCMS: Processing Mass Spectrometry Data for Metabolite Profiling Using Nonlinear Peak Alignment, Matching, and Identification. Anal. Chem. 2006, 78, 779–787. [Google Scholar] [CrossRef] [PubMed]
- De Livera, A.M.; Olshansky, G.; Simpson, J.A.; Creek, D.J. NormalizeMets: Assessing, selecting and implementing statistical methods for normalizing metabolomics data. Metabolomics 2018, 14, 54. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Wishart, D.S. Web-based inference of biological patterns, functions and pathways from metabolomic data using MetaboAnalyst. Nat. Protoc. 2011, 6, 743–760. [Google Scholar] [CrossRef] [PubMed]
- Paglia, G.; Williams, J.P.; Menikarachchi, L.; Thompson, J.W.; Tyldesley-Worster, R.; Halldórsson, S.; Rolfsson, O.; Moseley, A.; Grant, D.; Langridge, J.; et al. Ion Mobility Derived Collision Cross Sections to Support Metabolomics Applications. Anal. Chem. 2014, 86, 3985–3993. [Google Scholar] [CrossRef] [PubMed]
- Uppal, K.; Walker, D.I.; Jones, D.P. xMSannotator: An R Package for Network-Based Annotation of High-Resolution Metabolomics Data. Anal. Chem. 2017, 89, 1063–1067. [Google Scholar] [CrossRef]
- Kuhl, C.; Tautenhahn, R.; Böttcher, C.; Larson, T.R.; Neumann, S. CAMERA: An integrated strategy for compound spectra extraction and annotation of liquid chromatography/mass spectrometry data sets. Anal. Chem. 2012, 84, 283–289. [Google Scholar] [CrossRef]
- Schymanski, E.L.; Jeon, J.; Gulde, R.; Fenner, K.; Ruff, M.; Singer, H.P.; Hollender, J. Identifying Small Molecules via High Resolution Mass Spectrometry: Communicating Confidence. Environ. Sci. Technol. 2014, 48, 2097–2098. [Google Scholar] [CrossRef]
- Baldursson, B.T.; Kjartansson, H.; Konrádsdóttir, F.; Gudnason, P.; Sigurjonsson, G.F.; Lund, S.H. Healing Rate and Autoimmune Safety of Full-Thickness Wounds Treated With Fish Skin Acellular Dermal Matrix Versus Porcine Small-Intestine Submucosa:A Noninferiority Study. Int. J. Low. Extrem. Wounds 2015, 14, 37–43. [Google Scholar] [CrossRef]
- Krzyszczyk, P.; Schloss, R.; Palmer, A.; Berthiaume, F. The Role of Macrophages in Acute and Chronic Wound Healing and Interventions to Promote Pro-wound Healing Phenotypes. Front. Physiol. 2018, 9, 419. [Google Scholar] [CrossRef]
- Vannella, K.M.; Wynn, T.A. Mechanisms of Organ Injury and Repair by Macrophages. Annu. Rev. Physiol. 2017, 79, 593–617. [Google Scholar] [CrossRef]
- Arribas-López, E.; Zand, N.; Ojo, O.; Snowden, M.J.; Kochhar, T. The Effect of Amino Acids on Wound Healing: A Systematic Review and Meta-Analysis on Arginine and Glutamine. Nutrients 2021, 13, 2498. [Google Scholar] [CrossRef] [PubMed]
- Eming, S.A.; Murray, P.J.; Pearce, E.J. Metabolic orchestration of the wound healing response. Cell Metab. 2021, 33, 1726–1743. [Google Scholar] [CrossRef] [PubMed]
- Veith, A.P.; Henderson, K.; Spencer, A.; Sligar, A.D.; Baker, A.B. Therapeutic strategies for enhancing angiogenesis in wound healing. Adv. Drug Deliv. Rev. 2019, 146, 97–125. [Google Scholar] [CrossRef] [PubMed]
- Rasik, A.M.; Shukla, A. Antioxidant status in delayed healing type of wounds. Int. J. Exp. Pathol. 2000, 81, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Drury, A.N.; Szent-Györgyi, A. The physiological activity of adenine compounds with especial reference to their action upon the mammalian heart. J. Physiol. 1929, 68, 213–237. [Google Scholar] [CrossRef] [PubMed]
- Cronstein, B.N. Adenosine, an endogenous anti-inflammatory agent. J. Appl. Physiol. 1994, 76, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Frasson, A.P.; Menezes, C.B.; Goelzer, G.K.; Gnoatto, S.C.B.; Garcia, S.C.; Tasca, T. Adenosine reduces reactive oxygen species and interleukin-8 production by Trichomonas vaginalis-stimulated neutrophils. Purinergic Signal. 2017, 13, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Thakur, S.; Du, J.; Hourani, S.; Ledent, C.; Li, J.-M. Inactivation of Adenosine A2A Receptor Attenuates Basal and Angiotensin II-induced ROS Production by Nox2 in Endothelial Cells. J. Biol. Chem. 2010, 285, 40104–40113. [Google Scholar] [CrossRef]
- Almeida, C.G.; de Mendonça, A.; Cunha, R.A.; Ribeiro, J.A. Adenosine promotes neuronal recovery from reactive oxygen species induced lesion in rat hippocampal slices. Neurosci. Lett. 2003, 339, 127–130. [Google Scholar] [CrossRef]
- Montesinos, M.C.; Gadangi, P.; Longaker, M.; Sung, J.; Levine, J.; Nilsen, D.; Reibman, J.; Li, M.; Jiang, C.-K.; Hirschhorn, R.; et al. Wound Healing Is Accelerated by Agonists of Adenosine A2 (Gαs-linked) Receptors. J. Exp. Med. 1997, 186, 1615–1620. [Google Scholar] [CrossRef]
- Ohta, A.; Sitkovsky, M. Role of G-protein-coupled adenosine receptors in downregulation of inflammation and protection from tissue damage. Nature 2001, 414, 916–920. [Google Scholar] [CrossRef] [PubMed]
- Montesinos, M.C.; Desai, A.; Chen, J.-F.; Yee, H.; Schwarzschild, M.A.; Fink, J.S.; Cronstein, B.N. Adenosine Promotes Wound Healing and Mediates Angiogenesis in Response to Tissue Injury Via Occupancy of A2A Receptors. Am. J. Pathol. 2002, 160, 2009–2018. [Google Scholar] [CrossRef] [PubMed]
- Castro, C.M.; Corciulo, C.; Solesio, M.E.; Liang, F.; Pavlov, E.V.; Cronstein, B.N. Adenosine A2A receptor (A2AR) stimulation enhances mitochondrial metabolism and mitigates reactive oxygen species-mediated mitochondrial injury. Faseb J. 2020, 34, 5027–5045. [Google Scholar] [CrossRef] [PubMed]
- Pasquini, S.; Contri, C.; Borea, P.A.; Vincenzi, F.; Varani, K. Adenosine and Inflammation: Here, There and Everywhere. Int. J. Mol. Sci. 2021, 22, 7685. [Google Scholar] [CrossRef] [PubMed]
- Barletta, K.E.; Ley, K.; Mehrad, B. Regulation of neutrophil function by adenosine. Arter. Thromb. Vasc. Biol. 2012, 32, 856–864. [Google Scholar] [CrossRef] [PubMed]
- Ali, R.A.; Gandhi, A.A.; Meng, H.; Yalavarthi, S.; Vreede, A.P.; Estes, S.K.; Palmer, O.R.; Bockenstedt, P.L.; Pinsky, D.J.; Greve, J.M.; et al. Adenosine receptor agonism protects against NETosis and thrombosis in antiphospholipid syndrome. Nat. Commun. 2019, 10, 1916. [Google Scholar] [CrossRef]
- Wang, X.; Chen, D. Purinergic Regulation of Neutrophil Function. Front. Immunol. 2018, 9, 399. [Google Scholar] [CrossRef]
- Haywood, N.; Byler, M.R.; Zhang, A.; Rotar, E.P.; Money, D.; Gradecki, S.E.; Ta, H.Q.; Salmon, M.; Kron, I.L.; Laubach, V.E.; et al. Secondary Burn Progression Mitigated by an Adenosine 2A Receptor Agonist. J. Burn. Care Res. 2022, 43, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Shaked, G.; Gurfinkel, R.; Czeiger, D.; Douvdevani, A.; Sufaro, Y. Adenosine in burn blister fluid. Burns 2007, 33, 352–354. [Google Scholar] [CrossRef] [PubMed]
- Martínez, Y.; Li, X.; Liu, G.; Bin, P.; Yan, W.; Más, D.; Valdivié, M.; Hu, C.-A.A.; Ren, W.; Yin, Y. The role of methionine on metabolism, oxidative stress, and diseases. Amino Acids 2017, 49, 2091–2098. [Google Scholar] [CrossRef]
- Perez-Tamayo, R.; Ihnen, M. The effect of methionine in experimental wound healing; a morphologic study. Am. J. Pathol. 1953, 29, 233–249. [Google Scholar] [PubMed]
- Trocha, K.; Kip, P.; MacArthur, M.R.; Mitchell, S.J.; Longchamp, A.; Treviño-Villarreal, J.H.; Tao, M.; Bredella, M.A.; De Amorim Bernstein, K.; Mitchell, J.R.; et al. Preoperative Protein or Methionine Restriction Preserves Wound Healing and Reduces Hyperglycemia. J. Surg. Res. 2019, 235, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Rembe, J.D.; Fromm-Dornieden, C.; Stuermer, E.K. Effects of Vitamin B Complex and Vitamin C on Human Skin Cells: Is the Perceived Effect Measurable? Adv. Ski. Wound Care 2018, 31, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Findlay, C.W. Effect of Vitamin B12 on Wound Healing. Proc. Soc. Exp. Biol. Med. 1953, 82, 492–495. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, S.; Takano, M.; Sugano, N.; Ohtsu, M.; Tsunoda, K.; Koshi, R.; Yoshinuma, N. The effect of B vitamin supplementation on wound healing in type 2 diabetic mice. J. Clin. Biochem. Nutr. 2016, 58, 64–68. [Google Scholar] [CrossRef]
- Badedi, M.; Darraj, H.; Hummadi, A.; Solan, Y.; Zakri, I.; Khawaji, A.; Daghreeri, M.; Budaydi, A. Vitamin B(12) Deficiency and Foot Ulcers in Type 2 Diabetes Mellitus: A Case-Control Study. Diabetes Metab. Syndr. Obes. 2019, 12, 2589–2596. [Google Scholar] [CrossRef]
- Sindelar, M.; Patti, G.J. Chemical Discovery in the Era of Metabolomics. J. Am. Chem. Soc. 2020, 142, 9097–9105. [Google Scholar] [CrossRef]
- Patti, G.J.; Yanes, O.; Siuzdak, G. Innovation: Metabolomics: The apogee of the omics trilogy. Nat. Rev. Mol. Cell Biol. 2012, 13, 263–269. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kotronoulas, A.; de Lomana, A.L.G.; Einarsdóttir, H.K.; Kjartansson, H.; Stone, R., II; Rolfsson, Ó. Fish Skin Grafts Affect Adenosine and Methionine Metabolism during Burn Wound Healing. Antioxidants 2023, 12, 2076. https://doi.org/10.3390/antiox12122076
Kotronoulas A, de Lomana ALG, Einarsdóttir HK, Kjartansson H, Stone R II, Rolfsson Ó. Fish Skin Grafts Affect Adenosine and Methionine Metabolism during Burn Wound Healing. Antioxidants. 2023; 12(12):2076. https://doi.org/10.3390/antiox12122076
Chicago/Turabian StyleKotronoulas, Aristotelis, Adrián López García de Lomana, Helga Kristín Einarsdóttir, Hilmar Kjartansson, Randolph Stone, II, and Óttar Rolfsson. 2023. "Fish Skin Grafts Affect Adenosine and Methionine Metabolism during Burn Wound Healing" Antioxidants 12, no. 12: 2076. https://doi.org/10.3390/antiox12122076
APA StyleKotronoulas, A., de Lomana, A. L. G., Einarsdóttir, H. K., Kjartansson, H., Stone, R., II, & Rolfsson, Ó. (2023). Fish Skin Grafts Affect Adenosine and Methionine Metabolism during Burn Wound Healing. Antioxidants, 12(12), 2076. https://doi.org/10.3390/antiox12122076







