Exploring the Antioxidant Properties of Caffeoylquinic and Feruloylquinic Acids: A Computational Study on Hydroperoxyl Radical Scavenging and Xanthine Oxidase Inhibition
Abstract
:1. Introduction
2. Computational Details
2.1. Quantum Chemistry Calculations
2.2. Blind Docking Consensus Procedure
2.3. Molecular Docking Simulations
3. Results and Discussion
3.1. Evaluation of Radical Scavenging Mechanisms
3.1.1. Acid-Base Equilibrium at Physiological pH
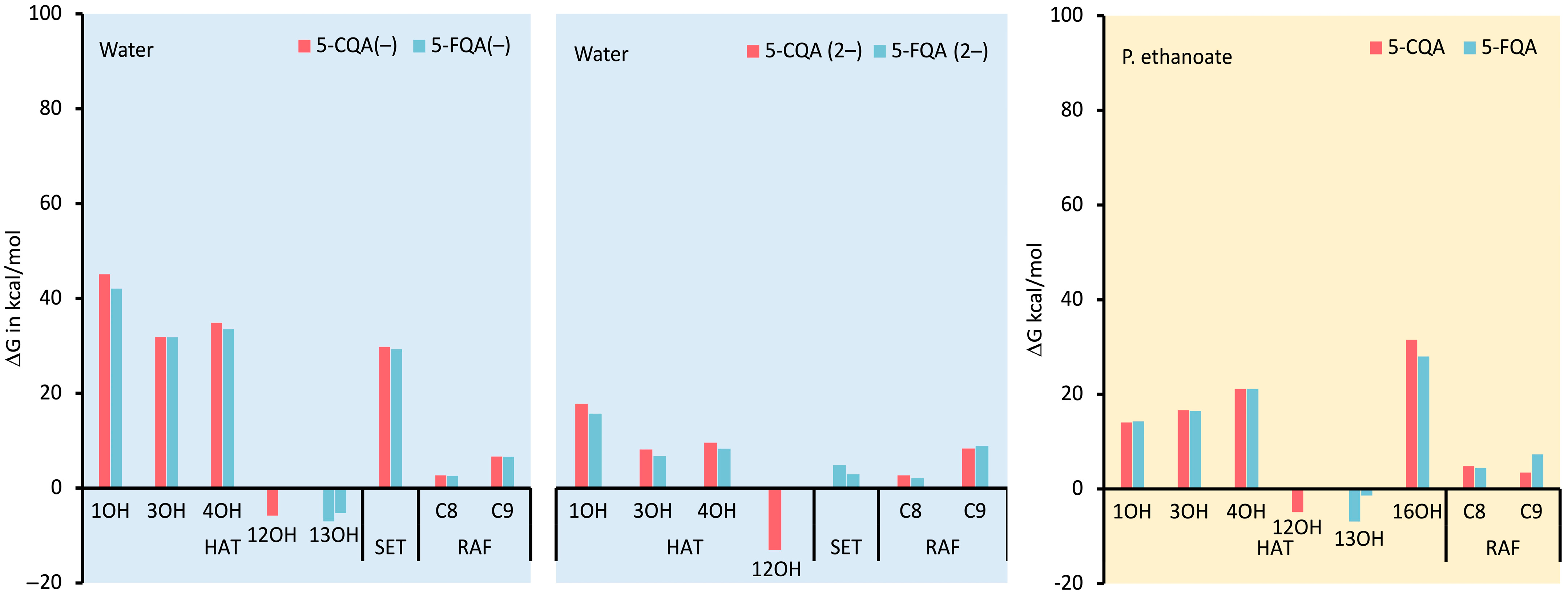
3.1.2. Thermodynamic Evaluation of the Antiradical Mechanisms
3.1.3. Reaction Kinetics under Physiological Conditions
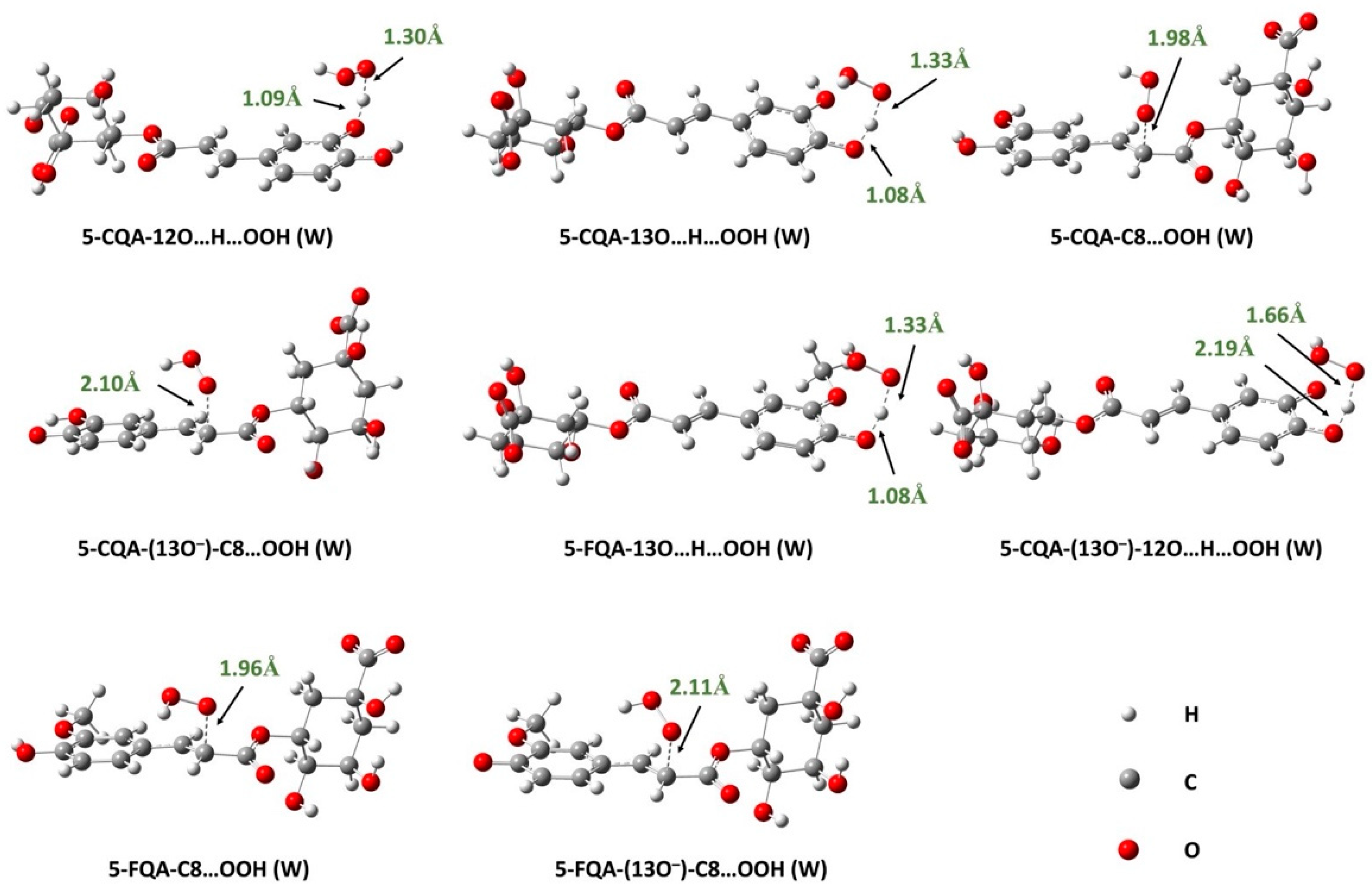
Effects of Polar Physiological Media
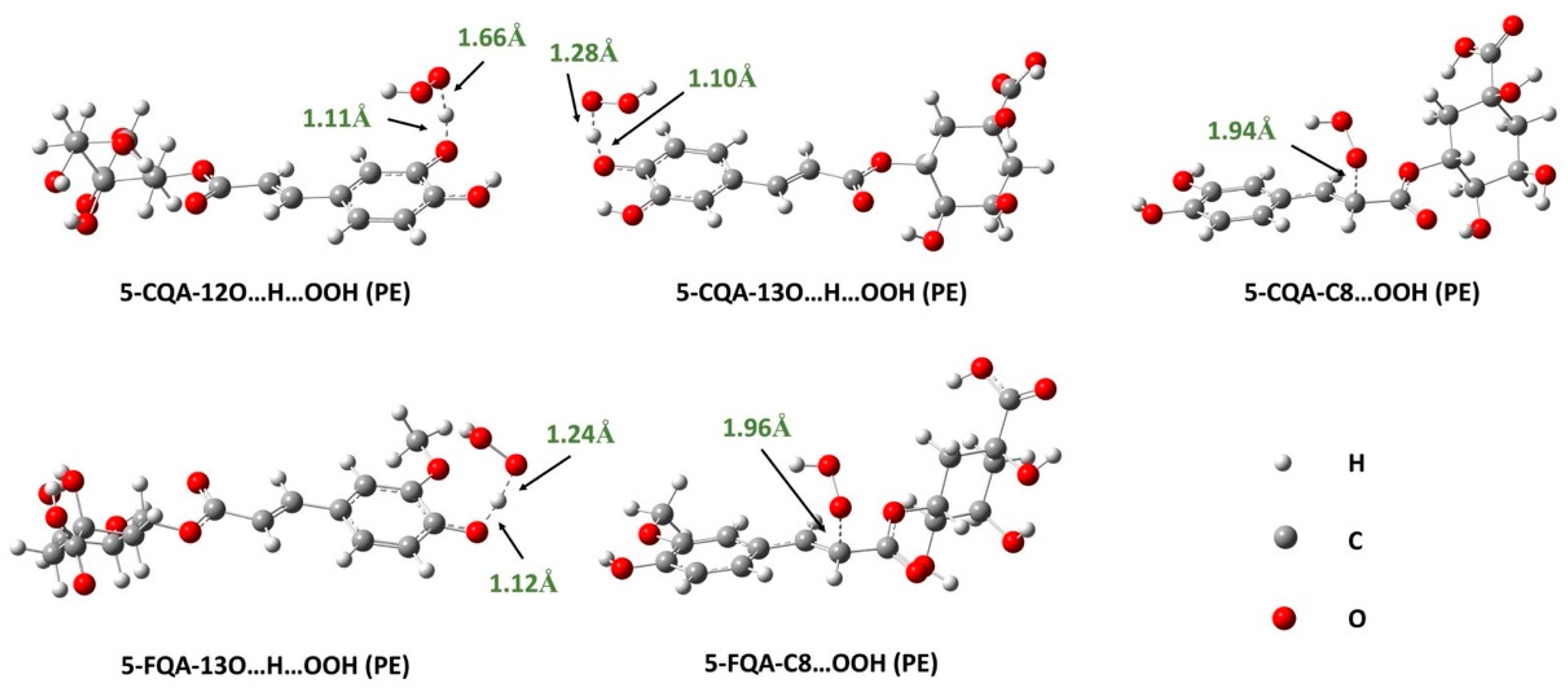
Effects of Lipid-Like Physiological Media
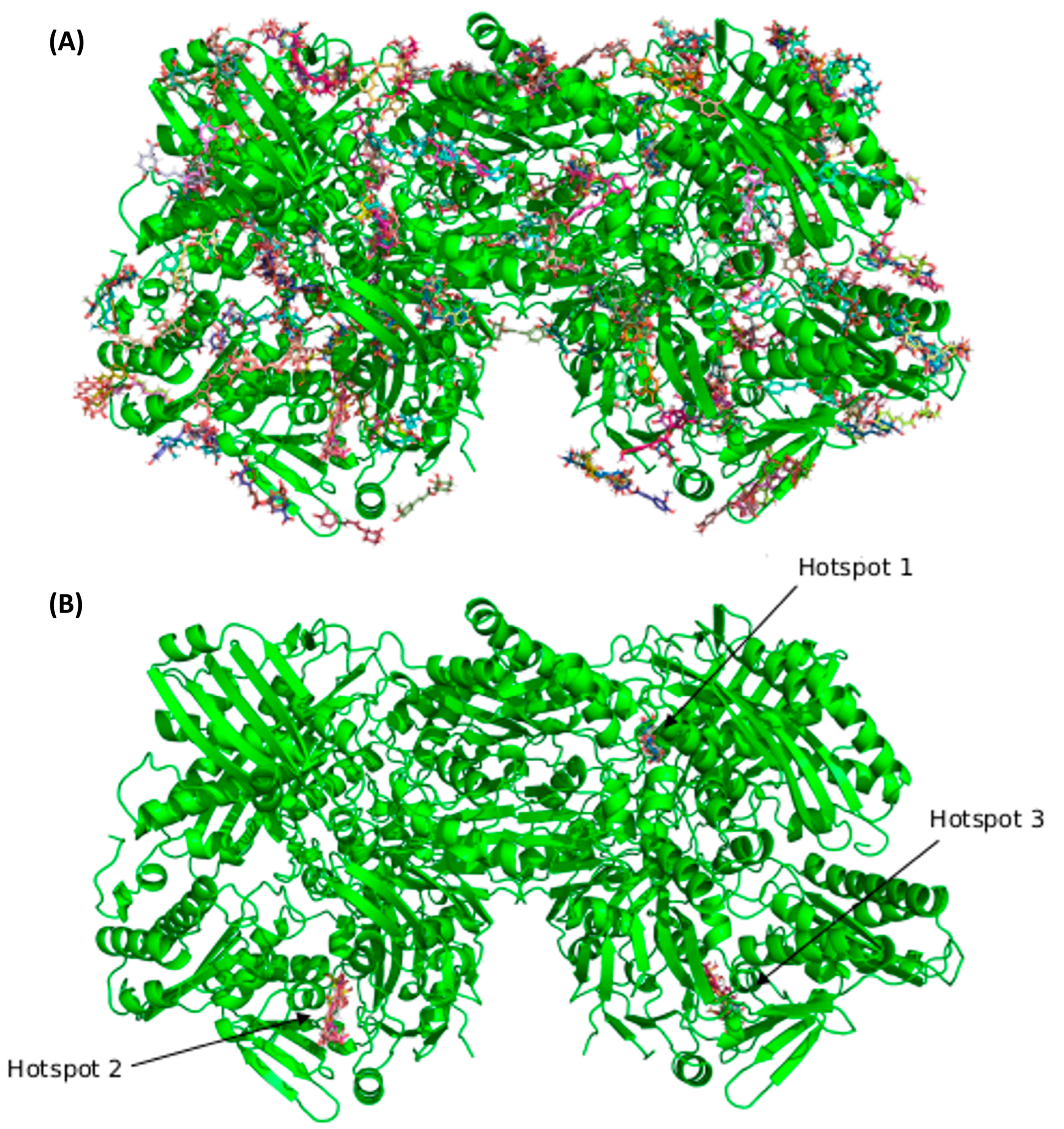
3.2. Blind Docking and Molecular Dynamics Simulations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barrea, L.; Pugliese, G.; Frias-Toral, E.; El Ghoch, M.; Castellucci, B.; Chapela, S.P.; Carignano, M.d.l.A.; Laudisio, D.; Savastano, S.; Colao, A.; et al. Coffee consumption, health benefits and side effects: A narrative review and update for dietitians and nutritionists. Crit. Rev. Food Sci. Nutr. 2023, 63, 1238–1261. [Google Scholar] [CrossRef]
- dePaula, J.; Farah, A. Caffeine Consumption through Coffee: Content in the Beverage, Metabolism, Health Benefits and Risks. Beverages 2019, 5, 37. [Google Scholar] [CrossRef]
- Ludwig, I.A.; Clifford, M.N.; Lean, M.E.J.; Ashihara, H.; Crozier, A. Coffee: Biochemistry and potential impact on health. Food Funct. 2014, 5, 1695–1717. [Google Scholar] [CrossRef] [PubMed]
- Ruggiero, E.; Di Castelnuovo, A.; Costanzo, S.; Persichillo, M.; De Curtis, A.; Cerletti, C.; Donati, M.B.; de Gaetano, G.; Iacoviello, L.; Bonaccio, M.; et al. Daily Coffee Drinking Is Associated with Lower Risks of Cardiovascular and Total Mortality in a General Italian Population: Results from the Moli-sani Study. J. Nutr. 2021, 151, 395–404. [Google Scholar] [CrossRef]
- Alcázar Magaña, A.; Kamimura, N.; Soumyanath, A.; Stevens, J.F.; Maier, C.S. Caffeoylquinic acids: Chemistry, biosynthesis, occurrence, analytical challenges, and bioactivity. Plant J. 2021, 107, 1299–1319. [Google Scholar] [CrossRef]
- Liu, W.; Li, J.; Zhang, X.; Zu, Y.; Yang, Y.; Liu, W.; Xu, Z.; Gao, H.; Sun, X.; Jiang, X.; et al. Current Advances in Naturally Occurring Caffeoylquinic Acids: Structure, Bioactivity, and Synthesis. J. Agric. Food Chem. 2020, 68, 10489–10516. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, K.; Nagel, C.W. Occurrence and content of hydroxycinnamic and hydroxybenzoic acid compounds in foods. Crit. Rev. Food Sci. Nutr. 1989, 28, 315–347. [Google Scholar] [CrossRef]
- Clifford, M.N. Chlorogenic acids and other cinnamates—Nature, occurrence, dietary burden, absorption and metabolism. J. Sci. Food Agric. 2000, 80, 1033–1043. [Google Scholar] [CrossRef]
- Lee, S.G.; Lee, H.; Nam, T.G.; Eom, S.H.; Heo, H.J.; Lee, C.Y.; Kim, D.-O. Neuroprotective Effect of Caffeoylquinic Acids from Artemisia princeps Pampanini against Oxidative Stress-Induced Toxicity in PC-12 Cells. J. Food Sci. 2011, 76, C250–C256. [Google Scholar] [CrossRef]
- Abdel Motaal, A.; Ezzat, S.M.; Tadros, M.G.; El-Askary, H.I. In vivo anti-inflammatory activity of caffeoylquinic acid derivatives from Solidago virgaurea in rats. Pharm. Biol. 2016, 54, 2864–2870. [Google Scholar] [CrossRef]
- Wu, C.; Zhang, X.; Zhang, X.; Luan, H.; Sun, G.; Sun, X.; Wang, X.; Guo, P.; Xu, X. The caffeoylquinic acid-rich Pandanus tectorius fruit extract increases insulin sensitivity and regulates hepatic glucose and lipid metabolism in diabetic db/db mice. J. Nutr. Biochem. 2014, 25, 412–419. [Google Scholar] [CrossRef]
- Mikulic-Petkovsek, M.; Samoticha, J.; Eler, K.; Stampar, F.; Veberic, R. Traditional Elderflower Beverages: A Rich Source of Phenolic Compounds with High Antioxidant Activity. J. Agric. Food Chem. 2015, 63, 1477–1487. [Google Scholar] [CrossRef]
- Szwajgier, D.; Borowiec, K.; Pustelniak, K. The Neuroprotective Effects of Phenolic Acids: Molecular Mechanism of Action. Nutrients 2017, 9, 477. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.-H.; Zhao, T.-R.; Liu, Y.-P.; Wang, Y.-F.; Cheng, G.-G.; Cao, J.-X. Phenolic constituents, antioxidant activity and neuroprotective effects of ethanol extracts of fruits, leaves and flower buds from Vaccinium dunalianum Wight. Food Chem. 2022, 374, 131752. [Google Scholar] [CrossRef] [PubMed]
- Olthof, M.R.; Katan, M.B.; Hollman, P.C.H. Chlorogenic Acid and Caffeic Acid Are Absorbed in Humans. J. Nutr. 2001, 131, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Clifford, M.N.; Knight, S.; Kuhnert, N. Discriminating between the Six Isomers of Dicaffeoylquinic Acid by LC-MSn. J. Agric. Food Chem. 2005, 53, 3821–3832. [Google Scholar] [CrossRef]
- Feng, R.; Lu, Y.; Bowman, L.L.; Qian, Y.; Castranova, V.; Ding, M. Inhibition of Activator Protein-1, NF-κB, and MAPKs and Induction of Phase 2 Detoxifying Enzyme Activity by Chlorogenic Acid. J. Biol. Chem. 2005, 280, 27888–27895. [Google Scholar] [CrossRef]
- Kwon, Y.I.; Vattem, D.A.; Shetty, K. Evaluation of clonal herbs of Lamiaceae species for management of diabetes and hypertension. Asia Pac. J. Clin. Nutr. 2006, 15, 107–118. [Google Scholar]
- Polito, L.; Bortolotti, M.; Battelli, M.G.; Bolognesi, A. Xanthine oxidoreductase: A leading actor in cardiovascular disease drama. Redox Biol. 2021, 48, 102195. [Google Scholar] [CrossRef]
- Gawlik-Dziki, U.; Dziki, D.; Świeca, M.; Nowak, R. Mechanism of action and interactions between xanthine oxidase inhibitors derived from natural sources of chlorogenic and ferulic acids. Food Chem. 2017, 225, 138–145. [Google Scholar] [CrossRef]
- Mehmood, A.; Li, J.; Rehman, A.U.; Kobun, R.; Llah, I.U.; Khan, I.; Althobaiti, F.; Albogami, S.; Usman, M.; Alharthi, F.; et al. Xanthine oxidase inhibitory study of eight structurally diverse phenolic compounds. Front. Nutr. 2022, 9, 966557. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Qian, J.; Li, Y.; Shen, Y.; Chen, Y.; Fu, G.; Xie, M. Inhibitory mechanism of xanthine oxidase activity by caffeoylquinic acids in vitro. Int. J. Biol. Macromol. 2021, 184, 843–856. [Google Scholar] [CrossRef]
- de Grey, A.D.N.J. HO2•: The forgotten radical. DNA Cell Biol. 2002, 21, 251–257. [Google Scholar] [CrossRef]
- Pryor, W.A. Oxy-radicals and related species: Their formation, lifetimes, and reactions. Annu. Rev. Physiol. 1986, 48, 657–667. [Google Scholar] [CrossRef]
- Galano, A.; Alvarez-Idaboy, J.R. A computational methodology for accurate predictions of rate constants in solution: Application to the assessment of primary antioxidant activity. J. Comput. Chem. 2013, 34, 2430–2445. [Google Scholar] [CrossRef] [PubMed]
- Leopoldini, M.; Russo, N.; Toscano, M. The molecular basis of working mechanism of natural polyphenolic antioxidants. Food Chem. 2011, 125, 288–306. [Google Scholar] [CrossRef]
- Galano, A.; Mazzone, G.; Alvarez-Diduk, R.; Marino, T.; Alvarez-Idaboy, J.R.; Russo, N. Food Antioxidants: Chemical Insights at the Molecular Level. Annu. Rev. Food Sci. Technol. 2016, 7, 335–352. [Google Scholar] [CrossRef] [PubMed]
- Litwinienko, G.; Ingold, K.U. Abnormal Solvent Effects on Hydrogen Atom Abstractions. 1. The Reactions of Phenols with 2,2-Diphenyl-1-picrylhydrazyl (dpph•) in Alcohols. J. Org. Chem. 2003, 68, 3433–3438. [Google Scholar] [CrossRef]
- Litwinienko, G.; Ingold, K.U. Abnormal Solvent Effects on Hydrogen Atom Abstraction. 2. Resolution of the Curcumin Antioxidant Controversy. The Role of Sequential Proton Loss Electron Transfer. J. Org. Chem. 2004, 69, 5888–5896. [Google Scholar] [CrossRef]
- Litwinienko, G.; Ingold, K.U. Abnormal Solvent Effects on Hydrogen Atom Abstraction. 3. Novel Kinetics in Sequential Proton Loss Electron Transfer Chemistry. J. Org. Chem. 2005, 70, 8982–8990. [Google Scholar] [CrossRef]
- Leopoldini, M.; Chiodo, S.G.; Russo, N.; Toscano, M. Detailed Investigation of the OH Radical Quenching by Natural Antioxidant Caffeic Acid Studied by Quantum Mechanical Models. J. Chem. Theory Comput. 2011, 7, 4218–4233. [Google Scholar] [CrossRef]
- Xue, Y.; Liu, Y.; Xie, Y.; Cong, C.; Wang, G.; An, L.; Teng, Y.; Chen, M.; Zhang, L. Antioxidant activity and mechanism of dihydrochalcone C-glycosides: Effects of C-glycosylation and hydroxyl groups. Phytochemistry 2020, 179, 112393. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Zhao, Y.; Truhlar, D.G. The Mo6 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four Mo6-class functionals and 12 other functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar] [CrossRef]
- Galano, A.; Alvarez-Idaboy, J.R. Kinetics of radical-molecule reactions in aqueous solution: A benchmark study of the performance of density functional methods. J. Comput. Chem. 2014, 35, 2019–2026. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. How Well Can New-Generation Density Functionals Describe the Energetics of Bond-Dissociation Reactions Producing Radicals? J. Phys. Chem. A 2008, 112, 1095–1099. [Google Scholar] [CrossRef]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal Solvation Model Based on Solute Electron Density and on a Continuum Model of the Solvent Defined by the Bulk Dielectric Constant and Atomic Surface Tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef] [PubMed]
- Boulebd, H. Is cannabidiolic acid an overlooked natural antioxidant? Insights from quantum chemistry calculations. New J. Chem. 2022, 46, 162–168. [Google Scholar] [CrossRef]
- Rebollar-Zepeda, A.M.; Campos-Hernández, T.; Ramírez-Silva, M.T.; Rojas-Hernández, A.; Galano, A. Searching for Computational Strategies to Accurately Predict pKas of Large Phenolic Derivatives. J. Chem. Theory Comput. 2011, 7, 2528–2538. [Google Scholar] [CrossRef] [PubMed]
- Galano, A.; Raúl Alvarez-Idaboy, J. Computational strategies for predicting free radical scavengers’ protection against oxidative stress: Where are we and what might follow? Int. J. Quantum Chem. 2019, 119, e25665. [Google Scholar] [CrossRef]
- Zhao, Y.; Schultz, N.E.; Truhlar, D.G. Design of density functionals by combining the method of constraint satisfaction with parametrization for thermochemistry, thermochemical kinetics, and noncovalent interactions. J. Chem. Theory Comput. 2006, 2, 364–382. [Google Scholar] [CrossRef]
- Evans, M.G.; Polanyi, M. Some applications of the transition state method to the calculation of reaction velocities, especially in solution. Trans. Faraday Soc. 1935, 31, 875–894. [Google Scholar] [CrossRef]
- Eyring, H. The activated complex in chemical reactions. J. Chem. Phys. 1935, 3, 107–115. [Google Scholar] [CrossRef]
- Truhlar, D.G.; Hase, W.L.; Hynes, J.T. Current status of transition-state theory. J. Phys. Chem. A 1983, 87, 2664–2682. [Google Scholar] [CrossRef]
- Furuncuoglu, T.; Ugur, I.; Degirmenci, I.; Aviyente, V. Role of chain transfer agents in free radical polymerization kinetics. Macromolecules 2010, 43, 1823–1835. [Google Scholar] [CrossRef]
- Vélez, E.; Quijano, J.; Notario, R.; Pabón, E.; Murillo, J.; Leal, J.; Zapata, E.; Alarcón, G. A computational study of stereospecifity in the thermal elimination reaction of menthyl benzoate in the gas phase. J. Phys. Org. Chem. 2009, 22, 971–977. [Google Scholar] [CrossRef]
- Pollak, E.; Pechukas, P. Symmetry numbers, not statistical factors, should be used in absolute rate theory and in Broensted relations. J. Am. Chem. Soc. 1978, 100, 2984–2991. [Google Scholar] [CrossRef]
- Fernández-Ramos, A.; Ellingson, B.A.; Meana-Pañeda, R.; Marques, J.M.; Truhlar, D.G. Symmetry numbers and chemical reaction rates. Theor. Chem. Acc. 2007, 118, 813–826. [Google Scholar] [CrossRef]
- Eckart, C. The penetration of a potential barrier by electrons. Phys. Rev. 1930, 35, 1303. [Google Scholar] [CrossRef]
- Corchado, J.C.; Coitino, E.L.; Chuang, Y.-Y.; Fast, P.L.; Truhlar, D.G. Interpolated variational transition-state theory by mapping. J. Phys. Chem. A 1998, 102, 2424–2438. [Google Scholar] [CrossRef]
- Collins, F.C.; Kimball, G.E. Diffusion-controlled reaction rates. J. Colloid Sci. 1949, 4, 425–437. [Google Scholar] [CrossRef]
- Tapia-Abellán, A.; Angosto-Bazarra, D.; Martínez-Banaclocha, H.; de Torre-Minguela, C.; Cerón-Carrasco, J.P.; Pérez-Sánchez, H.; Arostegui, J.I.; Pelegrin, P. MCC950 closes the active conformation of NLRP3 to an inactive state. Nat. Chem. Biol. 2019, 15, 560–564. [Google Scholar] [CrossRef] [PubMed]
- Roos, K.; Wu, C.; Damm, W.; Reboul, M.; Stevenson, J.M.; Lu, C.; Dahlgren, M.K.; Mondal, S.; Chen, W.; Wang, L.; et al. OPLS3e: Extending Force Field Coverage for Drug-Like Small Molecules. J. Chem. Theory Comput. 2019, 15, 1863–1874. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Stroganov, O.V.; Novikov, F.N.; Stroylov, V.S.; Kulkov, V.; Chilov, G.G. Lead Finder: An Approach To Improve Accuracy of Protein−Ligand Docking, Binding Energy Estimation, and Virtual Screening. J. Chem. Inf. Model. 2008, 48, 2371–2385. [Google Scholar] [CrossRef]
- Nosé, S. A unified formulation of the constant temperature molecular dynamics methods. J. Chem. Phys. 1984, 81, 511–519. [Google Scholar] [CrossRef]
- Martyna, G.J.; Tobias, D.J.; Klein, M.L. Constant pressure molecular dynamics algorithms. J. Chem. Phys. 1994, 101, 4177–4189. [Google Scholar] [CrossRef]
- Maegawa, Y.; Sugino, K.; Sakurai, H. Identification of free radical species derived from caffeic acid and related polyphenols. Free Radic. Res. 2007, 41, 110–119. [Google Scholar] [CrossRef]
- Ngoc, T.D.; Le, T.N.; Nguyen, T.V.A.; Mechler, A.; Hoa, N.T.; Nam, N.L.; Vo, Q.V. Mechanistic and Kinetic Studies of the Radical Scavenging Activity of 5-O-Methylnorbergenin: Theoretical and Experimental Insights. J. Phys. Chem. B 2022, 126, 702–707. [Google Scholar] [CrossRef]
- Castañeda-Arriaga, R.; Marino, T.; Russo, N.; Alvarez-Idaboy, J.R.; Galano, A. Chalcogen effects on the primary antioxidant activity of chrysin and quercetin. New J. Chem. 2020, 44, 9073–9082. [Google Scholar] [CrossRef]
- Boulebd, H.; Mechler, A.; Thi Hoa, N.; Vo, Q.V. Insights on the kinetics and mechanisms of the peroxyl radical scavenging capacity of caftaric acid: The important role of the acid–base equilibrium. New J. Chem. 2022, 46, 7403–7409. [Google Scholar] [CrossRef]
- Vo, Q.V.; Hoa, N.T.; Thong, N.M.; Mechler, A. The hydroperoxyl and superoxide anion radical scavenging activity of anthocyanidins in physiological environments: Theoretical insights into mechanisms and kinetics. Phytochemistry 2021, 192, 112968. [Google Scholar] [CrossRef] [PubMed]
- Vo, Q.V.; Thong, N.M.; Le Huyen, T.; Nam, P.C.; Tam, N.M.; Hoa, N.T.; Mechler, A. A thermodynamic and kinetic study of the antioxidant activity of natural hydroanthraquinones. RSC Adv. 2020, 10, 20089–20097. [Google Scholar] [CrossRef] [PubMed]
- Boulebd, H. Radical scavenging behavior of butylated hydroxytoluene against oxygenated free radicals in physiological environments: Insights from DFT calculations. Int. J. Chem. Kinet. 2022, 54, 50–57. [Google Scholar] [CrossRef]
- Boulebd, H. Modeling the peroxyl radical scavenging behavior of Carnosic acid: Mechanism, kinetics, and effects of physiological environments. Phytochemistry 2021, 192, 112950. [Google Scholar] [CrossRef] [PubMed]
- Boulebd, H.; Pereira, D.M.; Khodja, I.A.; Hoa, N.T.; Mechler, A.; Vo, Q.V. Assessment of the free radical scavenging potential of cannabidiol under physiological conditions: Theoretical and experimental investigations. J. Mol. Liq. 2022, 346, 118277. [Google Scholar] [CrossRef]
- Cao, H.; Pauff, J.M.; Hille, R. X-ray Crystal Structure of a Xanthine Oxidase Complex with the Flavonoid Inhibitor Quercetin. J. Nat. Prod. 2014, 77, 1693–1699. [Google Scholar] [CrossRef]


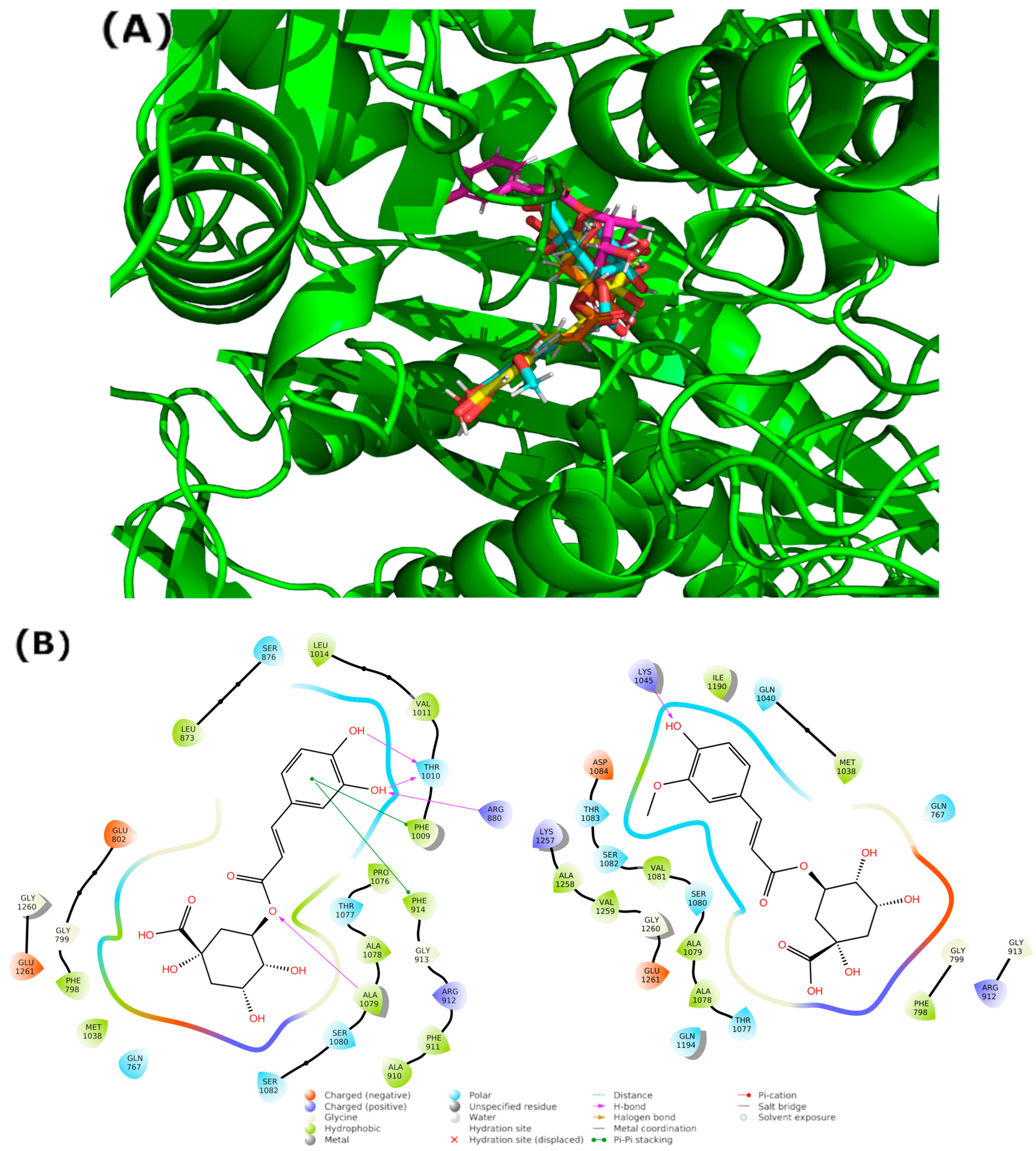
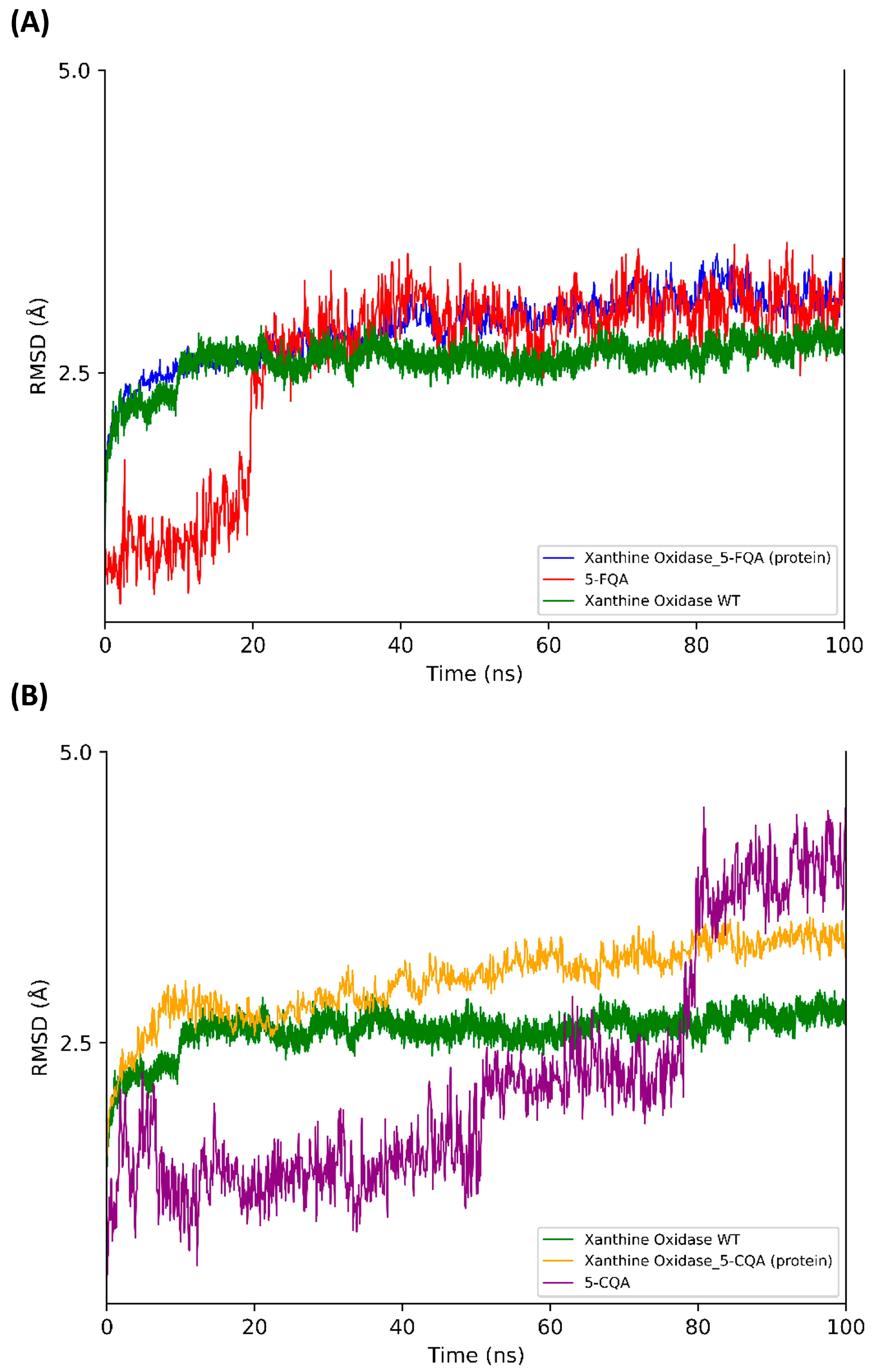
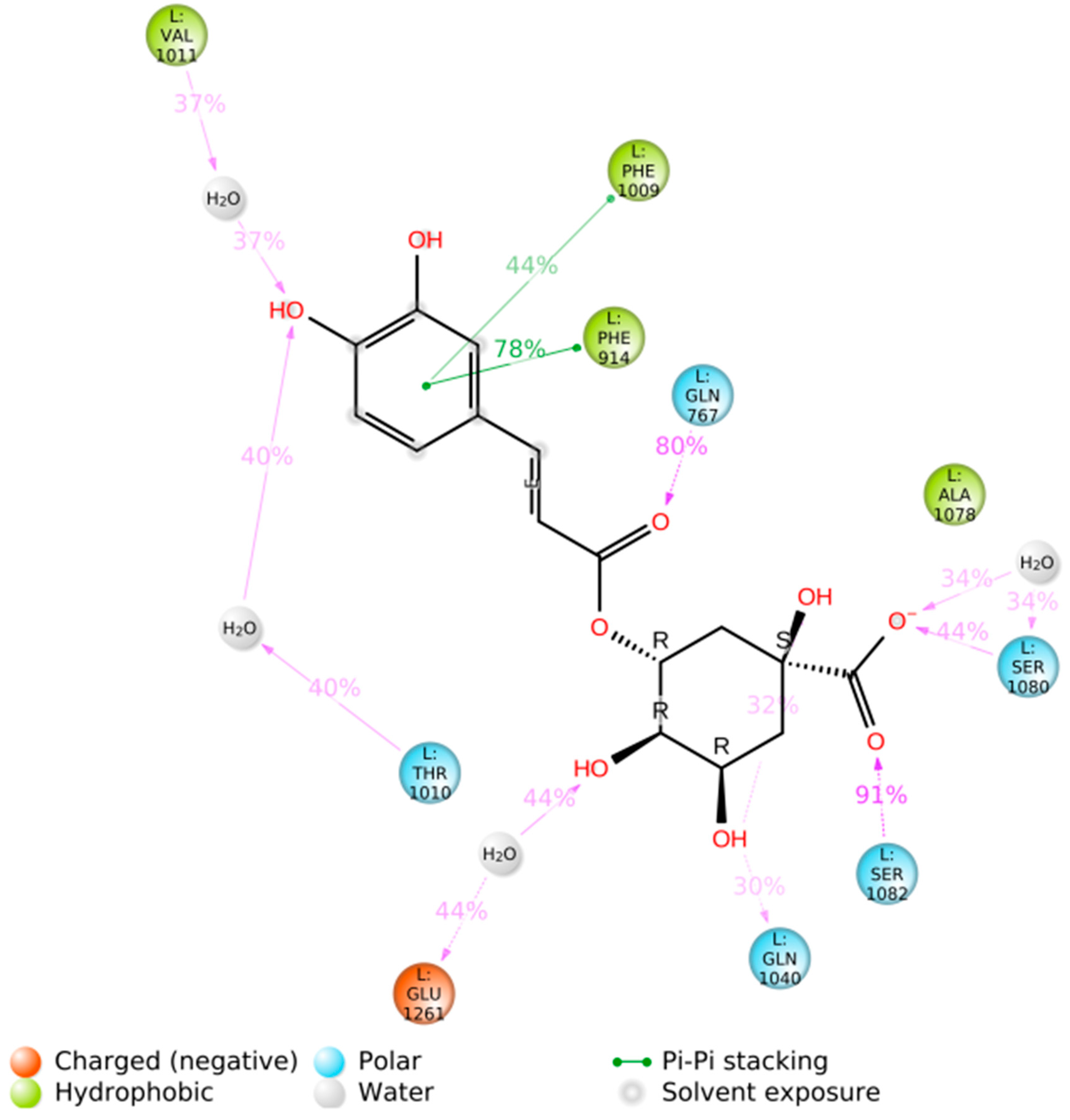

| Comp. | Mechanisms | State | ΔG≠ | κ | kapp | f a | kf b | Γ | koverall | |
|---|---|---|---|---|---|---|---|---|---|---|
| 5-CQA | HAT | 12OH | 5-CQA– | 16.8 | 205.7 | 6.60 × 102 | 0.918 | 6.06 × 102 | 0 | 2.68 × 108 |
| 13OH | 17.5 | 436.8 | 8.40 × 101 | 7.71 × 101 | 0 | |||||
| RAF | C8 | 19.6 | 1.4 | 2.50 × 10−2 | 2.30 × 10−2 | 0 | ||||
| HAT | 12OH | 5-CQA–2 | 2.0 | 1.2 | 2.30 × 109 | 0.082 | 1.89 × 108 | 70 | ||
| RAF | C8 | 14.2 | 1.0 | 2.40 × 102 | 1.97 × 101 | 0 | ||||
| SET | 4.8 | 4.0 c | 9.70 × 108 | 7.95 × 107 | 30 | |||||
| 5-FQA | HAT | 13OH | 5-FQA– | 17.4 | 838.8 | 1.70 × 102 | 0.988 | 1.68 × 102 | 0 | 2.28 × 107 |
| RAF | C8 | 17.5 | 1.4 | 1.20 × 100 | 1.19 × 100 | 0 | ||||
| RAF | C8 | 5-FQA–2 | 10.8 | 1.0 | 7.80 × 104 | 0.012 | 9.36 × 102 | 0 | ||
| SET | 3.7 | 1.1 c | 1.90 × 109 | 2.28 × 107 | 100 | |||||
| Comp. | Mechanisms | ΔG≠ | κ | kapp | Γ | koverall | |
|---|---|---|---|---|---|---|---|
| 5-CQA | HAT | 12OH | 8.7 | 0.7 | 1.80 × 106 | 86 | 2.09 × 106 |
| 13OH | 5.7 | 0.0 | 2.90 × 105 | 14 | |||
| RAF | C8 | 19.7 | 1.5 | 3.40 × 10−2 | 0 | ||
| 5-FQA | HAT | 13OH | 7.9 | 0.0 | 4.10 × 104 | 100 | 4.10 × 104 |
| RAF | C8 | 16.0 | 1.5 | 1.60 × 101 | 0 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boulebd, H.; Carmena-Bargueño, M.; Pérez-Sánchez, H. Exploring the Antioxidant Properties of Caffeoylquinic and Feruloylquinic Acids: A Computational Study on Hydroperoxyl Radical Scavenging and Xanthine Oxidase Inhibition. Antioxidants 2023, 12, 1669. https://doi.org/10.3390/antiox12091669
Boulebd H, Carmena-Bargueño M, Pérez-Sánchez H. Exploring the Antioxidant Properties of Caffeoylquinic and Feruloylquinic Acids: A Computational Study on Hydroperoxyl Radical Scavenging and Xanthine Oxidase Inhibition. Antioxidants. 2023; 12(9):1669. https://doi.org/10.3390/antiox12091669
Chicago/Turabian StyleBoulebd, Houssem, Miguel Carmena-Bargueño, and Horacio Pérez-Sánchez. 2023. "Exploring the Antioxidant Properties of Caffeoylquinic and Feruloylquinic Acids: A Computational Study on Hydroperoxyl Radical Scavenging and Xanthine Oxidase Inhibition" Antioxidants 12, no. 9: 1669. https://doi.org/10.3390/antiox12091669
APA StyleBoulebd, H., Carmena-Bargueño, M., & Pérez-Sánchez, H. (2023). Exploring the Antioxidant Properties of Caffeoylquinic and Feruloylquinic Acids: A Computational Study on Hydroperoxyl Radical Scavenging and Xanthine Oxidase Inhibition. Antioxidants, 12(9), 1669. https://doi.org/10.3390/antiox12091669







