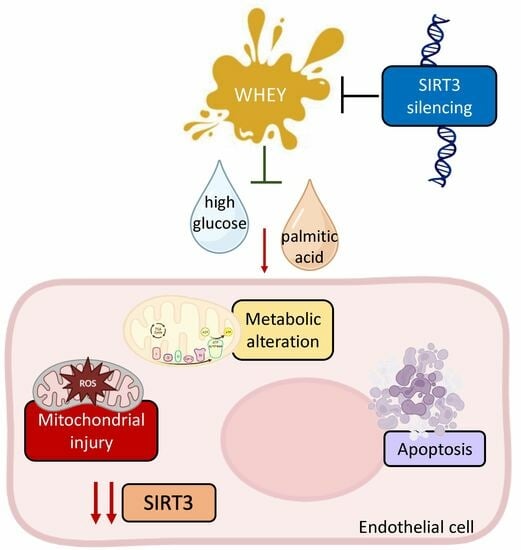
Whey Improves In Vitro Endothelial Mitochondrial Function and Metabolic Redox Status in Diabetic State
Abstract
1. Introduction
2. Materials and Methods
2.1. WH Extraction and Metabolic Characterization
2.2. Cell Line and Culture Conditions
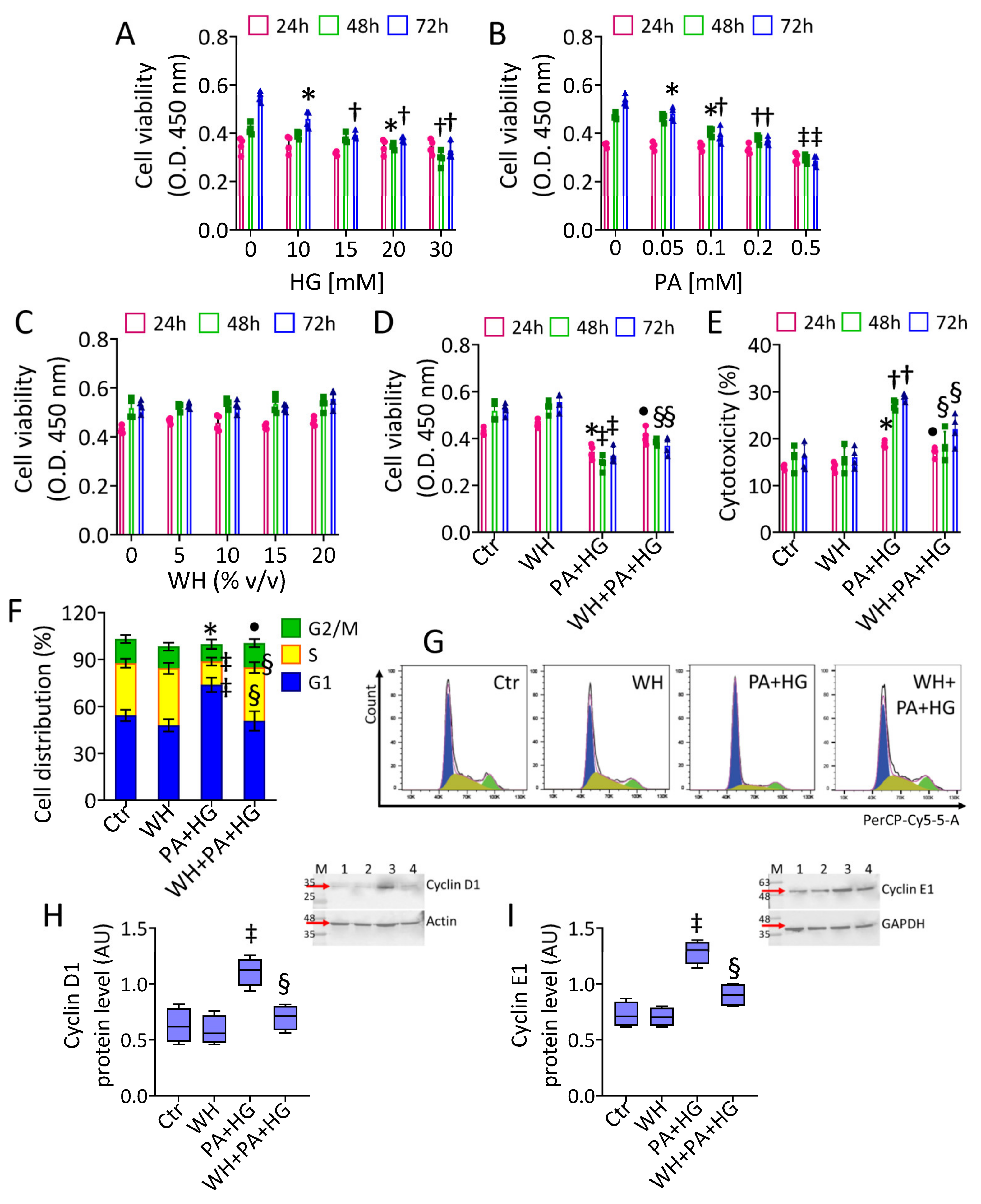
2.3. Viability and Cytotoxicity
2.4. HDAC3 and SIRT3 Assays
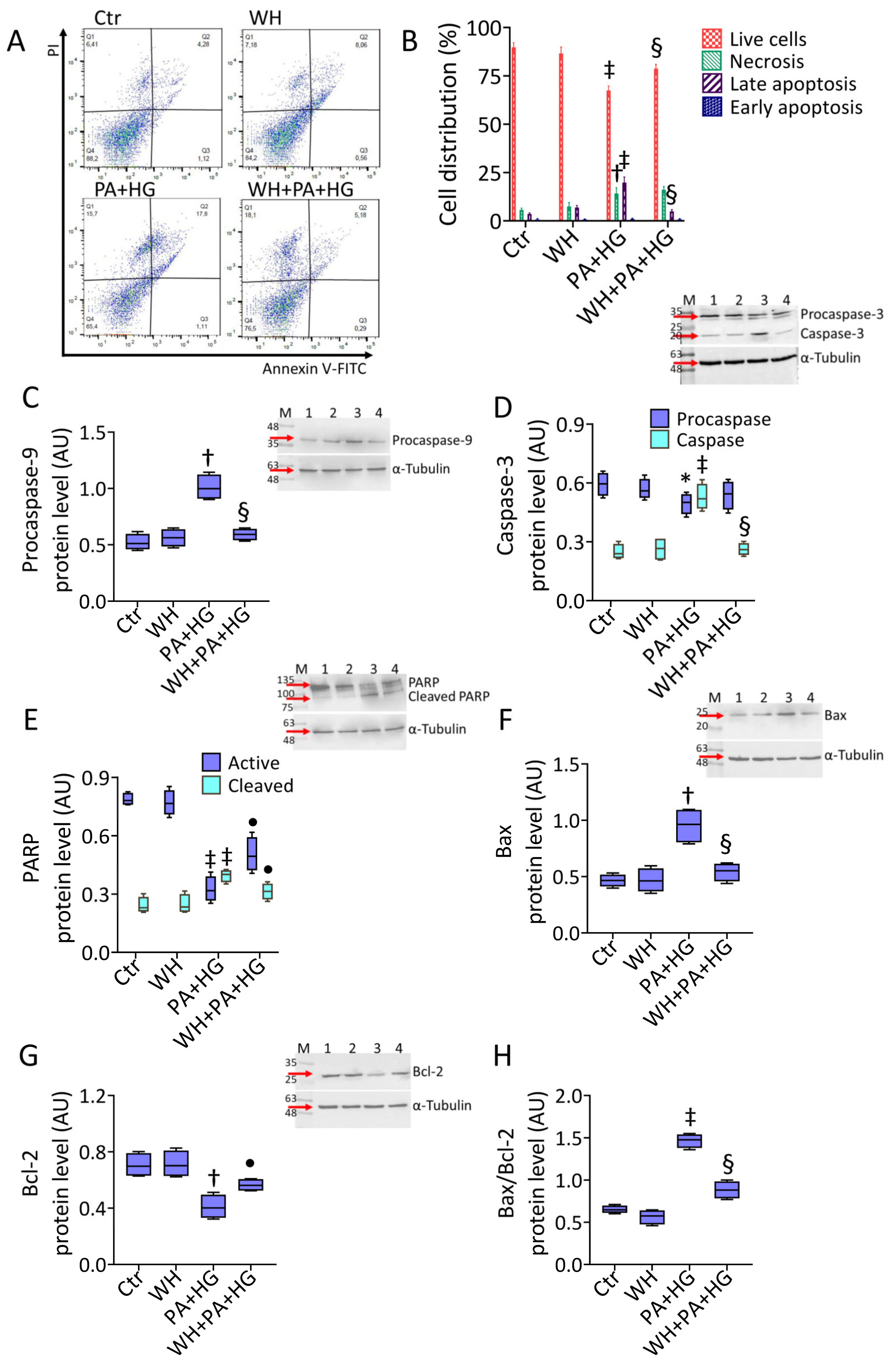
2.5. Cell Cycle and Death
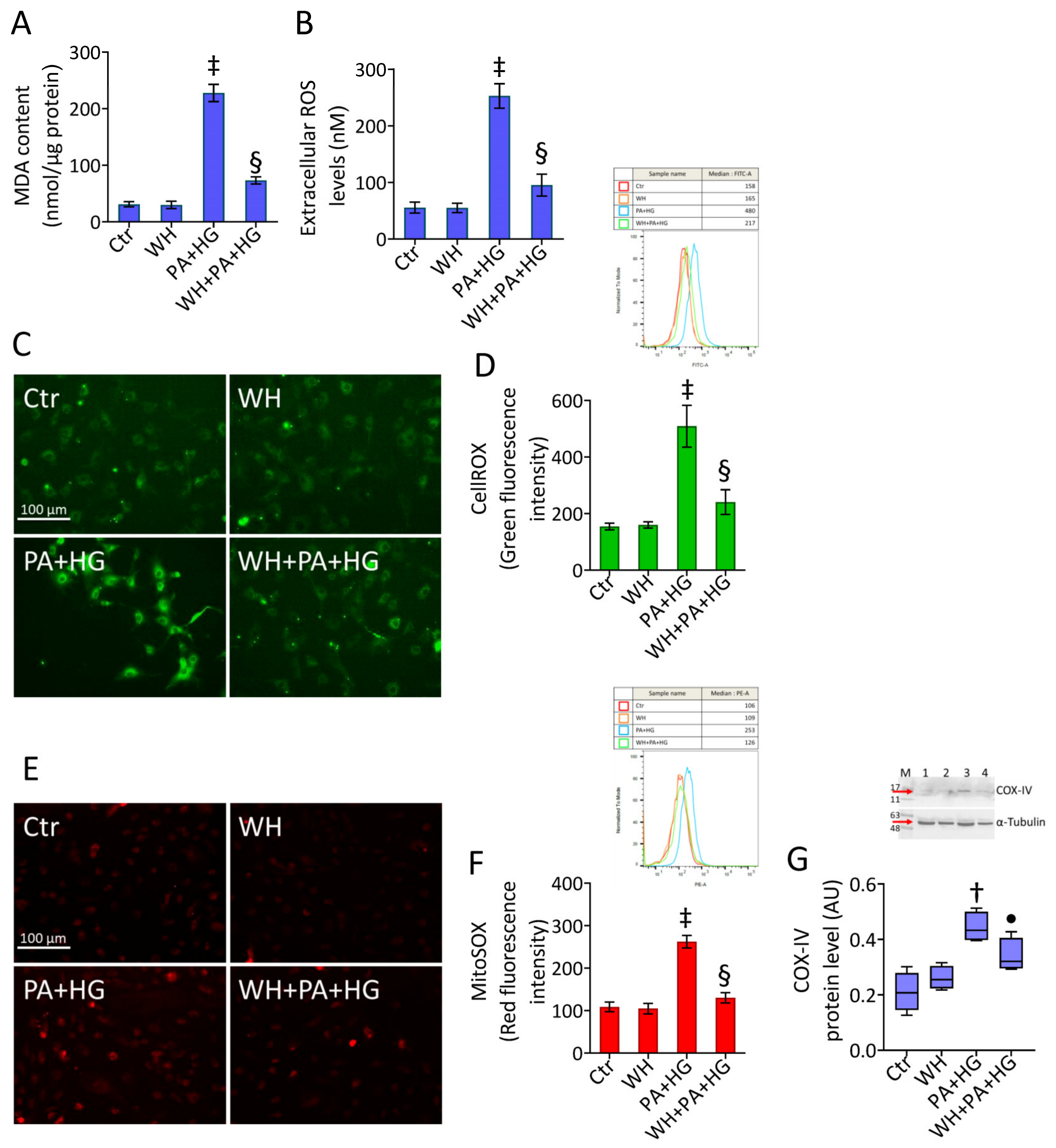
2.6. Oxidative State
2.7. Reactive Oxygen Species (ROS)
2.8. Mitochondrial Function
2.9. Mitochondrial Respiration
2.10. Western Blotting
2.11. Statistical Analysis
3. Results
3.1. WH Characterization
3.2. In Vitro T2DM and Cell Cycle Analysis
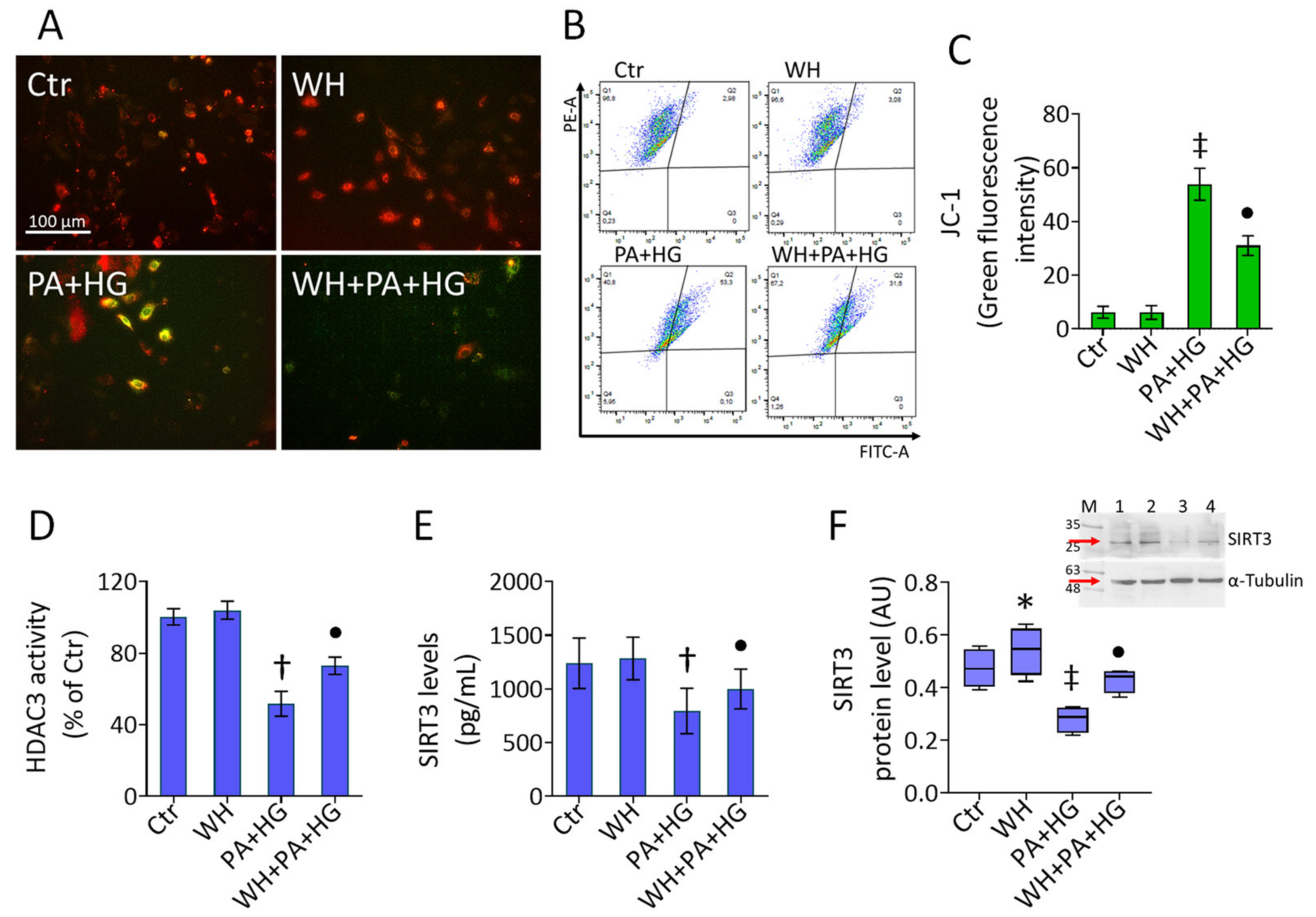
3.3. WH Effects on PA+HG-Induced Apoptosis
3.4. WH Prevented the PA+HG-Induced Oxidative Stress
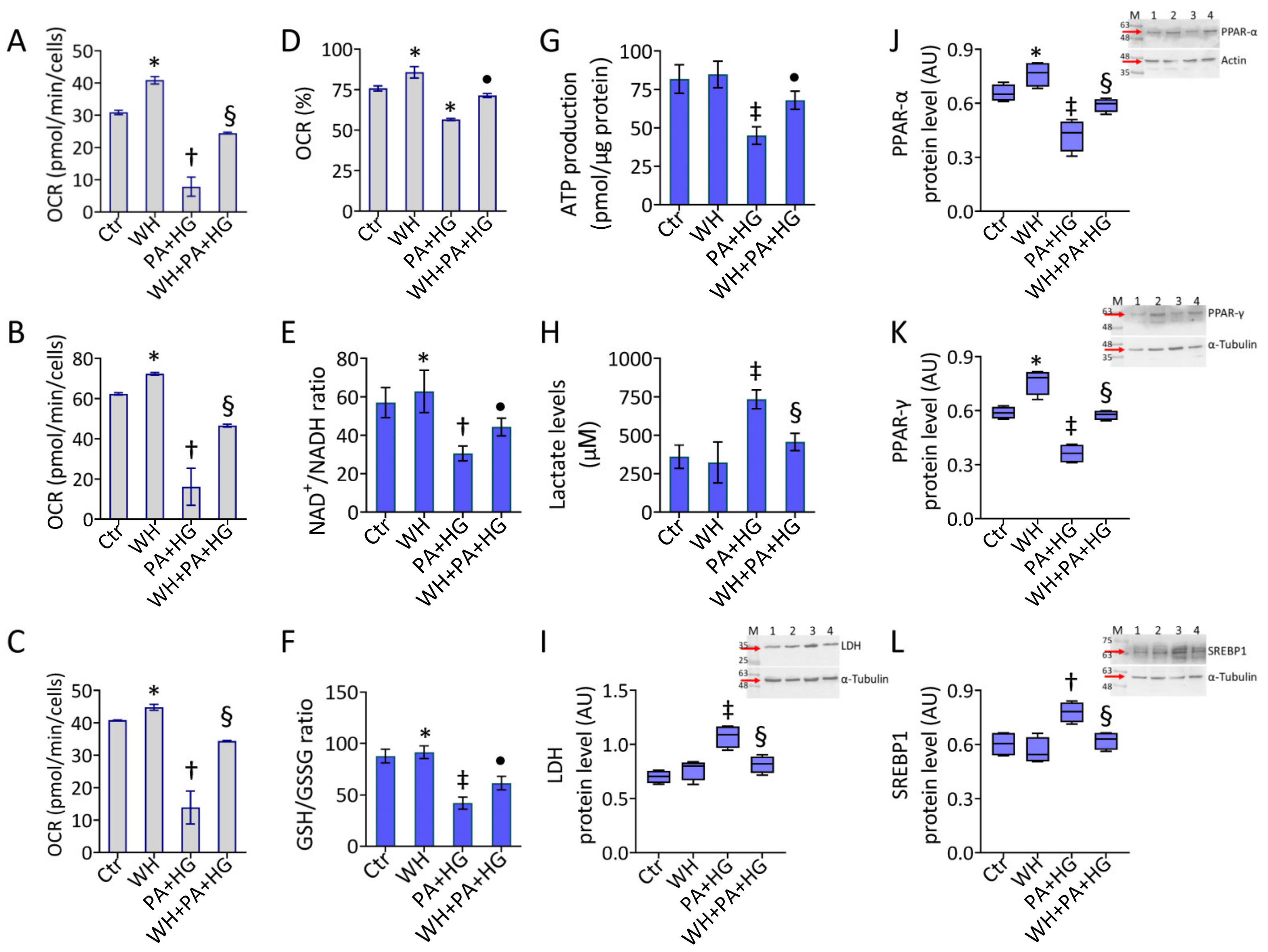
3.5. Mitochondrial Dysfunction in T2DM-EC
3.6. WH Effects on PA+HG-Mediated Mitochondrial Metabolic Dysfunction
3.7. SIRT3- Prevented the WH Protective Effects on Mitochondrial Dysfunction
3.8. SIRT3- Abolished the Beneficial Ability of WH against Oxidative Stress
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sardu, C.; Paolisso, P.; Sacra, C.; Mauro, C.; Minicucci, F.; Portoghese, M.; Rizzo, M.R.; Barbieri, M.; Sasso, F.C.; D’Onofrio, N.; et al. Effects of metformin therapy on coronary endothelial dysfunction in patients with prediabetes with stable angina and nonobstructive coronary artery stenosis: The CODYCE multicenter prospective study. Diabetes Care 2019, 42, 1946–1955. [Google Scholar] [CrossRef]
- Szydełko, J.; Matyjaszek-Matuszek, B. MicroRNAs as Biomarkers for Coronary Artery Disease Related to Type 2 Diabetes Mellitus-From Pathogenesis to Potential Clinical Application. Int. J. Mol. Sci. 2022, 24, 616. [Google Scholar] [CrossRef] [PubMed]
- Kwan, T.W.; Wong, S.S.; Hong, Y.; Kanaya, A.M.; Khan, S.S.; Hayman, L.L.; Shah, S.H.; Welty, F.K.; Deedwania, P.C.; Khaliq, A.; et al. Epidemiology of Diabetes and Atherosclerotic Cardiovascular Disease Among Asian American Adults: Implications, Management, and Future Directions: A Scientific Statement From the American Heart Association. Circulation 2023. Advance online publication. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, X.; Wang, Z.; Bancks, M.P.; Carnethon, M.R.; Greenland, P.; Feng, Y.Q.; Wang, H.; Zhong, V.W. Trends in Prevalence of Diabetes and Control of Risk Factors in Diabetes Among US Adults, 1999–2018. JAMA 2021, 326, 704–716. [Google Scholar] [CrossRef] [PubMed]
- Darenskaya, M.A.; Kolesnikova, L.I.; Kolesnikov, S.I. Oxidative Stress: Pathogenetic Role in Diabetes Mellitus and Its Complications and Therapeutic Approaches to Correction. Bull. Exp. Biol. Med. 2021, 171, 179–189. [Google Scholar] [CrossRef]
- Giordano, M.; Ciarambino, T.; Castellino, P.; Malatino, L.; Di Somma, S.; Biolo, G.; Paolisso, G.; Adinolfi, L.E. Diseases associated with electrolyte imbalance in the ED: Age-related differences. Am. J. Emerg. Med. 2016, 34, 1923–1926. [Google Scholar] [CrossRef] [PubMed]
- Tracy, E.P.; Hughes, W.; Beare, J.E.; Rowe, G.; Beyer, A.; LeBlanc, A.J. Aging-Induced Impairment of Vascular Function: Mitochondrial Redox Contributions and Physiological/Clinical Implications. Antioxid. Redox Signal. 2021, 35, 974–1015. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Z.; Ren, Y.; Wang, Y.; Fang, J.; Yue, H.; Ma, S.; Guan, F. Aging and age-related diseases: From mechanisms to therapeutic strategies. Biogerontology 2021, 22, 165–187. [Google Scholar] [CrossRef]
- Prasun, P. Mitochondrial dysfunction in metabolic syndrome. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165838. [Google Scholar] [CrossRef]
- Sangwung, P.; Petersen, K.F.; Shulman, G.I.; Knowles, J.W. Mitochondrial Dysfunction, Insulin Resistance, and Potential Genetic Implications. Endocrinology 2020, 161, bqaa017. [Google Scholar] [CrossRef]
- Zhang, J.; Xiang, H.; Liu, J.; Chen, Y.; He, R.R.; Liu, B. Mitochondrial Sirtuin 3: New emerging biological function and therapeutic target. Theranostics 2020, 10, 8315–8342. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, X.; Wang, P.; Shen, Y.; Yuan, K.; Li, M.; Liang, W.; Que, H. Sirt3 overexpression alleviates hyperglycemia-induced vascular inflammation through regulating redox balance, cell survival, and AMPK-mediated mitochondrial homeostasis. J. Recept. Signal Transduct. Res. 2019, 39, 341–349. [Google Scholar] [CrossRef]
- Martino, E.; Balestrieri, A.; Anastasio, C.; Maione, M.; Mele, L.; Cautela, D.; Campanile, G.; Balestrieri, M.L.; D’Onofrio, N. SIRT3 Modulates Endothelial Mitochondrial Redox State during Insulin Resistance. Antioxidants 2022, 11, 1611. [Google Scholar] [CrossRef] [PubMed]
- D’Onofrio, N.; Prattichizzo, F.; Martino, E.; Anastasio, C.; Mele, L.; La Grotta, R.; Sardu, C.; Ceriello, A.; Marfella, R.; Paolisso, G.; et al. MiR-27b attenuates mitochondrial oxidative stress and inflammation in endothelial cells. Redox Biol. 2023, 62, 102681. [Google Scholar] [CrossRef] [PubMed]
- Basdeki, E.D.; Argyris, A.A.; Efthymiou, O.; Athanasopoulou, E.; Sfikakis, P.P.; Protogerou, A.D.; Karatzi, K. Systematic breakfast consumption of medium-quantity and high-quality food choices is associated with better vascular health in individuals with cardiovascular disease risk factors. Nutrients 2023, 15, 1025. [Google Scholar] [CrossRef] [PubMed]
- Alexander, D.D.; Bylsma, L.C.; Vargas, A.J.; Cohen, S.S.; Doucette, A.; Mohamed, M.; Irvin, S.R.; Miller, P.E.; Watson, H.; Fryzek, J.P. Dairy consumption and CVD: A systematic review and meta-analysis. Br. J. Nutr. 2016, 115, 737–750. [Google Scholar] [CrossRef] [PubMed]
- Slurink, I.A.; Chen, L.; Magliano, D.J.; Kupper, N.; Smeets, T.; Soedamah-Muthu, S.S. Dairy product consumption and incident prediabetes in the australian diabetes, obesity, and lifestyle study with 12 years of follow-up. J. Nutr. 2023, 153, 1742–1752. [Google Scholar] [CrossRef]
- Aldhafiri, F.K. Investigating the Role of EPA and DHA on Cellular Oxidative Stress; Profiling Antidiabetic and Antihypertensive Potential. J. Pharm. Bioallied Sci. 2022, 14, 178–185. [Google Scholar] [CrossRef]
- Yang, H.M.; Kim, J.; Shin, D.; Kim, J.Y.; You, J.; Lee, H.C.; Jang, H.D.; Kim, H.S. Resistin impairs mitochondrial homeostasis via cyclase-associated protein 1-mediated fission, leading to obesity-induced metabolic diseases. Metabolism 2023, 138, 155343. [Google Scholar] [CrossRef]
- Wang, R.; Santos, J.M.; Dufour, J.M.; Stephens, E.R.; Miranda, J.M.; Washburn, R.L.; Hibler, T.; Kaur, G.; Lin, D.; Shen, C.L. Ginger Root Extract Improves GI Health in Diabetic Rats by Improving Intestinal Integrity and Mitochondrial Function. Nutrients 2022, 14, 4384. [Google Scholar] [CrossRef]
- Khalil, M.; Shanmugam, H.; Abdallah, H.; John Britto, J.S.; Galerati, I.; Gómez-Ambrosi, J.; Frühbeck, G.; Portincasa, P. The Potential of the Mediterranean Diet to Improve Mitochondrial Function in Experimental Models of Obesity and Metabolic Syndrome. Nutrients 2022, 14, 3112. [Google Scholar] [CrossRef]
- Kelly, J.; Karlsen, M.; Steinke, G. Type 2 diabetes remission and lifestyle medicine: A position statement from the American college of lifestyle medicine. Am. J. Lifestyle Med. 2020, 14, 406–419. [Google Scholar] [CrossRef]
- Tong, L.; Hao, H.; Zhang, Z.; Lv, Y.; Liang, X.; Liu, Q.; Liu, T.; Gong, P.; Zhang, L.; Cao, F.; et al. Milk-derived extracellular vesicles alleviate ulcerative colitis by regulating the gut immunity and reshaping the gut microbiota. Theranostics 2021, 11, 8570–8586. [Google Scholar] [CrossRef]
- García-Martínez, J.; Pérez-Castillo, Í.M.; Salto, R.; López-Pedrosa, J.M.; Rueda, R.; Girón, M.D. Beneficial Effects of Bovine Milk Exosomes in Metabolic Interorgan Cross-Talk. Nutrients 2022, 14, 1442. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Xie, Y.; Luo, J.; Chen, T.; Xi, Q.; Zhang, Y.; Sun, J. Milk exosome-derived miRNAs from water buffalo are implicated in immune response and metabolism process. BMC Vet. Res. 2020, 16, 123. [Google Scholar] [CrossRef] [PubMed]
- Servillo, L.; D’Onofrio, N.; Neglia, G.; Casale, R.; Cautela, D.; Marrelli, M.; Limone, A.; Campanile, G.; Balestrieri, M.L. Carnitine precursors and short-chain acylcarnitines in water buffalo milk. J. Agric. Food Chem. 2018, 66, 8142–8149. [Google Scholar] [CrossRef] [PubMed]
- Servillo, L.; D’Onofrio, N.; Giovane, A.; Casale, R.; Cautela, D.; Castaldo, D.; Iannaccone, F.; Neglia, G.; Campanile, G.; Balestrieri, M.L. Ruminant meat and milk contain -valerobetaine, another precursor of trimethylamine N-oxide (TMAO) like-butyrobetaine. Food Chem. 2018, 260, 193–199. [Google Scholar] [CrossRef]
- Salzano, A.; Cotticelli, A.; Marrone, R.; D’Occhio, M.J.; D’Onofrio, N.; Neglia, G.; Ambrosio, R.L.; Balestrieri, M.L.; Campanile, G. Effect of breeding techniques and prolonged post dry aging maturation process on biomolecule levels in raw buffalo meat. Vet. Sci. 2021, 8, 66. [Google Scholar] [CrossRef]
- D’Onofrio, N.; Cacciola, N.A.; Martino, E.; Borrelli, F.; Fiorino, F.; Lombardi, A.; Neglia, G.; Balestrieri, M.L.; Campanile, G. ROS-Mediated Apoptotic Cell Death of Human Colon Cancer LoVo Cells by Milk δ-Valerobetaine. Sci. Rep. 2020, 10, 8978. [Google Scholar] [CrossRef]
- D’Onofrio, N.; Martino, E.; Mele, L.; Colloca, A.; Maione, M.; Cautela, D.; Castaldo, D.; Balestrieri, M.L. Colorectal Cancer Apoptosis Induced by Dietary δ-Valerobetaine Involves PINK1/Parkin Dependent-Mitophagy and SIRT3. Int. J. Mol. Sci. 2021, 22, 8117. [Google Scholar] [CrossRef] [PubMed]
- Martino, E.; Balestrieri, A.; Mele, L.; Sardu, C.; Marfella, R.; D’Onofrio, N.; Campanile, G.; Balestrieri, M.L. Milk Exosomal miR-27b Worsen Endoplasmic Reticulum Stress Mediated Colorectal Cancer Cell Death. Nutrients 2022, 14, 5081. [Google Scholar] [CrossRef] [PubMed]
- D’Onofrio, N.; Mele, L.; Martino, E.; Salzano, A.; Restucci, B.; Cautela, D.; Tatullo, M.; Balestrieri, M.L.; Campanile, G. Synergistic Effect of Dietary Betaines on SIRT1-Mediated Apoptosis in Human Oral Squamous Cell Carcinoma Cal 27. Cancers 2020, 12, 2468. [Google Scholar] [CrossRef]
- Fonseka, P.; Kang, T.; Chee, S.; Chitti, S.V.; Sanwlani, R.; Ang, C.S.; Mathivanan, S. Temporal Quantitative Proteomics Analysis of Neuroblastoma Cells Treated with Bovine Milk-Derived Extracellular Vesicles Highlights the Anti-Proliferative Properties of Milk-Derived Extracellular Vesicles. Cells 2021, 10, 750. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Khomeiri, M.; Saeidi, M.; Davoodi, H.; Memarian, A. Anticancer potential of fermented milk with autochthonous lactic acid bacteria. J. Appl. Microbiol. 2023, 134, lxad041. [Google Scholar] [CrossRef]
- Mukhopadhya, A.; Tsiapalis, D.; McNamee, N.; Talbot, B.; O’Driscoll, L. Doxorubicin Loading into Milk and Mesenchymal Stem Cells’ Extracellular Vesicles as Drug Delivery Vehicles. Pharmaceutics 2023, 15, 718. [Google Scholar] [CrossRef] [PubMed]
- D’Onofrio, N.; Balestrieri, A.; Neglia, G.; Monaco, A.; Tatullo, M.; Casale, R.; Limone, A.; Balestrieri, M.L.; Campanile, G. Antioxidant and Anti-Inflammatory Activities of Buffalo Milk δ-Valerobetaine. J. Agric. Food Chem. 2019, 67, 1702–1710. [Google Scholar] [CrossRef]
- Salzano, A.; Licitra, F.; D’Onofrio, N.; Balestrieri, M.L.; Limone, A.; Campanile, G.; D’Occhio, M.J.; Neglia, G. Short communication: Space allocation in intensive Mediterranean buffalo production influences the profile of functional biomolecules in milk and dairy products. J. Dairy Sci. 2019, 102, 7717–7722. [Google Scholar] [CrossRef] [PubMed]
- Cacciola, N.A.; Salzano, A.; D’Onofrio, N.; Venneri, T.; Cicco, P.; Vinale, F.; Petillo, O.; Martano, M.; Maiolino, P.; Neglia, G.; et al. Buffalo Milk Whey Activates Necroptosis and Apoptosis in a Xenograft Model of Colorectal Cancer. Int. J. Mol. Sci. 2022, 23, 8464. [Google Scholar] [CrossRef]
- Cereda, E.; Turri, A.; Klersy, C.; Cappello, S.; Ferrari, A.; Filippi, A.R.; Brugnatelli, S.; Caraccia, M.; Chiellino, S.; Borioli, V.; et al. Whey protein isolate supplementation improves body composition, muscle strength, and treatment tolerance in malnourished advanced cancer patients undergoing chemotherapy. Cancer Med. 2019, 8, 6923–6932. [Google Scholar] [CrossRef]
- Rocha-Mendoza, D.; Kosmerl, E.; Krentz, A.; Zhang, L.; Badiger, S.; Miyagusuku-Cruzado, G.; Mayta-Apaza, A.; Giusti, M.; Jiménez-Flores, R.; García-Cano, I. Invited review: Acid whey trends and health benefits. J. Dairy Sci. 2021, 104, 1262–1275. [Google Scholar] [CrossRef]
- D’Onofrio, N.; Martino, E.; Balestrieri, A.; Mele, L.; Neglia, G.; Balestrieri, M.L.; Campanile, G. SIRT3 and Metabolic Reprogramming Mediate the Antiproliferative Effects of Whey in Human Colon Cancer Cells. Cancers 2021, 13, 5196. [Google Scholar] [CrossRef]
- Huang, S.; Chen, G.; Sun, J.; Chen, Y.; Wang, N.; Dong, Y.; Shen, E.; Hu, Z.; Gong, W.; Jin, L.; et al. Histone deacetylase 3 inhibition alleviates type 2 diabetes mellitus-induced endothelial dysfunction via Nrf2. Cell. Commun. Signal. 2021, 19, 35. [Google Scholar] [CrossRef] [PubMed]
- Alnahdi, A.; John, A.; Raza, H. Augmentation of Glucotoxicity, Oxidative Stress, Apoptosis and Mitochondrial Dysfunction in HepG2 Cells by Palmitic Acid. Nutrients 2019, 11, 1979. [Google Scholar] [CrossRef]
- Xue, W.J.; He, C.F.; Zhou, R.Y.; Xu, X.D.; Xiang, L.X.; Wang, J.T.; Wang, X.R.; Zhou, H.G.; Guo, J.C. High glucose and palmitic acid induces neuronal senescence by NRSF/REST elevation and the subsequent mTOR-related autophagy suppression. Mol. Brain 2022, 15, 61. [Google Scholar] [CrossRef]
- Winnik, S.; Auwerx, J.; Sinclair, D.A.; Matter, C.M. Protective effects of sirtuins in cardiovascular diseases: From bench to bedside. Eur. Heart J. 2015, 36, 3404–3412. [Google Scholar] [CrossRef] [PubMed]
- Giraldo-Grueso, M.; Echeverri, D. From endothelial dysfunction to arterial stiffness in diabetes mellitus. Curr. Diabetes Rev. 2020, 16, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Evangelista, I.; Nuti, R.; Picchioni, T.; Dotta, F.; Palazzuoli, A. Molecular Dysfunction and Phenotypic Derangement in Diabetic Cardiomyopathy. Int. J. Mol. Sci. 2019, 20, 3264. [Google Scholar] [CrossRef]
- Otto, M.; Bucher, C.; Liu, W.; Müller, M.; Schmidt, T.; Kardell, M.; Driessen, M.N.; Rossaint, J.; Gross, E.R.; Wagner, N.M. 12(S)-HETE mediates diabetes-induced endothelial dysfunction by activating intracellular endothelial cell TRPV1. J. Clin. Investig. 2020, 130, 4999–5010. [Google Scholar] [CrossRef]
- Shah, M.S.; Brownlee, M. Molecular and Cellular Mechanisms of Cardiovascular Disorders in Diabetes. Circ. Res. 2016, 118, 1808–1829. [Google Scholar] [CrossRef]
- Rahimi-Madiseh, M.; Malekpour-Tehrani, A.; Bahmani, M.; Rafeian-Kopaei, M. The research and development on the antioxidants in prevention of diabetic complications. Asian Pac. J. Trop. Med. 2016, 9, 825–831. [Google Scholar] [CrossRef]
- Wuni, R.; Lakshmipriya, N.; Abirami, K.; Ventura, E.F.; Anjana, R.M.; Sudha, V.; Shobana, S.; Unnikrishnan, R.; Krishnaswamy, K.; Vimaleswaran, K.S.; et al. Higher intake of dairy is associated with lower cardiometabolic risks and metabolic syndrome in Asian indians. Nutrients 2022, 14, 3699. [Google Scholar] [CrossRef]
- Britsemmer, J.H.; Krause, C.; Taege, N.; Geißler, C.; Lopez-Alcantara, N.; Schmidtke, L.; Naujack, A.M.; Wagner, J.; Wolter, S.; Mann, O.; et al. Fatty Acid Induced Hypermethylation in the Slc2a4 Gene in Visceral Adipose Tissue Is Associated to Insulin-Resistance and Obesity. Int. J. Mol. Sci. 2023, 24, 6417. [Google Scholar] [CrossRef]
- Lone, I.M.; Nun, N.B.; Ghnaim, A.; Schaefer, A.S.; Houri-Haddad, Y.; Iraqi, F.A. High-fat diet and oral infection induced type 2 diabetes and obesity development under different genetic backgrounds. Anim. Model. Exp. Med. 2023, 6, 131–145. [Google Scholar] [CrossRef]
- Bétry, C.; Lablanche, S.; Carvalho, M.; Amougay, H.; Du-Boullay, H.; Crand, A.; Lamy, C.; Borges, L.; Gorain, S.; Borel, J.C.; et al. Effect of a lifestyle intervention to prevent weight gain at initiation of insulin pump therapy in type 2 diabetes: A randomized, controlled, multicentre trial. Diabetes Res. Clin. Pract. 2023, 200, 110698. [Google Scholar] [CrossRef] [PubMed]
- Saslow, L.R.; Missel, A.L.; O’Brien, A.; Kim, S.; Hecht, F.M.; Moskowitz, J.T.; Bayandorian, H.; Pietrucha, M.; Raymond, K.; Richards, B.; et al. Psychological Support Strategies for Adults With Type 2 Diabetes in a Very Low-Carbohydrate Web-Based Program: Randomized Controlled Trial. JMIR Diabetes 2023, 8, e44295. [Google Scholar] [CrossRef] [PubMed]
- Kerasioti, E.; Kiskini, A.; Veskoukis, A.; Jamurtas, A.; Tsitsimpikou, C.; Tsatsakis, A.M.; Koutedakis, Y.; Stagos, D.; Kouretas, D.; Karathanos, V. Effect of a special carbohydrate-protein cake on oxidative stress markers after exhaustive cycling in humans. Food Chem. Toxicol. 2012, 50, 2805–2810. [Google Scholar] [CrossRef]
- Tseng, Y.M.; Lin, S.K.; Hsiao, J.K.; Chen, I.J.; Lee, J.H.; Wu, S.H.; Tsai, L.Y. Whey protein concentrate promotes the production of glutathione (GSH) by GSH reductase in the PC12 cell line after acute ethanol exposure. Food Chem. Toxicol. 2006, 44, 574–578. [Google Scholar] [CrossRef] [PubMed]
- Fekete, A.A.; Giromini, C.; Chatzidiakou, Y.; Givens, D.I.; Lovegrove, J.A. Whey protein lowers blood pressure and improves endothelial function and lipid biomarkers in adults with prehypertension and mild hypertension: Results from the chronic Whey2Go randomized controlled trial. Am. J. Clin. Nutr. 2016, 104, 1534–1544. [Google Scholar] [CrossRef]
- Lorenzo, A.; Santos, E.M.D.; Bello Moreira, A.S.; Huguenin, G.V.B.; Tibirica, E. Dietary supplementation with whey protein improves systemic microvascular function in heart failure patients: A pilot study. Braz. J. Med. Biol. Res. 2021, 54, e10577. [Google Scholar] [CrossRef]
- Gaffney, K.; Lucero, A.; Macartney-Coxson, D.; Clapham, J.; Whitfield, P.; Palmer, B.R.; Wakefield, S.; Faulkner, J.; Stoner, L.; Rowlands, D.S. Effects of whey protein on skeletal muscle micro-vascular and mitochondrial plasticity following 10 weeks of exercise training in men with type 2 diabetes. Appl. Physiol. Nutr. Metab. 2021, 46, 915–924. [Google Scholar] [CrossRef]
- Lesgards, J.-F. Benefits of Whey Proteins on Type 2 Diabetes Mellitus Parameters and Prevention of Cardiovascular Diseases. Nutrients 2023, 15, 1294. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; He, F.; Wu, C.; Li, P.; Li, N.; Deng, J.; Zhu, G.; Ren, W.; Peng, Y. Betaine in Inflammation: Mechanistic Aspects and Applications. Front. Immunol. 2018, 9, 1070. [Google Scholar] [CrossRef] [PubMed]
- Salzano, A.; Di Meo, M.C.; D’Onofrio, N.; Bifulco, G.; Cotticelli, A.; Licitra, F.; Iraci Fuintino, A.; Cascone, G.; Balestrieri, M.L.; Varricchio, E.; et al. Breed and Feeding System Impact the Bioactive Anti-Inflammatory Properties of Bovine Milk. Int. J. Mol. Sci. 2022, 23, 11088. [Google Scholar] [CrossRef] [PubMed]
- Berger, J.P.; Akiyama, T.E.; Meinke, P.T. PPARs: Therapeutic Targets for Metabolic Disease. Trends Pharmacol. Sci. 2005, 26, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Shen, W.J.; Bittner, S.; Kraemer, F.B.; Azhar, S. PPARs: Regulators of metabolism and as therapeutic targets in cardiovascular disease. Part II: PPAR-β/δ and PPAR-γ. Future Cardiol. 2017, 13, 279–296. [Google Scholar] [CrossRef]
- Sharma, S.; Sharma, D.; Dhobi, M.; Wang, D.; Tewari, D. An insight to treat cardiovascular diseases through phytochemicals targeting PPAR-α. Mol. Cell. Biochem. 2023; Epub ahead of print. [Google Scholar] [CrossRef]
- Werner, C.M.; Schirmer, S.H.; Gensch, C.; Pavlickova, V.; Pöss, J.; Wright, M.B.; Böhm, M.; Laufs, U. The dual PPARα/γ agonist aleglitazar increases the number and function of endothelial progenitor cells: Implications for vascular function and atherogenesis. Br. J. Pharmacol. 2014, 171, 2685–2703. [Google Scholar] [CrossRef]
- Lantier, L.; Williams, A.S.; Williams, I.M.; Yang, K.K.; Bracy, D.P.; Goelzer, M.; James, F.D.; Gius, D.; Wasserman, D.H. SIRT3 is crucial for maintaining skeletal muscle insulin action and protects against severe insulin resistance in high-fat-fed mice. Diabetes 2015, 64, 3081–3092. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Cao, M.; Xu, Y.; Li, Y. SIRT3 protects endothelial cells from high glucose-induced cytotoxicity. Int. J. Clin. Exp. Pathol. 2015, 8, 353–360. [Google Scholar]
- Gong, H.; Liu, J.; Xue, Z.; Wang, W.; Li, C.; Xu, F.; Du, Y.; Lyu, X. SIRT3 attenuates coronary atherosclerosis in diabetic patients by regulating endothelial cell function. J. Clin. Lab. Anal. 2022, 36, e24586. [Google Scholar] [CrossRef]
- Hua, Y.Y.; Zhang, Y.; Gong, W.W.; Ding, Y.; Shen, J.R.; Li, H.; Chen, Y.; Meng, G.L. Dihydromyricetin Improves Endothelial Dysfunction in Diabetic Mice via Oxidative Stress Inhibition in a SIRT3-Dependent Manner. Int. J. Mol. Sci. 2020, 21, 6699. [Google Scholar] [CrossRef]
- Wang, C.C.; Lee, A.S.; Liu, S.H.; Chang, K.C.; Shen, M.Y.; Chang, C.T. Spironolactone ameliorates endothelial dysfunction through inhibition of the AGE/RAGE axis in a chronic renal failure rat model. BMC Nephrol. 2019, 20, 351. [Google Scholar] [CrossRef] [PubMed]
- Bhatti, J.S.; Sehrawat, A.; Mishra, J.; Sidhu, I.S.; Navik, U.; Khullar, N.; Kumar, S.; Bhatti, G.K.; Reddy, P.H. Oxidative stress in the pathophysiology of type 2 diabetes and related complications: Current therapeutics strategies and future perspectives. Free Radic. Biol. Med. 2022, 184, 114–134. [Google Scholar] [CrossRef] [PubMed]
- Sifuentes-Franco, S.; Pacheco-Moisés, F.P.; Rodríguez-Carrizalez, A.D.; Miranda-Díaz, A.G. The role of oxidative stress, mitochondrial function, and autophagy in diabetic polyneuropathy. J. Diabetes Res. 2017, 2017, 1673081. [Google Scholar] [CrossRef]
- Serhiyenko, V.; Hotsko, M.; Serhiyenko, A.; Snitynska, O.; Serhiyenko, L.; Segin, V. The impact of alpha-lipoic acid on insulin resistance and inflammatory parameters in patients with type 2 diabetes mellitus and cardiac autonomic neuropathy. Am. J. Intern. Med. 2020, 8, 197–203. [Google Scholar] [CrossRef]


| Bioactive Compound | m/z | MS/MS Transition | Levels (mg/L) |
|---|---|---|---|
| l-Carnitine | 162.1 | 162.1 → 103 | 43.4 ± 1.6 |
| Acetyl-l-carnitine | 204.1 | 204.1 → 85 | 6.5 ± 3.1 |
| Propionyl- L-carnitine | 218.1 | 218.1 → 85 | 29.4 ± 2.2 |
| Glycine betaine | 118.1 | 118.1 → 59 | 13.2 ± 1.2 |
| δ-valerobetaine | 160.1 | 160.1 → 101 | 26.7 ± 2.3 |
| γ-butyrobetaine | 146.1 | 146.1 → 87 | 6.8 ± 0.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martino, E.; Luce, A.; Balestrieri, A.; Mele, L.; Anastasio, C.; D’Onofrio, N.; Balestrieri, M.L.; Campanile, G. Whey Improves In Vitro Endothelial Mitochondrial Function and Metabolic Redox Status in Diabetic State. Antioxidants 2023, 12, 1311. https://doi.org/10.3390/antiox12061311
Martino E, Luce A, Balestrieri A, Mele L, Anastasio C, D’Onofrio N, Balestrieri ML, Campanile G. Whey Improves In Vitro Endothelial Mitochondrial Function and Metabolic Redox Status in Diabetic State. Antioxidants. 2023; 12(6):1311. https://doi.org/10.3390/antiox12061311
Chicago/Turabian StyleMartino, Elisa, Amalia Luce, Anna Balestrieri, Luigi Mele, Camilla Anastasio, Nunzia D’Onofrio, Maria Luisa Balestrieri, and Giuseppe Campanile. 2023. "Whey Improves In Vitro Endothelial Mitochondrial Function and Metabolic Redox Status in Diabetic State" Antioxidants 12, no. 6: 1311. https://doi.org/10.3390/antiox12061311
APA StyleMartino, E., Luce, A., Balestrieri, A., Mele, L., Anastasio, C., D’Onofrio, N., Balestrieri, M. L., & Campanile, G. (2023). Whey Improves In Vitro Endothelial Mitochondrial Function and Metabolic Redox Status in Diabetic State. Antioxidants, 12(6), 1311. https://doi.org/10.3390/antiox12061311