Impact of Modern Oven Treatments on Lipid Oxidation and Vitamin E Content of Fillets from Sardine (Sardina pilchardus) at Different Reproductive Cycle Phases
Abstract
1. Introduction
2. Materials and Methods
2.1. Fish-Sampling Collection
2.2. Reagents and Chemicals
2.3. Biological Parameters, Gonadosomatic Index (GSI), and Mesenteric Fat
2.4. Thermal Treatments
2.5. Lipid Extraction
2.6. Vitamin E Analysis
2.7. Total Fatty Acids Profile
2.8. Primary and Secondary Products of Lipid Oxidation
2.9. Statistical Analysis
3. Results
3.1. Biological Parameters
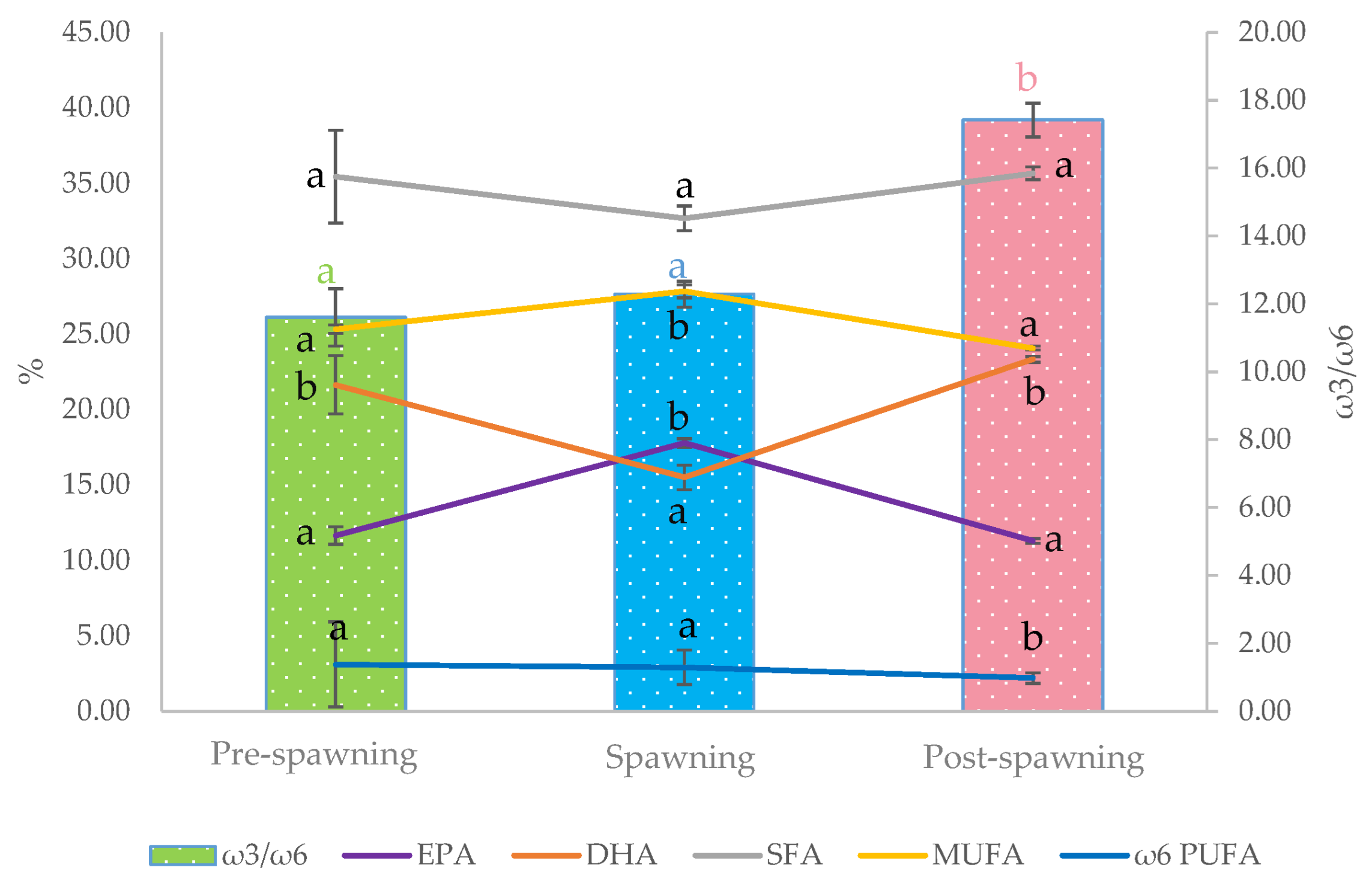
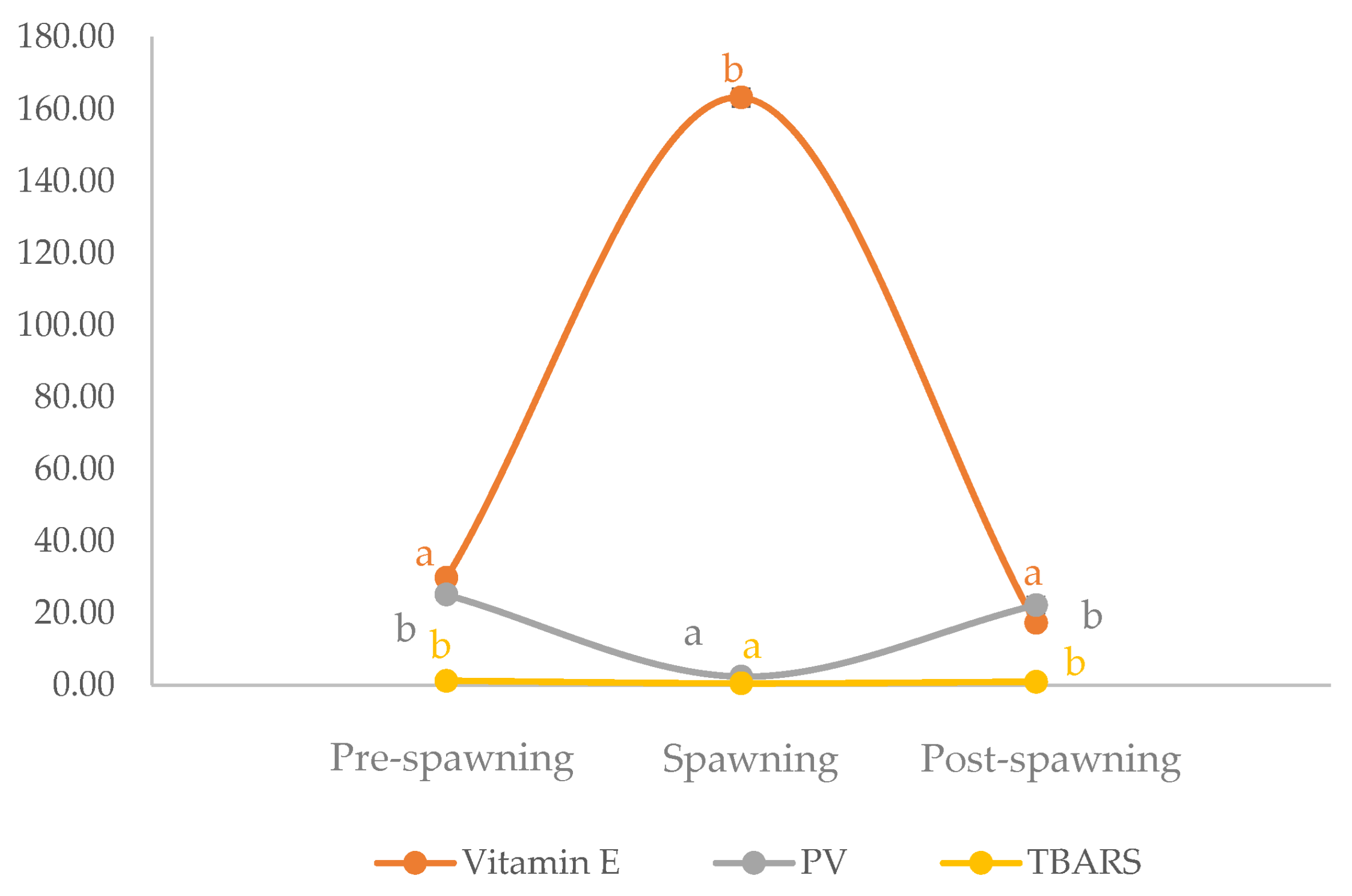
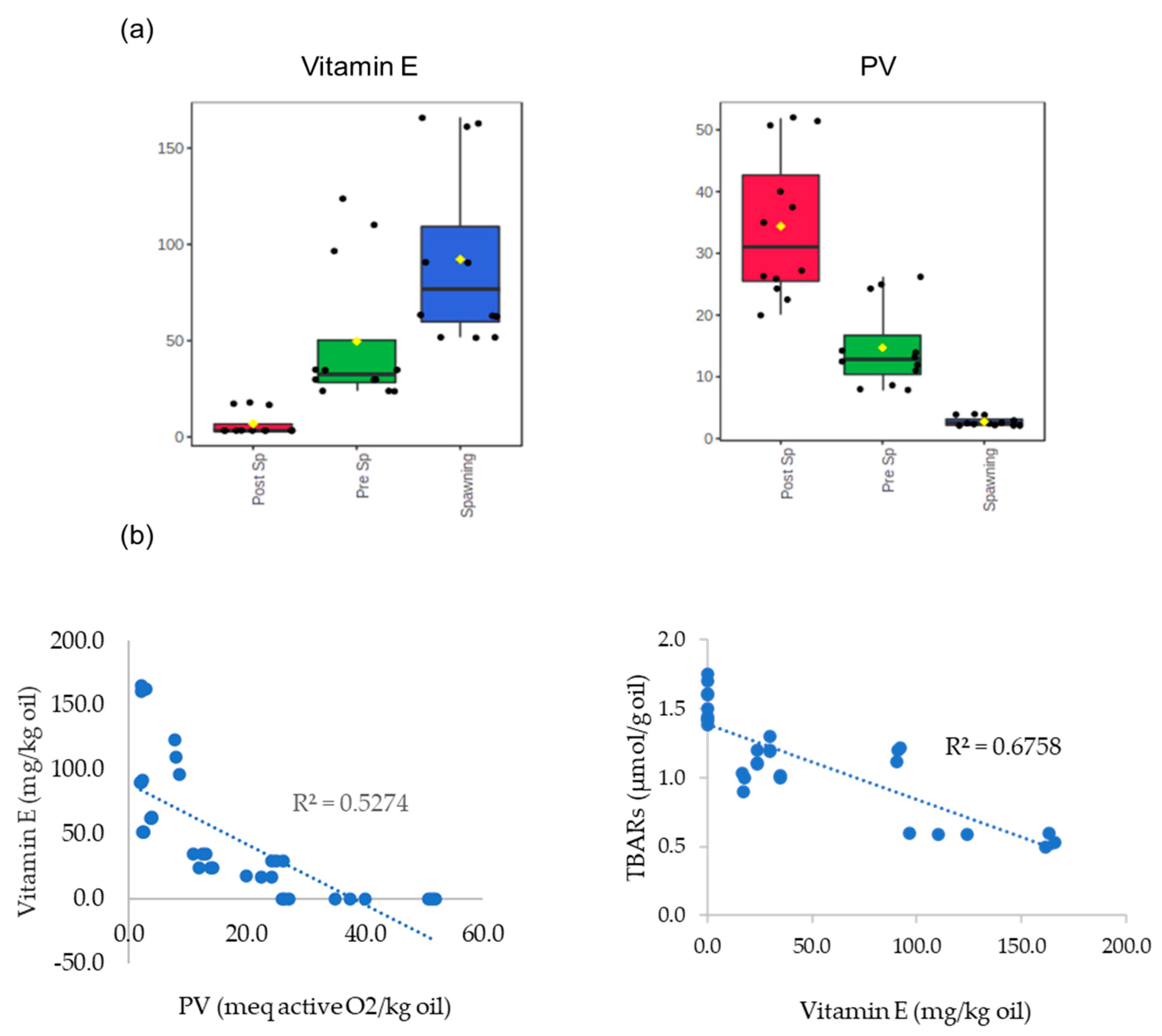
3.2. Assessment of the Fatty Acid Composition, Vitamin E Content, and Oxidative Status of Raw Fillets from Sardine at Different Phases of the Reproductive Cycle
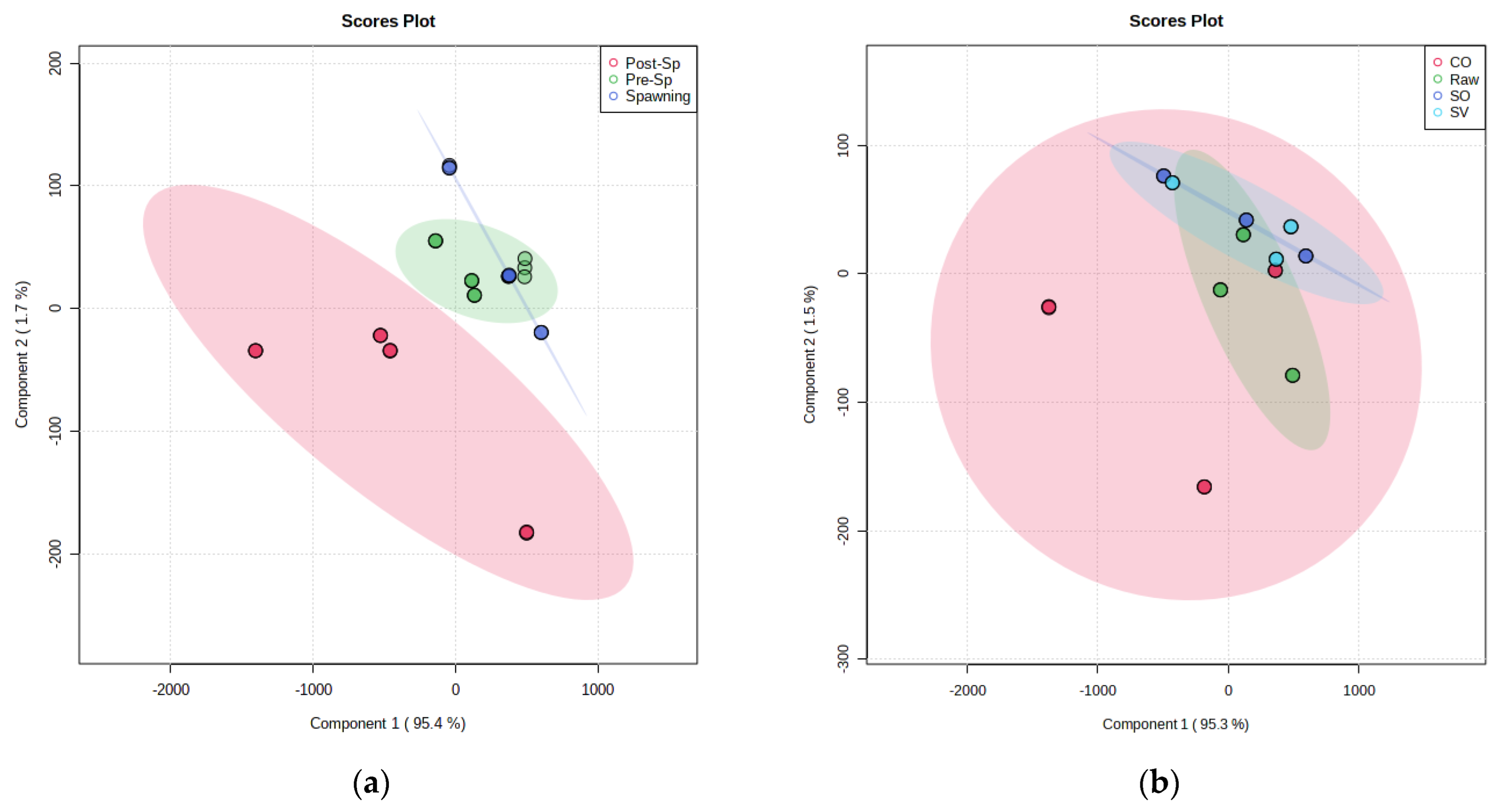
3.3. Influence of Thermal Treatments on Fatty Acid Composition, Vitamin E Content, and Oxidative Status of Fillets from Sardine at Different Phases of Reproductive Cycle
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Pre-Spawning | Spawning | Post-Spawning | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fatty Acids (%) | Raw | CO | SO | SV | Raw | CO | SO | SV | Raw | CO | SO | SV |
| C14:0 | 8.8 ± 1.3 | 7.8 ± 0.1 | 6.9 ± 0.1 | 8.2 ± 0.2 | 10.2 ± 0.3 | 10.2 ± 0.1 | 9.2 ± 0.0 | 10.3 ± 0.3 | 8.0 ± 0.3 | 7.0 ± 1.0 | 7.0 ± 0.5 | 7.0 ± 0.1 |
| C16:0 | 22.4 ± 1.7 | 22.1 ± 0.2 | 22.5 ± 0.1 | 21.3 ± 0.2 | 18.3 ± 0.5 | 18.2 ± 0.1 | 18.7 ± 0.1 | 18.6 ± 0.2 | 22.3 ± 0.2 | 22.9 ± 2.9 | 22.4 ± 1.1 | 21.6 ± 0.1 |
| C18:0 | 4.3 ± 0.1 | 4.0 ± 0.0 | 4.3 ± 0.1 | 4.2 ± 0.0 | 4.2 ± 0.0 | 4.3 ± 0.0 | 4.2 ± 0.0 | 4.2 ± 0.0 | 5.4 ± 0.1 | 4.8 ± 0.6 | 5.3 ± 0.1 | 5.3 ± 0.0 |
| ΣSFA | 35.4 ± 3.1 | 34.0 ± 0.4 | 33.7 ± 0.1 | 33.7 ± 0.4 | 32.7 ± 0.8 | 32.7 ± 0.2 | 32.1 ± 0.1 | 33.1 ± 0.5 | 35.7 ± 0.4 | 33.7 ± 4.4 | 34.7 ± 1.6 | 34.0 ± 0.1 |
| C16:1 Δ9 | 7.8 ± 0.1 | 6.7 ± 0.4 | 6.2 ± 0.1 | 7.2 ± 0.4 | 11.3 ± 0.5 | 11.8 ± 0.1 | 10.8 ± 0.2 | 11.8 ± 0.1 | 7.1 ± 0.1 | 6.0 ± 0.8 | 6.9 ± 0.4 | 6.5 ± 0.1 |
| C18:1 Δ9cis | 6.8 ± 0.1 | 8.8 ± 0.0 | 8.2 ± 0.1 | 7.8 ± 0.0 | 6.4 ± 0.1 | 7.1 ± 0.0 | 5.6 ± 0.0 | 6.5 ± 0.0 | 8.6 ± 0.1 | 7.6 ± 0.9 | 9.1 ± 0.1 | 7.9 ± 0.0 |
| C18:1 Δ11 | 3.6 ± 0.2 | 3.1 ± 0.0 | 2.9 ± 0.0 | 3.2 ± 0.0 | 5.4 ± 0.1 | 5.8 ± 0.0 | 5.7 ± 0.0 | 5.6 ± 0.0 | 3.0 ± 0.0 | 2.6 ± 0.3 | 3.0 ± 0.0 | 2.9 ± 0.0 |
| isomer C20:1 | 2.0 ± 0.1 | 2.6 ± 0.0 | 2.6 ± 0.0 | 3.0 ± 0.1 | 1.0 ± 0.1 | 1.3 ± 0.0 | 1.2 ± 0.0 | 0.9 ± 0.0 | 0.3 ± 0.0 | 0.3 ± 0.0 | 0.3 ± 0.0 | 0.4 ± 0.0 |
| C20:1 Δ11 | 1.9 ± 0.0 | 2.2 ± 0.0 | 2.0 ± 0.1 | 2.0 ± 0.0 | 2.3 ± 0.1 | 2.1 ± 0.0 | 2.2 ± 0.0 | 2.3 ± 0.0 | 3.9 ± 0.1 | 3.6 ± 0.5 | 3.4 ± 0.0 | 3.8 ± 0.0 |
| C22:1 Δ11 | 2.0 ± 0.2 | 2.4 ± 0.0 | 2.6 ± 0.0 | 3.1 ±0.1 | 0.5 ± 0.1 | 0.7 ± 0.0 | 0.8 ± 0.0 | 0.1 ± 0.1 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.1 |
| C22:1 Δ9 | 1.3 ± 0.6 | 1.0 ± 0.0 | 1.0 ± 0.0 | 1.0 ± 0.1 | 0.9 ± 0.0 | 0.9 ± 0.0 | 0.9 ± 0.0 | 1.0 ± 0.0 | 0.9 ± 0.1 | 0.9 ± 0.0 | 0.9 ± 0.0 | 1.0 ± 0.0 |
| ΣMUFA | 25.3 ± 0.3 | 26.7 ± 0.4 | 25.5 ± 02 | 27.4 ± 0.3 | 27.8 ± 0.4 | 29.7 ± 0.1 | 27.3 ± 0.2 | 28.2 ± 0.1 | 24.1 ± 0.1 | 27.8 ± 9.0 | 23.9 ± 0.4 | 22.4 ± 0.1 |
| C18:2 Δ9,12ω6 | 1.8 ± 0.1 | 1.9 ± 0.0 | 2.0 ± 0.0 | 2.0 ± 0.0 | 1.4 ± 0.0 | 1.2 ± 0.1 | 1.3 ± 0.1 | 1.2 ± 0.1 | 1.7 ± 0.1 | 1.7 ± 0.2 | 1.6 ± 0.0 | 1.7 ± 0.0 |
| C18:3 Δ9,12,15ω3 + C18:4 Δ6,9,11,15 ω3 | 1.0 ± 0.4 | 1.5 ± 0.2 | 1.0 ± 0.4 | 1.3 ± 0.2 | 0.8 ± 0.2 | 0.9 ± 0.2 | 1.2 ± 0.0 | 1.2 ± 0.0 | 2.0 ± 0.1 | 1.6 ± 0.2 | 1.8 ± 0.0 | 1.9 ± 0.0 |
| C20:2 Δ14 ω6 | 0.4 ± 0.0 | 0.4 ± 0.1 | 0.4 ± 0.0 | 0.4 ± 0.0 | 0.3 ± 0.0 | 0.3 ± 0.0 | 0.3 ± 0.0 | 0.3 ± 0.0 | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.2 ± 0.0 |
| C20:4 Δ5,8,11,14ω6 (ARA) | 1.3 ± 0.1 | 1.2 ± 0.0 | 1.2 ± 0.0 | 1.2 ± 0.0 | 1.6 ± 0.0 | 1.5 ± 0.0 | 1.5 ± 0.0 | 1.5 ± 0.0 | 0.5 ± 0.0 | 0.5 ± 0.0 | 0.5 ± 0.0 | 0.6 ± 0.0 |
| C20:5 Δ5,8,11,14,17ω3 (EPA) | 11.6 ± 0.6 | 10.7 ± 0.1 | 10.1 ± 0.1 | 11.4 ± 0.2 | 17.8 ± 0.3 | 18.2 ± 0.2 | 16.7 ± 0.1 | 18.0 ± 0.2 | 11.3 ± 0.2 | 9.9 ± 1.2 | 11.2 ± 0.3 | 11.1 ± 0.0 |
| C22:5 Δ7,10,13,16,19ω3 | 1.5 ± 0.1 | 1.5 ± 0.0 | 1.4 ± 0.0 | 1.6 ± 0.0 | 2.2 ± 0.1 | 2.3 ± 0.0 | 1.9 ± 0.0 | 2.2 ± 0.0 | 1.3 ± 0.0 | 1.1 ± 0.1 | 1.3 ± 0.1 | 1.3 ± 0.0 |
| C22:6 Δ4,7,10,13,16,19ω3 (DHA) | 21.6 ± 1.9 | 22.2 ± 0.5 | 24.8 ± 0.1 | 21.1 ± 0.5 | 15.5 ± 0.8 | 13.3 ± 0.2 | 17.8 ± 0.1 | 14.2 ± 0.2 | 23.3 ± 0.2 | 23.5 ± 3.0 | 24.7 ± 1.6 | 26.7 ± 0.1 |
| ΣPUFA | 39.3 ± 2.8 | 39.3 ± 0.5 | 40.8 ± 0.3 | 38.9 ± 0.5 | 39.5 ± 1.2 | 37.6 ± 0.3 | 40.6 ± 0.1 | 38.6 ± 0.5 | 40.3 ± 0.3 | 38.5 ± 4.8 | 41.4 ± 2.1 | 43.6 ± 0.2 |
| Σω3 PUFA | 35.8 ± 2.8 | 35.8 ± 0.6 | 37.2 ± 0.3 | 35.4 ± 0.5 | 36.2 ± 1.1 | 34.7 ± 0.2 | 37.5 ± 0.1 | 35.6 ± 0.4 | 37.9 ± 0.4 | 36.1 ± 4.5 | 39.1 ± 2.1 | 41.1 ± 2.3 |
| Σω6 PUFA | 3.1 ± 0.0 | 3.1 ± 0.0 | 3.2 ± 0.0 | 3.1 ± 0.0 | 2.9 ± 0.1 | 2.6 ± 0.1 | 2.8 ± 0.1 | 2.7 ± 0.1 | 2.2 ± 0.0 | 2.2 ± 0.3 | 2.1 ± 0.0 | 0.1 ± 0.0 |
References
- Zorica, B.; Čikeš Keč, V.; Vidjak, O.; Kraljević, V.; Brzulja, G. Seasonal Pattern of Population Dynamics, Spawning Activities, and Diet Composition of Sardine (Sardina pilchardus Walbaum) in the Eastern Adriatic Sea. Turk. J. Zool. 2017, 41, 892–900. [Google Scholar] [CrossRef]
- Servou, E.; Schismenou, E.; Somarakis, S. Quantitative Analysis of Ovarian Dynamics of European Sardine Sardina pilchardus (Walbaum, 1792) during Its Spawning Period. Fishes 2023, 8, 226. [Google Scholar] [CrossRef]
- Zorica, B.; Anđelić, I.; Keč, V.Č. Sardine (Sardina pilchardus) Spawning in the Light of Fat Content Analysis. Sci. Mar. 2019, 83, 207–213. [Google Scholar] [CrossRef]
- Cury, P.; Bakun, A.; Crawford, R.J.M.; Jarre, A.; Quiñones, R.A.; Shannon, L.J.; Verheye, H.M. Small Pelagics in Upwelling Systems: Patterns of Interaction and Structural Changes in “Wasp-Waist” Ecosystems. ICES J. Mar. Sci. 2000, 57, 603–618. [Google Scholar] [CrossRef]
- Palomera, I.; Olivar, M.P.; Salat, J.; Sabatés, A.; Coll, M.; García, A.; Morales-Nin, B. Small Pelagic Fish in the NW Mediterranean Sea: An Ecological Review. Prog. Oceanogr. 2007, 74, 377–396. [Google Scholar] [CrossRef]
- Massing, J.C.; Schukat, A.; Auel, H.; Auch, D.; Kittu, L.; Pinedo Arteaga, E.L.; Correa Acosta, J.; Hagen, W. Toward a Solution of the “Peruvian Puzzle”: Pelagic Food-Web Structure and Trophic Interactions in the Northern Humboldt Current Upwelling System Off Peru. Front. Mar. Sci. 2022, 8, 2062. [Google Scholar] [CrossRef]
- Soldo, B.; Šimat, V.; Vlahović, J.; Skroza, D.; Ljubenkov, I.; Generalić Mekinić, I. High Quality Oil Extracted from Sardine By-Products as an Alternative to Whole Sardines: Production and Refining. Eur. J. Lipid Sci. Technol. 2019, 121, 1800513. [Google Scholar] [CrossRef]
- Research and Markets Global Sardine Market (2021 to 2026)—Industry Trends, Share, Size, Growth, Opportunity and Forecasts. Available online: https://www.globenewswire.com/en/news-release/2022/01/27/2374217/28124/en/Global-Sardine-Market-2021-to-2026-Industry-Trends-Share-Size-Growth-Opportunity-and-Forecasts.html (accessed on 9 January 2023).
- Bastías, J.M.; Balladares, P.; Acuña, S.; Quevedo, R.; Muñoz, O. Determining the Effect of Different Cooking Methods on the Nutritional Composition of Salmon (Salmo salar) and Chilean Jack Mackerel (Trachurus murphyi) Fillets. PLoS ONE 2017, 12, e0180993. [Google Scholar] [CrossRef] [PubMed]
- Pauwels, E.K.J. The Protective Effect of the Mediterranean Diet: Focus on Cancer and Cardiovascular Risk. Med. Princ. Pract. 2011, 20, 103–111. [Google Scholar] [CrossRef]
- Bosetti, C.; Pelucchi, C.; la Vecchia, C. Diet and Cancer in Mediterranean Countries: Carbohydrates and Fats. Public Health Nutr. 2009, 12, 1595–1600. [Google Scholar] [CrossRef] [PubMed]
- Šimat, V.; Hamed, I.; Petrǐcevíc, S.; Bogdanovíc, T. Seasonal Changes in Free Amino Acid and Fatty Acid Compositions of Sardines, Sardina pilchardus (Walbaum, 1792): Implications for Nutrition. Foods 2020, 9, 867. [Google Scholar] [CrossRef]
- Jankajova, M.; Pella, D.; Zenuch, P.; Zenuchova, Z.; Toth, S.; Kalanin, P.; Fedacko, J. The Role of W3 PUFFA in Patients with Metabolic Syndrome and Endothelial Dysfunction. Clin. Med. 2023, 2023051570. [Google Scholar] [CrossRef]
- Wei, L.; Wu, Z.; Chen, Y.Q. Multi-Targeted Therapy of Cancer by Omega-3 Fatty Acids-an Update. Cancer Lett. 2022, 526, 193–204. [Google Scholar] [CrossRef]
- Saini, R.K.; Keum, Y.S. Omega-3 and Omega-6 Polyunsaturated Fatty Acids: Dietary Sources, Metabolism, and Significance—A Review. Life Sci. 2018, 203, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Tacon, A.G.J.; Metian, M. Fish Matters: Importance of Aquatic Foods in Human Nutrition and Global Food Supply. Rev. Fish. Sci. 2013, 21, 22–38. [Google Scholar] [CrossRef]
- Bouderoua, K.; Mourot, J.; Benmehdi-Tabet-Aoull, F.; Selselet-Attou, G. The Effects of Season and Site of Catch on Morphometric Characteristics, Mineral Content, and Fatty Acids of Sardines (Sardina pilchardus) Caught on the Algerian Coast. J. Aquat. Food Prod. Technol. 2011, 20, 412–420. [Google Scholar] [CrossRef]
- Moyad, M.A. An Introduction to Dietary/Supplemental Omega-3 Fatty Acids for General Health and Prevention: Part II. Urol. Oncol. 2005, 23, 36–48. [Google Scholar] [CrossRef]
- Alsumari, S.R.; ALNouri, D.M.; El-Sayed, M.M.A.; El-Din, M.F.S.; Arzoo, S. The Sociodemographic Characteristics and Dietary and Blood Plasma Fatty Acid Profiles of Elderly Saudi Women with Alzheimer Disease. Lipids Health Dis. 2019, 18, 77. [Google Scholar] [CrossRef] [PubMed]
- Das, U.N. Pro- and Anti-Inflammatory Bioactive Lipids Imbalance Contributes to the Pathobiology of Autoimmune Diseases. Eur. J. Clin. Nutr. 2022, 77, 637–651. [Google Scholar] [CrossRef]
- Marchand, N.E.; Choi, M.Y.; Oakes, E.G.; Cook, N.R.; Stevens, E.; Gomelskaya, N.; Kotler, G.; Manson, J.A.E.; Lasky-Su, J.; Mora, S.; et al. Over-the-Counter Fish Oil Supplementation and pro-Resolving and pro-Inflammatory Lipid Mediators in Rheumatoid Arthritis. Prostaglandins Leukot Essent Fat. Acids 2023, 190, 102542. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Hu, M.; Huang, J.; Yu, S.; Li, X.; Li, Y.; Mao, L. Anti-Obesity Effects of DHA and EPA in High Fat-Induced Insulin Resistant Mice. Food Funct. 2021, 12, 1614–1625. [Google Scholar] [CrossRef]
- Díaz-Rizzolo, D.A.; Serra, A.; Colungo, C.; Sala-Vila, A.; Sisó-Almirall, A.; Gomis, R. Type 2 Diabetes Preventive Effects with a 12-Months Sardine-Enriched Diet in Elderly Population with Prediabetes: An Interventional, Randomized and Controlled Trial. Clin. Nutr. 2021, 40, 2587–2598. [Google Scholar] [CrossRef] [PubMed]
- Suárez-Jiménez, G.M.; López-Saiz, C.M.; Ramírez-Guerra, H.E.; Ezquerra-Brauer, J.M.; Ruiz-Cruz, S.; Torres-Arreola, W. Role of Endogenous and Exogenous Tocopherols in the Lipid Stability of Marine Oil Systems: A Review. Int. J. Mol. Sci. 2016, 17, 1968. [Google Scholar] [CrossRef] [PubMed]
- Çankiriligil, E.C.; Berik, N. Effects of Deep-Frying to Sardine Croquettes’ Chemical Composition. Ege J. Fish. Aquat. Sci. 2017, 34, 293–302. [Google Scholar] [CrossRef]
- Choo, P.Y.; Azlan, A.; Khoo, H.E. Cooking Methods Affect Total Fatty Acid Composition and Retention of DHA and EPA in Selected Fish Fillets. Sci. Asia 2018, 44, 92–101. [Google Scholar] [CrossRef]
- Uran, H.; Gokoglu, N. Effects of Cooking Methods and Temperatures on Nutritional and Quality Characteristics of Anchovy (Engraulis encrasicholus). J. Food Sci. Technol. 2014, 51, 722–728. [Google Scholar] [CrossRef] [PubMed]
- Abraha, B.; Admassu, H.; Mahmud, A.; Tsighe, N.; Shui, X.W.; Fang, Y. Effect of Processing Methods on Nutritional and Physico-Chemical Composition of Fish: A Review. MOJ Food Process. Technol. 2018, 6, 376–382. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, D.; Liu, D.; Fang, Z.; Chen, J.; Hu, Y.; Ye, X. Effect of Cooking Styles on the Lipid Oxidation and Fatty Acid Composition of Grass Crap (Ctenopharynyodon idellus) Fillet. J. Food Biochem. 2013, 37, 212–219. [Google Scholar] [CrossRef]
- Pacetti, D.; Lucci, P.; Mozzon, M.; Gagliardi, R.; Fiorini, D.; Frega, N.G. Influence of Deep-Fat Frying Process on Phospholipid Molecular Species Composition of Sardina Pilchardus Fillet. Food Control 2015, 48, 155–162. [Google Scholar] [CrossRef]
- Orlando, P.; Giardinieri, A.; Lucci, P.; Nartea, A.; Balzano, M.; Pacetti, D.; Frega, N.G.; Silvestri, S.; Tiano, L. Impact of Traditional and Mild Oven Cooking Treatments on Antioxidant Compounds Levels and Oxidative Status of Atlantic Salmon (Salmo salar) Fillets. LWT 2020, 134, 110011. [Google Scholar] [CrossRef]
- Caballero-Huertas, M.; Vargas-Yánez, M.; Frigola-Tepe, X.; Viñas, J.; Muñoz, M. Unravelling the Drivers of Variability in Body Condition and Reproduction of the European Sardine along the Atlantic-Mediterranean Transition. Mar. Environ. Res. 2022, 179, 105697. [Google Scholar] [CrossRef]
- Pacetti, D.; Balzano, M.; Colella, S.; Santojanni, A.; Frega, N.G. Effect of Spawning on Furan Fatty Acid Profile of Edible Muscle and Organ Tissues from Sardine (Sardina pilchardus) and Anchovy (Engraulis encrasicolus). J. Agric. Food Chem. 2013, 61, 3969–3977. [Google Scholar] [CrossRef]
- Šimat, V.; Vlahović, J.; Soldo, B.; Skroza, D.; Ljubenkov, I.; Generalić Mekinić, I. Production and refinement of omega-3 rich oils from processing by-products of farmed fish species. Foods 2019, 8, 125. [Google Scholar] [CrossRef] [PubMed]
- Frapiccini, E.; Cocci, P.; Annibaldi, A.; Panfili, M.; Santojanni, A.; Grilli, F.; Marini, M.; Palermo, F.A. Assessment of Seasonal Relationship between Polycyclic Aromatic Hydrocarbon Accumulation and Expression Patterns of Oxidative Stress-Related Genes in Muscle Tissues of Red Mullet (M. barbatus) from the Northern Adriatic Sea. Environ. Toxicol. Pharmacol. 2021, 88, 103752. [Google Scholar] [CrossRef] [PubMed]
- Chainy, G.B.N.; Paital, B.; Dandapat, J. An Overview of Seasonal Changes in Oxidative Stress and Antioxidant Defence Parameters in Some Invertebrate and Vertebrate Species. Scientifica 2016, 2016, 6126570. [Google Scholar] [CrossRef] [PubMed]
- Birnie-Gauvin, K.; Costantini, D.; Cooke, S.J.; Willmore, W.G. A Comparative and Evolutionary Approach to Oxidative Stress in Fish: A Review. Fish Fish. 2017, 18, 928–942. [Google Scholar] [CrossRef]
- Alonso-Alvarez, C.; Bertrand, S.; Devevey, G.; Gaillard, M.; Prost, J.; Faivre, B.; Sorci, G. An Experimental Test of the Dose-Dependent Effect of Carotenoids and Immune Activation on Sexual Signals and Antioxidant Activity. Am. Soc. Nat. 2004, 164, 651–659. [Google Scholar] [CrossRef]
- Faheem, M.; Bhandari, R.K. Detrimental Effects of Bisphenol Compounds on Physiology and Reproduction in Fish: A Literature Review. Environ. Toxicol. Pharmacol. 2021, 81, 103497. [Google Scholar] [CrossRef]
- Izquierdo, M.S.; Scolamacchia, M.; Betancor, M.; Roo, J.; Caballero, M.J.; Terova, G.; Witten, P.E. Effects of Dietary DHA and α-Tocopherol on Bone Development, Early Mineralisation and Oxidative Stress in Sparus aurata (Linnaeus, 1758) Larvae. Br. J. Nutr. 2013, 109, 1796–1805. [Google Scholar] [CrossRef]
- Albo-Puigserver, M.; Sánchez, S.; Coll, M.; Bernal, M.; Sáez-Liante, R.; Navarro, J.; Palomera, I. Year-Round Energy Dynamics of Sardine and Anchovy in the North-Western Mediterranean Sea. Mar. Environ. Res. 2020, 159, 105021. [Google Scholar] [CrossRef]
- Fortibuoni, T.; Giovanardi, O.; Pranovi, F.; Raicevich, S.; Solidoro, C.; Libralato, S. Analysis of Long-Term Changes in a Mediterranean Marine Ecosystem Based on Fishery Landings. Front. Mar. Sci. 2017, 4, 33. [Google Scholar] [CrossRef]
- ICES (International Council for the Exploration of the Sea). Report of the Workshop on Small Pelagics (Sardina pilchardus, Engraulis encrasicolus) Maturity Stages (WKSPMAT), 10–14 November 2008, Mazara del Vallo, Italy; International Council for the Exploration of the Sea: Copenhagen, Denmark, 2008; 82p, ICES CM 2008/ACOM:40. [Google Scholar] [CrossRef]
- Mustać, B.; Sinovčić, G. Reproductive Cycle of Gilt Sardine, Sardinella Aurita, Valenciennes 1847, in the Eastern Middle Adriatic Sea. J. Appl. Ichthyol. 2012, 28, 46–50. [Google Scholar] [CrossRef]
- Ganias, K.; Somarakis, S.; Machias, A.; Theodorou, A. Pattern of Oocyte Development and Batch Fecundity in the Mediterranean Sardine. Fish Res. 2004, 67, 13–23. [Google Scholar] [CrossRef]
- Lloret, J.; Shulman, G.; Love, R.M. Condition and Health Indicators of Exploited Marine Fishes; John Wiley & Sons: Hoboken, NJ, USA, 2014; pp. 1–247. [Google Scholar] [CrossRef]
- Follesa, M.C.; Carbonara, P. Food and Agriculture Organization of the United Nations. Atlas of the Maturity Stages of Mediterranean Fishery Resources; Studies and Reviews n. 99; Food and Agriculture Organization: Rome, Italy, 2019; 268p, ISSN 1020-9549. [Google Scholar]
- Morello, E.B.; Arneri, E. Anchovy and Sardine in the Adriatic Sea-an Ecological Review. Oceanogr. Mar. Biol. 2009, 47, 209–256. [Google Scholar] [CrossRef]
- Holden, M.J.; Raitt, D.F.S. Manual De Ciencia Pesquera Parte 2—Métodos para Investigar los Recursos y su Aplicación; FAO Documento Técnico de Pesca. No.115; Food and Agriculture Organization: Rome, Italy, 1974; 255p. [Google Scholar]
- Giráldez, A.; Abad, R. Aspects on the Reproductive Biology of the Western Mediterranean Anchovy from the Coasts of Málaga (Alboran Sea). Sci. Mar. 1995, 59, 15–23. [Google Scholar]
- Sinovcic, G. Anchovy, Engraulis encrasicolus (LINNAEUS,1758): Biology, Population Dynamics and Fisheries Case Study. Acta Adriat. 2000, 41, 3–53. [Google Scholar]
- Sinovčić, G.; Zorica, B. Reproductive Cycle and Minimal Length at Sexual Maturity of Engraulis encrasicolus (L.) in the Zrmanja River Estuary (Adriatic Sea, Croatia). Estuar. Coast. Shelf Sci. 2006, 69, 439–448. [Google Scholar] [CrossRef]
- Mustać, B.; Sinovčić, G. Comparison of Mesenteric and Tissue Fat Content in Relation to Sexual Cycle of the Sardine, Sardina pilchardus (Walb., 1792), in the Eastern Middle Adriatic Fishery Grounds (Croatia). J. Appl. Ichthyol. 2009, 25, 595–599. [Google Scholar] [CrossRef]
- Čikeš Keč, V.; Zorica, B. Mesenteric Fat and Condition of Chub Mackerel, Scomber Colias in the Adriatic Sea. Croat. J. Fish. Ribar. 2012, 70, 19–30. [Google Scholar]
- Van der Lingen, C.D.; Hutchings, L. Estimating the Lipid Content of Pelagic Fish in the Southern Benguela by Visual Assessment of Their Mesenteric Fat. Afr. J. Mar. Sci. 2005, 27, 45–53. [Google Scholar] [CrossRef]
- Nartea, A.; Fanesi, B.; Falcone, P.M.; Pacetti, D.; Frega, N.G.; Lucci, P. Impact of Mild Oven Cooking Treatments on Carotenoids and Tocopherols of Cheddar and Depurple Cauliflower (Brassica oleracea L. Var. Botrytis). Antioxidants 2021, 10, 196. [Google Scholar] [CrossRef]
- Crowe, T.D.; White, P.J. Adaptation of the AOCS Official Method for Measuring Hydroperoxides from Small-Scale Oil Samples. JAOCS J. Am. Oil Chem. Soc. 2001, 78, 1267–1269. [Google Scholar] [CrossRef]
- Conte, L.; Milani, A.; Calligaris, S.; Rovellini, P.; Lucci, P.; Nicoli, M.C. Temperature Dependence of Oxidation Kinetics of Extra Virgin Olive Oil (EVOO) and Shelf-Life Prediction. Foods 2020, 9, 295. [Google Scholar] [CrossRef] [PubMed]
- Massimo, M.; Frega, N.G. Il Controllo Analitico Degli Oli e Grassi Alimentari; Mattioli 1885: London, UK, 2013; ISBN 9788862612845. [Google Scholar]
- Aakre, I.; Bøkevoll, A.; Chaira, J.; Bouthir, F.Z.; Frantzen, S.; Kausland, A.; Kjellevold, M. Variation in Nutrient Composition of Seafood from North West Africa: Implications for Food and Nutrition Security. Foods 2020, 9, 1516. [Google Scholar] [CrossRef]
- Mourente, G.; Bell, J.G.; Tocher, D.R. Does Dietary Tocopherol Level Affect Fatty Acid Metabolism in Fish? Fish Physiol. Biochem. 2007, 33, 269–280. [Google Scholar] [CrossRef]
- Gladyshev, M.I.; Sushchik, N.N.; Gubanenko, G.A.; Demirchieva, S.M.; Kalachova, G.S. Effect of Boiling and Frying on the Content of Essential Polyunsaturated Fatty Acids in Muscle Tissue of Four Fish Species. Food Chem. 2007, 101, 1694–1700. [Google Scholar] [CrossRef]
- Ahongo, Y.D.; Kerneis, T.; Goardon, L.; Labbé, L.; Bugeon, J.; Rescan, P.Y.; Lefèvre, F. Flesh Quality Recovery in Female Rainbow Trout (Oncorhynchus mykiss) after Spawning. Aquaculture 2021, 536, 736290. [Google Scholar] [CrossRef]
- Lee, S.; Choi, Y.; Jeong, H.S.; Lee, J.; Sung, J. Effect of Different Cooking Methods on the Content of Vitamins and True Retention in Selected Vegetables. Food Sci. Biotechnol. 2017, 27, 333–342. [Google Scholar] [CrossRef]
- Zotos, A.; Kotaras, A.; Mikras, E. Effect of Baking of Sardine (Sardina pilchardus) and Frying of Anchovy (Engraulis encrasicholus) in Olive and Sunflower Oil on Their Quality. Food Sci. Technol. Int. 2013, 19, 11–23. [Google Scholar] [CrossRef]
- García-Arias, M.T.; Álvarez Pontes, E.; García-Linares, M.C.; García-Fernández, M.C.; Sánchez-Muniz, F.J. Cooking–Freezing–Reheating (CFR) of Sardine (Sardina pilchardus) Fillets. Effect of Different Cooking and Reheating Procedures on the Proximate and Fatty Acid Compositions. Food Chem. 2003, 83, 349–356. [Google Scholar] [CrossRef]
- Nartea, A.; Bartolucci, E.; Lucci, P.; Frega, N.G.; Pacetti, D. Application of the Gas Chromatography Technique in the Characterization and Authentication of Food Products. In Chromatographic and Related Separation Techniques in Food Integrity and Authenticity; World Scientific: Singapore, 2021; pp. 43–69. [Google Scholar]
- Nartea, A.; Fanesi, B.; Giardinieri, A.; Campmajó, G.; Lucci, P.; Saurina, J.; Pacetti, D.; Fiorini, D.; Frega, N.G.; Núñez, O. Glucosinolates and Polyphenols of Colored Cauliflower as Chemical Discriminants Based on Cooking Procedures. Foods 2022, 11, 3041. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, A.F.M.; Izquierdo, M. The Importance of Vitamin E for Farmed Fish—A Review. Rev. Aquac. 2022, 14, 688–703. [Google Scholar] [CrossRef]
- Lenti, L.; Rigano, D.; Woo, S.L.; Nartea, A.; Pacetti, D.; Maggi, F.; Fiorini, D. A Rapid Procedure for the Simultaneous Determination of Eugenol, Linalool and Fatty Acid Composition in Basil Leaves. Foods 2022, 11, 3315. [Google Scholar] [CrossRef] [PubMed]
- Ratti, S.; Zarantoniello, M.; Chemello, G.; Giammarino, M.; Palermo, F.A.; Cocci, P.; Mosconi, G.; Tignani, M.V.; Pascon, G.; Cardinaletti, G.; et al. Spirulina-Enriched Substrate to Rear Black Soldier Fly (Hermetia illucens) Prepupae as Alternative Aquafeed Ingredient for Rainbow Trout (Oncorhynchus mykiss) Diets: Possible Effects on Zootechnical Performances, Gut and Liver Health Status, and Fillet Quality. Animals 2023, 13, 173. [Google Scholar] [CrossRef]

| Pre-Spawning | Spawning | Post-Spawning | ||||
|---|---|---|---|---|---|---|
| Individuals | Frequency of Mesenteric Fat (%) | GSI | Frequency of Mesenteric Fat (%) | GSI | Frequency of Mesenteric Fat (%) | GSI |
| Females | 100 (n = 53) | 0.851 | 6 (n = 63) | 7.061 | 61 (n = 46) | 0.582 |
| Males | 100 (n = 45) | 0.853 | 11 (n = 37) | 3.927 | 63 (n = 41) | 0.378 |
| Total | 100 (n = 98) | 0.852 | 8 (n = 100) | 5.905 | 62 (n = 87) | 0.486 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nartea, A.; Ismaiel, L.; Frapiccini, E.; Falcone, P.M.; Pacetti, D.; Frega, N.G.; Lucci, P.; Colella, S. Impact of Modern Oven Treatments on Lipid Oxidation and Vitamin E Content of Fillets from Sardine (Sardina pilchardus) at Different Reproductive Cycle Phases. Antioxidants 2023, 12, 1312. https://doi.org/10.3390/antiox12061312
Nartea A, Ismaiel L, Frapiccini E, Falcone PM, Pacetti D, Frega NG, Lucci P, Colella S. Impact of Modern Oven Treatments on Lipid Oxidation and Vitamin E Content of Fillets from Sardine (Sardina pilchardus) at Different Reproductive Cycle Phases. Antioxidants. 2023; 12(6):1312. https://doi.org/10.3390/antiox12061312
Chicago/Turabian StyleNartea, Ancuta, Lama Ismaiel, Emanuela Frapiccini, Pasquale Massimiliano Falcone, Deborah Pacetti, Natale Giuseppe Frega, Paolo Lucci, and Sabrina Colella. 2023. "Impact of Modern Oven Treatments on Lipid Oxidation and Vitamin E Content of Fillets from Sardine (Sardina pilchardus) at Different Reproductive Cycle Phases" Antioxidants 12, no. 6: 1312. https://doi.org/10.3390/antiox12061312
APA StyleNartea, A., Ismaiel, L., Frapiccini, E., Falcone, P. M., Pacetti, D., Frega, N. G., Lucci, P., & Colella, S. (2023). Impact of Modern Oven Treatments on Lipid Oxidation and Vitamin E Content of Fillets from Sardine (Sardina pilchardus) at Different Reproductive Cycle Phases. Antioxidants, 12(6), 1312. https://doi.org/10.3390/antiox12061312










